A Temperature-Controlled Fluorescence Fingerprint for Identifying Pseudo-nitzschia hasleana in Harmful Algal Blooms
Abstract
1. Introduction
2. Materials and Methods
3. Results
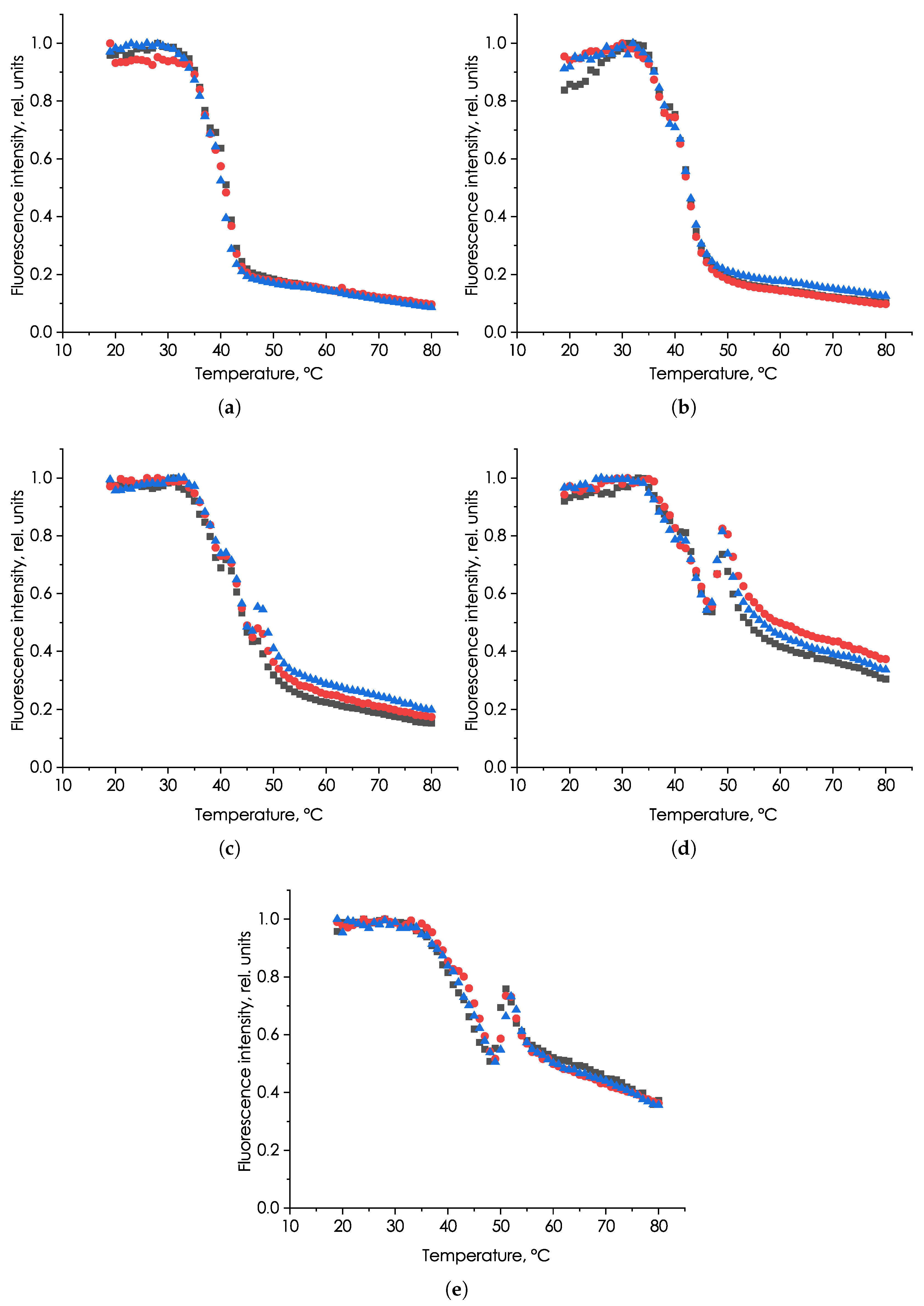
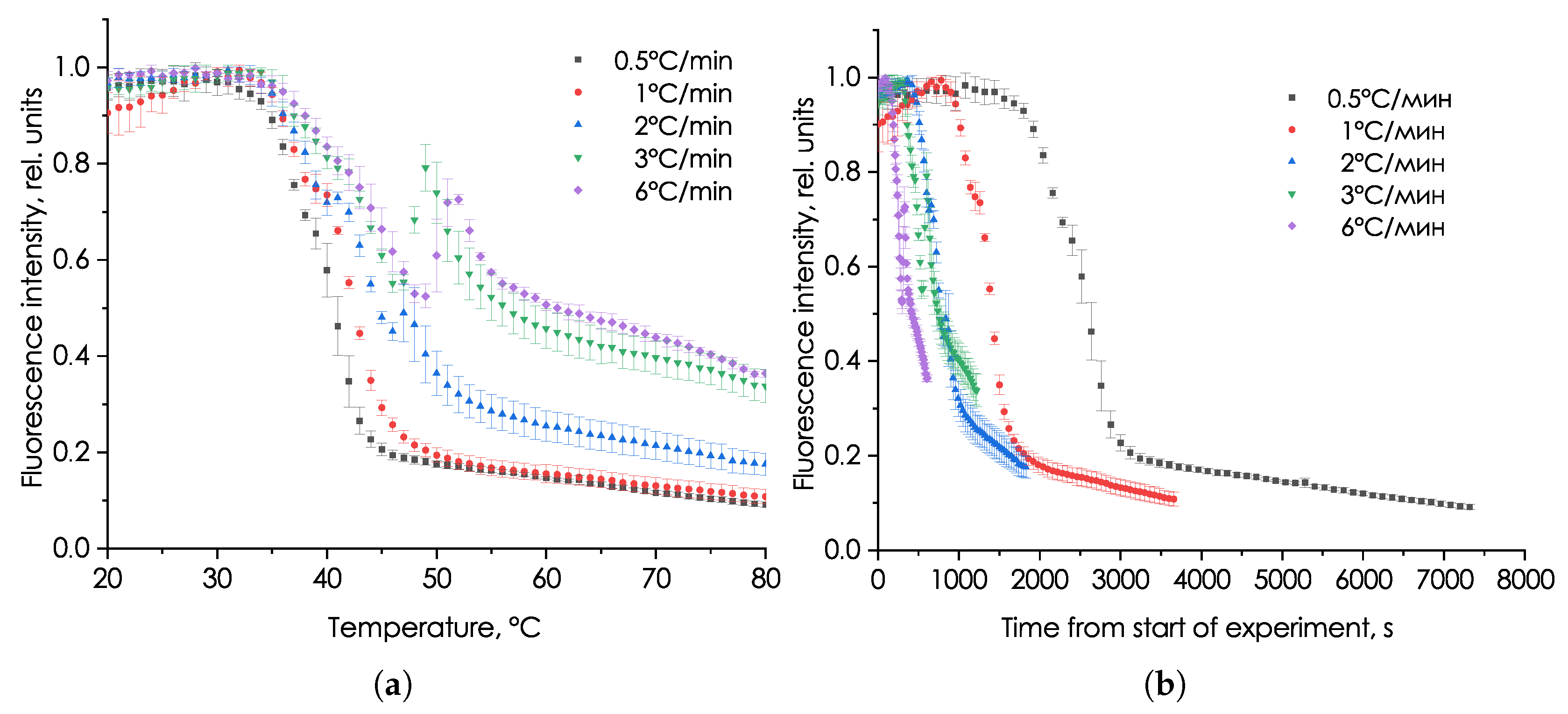
3.1. Study of the Influence of Heating Rate on the Characteristics of the Fluorescent Signal of P. hasleana
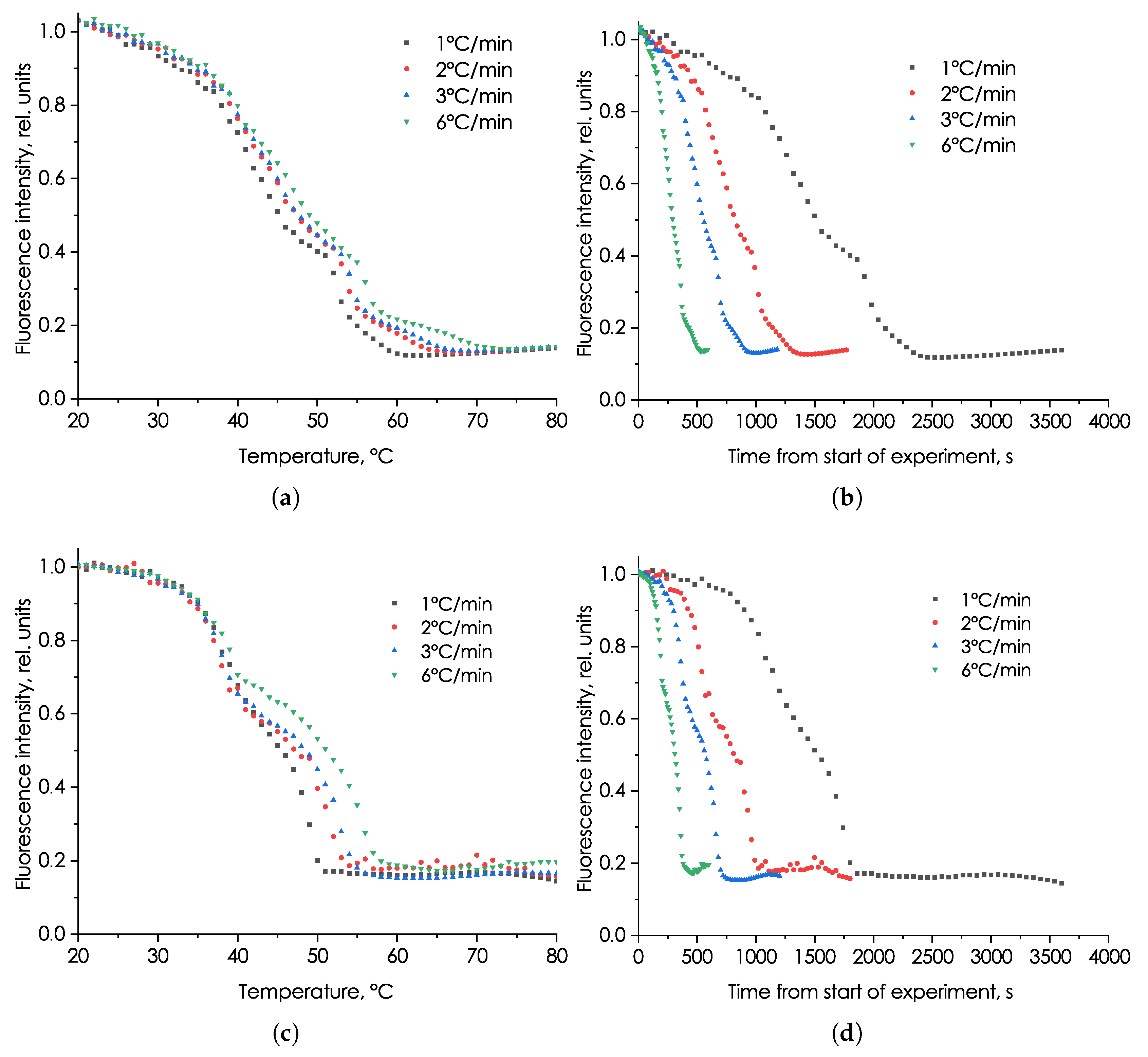
3.2. Study of the Effect of Heating Rate on the Fluorescent Signal Characteristics of Microalga Phaeodactylum tricornutum and Picochlorum maculatum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taniguchi, M.; Lindsey, J.S. Absorption and Fluorescence Spectral Database of Chlorophylls and Analogues. Photochem. Photobiol. 2021, 97, 136–165. [Google Scholar] [CrossRef]
- Sá, M.; Ferrer Ledo, N.; Gao, F.; Bertinetto, C.; Jansen, J.; Crespo, J.; Wijffels, R.; Barbosa, M.J.; Galinha, C. Perspectives of fluorescence spectroscopy for online monitoring in microalgae industry. Microb. Biotechnol. 2022, 15, 1824–1838. [Google Scholar] [CrossRef]
- Voznesenskiy, S.S.; Gamayunov, E.L.; Popik, A.Y.; Markina, Z.V.; Orlova, T.Y. Temperature dependence of the parameters of laser-induced fluorescence and species composition of phytoplankton: The theory and the experiments. Algal Res. 2019, 44, 101719. [Google Scholar] [CrossRef]
- Popik, A.Y.; Gamayunov, E.L.; Voznesenskiy, S.S.; Markina, Z.M.; Orlova, T.Y. The study of fluorescence features of microalgae from the genus Pseudo-nitzschia and the possibility of their detection in water. Algal Res. 2022, 64, 102662. [Google Scholar] [CrossRef]
- Popik, A.; Voznesenskiy, S.; Dunkai, T.; Gamayunov, E.; Orlova, T.; Leonov, A.; Zinov, A. Methodology of Measuring and Using Fluorescent Parameters of Microalgae. Bull. Russ. Acad. Sci. Phys. 2024, 88, S399–S407. [Google Scholar] [CrossRef]
- Santi Delia, A.; Caruso, G.; Melcarne, L.; Caruso, G.; Parisi, S.; Laganà, P. Biological Toxins from Marine and Freshwater Microalgae. In Microbial Toxins and Related Contamination in the Food Industry; Caruso, G., Caruso, G., Laganà, P.L., Santi Delia, A., Parisi, S., Barone, C., Melcarne, L., Mazzù, F., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 13–55. [Google Scholar] [CrossRef]
- Blossom, H.E.; Markussen, B.; Daugbjerg, N.; Krock, B.; Norlin, A.; Hansen, P.J. The cost of toxicity in microalgae: Direct evidence from the dinoflagellate Alexandrium. Front. Microbiol. 2019, 10, 1065. [Google Scholar] [CrossRef]
- Wang, S.Y.; Bi, W.H.; Li, X.Y.; Zhang, B.J.; Fu, G.W.; Jin, W.; Jiang, T.J.; Zhao, J.; Shi, W.J.; Zhang, Y.F. A detection method of typical toxic mixed red tide algae in Qinhuangdao based on three-dimensional fluorescence spectroscopy. Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 2023, 298, 122704. [Google Scholar] [CrossRef]
- Al-Hussieny, A.A. Algae Toxins and Their Treatment. In Progress in Microalgae Research; Zepka, L.Q., Jacob-Lopes, E., Deprá, M.C., Eds.; IntechOpen: Rijeka, Croatia, 2022; Chapter 6; pp. 1–16. [Google Scholar] [CrossRef]
- Anderson, D.M.; Cembella, A.D.; Hallegraeff, G.M. Progress in understanding harmful algal blooms: Paradigm shifts and new technologies for research, monitoring, and management. Annu. Rev. Mar. Sci. 2012, 4, 143–176. [Google Scholar] [CrossRef]
- Oyeku, O.G.; Mandal, S.K. Historical occurrences of marine microalgal blooms in Indian peninsula: Probable causes and implications. Oceanologia 2021, 63, 51–70. [Google Scholar] [CrossRef]
- Kholssi, R.; Lougraimzi, H.; Moreno-Garrido, I. Effects of global environmental change on microalgal photosynthesis, growth and their distribution. Mar. Environ. Res. 2023, 184, 105877. [Google Scholar] [CrossRef]
- Teoh, M.L.; Chu, W.L.; Phang, S.M. Effect of temperature change on physiology and biochemistry of algae: A review. Malays. J. Sci. 2010, 29, 82–97. [Google Scholar] [CrossRef]
- Singh, S.; Singh, P. Effect of temperature and light on the growth of algae species: A review. Renew. Sustain. Energy Rev. 2015, 50, 431–444. [Google Scholar] [CrossRef]
- Kouřil, R.; Ilík, P.; Tomek, P.; Nauš, J.; Poulíčková, A. Chlorophyll fluorescence temperature curve on Klebsormidium flaccidum cultivated at different temperature regimes. J. Plant Physiol. 2001, 158, 1131–1136. [Google Scholar] [CrossRef]
- Lípová, L.; Krchňák, P.; Komenda, J.; Ilík, P. Heat-induced disassembly and degradation of chlorophyll-containing protein complexes in vivo. Biochim. Et Biophys. Acta-Bioenerg. 2010, 1797, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Kochubey, S.M.; Shevchenko, V.V.; Kazantsev, T.A. Changes of the antenna of photosystem i induced by short-term heating. Biochem. (Moscow) Suppl. Ser. A Membr. Cell Biol. 2013, 7, 67–77. [Google Scholar] [CrossRef]
- Ladjimi, M.T.; Labavić, D.; Guilbert, M.; Anquez, F.; Pruvost, A.; Courtade, E.; Pfeuty, B.; Thommen, Q. Dynamical thermal dose models and dose time-profile effects. Int. J. Hyperth. 2019, 36, 721–729. [Google Scholar] [CrossRef]
- Sturm, S.; Engelken, J.; Gruber, A.; Vugrinec, S.; G Kroth, P.; Adamska, I.; Lavaud, J. A novel type of light-harvesting antenna protein of red algal origin in algae with secondary plastids. BMC Evol. Biol. 2013, 13, 159. [Google Scholar] [CrossRef]
- Engelken, J.; Brinkmann, H.; Adamska, I. Taxonomic distribution and origins of the extended LHC (light-harvesting complex) antenna protein superfamily. BMC Evol. Biol. 2010, 10, 233. [Google Scholar] [CrossRef]
- Pi, X.; Zhao, S.; Wang, W.; Liu, D.; Xu, C.; Han, G.; Kuang, T.; Sui, S.F.; Shen, J.R. The pigment-protein network of a diatom photosystem II–light-harvesting antenna supercomplex. Science 2019, 365, eaax4406. [Google Scholar] [CrossRef]
- Green, B.R.; Anderson, J.M.; Parson, W.W. Photosynthetic Membranes and Their Light-Harvesting Antennas. In Light-Harvesting Antennas in Photosynthesis. Advances in Photosynthesis and Respiration; Green, B.R., Parson, W.W., Eds.; Springer: Dordrecht, The Netherlands, 2003; Chapter 1; pp. 1–28. [Google Scholar] [CrossRef]
- Trainer, V.L.; Bates, S.S.; Lundholm, N.; Thessen, A.E.; Cochlan, W.P.; Adams, N.G.; Trick, C.G. Pseudo-nitzschia physiological ecology, phylogeny, toxicity, monitoring and impacts on ecosystem health. Harmful Algae 2012, 14, 271–300. [Google Scholar] [CrossRef]
- Lundholm, N.; Bates, S.S.; Baugh, K.A.; Bill, B.D.; Connell, L.B.; Léger, C.; Trainer, V.L. Cryptic and pseudo-cryptic diversity in diatoms-with descriptions of pseudo-nitzschia hasleana sp. Nov. And p. Fryxelliana sp. Nov.(1). J. Phycol. 2012, 48, 436–454. [Google Scholar] [CrossRef]
- Aleksanin, A.I.; Kim, V.; Orlova, T.Y.; Stonik, I.V.; Shevchenko, O.G. Phytoplankton of the Peter the Great Bay and its remote sensing problem. Oceanology 2012, 52, 219–230. [Google Scholar] [CrossRef]
- Stonik, I.V. Long-term variations in species composition of bloom-forming toxic pseudo-nitzschia diatoms in the north-western sea of japan during 1992–2015. J. Mar. Sci. Eng. 2021, 9, 568. [Google Scholar] [CrossRef]
- Martin-Jézéquel, V.; Tesson, B. Phaeodactylum tricornutum polymorphism: An overview. In Advances in Algal Cell Biology; De Gruyter Brill: Berlin, Germany, 2013; pp. 43–80. [Google Scholar] [CrossRef]
- Butler, T.; Kapoore, R.V.; Vaidyanathan, S. Phaeodactylum tricornutum: A Diatom Cell Factory. Trends Biotechnol. 2020, 38, 606–622. [Google Scholar] [CrossRef] [PubMed]
- Stonik, I.V.; Orlova, T.Y. The Seasonal Accumulation of Amnesic Toxin (Domoic Acid) in Commercial Bivalves Mytilus trossulus Gould, 1850 and Mizuhopecten yessoensis Jay, 1850 in Vostok Bay, Sea of Japan. Russ. J. Mar. Biol. 2020, 46, 56–58. [Google Scholar] [CrossRef]
- Bates, S.S.; Hubbard, K.A.; Lundholm, N.; Montresor, M.; Leaw, C.P. Pseudo-nitzschia, Nitzschia, and domoic acid: New research since 2011. Harmful Algae 2018, 79, 3–43. [Google Scholar] [CrossRef]
- Lefebvre, K.A.; Robertson, A. Domoic acid and human exposure risks: A review. Toxicon 2010, 56, 218–230. [Google Scholar] [CrossRef]
- Garcia, A.R.; Filipe, S.B.; Fernandes, C.; Estevão, C.; Ramos, G. Algal Culturing Techniques; Elsevier Academic Press: Amsterdam, The Netherlands, 2005; p. 578. [Google Scholar]
- Pospišil, P.; Skotnica, J.; Nauš, J. Low and high temperature dependence of minimum F0 and maximum FM chlorophyll fluorescence in vivo. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 1998, 1363, 95–99. [Google Scholar] [CrossRef]
- Kouřil, R.; Lazár, D.; Ilík, P.; Skotnica, J.; Krchňák, P.; Nauš, J. High-Temperature Induced Chlorophyll Fluorescence Rise in Plants at 40–50 °C: Experimental and Theoretical Approach. Photosynth. Res. 2004, 81, 49–66. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popik, A.; Voznesenskiy, S.; Dunkai, T.; Leonov, A.; Orlova, T. A Temperature-Controlled Fluorescence Fingerprint for Identifying Pseudo-nitzschia hasleana in Harmful Algal Blooms. Phycology 2025, 5, 52. https://doi.org/10.3390/phycology5040052
Popik A, Voznesenskiy S, Dunkai T, Leonov A, Orlova T. A Temperature-Controlled Fluorescence Fingerprint for Identifying Pseudo-nitzschia hasleana in Harmful Algal Blooms. Phycology. 2025; 5(4):52. https://doi.org/10.3390/phycology5040052
Chicago/Turabian StylePopik, Alexander, Sergey Voznesenskiy, Tatiana Dunkai, Andrei Leonov, and Tatiana Orlova. 2025. "A Temperature-Controlled Fluorescence Fingerprint for Identifying Pseudo-nitzschia hasleana in Harmful Algal Blooms" Phycology 5, no. 4: 52. https://doi.org/10.3390/phycology5040052
APA StylePopik, A., Voznesenskiy, S., Dunkai, T., Leonov, A., & Orlova, T. (2025). A Temperature-Controlled Fluorescence Fingerprint for Identifying Pseudo-nitzschia hasleana in Harmful Algal Blooms. Phycology, 5(4), 52. https://doi.org/10.3390/phycology5040052





