Enhancement of Lipids Content in Chlorella sp. Under Phosphorus Limitation and Heavy Metal Addition for Biodiesel Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalgal Cultivation and Biomass Determination
2.2. Total Lipid Extraction and Determination
2.3. Transesterification and Biodiesel Properties’ Analysis
2.4. Determination of Antioxidative Components
2.5. Energy Conversion Efficiency Analysis
2.6. Statistical Analysis
3. Results and Discussion
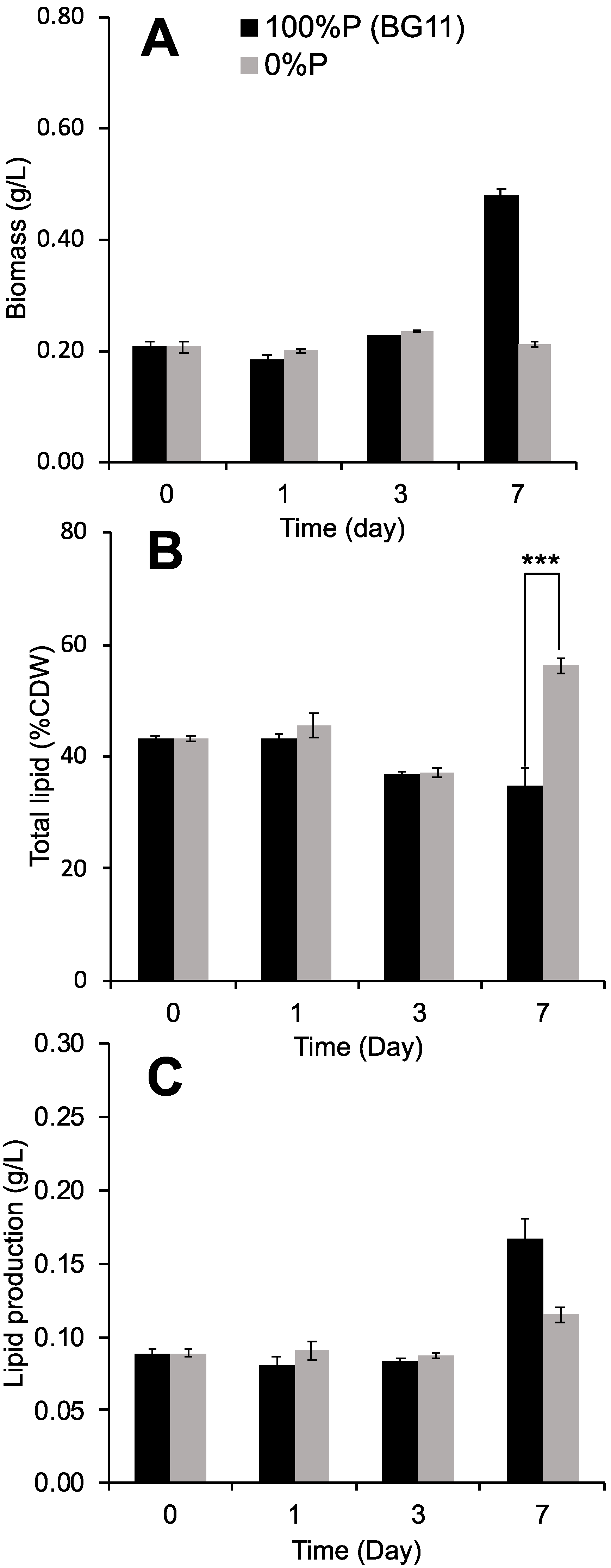
3.1. Effect of Phosphorus Deprivation on Lipid Production
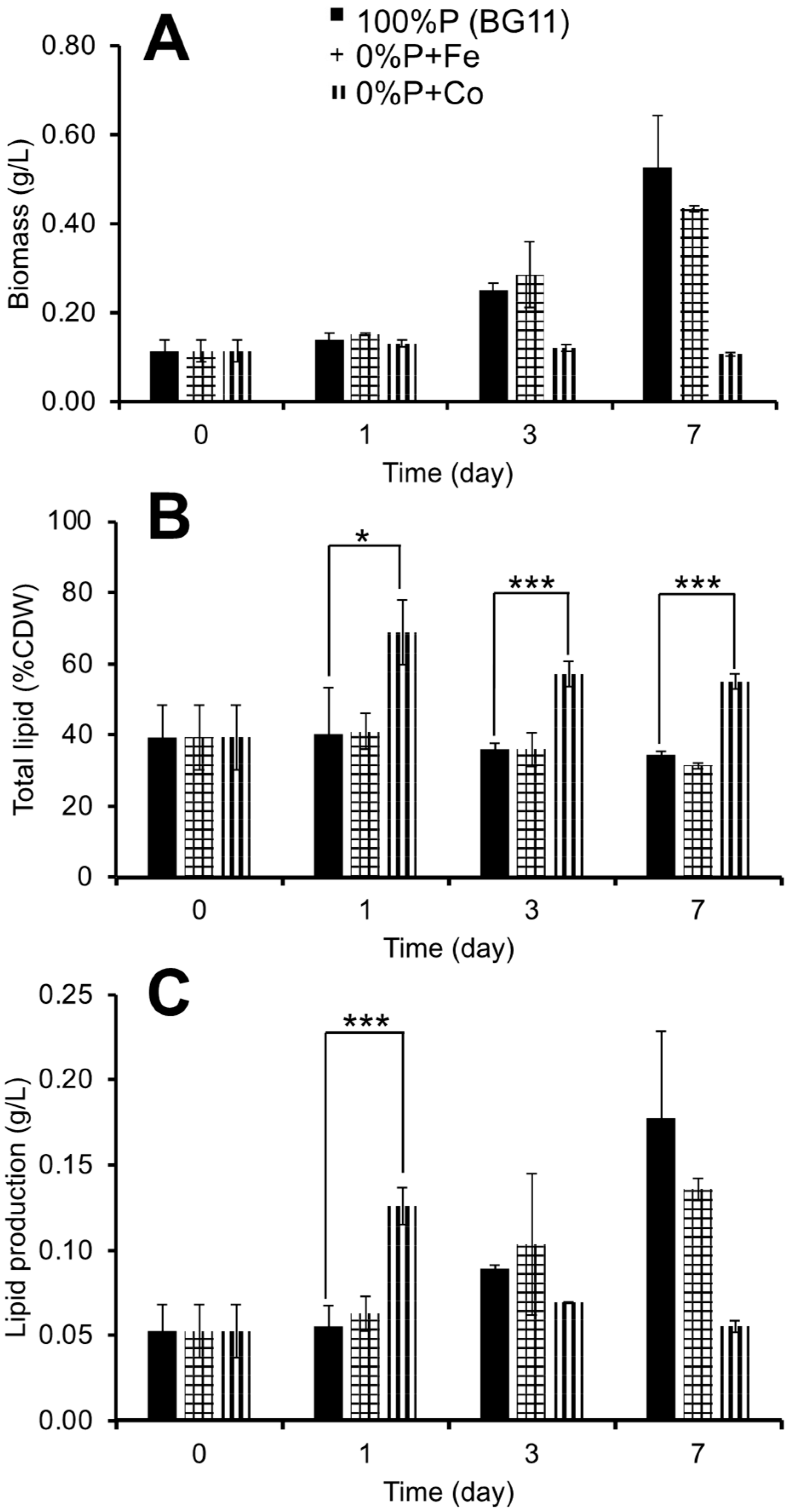
3.2. Effect of Phosphorus Deprivation and Heavy Metal Addition on Lipid Production
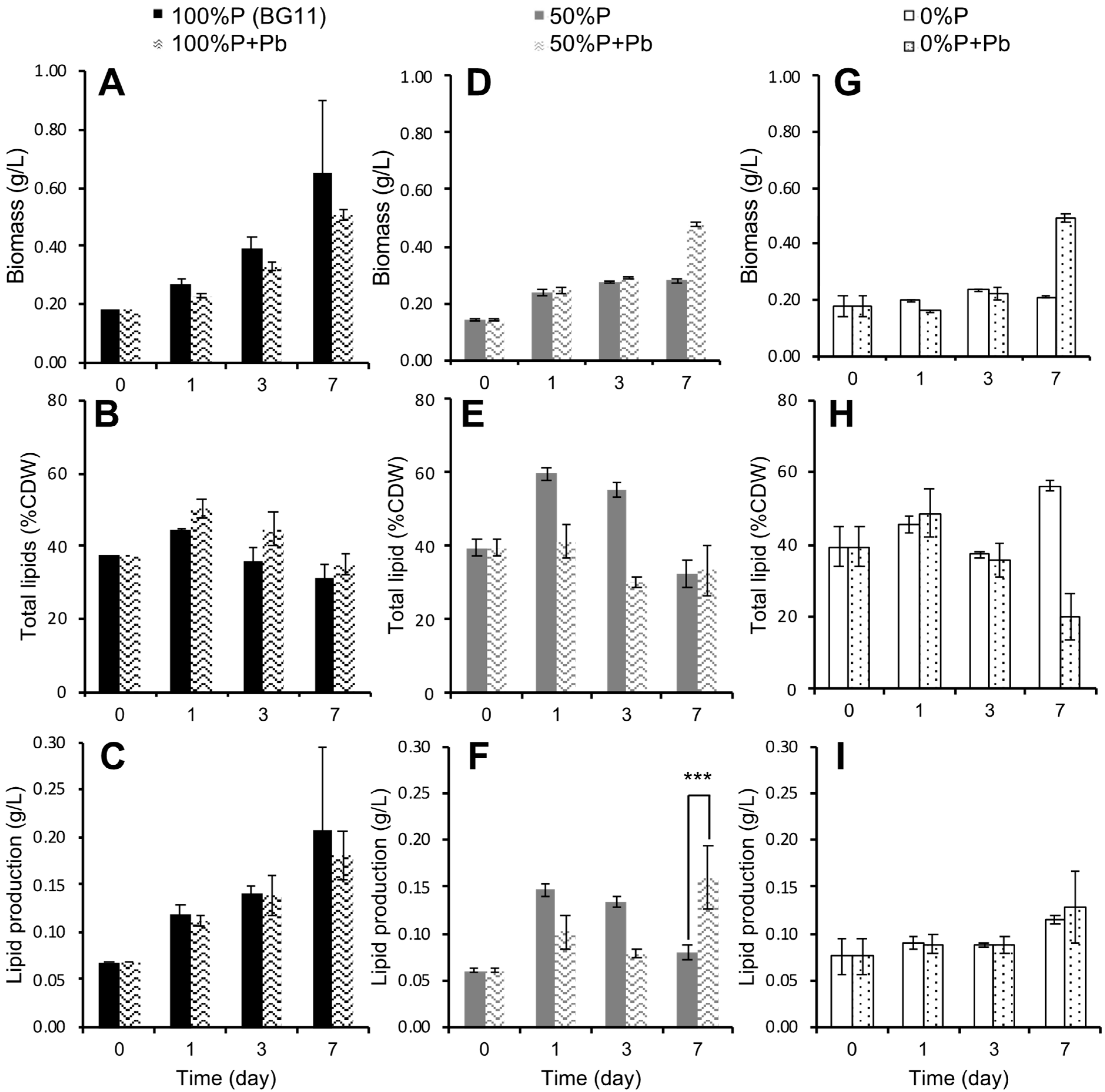
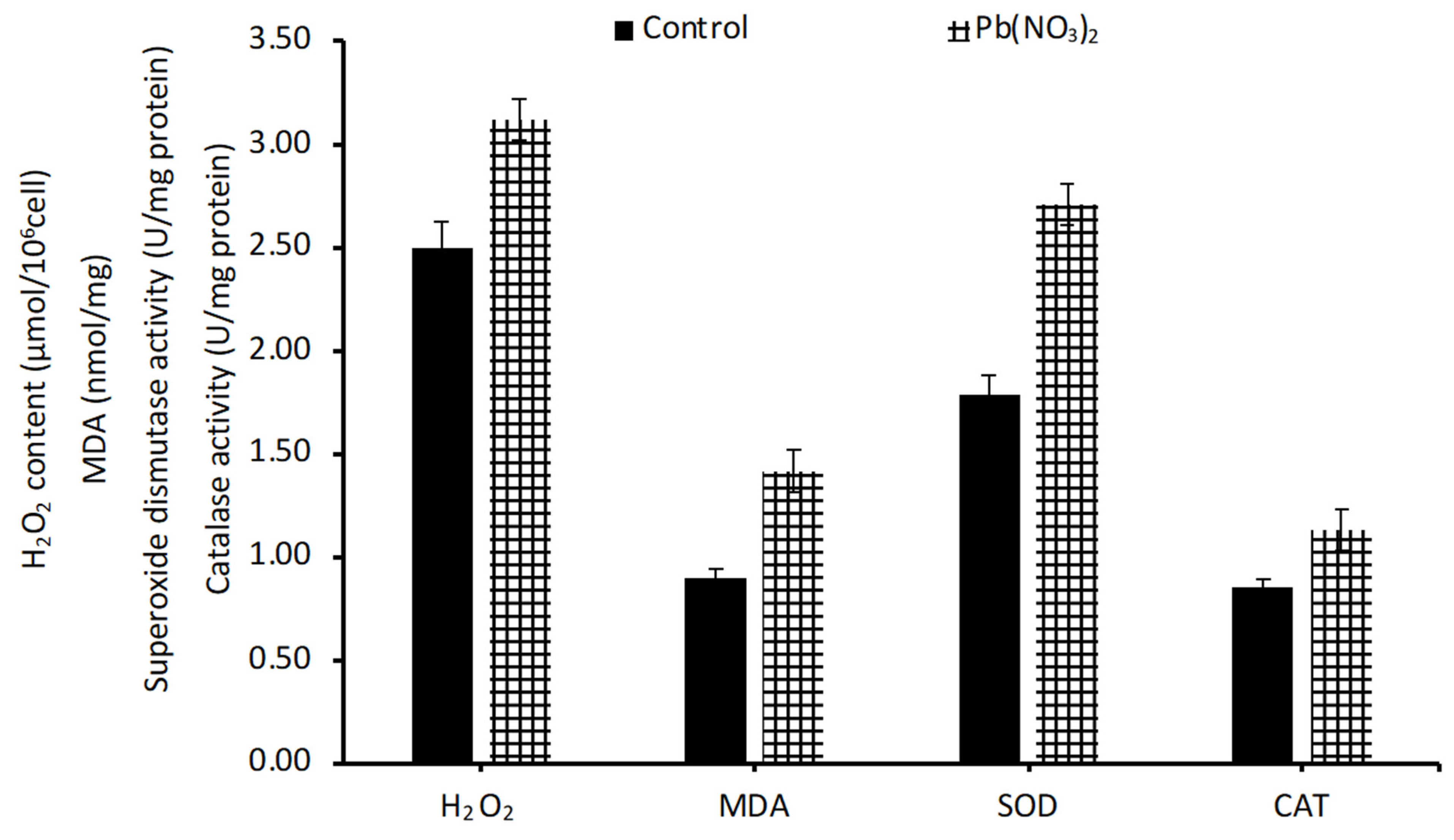
3.3. Effect of Phosphorus Limitation and Lead-Induced Oxidative Stress on Lipid Production
3.4. Fatty Acid Profiles and Biodiesel Properties Under Pb Stress Conditions
3.5. Total Energy Conversion Efficiency
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Bloyd, C. Thailand Alternative Fuels Update 2017; United State Department of Energy: Washington, DC, USA, 2017. [Google Scholar]
- Sakulsuraekkapong, J.; Thepa, S.; Pairintra, R. Improvement of biodiesel’s policy in Thailand. Energy Sources Part B Econ. Plan. Policy 2018, 13, 158–164. [Google Scholar] [CrossRef]
- Kumar, K.S.; Dahms, H.-U.; Won, E.-J.; Lee, J.-S.; Shin, K.-H. Microalgae–A promising tool for heavy metal remediation. Ecotoxicol. Environ. Saf. 2015, 113, 329–352. [Google Scholar]
- Praveenkumar, R.; Shameera, K.; Mahalakshmi, G.; Akbarsha, M.A.; Thajuddin, N. Influence of nutrient deprivations on lipid accumulation in a dominant indigenous microalga Chlorella sp., BUM11008: Evaluation for biodiesel production. Biomass Bioenergy 2012, 37, 60–66. [Google Scholar]
- Sharma, K.K.; Schuhmann, H.; Schenk, P.M. High lipid induction in microalgae for biodiesel production. Energies 2012, 5, 1532–1553. [Google Scholar] [CrossRef]
- El-Sheek, M.; Rady, A.J.P. Effect of phosphorus starvation on growth, photosynthesis and some metabolic processes in the unicellular green alga Chlorella kessleri. Phyton 1995, 35, 139–151. [Google Scholar]
- Liang, K.; Zhang, Q.; Gu, M.; Cong, W. Effect of phosphorus on lipid accumulation in freshwater microalga Chlorella sp. J. Appl. Phycol. 2013, 25, 311–318. [Google Scholar] [CrossRef]
- Stansell, G.R.; Gray, V.M.; Sym, S.D. Microalgal fatty acid composition: Implications for biodiesel quality. J. Appl. Phycol. 2012, 24, 791–801. [Google Scholar] [CrossRef]
- Yodsuwan, N.; Chamchuang, N.; Puchcha, Y.; Sirisansaneeyakul, S.J.K.J. Outdoor photoautotrophic cultivation of Chlorella sp. TISTR 8990 in nitrogen-and phosphorus-minimal media for lipid accumulations. Kasetsart J. 2015, 49, e91. [Google Scholar]
- Silitonga, A.S.; Masjuki, H.H.; Mahlia, T.M.I.; Ong, H.C.; Chong, W.T.; Boosroh, M.H. Overview properties of biodiesel diesel blends from edible and non-edible feedstock. Renew. Sustain. Energy Rev. 2013, 22, 346–360. [Google Scholar] [CrossRef]
- da Silva Ferreira, V.; Sant’Anna, C. The effect of physicochemical conditions and nutrient sources on maximizing the growth and lipid productivity of green microalgae. Phycol. Res. 2017, 65, 3–13. [Google Scholar] [CrossRef]
- Saavedra, R.; Muñoz, R.; Taboada, M.E.; Vega, M.; Bolado, S. Comparative uptake study of arsenic, boron, copper, manganese and zinc from water by different green microalgae. Bioresour. Technol. 2018, 263, 49–57. [Google Scholar] [CrossRef]
- Liu, Z.-Y.; Wang, G.-C.; Zhou, B.-C. Effect of iron on growth and lipid accumulation in Chlorella vulgaris. Bioresour. Technol. 2008, 99, 4717–4722. [Google Scholar] [CrossRef] [PubMed]
- Battah, M.; El-Ayoty, Y.; Abomohra, A.E.-F.; El-Ghany, S.A.; Esmael, A. Effect of Mn2+, Co2+ and H2O2 on biomass and lipids of the green microalga Chlorella vulgaris as a potential candidate for biodiesel production. Ann. Microbiol. 2015, 65, 155–162. [Google Scholar] [CrossRef]
- Carfagna, S.; Lanza, N.; Salbitani, G.; Basile, A.; Sorbo, S.; Vona, V. Physiological and morphological responses of lead or cadmium exposed Chlorella sorokiniana 211-8K (Chlorophyceae). SpringerPlus 2013, 2, 147. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Magnusson, M.; Brown, R.J.; Ayoko, G.A.; Nabi, M.N.; Heimann, K. Microalgal species selection for biodiesel production based on fuel properties derived from fatty acid profiles. Energies 2013, 6, 5676–5702. [Google Scholar] [CrossRef]
- Sivaramakrishnan, R.; Incharoensakdi, A. Enhancement of total lipid yield by nitrogen, carbon, and iron supplementation in isolated microalgae. J. Phycol. 2017, 53, 855–868. [Google Scholar] [CrossRef]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology 1979, 111, 1–61. [Google Scholar] [CrossRef]
- Halim, R.; Gladman, B.; Danquah, M.K.; Webley, P.A. Oil extraction from microalgae for biodiesel production. Bioresour. Technol. 2011, 102, 178–185. [Google Scholar] [CrossRef]
- IUPAC. Standard Methods for the Analysis of Oils, Fats and Derivatives; Pergamon Press: Paris, France, 1979; pp. 2503–2526. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- Limsuwatthanathamrong, M.; Sooksai, S.; Chunhabundit, S.; Noitung, S.; Ngamrojanavanich, N.; Petsom, A. Fatty acid profile and lipid composition of farm-raised and wild-caught sand worms, Perinereis nuntia, the diet for marine shrimp broodstock. Asian J. Anim. Sci. 2012, 6, 65–75. [Google Scholar]
- Sivaramakrishnan, R.; Incharoensakdi, A. Higher efficiency of microalgal biorefinery is achieved with integrated than one-way method. Fuel 2021, 300, 120988. [Google Scholar] [CrossRef]
- Francisco, É.C.; Neves, D.B.; Jacob-Lopes, E.; Franco, T.T. Microalgae as feedstock for biodiesel production: Carbon dioxide sequestration, lipid production and biofuel quality. J. Chem. Technol. Biotechnol. 2010, 85, 395–403. [Google Scholar] [CrossRef]
- Ramos, M.J.; Fernández, C.M.; Casas, A.; Rodríguez, L.; Pérez, Á. Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour. Technol. 2009, 100, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Sarin Sarin, A.; Arora, R.; Singh, N.P.; Sarin, R.; Malhotra, R.K.; Kundu, K. Effect of blends of Palm-Jatropha-Pongamia biodiesels on cloud point and pour point. Energy 2009, 34, 2016–2021. [Google Scholar] [CrossRef]
- Ramírez-Verduzco, L.F.; Rodríguez-Rodríguez, J.E.; Jaramillo-Jacob, A.d.R. Predicting cetane number, kinematic viscosity, density and higher heating value of biodiesel from its fatty acid methyl ester composition. Fuel 2012, 91, 102–111. [Google Scholar] [CrossRef]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Szechyńska-Hebda, M.; Skrzypek, E.; Dąbrowska, G.; Biesaga-Kościelniak, J.; Filek, M.; Wędzony, M. The role of oxidative stress induced by growth regulators in the regeneration process of wheat. Acta Physiol. Plant. 2007, 29, 327–337. [Google Scholar] [CrossRef]
- Ren, H.-Y.; Liu, B.-F.; Kong, F.; Zhao, L.; Xie, G.-J.; Ren, N.-Q. Energy conversion analysis of microalgal lipid production under different culture modes. Bioresour. Technol. 2014, 166, 625–629. [Google Scholar] [CrossRef]
- Lips, D.; Schuurmans, J.M.; Branco dos Santos, F.; Hellingwerf, K.J. Many ways towards ‘solar fuel’: Quantitative analysis of the most promising strategies and the main challenges during scale-up. Energy Environ. Sci. 2018, 11, 10–22. [Google Scholar] [CrossRef]
- Shuba Eyasu, S.; Kifle, D. Microalgae to biofuels: ‘Promising’ alternative and renewable energy, review. Renew. Sustain. Energy Rev. 2018, 81, 743–755. [Google Scholar] [CrossRef]
- Yang, J.; Li, X.; Hu, H.; Zhang, X.; Yu, Y.; Chen, Y. Growth and lipid accumulation properties of a freshwater microalga, Chlorella ellipsoidea YJ1, in domestic secondary effluents. Appl. Energy 2011, 88, 3295–3299. [Google Scholar] [CrossRef]
- Zhang, Q.; Hong, Y. Effects of stationary phase elongation and initial nitrogen and phosphorus concentrations on the growth and lipid-producing potential of Chlorella sp. HQ. J. Appl. Phycol. 2014, 26, 141–149. [Google Scholar] [CrossRef]
- Feng, P.; Deng, Z.; Fan, L.; Hu, Z. Lipid accumulation and growth characteristics of Chlorella zofingiensis under different nitrate and phosphate concentrations. J. Biosci. Bioeng. 2012, 114, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Han, F.; Xu, H.; Mu, J.; Chen, D.; Feng, B.; Zeng, H. Potential lipid accumulation and growth characteristic of the green alga Chlorella with combination cultivation mode of nitrogen (N) and phosphorus (P). Bioresour. Technol. 2014, 174, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Anto, S.; Pugazhendhi, A.; Mathimani, T. Lipid enhancement through nutrient starvation in Chlorella sp. and its fatty acid profiling for appropriate bioenergy feedstock. Biocatal. Agric. Biotechnol. 2019, 20, 101179. [Google Scholar] [CrossRef]
- Roopnarain, A.; Gray, V.M.; Sym, S.D. Phosphorus limitation and starvation effects on cell growth and lipid accumulation in Isochrysis galbana U4 for biodiesel production. Bioresour. Technol. 2014, 156, 408–411. [Google Scholar] [CrossRef]
- Carstensen, A.; Herdean, A.; Schmidt, S.B.; Sharma, A.; Spetea, C.; Pribil, M.; Husted, S. The impacts of phosphorus deficiency on the photosynthetic electron transport chain. Plant Physiol. 2018, 177, 271–284. [Google Scholar] [CrossRef]
- Kalisch, B.; Dörmann, P.; Hölzl, G. DGDG and glycolipids in plants and algae. In Lipids in Plant and Algae Development; Springer: Cham, Switzerland, 2016; pp. 51–83. [Google Scholar]
- Khozin-Goldberg, I.; Cohen, Z. The effect of phosphate starvation on the lipid and fatty acid composition of the fresh water eustigmatophyte Monodus subterraneus. Phytochemistry 2006, 67, 696–701. [Google Scholar] [CrossRef]
- Alipanah, L.; Winge, P.; Rohloff, J.; Najafi, J.; Brembu, T.; Bones, A.M. Molecular adaptations to phosphorus deprivation and comparison with nitrogen deprivation responses in the diatom Phaeodactylum tricornutum. PLoS ONE 2018, 13, e0193335. [Google Scholar] [CrossRef]
- Ghafari, M.; Rashidi, B.; Haznedaroglu, B.Z. Effects of macro and micronutrients on neutral lipid accumulation in oleaginous microalgae. Biofuels 2018, 9, 147–156. [Google Scholar] [CrossRef]
- Das, P.; Ibrahim Thaher, M.; Abdul Quadir Mohd Abdul Hakim, M.; Al-Jabri, H.M.S.J.; Alghasal, G.S.H.S. Optimization of iron dosage for microalgal biomass production as a feedstock for biofuel. Biofuels 2019, 12, 569–577. [Google Scholar] [CrossRef]
- Ren, H.-Y.; Liu, B.-F.; Kong, F.; Zhao, L.; Xie, G.-J.; Ren, N.-Q. Enhanced lipid accumulation of green microalga Scenedesmus sp. by metal ions and EDTA addition. Bioresour. Technol. 2014, 169, 763–767. [Google Scholar] [CrossRef]
- Rizwan, M.; Mujtaba, G.; Lee, K. Effects of iron sources on the growth and lipid/carbohydrate production of marine microalga Dunaliella tertiolecta. Biotechnol. Bioprocess Eng. 2017, 22, 68–75. [Google Scholar] [CrossRef]
- Singh, P.; Guldhe, A.; Kumari, S.; Rawat, I.; Bux, F. Investigation of combined effect of nitrogen, phosphorus and iron on lipid productivity of microalgae Ankistrodesmus falcatus KJ671624 using response surface methodology. Biochem. Eng. J. 2015, 94, 22–29. [Google Scholar] [CrossRef]
- Singh, P.; Kumari, S.; Guldhe, A.; Singh, G.; Bux, F. ACCase and rbcL gene expression as a function of nutrient and metal stress for enhancing lipid productivity in Chlorella sorokiniana. Energy Convers. Manag. 2017, 148, 809–819. [Google Scholar] [CrossRef]
- Li, M.; Zhu, Q.; Hu, C.-w.; Chen, L.; Liu, Z.-l.; Kong, Z.-m. Cobalt and manganese stress in the microalga Pavlova viridis (Prymnesiophyceae): Effects on lipid peroxidation and antioxidant enzymes. J. Environ. Sci. 2007, 19, 1330–1335. [Google Scholar] [CrossRef] [PubMed]
- Plekhanov, S.E.; Chemeris, Y.K. Early toxic effects of zinc, cobalt, and cadmium on photosynthetic activity of the green alga Chlorella pyrenoidosa Chick S-39. Biol. Bull. Russ. Acad. Sci. 2003, 30, 506–511. [Google Scholar] [CrossRef]
- Kashyap, M.; Anand, V.; Ghosh, A.; Kiran, B. Superintending Scenedesmus and Chlorella sp. with lead and cobalt tolerance governed via stress biomarkers. Water Supply 2021, 21, 2387–2399. [Google Scholar] [CrossRef]
- Wan, M.; Jin, X.; Rosenberg, J.N.; Yu, G.; Nie, Z.; Oyler, G.A.; Betenbaugh, M.J. The effect of iron on growth, lipid accumulation, and gene expression profile of the freshwater microalga Chlorella sorokiniana. Bioenerg. Biofuels 2014, 98, 9473–9481. [Google Scholar] [CrossRef]
- Bajguz, A. Suppression of Chlorella vulgaris growth by cadmium, lead, and copper stress and its restoration by endogenous brassinolide. Arch. Environ. Contam. Toxicol. 2011, 60, 406–416. [Google Scholar] [CrossRef]
- Piotrowska-Niczyporuk, A.; Bajguz, A.; Talarek, M.; Bralska, M.; Zambrzycka, E. The effect of lead on the growth, content of primary metabolites, and antioxidant response of green alga Acutodesmus obliquus (Chlorophyceae). Environ. Sci. Pollut. Res. 2015, 22, 19112–19123. [Google Scholar] [CrossRef]
- Cheng, P.; Wang, J.; Liu, T. Effect of cobalt enrichment on growth and hydrocarbon accumulation of Botryococcus braunii with immobilized biofilm attached cultivation. Bioresour. Technol. 2015, 177, 204–208. [Google Scholar] [CrossRef]
- Piotrowska-Niczyporuk, A.; Bajguz, A.; Zambrzycka-Szelewa, E. Response and the detoxification strategies of green alga Acutodesmus obliquus (Chlorophyceae) under lead stress. Environ. Exp. Bot. 2017, 144, 25–36. [Google Scholar] [CrossRef]
- Pham, T.-L.; Dao, T.-S.; Bui, H.N.; Pham, T.K.N.; Ngo, T.T.H.; Bui, H.M. Lipid production combined with removal and bioaccumulation of Pb by Scenedesmus sp. green alga. Pol. J. Environ. Stud. 2020, 29, 1785–1791. [Google Scholar] [CrossRef] [PubMed]
- Dat, J.; Vandenabeele, S.; Vranová, E.; Van Montagu, M.; Inzé, D.; Van Breusegem, F. Dual action of the active oxygen species during plant stress responses. Cell. Mol. Life Sci. 2000, 57, 779–795. [Google Scholar] [CrossRef]
- Piotrowska-Niczyporuk, A.; Bajguz, A. The effect of natural and synthetic auxins on the growth, metabolite content and antioxidant response of green alga Chlorella vulgaris (Trebouxiophyceae). Plant Growth Regul. 2014, 73, 57–66. [Google Scholar] [CrossRef]
- Sivaramakrishnan, R.; Incharoensakdi, A. Enhancement of lipid production in Scenedesmus sp. by UV mutagenesis and hydrogen peroxide treatment. Bioresour. Technol. 2017, 235, 366–370. [Google Scholar] [CrossRef]
- Sivaramakrishnan, R.; Incharoensakdi, A. UV mutagenesis followed by hydrogen peroxide treatment ameliorates lipid production and omega-3 fatty acids levels in Chlorella sp. Algal Res. 2023, 74, 103195. [Google Scholar] [CrossRef]
- Zezza, T.R.C.; Castilho, M.d.S.; Stradiotto, N.R. Determination of phosphorus in biodiesel using 1:12 phosphomolybdic modified electrode by cyclic voltammetry. Fuel 2012, 95, 15–18. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C.; Ceniceros, E.; Natarajan, M. Review of biodiesel composition, properties, and specifications. Renew. Sustain. Energy Rev. 2012, 16, 143–169. [Google Scholar] [CrossRef]
- Yaakob, Z.; Narayanan, B.N.; Padikkaparambil, S.; Unni, K.S.; Akbar, P.M. A review on the oxidation stability of biodiesel. Renew. Sustain. Energy Rev. 2014, 35, 136–153. [Google Scholar] [CrossRef]
- Deshmukh, S.; Kumar, R.; Bala, K. Microalgae biodiesel: A review on oil extraction, fatty acid composition, properties and effect on engine performance and emissions. Fuel Process. Technol. 2019, 191, 232–247. [Google Scholar] [CrossRef]
- Demirbas, A. Progress and recent trends in biodiesel fuels. Energy Convers. Manag. 2009, 50, 14–34. [Google Scholar] [CrossRef]
- Sajjadi, B.; Raman, A.A.A.; Arandiyan, H. A comprehensive review on properties of edible and non-edible vegetable oil-based biodiesel: Composition, specifications and prediction models. Renew. Sustain. Energy Rev. 2016, 63, 62–92. [Google Scholar] [CrossRef]
- Atadashi, I.M.; Aroua, M.K.; Aziz, A.A. High quality biodiesel and its diesel engine application: A review. Renew. Sustain. Energy Rev. 2010, 14, 1999–2008. [Google Scholar] [CrossRef]
- Thongtha, S.; Kittiwongwattana, C.; Incharoensakdi, A.; Phunpruch, S. Light emitting diode illumination enhances biomass, pigment and lipid production in halotolerant cyanobacterium Aphanothece halophytica. Phycology 2025, 5, 12. [Google Scholar] [CrossRef]
| Fatty Acid Compositions (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Types of fatty acids | BG-11 | BG-11 with Pb | ||||||
| 100% a | 50% a | 0% a | 100% a | 50% a | 0% a | |||
| Decanoic acid (C10:0) | 8.21 ± 0.37 | 7.66 ± 0.31 | n.d. * | 24.30 ± 0.80 | 11.16 ± 0.33 | 2.56 ± 0.11 | ||
| Myristic acid (C14:0) | 11.86 ± 0.36 | 10.88 ± 0.30 | ≤0.01 | 3.33 ± 0.11 | 11.13 ± 0.28 | 12.02 ± 0.35 | ||
| Palmitic acid (C16:0) | 42.27 ± 1.99 | 45.86 ± 1.86 | 49.24 ± 1.95 | 26.19 ± 1.05 | 38.59 ± 1.75 | 54.57 ± 2.05 | ||
| Palmitoleic acid (C16:1) | 15.52 ± 0.62 | 9.32 ± 0.39 | 33.36 ± 1.50 | 5.27 ± 0.15 | 17.15 ± 0.80 | 7.61 ± 0.33 | ||
| Stearic acid (C18:0) | 17.94 ± 0.81 | 16.68 ± 0.78 | 11.36 ± 0.35 | 25.24 ± 0.81 | 18.01 ± 0.82 | 11.89 ± 0.34 | ||
| Oleic acid (C18:1) | ≤0.01 | 6.92 ± 0.31 | ≤0.01 | 10.59 ± 0.35 | ≤0.01 | 2.30 ± 0.10 | ||
| Others b | 2.90 ± 0.10 | 2.68 ± 0.11 | 6.02 ± 0.24 | 5.08 ± 0.22 | 3.95 ± 0.12 | 9.05 ± 0.35 | ||
| SFA * | 83.28 ± 2.42 | 81.07 ± 2.18 | 60.59 ± 2.30 | 79.06 ± 2.31 | 78.88 ± 2.31 | 81.04 ± 2.51 | ||
| UFA * | 16.72 ± 0.65 | 18.93 ± 0.80 | 39.41 ± 1.81 | 20.94 ± 0.90 | 21.12 ± 0.82 | 18.96 ± 0.78 | ||
| Factors | EN | ASTM | Biodiesel properties | |||||
| SV (mg KOH/g) | NA | NA | 226.50 ± 10.61 | 224.14 ± 9.82 | 215.32 ± 9.85 | 236.64 ± 10.18 | 228.92 ± 10.73 | 219.49 ± 9.55 |
| IV (g I2/100g) | 120 | NA | 16.57 ± 0.76 | 17.95 ± 0.81 | 38.75 ± 1.74 | 19.35 ± 0.75 | 20.69 ± 0.83 | 17.74 ± 0.51 |
| CN | >51 | >47 | 66.67 ± 2.91 | 66.61 ± 2.88 | 62.93 ± 2.82 | 65.00 ± 2.68 | 65.49 ± 3.01 | 67.18 ± 3.09 |
| CP (°C) | NA | NA | 18.82 ± 0.85 | 19.13 ± 0.88 | 20.91 ± 0.84 | 8.78 ± 0.33 | 15.31 ± 0.66 | 23.71 ± 1.14 |
| HHV (MJ/kg) | NA | NA | 38.90 ± 1.56 | 38.97 ± 1.62 | 39.21 ± 1.81 | 38.56 ± 1.71 | 38.82 ± 1.86 | 39.09 ± 1.90 |
| Viscosity (mm2/s) | 3.5–5.0 | 1.9–6.0 | 3.47 ± 0.16 | 3.54 ± 0.17 | 3.70 ± 0.17 | 3.18 ± 0.16 | 3.37 ± 0.16 | 3.68 ± 0.18 |
| Density (g/cm3) | 0.86–0.90 | NA | 0.87 ± 0.04 | 0.87 ± 0.04 | 0.87 ± 0.05 | 0.87 ± 0.04 | 0.87 ± 0.04 | 0.87 ± 0.04 |
| Conditions | Total Lipids (g/L) | HV of Lipids (kJ) | Input Light Energy (kJ) | TECE (%) |
|---|---|---|---|---|
| 100% P (BG11) | 0.09 ± 0.001 | 3.14 ± 0.06 | 18.88 | 15.84 ± 0.71 |
| 0% P | 0.09 ± 0.003 | 3.25 ± 0.10 | 18.88 | 17.19 ± 0.51 |
| 0% P + Co | 0.06 ± 0.01 | 2.35 ± 0.19 | 18.88 | 12.44 ± 1.03 |
| 0% P + Fe | 0.10 ± 0.03 | 3.71 ± 1.19 | 18.88 | 19.66 ± 6.28 |
| 100% P + Pb | 0.14 ± 0.008 | 5.04 ± 0.29 | 18.88 | 26.70 ± 1.52 |
| 50% P + Pb | 0.09 ± 0.001 | 3.16 ± 0.07 | 18.88 | 16.73 ± 0.36 |
| 0% P + Pb | 0.08 ± 0.01 | 2.85 ± 0.37 | 18.88 | 15.08 ± 1.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pattharaprachayakul, N.; Sivaramakrishnan, R.; Incharoensakdi, A. Enhancement of Lipids Content in Chlorella sp. Under Phosphorus Limitation and Heavy Metal Addition for Biodiesel Production. Phycology 2025, 5, 49. https://doi.org/10.3390/phycology5030049
Pattharaprachayakul N, Sivaramakrishnan R, Incharoensakdi A. Enhancement of Lipids Content in Chlorella sp. Under Phosphorus Limitation and Heavy Metal Addition for Biodiesel Production. Phycology. 2025; 5(3):49. https://doi.org/10.3390/phycology5030049
Chicago/Turabian StylePattharaprachayakul, Napisa, Ramachandran Sivaramakrishnan, and Aran Incharoensakdi. 2025. "Enhancement of Lipids Content in Chlorella sp. Under Phosphorus Limitation and Heavy Metal Addition for Biodiesel Production" Phycology 5, no. 3: 49. https://doi.org/10.3390/phycology5030049
APA StylePattharaprachayakul, N., Sivaramakrishnan, R., & Incharoensakdi, A. (2025). Enhancement of Lipids Content in Chlorella sp. Under Phosphorus Limitation and Heavy Metal Addition for Biodiesel Production. Phycology, 5(3), 49. https://doi.org/10.3390/phycology5030049






