Abstract
The nutrient-rich composition of seaweeds and lichens makes them well-suited for agricultural applications. Their use as alternatives to synthetic fertilizers contributes to sustainable agricultural production, enabling farmers to adopt ecological practices while maintaining or increasing crop productivity. This review aims to highlight the status and trends of research, along with a literature analysis on the application of these biomasses in sustainable agriculture. A bibliometric analysis was performed based on two databases (Scopus and Web of Science) to overview the main research topics regarding the use of biomasses studied in agriculture, thus providing useful information for future research. The biochemical composition and agricultural applications of these biomasses have been highlighted. The analysis shows that these biomasses are rich of nutrient compounds, revealing their roles and mechanisms of action on the chemical, nutritional properties, and soil microbial activities and their effect on plant growth, using various extraction and application methods. It also highlighted the potential of seaweeds for protection against biotic and abiotic stresses. In light of all the data presented in this review, it is possible to stimulate farmers’ interest in using seaweeds and lichens as natural fertilizers, with a focus on sustainable and ecological agriculture mainly in developing countries.
1. Introduction
The current trend in scientific research directed at agriculture is to discover new sources of nutrients as alternatives to chemical fertilizers [1]. In order to overcome the high cost and the environmental impact of usual fertilizers, natural products offer multiple benefits for improving the nutritional value of plants while being environmentally sustainable. Seaweeds and lichens are among natural sources with biofertilization potential [2,3,4]. Seaweeds have gained importance as a treasure trove of nutrients, including minerals, proteins, pigments, growth hormones, fatty acids, polyphenols, and oligosaccharides, which were found to impact plant development from seed germination [5,6,7] to vegetative growth [3,8,9,10,11,12]. The use of seaweed as biofertilizer products has been developed and applied in various forms, including liquid extracts, where the constituents of the seaweed are partially decomposed or isolated, allowing for more rapid and effective absorption by plants, or in the form of powders that promote plant growth through a gradual release of mineral nutrients [13,14], such as the liquid extract of Sargassum ilicifolium stimulated the development of Trigonella foenum-graecum, influencing growth parameters such as shoots and roots length, as well as the contents of photosynthetic pigments, carbohydrates, proteins, amino acids, polyphenols, and nitrogen [15,16]. Green seaweed species can also be applied as liquid extracts for agricultural crops. For example, the liquid extract of Ulva lactuca was applied to the growth of Vigna radiata plants, improving germination rate, vegetative growth, and several biochemical parameters [6]. Thus, aqueous extracts of Enteromorpha flexuosa were reported by Omar et al. [12] as a natural fertilizer for the growth of Zea mays, including an increase in shoot and root length, dry weight of aerial and root parts, photosynthetic pigments, carbohydrate and protein content, and nutrient absorption. Similarly, the application of Sargassum muticum powder has shown positive effects on the morphological and biochemical characterization of Capsicum annuum plants, as well as the nutritional quality of their fruits [11]. However, modern agricultural practices tend to use extracts of bioactive compounds derived from seaweeds. For example, polysaccharide extracts (carrageenan or agar) obtained mainly from red algae such as Calliblepharis jubata, Chondracanthus teedei, and Gracilaria gracilis, have been used for their positive effects on plant growth [5]. Thus, the phenolic compounds extracted from seaweeds can serve as biofertilizers, such as the extraction of phenolic compounds from the brown seaweed Ecklonia maxima used to improve the germination of corn plants [10]. Furthermore, seaweed extracts can be applied directly to the soil or by foliar spray, depending on the agricultural objectives pursued. For example, the application of liquid extracts of Ulva Lactuca and Padina gymnospora as a soil soak has shown promising effects on the growth of Solanum lycopersicum plants [17]. However, the foliar application appears more advantageous to defend plants against unfavorable conditions. Foliar spraying of the liquid algae extract of Enteromoropha intestinalis can be considered an effective means to improve salt stress tolerance in tomato varieties [18]. Thus, foliar treatment with Ascophyllum nodosum extract on carrot plants increased their resistance to Alternaria radicina and Botrytis cinerea fungal pathogens [19].
Lichens are an effective source of macronutrients (nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), and magnesium (Mg)), micronutrients (iron (Fe), copper (Cu), manganese (Mn), and zinc (Zn)), amino acids (proline, methionine, cysteine, asparagine, and alanine), organic acids (oxalic acid, lactic acid, tartaric acid, and malic acid), as well as plant growth regulators (gibberellic acid, indole acetic acid, and abscisic acid) [4,20,21,22]. In the same way, lichens produce substances such as norstic acid, psoromic acid, usnic acid, and iso-usnic acid [23], which interact with minerals such as K, Cu, and Fe, forming complexes that lead to the bioaccumulation of trace elements in lichens [24]. These properties give lichens the potential to be used as natural organic fertilizers for plant growth. Species such as Dermatocarpon miniatum and Parmelia saxatilis have been successfully demonstrated as natural organic fertilizers [4]. This is evidenced by the improved growth of Genipa americana plants in soils covered with lichen Cladonia salzmannii Nyl compared to lichen-free soil [25]. However, to date, there has been no direct application of lichens as an organic nutrient to improve soil fertility.
In fact, the objective of this review is to provide a scientific overview of the application of seaweeds and lichens in agriculture by offering a critical bibliometric and bibliographic analysis of research on their potential as biofertilizers. It also explores their role as sustainable alternatives for agricultural crop improvement.
2. Bibliometric Analysis of Research on the Use of Seaweeds and Lichens for Agricultural Purposes: Insights from Scopus and Web of Science
2.1. Methods
2.1.1. Descriptive Sources
To gain an overview of the main research in the literature and emerging trends concerning the application of seaweeds and lichens as organic fertilizer, a bibliometric analysis was conducted using Scopus and the Web of Science (WOS) Core Collection. The keywords used for seaweed research were “seaweed” OR “seaweeds” OR “Macroalgae” OR “marine algae” and “organic fertilizer” OR “fertilization” OR “biofertilization” OR “biostimulant” OR “fertilizer” OR “soil amendment” OR “inoculation” AND “plant growth” OR “plants growth” OR “plant development” OR “crop development” OR “growth promotion”). The search resulted in 383 documents published across 215 sources covering the period from 1968 to 2024. The keywords used in our search for lichens were “lichen” OR “lichens” and “organic fertilizer” OR “fertilization” OR “biofertilization” OR “biostimulant” OR “fertilizer” and “plant growth” OR “plants growth” OR “plant development” OR “crop development” OR “growth promotion”. The search resulted in a dataset with 24 documents derived from 19 distinct sources obtained in a period from 1989 to 2024. The search type was set to “article” and “review”, and the search language was set to “English”.
2.1.2. Methodologies Analysis
The dataset extracted from the Scopus and WOS databases was applied to analysis packages using R software (version 4.1.2) [26], bibliometrix [27], and VOSviewer [28] to visualize key parameters, including frequency of publications per year, most frequent keywords, most involved countries, and trends topic emergent in the subject of the seaweeds and lichens applications as fertilizer organic. These parameters were expressed by several methods, such as annual scientific production, word cloud analysis, co-occurrence network keywords analysis, countries’ scientific production, and topic trends analysis.
2.2. Results of Bibliometric Analysis on the Application of Seaweeds in Agriculture
2.2.1. Annual Evolution of the Number of Publications
The annual publication frequency of research articles on the application of seaweeds in agriculture is shown in Figure 1. Publications on this topic began to multiply in 1998 but were very few until 2008. From 2015 onwards, the number of articles published annually increased slightly, reaching an average of 13 articles per year. The number of publications peaked in 2022 with 57 articles and remained high until 2024. This confirms the continuing and growing trend of research into the use of algae as natural biofertilizing resources.
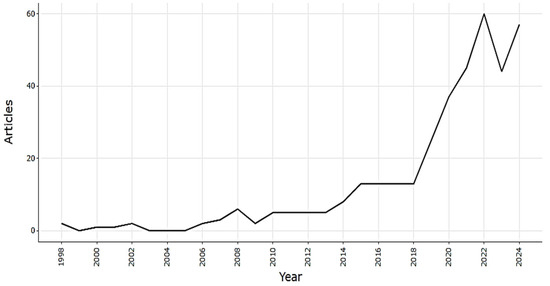
Figure 1.
Annual scientific production on application of seaweeds as biofertilizer from 1998 to 2024.
2.2.2. Keyword Analysis
The visualization mode used in the analysis of keywords relating to the application of seaweed in agriculture is mainly presented in the form of a word cloud map (Figure 2), which highlights the frequency of appearance of keywords in a set of publications extracted from the WOS and Scopus databases. The results of this analysis reveal a high frequency of scientific articles concerning the use of seaweed to fertilize the soil, thereby promoting plant germination and growth as well as the development of their mechanisms due to their richness in nutrients and phytohormones. In addition, the little words in the cloud suggest potential avenues of research to improve the sustainability of seaweed application in agricultural crops. In this context, our findings strongly suggest the need to develop different modes of seaweed application, such as in the form of extracts or compost. Similarly, it is crucial to further explore the positive role of algae on the soil microbial community, stimulating its activity and contributing to the assimilation of nutrients by plants.
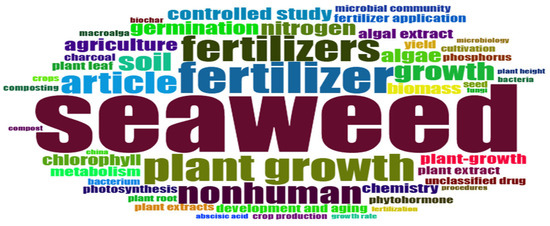
Figure 2.
Word cloud analysis—the most frequent keywords highlight the role of seaweed in agriculture.
2.2.3. Three Fields Plot Analysis
Analysis of the “Three Fields Plot” map (Figure 3) highlights the relationships between the most frequently cited keywords, the authors, and the countries most involved in the evolution of the impact of seaweed as a valuable source of agricultural nutrients, offering an alternative and ecological solution. In line with the word cloud map analysis, the most frequently cited keywords were “seaweed”, “fertilizer”, and “plant growth”. However, the analysis also revealed that the impact of seaweed on soil properties is being studied, albeit to a lesser extent. The most cited authors in this field are Liu Z., Van S.J., Michalak I., and Rouphael Y. These researchers appear to be conducting in-depth research into the subject under analysis. The countries most involved in algae biofertilization are Italy, Portugal, India, and China. They represent key research centers for this ecological approach.
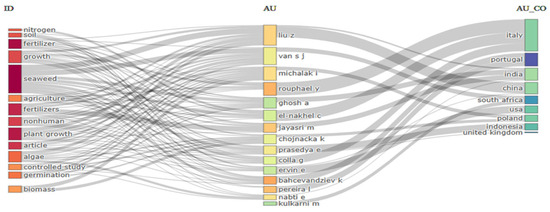
Figure 3.
Three Fields Plot map analysis—network analysis of highly cited keywords, authors, and country in application for seaweeds for agriculture application. The darkness of the color bars shows the frequency of occurrence of each keyword, author, or country, while the height of the bars reflects the relative importance or strength of the relationship between entities.
2.3. Results of Bibliometric Analysis on the Application of Lichens in Agriculture
2.3.1. Annual Scientific Publication
The number of publications may reflect the evolution of lichen valorization processes for agricultural crop improvement. As shown in Figure 4, the total number of articles published in this field has shown little irregular growth, with an annual growth rate of 4.04%. The first publication was in 1989, and from then until 2008, the number of papers published annually irregularly did not exceed one paper, corresponding to a long embryonic period. The attention of scientific researchers to lichen as an organic fertilizer began to fluctuate between 2011 and 2024; however, the annual publication frequency remains very low, not exceeding 4 papers per year. This could encourage a number of scientific research projects to take greater advantage of this new ecological approach.
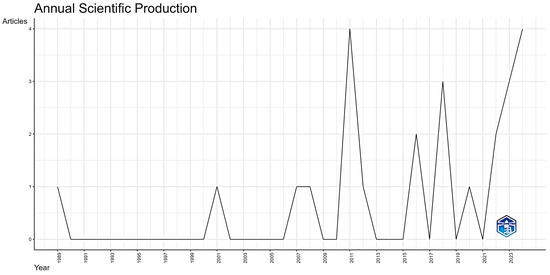
Figure 4.
Annual scientific production on application of lichen as organic biofertilizer from 1989 to 2024.
2.3.2. Keyword Analysis
The frequency of keywords appearing in the literature on the application of lichen as an organic fertilizer is expressed by the keyword co-occurrence network diagram drawn using VOSviewer software (version 1.6.20) (Figure 5). The area of the circular keyword zone in the graph represents the frequency of occurrences of the keyword; the larger it is, the more often the keyword in question appears. The different colors of the circles represent the different themes used in the search area. The relationship between co-occurrences is expressed by the lines connecting the circles, shedding light on the directions of scientific research evaluating lichen species as organic fertilizers.
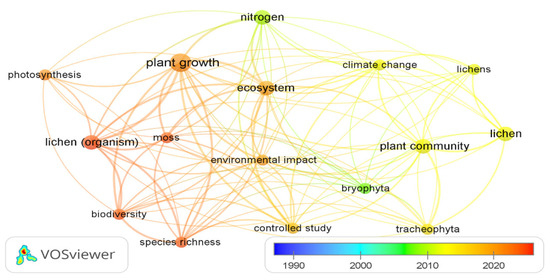
Figure 5.
Co-occurrence network diagram of high-frequency keywords cooperation in the research fields of lichen-based organic fertilizer.
The co-occurrence network analysis was carried out on 16 keywords with a high frequency of occurrence (more than 2 times) among the 460 keywords. The most frequent keywords were plant growth, nitrogen, lichen (organism), ecosystem, lichens, plant community, etc. Among these, those that have appeared significantly in the literature in recent years are plant growth, photosynthesis, moss, lichen (organism), biodiversity, and species richness. The results of this analysis reveal the high frequency of scientific articles highlighting the importance of the application of lichen as an agricultural fertilizer due to its role in plant growth by releasing essential nutrients, such as nitrogen, and thus promoting photosynthesis. In addition, Figure 5 suggests potential avenues of research to enhance the role of lichen in plant communities, particularly bryophytes, and tracheophytes, thereby influencing ecosystem structure and diversity.
2.3.3. Most Countries Production
The most productive countries in terms of scientific papers on the subject of lichens in agriculture are shown in the heat map in Figure 6. China is the world’s most productive country in this field, having focused its research on the application of lichen as a fertilizing amendment, with a total of 7 scientific publications, followed by Brazil and Sweden. Other countries do not produce more than 3 papers. In 2024, China, Brazil, Sweden, and the United States were more involved in researching and using lichen in agriculture as part of the development of organic and sustainable agriculture.
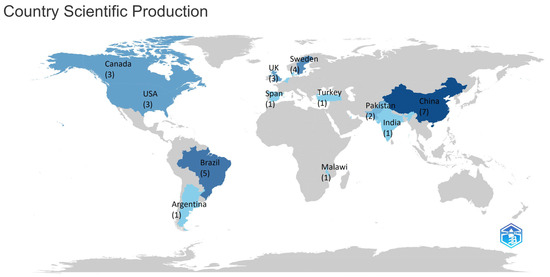
Figure 6.
Map showing the 16 most prolific countries in terms of publications on the application of lichens to agricultural crops between 1989 and 2024 (number of publications). Countries less frequently involved in this field are shown in gray.
2.3.4. Trend Topics Analysis
The trend analysis highlighted the frequency and distribution of keywords over time on the theme of lichen biomass application in agriculture (Figure 7). The most frequent keyword is “nitrogen”, appearing from 2008 to 2018, indicating a high frequency of studies on the nitrogen richness of lichens. The word “fertilization” appeared frequently between 2018 and 2024, during which time researchers studied the effect of lichens on soil fertilization and plant growth.
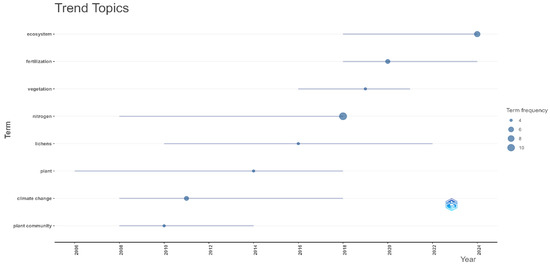
Figure 7.
Trend Topics analysis—frequency and distribution of keywords over time in the research on using lichens in agriculture application.
2.4. Future Orientations Related to the Bibliometric Approach
The bibliometric analysis used in this study, which focused on biofertilization using seaweeds, revealed an average annual growth rate of 13.75% in scientific output. This percentage highlights the need for further research in order to fill gaps that were not revealed by the used visualization parameters, such as the application of different species of seaweed harvested in different geographical areas, in order to globalize this agricultural approach. Another gap concerns the need for further research into biofertilization mechanisms on different soil types and through different agricultural applications in order to maximize the benefits of seaweed components in the context of sustainable agriculture. Similarly, further research is needed to develop relevant solutions for the management of self-sustained seaweed cultivation in order to minimize the negative impact on ecosystem sources.
The limited number of publications on the application of lichens in agriculture (24 documents) restricts analytical power and the ability to identify emerging trends in this ecological practice. In this regard, the present bibliometric analysis could, in the future, stimulate researchers’ interest in developing the use of lichens in agriculture, focusing on three main topics for further study: (1) their effect on the physical, chemical, and biological characteristics of soil; (2) their impact on the growth of different types of agricultural crops; and (3) their role in protecting against biotic and abiotic stresses. These areas should remain the focal point of future research, as they reflect a wide range of agricultural interests aimed at maximizing organic biomass diversity to sustainable and ecological agriculture and reducing dependence on chemical fertilizers, thereby contributing to the preservation of environmental health.
3. Bibliographical Analysis of the Application of Seaweeds as a Natural Fertilizer to Enhance the Crop Production
3.1. African Seaweed: A Resource for Sustainable Agriculture
The African continent, with its varied climatic zones and coastal areas, presents a great diversity of seaweed species. More than 2200 species of seaweed have been recorded in Africa, including over 1400 species of red algae, some 400 species of green algae, and almost 400 species of brown algae [29,30]. Species such as Eucheuma spp. and Kappaphycus spp. in Tanzania account for 92% of algal production and 4.7% in Madagascar [31,32]. Indeed, Tanzania began exploiting the economic potential of algae in 1989, starting commercial cultivation using Eucheuma strains as biofertilizers [32]. Similarly, Africa is the world’s third-largest producer of red algae, with annual production of around 120,000 tons of fresh material [33].
Morocco, with its Atlantic and Mediterranean coasts, as well as the unique hydro-climatic and salinity conditions of its marine currents, offers a wealth of considerable taxonomic richness, which supports the development of marine algae [34]. The city of El Jadida is the richest region in terms of algae diversity. Studies carried out by Bahammou et al. [35,36] highlighted this richness by constructing a checklist of the different types of algae. The study identified 33 species of green algae, dominated by Cladophoraceae and Ulvaceae, as well as 29 brown algae, the majority of which belong to the Sargassaceae, followed by the Chordariaceae. In addition, 156 species of red algae have been recorded, with the majority belonging to the order Ceramiales, followed by Gigartinales, Gelidiales, and Corallinales. In addition, the Essaouira coastline is considered a significant source of algae collection, listing 39 genera of brown algae and 104 genera of red algae [37,38]. Another region in Morocco with a great diversity of seaweed species is Al-Hoceima National Park, as noted by Moussa et al. [39]. Several other Moroccan regions also boast a diversity of macroalgae, including Saidia, Melilla, Tamernoute, and M’diq in the Mediterranean Sea [40,41].
3.2. Chemical Composition of Algae
Seaweeds are rich in biocompounds. They can be used as sources of oligosaccharides, mineral elements, polyphenols, phytohormones, vitamins, chlorophyll, phycobiliproteins, fucoxanthin, phenol, flavonoids, sterols, and terpenes. These biocompounds play a potential role in agricultural crop improvement [42,43,44].
3.2.1. Oligosaccharides
Seaweeds are an important source of complex polysaccharides, which differ from one species to another. Their acid or enzymatic hydrolysis yields oligosaccharides [45], such as alginate oligosaccharides derived from alginate, a polysaccharide found mainly in brown algae, extracted in particular from Laminaria japonica [46] and Sargassum polycystum [47]. Fuco-oligosaccharides are derived from the hydrolysis of fucoidan extracted from several species of brown algae, including Saccharina japonica [48], Sargassum horneri [49], Ascophyllum nodosum [50], and Sargassum siliquosum [51]. Laminaran oligosaccharides are derived from the polysaccharide laminarin, predominantly found in brown algae, mainly Laminaria digitata, Sargassum polycystum, Turbinaria ornate, and Padina boryana [52,53]. Green algae, particularly species of the Ulva genus, produce the ulvan polysaccharide, which hydrolyzes to form ulvan-oligosaccharides [54,55]. Agar-oligosaccharides and carrageenan-oligosaccharides are obtained by enzymatic or chemical hydrolysis of agar and carrageenan polysaccharides from the red algae [56], such as the utilization of Gracilaria fisheri [57], Gracilaria verrucosa, Gelidium latifolium [58], and Gelidium sesquipedale [59] for agar extraction. Carrageenan is mainly extracted from Hypnea bryoides [60].
The research on oligosaccharides derived from seaweed began in 1995 and has been progressing rapidly since 2005 [61]. They are studied for their potential effects on plant growth due to their ability to improve nitrogen assimilation and stimulate basal plant metabolism [62,63,64,65]. For example, the alginate oligosaccharides can stimulate the growth of Hordeum vulgare seedlings and roots, as well as the rate of photosynthesis. In addition, this oligosaccharide induces the expression of development-related genes, notably those encoding an auxin response factor and a protein kinase [64]. Laminaran oligosaccharide is mentioned by Krishna et al. [65] for its agricultural applications, promoting plant growth. Foliar spraying with oligocarrageenan extracted from the red alga Kappaphycus alvarezii increased ear height, diameter, fresh and dry weight, and yield in Zea mays [66]. Improved seedling emergence was also stimulated by the application of ulvan oligosaccharide to Phaseolus vulgaris plants [67]. In addition, the oligosaccharides delivered from seaweeds can protect plants against abiotic stress, such as the application of oligo-alginate to defend against salt stress affecting Eucomis autumnalis plants, improving their physiological activity [68]. Thus, to protect against biotic stress, such as a reduction in infection caused by the pathogen Botrytis cinera and Aspergillus flavus affecting plants, using laminarin oligosaccharides [69]. Reduction in Fusarium infection caused by Fusarium oxysporum in Phaseolus vulgaris plants due to the increase in their phenolic compounds induced by ulvan oligosaccharide treatment [67].
3.2.2. Major Mineral and Trace Elements
Overall, seaweeds presented high levels of nitrogen, phosphorus, potassium, calcium, magnesium, manganese, zinc, and copper [70,71]. Their content varies considerably between algae species (Table 1). In fact, several species, such as Fucus vesiculosus, Laminaria digitata, Undaria pinnatifida, Chondrus crispus, and Porphyra tenera are reported to be rich in essential minerals and trace elements, notably sodium, potassium, calcium, magnesium, iron, zinc, manganese, and copper as reported by Rupérez [71]. Indeed, the application of seaweed-based biofertilizers improves soil nutrient levels in nitrogen, phosphorus, potassium, and other minerals necessary for plant growth, as confirmed by Nabti et al. [3], revealing that the application of liquid biofertilizers from seaweed can reduce the application rates of chemical fertilizers (nitrogen, phosphorus, and potassium). Crouch et al. [13] have exploited a biofertilizing product, “Kelpak”, based on Ecklonia maxima seaweed extract, to supply Ca, K, and Mg nutrients to lettuce plants. Similarly, the richness of macroalgae in mineral elements is able to act as an enzyme activator, as in the case of molybdenum, which acts on the antioxidant activity of stressed plants [72].

Table 1.
Content of macro and microelements in different seaweed species (mg·100 g−1 DW).
3.2.3. Phytohormones
Seaweeds produce several phytohormones, including auxins, cytokinin, gibberellins, abscisic acid, and ethylene, whose concentrations vary according to algal species, season, and harvesting location (Table 2). As revealed by the two studies conducted by Saebmehr et al. [81,82] on Sargassum muticum and Gracilaria corticata seaweeds harvested from Bushehr (north of the Persian Gulf). They showed significant seasonal variations in their abundance of phytohormones. Abscisic acid yields were very low for several months, but in November, they intensified to 20.667% for Sargassum muticum and 66.20% for Gracilaria corticata. A maximum auxin concentration was noted for Sargassum muticum in May, while Gracilaria corticata reached its maximum concentration in January. Gibberelline levels increased by 58.561% in Sargassum muticum in July and 84.467% in Gracilaria corticata in May. This fraction of the phytohormonal composition of algae gives them the potential to be used for promoting plant growth in agricultural crops by stimulating the evolution of several processes, such as seed germination, cell division, plant differentiation and elongation, and the formation of root architecture [8,83], as well as improving crop metabolic activity and stimulating nutrient mobilization [84,85]. They also enhance the resistance capacity of many crops to biotic and abiotic stresses, such as the development of specific mechanisms for salinity tolerance and drought and cold resistance [86,87,88]. In addition, they may have antioxidant activities against several bacterial and fungal pathogens [89].

Table 2.
Content of phytohormones in different seaweed species.
3.3. Extraction of Seaweed Compounds for Agricultural Applications
3.3.1. Aqueous Extract
Various methods for extracting bioactive compounds from macroalgae have been studied as biofertilizers. Among the most widely used methods is aqueous extraction, which is widely used to obtain liquid extracts rich in soluble nutrients and can be performed using a number of techniques [6,12,15,92]. Boiling extraction is widely utilized, where algae biomass is boiled in distilled water. This method has produced positive results in terms of improved plant growth. For example, the application of the aqueous extract of Ulva lactuca, obtained by boiling, gives results comparable to those of Hoagland’s nutrient medium. It improves plant growth and fresh biomass, as well as carbohydrate, protein, free amino acid, polyphenol, and nitrogen content in different plants Corinderum sativum, Trigonella foenum graecum, and Spinacia oleracea [16]. Likewise, a better seed vigor index and seed endurance index were shown in Corinderum sativum treated with boiling liquid extracts of Ulva Lactuca at concentrations of 6 and 8% [15]. Similarly, extraction by soaking, which consists of infusing seaweed in distilled water for a prolonged period (generally between 24 h and 2 days), has been shown to improve plant growth, with notable effects on mineral nutrition and biochemical parameters [6]. A study carried out by Godlewska et al. [92], comparing boiling and soaking extraction, suggests that both seaweed extraction methods have biofertilizing potential, promoting plant growth and improving mineral nutrition. The results of this study show that both methods offer extraction rich in micro- and macro-elements, having a positive effect on Lepidium sativum plant growth, increasing the length of plants treated with 10% boiled extract, and improving nutrient content in the group treated with 10% soaking extract. In addition, in terms of antimicrobial properties, the extract obtained by boiling showed inhibitory activity against Escherichia coli, while the extract obtained by soaking showed no inhibitory effect against Escherichia coli and Staphylococcus aureus. Similarly, the application of seaweed extracts such as Polysiphonia lanosa revealed an antibacterial effect against plant pathogens like Xanthomonas fragariae and Xanthomonas arboricola [93]. In addition, the liquid extract can provide protection against oxidative stress, such as improved tolerance to drought stress in Triticum aestivum treated with Sargassum latifolium and Ulva Lactuca liquid extracts [94].
3.3.2. Organic Extracts
Marine algae produce a wide range of active metabolites, including alkaloids, cyclic kernels, polysaccharides, diterpenoids, sterols, quinones, lipids, and glycerol. Their extracts have potential as biofertilizing and protective agents in plants.
- (a)
- Polysaccharide Extraction
Polysaccharide yields vary among seaweed species, as shown by He et al. [95], who evaluated their proportions in different types of seaweed. They found variable yields in green algae such as Ulva lactuca and Ulva intestinalis, brown algae like Durvillaea antarctica, and red algae including Gracilaria lemaneiformis and Sarcodia ceylonensis, with respective polysaccharide levels of 11.09% ± 0.87, 29.15% ± 0.19, 14.21% ± 1.03, 13.32% ± 0.93, and 12.49% ± 0.79. In addition, Furcellaria lumbricalis and Polysiphonia lanosa show high levels of polysaccharides, namely 19.71 and 49.20%, respectively, as reported by Jiao et al. [96]. On the other hand, the species Fucus virsoides and Cystoseira barbata showed polysaccharide contents of 15.07 and 7.80%, respectively [97]. This polysaccharide fraction can be used in agriculture as a strong and environmentally friendly biostimulant to improve plant growth and yield by boosting their antioxidant activities and their content of bioactive compounds. For example, the application of a Halimeda dilatata polysaccharide extract obtained by organic solvent acetone and chloroform to mung bean plants has improved many agronomic factors such as shoot and root elongation, leaf area, fresh and dry weight, total chlorophyll, carbohydrate, and protein content, as well as flower number, pod length and weight, and seed yield [9]. In addition, Mzibra et al. [98] show that polysaccharide extracts from algal species of different types, green algae (Ulva rigida and Codium decorticatum), red algae (Gigartina sp. and Chondracanthus acicularis), and brown algae (Fucus spiralis and Bifurcaria bifurcata) obtained by aqueous extraction followed by ethanol precipitation, have positive effects on seed germination, biomass, and chlorophyll content of the Solanum lycopersicum plant. The treatment of Zea mays plants with a soluble polysaccharide extract from Ulva fasciata at a concentration of 5 mg/mL increased carbohydrate, protein, phenol, ascorbic acid, peroxidase, and catalase contents [99]. Red algae are widely used to extract polysaccharides that promote plant development. For example, the carrageenan extract of Calliblepharis jubata and Chondracanthus teedei, obtained by alkaline extraction, and the agar extract of Gracilaria gracilis, obtained by aqueous extraction under pressure, have shown a beneficial effect on the germination and growth of Brassica oleracea plant [5]. In addition, the polysaccharide extracts from seaweeds have the ability to act as an elicitor of defense responses in plants, such as the application of Ulva fasciata polysaccharide extract to reduce the severity of anthracnose in Phaseolus vulgaris L. [100], as well as the control of Verticillium wilt of olive caused by Verticillium dahliae [101].
- (b)
- Polyphenol Extraction
Concerning phenolic compounds, their optimal extraction begins as soon as the algae are harvested, and they must be frozen in liquid nitrogen to limit any loss of these compounds. The seaweed must then be carefully dried to avoid the loss of phenolic compounds [102]. Several extraction methods using solvents for the extraction of phenolic compounds have been employed, such as maceration, Soxhlet extraction, and microwave-assisted extraction [103]. A study carried out by Aremu et al. [10] extracted the phenolic compounds (eckol and phloroglucinol) from brown algal Ecklonia maxima by solvent–solvent extraction with various polarity (methanol, hexane, dichloromethane, and ethyl acetate). Phenolic compounds were identified and isolated using spectroscopic techniques. The phenolic extracts obtained were applied to the soil to enhance the growth of Eucomis autumnalis. After 4 months of growth, the eckol extracts improved bulb size and root production, while the phloroglucinol extract significantly increased bulb number. In addition, these compounds induced increased levels of bioactive phytochemicals, such as hydroxybenzoic acid, ferulic acid, and indole-3-acetic acid. Indeed, the aqueous extracts of different algae species contain high levels of phenolic compounds, including phenolic acids, lignins, flavonoids, tocopherols, and tannins [104]. For example, a total polyphenol content of 34.26 mg of gallic acid per gram of extract (mg GAE/g) was measured in Padina durvillaei and 27.29 mg GAE/g in Ulva lactuca [83]. This component plays an important role in plant growth and antibacterial activity [83,92].
- (c)
- Phytohormones Extraction
The phytohormones are key components in the regulation of plant metabolism, and their extraction from seaweed has been the subject of various studies [11,104,105]. For example, Sanderson et al. [105] described the extraction and identification of auxins from Ascophyllum nodosum powder using a liquid–liquid extraction method. Similarly, the extraction of indole-3-butyric acid from Sargassum muticum was carried out using methanol, followed by a liquid–liquid extraction with ethyl acetate, used to assess the effects of this seaweed on the growth of Capsicum annuum plant [11]. Indeed, this fraction can bring benefits to agriculture. For example, seaweed extract prepared by alkaline hydrolysis of Durvillea potatorum seaweed is commercialized as a liquid organic fertilizer in Australia under the trade name Seasol (Seasol International). This liquid organic fertilizer contains glucosides and cytokinins [106]. Similarly, the liquid extract of Padina durvillaei and Ulva lactuca is rich in gibberellin, abscisic acid, indoleacetic acid, cytokinins, jasmonic acid, and salicylic acid. Together, these phytohormones help regulate plant cell metabolism and stimulate plant production and growth [2,83]. However, the direct application of phenolic and phytohormones extracted from seaweeds on plant development and protection is still poorly documented.
3.4. Agriculture Application
3.4.1. Effect of Seaweed on Soil Properties
- Chemical properties and availability of nutrients
The application of seaweed as a soil amendment or liquid biofertilizer can improve soil properties, including pH and nutrient availability. Sulakhudin et al. [107] revealed that the application of the Eucheuma cottonii seaweed resulted in a high cation exchange capacity at the soil level. This is coherent with the results of Ammar et al. [108] who showed that the addition of Sargassum sp. and Gracilaria verrucosa induces significant chemical changes in the soil by increasing its organic matter, rebalancing the pH, and decreasing the C/N ratio in sandy and clayey soils. These changes are considered vital signs of soil fertility. Seaweed application can lead to variations in soil pH over time. However, a study conducted by Kumari et al. [109] showed that the application of seaweed granules or powder can adjust soil pH to a range favorable to microorganisms, typically between 6.0 and 7.3. In this pH range, soil microorganisms are particularly active, promoting the breakdown of organic matter and the conversion of nutrients into bioavailable forms, which enhances the assimilation of nutrients released by the algae. Consequently, maintaining a soil pH close to this range is crucial to maximize the positive impact of algae on plant growth and soil fertility. This was confirmed by Arthur et al. [110], who demonstrated that a liquid extract of Ecklonia maxima promoted rooting in beans and mung plants at a pH of 6.5. Similarly, a study using Sargassum spp. as a biofertilizer for tomato plants grown in the Bahamas, where soils are alkaline with a pH between 7.5 and 8.5, showed that plant growth parameters were negatively affected in the alkaline pH range. Consequently, pH adjustment following algae application is an excellent indicator demonstrating the biofertilizing effect of algae in terms of soil nutrient bioavailability. At pH levels between 6 and 7, nutrient availability is optimal [111]. In contrast, soils with pH levels above 7.5 lead to nutrient deficits [112].
- Microbial Activity
- (a)
- Bacteria
The application of seaweed as a biofertilizer has a significant impact on the biological properties of the soils, namely by promoting the growth and diversity of the bacterial community beneficial to plant growth [2]. As reported by Hussain et al. [113], the application of seaweed extracts from the brown algae Durvillaea potatorum and Ascophyllum nodosum to soil cultivated with tomato resulted in an increase in the total number of bacteria, improved nitrogen availability, and a significant enhancement in the diversity of bacterial communities beneficial to plant growth. Chen et al. [114] showed that the application of fermented Ascophyllum nodosum biofertilizer has significant effects on rhizosphere soil. It leads to the intensification of microbial activity reflected by the increase in several soil enzymes, such as dehydrogenase, nitrite reductase, urease, and cellulase. The improvement of soil biological fertility results in optimal growth of maize seedlings. In line with the findings of Wang et al. [115], the application of a fermented biofertilizer based on the brown alga Sargassum horneri resulted in significant changes in the soil microbial community, including an increase in bacterial α-diversity. These variations were correlated with the activity of enzymes such as invertase, dehydrogenase, protease, polyphenol oxidase, and urease. In fact, a synergistic relationship is produced between microbial diversity and enzyme activity, as a more diverse and active bacterial community stimulates enzyme production, which in turn promotes the decomposition of organic matter and the availability of nutrients for plants, highlighting the potential of this biofertilizer as a sustainable substitute for chemical fertilizers, improving the productivity of tomato crops. A study conducted by Zhou et al. [116] also showed that the application of seaweed on soils can enhance the activity of key soil enzymes such as dehydrogenase, nitrite reductase, and urease, which potentially promote plant growth. Qiqin et al. [117] identified the dominant bacteria in soil grown with Abelmoschus moschatus treated with seaweed biofertilizer. They found a potential abundance of Acidobacteria and Cyanobacteria at the phylum level and Methylobacterium and Micromonospora at the genus level, which are involved in the degradation of soil organic matter. This suggests that the long-term application of seaweed fertilizers is one of the most effective ways of developing sustainable green agriculture.
- (b)
- Fungi
Several studies show that the application of algae to agricultural soil can stimulate fungi dominance involved in rhizosphere improvement and plant nutrition. For example, a study by Espinosa-Antón et al. [118] shows that the application of Ulva ohnoi seaweed extract or powder to soil promotes the development of fungi due to its high cellulose content in the lignocellulosic fraction, which is a beneficial nutrient source for fungi proliferation. While Wang et al. [119] showed that the number of bacteria and fungi in soil fertilized with Lessonia nigrescens and Lessonia flavicans algae was 172% for bacteria and 67% for fungi, higher than in soil not fertilized with algae. These results suggest that the predominance of fungi or bacteria depends on the algal species used as soil improvers.
3.4.2. Effect of Seaweed on Plant Growth
Since ancient times, seaweed has been valued for its potential applications in agriculture, with optimal concentrations and application rates depending on the plant species. [2,3,13]. Initially, they were used as a powdered organic amendment, applied directly to the soil. In this form, they promote the gradual release of mineral nutrients to plants and improve soil structure and moisture retention. Recently, due to scientific advances, liquid forms have also been used for seed soaking, foliar spraying, or soil irrigation [13]. A study conducted by Espinosa-Antón et al. [118] showed that the application of Ulva ohnoi seaweed powder to the soil cultivated by tomato plants improved their growth morpho physiology and increased mineral availability and chlorophyll content [119]. The seaweed extract treatment showed a positive effect on the root, mineral content, and soil microbes. In many fields, it is advisable to combine different modes of application, such as soaking seeds, followed by foliar spraying of the extract at the vegetative growth stage [120], or combining the foliar spray and root dripped [16]. These different methods have shown a positive impact on seed germination [5,6,121,122,123] and plant growth and development [5,6,124,125,126,127,128,129] (Table 3).
- Brown algae
Several species of brown algae, including the genus Sargassum, have been successfully exploited as biofertilizers for different crops demonstrated by numerous studies (Table 3). For example, the application of Sargassum muticum powder to the growth of Capsicum annuum [11]. The soaking of Triticum aestivum seeds in the 20% liquid extract of Sargassum vulgare improved germination and morphological growth, as well as the chlorophyll content of the crop [128]. Similarly, the liquid extract of Sargassum ilicifolium applied as a foliar spray and as irrigation has shown high levels of carbohydrates, proteins, free amino acids, polyphenols, and nitrogen, thus improving the growth of Trigonella foenum-graecum (fenugreek), as reported by Pise and Sabale [16]. Furthermore, the foliar application of Sargussum wightti extract in combination with the recommended rate of chemical fertilizer was proposed by Jayasinghe et al. [129] to improve the growth, yield, and quality of Capsicum annum plants. The study conducted by Patel et al. [15] has also shown beneficial effects on the germination and growth of various seeds such as Trigonella foenum-graecum, Coriandrum sativum, and Spinacia oleracea, as well as on Solanum melongena, Solanum lycopersicum, and Capsicum annuum by their soaking in the seaweed extract of species Sargassum johnstonii, due to its richness in micronutrients and plant growth hormones. This positive effect is expressed in various growth parameters, such as seed germination rate, shoot height, root length, seedling length, and seed vigor index. A similar effect was observed with Padina pavonica [130], Padina gymnospora, and Padina boergesenii [131]. Another brown algal species considered a potential biofertilizer is Ascophyllum nodosum, which is characterized by a mixture of phytohormones (cytokinins, auxins, …). When applied as a foliar spray, it has shown a significant effect on the morphological and biochemical of the Allium cepa L. plant [132] and in various other cultivars such as Phulkara, Nasarpuri, Lambada, and Red Bone [133]. Similarly, the application of Fucus spiralis extracts as foliar spray promotes the vegetative growth of bean plants [134] by activating the enzyme nitrate reductase, essential for plant nitrogen nutrition.
- Green algae
Concerning green algae, mainly Ulva species, have an important potential for biofertilization and biostimulation of different agricultural crops, such as soaking Glycine max L. seeds in Ulva lactuca liquid extract has been shown to improve germination, as reported by Ramarajan et al. [121]. Similarly, Vigna radiata L. seeds showed improved germination following the same treatment, as demonstrated by Castellanos et al. [135]. The beneficial effect of green algae on agriculture application was also observed in the study conducted by Karthik et al. [136] when they soaked the seeds of Phaseolus vulgaris, Raphanus sativus, and Vigna radiata in the liquid extract of Ulva reticulata, followed by foliar treatment. Similarly, Omar et al. [12] applied Enteromorpha flexuosa in the form of seed soaking and incorporating seaweed powder into the soil. This approach demonstrated the effectiveness of this green alga, rich in nitrogen, phosphorus, potassium, and phytohormones, in stimulating the development of Zea mays L. and Helianthus annuus L. plants by increasing shoot and root length, photosynthetic pigments, carbohydrates, proteins, and nutrient contents. Similarly, soaking the seeds in Codium decorticatum extract enables maximum seed germination due to its richness in various compounds such as proteins, carbohydrates, nitrogen, potassium, sodium, manganese, calcium, and growth regulators [137]. The same findings were found by El-Din [128], applying Codium tomentosum extract for the growth of Triticum aestivum L. seeds presoaked in the extract. However, Hernández-Herrera et al., [138] applied the polysaccharide extract of Ulva lactuca in vitro to promote germination and stimulated the growth of Solanum lycopersicum L., Phaseolus vulgaris L., and Vigna radiata L. plants.
- Red algae
Interesting studies have been carried out on the use of red algae products as biofertilizers. Pise and Sabale [16] reported that soaking Trigonella foenum-graecum seeds in a liquid extract of the alga Gracilaria corticata, followed by foliar spraying and localized root irrigation with the seaweed extract, promoted shoot growth and increased fresh biomass, as well as the contents of carbohydrates, proteins, free amino acids, polyphenols, and nitrogen. Similarly, Satish et al. [131] demonstrated that treatment with the red alga Gracilaria salicornia could be an alternative to phytohormones, revealing by different modes of application, the incorporation of extract in the in vitro culture medium for the propagation of shoots of the Solanum melongena L. plant, as well as the use in the rooting medium to promote root growth. Similarly, polysaccharide extracts from red algae play a crucial role as effective growth promoters for agricultural crops. Carrageenan extracted from Calliblepharis jubata and agar from Gracilaria gracilis have shown beneficial effects on Brassica oleracea plant growth [5].

Table 3.
Effect of seaweed on various agricultural crops.
Table 3.
Effect of seaweed on various agricultural crops.
| Seaweeds | Crops | Method of Application | Effects | Ref. |
|---|---|---|---|---|
| Sargassum wightii Caulerpa chemnitzia | Vigna sinensis | Soaking the seeds for 24 h | The 20% of liquid extracts induced
| [139] |
| Padina gymnospora | Capsicum annum | Soaking the seeds for 24 h | An 8% aqueous extract enhances the germination process of Capsicum annuum, achieving a germination percentage of 70% after 15 days, which is significantly higher than the control group (25%). | [122] |
| Ulva fasciata | Raphanus sativus | Soil incorporation | The application of 10 g dry weight of Ulva fasciata per 500 g of soil resulted in the greatest
| [125] |
| Ecklonia maxima | Eucomis autumnalis | Soil drenching | The application of phenolic compound extract isolated from seaweed at a concentration of 10−6 M improved bulb size and number, fresh weight, root production, and the content of bioactive phytochemicals. | [123] |
| Sargassum wightii | Triticum aestivum | Soaking the seeds for 24 h | The application of a 20% liquid extract resulted in
| [124] |
| Sargassum muticum | Capsicum Annuumm | Soil incorporation | Application of 20 g·kg−1 of seaweed powder improves plant development compared with NPK.
| [11] |
| Lessonia nigrescens Lessonia flavicans | Malus hupehensis | Soil incorporation | Plants treated with 40 g kg−1 of algae showed
| [119] |
3.4.3. Effect Against Biotic Stress
Seaweeds are used to protect plants against bacteria and pests and to improve stress management (drought, salinity, etc.) [140]. In particular, their methanolic extracts are considered a source of bioactive components with antimicrobial activity, such as polysaccharides, peptides, amino acids, lipids, and polyphenols. A study by Arumugam et al. [141] shows that the methanolic extract of Gracilaria edulis acts perfectly against Staphylococcus aureus bacteria due to their richness in esters and polyunsaturated alcohols. However, Ulva lactuca extract has low antimicrobial activity against Pseudomonas aeruginosa. Čmiková et al. [142] point out that the fucoidan present in the algae Laminaria japonica and Undaria pinnatifida is responsible for the strong antimicrobial activity on bacteria (Bacillus subtilis, Staphylococcus aureus subsp. aureus, and Enterococcus faecalis). Another study conducted by Chandrasekaran et al. [143], comparing different biofertilizer extracts, showed that the extract of Ulva fasciata obtained by ethyl acetate showed the greatest antibacterial activity against all tested bacterial strains (Bacillus subtilis, Streptococcus pyogenes, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Salmonella typhimurium, Vibrio cholerae, Shigella flexneri, Proteus mirabilis, and P. vulgaris). Nagayama et al. [144] also reported that the antibacterial activity of Bifurcaria bifurcata is essentially linked to the interactions of bacterial proteins with algal tannin groups, which are polar, highly reactive, and display significant antioxidant activity. Various other studies evaluating the antibacterial effect of algae, such as the methanolic extract of Polysiphonia lanosa against Xanthomonas arboricola [93], Padina tetrastromatica and Sargassum ilicifolium against Vibrio parahaemolyticus [145], Bifurcaria bifurcata and Ulva fasciata collected from the Moroccan Atlantic coastline (Agadir) against pathogenic bacterial strains such as Escherichia coli and Bacillus subtilis [146]. The fatty acids extracted from Spatoglossum asperum were found to inhibit the growth of Macrophomina phaseolina, Rhizoctonia solani, and Fusarium solani [147]. In addition, the seaweeds liquid extracts also act against different types of fungi, such as the application of Halimeda tuna extract against Aspergillus niger, Aspergillus favus, Alternaria alternata, Penicillium sp., and Rhizopus sp. [148]. Similarly, Ascophyllum nodosum liquid extract applied by spraying has a potential elicitor effect against carrot leaf diseases caused by Alternaria cucumerinium, Alternaria radicina, Didymella applanata, and Botrytis cinerea [19].
3.4.4. Effect Against Abiotic Stress
Seaweeds are potentially an effective solution for mitigating the negative effects of plant drought, acting in different ways depending on the species. For example, the application of Laminaria ochroleuca extract reduced lipid peroxidation in plants. The positive effect of Ascophyllum nodosum extracts was mainly attributed to the accumulation of proline in plant tissues. The application of Ulva lactuca resulted in a higher concentration of soluble sugars in plants. In contrast, the application of Fucus spiralis contributed to the accumulation of both proline and soluble sugars [149]. Kasim et al. [94] reported that presoaked seeds of Triticum aestivum in seaweed extracts (Sargassum latifolium, Ulva lactuca, and their mixture) enhanced plant defense against drought stress by activating antioxidant systems, such as catalase, peroxidase, and ascorbate, also providing essential hormones and micronutrients for wheat growth. Furthermore, the foliar application of Ascophyllum nodosum extract, drench, or both promoted the growth of spinach grown under drought conditions by improving leaf water relations, maintaining cell turgor pressure, and reducing stomatal limitation. However, it may have a negative impact on nutritional quality, notably by reducing the ability to chelate ferrous ions under severe drought conditions [150]. Thus, algae are among the most promising treatments for promoting growth in plants grown under salt stress. This is confirmed by the decrease in Na levels in plants grown under salt stress and treated with seaweed [151]. Latique et al. [152] applied the liquid seaweed extract of Fucus spiralis by spraying to durum wheat plants subjected to salt stress and, through this study, they confirmed that algae treatment has proven to be an effective technique for improving the growth of wheat seedlings in salt stress conditions. Furthermore, seaweed extracts can enhance photosynthesis and the antioxidant system in saline-stressed plants by inducing the accumulation of secondary metabolites (proline, total phenols, flavonoids, and antioxidant compounds), which are the main factors modulating plant response to salinity tolerance [153].
3.5. Industrial Development of Seaweed-Based Biofertilizers
The field application of seaweed-based biofertilizers to agricultural crops for human consumption is now widely practiced on a commercial scale. Of these, brown algae are the most widely used in the production of commercial biofertilizers, such as Ascophyllum nodosum, Ecklonia maxima, Durvillea potatorum, and Macrocystis pyrifera [2,154]. Most commercialized products are Ascophyllum nodosum under various product names, such as Seasol (Seasol International), A. nodosum extract (Acadian Seaplants Ltd- In Nova Scotia, Canada.), Super Fifty (BioAtlantis Ltd-County Kerry, Ireland), and Alga Special (AS) (L. Gobbi s.r.l.) [155]. Indeed, Ascophyllum nodosum extract is the most widely exploited, particularly in Canada’s Maritime provinces, where it is a major economic resource [156]. The experiment of Frioni et al. [157] was conducted in 2013 in central Italy (Deruta, Umbria, clay soil type, southern exposure, north–south row orientation) on 48 vines of 15-year-old Vitis vinifera L. An application of 1.5 kg/ha of product five times during the season, starting two weeks before veraison, resulted in acceleration of veraison, improved anthocyanin accumulation, and increased phenol content. This suggests that the modulated application of this product may be a simple way of improving the chromatic and chemical properties of grapes and wines. As part of a three-year field experiment conducted by Szczepanek et al. [158] in Poland, the effects of the commercial biostimulant Kelpak derived from Ecklonia maxima Osbeck, due to their richness in phytohormones, brassinosteroids, alginates, amino acids as well as macro- and microelements, on the growth of the Triticum aestivum L. plant, were evaluated. It was applied at a rate of 1.5 L/ha at the start of tillering and stem elongation. The results indicated a positive response in yields, such as increased grain quantity per ear, grain weight, and overall grain yield under sequential treatment. Ashour et al. [159] used the commercial biofertilizer True-Algae-Max (TAM), which contains a combination of three seaweed species: Ulva lactuca (Chlorophyceae), Jania rubens, and Pterocladia capillacea (Rhodophyceae), and studied its ability to improve the yield and nutritional characteristics of a strawberry plant cultivated in the village of Omar Makram (Egypt). The results showed that treatments with 50% TAM, combined with 50% NPK chemical fertilizer, resulted in a yield of 982.33 g per plant, a 25% increase over the control group. These findings suggest that TAM could serve as a sustainable alternative to reduce the use of chemical fertilizers while enhancing crop yields. Along with the use of Ascophyllum nodosum as a commercial biofertilizer, it also offers effective protection against various biotic and abiotic stresses. For example, Ascophyllum nodosum extract supplied by Acadian Seaplants Ltd. (Dartmouth, Nova Scotia, Canada) was used by Ali et al. [160] on tomato and bell pepper crops in farmers’ fields in Valencia to protect them against biotic stress induced by the bacteria Xanthomonas campestris and Alternaria solani. Foliar application of this extract at 0.5% reduced disease by 60% and increased yields by 57%, achieving fruit yields of 258.15 kg for tomato and 364.10 kg for bell pepper per season, compared with 167.79 kg and 212.04 kg, respectively, in control plots. Similarly, the commercial extract of Ascophyllum nodosum, marketed as Amino Seaweed (SV Group, Bangkok, Thailand), was used by Ahmed et al. [161] to evaluate its effects on growth, physiology, yield, and water use efficiency in tomato under water stress. The results showed that the application of 5 mL/L of this product in soil drenches resulted in a 225% yield increase, with a yield of 523.3 g of fruit per plant, compared with 265.6 g of fruit per plant for plants not treated with algal extract.
In light of the results of the literature review, the biofertilization and bioprotective properties of seaweeds make them invaluable for promoting sustainable, environmentally friendly agricultural practices. Their ability to enhance plant growth under normal conditions or in the event of biotic or abiotic stress means that agricultural crop yields can be optimized.
4. Bibliographical Analysis of the Potential Impact of Lichens as a Source of Organic Fertilizer
4.1. Diversity of Lichens in Africa
Lichen species diversity is potentially threatened by global climate change, particularly in southern Africa [162], which represents a major center of global lichen distribution. Since 1770, biologists have attempted to study the distribution of lichen communities in southern Africa [163]. In Morocco, the first catalog of lichen distribution was provided by Gattefossé and Werner in 1931, listing 542 species. In 1996, a new catalog was published, including 210 genera and 1100 taxa [164]. In 2013, another catalog was updated, showing the evolution of lichen species diversity, which then stood at 1169 species, divided into 21 orders, 75 families, and 241 genera [165]. Finally, the most recent update of the lichen catalog in Morocco shows that the country’s lichen biodiversity now includes 1237 species [166]. However, although they are ecologically important, the study of their distribution is limited.
4.2. Biochemical Characterization of Lichen
Lichens produce primary and secondary substances. Among primary substances are chitin, lichenin, isolichenin, hemicellulose, pectins, disaccharides, polyalcohols, amino acids, vitamins, enzymes, and pigments. They are related to structural, functional, and cellular functions and are similar to those found in plants [167,168]. They also contain essential nutrients, including macro (N, P, K, Ca, Mg, and S)- and micro (Fe, Cu, Mn, Zn, and B)-elements [4]. Secondary substances include large quantities of organic acids (butyric, propionic, malic, malonic, citric, maleic, and succinic acids, etc.), amino acids (histidine, alanine, cysteine, glutamate, aspartate, asparagine, glutamine, and glycine, etc.), and hormones (gibberellic acid, salicylic acid, indole acetic acid, and abscisic acid) [169,170]. Lichens are capable of producing unique secondary substances not generally found in plants [171].
4.2.1. Polysaccharides
Polysaccharides represent a major fraction of the compounds present in lichens, with a predominance of α-glucans, β-glucans, and galactomannans, representing 57% of all lichenic constituents [168,171]. These compounds originate mainly from the mycobiont. That is confirmed by the separation of photobiont from mycobiont, the latter producing polysaccharides similar to those of the parent lichen. While the photobiont generates distinct polysaccharides, as reported by Akbulut et al. [168] and Silva et al. [170].
4.2.2. Chlorophyll
The lichen photobiont produces chlorophyll, enabling photosynthesis and supporting lichen growth and development, even under extreme conditions. The thallus of the mycobiont, which acts as a shelter, in turn protects the photobiont from excessive radiation by filtering it through various substances. This interaction was confirmed by separate cultivation experiments of the lichenized fungus and its photobiont partner, where chlorophyll content decreased significantly in the photobiont grown alone [172]. However, chlorophyll content in lichens varies according to the location of the species and decreases with increasing air pollution [173].
4.2.3. Phytohormones
Several studies have shown that lichens represent an important source of phytohormones, such as auxin (indole-3-acetic acid, IAA), gibberellic acid (GA3), abscisic acid (ABA) and cytokinin (zeatin). Their production and release vary according to species [169,174]. Pichler et al. [174] show that IAA represents the most abundant phytohormone produced and released by mycobionts of Cladonia grayi, Xanthoria parietina, and Tephromela atra lichens. In contrast, salicylic acid was released only by Xanthoria parietina and Tephromela atra, while jasmonic acid was released by Cladonia grayi only. These compounds were also released in large quantities into the extracellular space, where they can be perceived by other microorganisms, potentially influencing their gene expression, metabolism, and physiology [174].
4.2.4. Minerals Elements
Lichens efficiently accumulate a variety of essential mineral elements such as N, K, Ca, Mn, P, B, S, Zn, Mg, Fe, and Cr [175,176,177,178]. Previous studies have shown that the mycobiont, due to its direct interface with the external environment, is primarily responsible for the acquisition of these mineral elements [179,180]. A mineralogical analysis performed in the study conducted by Clark et al. [180] on Xanthoparmelia chlorochroa showed a particular spatial distribution of mineral constituents in the thallus, indicating that mycobiont is responsible for their absorption. It would also help to create a favorable environment, thereby enhancing photosynthetic processes in the photobiont. Moreover, the ability of lichens to accumulate minerals is enhanced under humid conditions, as shown in a study by Adamo et al. [176]. Once minerals have been absorbed, they can be transferred to surrounding plant communities via litter, leaching, or incorporation by bacteria, thus contributing to soil fertility [181]. Nitrogen is mainly acquired by the lichen photobiont from a variety of sources, with ammonium uptake generally higher and more passive than nitrate uptake. Cyanobacterial lichens, on the other hand, show a lower rate of nitrogen uptake. However, all lichens could assimilate amino acids [182]. Furthermore, lichen-associated bacteria possess nitrogen-fixing potential [22]. However, increasing nitrogen sources and availability to high levels can reduce lichen abundance and diversity [183].
The richness of lichen species such as Usnea antarctica, Hydropunctaria maura, Wahlenbergiella mucosa, Stictu sp., and Pseudevernia furfuracea in photosynthetic pigments, mineral compounds, and other bioactive compounds revealed by several literature references [167,169,176,177,178,184], suggesting that their nutritional potential was significantly higher than that of various organic sources [4,20,185]. However, their application as biofertilizers has rarely been studied.
4.3. Effect of Lichen on Agricultural Soil
4.3.1. Effect of Lichen on the Physical, Chemical, and Nutritional Characteristics of the Soil
Lichens have a positive influence on the physical and chemical properties and nutritional quality of soils, depending on the lichen species [25,185,186]. Lichens can increase water content by regulating the soil surface microclimate (lowering soil temperature), reducing soil evaporation, and increasing water retention. Ghilouf et al. [187] show that Squamarina cartilaginea, Diploschistes diacapsis, and Fulgensia bracteata significantly increased soil water and nutrient content. They can also influence soil pH and organic matter. The secretion of organic matter by lichens and its accumulation under lichenic cover leads to a drop in soil pH by releasing hydrogen ions associated with organic anions [25,188].
This positive impact is also linked to an increase in carbon and nitrogen nutrients in the soil, with bioavailable forms of inorganic nitrogen such as NH4+ and NO3− increasing under lichen cover [187]. In addition, Santiago et al. [25] demonstrated that soil covered with Cladonia salzmannii lichen showed improvements in calcium content, with a reduction in aluminum content. Indeed, there is a close relationship between lichens and soil minerals. The production of polysaccharide compounds by lichen hyphae, as well as the activity of lichen-specific bacterial colonies, is essential in the formation of soil organo-mineral aggregates [189]. The impact of lichens on soil properties depends on the species. Ghiloufi et al. [187] revealed that the lichens Squamarina cartilaginea, Diploschistes diacapsis, and Fulgensia bracteata were particularly effective in improving soil characteristics, reducing soil pH, and increasing soil water and nutrient content compared to Psora decipiens and Endocarpon pusillum due to their lower cover and their thinner thallus (<1 mm thick), which results in low water retention.
4.3.2. Interactions of Lichen with Soil Microbial Community
Changes in soil physico-chemical properties caused by lichen cover can induce alterations in the microbial community. Knowing that soil pH has been identified as the main factor influencing microbial diversity, including bacteria and fungi [190,191]. Similarly, soil moisture, as well as carbon (C) and nitrogen (N) concentrations, exert a beneficial effect on the biomasses of microbial communities [25,192]. Substances secreted by lichens, such as usnic and perlatolic acids, are also a source of carbon beneficial to microbial biomass in the soil [25,193]. Santiago et al. [25] observed that soils covered with Cladonia salzmannii had higher microbial biomass (1462.3 µg C g soil−1) than bare sandy soils (1268.6 µg C g soil−1), although the activity of the microbial community did not show a dramatic response to lichens as evidenced by the identification of carbon dioxide respiration rates between lichen-covered and bare soils. In fact, microbial communities accumulate carbon in their biomass and therefore show lower microbial respiration values, which reduce carbon emissions to the atmosphere. These results suggest that soil cover by C. salzmannii could contribute to better carbon sequestration. In addition, fungi were also evaluated, showing that the highest values of percentage root colonization by arbuscular mycorrhizae and spore number were observed in areas covered by lichens, suggesting an interaction between arbuscular mycorrhizae fungi and C. salzmannii cover [25]. All these factors induced by the lichen have contributed to the development of the Genipa Americana plant cultivated in the soil studied, improving their length, fresh and dry weight, and Ca, Mg, and Na content. However, other studies showed that microbial biomass in soil did not react significantly after the application of some lichens [194,195]. This effect was related to the negative correlation of the microbial community abundance with higher lichen soil cover, as suggested by Ghiloufi et al. [187]. The species of Squamarina cartilaginea, Diploschistes diacapsis, and Fulgensia bracteata were found to significantly reduce the abundance of soil microbes, which could be explained by their ability to reduce light availability for microorganisms. This light reduction would explain the lower microbial abundance observed under these lichens compared with P. decipiens and E. pusillum, which have a lower cover and finer, discontinuous thallus, allowing better light transmission. This inhibition of microbial growth may also be due to their antibacterial activity, as shown by the in vitro results of Mendili et al. [196], which deduced that the application of Flavoparmelia caperata powder showed the greatest inhibition diameter (25.5 mm) against Staphylococcus aureus. This antimicrobial effect is likely due to the production of secondary metabolites, including usnic acid, psoromic, lecanoric, and parietin [197,198]. Consequently, this differentiation in lichen effect is mainly associated with differences in lichen cover, their morphological and physiological characteristics, and the chemical substances they secrete.
The interaction between the biotic attributes of lichen communities, including lichen species richness, regularity, and spatial configuration, strongly influences soil microbial biomass and the functionality of their biodiversity, as shown in a study by Castillo-Monroy et al. [199], which assessed the influence of biocrusts formed by lichen communities Acarospora nodulosa, Collema crispum, Diploschistes diacapsis, Squamarina lentigera, Fulgensia subbractaceatan, Lepraria membranaceum, Psora decipiens, Cladonia convoluta, Squamarina cartilaginea, and Toninia sedifolia on subsurface microbial function. They showed that microcosms accompanied by a random distribution of lichen species, with intermediate species richness and maximum equitability, display a higher microbial catabolic profile than those observed in soils where lichens are distributed in clusters. Consequently, the distribution of lichens in microcosms promotes the improvement and diversification of the microbial community.
4.4. Potential Effect of Lichens on Plant Growth: Future Ecological Opportunity for Sustainable Agriculture
The positive impacts resulting from the application of lichens to the soil potentially favored plant development (Figure 8) [4,25,187]. Santiago et al. [25] showed that Genipa americana plants grown in soil covered with lichen Cladonia salzmannii showed improved vegetative development. In addition, inoculation of lichen-covered soils with arbuscular mycorrhizae significantly enhanced Genipa americana growth, increasing fresh and dry plant weights and nutrient contents. This is due to the bioactive compounds secreted by the lichen, whose barbaric acid strengthens the association between plant roots and arbuscular mycorrhizae. Another study applied Bacillus licheniformis isolated from Collema undulatum lichen, by inoculation to corn plant growth. Results showed that this innovative practice promoted elongation of stems (7 cm), leaves (57 cm), and roots (22.5 cm), compared with the negative control (18, 10, and 9.8 cm, respectively). Increased chlorophyll levels were also seen at 40 SPAD (Soil Plant Analysis Development) compared with the negative control (13 SPAD). Similarly, the production of phytohormones by this lichenin bacterium, notably indole-3-acetic acid, stimulated phosphate, potassium solubilization, and the synthesis of extracellular enzymes such as cellulases, proteases, amylases, and β-1,3-glucanases [200]. Gunes et al. [4] also revealed that certain lichens, such as Dermatcarpon miniatum, Parmelia saxatilis, and Umbilicaria nylanderiana, possess high nutritional potential, with a higher mineral composition than of certain organic fertilizer sources, which could be particularly beneficial in sustainable organic agriculture.
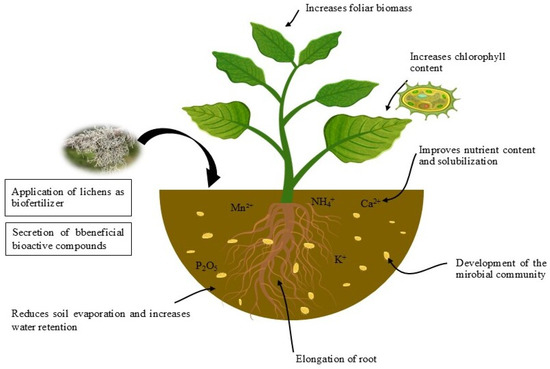
Figure 8.
Schematic improved soil fertility through lichens.
These discoveries suggest that lichens could play a key role as innovative biofertilizers in improving soil fertility. However, research into the direct use of lichens in agricultural crops is still rare.
4.5. Agronomic Validation of Lichens: Suggestions for Experimental Axes Towards a New Class of Biofertilizers
The present bibliometric and bibliographic study on the use of lichens in agriculture highlights their richness in bioactive secondary metabolites [172,175,176,178,179,182]. However, research on the evaluation of lichens as biofertilizers in both controlled and field conditions remains very limited. With this in mind, a rigorous agronomic validation process of lichens is essential in order to determine their effectiveness as innovative, environmentally friendly, and sustainable biofertilizers and to enable their integration into organic product ranges that can serve as an alternative to chemical fertilizers. Indeed, future scientific research should be based on credible analogies and plausible experimental avenues. Evaluating the effects of different lichen species on plant growth under controlled conditions, from germination to root, aerial, and biomass development. Similarly, developing formulations suitable for agricultural applications is a promising avenue of research, such as encapsulation, soil incorporation, or foliar spraying. This research will then need to be followed up with field trials to evaluate the effect of lichens on crop yield, nutrient mobility in the soil, microbial biodiversity, and their synergies with beneficial microorganisms present in the soil, such as PGPR and mycorrhizal fungi.
5. Conclusions and Future Perspectives
Seaweeds and lichens are natural resources rich in bioactive compounds. These biomasses can be exploited as natural fertilizers for agricultural crops, laying the foundations for sustainable environmental development without endangering the natural habitat. Based on the bibliometric and bibliographic analysis carried out in this review, the application of seaweed-based biofertilizers in agriculture is widely studied and well developed worldwide. They are used in a different form, solid or liquid. However, the use of lichens for agricultural purposes remains marginal despite emerging evidence in the scientific literature of their potential beneficial effects on plant growth.
Future perspectives can contribute to maximizing the diversity of organic biomass used in agriculture while reinforcing the transition to a more sustainable, ecological, and efficient model, such as the optimization of algal biofertilizer application methods, in particular the enrobing of seeds with seaweeds, which would enable action to be taken at germination and in the early stages of development, while providing protection against abiotic stress and unintended bacterial or fungal contamination.
Concerning lichens, to exploit this innovative reservoir of nutritive and bioactive compounds, scientific research needs to be extended, in particular on the direct applications of different lichen species, as well as deepening the understanding of their mechanisms of action, while defining optimal formulations and validating their effects on various types of crops. Thus, further data needs to be collected to strengthen the arguments in favor of large-scale industrial applications. Similarly, the combination of algae and lichens suggests a highly interesting synergistic potential for enhancing plant growth.
Author Contributions
O.O.; Methodology, investigation, and writing. Y.E.; Methodology, investigation, and writing—original draft. B.O.; Conceptualization, formal analysis, resources, writing and editing, and supervision. F.E.K.; Conceptualization, formal analysis, resources, and writing and editing. R.M.; Conceptualization, formal analysis, resources, writing and editing, and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank the teams at the Laboratoire des Sciences de l’Eau, Biotechnologie Microbienne et Durabilité des Ressources Naturelles (AQUABIOTECH) (Cadi Ayyad University, Morocco) for their scientific and technical support for this work. Rosário Martins thanks the Portuguese Foundation for Science and Technology FCT/MCTES to CIIMAR (UIDB/04423/2020 and UIDP/04423/2020) for support.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Savci, S. Investigation of Effect of Chemical Fertilizers on Environment. APCBEE Procedia 2012, 1, 287–292. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Nabti, E.; Jha, B.; Hartmann, A. Impact of seaweeds on agricultural crop production as biofertilizer. Int. J. Environ. Sci. Technol. 2017, 14, 1119–1134. [Google Scholar] [CrossRef]
- Gunes, A.; Asian, A.; Turan, M. Biochemical properties of some lichen species as a source of organic fertilizer. Comptes Rendus L’Academie Bulg. Sci. 2016, 69, 539–548. [Google Scholar]
- Pacheco, D.; Cotas, J.; Rocha, C.P.; Araújo, G.S.; Figueirinha, A.; Gonçalves, A.M.M.; Bahcevandziev, K.; Pereira, L. Seaweeds’ carbohydrate polymers as plant growth promoters. Carbohydr. Polym. Technol. Appl. 2021, 2, 100097. [Google Scholar] [CrossRef]
- Pandya, M.; Mehta, S. Effect of Ulva lactuca L. Seaweed Biostimulant on Seed germination, Growth, and some Biochemical properties of Vigna radiata L. Int. J. Environ. Agric. Biotechnol. 2021, 6, 042–053. [Google Scholar] [CrossRef]
- Rao, G.M.N.; Chatterjee, R. Effect of Seaweed Liquid Fertilizer from Gracilaria Textorii and Hypnea Musciformis on Seed Germination and Productivity of Some Vegetable Crops. Univers. J. Plant Sci. 2014, 2, 115–120. [Google Scholar] [CrossRef]
- Ramya, S.S.; Vijayanand, N.; Rathinavel, S. Foliar application of liquid biofertilizer of brown alga Stoechospermum marginatum on growth, biochemical and yield of Solanum melongena. Int. J. Recycl. Org. 2015, 4, 167–173. [Google Scholar] [CrossRef]
- Vinoth, S.; Gurusaravanan, P.; Arun, M.; Saradhadevi, M.; Senthilkumar, N.; Gowtham, P.; Sivakumar, S.R. Biostimulant activity of sulfated polysaccharide extract from red seaweed Halymenia dilatata on yield of Mung bean in greenhouse conditions. J. Appl. Phycol. 2021, 33, 3309–3317. [Google Scholar] [CrossRef]
- Aremu, A.O.; Masondo, N.A.; Rengasamy, K.R.R.; Amoo, S.O.; Gruz, J.; Bíba, O.; Šubrtová, M.; Pěnčík, A.; Novák, O.; Doležal, K.; et al. Physiological role of phenolic biostimulants isolated from brown seaweed Ecklonia maxima on plant growth and development. Planta 2015, 241, 1313–1324. [Google Scholar] [CrossRef]
- Ouala, O.; Essadki, Y.; Redouane, E.M.; Cherifi, O.; El Khalloufi, F.; Oudra, B. Application of Sargassum muticum as a nature-based fertilizer to enhance the growth of Capsicum annuum L. plants. J. Taibah Univ. Sci. 2025, 19, 2484925. [Google Scholar] [CrossRef]
- Omar, H.H.; Abdullatif, B.M.; Al-Kazan, M.M.; El-Gendy, A.M. Various Applications of Seaweed Improves Growth and Biochemical Constituents of Zea mays L. and Helianthus annuus L. J. Plant Nutr. 2015, 38, 28–40. [Google Scholar] [CrossRef]
- Crouch, I.J.; Van Staden, J. Commercial Seaweed Products as Biostimulants in Horticulture. J. Home Consum. Hortic. 1993, 1, 19–76. [Google Scholar] [CrossRef]
- Akila, N.; Jeyadoss, T. The potential of seaweed liquid fertilizer on the growth and antioxidant enhancement of Helianthus annuus L. Orient. J. Chem. 2010, 26, 1353. [Google Scholar]
- Patel, H.D.; Brahmbhatt, N.; Patel, J.; Patel, R.; Thaker, P. Effect of Seaweed Extract on different Vegetables as a Bio Fertilizer in Farming. Int. J. Res. 2019, 7, 2062–2067. [Google Scholar] [CrossRef]
- Pise, N.M.; Sabale, A.B. Effect of seaweed concentrates on the growth and biochemical constituents of Trigonella foenum-graecum L. J. Phytol. 2010, 2010, 50–56. [Google Scholar]
- Hernández-Herrera, R.M.; Santacruz-Ruvalcaba, F.; Ruiz-López, M.A.; Norrie, J.; Hernández-Carmona, G. Effect of liquid seaweed extracts on growth of tomato seedlings (Solanum lycopersicum L.). J. Appl. Phycol. 2014, 26, 619–628. [Google Scholar] [CrossRef]
- Toueileb, S.; Ett, B.L.; Maleinine, D. Effets de l’application foliaire d’ un extrait liquide d’ algue marine (Enteromorophaintestinalis Linnaeus Link.) sur la croissance végétative et la physiologie des jeunes plantes de tomate cultivées sous stress salin. J. New Sci. 2020, 76, 4483–4492. [Google Scholar]
- Jayaraj, J.; Wan, A.; Rahman, M.; Punja, Z.K.Ã. Seaweed extract reduces foliar fungal diseases on carrot. Crop. Prot. 2008, 27, 1360–1366. [Google Scholar] [CrossRef]
- Bano, A.; Waqar, A.; Khan, A.; Tariq, H. Phytostimulants in sustainable agriculture. Front. Sustain. Food Syst. 2022, 6, 801788. [Google Scholar] [CrossRef]
- Aslan, A.; Budak, G.; Karabulut, A. The amounts Fe, Ba, Sr, K, Ca and Ti in some lichens growing in Erzurum province (Turkey). J. Quant. Spectrosc. Radiat. Transf. 2004, 88, 423–431. [Google Scholar] [CrossRef]
- Swamy, C.T.; Gayathri, D.; Devaraja, T.N.; Bandekar, M.; D’Souza, S.E.; Meena, R.M.; Ramaiah, N. Plant growth promoting potential and phylogenetic characteristics of a lichenized nitrogen fixing bacterium, Enterobacter cloacae. J. Basic Microbiol. 2016, 56, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Jannah, M.; A’yun, Q.; Afifah, N.; Prasetya, E.; Hariri, M.R. Usnea in West Java: A potential source of bioactive secondary metabolites. Berk. Penelit. Hayati 2022, 28, 26–31. [Google Scholar] [CrossRef]
- Huneck, S. The significance of lichens and their metabolites. Naturwissenschaften 1999, 86, 559–570. [Google Scholar] [CrossRef]
- Santiago, R.; Silva, N.H.; Silva, F.P.; Martins, M.C.B.; de Vasconcelos, T.L.; Yano-Melo, A.M.; Pereira, E.C. Interactions of the lichen Cladonia salzmannii nyl. With soil, microbiota, mycorrhizae and genipa Americana. J. Soil Sci. Plant Nutr. 2018, 18, 833–850. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Aria, M.; Cuccurullo, C. bibliometrix: An R-tool for comprehensive science mapping analysis. J. Informetr. 2017, 11, 959–975. [Google Scholar] [CrossRef]
- van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M.; Morrison, L.; Rindi, F.; Miranda, S.V.; Mathieson, A.C.; Parker, B.C.; Langangen, A.; John, D.M.; Bárbara, I.; et al. AlgaeBase: An on-line resource for algae. Cryptogam. Algol. 2014, 35, 105–115. [Google Scholar] [CrossRef]
- Bolton, J.J.; De Clerck, O.; John, D.M. Seaweed diversity patterns in sub-Saharan Africa. In Proceedings of the Marine Biodiversity in Sub-Saharan Africa: The Known and the Unknown, Cape Town, South Africa, 24–26 September 2003; pp. 23–26. [Google Scholar]
- Amosu, A.O.; Robertson-Andersson, D.V.; Kean, E.; Maneveldt, G.W.; Cyster, L. Biofiltering and uptake of dissolved nutrients by Ulva armoricana (Chlorophyta) in a land-based aquaculture system. Int. J. Agric. Biol. 2016, 18, 298–304. [Google Scholar] [CrossRef]
- Msuya, F.E.; Buriyo, A.; Omar, I.; Pascal, B.; Narrain, K.; Ravina, J.J.M.; Mrabu, E.; Wakibia, J.G. Cultivation and utilisation of red seaweeds in the Western Indian Ocean (WIO) Region. J. Appl. Phycol. 2014, 26, 699–705. [Google Scholar] [CrossRef]
- Msuya, F.E.; Bolton, J.; Pascal, F.; Narrain, K.; Nyonje, B.; Cottier-Cook, E.J. Seaweed farming in Africa: Current status and future potential. J. Appl. Phycol. 2022, 34, 985–1005. [Google Scholar] [CrossRef]
- Kazzaz, M.; Riadi, H. Inventaire préliminaire de la phycoflore benthique du littoral marocain. II. Rhodophyceae. Acta Bot. Barcinonensia 2000, 46, 53–88. [Google Scholar]
- Bahammou, N.; Cherifi, O.; Bouamama, H.; Rezzoum, N.; Sabri, H.; Boundir, Y. Checklist of rhodophyceae and the first report of Aglaothamnion tripinnatum and Gaillona gallica in the Moroccan coastline. Egypt. J. Aquat. Res. 2021, 47, 101–107. [Google Scholar] [CrossRef]
- Bahammou, N.; Sabri, H.; Cherifi, O.; Bouamama, H. Saesonal variation, the ecological index and the potential use of Chlorophyceae and Phaeophyceae of the Moroccan coast Sidi Bouzid Eljadida in the agricultural field. J. Anal. Sci. Appl. Biotechnol. 2021, 3, 56–64. [Google Scholar] [CrossRef]
- Sabri, H.; Haroun, R.; Bahammou, N.; Hasni, M.; Boundir, Y.; Maarouf, A.; Cherifi, O. Intertidal benthic red algae (Rhodophyta) from Essaouira coastline (Morocco). Egypt. J. Aquat. Res. 2021, 47, 277–282. [Google Scholar] [CrossRef]
- Sabri, H.; Cherifi, O.; Maarouf, A.; Bahammou, N.; Boundir, Y. Original Paper the checklist and the ecological index of the brown seaweeds from essaouira coastline (MOROCCO). J. Appl. Sci. Environ. Stud. JASES 2021, 4, 406–417. [Google Scholar]
- Moussa, H.; Hassoun, M.; Salhi, G.; Zbakh, H.; Riadi, H. Checklist of seaweeds of Al-Hoceima National Park of Morocco (Mediterranean Marine Protected Area). Acta Bot. Malacit. 2018, 43, 91–109. [Google Scholar] [CrossRef]
- Benhissoune, S.; Boudouresque, C.F.; Verlaque, M. A checklist of the seaweeds of the Mediterranean and Atlantic coasts of Morocco. II. Phaeophyceae. Bot. Mar. 2002, 45, 217–230. [Google Scholar] [CrossRef]
- Benhissoune, S.; Boudouresque, C.F.; Perret-Boudouresque, M.; Verlaque, M. A checklist of the seaweeds of the Mediterranean and Atlantic coasts of Morocco. IV. Rhodophyceae—Ceramiales. Bot. Mar. 2003, 46, 55–68. [Google Scholar] [CrossRef]
- Marinho-Soriano, E.; Fonseca, P.C.; Carneiro, M.A.A.; Moreira, W.S.C. Seasonal variation in the chemical composition of two tropical seaweeds. Bioresour. Technol. 2006, 97, 2402–2406. [Google Scholar] [CrossRef]
- Peng, Y.; Xie, E.; Zheng, K.; Fredimoses, M.; Yang, X.; Zhou, X.; Wang, Y.; Yang, B.; Lin, X.; Liu, J.; et al. Nutritional and chemical composition and antiviral activity of cultivated seaweed sargassum naozhouense Tseng et Lu. Mar. Drugs 2013, 11, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Yaich, H.; Garna, H.; Besbes, S.; Paquot, M.; Blecker, C.; Attia, H. Chemical composition and functional properties of Ulva lactuca seaweed collected in Tunisia. Food Chem. 2011, 128, 895–901. [Google Scholar] [CrossRef]
- Zheng, L.X.; Liu, Y.; Tang, S.; Zhang, W.; Cheong, K.L. Preparation methods, biological activities, and potential applications of marine algae oligosaccharides: A review. Food Sci. Hum. Wellness 2023, 12, 359–370. [Google Scholar] [CrossRef]
- Li, S.Y.; Wang, Z.P.; Wang, L.N.; Peng, J.X.; Wang, Y.N.; Han, Y.T.; Zhao, S.F. Combined enzymatic hydrolysis and selective fermentation for green production of alginate oligosaccharides from Laminaria japonica. Bioresour. Technol. 2019, 281, 84–89. [Google Scholar] [CrossRef]
- Yudiati, E.; Santosa, G.W.; Tontowi, M.R.; Sedjati, S.; Supriyantini, E.; Khakimah, M. Optimization of alginate alkaline extraction technology from Sargassum polycystum and its antioxidant properties. IOP Conf. Ser. Earth Environ. Sci. 2018, 139, 012052. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Chen, X.Q.; Aweya, J.J.; Cheong, K.L. Catabolism of Saccharina japonica polysaccharides and oligosaccharides by human fecal microbiota. LWT 2020, 130, 109635. [Google Scholar] [CrossRef]
- Ermakova, S.; Sokolova, R.; Kim, S.M.; Um, B.H.; Isakov, V.; Zvyagintseva, T. Fucoidans from brown seaweeds Sargassum hornery, eclonia cava, costaria costata: Structural characteristics and anticancer activity. Appl. Biochem. Biotechnol. 2011, 164, 841–850. [Google Scholar] [CrossRef]
- Rajauria, G.; Ravindran, R.; Garcia-Vaquero, M.; Rai, D.K.; Sweeney, T.; O’Doherty, J. Purification and Molecular Characterization of Fucoidan Isolated from Ascophyllum nodosum Brown Seaweed Grown in Ireland. Mar. Drugs 2023, 21, 315. [Google Scholar] [CrossRef]
- Wang, S.H.; Huang, C.Y.; Chen, C.Y.; Chang, C.C.; Huang, C.Y.; Dong, C.D.; Chang, J.S. Isolation and purification of brown algae fucoidan from Sargassum siliquosum and the analysis of anti-lipogenesis activity. Biochem. Eng. J. 2021, 165, 107798. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Lee, J.S.; Kim, W.S.; Jeon, Y.J. The potential of brown-algae polysaccharides for the development of anticancer agents: An update on anticancer effects reported for fucoidan and laminaran. Carbohydr. Polym. 2017, 177, 451–459. [Google Scholar] [CrossRef]
- Mohd Fauziee, N.A.; Chang, L.S.; Wan Mustapha, W.A.; Md Nor, A.R.; Lim, S.J. Functional polysaccharides of fucoidan, laminaran and alginate from Malaysian brown seaweeds (Sargassum polycystum, Turbinaria ornata and Padina boryana). Int. J. Biol. Macromol. 2021, 167, 1135–1145. [Google Scholar] [CrossRef]
- Tziveleka, L.A.; Ioannou, E.; Roussis, V. Ulvan, a bioactive marine sulphated polysaccharide as a key constituent of hybrid biomaterials: A review. Carbohydr. Polym. 2019, 218, 355–370. [Google Scholar] [CrossRef]
- Lahaye, M.; Ray, B. Cell-wall polysaccharides from the marine green. Carbohydr. Res. 1996, 283, 161–173. [Google Scholar] [CrossRef]
- Cheong, K.L.; Qiu, H.M.; Du, H.; Liu, Y.; Khan, B.M. Oligosaccharides derived from red seaweed: Production, properties, and potential health and cosmetic applications. Molecules 2018, 23, 2451. [Google Scholar] [CrossRef]
- K-da, S.; Peerakietkhajorn, S.; Siringoringo, B.; Muangnil, P.; Wichienchot, S.; Khuituan, P. Oligosaccharides from Gracilaria fisheri ameliorate gastrointestinal dysmotility and gut dysbiosis in colitis mice. J. Funct. Foods 2020, 71, 104021. [Google Scholar] [CrossRef]
- Meinita, M.D.N.; Marhaeni, B.; Hong, Y.K.; Jeong, G.T. Enzymatic saccharification of agar waste from Gracilaria verrucosa and Gelidium latifolium for bioethanol production. J. Appl. Phycol. 2017, 29, 3201–3209. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Gómez-Mascaraque, L.G.; Ballester, A.R.; Martínez-Abad, A.; Brodkorb, A.; López-Rubio, A. Production of unpurified agar-based extracts from red seaweed Gelidium sesquipedale by means of simplified extraction protocols. Algal Res. 2019, 38, 101420. [Google Scholar] [CrossRef]
- Al-Alawi, A.A.; Al-Marhubi, I.M.; Al-Belushi, M.S.M.; Soussi, B. Characterization of Carrageenan Extracted from Hypnea bryoides in Oman. Mar. Biotechnol. 2011, 13, 893–899. [Google Scholar] [CrossRef]
- Zhu, B.; Ni, F.; Xiong, Q.; Yao, Z. Marine oligosaccharides originated from seaweeds: Source, preparation, structure, physiological activity and applications. Crit. Rev. Food Sci. Nutr. 2021, 61, 60–74. [Google Scholar] [CrossRef]
- González, A.; Castro, J.; Vera, J.; Moenne, A. Seaweed Oligosaccharides Stimulate Plant Growth by Enhancing Carbon and Nitrogen Assimilation, Basal Metabolism, and Cell Division. J. Plant Growth Regul. 2013, 32, 443–448. [Google Scholar] [CrossRef]
- El-Mohdy, H.L.A. Radiation-induced degradation of sodium alginate and its plant growth promotion effect. Arab. J. Chem. 2017, 10, S431–S438. [Google Scholar] [CrossRef]
- Yang, J.; Shen, Z.; Sun, Z.; Wang, P.; Jiang, X. Growth Stimulation Activity of Alginate-Derived Oligosaccharides with Different Molecular Weights and Mannuronate/Guluronate Ratio on Hordeum vulgare L. J. Plant Growth Regul. 2021, 40, 91–100. [Google Scholar] [CrossRef]
- Krishna Perumal, P.; Huang, C.Y.; Chen, C.W.; Anisha, G.S.; Singhania, R.R.; Dong, C.D.; Patel, A.K. Advances in oligosaccharides production from brown seaweeds: Extraction, characterization, antimetabolic syndrome, and other potential applications. Bioengineered 2023, 14, 2252659. [Google Scholar] [CrossRef] [PubMed]
- San, P.T.; Khanh, C.M.; Khanh, H.H.N.; Khoa, T.A.; Hoang, N.; Nhung, L.T.; Trinh, N.T.K.; Nguyen, T.D. k-Oligocarrageenan Promoting Growth of Hybrid Maize: Influence of Molecular Weight. Molecules 2020, 25, 3825. [Google Scholar] [CrossRef]
- de Borba, M.C.; de Freitas, M.B.; Stadnik, M.J. Ulvan enhances seedling emergence and reduces Fusarium wilt severity in common bean (Phaseolus vulgaris L.). Crop Prot. 2019, 118, 66–71. [Google Scholar] [CrossRef]
- Salachna, P.; Grzeszczuk, M.; Meller, E.; Soból, M. Oligo-Alginate with low molecular mass improves growth and physiological activity of eucomis autumnalis under salinity stress. Molecules 2018, 23, 812. [Google Scholar] [CrossRef]
- Yi, H.; Xu, G.; Cheng, H.; Wang, J.; Wan, Y.; Chen, H. An Overview of Utilization of Steel Slag. Procedia Environ. Sci. 2012, 16, 791–801. [Google Scholar] [CrossRef]
- Kumar, M.; Kumari, P.; Trivedi, N.; Shukla, M.K.; Gupta, V.; Reddy, C.R.K.; Jha, B. Minerals, PUFAs and antioxidant properties of some tropical seaweeds from Saurashtra coast of India. J. Appl. Phycol. 2011, 23, 797–810. [Google Scholar] [CrossRef]
- Rupérez, P. Mineral content of edible marine seaweeds. Food Chem. 2002, 79, 23–26. [Google Scholar] [CrossRef]
- Sagi, M.; Omarov, R.T.; Lips, S.H. The Mo-hydroxylases xanthine dehydrogenase and aldehyde oxidase in ryegrass as affected by nitrogen and salinity. Plant Sci. 1998, 135, 125–135. [Google Scholar] [CrossRef]
- Shuuluka, D.; Bolton, J.J.; Anderson, R.J. Protein content, amino acid composition and nitrogen-to-protein conversion factors of Ulva rigida and Ulva capensis from natural populations and Ulva lactuca from an aquaculture system, in South Africa. J. Appl. Phycol. 2013, 25, 677–685. [Google Scholar] [CrossRef]
- Mathew, S.; Venkateshwarlu, G.; Ravishankar, C.N. Nutritional profiling of the edible seaweeds Gracilaria edulis, Ulva lactuca and Sargassum sp. Indian J. Fish. 2016, 63, 81–87. [Google Scholar] [CrossRef]
- El-said, G.F.; El-sikaily, A. Chemical composition of some seaweed from Mediterranean. Environ. Monit. Assess. 2013, 185, 6089–6099. [Google Scholar] [CrossRef]
- Milledge, J.J.; Nielsen, B.V. High-value products from macroalgae: The potential uses of the invasive brown seaweed, Sargassum muticum. Rev. Environ. Sci. Bio/Technol. 2016, 15, 67–88. [Google Scholar] [CrossRef]
- Taboada, M.C.; Millán, R.; Miguez, M.I. Nutritional value of the marine algae wakame (Undaria pinnatifida) and nori (Porphyra purpurea) as food supplements. J. Appl. Phycol. 2013, 25, 1271–1276. [Google Scholar] [CrossRef]
- Milledge, J.J.; Staple, A.; Harvey, P.J. Slow Pyrolysis as a Method for the Destruction of Japanese Wireweed, Sargassum muticum. Environ. Nat. Resour. Res. 2015, 5, 28–37. [Google Scholar] [CrossRef]
- Balboa, E.M.; Gallego-fábrega, C.; Moure, A.; Domínguez, H. Study of the seasonal variation on proximate composition of oven-dried Sargassum muticum biomass collected in Vigo Ria, Spain. J. Appl. Phycol. 2016, 28, 1943–1953. [Google Scholar] [CrossRef]
- Mawi, S.; Krishnan, S.; Din, M.F.; Arumugam, N.; Chelliapan, S. Bioremediation potential of macroalgae Gracilaria edulis and Gracilaria changii co-cultured with shrimp wastewater in an outdoor water recirculation system. Environ. Technol. Innov. 2019, 17, 100571. [Google Scholar] [CrossRef]
- Saebmehr, H.; Rafiee, F.; Givianrad, M.H.; Mostafavi, G. Determination and quantification of two regulatory phytohormones extracted from Sargassum muticum and Gracilaria corticata seaweeds in the south of Iran. Nova Biol. Reper. 2022, 9, 115–123. [Google Scholar] [CrossRef]
- Saebmehr, H.; Rafiee, F.; Givianrad, M.H.; Mostafavi, G. Extraction of abscisic acid and gibberellin from Sargassum muticum (Phaeophyceae) and Gracilaria corticata (Rhodophyta) harvested from Persian Gulf. Iran. J. Fish. Sci. 2022, 21, 590–604. [Google Scholar] [CrossRef]
- Ben, I.; Karen, A.; Ledezma, D.; Mart, E.; Leyva, S.; Carrera, E.; Ruiz, I.O. Identification and Quantification of Plant Growth Regulators and Antioxidant Compounds in Aqueous Extracts of Padina durvillaei and Ulva lactuca. Agronomy 2020, 10, 866. [Google Scholar] [CrossRef]
- Urbanek Krajnc, A.; Turinek, M.; Ivančič, A. Morphological and physiological changes during adventitious root formation as affected by auxin metabolism: Stimulatory effect of auxin containing seaweed extract treatment. Agricultura 2013, 1, 17–27. [Google Scholar]
- Sakakibara, H. Cytokinins: Activity, biosynthesis, and translocation. Annu. Rev. Plant Biol. 2006, 57, 431–449. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Matloob, A.; Khan, F.A.; Khaliq, A.; Saud, S.; Hassan, S.; Shan, D.; Khan, F.; Ullah, N.; et al. Phytohormones and plant responses to salinity stress: A review. Plant Growth Regul. 2015, 75, 391–404. [Google Scholar] [CrossRef]
- Zhang, X.; Ervin, E.H. Cytokinin-containing seaweed and humic acid extracts associated with creeping bentgrass leaf cytokinins and drought resistance. Crop Sci. 2004, 44, 1737–1745. [Google Scholar] [CrossRef]
- Zhang, X.; Ervin, E.H. Impact of seaweed extract-based cytokinins and zeatin riboside on creeping bentgrass heat tolerance. Crop Sci. 2008, 48, 364–370. [Google Scholar] [CrossRef]
- Mahendran, S.; Maheswari, P.; Sasikala, V. In vitro antioxidant study of polyphenol from red seaweeds dichotomously branched gracilaria Gracilaria edulis and robust sea moss Hypnea valentiae. Toxicol. Rep. 2021, 8, 1404–1411. [Google Scholar] [CrossRef]
- Górka, B.; Wieczorek, P.P. Simultaneous determination of nine phytohormones in seaweed and algae extracts by HPLC-PDA. J. Chromatogr. B 2017, 1057, 32–39. [Google Scholar] [CrossRef]
- Yalçln, S.; Okudan, E.Å.; Karakaş, Ö.; Önem, A.N. Determination of Major Phytohormones in Fourteen Different Seaweeds Utilizing SPE-LC-MS/MS. J. Chromatogr. Sci. 2020, 58, 98–108. [Google Scholar] [CrossRef]
- Godlewska, K.; Michalak, I.; Tuhy, L.; Chojnacka, K. Plant Growth Biostimulants Based on Different Methods of Seaweed Extraction with Water. Biomed Res. Int. 2016, 2016, 5973760. [Google Scholar] [CrossRef]
- Hughes, H.; Mcloughlin, P.; Tan, S.P.; Keeffe, E.O. Antibacterial Activity of Seaweed Extracts against Plant Pathogenic Bacteria Antibacterial Activity of Seaweed Extracts against Plant Pathogenic Bacteria. J. Bacteriol. Mycol. 2019, 6, 1105. [Google Scholar]
- Kasim, W.A.; Hamada, E.A.M.; El-din, N.G.S.; Eskander, S.; Agri, I.J.; Agri, R. Influence of seaweed extracts on the growth, some metabolic activities and yield of wheat grown under drought stress. Int. J. Agron. Agric. Res. 2015, 7, 173–189. [Google Scholar]
- He, J.; Xu, Y.; Chen, H.; Sun, P. Extraction, Structural Characterization, and Potential Antioxidant Activity of the Polysaccharides from Four Seaweeds. Int. J. Mol. Sci. 2016, 17, 1988. [Google Scholar] [CrossRef]
- Jiao, G.; Yu, G.; Wang, W.; Zhao, X.; Zhang, J.; Ewart, S.H. Properties of polysaccharides in several seaweeds from Atlantic Canada and their potential anti-influenza viral activities. J. Ocean Univ. China 2012, 11, 205–212. [Google Scholar] [CrossRef]
- Dobrinčić, A.; Dobroslavić, E.; Pedisić, S.; Balbino, S.; Elez Garofulić, I.; Čož-Rakovac, R.; Dragović-Uzelac, V. The effectiveness of the Fucus virsoides and Cystoseira barbata fucoidan isolation as a function of applied pre-treatment and extraction conditions. Algal Res. 2021, 56, 102286. [Google Scholar] [CrossRef]
- Mzibra, A.; Aasfar, A.; El Arroussi, H.; Khouloud, M.; Dhiba, D.; Kadmiri, I.M.; Bamouh, A. Polysaccharides extracted from Moroccan seaweed: A promising source of tomato plant growth promoters. J. Appl. Phycol. 2018, 30, 2953–2962. [Google Scholar] [CrossRef]
- Hamouda, R.A.; Hussein, M.H.; El-Naggar, N.E.A.; Karim-Eldeen, M.A.; Alamer, K.H.; Saleh, M.A.; Al Masoudi, L.M.; Sharaf, E.M.; El-Azeem, R.M.A. Promoting Effect of Soluble Polysaccharides Extracted from Ulva spp. on Zea mays L. Growth. Molecules 2022, 27, 1394. [Google Scholar] [CrossRef]
- Paulert, R.; Talamini, V.; Cassolato, J.E.F.; Duarte, M.E.R.; Noseda, M.D.; Smania, A.; Stadnik, M.J. Effects of sulfated polysaccharide and alcoholic extracts from green seaweed Ulva fasciata on anthracnose severity and growth of common bean (Phaseolus vulgaris L.). J. Plant Dis. Prot. 2009, 116, 263–270. [Google Scholar] [CrossRef]
- Ben Salah, I.; Aghrouss, S.; Douira, A.; Aissam, S.; El, Z.; Filali-maltouf, A.; El Modafar, C. Seaweed polysaccharides as bio-elicitors of natural defenses in olive trees against verticillium wilt of olive. J. Plant Interact. 2018, 13, 248–255. [Google Scholar] [CrossRef]
- Kisiriko, M.; Anastasiadi, M.; Terry, L.A.; Yasri, A.; Beale, M.H.; Ward, J.L. Phenolics from medicinal and aromatic plants: Characterisation and potential as biostimulants and bioprotectants. Molecules 2021, 26, 6343. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Mori, I.C.; Ikeda, Y.; Matsuura, T.; Hirayama, T.; Mikami, K. Phytohormones in red seaweeds: A technical review of methods for analysis and a consideration of genomic data. Bot. Mar. 2017, 60, 153–170. [Google Scholar] [CrossRef]
- Sanderson, K.J.; Jameson, P.E.; Zabkiewicz, J.A. Auxin in a Seaweed Extract: Identification and Quantitation of Indole-3-acetic acid by Gas Chromatography-Mass Spectrometry. J. Plant Physiol. 1987, 129, 363–367. [Google Scholar] [CrossRef]
- Tay, S.A.B.; Macleod, J.K.; Palni, L.M.S.; Letham, D.S. Detection of cytokinins in a seaweed extract. Phytochemistry 1985, 24, 2611–2614. [Google Scholar] [CrossRef]
- Sulakhudin, S.; Hatta, M.; Suryadi, U.E. Application of coastal sediments and foliar seaweed extract and its influence to soil properties, growth and yield of shallot in peatland. AGRIVITA J. Agric. Sci. 2019, 41, 450–460. [Google Scholar] [CrossRef]
- Ammar, E.E.; Aioub, A.A.A.; Elesawy, A.E.; Karkour, A.M.; Mouhamed, M.S.; Amer, A.A.; EL-Shershaby, N.A. Algae as Bio-fertilizers: Between current situation and future prospective: The role of Algae as a Bio-fertilizer in serving of ecosystem. Saudi J. Biol. Sci. 2022, 29, 3083–3096. [Google Scholar] [CrossRef]
- Kumari, R.; Kaur, I.; Bhatnagar, A.K. Enhancing soil health and productivity of Lycopersicon esculentum Mill. using Sargassum johnstonii Setchell Gardner as a soil conditioner and fertilizer. J. Appl. Phycol. 2013, 25, 1225–1235. [Google Scholar] [CrossRef]
- Arthur, G.D.; Aremu, A.O.; Moyo, M.; Stirk, W.A.; Van Staden, J. Growth-promoting effects of a seaweed concentrate at various pH and water hardness conditions. S. Afr. J. Sci. 2013, 109, 6. [Google Scholar] [CrossRef]
- Adderley, A.; Wallace, S.; Stubbs, D.; Connor, C.B.O.; Ferguson, J. Sargassum sp. as a biofertilizer: Is it really a key towards sustainable agriculture for The Bahamas? Bull. Natl. Res. Cent. 2023, 47, 112. [Google Scholar] [CrossRef]
- Msimbira, L.A.; Smith, D.L. The Roles of Plant Growth Promoting Microbes in Enhancing Plant Tolerance to Acidity and Alkalinity Stresses. Front. Sustain. Food Syst. 2020, 4, 106. [Google Scholar] [CrossRef]
- Hussain, H.I.; Kasinadhuni, N.; Arioli, T. The effect of seaweed extract on tomato plant growth, productivity and soil. J. Appl. Phycol. 2021, 33, 1305–1314. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Huang, Z.; Su, G.; Li, X.; Sun, Z.; Qin, Y. Impact of short-term application of seaweed fertilizer on bacterial diversity and community structure, soil nitrogen contents, and plant growth in maize rhizosphere soil. Folia Microbiol. 2020, 65, 591–603. [Google Scholar] [CrossRef]
- Wang, M.; Chen, L.; Li, Y.; Chen, L.; Liu, Z.; Wang, X. Responses of soil microbial communities to a short-term application of seaweed fertilizer revealed by deep amplicon sequencing. Appl. Soil Ecol. 2018, 125, 288–296. [Google Scholar] [CrossRef]
- Zhou, G.; Qiu, X.; Zhang, J.; Tao, C. Bioresource Technology E ffects of seaweed fertilizer on enzyme activities, metabolic characteristics, and bacterial communities during maize straw composting. Bioresour. Technol. 2019, 286, 121375. [Google Scholar] [CrossRef]
- Qiqin, L.; Huaguang, Z.; Minxiu, S.; Qian, L.; Haijun, F.; Haimin, C.; Rui, Y. Improvement of Soil Structure and Bacterial Composition by Long-Term Application of Seaweed Fertilizer. J. Soil Sci. Plant Nutr. 2023, 23, 5122–5132. [Google Scholar] [CrossRef]
- Espinosa-Antón, A.A.; Zamora-Natera, J.F.; Zarazúa-Villaseñor, P.; Santacruz-Ruvalcaba, F.; Sánchez-Hernández, C.V.; Águila Alcántara, E.; Torres-Morán, M.I.; Velasco-Ramírez, A.P.; Hernández-Herrera, R.M. Application of Seaweed Generates Changes in the Substrate and Stimulates the Growth of Tomato Plants. Plants 2023, 12, 1520. [Google Scholar] [CrossRef]
- Wang, Y.; Xiang, L.; Wang, S.; Wang, X.; Chen, X.; Mao, Z. Effects of seaweed fertilizer on the Malus hupehensis Rehd. seedlings growth and soil microbial numbers under continue cropping. Acta Ecol. Sin. 2017, 37, 180–186. [Google Scholar] [CrossRef]
- Mahmoud, S.H.; Salama, D.M.; El-Tanahy, A.M.M.; Abd El-Samad, E.H. Utilization of seaweed (Sargassum vulgare) extract to enhance growth, yield and nutritional quality of red radish plants. Ann. Agric. Sci. 2019, 64, 167–175. [Google Scholar] [CrossRef]
- Ramarajan, S.; Ganthi, S. Effect of Seaweed Liquid Fertilizer on the Germination and Pigment Concentration of Soybean. J. Crop Sci. Technol. 2012, 1, 1–15. [Google Scholar]
- Thriunavukkarasu, R.; Joseph, J.; Aruni, W. Effect of seaweed on seed germination and biochemical constituents of Capsicum annuum. Biocatal. Agric. Biotechnol. 2020, 29, 101761. [Google Scholar]
- Ahmed, D.A.E. Efficacy of two seaweeds dry mass in bioremediation of heavy metal polluted soil and growth of radish (Raphanus sativus L.) plant. Environ. Sci. Pollut. Res. 2020, 28, 12831–12846. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.S.; Borza, T.; Critchley, A.T.; Prithiviraj, B. Carrageenans from Red Seaweeds As Promoters of Growth and Elicitors of Defense Response in Plants. Front. Mar. Sci. 2016, 3, 81. [Google Scholar] [CrossRef]
- Kumar, G.; Sahoo, D. Effect of seaweed liquid extract on growth and yield of Triticum aestivum var. Pusa Gold. J. Appl. Phycol. 2011, 23, 251–255. [Google Scholar] [CrossRef]
- Paul, J. Influence of seaweed liquid fertilizer of Gracilaria dura (AG.) J. AG.(red seaweed) on Vigna radiata (l.) r. Wilczek., in Thoothukudi, Tamil Nadu, India. World J. Pharm. Res. 2014, 3, 968–978. [Google Scholar]
- Yamamoto, M.; Fukushima, M.; Kiso, E.; Kato, T.; Shibuya, M.; Horiya, S.; Nishida, A.; Otsuka, K.; Komai, T. Application of Iron Humates to Barren Ground in a Coastal Area forRestoring Seaweed Beds. J. Chem. Eng. Jpn. 2010, 43, 627–634. [Google Scholar] [CrossRef]
- El-Din, S.M. Utilization of seaweed extracts as bio-fertilizers to stimulate the growth of wheat seedlings. Egypt. J. Exp. Biol. 2015, 11, 31–39. [Google Scholar]
- Jayasinghe, P.S.; Pahalawattaarachchi, V.; Kkds, R. Fertilizer on plant growth of Capsicum annum. Discovery 2016, 52, 723–734. [Google Scholar]
- Patel, R.V.; Pandya, K.Y.; Brahmbhatt, N. Significance of Green And Brown Seaweed Liquid Fertilizer on Seed Germination of Solanum Melongena, Solanum Lycopersicum. Int. J. Recent Sci. Res. 2018, 2, 24065–24072. [Google Scholar] [CrossRef]
- Satish, L.; Rameshkumar, R.; Rathinapriya, P.; Pandian, S.; Rency, A.S.; Sunitha, T.; Ramesh, M. Effect of seaweed liquid extracts and plant growth regulators on in vitro mass propagation of brinjal (Solanum melongena L.) through hypocotyl and leaf disc explants. J. Appl. Phycol. 2015, 27, 993–1002. [Google Scholar] [CrossRef]
- Hidangmayum, A.; Sharma, R. Effect of different concentrations of commercial seaweed liquid extract of Ascophyllum nodosum as a plant bio stimulant on growth, yield and biochemical constituents of onion (Allium cepa L.). J. Pharmacogn. Phytochem. 2017, 6, 658–663. [Google Scholar]
- Abbas, M.; Anwar, J.; Zafar-ul-hye, M.; Khan, R.I. horticulturae Effect of Seaweed Extract on Productivity and Quality Attributes of Four Onion Cultivars. Horticulturae 2020, 6, 28. [Google Scholar] [CrossRef]
- Latique, S.; Chernane, H.; Mansori, M.; Kaoua, E. Seaweed Liquid Fertilizer Effect On Physiological And Biochemical Parameters Of Bean Plant (Phaesolus Vulgaris Variety Paulista) Under Hydroponic System. Eur. Sci. J. 2013, 9, 30. [Google Scholar]
- Castellanos-Barriga, L.G.; Santacruz-Ruvalcaba, F.; Hernández-Carmona, G.; Ramírez-Briones, E.; Hernández-Herrera, R.M. Effect of seaweed liquid extracts from Ulva lactuca on seedling growth of mung bean (Vigna radiata). J. Appl. Phycol. 2017, 29, 2479–2488. [Google Scholar] [CrossRef]
- Karthik, T.; Sarkar, G.; Babu, S.; Amalraj, L.D.; Jayasri, M.A. Preparation and evaluation of liquid fertilizer from Turbinaria ornata and Ulva reticulata. Biocatal. Agric. Biotechnol. 2020, 28, 101712. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Durgadevi, S.; Arulmozhi, P.; Rajalakshmi, S.; Gopalakrishnan, T.; Parameswari, N. Acta Ecologica Sinica Effect of seaweed liquid fertilizer on yield and quality of Capsicum. Acta Ecol. Sin. 2019, 39, 406–410. [Google Scholar] [CrossRef]
- Hernández-Herrera, R.M.; Santacruz-Ruvalcaba, F.; Zañudo-Hernández, J.; Hernández-Carmona, G. Activity of seaweed extracts and polysaccharide-enriched extracts from Ulva lactuca and Padina gymnospora as growth promoters of tomato and mung bean plants. J. Appl. Phycol. 2016, 28, 2549–2560. [Google Scholar] [CrossRef]
- Sivasankari, S.; Venkatesalu, V.; Anantharaj, M.; Chandrasekaran, M. Effect of seaweed extracts on the growth and biochemical constituents of Vigna sinensis. Bioresour. Technol. 2006, 97, 1745–1751. [Google Scholar] [CrossRef]
- Patel, A.M.J.S. Seaweed extract: Biostimulator of plant defense and plant productivity. Int. J. Environ. Sci. Technol. 2020, 17, 553–558. [Google Scholar] [CrossRef]
- Arumugam, R.; Anantharaman, P. Antibacterial activity of Seaweeds of Pudumadam coast Antibacterial Activity of Some Selected Seaweeds from Pudumadam Coastal Regions. Glob. J. Pharmacol. 2016, 3, 50–52. [Google Scholar]
- Čmiková, N.; Galovičová, L.; Miškeje, M.; Borotová, P.; Kluz, M.; Kačániová, M. Determination of Antioxidant, Antimicrobial Activity, Heavy Metals and Elements Content of Seaweed Extracts. Plants 2022, 11, 1493. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Venkatesalu, V.; Raj, G.A. Antibacterial activity of Ulva fasciata against Multidrug Resistant Bacterial Strains. Int. Lett. Nat. Sci. 2014, 14, 40–51. [Google Scholar] [CrossRef]
- Nagayama, K.; Iwamura, Y.; Shibata, T.; Hirayama, I.; Nakamura, T. Bactericidal activity of phlorotannins from the brown alga Ecklonia kurome. J. Antimicrob. Chemother. 2002, 50, 889–893. [Google Scholar] [CrossRef]
- Aftabuddin, S.; Abdul, M.; Siddique, M.; Habib, A.; Akter, S.; Hossen, S.; Tanchangya, P.; Al, M.A. Effects of seaweeds extract on growth, survival, antibacterial activities, and immune responses of Penaeus monodon against Vibrio parahaemolyticus. Ital. J. Anim. Sci. 2021, 20, 243–255. [Google Scholar] [CrossRef]
- Fayzi, L.; Askarne, L.; Cherifi, O.; Boufous, E.H.; Cherifi, K. Comparative Antibacterial Activity of Some Selected Seaweed Extracts from Agadir Coastal Regions in Morocco. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 390–399. [Google Scholar] [CrossRef]
- Indira, K.; Balakrishnan, S.; Srinivasan, M.; Bragadeeswaran, S.; Balasubramanian, T. Evaluation of in vitro antimicrobial property of seaweed (Halimeda tuna) from Tuticorin coast, Tamil Nadu, Southeast coast of India. Afr. J. Biotechnol. 2013, 12, 284–289. [Google Scholar] [CrossRef]
- Ara, J.; Sultana, V.; Qasim, R.; Ehteshamul-haque, S.; Ahmad, V.U. Biological Activity of Spatoglossum asperum: A Brown Alga. Phytotherapy Res. 2005, 623, 618–623. [Google Scholar] [CrossRef] [PubMed]
- El Boukhari, M.E.M.; Barakate, M.; Drissi, B.; Bouhia, Y.; Lyamlouli, K. Seaweed Extract Biostimulants Differentially act in Mitigating Drought Stress on Faba Bean (Vicia faba L.). J. Plant Growth Regul. 2023, 42, 5642–5652. [Google Scholar] [CrossRef]
- Xu, C.; Leskovar, D.I. Effects of A. nodosum seaweed extracts on spinach growth, physiology and nutrition value under drought stress. Sci. Hortic. 2015, 183, 39–47. [Google Scholar] [CrossRef]
- Yarsi, G. Effects of mycorrhiza, seaweed and bionutrient applied to reduce the salt stress on nutrient content, plant growth, malondialdehyde (MDA) and proline in pepper. J. Elem. 2023, 28. [Google Scholar] [CrossRef]
- Latique, S.; Aymen, E.M.; Halima, C.; Chérif, H.; Mimoun, E.K. Alleviation of Salt Stress in Durum Wheat (Triticum durum L.) Seedlings Through the Application of Liquid Seaweed Extracts of Fucus spiralis. Commun. Soil Sci. Plant Anal. 2017, 48, 2582–2593. [Google Scholar] [CrossRef]
- Mireya, R.; Vanessa, C.; Andrea, P.; Ocampo-alvarez, H.; Santacruz-ruvalcaba, F. Seaweed Extract Improves Growth and Productivity of Tomato Plants under Salinity Stress. Agronomy 2022, 12, 2495. [Google Scholar] [CrossRef]
- Stirk, W.A.; Tarkowská, D.; Turečová, V.; Strnad, M.; van Staden, J. Abscisic acid, gibberellins and brassinosteroids in Kelpak®, a commercial seaweed extract made from Ecklonia maxima. J. Appl. Phycol. 2014, 26, 561–567. [Google Scholar] [CrossRef]
- Pereira, L.; Morrison, L.; Shukla, P.S.; Critchley, A.T. A concise review of the brown macroalga Ascophyllum nodosum (Linnaeus) Le Jolis. J. Appl. Phycol. 2020, 32, 3561–3584. [Google Scholar] [CrossRef]
- Ugarte, R.; Sharp, G. Management and production of the brown algae Ascophyllum nodosum in the Canadian maritimes. J. Appl. Phycol. 2012, 24, 409–416. [Google Scholar] [CrossRef]
- Frioni, T.; Sabbatini, P.; Tombesi, S.; Norrie, J.; Poni, S.; Gatti, M.; Palliotti, A. Effects of a biostimulant derived from the brown seaweed Ascophyllum nodosum on ripening dynamics and fruit quality of grapevines. Sci. Hortic. 2018, 232, 97–106. [Google Scholar] [CrossRef]
- Szczepanek, M.; Wszelaczynska, E.; Poberezny, J. Effect of Seaweed Biostimulant Application in Sprng Wheat. AgroLife Sci. J. 2018, 7, 131–136. [Google Scholar]
- Ashour, M.; Al-Souti, A.S.; Hassan, S.M.; Ammar, G.A.G.; Goda, A.M.A.S.; El-Shenody, R.; Abomohra, A.E.F.; El-Haroun, E.; Elshobary, M.E. Commercial Seaweed Liquid Extract as Strawberry Biostimulants and Bioethanol Production. Life 2023, 13, 85. [Google Scholar] [CrossRef]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulatory activities of Ascophyllum nodosum extract in tomato and sweet pepper crops in a tropical environment. PLoS ONE 2019, 14, e0216710. [Google Scholar] [CrossRef]
- Ahmed, M.; Ullah, H.; Himanshu, S.K.; García-Caparrós, P.; Tisarum, R.; Cha-um, S.; Datta, A. Ascophyllum nodosum seaweed extract and potassium alleviate drought damage in tomato by improving plant water relations, photosynthetic performance, and stomatal function. J. Appl. Phycol. 2024, 36, 2255–2268. [Google Scholar] [CrossRef]
- Belnap, J.; Lange, O.L. (Eds.) Biological Soil Crusts: Structure, Function, and Management; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; Volume 150. [Google Scholar]
- Ward, D.A.; Adhikari, S.; Struwig, M.; Skikne, S.; Fryday, A.; Smith, D.; Rajakaruna, N. Lichen morphospecies diversity and community composition across the Tswalu Kalahari Reserve, South Africa. S. Afr. J. Bot. 2024, 174, 978–987. [Google Scholar] [CrossRef]
- Aoussar, N.; Manzali, R.; Nattah, I.; Rhallabi, N.; Vasiljevic, P.; Bouksaim, M.; Douira, A.; Manojlović, N.; Mellouki, F. Chemical composition and antioxidant activity of two lichens species (Pseudevernia furfuracea L. and Evernia prunastri L.) collected from Morocco. J. Mater. Environ. Sci. 2017, 8, 1968–1976. [Google Scholar]
- Ajaj, A.; Ouazzani Touhami, A.; Benkirane, R. Contribution to the update catalogue of lichenized and lichenicolous fungi in Morocco. J. Anim. Plant Sci. 2013, 19, 2961–3025. [Google Scholar]
- Seaward, M.R.D.; Amrani, S. Checklist of lichens and lichenicolous fungi of Morocco. Herzogia 2023, 35, 564–612. [Google Scholar] [CrossRef]
- Badridze, G.; Chkhubianishvili, E.; Rapava, L.; Kikvidze, M.; Chigladze, L.; Tsiklauri, N.; Tsilosani, K.; Kupradze, I.; Chanishvili, S. Active metabolites of some lichens growing in Georgia. J. Biodivers. Environ. Sci. 2019, 15, 1–15. [Google Scholar]
- Akbulut, G.; Yildiz, A.; Kodu, D.; Code, R. An Overview to Lichens: The Nutrient Composition of Some Species Lichens, composed of fungi (microbiont) and algae (photobiont), are chemically and structurally very different from vascular plants. They represent a type of symbiosis, generally reg. Kafkas Üniversitesi Fen Bilim. Enstitüsü Derg. 2010, 3, 79–86. [Google Scholar]
- Ergün, N. Auxin (Indole-3-acetic acid), Gibberellic acid (GA 3), Abscisic Acid (ABA) and Cytokinin (Zeatin) Production by Some Species of Mosses and Lichens. Turk. J. Bot. 2002, 26, 13–18. [Google Scholar]
- Silva, M.d.L.C.d.; Iacomini, M.; Jablonski, E.; Gorin, P.A. Carbohydrate, glycopeptide and protein components of the lichen Sticta sp. and effect of storage. Phytochemistry 1993, 33, 547–552. [Google Scholar] [CrossRef]
- Yildiz, A.; Aksoy, A.; Akbulut, G.; Demirezen, D.; Islek, C.; Altuner, E.M.; Duman, F. Kayseri İlinde Pseudevernia furfuracea Türünde Klorofil Yikimi ile Aǧir Metal Miktari Arasindaki İlişki. Ekoloji 2011, 20, 82–88. [Google Scholar] [CrossRef]
- Kranner, I.; Cram, W.J.; Zorn, M.; Wornik, S.; Yoshimura, I.; Stabentheiner, E.; Pfeifhofer, H.W. Antioxidants and photoprotection in a lichen as compared with its isolated symbiotic partners. Proc. Natl. Acad. Sci. USA 2005, 102, 3141–3146. [Google Scholar] [CrossRef]
- Karakaş, V.E.; Öztürk, Ş.; Oran, S. Original Research Article Comparison of Photosynthetic Pigment Contents in Lichen Samples were Collected from Different Localities in Bursa. J. Biol. Environ. Sci. 2017, 11, 121–127. [Google Scholar]
- Pichler, G.; Stöggl, W.; Trippel, D.; Candotto Carniel, F.; Muggia, L.; Ametrano, C.G.; Çimen, T.; Holzinger, A.; Tretiach, M.; Kranner, I. Phytohormone release by three isolated lichen mycobionts and the effects of indole-3-acetic acid on their compatible photobionts. Symbiosis 2020, 82, 95–108. [Google Scholar] [CrossRef]
- Demirbas, A. Trace element concentrations in ashes from various types of Lichen biomass species. Energy Sources 2004, 26, 499–506. [Google Scholar] [CrossRef]
- Adamo, P.; Giordano, S.; Vingiani, S.; Cobianchi, R.C.; Violante, P. Trace element accumulation by moss and lichen exposed in bags in the city of Naples (Italy). Environ. Pollut. 2003, 122, 91–103. [Google Scholar] [CrossRef]
- Sorbo, S.; Aprile, G.; Strumia, S.; Castaldo Cobianchi, R.; Leone, A.; Basile, A. Trace element accumulation in Pseudevernia furfuracea (L.) Zopf exposed in Italy’s so called Triangle of Death. Sci. Total Environ. 2008, 407, 647–654. [Google Scholar] [CrossRef]
- Malaspina, P.; Giordani, P.; Modenesi, P.; Abelmoschi, M.L.; Magi, E.; Soggia, F. Bioaccumulation capacity of two chemical varieties of the lichen Pseudevernia furfuracea. Ecol. Indic. 2014, 45, 605–610. [Google Scholar] [CrossRef]
- Palmqvist, K. Carbon economy in lichens. New Phytol. 2000, 148, 11–36. [Google Scholar] [CrossRef]
- Clark, B.M.; Clair, L.L.S.; Mangelson, N.F.; Rees, L.B.; Grant, P.G.; Bench, G.S. Characterization of mycobiont adaptations in the foliose lichen Xanthoparmelia chlorochroa (Parmeliaceae). Am. J. Bot. 2001, 88, 1742–1749. [Google Scholar] [CrossRef]
- Pike, L.H. The Importance of Epiphytic Lichens in Mineral Cycling. Bryologist 1978, 81, 247–257. [Google Scholar] [CrossRef]
- Dahlman, L.; Persson, J.; Näsholm, T.; Palmqvist, K. Carbon and nitrogen distribution in the green algal lichens Hypogymnia physodes and Platismatia glauca in relation to nutrient supply. Planta 2003, 217, 41–48. [Google Scholar] [CrossRef]
- Johansson, O.; Palmqvist, K.; Olofsson, J. Nitrogen deposition drives lichen community changes through differential species responses. Glob. Chang. Biol. 2012, 18, 2626–2635. [Google Scholar] [CrossRef]
- Balarinová, K.; Barták, M.; Hazdrová, J.; Hájek, J.; Jílková, J. Changes in photosynthesis, pigment composition and glutathione contents in two Antarctic lichens during a light stress and recovery. Photosynthetica 2014, 52, 538–547. [Google Scholar] [CrossRef]
- Haugwitz, M.S.; Michelsen, A. Long-term addition of fertilizer, labile carbon, and fungicide alters the biomass of plant functional groups in a subarctic-alpine community. Plant Ecol. 2011, 212, 715–726. [Google Scholar] [CrossRef]
- Tunç, E.; Doğan, B. The Effect of Lichens on Soil Agregate Stability. Int. J. Energy Eng. Sci. 2022, 7, 40–51. [Google Scholar]
- Ghiloufi, W.; Yun, J.; Kim, J.; Lee, J.; Kang, H. The influences of lichens on soil physico-chemical properties, enzymes and microbes are species specific: Insights from South Mediterranean arid ecosystem. Appl. Soil Ecol. 2023, 181, 104656. [Google Scholar] [CrossRef]
- Ji, C.J.; Yang, Y.H.; Han, W.X.; He, Y.F.; Smith, J.; Smith, P. Climatic and Edaphic Controls on Soil pH in Alpine Grasslands on the Tibetan Plateau, China: A Quantitative Analysis. Pedosphere 2014, 24, 39–44. [Google Scholar] [CrossRef]
- Asta, J.; Orry, F.; Toutain, F.; Souchier, B.; Villemin, G. Micromorphological and ultrastructural investigations of the lichen—Soil interface. Soil Biol. Biochem. 2001, 33, 323–337. [Google Scholar] [CrossRef]
- Sauze, J.; Ogée, J.; Maron, P.A.; Crouzet, O.; Nowak, V.; Wohl, S.; Kaisermann, A.; Jones, S.P.; Wingate, L. The interaction of soil phototrophs and fungi with pH and their impact on soil CO2, CO18O and OCS exchange. Soil Biol. Biochem. 2017, 115, 371–382. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef]
- Liu, M.; Sui, X.; Hu, Y.; Feng, F. Microbial community structure and the relationship with soil carbon and nitrogen in an original Korean pine forest of Changbai Mountain, China. BMC Microbiol. 2019, 19, 218. [Google Scholar] [CrossRef]
- Stark, S.; Hyvärinen, M. Are phenolics leaching from the lichen Cladina stellaris sources of energy rather than allelopathic agents for soil microorganisms? Soil Biol. Biochem. 2003, 35, 1381–1385. [Google Scholar] [CrossRef]
- Sedia, E.G.; Ehrenfeld, J.G. Differential effects of lichens, mosses and grasses on respiration and nitrogen mineralization in soils of the New Jersey Pinelands. Oecologia 2005, 144, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Ohtonen, R.; Väre, H. Vegetation composition determines microbial activities in a boreal forest soil. Microb. Ecol. 1998, 36, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Mendili, M.; Essghaier, B.; Seaward, M.R.D.; Khadhri, A. In vitro evaluation of lysozyme activity and antimicrobial effect of extracts from four Tunisian lichens: Diploschistes ocellatus, Flavoparmelia caperata, Squamarina cartilaginea and Xanthoria parietina. Arch. Microbiol. 2021, 203, 1461–1469. [Google Scholar] [CrossRef]
- Galanty, A.; Paśko, P.; Podolak, I. Enantioselective activity of usnic acid: A comprehensive review and future perspectives. Phytochem. Rev. 2019, 18, 527–548. [Google Scholar] [CrossRef]
- Basile, A.; Rigano, D.; Loppi, S.; Di Santi, A.; Nebbioso, A.; Sorbo, S.; Conte, B.; Paoli, L.; De Ruberto, F.; Molinari, A.M.; et al. Antiproliferative, antibacterial and antifungal activity of the lichen Xanthoria parietina and its secondary metabolite parietin. Int. J. Mol. Sci. 2015, 16, 7861–7875. [Google Scholar] [CrossRef]
- Castillo-Monroy, A.P.; Bowker, M.A.; García-Palacios, P.; Maestre, F.T. Aspects of soil lichen biodiversity and aggregation interact to influence subsurface microbial function. Plant Soil 2014, 386, 303–316. [Google Scholar] [CrossRef]
- Medison, R.G.; Jiang, J.; Medison, M.B.; Tan, L.T.; Kayange, C.D.M.; Sun, Z.; Zhou, Y. Evaluating the potential of Bacillus licheniformis YZCUO202005 isolated from lichens in maize growth promotion and biocontrol. Heliyon 2023, 9, e20204. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).