Photon Fluence Rate and Temperature Effects on Temperate Atlantic Kelp Species
Abstract
1. Introduction
2. Materials and Methods
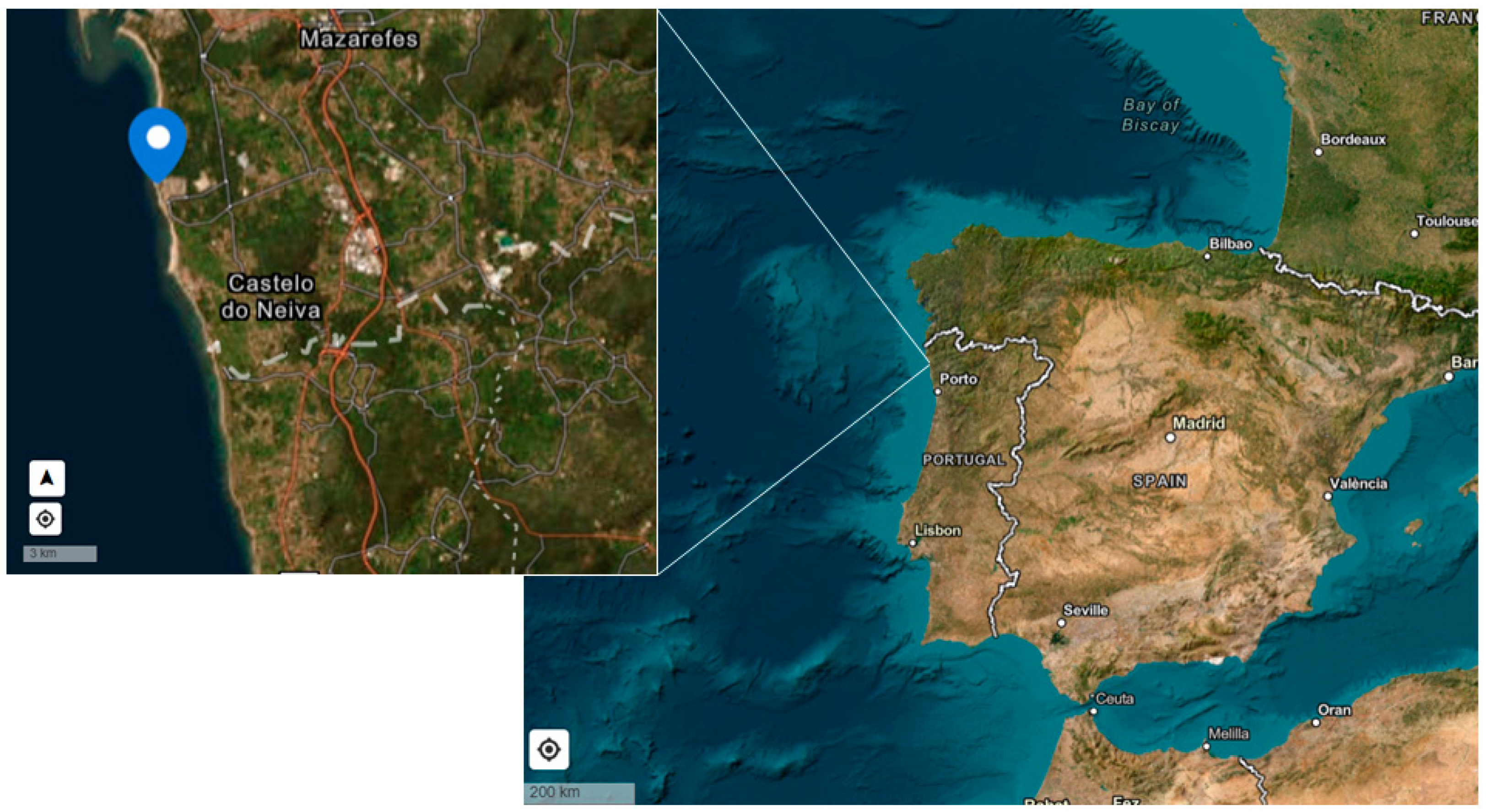
2.1. Biological Material
2.2. Photosynthesis and Respiration
3. Results
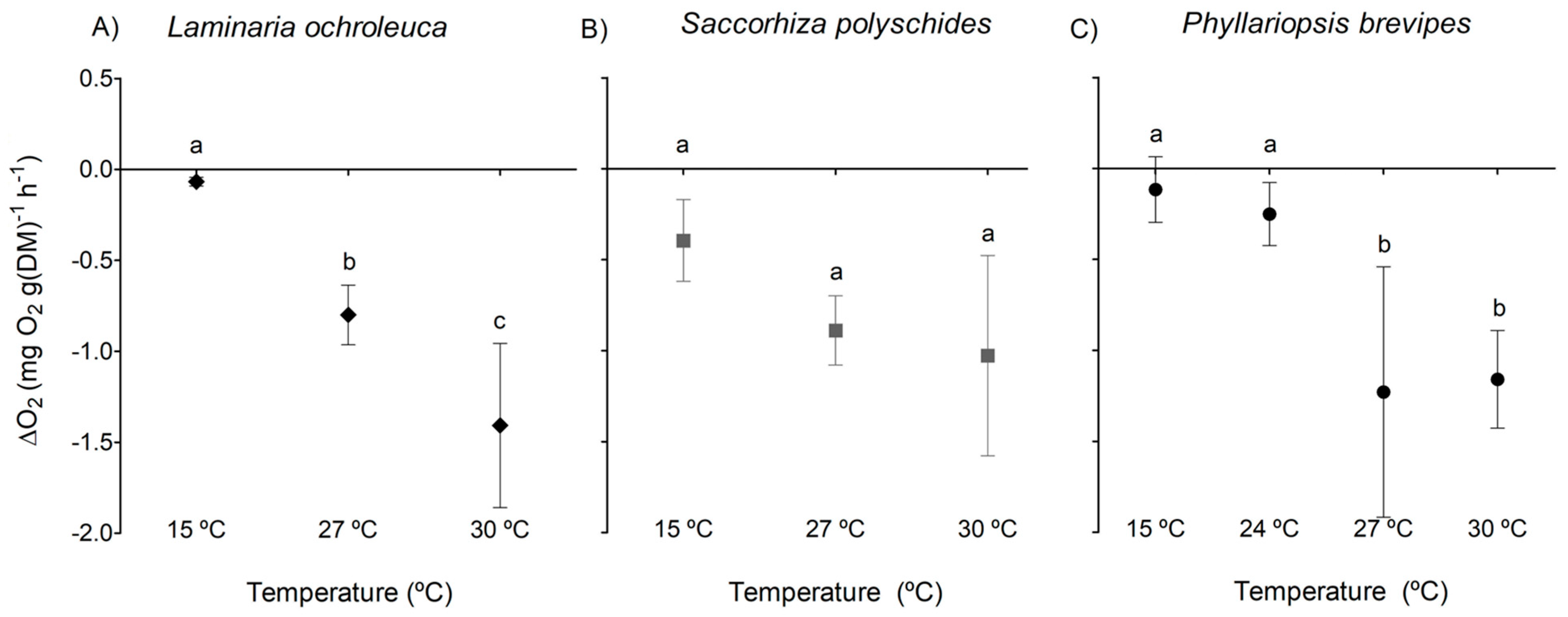
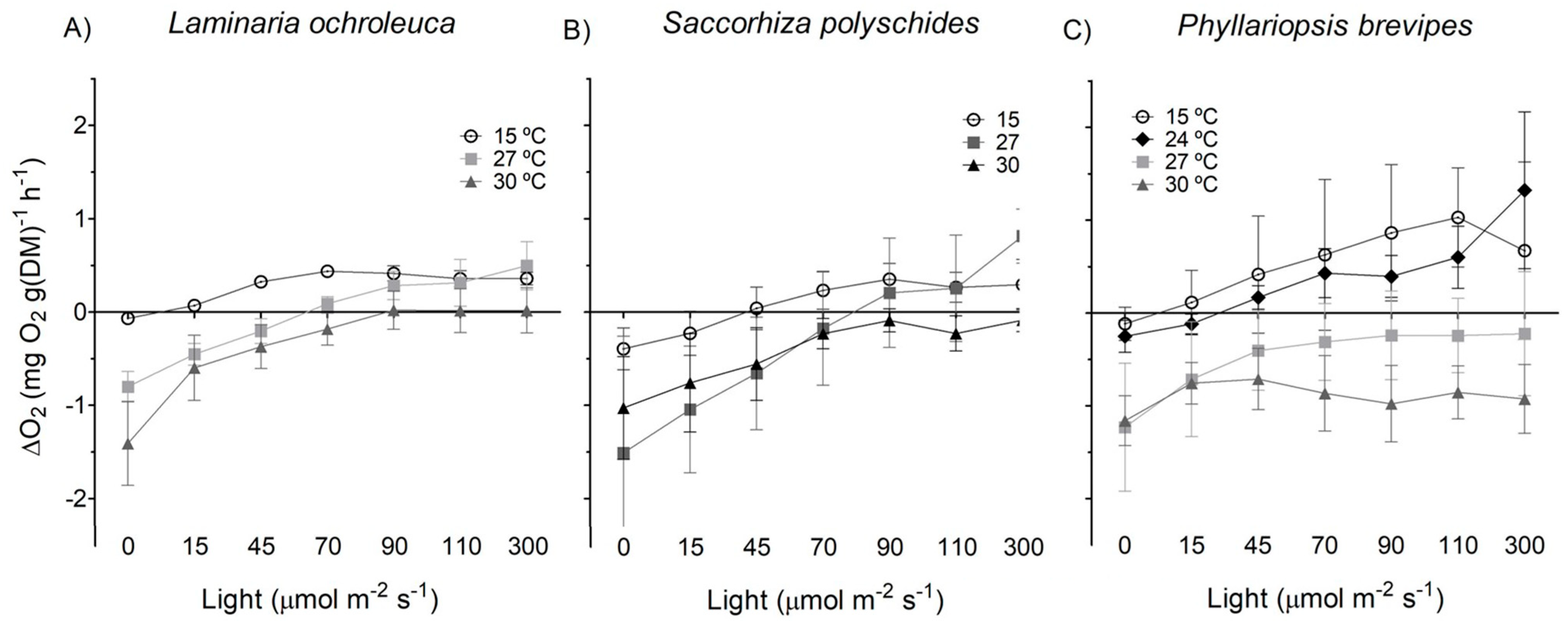
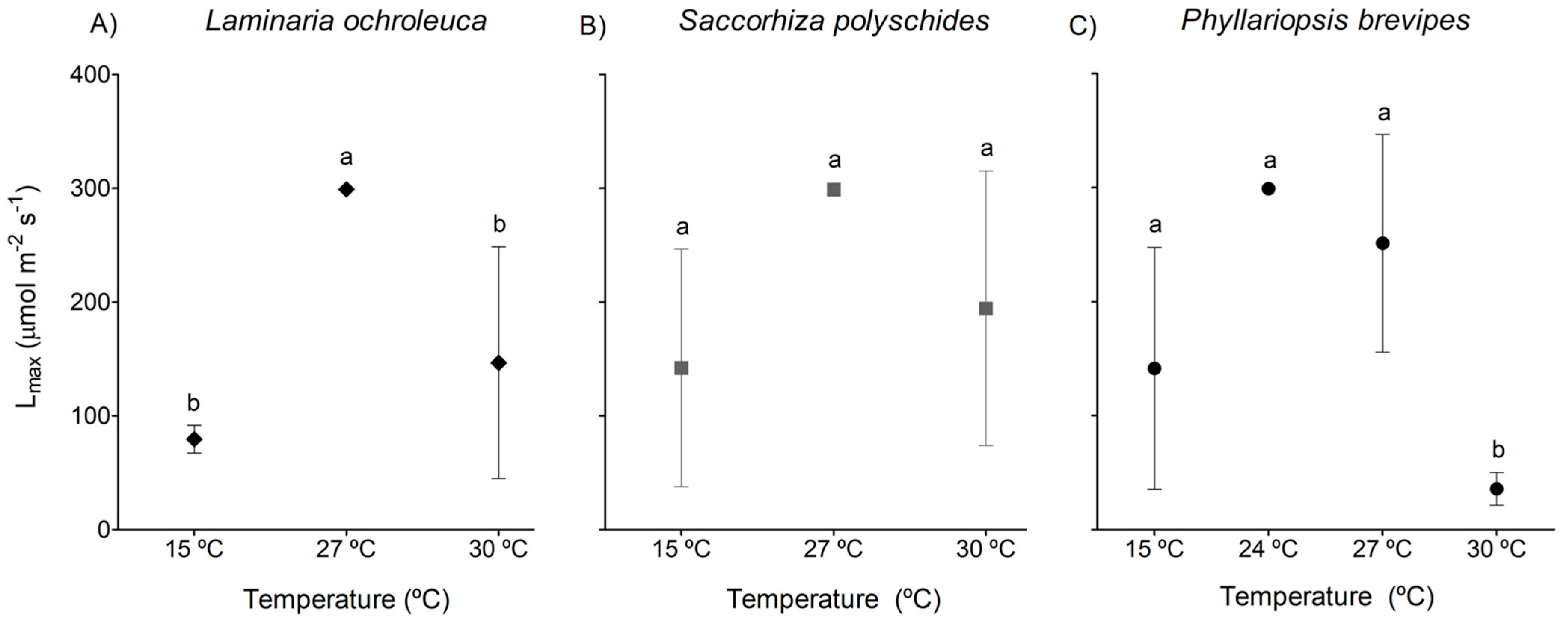
3.1. Photosynthesis and Respiration
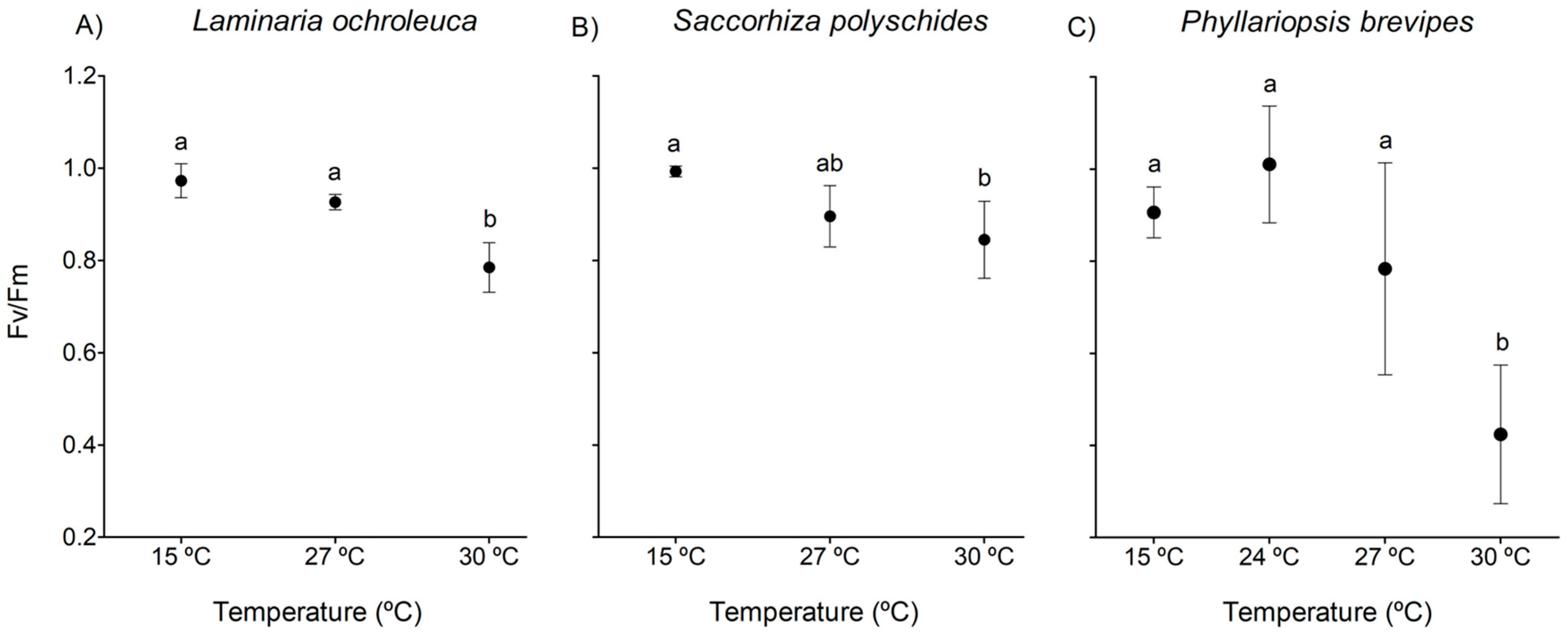
3.2. Chlorophyll Fluorescence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DM | Dry Mass |
| Fv/Fm | Maximum quantum yield of photosystem II |
| LMax | Photon fluence rate at which maximum production was attained |
| NPP | Net Primary Production |
| RMax | Maximum respiration rate |
References
- Wernberg, T.; Krumhansl, K.A.; Filbee-Dexter, K.; Pedersen, M. Status and Trends for the World’s Kelp Forests. In World Seas: An Environmental Evaluation, 2nd ed.; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Jayathilake, D.R.M.; Costello, M.J. A modelled global distribution of the kelp biome. Biol. Conserv. 2020, 252, 108815. [Google Scholar] [CrossRef]
- Dayton, P. Ecology of Kelp Communities. Annu. Rev. Ecol. Evol. Syst. 1985, 16, 215–245. [Google Scholar] [CrossRef]
- Lenoir, J.; Gégout, J.C.; Marquet, P.A.; de Ruffray, P.; Brisse, H. A significant upward shift in plant species optimum elevation during the 20th century. Science 2008, 320, 1768–1771. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, I.; Vogt, J.; Pehlke, C.; Hanelt, D. Prevailing sea surface temperatures inhibit summer reproduction of the kelp aminaria digitata at Helgoland (North Sea). J. Phycol. 2013, 49, 1061–1073. [Google Scholar] [CrossRef] [PubMed]
- Markager, S.; Sand-Jensen, K. Light requirements and depth zonation of marine macroalgae. Mar. Ecol. Prog. Ser. 1992, 88, 83–92. [Google Scholar] [CrossRef]
- Dieter, H.; Melchersmann, B.; Wiencke, C.; Nultsch, W. Effects of high light stress on photosynthesis of polar macroalgae in relation to depth distribution. Mar. Ecol. Prog. Ser. 1997, 149, 255–266. [Google Scholar] [CrossRef]
- Franco, J.N.; Tuya, F.; Bertocci, I.; Rodríguez, L.; Martínez, B.; Sousa-Pinto, I.; Arenas, F. The ‘golden kelp’ Laminaria ochroleuca under global change: Integrating multiple eco-physiological responses with species distribution models. J. Ecol. 2018, 106, 47–58. [Google Scholar] [CrossRef]
- Franco, J.N.; Wernberg, T.; Bertocci, I.; Duarte, P.; Jacinto, D.; Vasco-Rodrigues, N.; Tuya, F. Herbivory drives kelp recruits into ‘hiding’ in a warm ocean climate. Mar. Ecol. Prog. Ser. 2015, 536, 1–9. [Google Scholar] [CrossRef]
- Schoenrock, K.; O’Callaghan, T.; O’Callaghan, R.; Krueger-Hadfield, S. First record of Laminaria ochroleuca Bachelot de la Pylaie in Ireland in Béal an Mhuirthead, county Mayo. Mar. Biodivers. Rec. 2019, 12, 9. [Google Scholar] [CrossRef]
- Lüning, K.; Yarish, C.; Kirkman, H. Seaweeds: Their Environment, Biogeography, and Ecophysiology; Wiley: Hoboken, NJ, USA, 1990. [Google Scholar]
- Birkett, D.; Maggs, C.; Dring, M.; Boaden, P. An Overview of Dynamic and Sensitivity Characteristics for Conservation Management of Marine SACs. 1998. Available online: https://www.researchgate.net/publication/242700157_An_Overview_of_Dynamic_and_Sensitivity_Characteristics_for_Conservation_Management_of_Marine_SACs (accessed on 1 June 2025).
- Henry, E. The life history of Phyllariopsis brevipes (=Phyllaria reniformis) (Phyllariaceae, Laminariales, Phaeophyceae), a kelp with dioecious but sexually monomorphic gametophytes. Phycologia 1987, 26, 17–22. [Google Scholar] [CrossRef]
- Dieck, I. Temperature tolerance and survival in darkness of kelp gametophytes (Laminariales, Phaeophyta)—Ecological and biogeographical implications. Mar. Ecol. Prog. Ser. 1993, 100, 253. [Google Scholar] [CrossRef]
- Pereira, T.R.; Engelen, A.H.; Pearson, G.A.; Serrão, E.A.; Destombe, C.; Valero, M. Temperature effects on the microscopic haploid stage development of Laminaria ochroleuca and Sacchoriza polyschides, kelps with contrasting life histories. Cah. Biol. Mar. 2011, 52, 395–403. [Google Scholar]
- García-Sánchez, M.; Delgado Huertas, A.; Fernández, J.; Flores-Moya, A. Photosynthetic use of inorganic carbon in deep-water kelps from the Strait of Gibraltar. Photosynth. Res. 2016, 2016, 295–305. [Google Scholar] [CrossRef]
- Tempera, F.; Milla-Figueras, D.; Sinde-Mano, A.L.; Atchoi, E.; Afonso, P. Range Extension of Mesophotic Kelps (Ochrophyta: Laminariales and Tilopteridales) in the Central North Atlantic: Opportunities for Marine Forest Research and Conservation. J. Phycol. 2021, 57, 1140–1150. [Google Scholar] [CrossRef] [PubMed]
- Pearson, G.; Lago-Leston, A.; Mota, C. Frayed at the edges: Selective pressure and adaptive response to abiotic stressors are mismatched in low diversity edge populations. J. Ecol. 2009, 97, 450–462. [Google Scholar] [CrossRef]
- Dieck, I.T. North Pacific and North Atlantic digitate Laminaria species (Phaeophyta): Hybridization experiments and temperature responses. Phycologia 1992, 31, 147–163. [Google Scholar]
- Biškup, S.; Bertocci, I.; Arenas, F.; Tuya, F. Functional responses of juvenile kelps, Laminaria ochroleuca and Saccorhiza polyschides, to increasing temperatures. Aquat. Bot. 2014, 113, 117–122. [Google Scholar] [CrossRef]
- Pereira, T.; Engelen, A.; Pearson, G.; Valero, M.; Serrao, E. Response of kelps from different latitudes to consecutive heat shock. J. Exp. Mar. Biol. Ecol. 2015, 463, 57–62. [Google Scholar] [CrossRef]
- King, N.; Leathers, T.; Smith, K.; Smale, D. The influence of pre-exposure to marine heatwaves on the critical thermal maxima (CTmax) of marine foundation species. Funct. Ecol. 2024. [Google Scholar] [CrossRef]
- Leathers, T.; King, N.G.; Foggo, A.; Smale, D.A. Marine heatwave duration and intensity interact to reduce physiological tipping points of kelp species with contrasting thermal affinities. Ann. Bot. 2024, 133, 51–60. [Google Scholar] [CrossRef]
- Strasser, F.-E.; Barreto, L.M.; Kaidi, S.; Sabour, B.; Serrão, E.A.; Pearson, G.A.; Martins, N. Population level variation in reproductive development and output in the golden kelp Laminaria ochroleuca under marine heat wave scenarios. Front. Mar. Sci. 2022, 9, 943511. [Google Scholar] [CrossRef]
- Izquierdo, J.; Pérez-ruzafa, I.; Gallardo, T. Effect of temperature and photon fluence rate on gametophytes and young sporophytes of Laminaria ochroleuca Pylaie. Helgol. Mar. Res. 2001, 55, 285–292. [Google Scholar] [CrossRef]
- Smale, D.; Wernberg, T.; Yunnie, A.L.E.; Vance, T. The rise of Laminaria ochroleuca in the Western English Channel (UK) and comparisons with its competitor and assemblage dominant Laminaria hyperborea. Mar. Ecol. 2015, 36, 1033–1044. [Google Scholar] [CrossRef]
- Smale, D.A.; Moore, P.J. Variability in kelp forest structure along a latitudinal gradient in ocean temperature. J. Exp. Mar. Biol. Ecol. 2017, 486, 255–264. [Google Scholar] [CrossRef]
- Norton, T.A. Experiments on the Factors Influencing the Geographical Distributions of Saccorhiza polyschides and Saccorhiza dermatodea. New Phytol. 1977, 78, 625–635. [Google Scholar] [CrossRef]
- Fernández, C. The retreat of large brown seaweeds on the north coast of Spain: The case of Saccorhiza polyschides. Eur. J. Phycol. 2011, 46, 352–360. [Google Scholar] [CrossRef]
- Chefaoui, R.M.; Duarte, C.M.; Serrão, E.A. Dramatic loss of seagrass habitat under projected climate change in the Mediterranean Sea. Glob. Change Biol. 2018, 24, 4919–4928. [Google Scholar] [CrossRef]
- Pereira, T.R.; Engelen, A.H.; Pearson, G.A.; Valero, M.; Serrão, E.A. Population dynamics of temperate kelp forests near their low-latitude limit. Aquat. Bot. 2017, 139, 8–18. [Google Scholar] [CrossRef]
- Flores-Moya, A.; Fernández, J.A.; Niell, F.X. Reproductive phenology, growth and primary production of Phyllariopsis purpurascens (Phyllariaceae, Phaeophyta) from the Straits of Gibraltar. Eur. J. Phycol. 1993, 28, 223–230. [Google Scholar] [CrossRef]
- Lemos, R.; Pires, H. The upwelling regime off the West Portuguese Coast, 1941–2000. Int. J. Climatol. 2004, 24, 511–524. [Google Scholar] [CrossRef]
- Gómez-Gesteira, M.; de Castro, M.; Alvarez, I.; Lorenzo, M.N.; Gesteira, J.L.; Crespo, A.J. Spatio-temporal upwelling trends along the Canary Upwelling System (1967–2006). Ann. N. Y. Acad. Sci. 2008, 1146, 320–337. [Google Scholar] [CrossRef] [PubMed]
- Oliver, E.C.J.; Donat, M.G.; Burrows, M.T.; Moore, P.J.; Smale, D.A.; Alexander, L.V.; Benthuysen, J.A.; Feng, M.; Sen Gupta, A.; Hobday, A.J.; et al. Longer and more frequent marine heatwaves over the past century. Nat. Commun. 2018, 9, 1324. [Google Scholar] [CrossRef] [PubMed]

| Species | p-Value | F | df |
|---|---|---|---|
| Laminaria ochroleuca | 0.0001 | 23.419 | 2 |
| Saccorhiza polyschides | 0.197 | 1.956 | 2 |
| Phyllariopsis brevipes | 0.002 | 9.051 | 3 |
| Species | p-Value | F | df |
|---|---|---|---|
| Laminaria ochroleuca | 0.006 | 9.622 | 2 |
| Saccorhiza polyschides | 0.012 | 7.427 | 2 |
| Phyllariopsis brevipes | 0.011 | 5.861 | 3 |
| Species | p-Value | F | df |
|---|---|---|---|
| Laminaria ochroleuca | 0.002 | 14.425 | 2 |
| Saccorhiza polyschides | 0.1 | 3.000 | 2 |
| Phyllariopsis brevipes | 0.001 | 10.743 | 3 |
| Species | p-Value | F | df |
|---|---|---|---|
| Laminaria ochroleuca | <0.001 | 25.621 | 2 |
| Saccorhiza polyschides | 0.023 | 5.922 | 2 |
| Phyllariopsis brevipes | 0.001 | 11.010 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinheiro, T.F.; Chemello, S.; Sousa-Pinto, I.; Pereira, T.R. Photon Fluence Rate and Temperature Effects on Temperate Atlantic Kelp Species. Phycology 2025, 5, 27. https://doi.org/10.3390/phycology5020027
Pinheiro TF, Chemello S, Sousa-Pinto I, Pereira TR. Photon Fluence Rate and Temperature Effects on Temperate Atlantic Kelp Species. Phycology. 2025; 5(2):27. https://doi.org/10.3390/phycology5020027
Chicago/Turabian StylePinheiro, Tomás F., Silvia Chemello, Isabel Sousa-Pinto, and Tânia R. Pereira. 2025. "Photon Fluence Rate and Temperature Effects on Temperate Atlantic Kelp Species" Phycology 5, no. 2: 27. https://doi.org/10.3390/phycology5020027
APA StylePinheiro, T. F., Chemello, S., Sousa-Pinto, I., & Pereira, T. R. (2025). Photon Fluence Rate and Temperature Effects on Temperate Atlantic Kelp Species. Phycology, 5(2), 27. https://doi.org/10.3390/phycology5020027





