Bio-Flocculation: A Green Tool in Biorefineries for Recovering High Added-Value Compounds from Microalgae
Abstract
1. Introduction
2. Flocculation Methods
2.1. Physical Flocculation
2.2. Chemical Flocculation
2.3. Bio-Flocculation
2.3.1. Flocculation by Bio-Flocculant Molecules
2.3.2. Fungi-Mediated Flocculation
2.3.3. Bacteria-Mediated Flocculation
2.3.4. Alga-Mediated Flocculation
2.3.5. Self-Flocculation
Genetic Improvement of Self-Flocculation
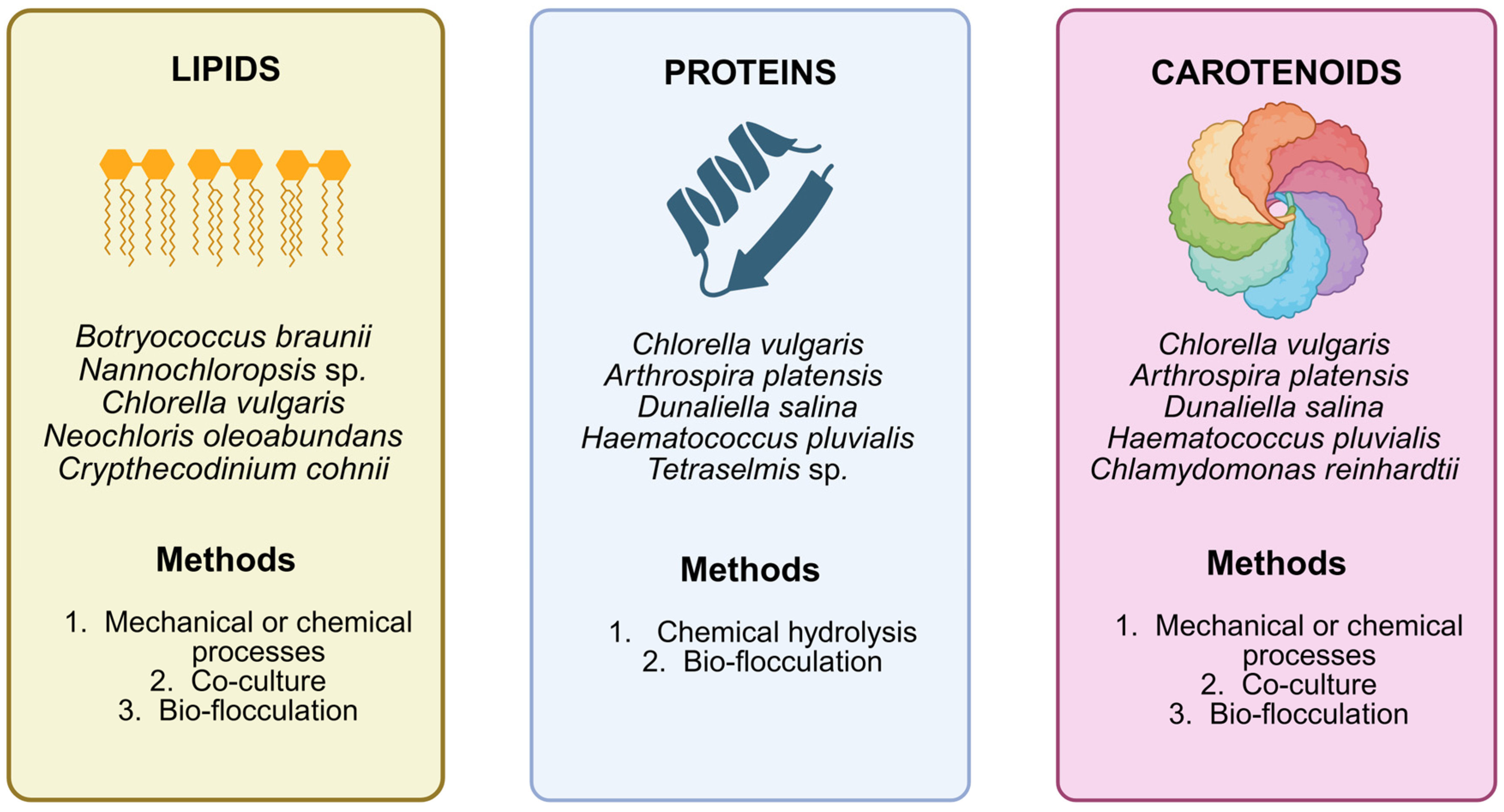
3. Valuable Microalgae Compounds and Bio-Flocculation Implications
3.1. Lipids
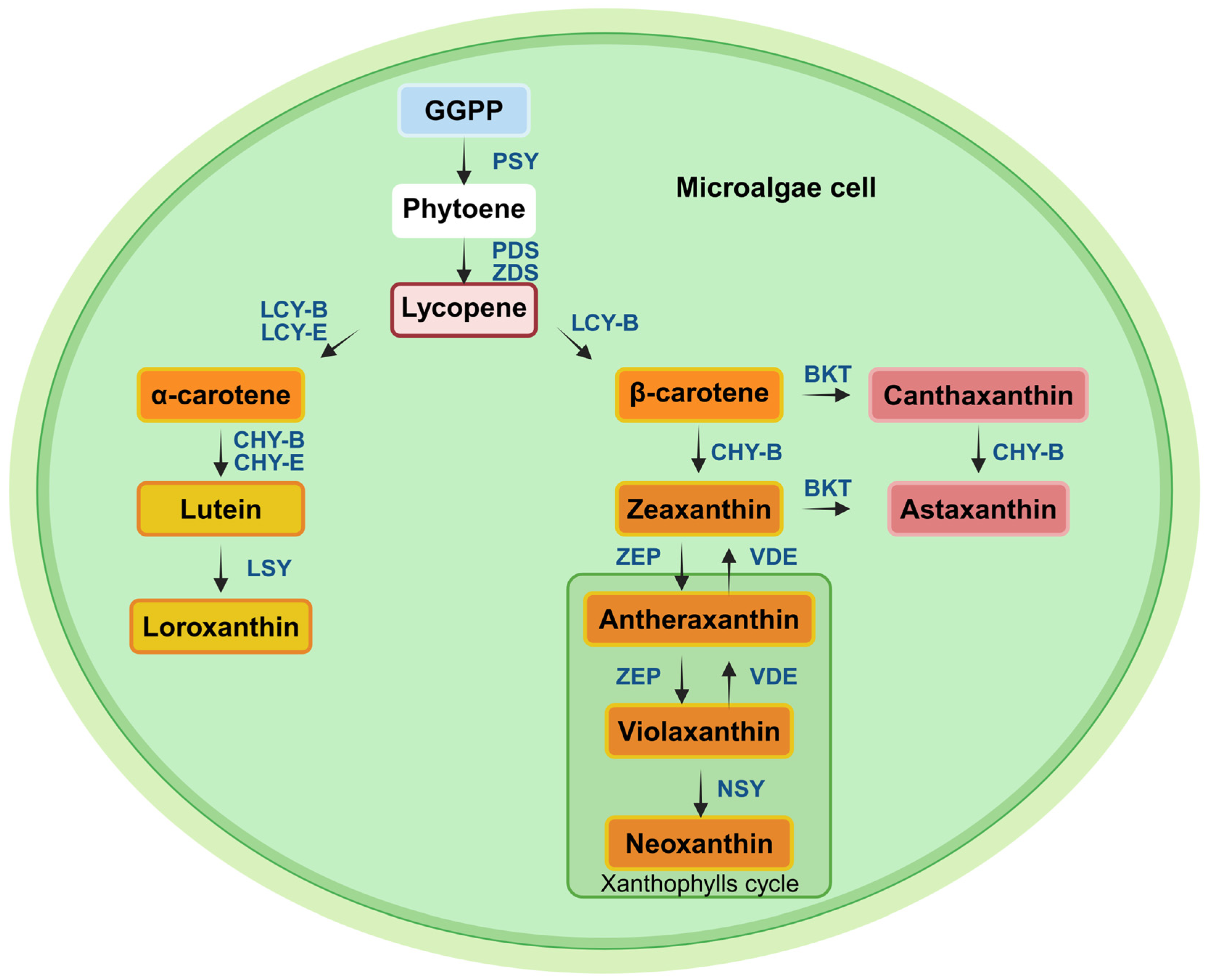
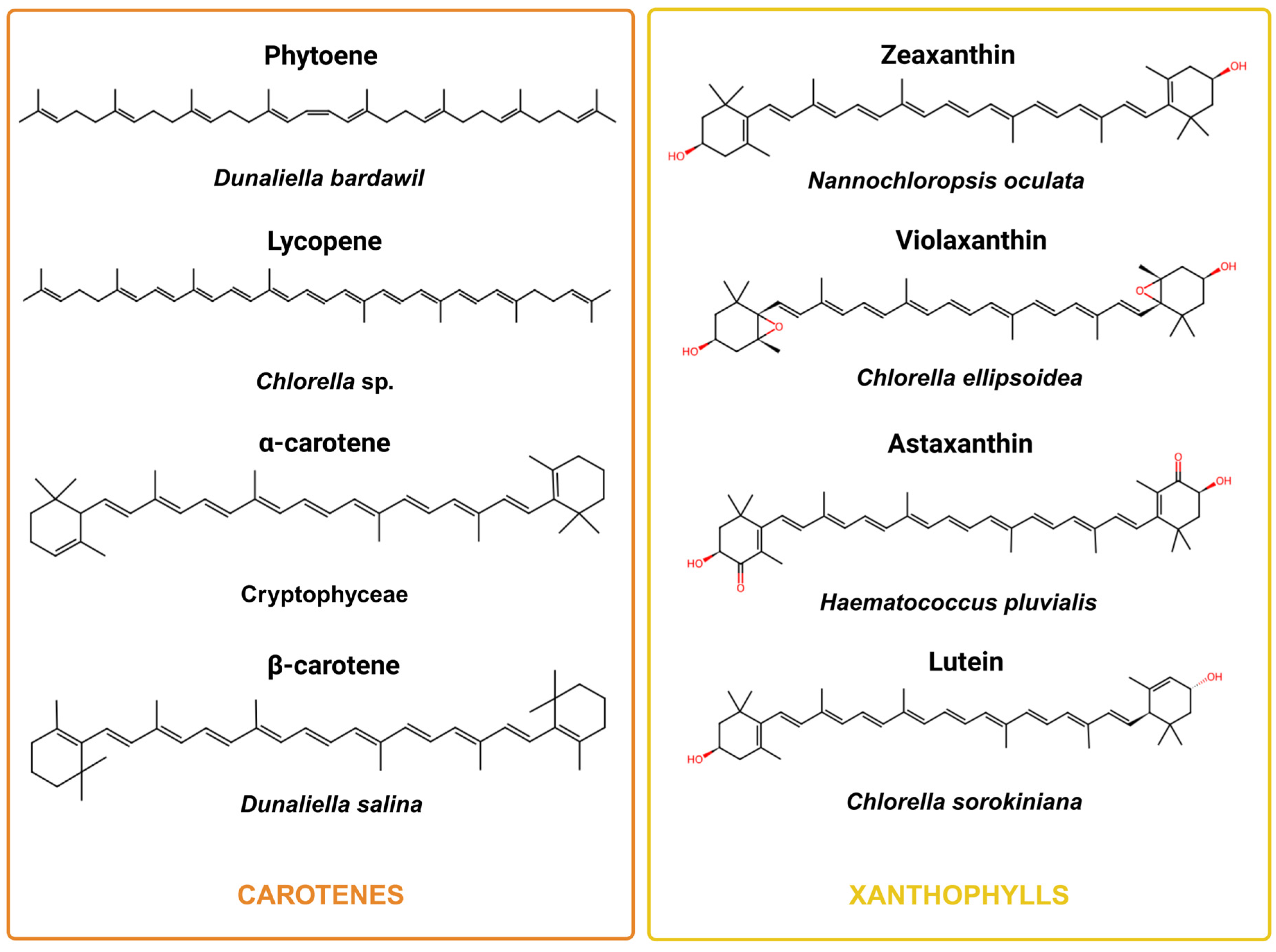
3.2. Carotenoids
3.3. Proteins
3.4. Other Valuable Compounds
4. Conclusions
5. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Zhang, Y.; Chen, L.; Cheng, W.; Liu, T. Combined Production of Fucoxanthin and EPA from Two Diatom Strains Phaeodactylum tricornutum and Cylindrotheca fusiformis Cultures. Bioprocess Biosyst. Eng. 2018, 41, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Darwesh, O.M.; Matter, I.A.; Eida, M.F.; Moawad, H.; Oh, Y.K. Influence of Nitrogen Source and Growth Phase on Extracellular Biosynthesis of Silver Nanoparticles Using Cultural Filtrates of Scenedesmus obliquus. Appl. Sci. 2019, 9, 1465. [Google Scholar] [CrossRef]
- Caspeta, L.; Buijs, N.A.A.; Nielsen, J. The Role of Biofuels in the Future Energy Supply. Energy Environ. Sci. 2013, 6, 1077–1082. [Google Scholar] [CrossRef]
- Abas, N.; Kalair, A.; Khan, N. Review of Fossil Fuels and Future Energy Technologies. Futures 2015, 69, 31–49. [Google Scholar] [CrossRef]
- Ray, A.; Banerjee, S.; Das, D. Microalgal Bio-Flocculation: Present Scenario and Prospects for Commercialization. Environ. Sci. Pollut. Res. Int. 2021, 28, 26294–26312. [Google Scholar] [CrossRef]
- Malik, S.; Khan, F.; Atta, Z.; Habib, N.; Haider, M.N.; Wang, N.; Alam, A.; Jambi, E.J.; Gull, M.; Mehmood, M.A.; et al. Microalgal Flocculation: Global Research Progress and Prospects for Algal Biorefinery. Biotechnol. Appl. Biochem. 2020, 67, 52–60. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from Microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Zhu, L.; Nugroho, Y.K.; Shakeel, S.R.; Li, Z.; Martinkauppi, B.; Hiltunen, E. Using Microalgae to Produce Liquid Transportation Biodiesel: What Is Next? Renew. Sustain. Energy Rev. 2017, 78, 391–400. [Google Scholar] [CrossRef]
- Goh, B.H.H.; Ong, H.C.; Cheah, M.Y.; Chen, W.H.; Yu, K.L.; Mahlia, T.M.I. Sustainability of Direct Biodiesel Synthesis from Microalgae Biomass: A Critical Review. Renew. Sustain. Energy Rev. 2019, 107, 59–74. [Google Scholar] [CrossRef]
- Mathimani, T.; Senthil Kumar, T.; Chandrasekar, M.; Uma, L.; Prabaharan, D. Assessment of Fuel Properties, Engine Performance and Emission Characteristics of Outdoor Grown Marine Chlorella vulgaris BDUG 91771 Biodiesel. Renew. Energy 2017, 105, 637–646. [Google Scholar] [CrossRef]
- Li, T.; Hu, J.; Zhu, L. Self-flocculation as an Efficient Method to Harvest Microalgae: A Mini-review. Water 2021, 13, 2585. [Google Scholar] [CrossRef]
- Milledge, J.J.; Heaven, S. A Review of the Harvesting of Micro-Algae for Biofuel Production. Rev. Environ. Sci. Biotechnol. 2013, 12, 165–178. [Google Scholar] [CrossRef]
- Branyikova, I.; Filipenska, M.; Urbanova, K.; Ruzicka, M.C.; Pivokonsky, M.; Branyik, T. Physicochemical Approach to Alkaline Flocculation of Chlorella vulgaris Induced by Calcium Phosphate Precipitates. Colloids Surf. B Biointerfaces 2018, 166, 54–60. [Google Scholar] [CrossRef]
- Lee, Y.C.; Lee, K.; Oh, Y.K. Recent Nanoparticle Engineering Advances in Microalgal Cultivation and Harvesting Processes of Biodiesel Production: A Review. Bioresour. Technol. 2015, 184, 63–72. [Google Scholar] [CrossRef]
- Li, S.; Hu, T.; Xu, Y.; Wang, J.; Chu, R.; Yin, Z.; Mo, F.; Zhu, L. A Review on Flocculation as an Efficient Method to Harvest Energy Microalgae: Mechanisms, Performances, Influencing Factors and Perspectives. Renew. Sustain. Energy Rev. 2020, 131, 110005. [Google Scholar] [CrossRef]
- Vandamme, D.; Pontes, S.C.V.; Goiris, K.; Foubert, I.; Pinoy, L.J.J.; Muylaert, K. Evaluation of Electro-Coagulation-Flocculation for Harvesting Marine and Freshwater Microalgae. Biotechnol. Bioeng. 2011, 108, 2320–2329. [Google Scholar] [CrossRef]
- El-Baz, F.K.; Gad, M.S.; Abdo, S.M.; Abed, K.A.; Matter, I.A. Performance and Exhaust Emissions of a Diesel Engine Burning Algal Biodiesel Blends. Int. J. Mech. Mechatron. Eng. 2016, 16, 151–158. [Google Scholar]
- Van Haver, L.; Nayar, S. Polyelectrolyte Flocculants in Harvesting Microalgal Biomass for Food and Feed Applications. Algal Res. 2017, 24, 167–180. [Google Scholar] [CrossRef]
- Mathimani, T.; Mallick, N. A Comprehensive Review on Harvesting of Microalgae for Biodiesel—Key Challenges and Future Directions. Renew. Sustain. Energy Rev. 2018, 91, 1103–1120. [Google Scholar] [CrossRef]
- Wan, C.; Alam, M.A.; Zhao, X.Q.; Zhang, X.Y.; Guo, S.L.; Ho, S.H.; Chang, J.S.; Bai, F.W. Current Progress and Future Prospect of Microalgal Biomass Harvest Using Various Flocculation Technologies. Bioresour. Technol. 2015, 184, 251–257. [Google Scholar] [CrossRef]
- Son, J.; Sung, M.; Ryu, H.; Oh, Y.K.; Han, J.I. Microalgae Dewatering Based on Forward Osmosis Employing Proton Exchange Membrane. Bioresour. Technol. 2017, 244, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Laamanen, C.A.; Ross, G.M.; Scott, J.A. Flotation Harvesting of Microalgae. Renew. Sustain. Energy Rev. 2016, 58, 75–86. [Google Scholar] [CrossRef]
- Demir, I.; Besson, A.; Guiraud, P.; Formosa-Dague, C. Towards a Better Understanding of Microalgae Natural Flocculation Mechanisms to Enhance Flotation Harvesting Efficiency. Water Sci. Technol. 2020, 82, 1009–1024. [Google Scholar] [CrossRef]
- Sukenik, A.; Bilanovic, D.; Shelef, G. Flocculation of Microalgae in Brackish and Sea Waters. Biomass 1988, 15, 187–199. [Google Scholar] [CrossRef]
- Vandamme, D.; Foubert, I.; Muylaert, K. Flocculation as a Low-Cost Method for Harvesting Microalgae for Bulk Biomass Production. Trends Biotechnol. 2013, 31, 233–239. [Google Scholar] [CrossRef]
- Salim, S.; Bosma, R.; Vermuë, M.H.; Wijffels, R.H. Harvesting of Microalgae by Bio-Flocculation. J. Appl. Phycol. 2011, 23, 849–855. [Google Scholar] [CrossRef]
- Wong, Y.K.; Ho, Y.H.; Leung, H.M.; Ho, K.C.; Yau, Y.H.; Yung, K.K.L. Enhancement of Chlorella vulgaris Harvesting via the Electro-Coagulation-Flotation (ECF) Method. Environ. Sci. Pollut. Res. 2017, 24, 9102–9110. [Google Scholar] [CrossRef]
- Estrada-Graf, A.; Hernández, S.; Morales, M. Biomitigation of CO2 from Flue Gas by Scenedesmus Obtusiusculus AT-UAM Using a Hybrid Photobioreactor Coupled to a Biomass Recovery Stage by Electro-Coagulation-Flotation. Environ. Sci. Pollut. Res. 2020, 27, 28561–28574. [Google Scholar] [CrossRef]
- Kim, D.Y.; Lee, K.; Lee, J.; Lee, Y.H.; Han, J.I.; Park, J.Y.; Oh, Y.K. Acidified-Flocculation Process for Harvesting of Microalgae: Coagulant Reutilization and Metal-Free-Microalgae Recovery. Bioresour. Technol. 2017, 239, 190–196. [Google Scholar] [CrossRef]
- Xu, H.; Tang, Z.; Yang, D.; Dai, X.; Chen, H. Enhanced Growth and Auto-Flocculation of Scenedesmus quadricauda in Anaerobic Digestate Using High Light Intensity and Nanosilica: A Biomineralization-Inspired Strategy. Water Res. 2023, 235, 119893. [Google Scholar] [CrossRef]
- Cerff, M.; Morweiser, M.; Dillschneider, R.; Michel, A.; Menzel, K.; Posten, C. Harvesting Fresh Water and Marine Algae by Magnetic Separation: Screening of Separation Parameters and High Gradient Magnetic Filtration. Bioresour. Technol. 2012, 118, 289–295. [Google Scholar] [CrossRef]
- Lee, K.; Lee, S.Y.; Na, J.G.; Jeon, S.G.; Praveenkumar, R.; Kim, D.M.; Chang, W.S.; Oh, Y.K. Magnetophoretic Harvesting of Oleaginous Chlorella sp. by Using Biocompatible Chitosan/Magnetic Nanoparticle Composites. Bioresour. Technol. 2013, 149, 575–578. [Google Scholar] [CrossRef]
- Lim, J.K.; Chieh, D.C.J.; Jalak, S.A.; Toh, P.Y.; Yasin, N.H.M.; Ng, B.W.; Ahmad, A.L. Rapid Magnetophoretic Separation of Microalgae. Small 2012, 8, 1683–1692. [Google Scholar] [CrossRef]
- Sumathi, Y.; Kumar, P.; Singhania, R.R.; Chen, C.W.; Gurunathan, B.; Dong, C.D.; Patel, A.K. Harnessing Fe3O4 Nanoparticles for Sustainable Harvesting of Astaxanthin-Producing Microalgae: Advancing Industrial-Scale Biorefinery. Sep. Purif. Technol. 2025, 353, 128408. [Google Scholar] [CrossRef]
- Tripathi, G.; Pandey, V.K.; Ahmad, S.; Irum; Khujamshukurov, N.A.; Farooqui, A.; Mishra, V. Utilizing Novel Aspergillus Species for Bio-Flocculation: A Cost-Effective Approach to Harvest Scenedesmus Microalgae for Biofuel Production. Curr. Res. Microb. Sci. 2024, 7, 100272. [Google Scholar] [CrossRef]
- Xu, H.; Liu, C.; Wang, A.; Yue, B.; Lin, T.; Ding, M. Microalgae Treatment of Food Processing Wastewater for Simultaneous Biomass Resource Recycling and Water Reuse. J. Environ. Manag. 2024, 369, 122394. [Google Scholar] [CrossRef]
- Lu, Z.; Beal, C.M.; Johnson, Z.I. Comparative Performance and Technoeconomic Analyses of Two Microalgae Harvesting Systems Evaluated at a Commercially Relevant Scale. Algal Res. 2022, 64, 102667. [Google Scholar] [CrossRef]
- Chekli, L.; Corjon, E.; Tabatabai, S.A.A.; Naidu, G.; Tamburic, B.; Park, S.H.; Shon, H.K. Performance of Titanium Salts Compared to Conventional FeCl3 for the Removal of Algal Organic Matter (AOM) in Synthetic Seawater: Coagulation Performance, Organic Fraction Removal and Floc Characteristics. J. Environ. Manag. 2017, 201, 28–36. [Google Scholar] [CrossRef]
- Maia, C.; Pôjo, V.; Tavares, T.; Pires, J.C.M.; Malcata, F.X. Surfactant-Mediated Microalgal Flocculation: Process Efficiency and Kinetic Modelling. Bioengineering 2024, 11, 722. [Google Scholar] [CrossRef]
- Yudong, N.; Tao, Z.; Haihua, W.; Haixing, C. Upcycling Harmful Algal Blooms into Short-Chain Organic Matters Assisted with Cellulose-Based Flocculant. Bioresour. Technol. 2024, 397, 130425. [Google Scholar] [CrossRef]
- Papazi, A.; Makridis, P.; Divanach, P. Harvesting Chlorella Minutissima Using Cell Coagulants. J. Appl. Phycol. 2010, 22, 349–355. [Google Scholar] [CrossRef]
- Rwehumbiza, V.M.; Harrison, R.; Thomsen, L. Alum-Induced Flocculation of Preconcentrated Nannochloropsis salina: Residual Aluminium in the Biomass, FAMEs and Its Effects on Microalgae Growth upon Media Recycling. Chem. Eng. J. 2012, 200–202, 168–175. [Google Scholar] [CrossRef]
- Beach, E.S.; Eckelman, M.J.; Cui, Z.; Brentner, L.; Zimmerman, J.B. Preferential Technological and Life Cycle Environmental Performance of Chitosan Flocculation for Harvesting of the Green Algae Neochloris oleoabundans. Bioresour. Technol. 2012, 121, 445–449. [Google Scholar] [CrossRef]
- Zheng, H.; Gao, Z.; Yin, J.; Tang, X.; Ji, X.; Huang, H. Harvesting of Microalgae by Flocculation with Poly (γ-Glutamic Acid). Bioresour. Technol. 2012, 112, 212–220. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.P.; Guo, J.S.; Fang, F.; Yan, P. Flocculation-Enhanced Photobiological Hydrogen Production by Microalgae: Flocculant Composition, Hydrogenase Activity and Response Mechanism. Chem. Eng. J. 2024, 485, 150065. [Google Scholar] [CrossRef]
- Tran, D.T.; Le, B.H.; Lee, D.J.; Chen, C.L.; Wang, H.Y.; Chang, J.S. Microalgae Harvesting and Subsequent Biodiesel Conversion. Bioresour. Technol. 2013, 140, 179–186. [Google Scholar] [CrossRef]
- Swar, S.S.; Boonnorat, J.; Ghimire, A. Algae-Based Treatment of a Landfill Leachate Pretreated by Coagulation-Flocculation. J. Environ. Manag. 2023, 342, 118223. [Google Scholar] [CrossRef]
- Marinho, Y.F.; de Oliveira, A.P.S.; Oliveira, C.Y.B.; Napoleão, T.H.; Guedes Paiva, P.M.; de Sant’Anna, M.C.S.; Malafaia, C.B.; Gálvez, A.O. Usage of Moringa Oleifera Residual Seeds Promotes Efficient Flocculation of Tetradesmus dimorphus Biomass. Biomass Convers. Biorefin 2022, 13, 1–9. [Google Scholar] [CrossRef]
- Lama, S.; Pappa, M.; Brandão Watanabe, N.; Formosa-Dague, C.; Marchal, W.; Adriaensens, P.; Vandamme, D. Interference of Extracellular Soluble Algal Organic Matter on Flocculation–Sedimentation Harvesting of Chlorella sp. Bioresour. Technol. 2024, 411, 131290. [Google Scholar] [CrossRef]
- Chen, F.; Liu, Z.; Li, D.; Liu, C.; Zheng, P.; Chen, S. Using Ammonia for Algae Harvesting and as Nutrient in Subsequent Cultures. Bioresour. Technol. 2012, 121, 298–303. [Google Scholar] [CrossRef]
- Imbimbo, P.; Ferrara, A.; Giustino, E.; Liberti, D.; Monti, D.M. Microalgae Flocculation: Assessment of Extraction Yields and Biological Activity. Int. J. Mol. Sci. 2024, 25, 10238. [Google Scholar] [CrossRef] [PubMed]
- Endrawati, H.; Widianingsih, W.; Nuraini, R.A.T.; Hartati, R.; Redjeki, S.; Riniatsih, I.; Mahendrajaya, R.T. The Effect of Chitosan Concentration on Flocculation Efficiency Microalgae Porphyridium cruentum (Rhodhophyta). IOP Conf. Ser. Earth Environ. Sci. 2021, 919, 012052. [Google Scholar] [CrossRef]
- Gani, P.; Apandi, N.M.; Mohamed Sunar, N.; Matias-Peralta, H.M.; Kean Hua, A.; Mohd Dzulkifli, S.N.; Parjo, U.K. Outdoor Phycoremediation and Biomass Harvesting Optimization of Microalgae Botryococcus sp. Cultivated in Food Processing Wastewater Using an Enclosed Photobioreactor. Int. J. Phytoremediat. 2022, 24, 1431–1443. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.P.; Tran, T.N.T.; Le, T.V.A.; Nguyen Phan, T.X.; Show, P.L.; Chia, S.R. Auto-Flocculation through Cultivation of Chlorella vulgaris in Seafood Wastewater Discharge: Influence of Culture Conditions on Microalgae Growth and Nutrient Removal. J. Biosci. Bioeng. 2019, 127, 492–498. [Google Scholar] [CrossRef]
- Pérez, L.; Salgueiro, J.L.; Maceiras, R.; Cancela, Á.; Sánchez, Á. An Effective Method for Harvesting of Marine Microalgae: PH Induced Flocculation. Biomass Bioenergy 2017, 97, 20–26. [Google Scholar] [CrossRef]
- Schlesinger, A.; Eisenstadt, D.; Bar-Gil, A.; Carmely, H.; Einbinder, S.; Gressel, J. Inexpensive Non-Toxic Flocculation of Microalgae Contradicts Theories; Overcoming a Major Hurdle to Bulk Algal Production. Biotechnol. Adv. 2012, 30, 1023–1030. [Google Scholar] [CrossRef]
- Verfaillie, A.; Blockx, J.; Praveenkumar, R.; Thielemans, W.; Muylaert, K. Harvesting of Marine Microalgae Using Cationic Cellulose Nanocrystals. Carbohydr. Polym. 2020, 240, 116165. [Google Scholar] [CrossRef]
- Mixson, S.M.; Stikeleather, L.F.; Simmons, O.D.; Wilson, C.W.; Burkholder, J.A.M. PH-Induced Flocculation, Indirect Electrocoagulation, and Hollow Fiber Filtration Techniques for Harvesting the Saltwater Microalga Dunaliella. J. Appl. Phycol. 2014, 26, 1701–1709. [Google Scholar] [CrossRef]
- Spilling, K.; Seppälä, J.; Tamminen, T. Inducing Autoflocculation in the Diatom Phaeodactylum tricornutum Through CO2 Regulation. J. Appl. Phycol. 2011, 23, 959–966. [Google Scholar] [CrossRef]
- ’t Lam, G.P.; Vermuë, M.H.; Olivieri, G.; van den Broek, L.A.M.; Barbosa, M.J.; Eppink, M.H.M.; Wijffels, R.H.; Kleinegris, D.M.M. Cationic Polymers for Successful Flocculation of Marine Microalgae. Bioresour. Technol. 2014, 169, 804–807. [Google Scholar] [CrossRef]
- Wu, Z.; Zhu, Y.; Huang, W.; Zhang, C.; Li, T.; Zhang, Y.; Li, A. Evaluation of Flocculation Induced by PH Increase for Harvesting Microalgae and Reuse of Flocculated Medium. Bioresour. Technol. 2012, 110, 496–502. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Xu, X.; Yan, F.; Du, W.; Dai, R. Simultaneous Removal of Cyanobacteria and Algal Organic Matter by Mn(VII)/CaSO3 Enhanced Coagulation: Performance and Mechanism. J. Hazard. Mater. 2025, 485, 136839. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.R.; Wang, F.; Wang, S.K.; Liu, C.Z.; Guo, C. Efficient Harvesting of Marine Microalgae Nannochloropsis maritima Using Magnetic Nanoparticles. Bioresour. Technol. 2013, 138, 387–390. [Google Scholar] [CrossRef]
- Van Den Hende, S.; Carré, E.; Cocaud, E.; Beelen, V.; Boon, N.; Vervaeren, H. Treatment of Industrial Wastewaters by Microalgal Bacterial Flocs in Sequencing Batch Reactors. Bioresour. Technol. 2014, 161, 245–254. [Google Scholar] [CrossRef]
- Kim, D.G.; La, H.J.; Ahn, C.Y.; Park, Y.H.; Oh, H.M. Harvest of Scenedesmus sp. with Bioflocculant and Reuse of Culture Medium for Subsequent High-Density Cultures. Bioresour. Technol. 2011, 102, 3163–3168. [Google Scholar] [CrossRef]
- Chen, W.; Wang, T.; Dou, Z.; Xie, X. Microalgae Harvesting by Self-Driven 3D Microfiltration with Rationally Designed Porous Superabsorbent Polymer (PSAP) Beads. Environ. Sci. Technol. 2021, 55, 15446–15455. [Google Scholar] [CrossRef]
- Al-Humairi, S.T.; Lee, J.G.M.; Harvey, A.P.; Salman, A.D.; Juzsakova, T.; Van, B.; Le, P.C.; La, D.D.; Mungray, A.K.; Show, P.L.; et al. A Foam Column System Harvesting Freshwater Algae for Biodiesel Production: An Experiment and Process Model Evaluations. Sci. Total Environ. 2023, 862, 160702. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Q.; Pan, Y.; Chen, Q.; Sun, Z.; Hu, P. Development of an Effective Flocculation Method by Utilizing the Auto-Flocculation Capability of Phaeodactylum tricornutum. Algal Res. 2021, 58, 102413. [Google Scholar] [CrossRef]
- Alam, A.; Vandamme, D.; Chun, W.; Zhao, X.; Foubert, I.; Wang, Z.; Muylaert, K.; Yuan, Z. Bioflocculation as an Innovative Harvesting Strategy for Microalgae. Rev. Environ. Sci. Biotechnol. 2016, 15, 573–583. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Harun, M.R.; Lau, S.Y.; Sewu, D.D.; Danquah, M.K. Microalgal Biomass Generation via Electroflotation: A Cost-Effective Dewatering Technology. Appl. Sci. 2020, 10, 9053. [Google Scholar] [CrossRef]
- Tripathi, G.; Dubey, P.; Shamim, A.; Farooqui, A.; Mishra, V. Bio-Flocculation: A Cost Effective and Energy Efficient Harvesting Technique for Algal Biofuel Production and Wastewater Treatment. Bioresour. Technol. Rep. 2024, 28, 101969. [Google Scholar] [CrossRef]
- Ogbonna, C.N.; Nwoba, E.G. Bio-Based Flocculants for Sustainable Harvesting of Microalgae for Biofuel Production. A Review. Renew. Sustain. Energy Rev. 2021, 139, 110690. [Google Scholar] [CrossRef]
- Gorin, K.V.; Sergeeva, Y.E.; Butylin, V.V.; Komova, A.V.; Pojidaev, V.M.; Badranova, G.U.; Shapovalova, A.A.; Konova, I.A.; Gotovtsev, P.M. Methods Coagulation/Flocculation and Flocculation with Ballast Agent for Effective Harvesting of Microalgae. Bioresour. Technol. 2015, 193, 178–184. [Google Scholar] [CrossRef]
- Oh, H.-M.; Lee, S.J.; Park, M.-H.; Kim, H.-S.; Kim, H.-C.; Yoon, J.-H.; Kwon, G.-S.; Yoon, B.-D. Harvesting of Chlorella vulgaris Using a Bioflocculant from Paenibacillus sp. AM49. Biotechnol. Lett. 2001, 23, 1229–1234. [Google Scholar] [CrossRef]
- Ayad, H.I.; Matter, I.A.; Gharieb, M.M.; Darwesh, O.M. Bioflocculation Harvesting of Oleaginous Microalga Chlorella sp. Using Novel Lipid-Rich Cellulolytic Fungus Aspergillus terreus (MD1) for Biodiesel Production. Biomass Convers. Biorefin 2023, 14, 30315–30327. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K.; Dmytryk, A.; Wilk, R.; Gramza, M.; Rój, E. Evaluation of Supercritical Extracts of Algae as Biostimulants of Plant Growth in Field Trials. Front. Plant Sci. 2016, 7, 1591. [Google Scholar] [CrossRef]
- Roselet, F.; Burkert, J.; Abreu, P.C. Flocculation of Nannochloropsis oculata Using a Tannin-Based Polymer: Bench Scale Optimization and Pilot Scale Reproducibility. Biomass Bioenergy 2016, 87, 55–60. [Google Scholar] [CrossRef]
- Farid, M.S.; Shariati, A.; Badakhshan, A.; Anvaripour, B. Using Nano-Chitosan for Harvesting Microalga Nannochloropsis sp. Bioresour. Technol. 2013, 131, 555–559. [Google Scholar] [CrossRef]
- Al-Hothaly, K.A. An Optimized Method for the Bio-Harvesting of Microalgae, Botryococcus braunii, Using Aspergillus sp. in Large-Scale Studies. MethodsX 2018, 5, 788–794. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Mathur, M.; Kumar, P.; Prajapati, S.K.; Malik, A. A Rapid Method for Fungal Assisted Algal Flocculation: Critical Parameters & Mechanism Insights. Algal Res. 2017, 21, 42–51. [Google Scholar] [CrossRef]
- Chen, J.; Leng, L.; Ye, C.; Lu, Q.; Addy, M.; Wang, J.; Liu, J.; Chen, P.; Ruan, R.; Zhou, W. A Comparative Study between Fungal Pellet- and Spore-Assisted Microalgae Harvesting Methods for Algae Bioflocculation. Bioresour. Technol. 2018, 259, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Chu, R.; Li, S.; Zhu, L.; Yin, Z.; Hu, D.; Liu, C.; Mo, F. A Review on Co-Cultivation of Microalgae with Filamentous Fungi: Efficient Harvesting, Wastewater Treatment and Biofuel Production. Renew. Sustain. Energy Rev. 2021, 139, 110689. [Google Scholar] [CrossRef]
- Jing, R.; Liu, X.; Yang, Q.; Wang, Y.; Zhang, N.; Zhang, S. Indigenous Fungi Derived from a Municipal Wastewater Treatment Plant with Rapid Flocculation of Chlorella sp.: Convenience, Mechanisms, and Conditions Optimization. J. Water Process Eng. 2025, 73, 107693. [Google Scholar] [CrossRef]
- Yang, H.; He, G. Influence of Nutritional Conditions on Exopolysaccharide Production by Submerged Cultivation of the Medicinal Fungus Shiraia bambusicola. World J. Microbiol. Biotechnol. 2008, 24, 2903–2907. [Google Scholar] [CrossRef]
- Mahapatra, S.; Banerjee, D. Fungal Exopolysaccharide: Production, Composition and Applications. Microbiol. Insights 2013, 6, 1–16. [Google Scholar] [CrossRef]
- Civzele, A.; Mezule, L. Fungal—Assisted Microalgae Flocculation and Simultaneous Lignocellulolytic Enzyme Production in Wastewater Treatment Systems. Biotechnol. Rep. 2025, 45, e00875. [Google Scholar] [CrossRef]
- Powell, R.J.; Hill, R.T. Mechanism of Algal Aggregation by Bacillus sp. Strain RP1137. Appl. Environ. Microbiol. 2014, 80, 4042–4050. [Google Scholar] [CrossRef]
- Wan, C.; Zhao, X.Q.; Guo, S.L.; Asraful Alam, M.; Bai, F.W. Bioflocculant Production from Solibacillus silvestris W01 and Its Application in Cost-Effective Harvest of Marine Microalga Nannochloropsis oceanica by Flocculation. Bioresour. Technol. 2013, 135, 207–212. [Google Scholar] [CrossRef]
- Lakshmikandan, M.; Murugesan, A.G.; Ameen, F.; Maneeruttanarungroj, C.; Wang, S. Efficient Bioflocculation and Biodiesel Production of Microalgae Asterococcus limneticus on Streptomyces Two-Stage Co-Cultivation Strategy. Biomass Bioenergy 2023, 175, 106886. [Google Scholar] [CrossRef]
- Ndikubwimana, T.; Zeng, X.; Liu, Y.; Chang, J.S.; Lu, Y. Harvesting of Microalgae Desmodesmus sp. F51 by Bioflocculation with Bacterial Bioflocculant. Algal Res. 2014, 6, 186–193. [Google Scholar] [CrossRef]
- Cho, K.; Hur, S.P.; Lee, C.H.; Ko, K.; Lee, Y.J.; Kim, K.N.; Kim, M.S.; Chung, Y.H.; Kim, D.; Oda, T. Bioflocculation of the Oceanic Microalga Dunaliella Salina by the Bloom-Forming Dinoflagellate Heterocapsa circularisquama, and Its Effect on Biodiesel Properties of the Biomass. Bioresour. Technol. 2016, 202, 257–261. [Google Scholar] [CrossRef]
- Lei, X.; Chen, Y.; Shao, Z.; Chen, Z.; Li, Y.; Zhu, H.; Zhang, J.; Zheng, W.; Zheng, T. Effective Harvesting of the Microalgae Chlorella vulgaris via Flocculation-Flotation with Bioflocculant. Bioresour. Technol. 2015, 198, 922–925. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, Y.; Liu, L.; Jiang, X.; Zhang, K.; Zheng, T.; Wang, H. First Evidence of Bioflocculant from Shinella albus with Flocculation Activity on Harvesting of Chlorella vulgaris Biomass. Bioresour. Technol. 2016, 218, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Vogelaar, J.C.T.; De Keizer, A.; Spijker, S.; Lettinga, G. Bioflocculation of Mesophilic and Thermophilic Activated Sludge. Water Res. 2005, 39, 37–46. [Google Scholar] [CrossRef]
- Lee, M.; Woo, S.G.; Ten, L.N. Shinella daejeonensis sp. Nov., a Nitrate-Reducing Bacterium Isolated from Sludge of a Leachate Treatment Plant. Int. J. Syst. Evol. Microbiol. 2011, 61, 2123–2128. [Google Scholar] [CrossRef]
- Matsui, T.; Shinzato, N.; Tamaki, H.; Muramatsu, M.; Hanada, S. Shinella yambaruensis sp. Nov., a 3-Methy-Sulfolane-Assimilating Bacterium Isolated from Soil. Int. J. Syst. Evol. Microbiol. 2009, 59, 536–539. [Google Scholar] [CrossRef]
- Lal, A.; Das, D. Biomass Production and Identification of Suitable Harvesting Technique for Chlorella sp. MJ 11/11 and Synechocystis PCC 6803. 3 Biotech. 2016, 6, 41. [Google Scholar] [CrossRef][Green Version]
- Xiao, R.; Zheng, Y. Overview of Microalgal Extracellular Polymeric Substances (EPS) and Their Applications. Biotechnol. Adv. 2016, 34, 1225–1244. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The Biofilm Matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Delattre, C.; Pierre, G.; Laroche, C.; Michaud, P. Production, Extraction and Characterization of Microalgal and Cyanobacterial Exopolysaccharides. Biotechnol. Adv. 2016, 34, 1159–1179. [Google Scholar] [CrossRef]
- Paulsen, B.S.; Aslaksen, T.; Freire-Nordi, C.S.; Vieira, A.A.H. Extracellular Polysaccharides from Ankistrodesmus densus (Chlorophyceae). J. Phycol. 1998, 34, 638–641. [Google Scholar] [CrossRef]
- Saadaoui, I.; Cherif, M.; Siddiqui, S.A.; Al Jabri, H.; Sayadi, S. Algal-Algal Bioflocculation Enhances the Recovery Efficiency of Picochlorum sp. QUCCCM130 with Low Auto-Settling Capacity. Algal Res. 2023, 71, 103038. [Google Scholar] [CrossRef]
- Alam, M.A.; Wan, C.; Guo, S.L.; Zhao, X.Q.; Huang, Z.Y.; Yang, Y.L.; Chang, J.S.; Bai, F.W. Characterization of the Flocculating Agent from the Spontaneously Flocculating Microalga Chlorella vulgaris JSC-7. J. Biosci. Bioeng. 2014, 118, 29–33. [Google Scholar] [CrossRef]
- Deryabin, D.G.; Efremova, L.V.; Vasilchenko, A.S.; Saidakova, E.V.; Sizova, E.A.; Troshin, P.A.; Zhilenkov, A.V.; Khakina, E.E. A Zeta Potential Value Determines the Aggregate’s Size of Penta-Substituted [60]fullerene Derivatives in Aqueous Suspension Whereas Positive Charge Is Required for Toxicity against Bacterial Cells. J. Nanobiotechnol. 2015, 13, 50. [Google Scholar] [CrossRef]
- Salim, S.; Vermuë, M.H.; Wijffels, R.H. Ratio between Autoflocculating and Target Microalgae Affects the Energy-Efficient Harvesting by Bio-Flocculation. Bioresour. Technol. 2012, 118, 49–55. [Google Scholar] [CrossRef]
- Zhao, F.; Xiao, J.; Ding, W.; Cui, N.; Yu, X.; Xu, J.W.; Li, T.; Zhao, P. An Effective Method for Harvesting of Microalga: Coculture-Induced Self-Flocculation. J. Taiwan. Inst. Chem. Eng. 2019, 100, 117–126. [Google Scholar] [CrossRef]
- Guo, S.L.; Zhao, X.Q.; Tang, Y.; Wan, C.; Alam, M.A.; Ho, S.H.; Bai, F.W.; Chang, J.S. Establishment of an Efficient Genetic Transformation System in Scenedesmus Obliquus. J. Biotechnol. 2013, 163, 61–68. [Google Scholar] [CrossRef]
- Muir, E.; Grossman, A.R.; Chisti, Y.; Fedrizzi, B.; Guieysse, B.; Plouviez, M. Self-Aggregation for Sustainable Harvesting of Microalgae. Algal Res. 2024, 83, 103685. [Google Scholar] [CrossRef]
- Rai, A.; Sirotiya, V.; Mourya, M.; Khan, M.J.; Ahirwar, A.; Sharma, A.K.; Kawatra, R.; Marchand, J.; Schoefs, B.; Varjani, S.; et al. Sustainable Treatment of Dye Wastewater by Recycling Microalgal and Diatom Biogenic Materials: Biorefinery Perspectives. Chemosphere 2022, 305, 135371. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Bai, F.W. Yeast Flocculation: New Story in Fuel Ethanol Production. Biotechnol. Adv. 2009, 27, 849–856. [Google Scholar] [CrossRef]
- Bauer, F.F.; Govender, P.; Bester, M.C. Yeast Flocculation and Its Biotechnological Relevance. Appl. Microbiol. Biotechnol. 2010, 88, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Salim, S.; Kosterink, N.R.; Tchetkoua Wacka, N.D.; Vermuë, M.H.; Wijffels, R.H. Mechanism behind Autoflocculation of Unicellular Green Microalgae Ettlia Texensis. J. Biotechnol. 2014, 174, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Aljuboori, A.H.R.; Uemura, Y.; Thanh, N.T. Flocculation and Mechanism of Self-Flocculating Lipid Producer Microalga Scenedesmus Quadricauda for Biomass Harvesting. Biomass Bioenergy 2016, 93, 38–42. [Google Scholar] [CrossRef]
- Tan, D.; Zhang, H.; Sun, S.; Dong, F.; Sun, H.; Li, B. Rapid Flocculation-Sedimentation of Microalgae with Organosilane-Functionalized Halloysite. Appl. Clay Sci. 2019, 177, 37–42. [Google Scholar] [CrossRef]
- Chen, Z.; Shao, S.; He, Y.; Luo, Q.; Zheng, M.; Zheng, M.; Chen, B.; Wang, M. Nutrients Removal from Piggery Wastewater Coupled to Lipid Production by a Newly Isolated Self-Flocculating Microalga Desmodesmus sp. PW1. Bioresour. Technol. 2020, 302, 122806. [Google Scholar] [CrossRef]
- Cheng, P.; Chen, D.; Liu, W.; Cobb, K.; Zhou, N.; Liu, Y.; Liu, H.; Wang, Q.; Chen, P.; Zhou, C.; et al. Auto-Flocculation Microalgae Species Tribonema sp. and Synechocystis sp. with T-IPL Pretreatment to Improve Swine Wastewater Nutrient Removal. Sci. Total Environ. 2020, 725, 138263. [Google Scholar] [CrossRef]
- Goswami, R.K.; Mehariya, S.; Karthikeyan, O.P.; Verma, P. Fungi-Assisted Bio-Flocculation of Picochlorum sp.: A Novel Bio-Assisted Treatment System for Municipal Wastewater. J. Water Process Eng. 2024, 57, 104666. [Google Scholar] [CrossRef]
- Kilian, O.; Benemann, C.S.E.; Niyogi, K.K.; Vick, B. High-Efficiency Homologous Recombination in the Oil-Producing Alga Nannochloropsis sp. Proc. Natl. Acad. Sci. USA 2011, 108, 21265–21269. [Google Scholar] [CrossRef]
- Trentacoste, E.M.; Shrestha, R.P.; Smith, S.R.; Glé, C.; Hartmann, A.C.; Hildebrand, M.; Gerwick, W.H. Metabolic Engineering of Lipid Catabolism Increases Microalgal Lipid Accumulation Without Compromising Growth. Proc. Natl. Acad. Sci. USA 2013, 110, 19748–19753. [Google Scholar] [CrossRef]
- Valverde, F.; Romero-Campero, F.J.; León, R.; Guerrero, M.G.; Serrano, A. New Challenges in Microalgae Biotechnology. Eur. J. Protistol. 2016, 55, 95–101. [Google Scholar] [CrossRef]
- Liu, Z.; Hao, N.; Hou, Y.; Wang, Q.; Liu, Q.; Yan, S.; Chen, F.; Zhao, L. Technologies for Harvesting the Microalgae for Industrial Applications: Current Trends and Perspectives. Bioresour. Technol. 2023, 387, 129631. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.L.; Zhao, X.Q.; Wan, C.; Huang, Z.Y.; Yang, Y.L.; Asraful Alam, M.; Ho, S.H.; Bai, F.W.; Chang, J.S. Characterization of Flocculating Agent from the Self-Flocculating Microalga Scenedesmus Obliquus AS-6-1 for Efficient Biomass Harvest. Bioresour. Technol. 2013, 145, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Santos, E.; Vila, M.; Vigara, J.; León, R. A New Approach to Express Transgenes in Microalgae and Its Use to Increase the Flocculation Ability of Chlamydomonas reinhardtii. J. Appl. Phycol. 2016, 28, 1611–1621. [Google Scholar] [CrossRef]
- Bhatia, S.; Pooja; Yadav, S.K. CRISPR-Cas for Genome Editing: Classification, Mechanism, Designing and Applications. Int. J. Biol. Macromol. 2023, 238, 124054. [Google Scholar] [CrossRef]
- Pickar-Oliver, A.; Gersbach, C.A. The next Generation of CRISPR–Cas Technologies and Applications. Nat. Rev. Mol. Cell Biol. 2019, 20, 490–507. [Google Scholar] [CrossRef]
- Patel, A.K.; Singhania, R.R.; Awasthi, M.K.; Varjani, S.; Bhatia, S.K.; Tsai, M.L.; Hsieh, S.L.; Chen, C.W.; Dong, C. Di Emerging Prospects of Macro- and Microalgae as Prebiotic. Microb. Cell Fact. 2021, 20, 112. [Google Scholar] [CrossRef]
- Lee, T.M.; Lin, J.Y.; Tsai, T.H.; Yang, R.Y.; Ng, I.S. Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) Technology and Genetic Engineering Strategies for Microalgae towards Carbon Neutrality: A Critical Review. Bioresour. Technol. 2023, 368, 128350. [Google Scholar] [CrossRef]
- Dhokane, D.; Shaikh, A.; Yadav, A.; Giri, N.; Bandyopadhyay, A.; Dasgupta, S.; Bhadra, B. CRISPR-Based Bioengineering in Microalgae for Production of Industrially Important Biomolecules. Front. Bioeng. Biotechnol. 2023, 11, 1267826. [Google Scholar] [CrossRef]
- Aditi; Bhardwaj, R.; Yadav, A.; Swapnil, P.; Meena, M. Characterization of Microalgal β-Carotene and Astaxanthin: Exploring Their Health-Promoting Properties under the Effect of Salinity and Light Intensity. Biotechnol. Biofuels Bioprod. 2025, 18, 18. [Google Scholar] [CrossRef]
- Arshad, S.; Qadir, M.L.; Hussain, N.; Ali, Q.; Han, S.; Ali, D. Advances in CRISPR/Cas9 Technology: Shaping the Future of Photosynthetic Microorganisms for Biofuel Production. Funct. Plant Biol. 2025, 52, FP24255. [Google Scholar] [CrossRef]
- Nguyen, T.H.T.; Park, S.; Jeong, J.; Shin, Y.S.; Sim, S.J.; Jin, E.S. Enhancing Lipid Productivity by Modulating Lipid Catabolism Using the CRISPR-Cas9 System in Chlamydomonas. J. Appl. Phycol. 2020, 32, 2829–2840. [Google Scholar] [CrossRef]
- Ajjawi, I.; Verruto, J.; Aqui, M.; Soriaga, L.B.; Coppersmith, J.; Kwok, K.; Peach, L.; Orchard, E.; Kalb, R.; Xu, W.; et al. Lipid Production in Nannochloropsis gaditana Is Doubled by Decreasing Expression of a Single Transcriptional Regulator. Nat. Biotechnol. 2017, 35, 647–652. [Google Scholar] [CrossRef]
- Lin, W.R.; Ng, I.S. Development of CRISPR/Cas9 System in Chlorella vulgaris FSP-E to Enhance Lipid Accumulation. Enzym. Microb. Technol. 2020, 133, 109458. [Google Scholar] [CrossRef]
- Kneip, J.S.; Kniepkamp, N.; Jang, J.; Mortaro, M.G.; Jin, E.; Kruse, O.; Baier, T. CRISPR/Cas9-Mediated Knockout of the Lycopene ε-Cyclase for Efficient Astaxanthin Production in the Green Microalga Chlamydomonas Reinhardtii. Plants 2024, 13, 1393. [Google Scholar] [CrossRef]
- Hu, L.; Feng, S.; Liang, G.; Du, J.; Li, A.; Niu, C. CRISPR/Cas9-Induced β-Carotene Hydroxylase Mutation in Dunaliella salina CCAP19/18. AMB Express 2021, 11, 83. [Google Scholar] [CrossRef]
- Græsholt, C.; Brembu, T.; Volpe, C.; Bartosova, Z.; Serif, M.; Winge, P.; Nymark, M. Zeaxanthin Epoxidase 3 Knockout Mutants of the Model Diatom Phaeodactylum tricornutum Enable Commercial Production of the Bioactive Carotenoid Diatoxanthin. Mar. Drugs 2024, 22, 185. [Google Scholar] [CrossRef]
- Ambily, B.; Limna Mol, V.P.; Sini, H.; Nevin, K.G. CRISPR-Based Microalgal Genome Editing and the Potential for Sustainable Aquaculture: A Comprehensive Review. J. Appl. Phycol. 2024, 37, 265–285. [Google Scholar] [CrossRef]
- Fernandes, T.; Cordeiro, N. Microalgae as Sustainable Biofactories to Produce High-Value Lipids: Biodiversity, Exploitation, and Biotechnological Applications. Mar. Drugs 2021, 19, 573. [Google Scholar] [CrossRef]
- Wen, Z.; Liu, J.; Chen, F. Biofuel from Microalgae. Compr. Biotechnol. Second Ed. 2011, 3, 127–133. [Google Scholar] [CrossRef]
- Manning, S.R. Microalgal Lipids: Biochemistry and Biotechnology. Curr. Opin. Biotechnol. 2022, 74, 1–7. [Google Scholar] [CrossRef]
- Xin, Y.; Wu, S.; Miao, C.; Xu, T.; Lu, Y. Towards Lipid from Microalgae: Products, Biosynthesis, and Genetic Engineering. Life 2024, 14, 447. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Wang, Y.; Zuo, M.; Wang, X.; Shen, B. Structural Analysis of Isolated Components in Microalgal Lipids: Neutral Lipids, Phospholipids, and Glycolipids. Bioresour. Technol. Rep. 2025, 29, 102083. [Google Scholar] [CrossRef]
- Mutanda, T.; Naidoo, D.; Bwapwa, J.K.; Anandraj, A. Biotechnological Applications of Microalgal Oleaginous Compounds: Current Trends on Microalgal Bioprocessing of Products. Front. Energy Res. 2020, 8, 598803. [Google Scholar] [CrossRef]
- Wang, M.; Ye, X.; Bi, H.; Shen, Z. Microalgae Biofuels: Illuminating the Path to a Sustainable Future amidst Challenges and Opportunities. Biotechnol. Biofuels Bioprod. 2024, 17, 10. [Google Scholar] [CrossRef]
- Zhang, L.; Tsui, T.-H.; Tong, Y.W.; Liu, R.; Baganz, F. Harvesting of Oleaginous Microbial Cells and Extraction of Microbial Lipids. In Microbial Lipids and Biodiesel Technologies; Springer Nature: Singapore, 2025; pp. 223–239. [Google Scholar] [CrossRef]
- Zhang, L.; Tsui, T.-H.; Tong, Y.W.; Liu, R.; Aggarangsi, P. Microbial Lipid Technology Based on Oleaginous Microalgae. In Microbial Lipids and Biodiesel Technologies; Springer Nature: Singapore, 2025; pp. 77–100. [Google Scholar] [CrossRef]
- Muradov, N.; Taha, M.; Miranda, A.F.; Wrede, D.; Kadali, K.; Gujar, A.; Stevenson, T.; Ball, A.S.; Mouradov, A. Fungal-Assisted Algal Flocculation: Application in Wastewater Treatment and Biofuel Production. Biotechnol. Biofuels 2015, 8, 24. [Google Scholar] [CrossRef]
- Jaiswal, K.K.; Kumar, V.; Gururani, P.; Vlaskin, M.S.; Parveen, A.; Nanda, M.; Kurbatova, A.; Gautam, P.; Grigorenko, A.V. Bio-Flocculation of Oleaginous Microalgae Integrated with Municipal Wastewater Treatment and Its Hydrothermal Liquefaction for Biofuel Production. Environ. Technol. Innov. 2022, 26, 102340. [Google Scholar] [CrossRef]
- Zhu, L.; Li, Z.; Hiltunen, E. Theoretical Assessment of Biomethane Production from Algal Residues after Biodiesel Production. Wiley Interdiscip. Rev. Energy Environ. 2018, 7, e273. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Yang, J. Bio-Flocculation Property Analyses of Oleaginous Microalgae Auxenochlorella protothecoides Utex 2341. Sustainability 2021, 13, 2885. [Google Scholar] [CrossRef]
- Tjandraatmadja, G.; Diaper, C. Sources of Critical Contaminants in Domestic Wastewater—A Literature Review. In CSIRO: Water for a Healthy Country National Research Flagship; CSIRO: Pullenvale, Australia, 2006; pp. 1–88. ISSN 1835-095X. [Google Scholar]
- Rengel, R.; Giraldez, I.; Díaz, M.J.; García, T.; Vigara, J.; León, R. Simultaneous Production of Carotenoids and Chemical Building Blocks Precursors from Chlorophyta Microalgae. Bioresour. Technol. 2022, 351, 127035. [Google Scholar] [CrossRef]
- Tanaka, K.; Lan, J.C.-W.; Kondo, A.; Hasunuma, T. Metabolic Engineering and Cultivation Strategies for Efficient Production of Fucoxanthin and Related Carotenoids. Appl. Microbiol. Biotechnol. 2025, 109, 57. [Google Scholar] [CrossRef]
- Lin, W.; Chen, L.; Tan, Z.; Deng, Z.; Liu, H. Application of Filamentous Fungi in Microalgae-Based Wastewater Remediation for Biomass Harvesting and Utilization: From Mechanisms to Practical Application. Algal Res. 2022, 62, 102614. [Google Scholar] [CrossRef]
- Úbeda, B.; Gálvez, J.Á.; Michel, M.; Bartual, A. Microalgae Cultivation in Urban Wastewater: Coelastrum Cf. Pseudomicroporum as a Novel Carotenoid Source and a Potential Microalgae Harvesting Tool. Bioresour. Technol. 2017, 228, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Indahsari, H.S.; Tassakka, A.C.M.A.R.; Dewi, E.N.; Yuwono, M.; Suyono, E.A. Effects of Salinity and Bioflocculation during Euglena sp. Harvest on the Production of Lipid, Chlorophyll, and Carotenoid with Skeletonema sp. as a Bioflocculant. J. Pure Appl. Microbiol. 2022, 16, 2901–2911. [Google Scholar] [CrossRef]
- Elshobary, M.E.; Ebaid, R.; Alquraishi, M.; Ende, S.S.W. Synergistic Microalgal Cocultivation: Boosting Flocculation, Biomass Production, and Fatty Acids Profile of Nannochloropsis oculata and Phaeodactylum tricornutum. Biomass Bioenergy 2025, 193, 107595. [Google Scholar] [CrossRef]
- Udaypal, U.; Goswami, R.K.; Verma, P. Strategies for Improvement of Bioactive Compounds Production Using Microalgal Consortia: An Emerging Concept for Current and Future Perspective. Algal Res. 2024, 82, 103664. [Google Scholar] [CrossRef]
- Naseema Rasheed, R.; Pourbakhtiar, A.; Mehdizadeh Allaf, M.; Baharlooeian, M.; Rafiei, N.; Alishah Aratboni, H.; Morones-Ramirez, J.R.; Winck, F.V. Microalgal Co-Cultivation -Recent Methods, Trends in Omic-Studies, Applications, and Future Challenges. Front. Bioeng. Biotechnol. 2023, 11, 1193424. [Google Scholar] [CrossRef]
- Malik, S.; Ashraf, M.U.F.; Shahid, A.; Javed, M.R.; Khan, A.Z.; Usman, M.; Manivannan, A.; Mehmood, M.A.; Ashraf, G.A. Characterization of a Newly Isolated Self-Flocculating Microalga Bracteacoccus pseudominor BERC09 and Its Evaluation as a Candidate for a Multiproduct Algal Biorefinery. Chemosphere 2022, 304, 135346. [Google Scholar] [CrossRef]
- Galán-González, J.; Quintero-Zapata, I.; Elías-Santos, M.; Galán-Wong, L.J.; López-Chuken, U.J.; Guajardo-Barbosa, C.; Beltrán-Rocha, J.C. Harvest by Autoflocculation, Biomass, and Carotenoid Production in Sequential Batch Culture of Haematococcus pluvialis Under High Ionic Strength and Macroelements Content. J. Mar. Sci. Technol. 2024, 32, 390–398. [Google Scholar] [CrossRef]
- García-Encinas, J.P.; Ruiz-Cruz, S.; Juárez, J.; Ornelas-Paz, J.d.J.; Del Toro-Sánchez, C.L.; Márquez-Ríos, E. Proteins from Microalgae: Nutritional, Functional and Bioactive Properties. Foods 2025, 14, 921. [Google Scholar] [CrossRef]
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.T.; Show, P.L. Microalgae: A Potential Alternative to Health Supplementation for Humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- Nunes, E.; Odenthal, K.; Nunes, N.; Fernandes, T.; Fernandes, I.A.; Pinheiro de Carvalho, M.A.A. Protein Extracts from Microalgae and Cyanobacteria Biomass. Techno-Functional Properties and Bioactivity: A Review. Algal Res. 2024, 82, 103638. [Google Scholar] [CrossRef]
- Menon, R.; Raja, T.; Kumar, S.; Gokhale, T. Chapter 6: Biorefinery Approach to Obtain Sustainable Biofuels and High-Value Chemicals from Microalgae. In Woodhead Series in Bioenergy. Microalgal Biofuels; Woodhead Publishing: Cambridge, UK, 2025; pp. 109–137. ISBN 9780443241109. [Google Scholar] [CrossRef]
- Ashraf, A.; Guo, Y.; Yang, T.; Ud Din, A.S.; Ahmad, K.; Li, W.; Hou, H. Microalgae-Derived Peptides: Exploring Bioactivities and Functional Food Innovations. J. Agric. Food Chem. 2025, 73, 1000–1013. [Google Scholar] [CrossRef]
- Song, Y.; Hu, Z.; Liu, S.; Luo, S.; He, R.; Yang, X.; Li, S.; Yang, X.; An, Y.; Lu, Y. Utilization of Microalgae and Duckweed as Sustainable Protein Sources for Food and Feed: Nutritional Potential and Functional Applications. J. Agric. Food Chem. 2025, 73, 4466–4482. [Google Scholar] [CrossRef] [PubMed]
- Elisha, C.; Bhagwat, P.; Pillai, S. Emerging Production Techniques and Potential Health Promoting Properties of Plant and Animal Protein-Derived Bioactive Peptides. Crit. Rev. Food Sci. Nutr. 2024, 29, 1–30. [Google Scholar] [CrossRef]
- Quezada-Rivera, J.J.; Ponce-Alonso, J.; Davalos-Guzman, S.D.; Soria-Guerra, R.E. Chapter 5: Chlamydomonas reinhardtii for the Production of Recombinant Proteins: Current Knowledge and Perspectives. In Fundamentals of Recombinant Protein Production, Purification and Characterization; Academic Press: Cambridge, MA, USA, 2025; pp. 103–142. ISBN 9780323983884. [Google Scholar] [CrossRef]
- Ma, K.; Deng, L.; Wu, H.; Fan, J. Towards Green Biomanufacturing of High-Value Recombinant Proteins Using Promising Cell Factory: Chlamydomonas reinhardtii Chloroplast. Bioresour. Bioprocess. 2022, 9, 83. [Google Scholar] [CrossRef]
- Rivera-Serrano, B.V.; Cabanillas-Salcido, S.L.; Cordero-Rivera, C.D.; Jiménez-Camacho, R.; Norzagaray-Valenzuela, C.D.; Calderón-Zamora, L.; De Jesús-González, L.A.; Reyes-Ruiz, J.M.; Farfan-Morales, C.N.; Romero-Utrilla, A.; et al. Antiviral Effect of Microalgae Phaeodactylum tricornutum Protein Hydrolysates against Dengue Virus Serotype 2. Mar. Drugs 2024, 22, 369. [Google Scholar] [CrossRef]
- Paterson, S.; Alonso-Pintre, L.; Morato-López, E.; González de la Fuente, S.; Gómez-Cortés, P.; Hernández-Ledesma, B. Microalga Nannochloropsis gaditana as a Sustainable Source of Bioactive Peptides: A Proteomic and In Silico Approach. Foods 2025, 14, 252. [Google Scholar] [CrossRef]
- Stirk, W.A.; Ordog, V.; Novák, O.; Rolčík, J.; Strnad, M.; Balint, P.; van Staden, J. Auxin and Cytokinin Relationships in Twenty-Four Microalgae Strains. J. Phycol. 2013, 49, 459–467. [Google Scholar] [CrossRef]
- Stirk, W.A.; Bálint, P.; Tarkowská, D.; Novák, O.; Strnad, M.; Ördög, V.; van Staden, J. Hormone Profiles in Microalgae: Gibberellins and Brassinosteroids. Plant Physiol. Biochem. 2013, 70, 348–353. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, J. Phytohormones in Microalgae: A New Opportunity for Microalgal Biotechnology? Trends Plant Sci. 2015, 20, 273–282. [Google Scholar] [CrossRef]
- Puente-Padilla, B.L.; Romero-Villegas, G.I.; Sánchez-Estrada, A.; Cira-Chávez, L.A.; Estrada-Alvarado, M.I. Effect of Marine Microalgae Biomass (Nannochloropsis gaditana and Thalassiosira sp.) on Germination and Vigor on Bean (Phaseolus vulgaris L.) Seeds “Higuera”. Life 2025, 15, 386. [Google Scholar] [CrossRef] [PubMed]
- Cock, I.E.; Cheesman, M.J. A Review of the Antimicrobial Properties of Cyanobacterial Natural Products. Molecules 2023, 28, 7127. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, R.; Yadav, A.; Sahoo, A.; Kumari, P.; Singh, L.A.; Swapnil, P.; Meena, M.; Kumar, S. Microalgal-Based Sustainable Bio-Fungicides: A Promising Solution to Enhance Crop Yield. Discov. Sustain. 2025, 6, 39. [Google Scholar] [CrossRef]
- Parmar, P.; Kumar, R.; Neha, Y.; Srivatsan, V. Microalgae as next Generation Plant Growth Additives: Functions, Applications, Challenges and Circular Bioeconomy Based Solutions. Front. Plant Sci. 2023, 14, 1073546. [Google Scholar] [CrossRef]
- Nur, M.M.A.; Mahreni; Murni, S.W.; Setyoningrum, T.M.; Hadi, F.; Widayati, T.W.; Jaya, D.; Sulistyawati, R.R.E.; Puspitaningrum, D.A.; Dewi, R.N.; et al. Innovative Strategies for Utilizing Microalgae as Dual-Purpose Biofertilizers and Phycoremediators in Agroecosystems. Biotechnol. Rep. 2025, 45, e00870. [Google Scholar] [CrossRef]
- Guehaz, K.; Boual, Z.; Abdou, I.; Telli, A.; Belkhalfa, H. Microalgae’s Polysaccharides, Are They Potent Antioxidants? Critical Review. Arch. Microbiol. 2024, 206, 14. [Google Scholar] [CrossRef]
- Babich, O.; Ivanova, S.; Michaud, P.; Budenkova, E.; Kashirskikh, E.; Anokhova, V.; Sukhikh, S. Synthesis of Polysaccharides by Microalgae Chlorella sp. Bioresour. Technol. 2024, 406, 131043. [Google Scholar] [CrossRef]
- Pointcheval, M.; Massé, A.; Floc’hlay, D.; Chanonat, F.; Estival, J.; Durand, M.J. Antimicrobial Properties of Selected Microalgae Exopolysaccharide-Enriched Extracts: Influence of Antimicrobial Assays and Targeted Microorganisms. Front. Microbiol. 2025, 16, 1536185. [Google Scholar] [CrossRef]
- Six, A.; Dimitriades-Lemaire, A.; Lancelon-Pin, C.; Putaux, J.L.; Dauvillée, D.; Petroutsos, D.; Alvarez Diaz, P.; Sassi, J.F.; Li-Beisson, Y.; Fleury, G. Red Light Induces Starch Accumulation in Chlorella vulgaris Without Affecting Photosynthesis Efficiency, Unlike Abiotic Stress. Algal Res. 2024, 80, 103515. [Google Scholar] [CrossRef]
- Sarkar, P.; Bandyopadhyay, T.K.; Gopikrishna, K.; Nath Tiwari, O.; Bhunia, B.; Muthuraj, M. Algal Carbohydrates: Sources, Biosynthetic Pathway, Production, and Applications. Bioresour. Technol. 2024, 413, 131489. [Google Scholar] [CrossRef]

| Microalgae | Bio-Flocculant Used | Recovery Efficiency (%) | Reference |
|---|---|---|---|
| Chlorella vulgaris | Paenibacillus sp. AM49 | 90 | [74] |
| Chlorella sp. | Aspergillus terreus | 80–90 | [75] |
| Scenedesmus sp. | Aspergillus sp. | 80–90 | [71] |
| Dunaliella salina | Heterocapsa circularisquama | 80–90 | [76] |
| Nannochloropsis oculata | Tannin-based polymer | 99 | [77] |
| Nannochloropsis sp. | Nano-chitosan | 85 | [78] |
| Botryococcus braunii | Aspergillus sp. | 97 | [79] |
| Biocompounds | Microalgae Species | Applications | Harvesting Method | Reference |
|---|---|---|---|---|
| Phytohormones and Biostimulant molecules | Chlamydomonas reinhardtii, Chlorella vulgaris, Scenedesmus obliquus, Thalassiosira sp., Nannochloropsis oceanica, Haematococcus pluvialis, Arthrospira platensis, Synechococcus sp., Anabaena sp. | Production of auxins, gibberellins, and cytokinins, plant growth stimulants, root development, and stress resistance. Applications in agricultural biostimulants. | Bio-flocculation Aqueous extraction | [173,174,175,176]. |
| Biocidal molecules | Chlorella vulgaris, Nannochloropsis oculata, Oscillatoria agardhii, Nostoc (genus) | Antibacterial, antifungal, and insecticidal activity. Ecological alternatives to synthetic agrochemicals in agriculture. | Bio-flocculation | [177,178] |
| Enriched biomass and Biofertilization | Scenedesmus obliquus, Chlorella vulgaris, Anabaena cylindrica | Improves soil fertility, plant nutrition, soil structure, and beneficial microbial activity. Used as a biofertilizer. | Bio-flocculation | [179,180] |
| Polysaccharides | Chlorella vulgaris, Porphyridium cruentum, Arthrospira platensis | Production of polysaccharides with immunostimulant, antioxidant, antiviral, and anti-inflammatory activities. Applications in food and pharmaceuticals. | Bio-flocculation Chemical extraction | [181,182,183] |
| Starch and other carbohydrates | Chlamydomonas reinhardtii, Nannochloropsis gaditana, Chlorella vulgaris, Botryococcus braunii | Accumulation of starch and carbohydrates for the production of biofuels and bioplastics. Sustainable alternatives to conventional materials. | Bio-flocculation Chemical extraction Enzymatic hydrolysis Acid hydrolysis | [184,185] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heredia-Martínez, L.G.; Gutiérrez-Diánez, A.M.; Díaz-Santos, E. Bio-Flocculation: A Green Tool in Biorefineries for Recovering High Added-Value Compounds from Microalgae. Phycology 2025, 5, 19. https://doi.org/10.3390/phycology5020019
Heredia-Martínez LG, Gutiérrez-Diánez AM, Díaz-Santos E. Bio-Flocculation: A Green Tool in Biorefineries for Recovering High Added-Value Compounds from Microalgae. Phycology. 2025; 5(2):19. https://doi.org/10.3390/phycology5020019
Chicago/Turabian StyleHeredia-Martínez, Luis G., Alba María Gutiérrez-Diánez, and Encarnación Díaz-Santos. 2025. "Bio-Flocculation: A Green Tool in Biorefineries for Recovering High Added-Value Compounds from Microalgae" Phycology 5, no. 2: 19. https://doi.org/10.3390/phycology5020019
APA StyleHeredia-Martínez, L. G., Gutiérrez-Diánez, A. M., & Díaz-Santos, E. (2025). Bio-Flocculation: A Green Tool in Biorefineries for Recovering High Added-Value Compounds from Microalgae. Phycology, 5(2), 19. https://doi.org/10.3390/phycology5020019







