Bioactivity Screening of Extracts from Icelandic Seaweeds for Potential Application in Cosmeceuticals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seaweed Collection and Pre-Processing
2.2. Chemicals
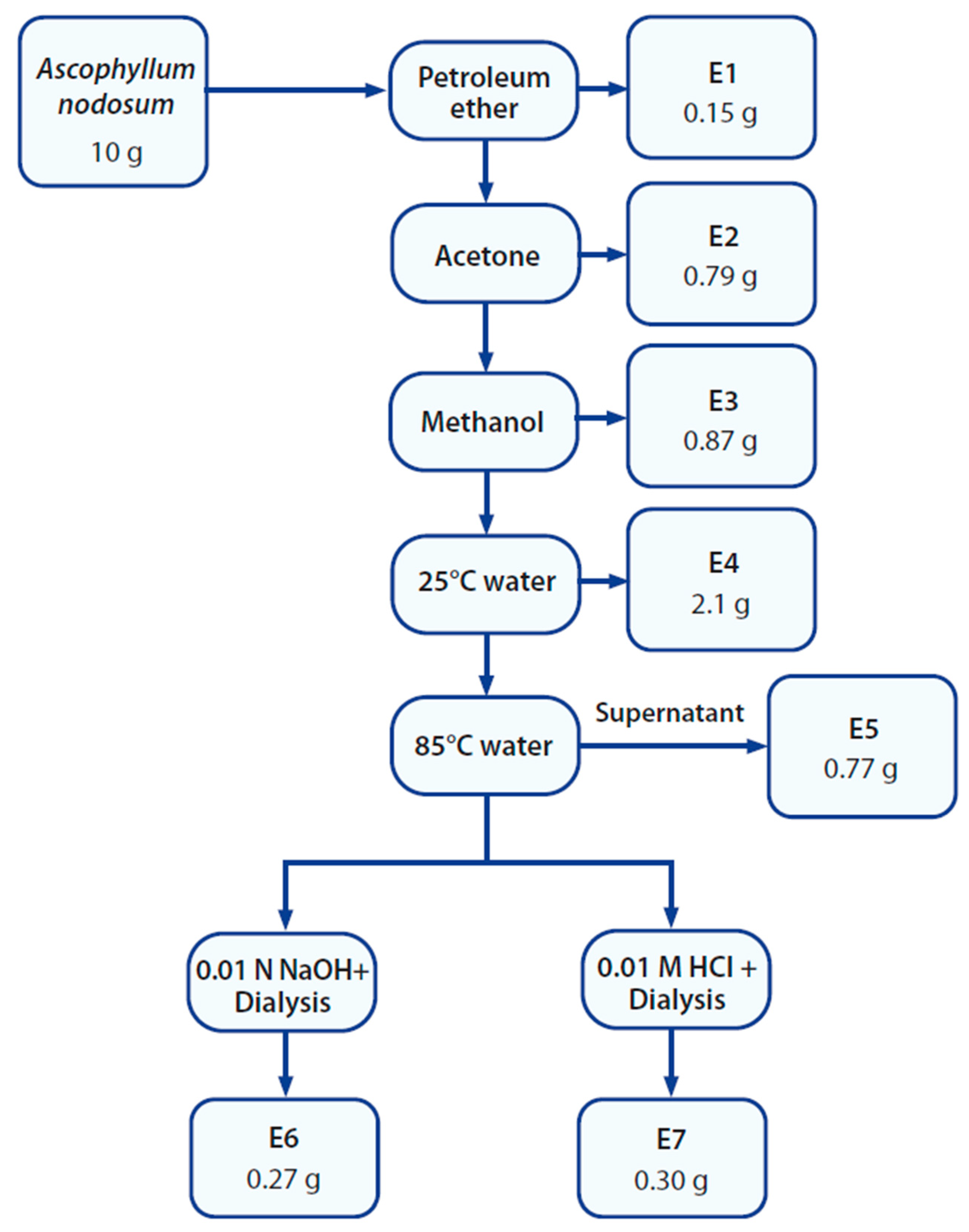
2.3. Preparation of Extracts
2.4. Bioassays
2.4.1. Total Polyphenol Content (TPC) Assay
2.4.2. DPPH Assay
2.4.3. ORAC Assay
2.4.4. Reducing Power Assay
2.4.5. Collagenase Inhibition Assay
2.4.6. Elastase Inhibition Assay
3. Results and Discussion
3.1. Extraction Yield
3.2. Evaluation of Skin-Protecting Properties of Icelandic Seaweed Species
3.2.1. Total Polyphenol Content (TPC)
3.2.2. Antioxidant Activity
3.2.3. Inhibition of Skin-Degrading Enzymes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Couteau, C.; Coiffard, L. Phycocosmetics and other marine cosmetics, specific cosmetics formulated using marine resources. Mar. Drugs 2020, 18, 322. [Google Scholar] [CrossRef] [PubMed]
- Tobin, D.J. Introduction to skin aging. J. Tissue Viability 2017, 26, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Russell-Goldman, E.; Murphy, G.F. The pathobiology of skin aging: New insights into an old dilemma. Am. J. Pathol. 2020, 190, 1356–1369. [Google Scholar] [CrossRef]
- Wong, Q.Y.A.; Chew, F.T. Defining skin aging and its risk factors: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 22075. [Google Scholar] [CrossRef]
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin anti-aging strategies. Derm.-Endocrinol. 2012, 4, 308–319. [Google Scholar] [CrossRef]
- Farage, M.A.; Miller, K.W.; Elsner, P.; Maibach, H.I. Characteristics of the aging skin. Adv. Wound Care 2013, 2, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Kilgman, A. Cosmeceuticals: A broad-spectrum category between cosmetics and drugs. In Cosmeceuticals and Active Cosmetics, 2nd ed.; Elsner, P., Maibach, H.I., Eds.; CRC Press: Boca Raton, FL, USA, 2005; pp. 1–9. [Google Scholar]
- Ferreira, M.S.; Resende, D.; Lobo, J.M.S.; Sousa, E.; Almeida, I.F. Marine ingredients for sensitive skin: Market overview. Mar. Drugs 2021, 19, 464. [Google Scholar] [CrossRef] [PubMed]
- Kalasariya, H.S.; Patel, N.B.; Yadav, A.; Perveen, K.; Yadav, V.K.; Munshi, F.M.; Yadav, K.K.; Alam, S.; Jung, Y.K.; Jeon, B.H. Characterization of fatty acids, polysaccharides, amino acids, and minerals in marine macroalga Chaetomorpha crassa and evaluation of their potentials in skin cosmetics. Molecules 2021, 26, 7515. [Google Scholar] [CrossRef]
- Kalasariya, H.S.; Yadav, V.K.; Yadav, K.K.; Tirth, V.; Algahtani, A.; Islam, S.; Gupta, N.; Jeon, B.H. Seaweed-based molecules and their potential biological activities: An eco-sustainable cosmetics. Molecules 2021, 26, 5313. [Google Scholar] [CrossRef]
- Jesumani, V.; Du, H.; Aslam, M.; Pei, P.; Huang, N. Potential use of seaweed bioactive compounds in skincare—A review. Mar. Drugs 2019, 17, 688. [Google Scholar] [CrossRef]
- Kim, S.K. Marine cosmeceuticals. J. Cosmet. Dermatol. 2014, 13, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.K.A.; Kim, E.-A.; Son, K.-T.; Jeon, Y.-J. Bioactive properties and potentials cosmeceutical applications of phlorotannins isolated from brown seaweeds: A review. J. Photochem. Photobiol. B Biol. 2016, 162, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Ravichandran, Y.D.; Khan, S.B.; Kim, Y.T. Prospective of the cosmeceuticals derived from marine organisms. Biotechnol. Bioprocess Eng. 2008, 13, 511–523. [Google Scholar] [CrossRef]
- Alves, A.; Sousa, E.; Kijjoa, A.; Pinto, M. Marine-derived compounds with potential use as cosmeceuticals and nutricosmetics. Molecules 2020, 25, 2536. [Google Scholar] [CrossRef] [PubMed]
- Siavash, H.C.; Fadzilah, A.A.M.; Mohamad, R.S. Cosmeceutical value of herbal extracts as natural ingredients and novel technologies in anti-aging. J. Med. Plants Res. 2011, 5, 3074–3077. [Google Scholar]
- El-Beltagi, H.S.; Mohamed, A.A.; Mohamed, H.I.; Ramadan, K.M.A.; Barqawi, A.A.; Mansour, A.T. Phytochemical and potential properties of seaweeds and their recent applications: A review. Mar. Drugs 2022, 20, 342. [Google Scholar] [CrossRef]
- Agrawal, S.; Adholeya, A.; Barrow, C.J.; Deshmukh, S.K. Marine fungi: An untapped bioresource for future cosmeceuticals. Phytochem. Lett. 2018, 23, 15–20. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, J.; Seca, A.M.L.; Pinto, D.; Michalak, I.; Trincone, A.; Mishra, A.P.; Nigam, M.; Zam, W.; Martins, N. Current trends on seaweeds: Looking at chemical composition, phytopharmacology, and cosmetic applications. Molecules 2019, 24, 4182. [Google Scholar] [CrossRef]
- Jayawardhana, H.H.A.C.K.; Jayawardena, T.U.; Sanjeewa, K.K.A.; Liyanage, N.M.; Nagahawatta, D.P.; Lee, H.-G.; Kim, J.-I.; Jeon, Y.-J. Marine Algal Polyphenols as Skin Protective Agents: Current Status and Future Prospectives. Mar. Drugs 2023, 21, 285. [Google Scholar] [CrossRef]
- López-Hortas, L.; Flórez-Fernández, N.; Torres, M.D.; Ferreira-Anta, T.; Casas, M.P.; Balboa, E.M.; Falqué, E.; Domínguez, H. Applying seaweed compounds in cosmetics, cosmeceuticals and nutricosmetics. Mar. Drugs 2021, 19, 552. [Google Scholar] [CrossRef]
- Grozdanić, N.; Zdunić, G.; Šavikin, K.; Đuričić, I.; Kosanić, M.; Macic, V.; Matić, I.; Stanojkovic, T. Seasonal variation in biopharmaceutical activity and fatty acid content of endemic Fucus virsoides algae from Adriatic Sea. Acta Pol. Pharm.-Drug Res. 2019, 76, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.; Fernandes, F.; Pereira, D.M.; Azevedo, I.C.; Sousa-Pinto, I.; Andrade, P.B.; Valentão, P. Fatty acid patterns of the kelps Saccharina latissima, Saccorhiza polyschides and Laminaria ochroleuca: Influence of changing environmental conditions. Arab. J. Chem. 2020, 13, 45–58. [Google Scholar] [CrossRef]
- Sappati, P.K.; Nayak, B.; VanWalsum, G.P.; Mulrey, O.T. Combined effects of seasonal variation and drying methods on the physicochemical properties and antioxidant activity of sugar kelp (Saccharina latissima). J. Appl. Phycol. 2019, 31, 1311–1332. [Google Scholar] [CrossRef]
- Manns, D.; Nielsen, M.M.; Bruhn, A.; Saake, B.; Meyer, A.S. Compositional variations of brown seaweeds Laminaria digitata and Saccharina latissima in Danish waters. J. Appl. Phycol. 2017, 29, 1493–1506. [Google Scholar] [CrossRef]
- Konstantin, B.; Anastasia, P.; Nikolay, I.; Daria, P. Seasonal variations in the chemical composition of Arctic brown macroalgae. Algal Res. 2023, 72, 103112. [Google Scholar] [CrossRef]
- Jang, H.; Lee, J.; Park, Y.-K.; Lee, J.-Y. Exploring the health benefits and concerns of brown seaweed consumption: A comprehensive review of bioactive compounds in brown seaweed and its potential therapeutic effects. J. Agric. Food Res. 2024, 17, 101215. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef]
- Ganske, F.; Dell, E. ORAC Assay on the FLUOstar OPTIMA to Determine Antioxidant Capacity. BMG LABTECH Application Note 148. Rev. 12/2006. Available online: https://www.pharmaceutical-technology.com/wp-content/uploads/sites/24/2017/09/196.pdf?utm_campaign=Verdict%20Whitepaper%20downloads&utm_medium=email&_hsenc=p2ANqtz--_qMD3rndRfHPZPZhZGZn0XEu7TPzioOoC3hswqewfmfzBvkkUpX20GN_3njY2Murs1l5psfOP8P8nGLXFmzX7ktsxGQ&_hsmi=75927857&utm_content=75927857&utm_source=hs_automation (accessed on 1 August 2024).
- Yano, S.; Mori, M.; Teramoto, N.; Iisaka, M.; Suzuki, N.; Noto, M.; Kaimoto, Y.; Kakimoto, M.; Yamada, M.; Shiratsuchi, E.; et al. Preparation of photocrosslinked fish elastin polypeptide/microfibrillated cellulose composite gels with elastic properties for biomaterial applications. Mar. Drugs 2015, 13, 338–353. [Google Scholar] [CrossRef]
- El Maghraby, D.M.; Fakhry, E.M. Lipid content and fatty acid composition of Mediterranean macro-algae as dynamic factors for biodiesel production. Oceanologia 2015, 57, 86–92. [Google Scholar] [CrossRef]
- Rodrigues, D.; Freitas, A.C.; Pereira, L.; Rocha-Santos, T.A.; Vasconcelos, M.W.; Roriz, M.; Rodríguez-Alcalá, L.M.; Gomes, A.M.; Duarte, A.C. Chemical composition of red, brown and green macroalgae from Buarcos bay in Central West Coast of Portugal. Food Chem. 2015, 183, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Application of novel extraction technologies for bioactives from marine algae. J. Agric. Food Chem. 2013, 61, 4667–4675. [Google Scholar] [CrossRef]
- Tiwari, B.K. Ultrasound: A clean, green extraction technology. TrAC Trends Anal. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Cikoš, A.-M.; Jokić, S.; Šubarić, D.; Jerković, I. Overview on the application of modern methods for the extraction of bioactive compounds from marine macroalgae. Mar. Drugs 2018, 16, 348. [Google Scholar] [CrossRef]
- Bordoloi, A.; Goosen, N. Green and integrated processing approaches for the recovery of high-value compounds from brown seaweeds. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2020; Volume 95, pp. 369–413. [Google Scholar]
- Gomez, L.; Alvarez, C.; Zhao, M.; Tiwari, U.; Curtin, J.; Garcia-Vaquero, M.; Tiwari, B. Innovative processing strategies and technologies to obtain hydrocolloids from macroalgae for food applications. Carbohydr. Polym. 2020, 248, 116784. [Google Scholar] [CrossRef] [PubMed]
- van Sittert, D.; Lufu, R.; Mapholi, Z.; Goosen, N.J. Ultrasound-assisted extraction of alginate from Ecklonia maxima with and without the addition of alkaline cellulase–factorial and kinetic analysis. J. Appl. Phycol. 2024, 36, 2781–2793. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; Smyth, T.J.; O’Donnell, C.P. Optimization of ultrasound assisted extraction of bioactive components from brown seaweed Ascophyllum nodosum using response surface methodology. Ultrason. Sonochemistry 2015, 23, 308–316. [Google Scholar] [CrossRef]
- Chemat, F.; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochemistry 2011, 18, 813–835. [Google Scholar] [CrossRef]
- Wang, T.; Jónsdóttir, R.; Ólafsdóttir, G. Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chem. 2009, 116, 240–248. [Google Scholar] [CrossRef]
- Castejón, N.; Thorarinsdottir, K.A.; Einarsdóttir, R.; Kristbergsson, K.; Marteinsdóttir, G. Exploring the potential of Icelandic seaweeds extracts produced by aqueous pulsed electric fields-assisted extraction for cosmetic applications. Mar. Drugs 2021, 19, 662. [Google Scholar] [CrossRef] [PubMed]
- Koivikko, R.; Loponen, J.; Honkanen, T.; Jormalainen, V. Contents of soluble, cell-wall-bound and exuded phlorotannins in the brown alga Fucus vesiculosus, with implications on their ecological functions. J. Chem. Ecol. 2005, 31, 195–212. [Google Scholar] [CrossRef] [PubMed]
- Waterman, P.G.; Mole, S. Analysis of Phenolic Plant Metabolites; Blackwell Scientific Publications: Oxford, UK, 1994. [Google Scholar]
- Susano, P.; Silva, J.; Alves, C.; Martins, A.; Gaspar, H.; Pinteus, S.; Mouga, T.; Goettert, M.I.; Petrovski, Ž.; Branco, L.B.; et al. Unravelling the dermatological potential of the brown seaweed Carpomitra costata. Mar. Drugs 2021, 19, 135. [Google Scholar] [CrossRef] [PubMed]
- Connan, S.; Goulard, F.; Stiger, V.; Deslandes, E.; Gall, E.A. Interspecific and temporal variation in phlorotannin levels in an assemblage of brown algae. Bot. Mar. 2004, 47, 410–416. [Google Scholar] [CrossRef]
- Schiener, P.; Black, K.D.; Stanley, M.S.; Green, D.H. The seasonal variation in the chemical composition of the kelp species Laminaria digitata, Laminaria hyperborea, Saccharina latissima and Alaria esculenta. J. Appl. Phycol. 2015, 27, 363–373. [Google Scholar] [CrossRef]
- Gager, L.; Connan, S.; Molla, M.; Couteau, C.; Arbona, J.-F.; Coiffard, L.; Cérantola, S.; Stiger-Pouvreau, V. Active phlorotannins from seven brown seaweeds commercially harvested in Brittany (France) detected by 1H NMR and in vitro assays: Temporal variation and potential valorization in cosmetic applications. J. Appl. Phycol. 2020, 32, 2375–2386. [Google Scholar] [CrossRef]
- Connan, S.; Deslandes, E.; Gall, E.A. Influence of day–night and tidal cycles on phenol content and antioxidant capacity in three temperate intertidal brown seaweeds. J. Exp. Mar. Biol. Ecol. 2007, 349, 359–369. [Google Scholar] [CrossRef]
- Heffernan, N.; Brunton, N.P.; FitzGerald, R.J.; Smyth, T.J. Profiling of the Molecular Weight and Structural Isomer Abundance of Macroalgae-Derived Phlorotannins. Mar. Drugs 2015, 13, 509–528. [Google Scholar] [CrossRef]
- Roleda, M.Y.; Marfaing, H.; Desnica, N.; Jónsdóttir, R.; Skjermo, J.; Rebours, C.; Nitschke, U. Variations in polyphenol and heavy metal contents of wild-harvested and cultivated seaweed bulk biomass: Health risk assessment and implication for food applications. Food Control 2019, 95, 121–134. [Google Scholar] [CrossRef]
- Meng, W.; Sun, H.; Mu, T.; Garcia-Vaquero, M. Extraction, purification, chemical characterization and antioxidant properties in vitro of polyphenols from the brown macroalga Ascophyllum nodosum. Algal Res. 2023, 70, 102989. [Google Scholar] [CrossRef]
- Blanc, N.; Hauchard, D.; Audibert, L.; Gall, E.A. Radical-scavenging capacity of phenol fractions in the brown seaweed Ascophyllum nodosum: An electrochemical approach. Talanta 2011, 84, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Gu, L. Phlorotannins from brown algae (Fucus vesiculosus) inhibited the formation of advanced glycation endproducts by scavenging reactive carbonyls. J. Agric. Food Chem. 2012, 60, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Farasat, M.; Khavari-Nejad, R.A.; Nabavi, S.M.; Namjooyan, F. Antioxidant properties of two edible green seaweeds from Northern Coasts of the Persian Gulf. J. Nat. Pharm. Prod. 2013, 8, 47–52. [Google Scholar]
- Makori, S.I.; Mu, T.-H.; Sun, H.-N. Profiling of polyphenols, flavonoids and anthocyanins in potato peel and flesh from four potato varieties. Potato Res. 2022, 65, 193–208. [Google Scholar] [CrossRef]
- Afrin, F.; Ahsan, T.; Mondal, M.N.; Rasul, M.G.; Afrin, M.; Silva, A.A.; Yuan, C.; Shah, A.K.M.A. Evaluation of antioxidant and antibacterial activities of some selected seaweeds from Saint Martin’s Island of Bangladesh. Food Chem. Adv. 2023, 3, 100393. [Google Scholar] [CrossRef]
- O’Sullivan, A.M.; O’Callaghan, Y.C.; O’Grady, M.N.; Queguineur, B.; Hanniffy, D.; Troy, D.J.; Kerry, J.P.; O’Brien, N.M. In vitro and cellular antioxidant activities of seaweed extracts prepared from five brown seaweeds harvested in spring from the West Coast of Ireland. Food Chem. 2011, 126, 1064–1070. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Zakharov, D.V.; Flisyuk, E.V.; Terninko, I.I.; Generalova, Y.E.; Smekhova, I.E.; Shikov, A.N. The biochemical composition and antioxidant properties of Fucus vesiculosus from the Arctic region. Mar. Drugs 2022, 20, 193. [Google Scholar] [CrossRef]
- Sanger, G.; Wonggo, D.; Lita, Y.; Dotulong, V. Pigments constituents, phenolic content and antioxidant activity of brown seaweed Sargassum sp. IOP Conf. Ser. Earth Environ. Sci. 2022, 1033, 012057. [Google Scholar] [CrossRef]
- Garcia-Perez, P.; Lourenço-Lopes, C.; Silva, A.; Pereira, A.G.; Fraga-Corral, M.; Zhao, C.; Xiao, J.; Simal-Gandara, J.; Prieto, M.A. Pigment composition of nine brown algae from the Iberian Northwestern Coastline: Influence of the extraction solvent. Mar. Drugs 2022, 20, 113. [Google Scholar] [CrossRef]
- Ajisaka, K.; Yokoyama, T.; Matsuo, K. Structural characteristics and antioxidant activities of fucoidans from five brown seaweeds. J. Appl. Glycosci. 2016, 63, 31–37. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Gorshenina, E.V.; Daurtseva, A.V.; Flisyuk, E.V.; Generalova, Y.E.; Terninko, I.I.; Shikov, A.N. Ascophyllum nodosum (Linnaeus) Le Jolis from Arctic: Its biochemical composition, antiradical potential, and human health risk. Mar. Drugs 2024, 22, 48. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Shikov, A.N. In vitro anti-inflammatory activities of fucoidans from five species of brown seaweeds. Mar. Drugs 2022, 20, 606. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S. Extraction and Characterization of Bioactive Natural Products from Brown Seaweed Alaria esculenta. Master’s Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2022. [Google Scholar]
- Yan, X.; Nagata, T.; Fan, X. Antioxidative activities in some common seaweeds. Plant Foods Hum. Nutr. 1998, 52, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Amaro, H.M.; Pagels, F.; Tavares, T.G.; Costa, I.; Sousa-Pinto, I.; Guedes, A.C. Antioxidant and anti-inflammatory potential of seaweed extracts as functional ingredients. Hydrobiology 2022, 1, 469–482. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N. The efficacy of two methods for extracting fucoidan from frozen Arctic algae thalli: Chemical composition, kinetic study and process optimization. J. Appl. Phycol. 2024, 36, 1413–1432. [Google Scholar] [CrossRef]
- Abu, R.; Jiang, Z.; Ueno, M.; Isaka, S.; Nakazono, S.; Okimura, T.; Cho, K.; Yamaguchi, K.; Kim, D.; Oda, T. Anti-metastatic effects of the sulfated polysaccharide ascophyllan isolated from Ascophyllum nodosum on B16 melanoma. Biochem. Biophys. Res. Commun. 2015, 458, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Abu, R.; Jiang, Z.; Ueno, M.; Okimura, T.; Yamaguchi, K.; Oda, T. In vitro antioxidant activities of sulfated polysaccharide ascophyllan isolated from Ascophyllum nodosum. Int. J. Biol. Macromol. 2013, 59, 305–312. [Google Scholar] [CrossRef]
- Jiang, Z.; Hama, Y.; Yamaguchi, K.; Oda, T. Inhibitory effect of sulphated polysaccharide porphyran on nitric oxide production in lipopolysaccharide-stimulated RAW264. 7 macrophages. J. Biochem. 2012, 151, 65–74. [Google Scholar] [CrossRef]
- Jiang, Z.; Okimura, T.; Yamaguchi, K.; Oda, T. The potent activity of sulfated polysaccharide, ascophyllan, isolated from Ascophyllum nodosum to induce nitric oxide and cytokine production from mouse macrophage RAW264. 7 cells: Comparison between ascophyllan and fucoidan. Nitric Oxide 2011, 25, 407–415. [Google Scholar] [CrossRef]
- Jiang, Z.; Okimura, T.; Yokose, T.; Yamasaki, Y.; Yamaguchi, K.; Oda, T. Effects of sulfated fucan, ascophyllan, from the brown alga Ascophyllum nodosum on various cell lines: A comparative study on ascophyllan and fucoidan. J. Biosci. Bioeng. 2010, 110, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Nakayasu, S.; Soegima, R.; Yamaguchi, K.; Oda, T. Biological activities of fucose-containing polysaccharide ascophyllan isolated from the brown alga Ascophyllum nodosum. Biosci. Biotechnol. Biochem. 2009, 73, 961–964. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Du, J.-Y.; Jiang, Z.; Okimura, T.; Oda, T.; Yu, Q.; Jin, J.-O. Ascophyllan purified from Ascophyllum nodosum induces Th1 and Tc1 immune responses by promoting dendritic cell maturation. Mar. Drugs 2014, 12, 4148–4164. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K.; Kim, D.; Jiang, Z.; Ueno, M.; Okimura, T.; Yamaguchi, K.; Oda, T. Immunostimulatory activities of the sulfated polysaccharide ascophyllan from Ascophyllum nodosum in in vivo and in vitro systems. Biosci. Biotechnol. Biochem. 2012, 76, 1573–1576. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lee, W.; Oh, J.Y.; Cui, Y.R.; Ryu, B.; Jeon, Y.-J. Protective effect of sulfated polysaccharides from celluclast-assisted extract of Hizikia fusiforme against ultraviolet B-Induced skin damage by regulating NF-κB, AP-1, and MAPKs signaling pathways in vitro in human dermal fibroblasts. Mar. Drugs 2018, 16, 239. [Google Scholar] [CrossRef]
- Moon, H.J.; Lee, S.R.; Shim, S.N.; Jeong, S.H.; Stonik, V.A.; Rasskazov, V.A.; Zvyagintseva, T.; Lee, Y.H. Fucoidan inhibits UVB-induced MMP-1 expression in human skin fibroblasts. Biol. Pharm. Bull. 2008, 31, 284–289. [Google Scholar] [CrossRef]
- Moon, H.J.; Lee, S.H.; Ku, M.J.; Yu, B.C.; Jeon, M.J.; Jeong, S.H.; Stonik, V.A.; Zvyagintseva, T.N.; Ermakova, S.P.; Lee, Y.H. Fucoidan inhibits UVB-induced MMP-1 promoter expression and down regulation of type I procollagen synthesis in human skin fibroblasts. Eur. J. Dermatol. 2009, 19, 129–134. [Google Scholar] [CrossRef]
- Kim, M.-M.; Van Ta, Q.; Mendis, E.; Rajapakse, N.; Jung, W.-K.; Byun, H.-G.; Jeon, Y.-J.; Kim, S.-K. Phlorotannins in Ecklonia cava extract inhibit matrix metalloproteinase activity. Life Sci. 2006, 79, 1436–1443. [Google Scholar] [CrossRef]
| Species | Collection Date | Collection Site | Harvesting |
|---|---|---|---|
| Alaria esculenta | 25 April 2021 | Breiðafjörður 1 | Wild |
| Ascophyllum nodosum | 25 April 2021 | Breiðafjörður | Wild |
| Laminara digitata | 21 August 2021 | Breiðafjörður | Wild |
| Laminara hyperborea | 21 August 2021 | Breiðafjörður | Wild |
| Palmaria palmata | 28 May 2021 | Grindavík 2 | Wild |
| Schizymenia jonssonii | 22 August 2021 | Reykjanes 3 | Cultivated |
| Saccharina latissima | 25 April 2021 | Breiðafjörður | Wild |
| Assays | ||||||
|---|---|---|---|---|---|---|
| Seaweed Species | Yield (%) Dry Weight | TPC (g GAE/100 g ±SD) (n = 8) | TPC (g PGE/100 g ±SD) (n = 8) | ORAC (µmol TEq/g ±SD) (n = 3) | DPPH IC50 Value (mg/mL ±SEM) (n = 3) | RP (mg AAEq/g ±SD) (n = 8) |
| A. esculenta | 36 | 1.0 c ± 0.05 | 0.70 bc ± 0.04 | 107 b ± 7 | 3.0 ± 0.14 | 5.2 cd ± 1.0 |
| A. nodosum | 29 | 10 a ± 0.8 | 11 a ± 0.9 | 869 a ± 60 | 0.18 b ± 0.01 | 130 a ± 8.1 |
| L. digitata | 50 | 0.30 d ± 0.03 | 0.30 c ± 0.06 | N/A | N/A | 1.7 d ± 0.07 |
| L. hyperborea | 57 | 1.6 b ± 0.4 | 1.1 b ± 0.3 | 59 b ± 2 | 1.4 b ± 0.06 | 18 b ± 2.8 |
| P. palmata | 32 | 0.70 cd ± 0.06 | 0.50 c ± 0.04 | 39 b ± 4 | N/A | 2.4 d ± 0.05 |
| S. jonssonii | 32 | 0.90 c ± 0.2 | 0.60 bc ± 0.1 | 49 b ± 8 | 4.9 a ± 1.23 | 3.7 d ± 0.5 |
| S. latissima | 41 | 0.60 cd ± 0.06 | 0.40 c ± 0.05 | 45 b ± 3 | 2.8 ± 0.46 | 8.5 c ± 0.4 |
| Assays | ||||||
|---|---|---|---|---|---|---|
| A. nodosum Extract | Yield (%) Dry Weight | TPC (g GAE/100 g ±SD) (n = 8) | TPC (g PGE/100 g ±SD) (n = 8) | ORAC (µmol TEq/g ±SD) (n = 3) | DPPH IC50 Value (mg/mL ±SEM) (n = 3) | RP (mg AAEq/g ±SD) (n = 16) |
| E1 (Petroleum ether extract) | 1 | 2.2 c ± 0.1 | 1.6 cd ± 0.0 | 150 f ± 4.6 | 0.20 c ± 0.0 | 34 c ± 3.0 |
| E2 (Acetone extract) | 5 | 110 a ± 3.2 | 83 a ± 2.4 | 2640 a ± 38 | 0.050 c ± 0.0 | 410 a ± 43 |
| E3 (Methanol extract) | 6 | 77 b ± 7.5 | 58 b ± 5.7 | 2120 b ± 255 | 0.80 bc ± 0.1 | 300 b ± 26 |
| E4 (Water extract) | 14 | 4.5 c ± 0.2 | 4.8 c ± 0.3 | 1580 c ± 57 | 1.5 ± 0.3 | 47 c ± 7.2 |
| E5 (85 °C water extract) | 5 | 3.3 c ± 0.4 | 3.6 cd ± 0.5 | 1070 d ± 43 | 0.080 bc ± 0.04 | 33 c ± 13 |
| E6 (0.01 M NaOH extract) | 2 | 3.0 c ± 0.3 | 2.8 cd ± 0.3 | 547 e ± 17 | 6.1 ab ± 0.3 | 28 c ± 6.3 |
| E7 (0.01 M HCl extract) | 2 | 1.3 c ± 0.1 | 1.7 d ± 0.1 | 359 ef ± 59 | 4.1 a ± 0.4 | 14 c ± 2.6 |
| Inhibition of Collagenase | Inhibition of Elastase | |
|---|---|---|
| A. nodosum Extract | IC50 Value (mg/mL ± SEM) | IC50 Value (mg/mL ± SEM) |
| E1 (Petroleum ether extract) | N/A | 0.38 ab ± 0.02 |
| E2 (Acetone extract) | 0.02 b ± 0.00 | 0.004 d ± 0.00 |
| E3 (Methanol extract) | 0.03 b ± 0.00 | <0.01 ± NA |
| E4 (Water extract) | 0.14 b ± 0.02 | 0.17 c ± 0.01 |
| E5 (85 °C water extract) | 0.57 a ± 0.23 | 0.24 b ± 0.02 |
| E6 (0.01 M NaOH extract) | N/A | 0.33 a ± 0.03 |
| E7 (0.01 M HCl extract) | N/A | 0.32 a ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jensen, S.; Helgadóttir, J.K.; Jónsdóttir, R. Bioactivity Screening of Extracts from Icelandic Seaweeds for Potential Application in Cosmeceuticals. Phycology 2024, 4, 576-588. https://doi.org/10.3390/phycology4040031
Jensen S, Helgadóttir JK, Jónsdóttir R. Bioactivity Screening of Extracts from Icelandic Seaweeds for Potential Application in Cosmeceuticals. Phycology. 2024; 4(4):576-588. https://doi.org/10.3390/phycology4040031
Chicago/Turabian StyleJensen, Sophie, Júlía Karítas Helgadóttir, and Rósa Jónsdóttir. 2024. "Bioactivity Screening of Extracts from Icelandic Seaweeds for Potential Application in Cosmeceuticals" Phycology 4, no. 4: 576-588. https://doi.org/10.3390/phycology4040031
APA StyleJensen, S., Helgadóttir, J. K., & Jónsdóttir, R. (2024). Bioactivity Screening of Extracts from Icelandic Seaweeds for Potential Application in Cosmeceuticals. Phycology, 4(4), 576-588. https://doi.org/10.3390/phycology4040031






