Harnessing Symbiotic Mixotrophic Microalgal–Bacterial Biofilms for N and P Elimination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
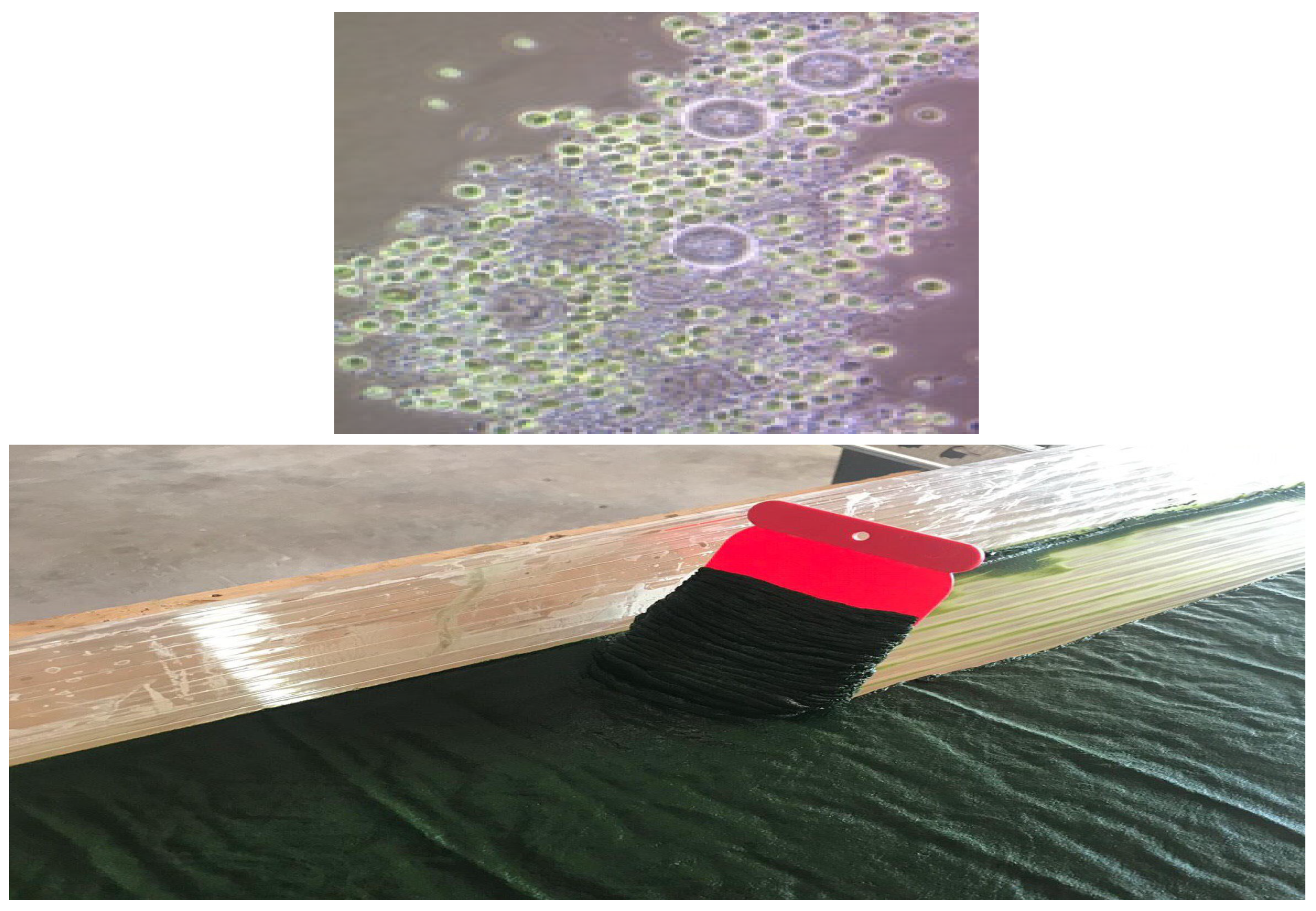
2.2. Microalgal Biofilm Cultivation
Cultivation of Chlorella vulgaris BEA 0753B (C. vulgaris)
2.3. Analytical Procedures
2.4. Photosynthesis Inhibition and ROS Test
2.5. Biomass Separation and Characterization
3. Results
3.1. Biofilm Growth
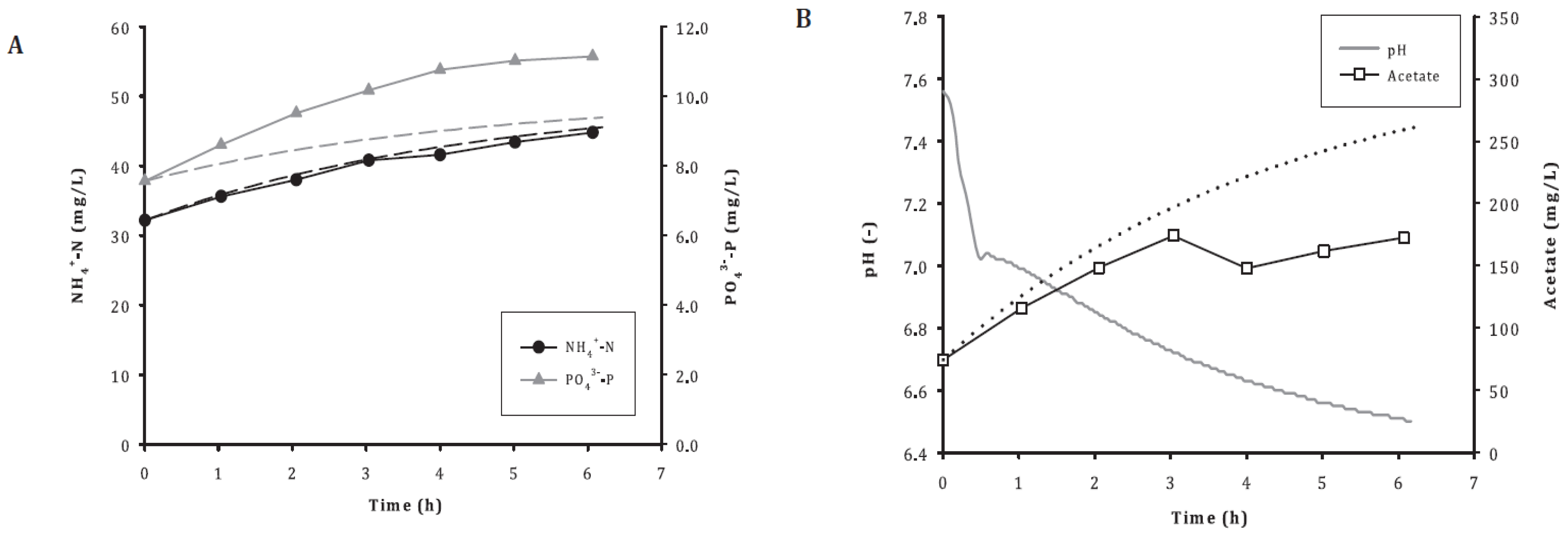
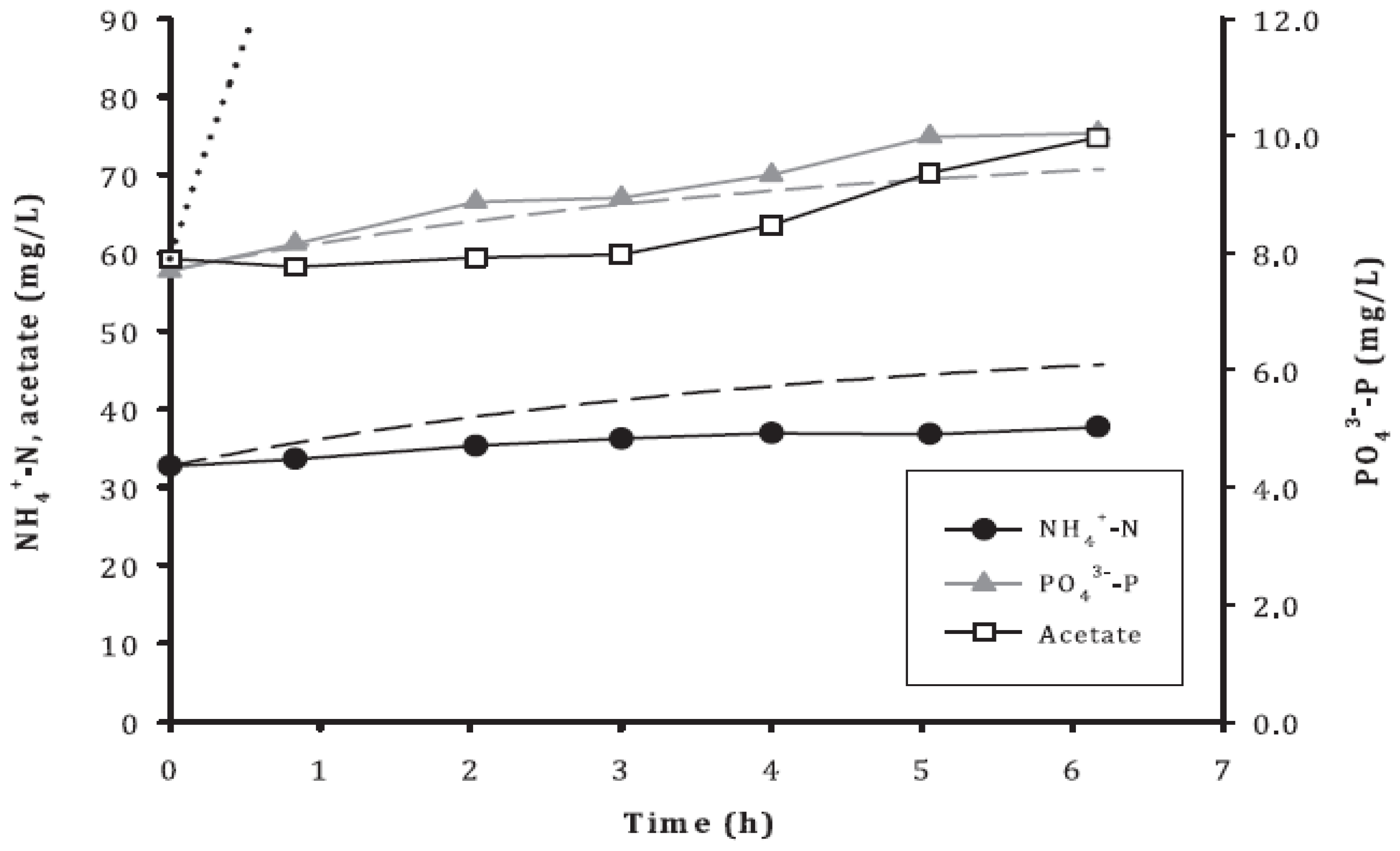
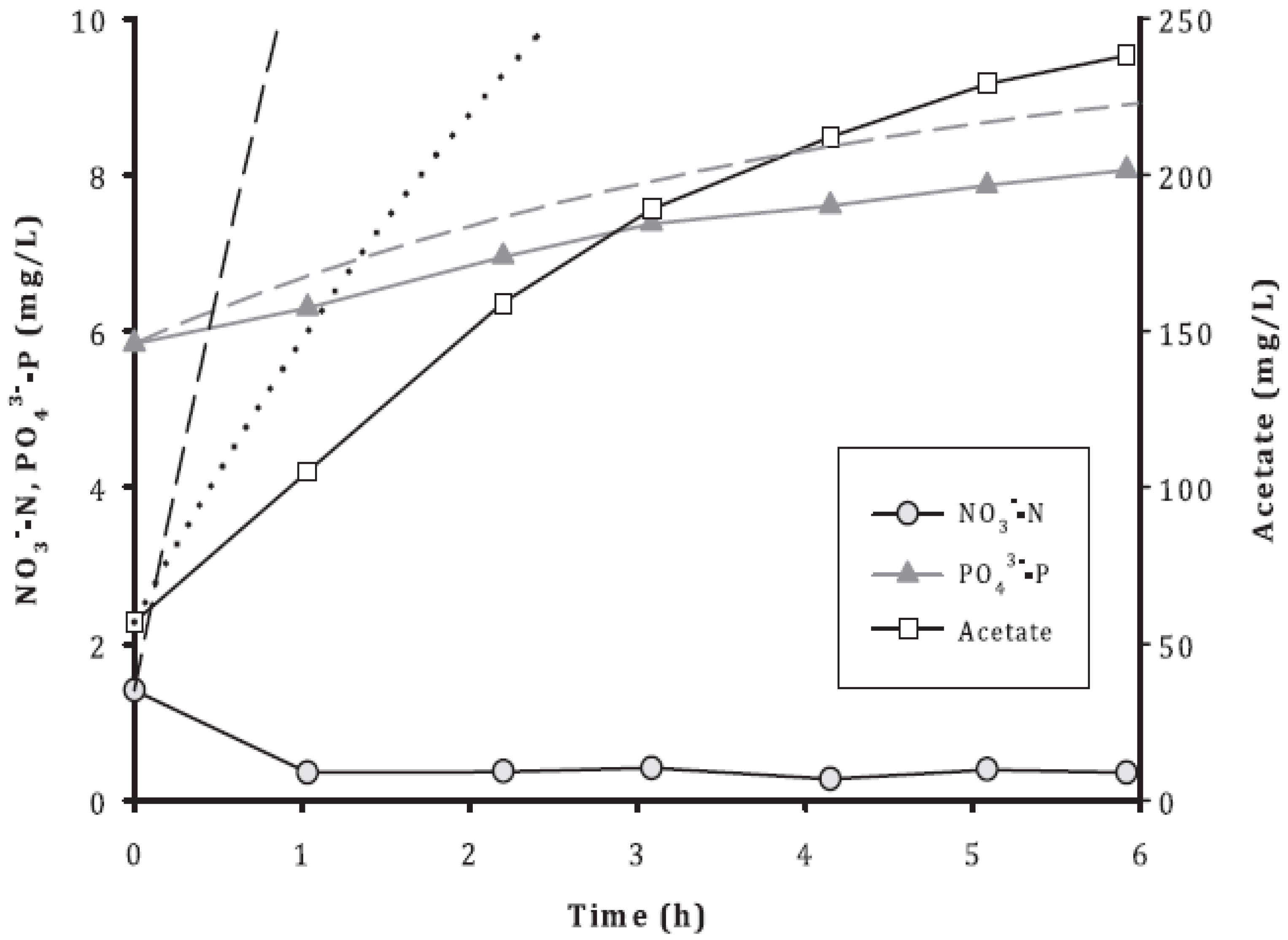
3.2. Removal of Acetate, NO3−, and PO43−
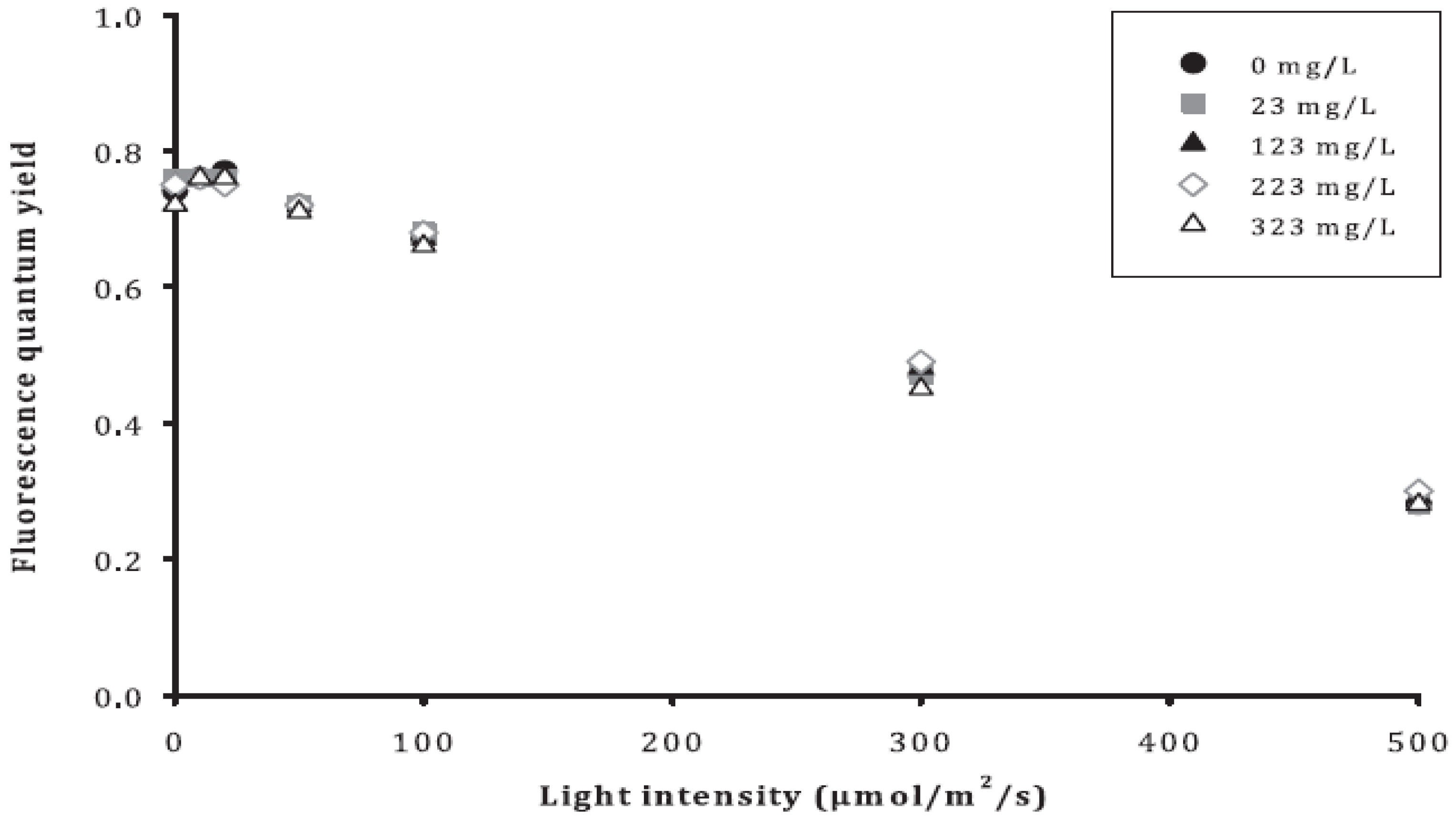
3.3. Symbiosis and ROS Test
3.4. Darkness and Symbiosis
3.5. Acetate Depletion and Symbiosis
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdel-Raouf, N.; Al-Homaidan, A.A.; Ibraheem, I.B.M. Microalgae and wastewater treatment. Saudi J. Biol. Sci. 2012, 19, 257–275. [Google Scholar] [CrossRef]
- Kuo, C.M.; Jian, J.F.; Lin, T.H.; Chang, Y.B.; Wan, X.H.; Lai, J.T.; Chang, J.S.; Lin, C.S. Simultaneous microalgal biomassproduction and CO2 fixation by cultivating Chlorella sp. GD with aquaculture wastewater and boiler flue gas. Bioresour. Technol. 2016, 221, 241–250. [Google Scholar] [CrossRef]
- Collotta, M.; Champagne, P.; Mabee, W.; Tomasoni, G. Wastewater and waste CO2 for sustainable biofuels from microalgae. Algal Res. 2018, 29, 12–21. [Google Scholar] [CrossRef]
- De-Bashan, L.E.; Bashan, Y. Joint immobilization of plant growth-promoting bacteria and green microalgae in alginate beads as an experimental model for studying plant-bacterium interactions. Appl. Environ. Microbiol. 2008, 74, 6797–6802. [Google Scholar] [CrossRef]
- Babu, M.; Hes, E.; van der Steen, N.; Hooijmans, C.; Gijzen, H. Nitrification rates of algal–bacterial biofilms in wastewater stabilization ponds under light and dark conditions. Ecol. Eng. 2010, 36, 1741–1746. [Google Scholar] [CrossRef]
- Craggs, R.J.; Tanner, C.C.; Sukias, J.P.; Davies-Colley, R.J. Nitrification potential of attached biofilms in dairy farm waste stabilisation ponds. Water Sci. Technol. 2000, 42, 195–202. [Google Scholar] [CrossRef]
- Higgins, P.G.; Prior, K.; Harmsen, D.; Seifert, H. Development and evaluation of a core genome multilocus typing scheme for whole-genome sequence-based typing of Acinetobacter baumannii. PLoS ONE 2017, 12, e0179228. [Google Scholar] [CrossRef]
- Kim, K.; Han, J.-I. Performance of direct alkaline sulfide fuel cell without sulfur deposition on anode. Int. J. Hydrog. Energy 2014, 39, 7142–7146. [Google Scholar] [CrossRef]
- Babu, M.A.; van der Steen, N.P.; Hooijmans, C.M.; Gijzen, H.J. Nitrogen mass balances for pilot-scale biofilm stabilization ponds under tropical conditions. Bioresour. Technol. 2011, 102, 3754–3760. [Google Scholar] [CrossRef]
- Johnson, L.M.; Smith, R.J.; Thompson, K.W.; Chen, H.; Lee, S. Characterization of Anoxic Microniches and Microbial Processes in Aerated Activated Sludge. Environ. Sci. Technol. 2023, 58, 3025–3032. [Google Scholar]
- Gupta, A.; Lee, S.M.; Kim, D.; Park, J. Synergistic Effects of Marine Bacteria on Microalgal Growth: Insights into a Balanced Inorganic/Organic Carbon Ratio. J. Appl. Phycol. 2023, 35, 87–95. [Google Scholar]
- Xue, F.; Miao, C.; Ma, Y.; Shi, M.; Zheng, H.; Chen, F.; Ye, N. Effects of Organic Carbon Sources on Growth, Photosynthesis, and Respiration of Phaeodactylum tricornutum. Biotechnol. Bioeng. 2016, 113, 536–545. [Google Scholar]
- Boelee, N.C.; Temmink, H.; Janssen, M.; Buisman, C.J.N.; Wijffels, R.H. Scenario Analysis of Nutrient Removal from Municipal Wastewater by Microalgal Biofilms. Water 2012, 4, 460–473. [Google Scholar] [CrossRef]
- Pikula, K.; Zakharenko, A.; Aruoja, V.; Golokhvast, K.; Tsatsakis, A. Oxidative stress and its biomarkers in microalgal ecotoxicology. Curr. Opin. Toxicol. 2019, 13, 8–15. [Google Scholar] [CrossRef]
- De Godos, I.; González, C.; Becares, E.; García-Encina, P.; Muñoz, R. Simultaneous Nutrients and Carbon Removal During Pretreated Swine Slurry Degradation in a Tubular Biofilm Photobioreactor. Appl. Microbiol. Biotechnol. 2009, 82, 187–194. [Google Scholar] [CrossRef]
- Holmes, D.E.; Dang, Y.; Smith, J.A. Nitrogen cycling during wastewater treatment. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 106. [Google Scholar] [CrossRef]
- González, C.; Marciniak, J.; Villaverde, S.; León, C.; García, P.A.; Muñoz, R. Efficient Nutrient Removal from Swine Manure in a Tubular Biofilm Photo-Bioreactor Using Algae-Bacteria Consortia. Water Sci. Technol. 2008, 58, 95–102. [Google Scholar] [CrossRef]
- Tchobanoglous, G.L.; Burton, F.; Stensel, D.H. Metcalf & Eddy: Wastewater Engineering: Treatment and Reuse; McGraw Hill Companies: New York, NY, USA, 2014; Issue 7. [Google Scholar]
- Flameling, I.A.; Kromkamp, J. Light dependence of quantum yields for PSII charge separation and oxygen evolution in eucaryotic algae. Limnol. Oceanogr. 1998, 43, 284–297. [Google Scholar] [CrossRef]
- Smith, J.K.; Johnson, A.B.; Williams, C.D. Kinetics of Hydrogen Peroxide Decomposition in High-Temperature Water. J. Radioanal. Nucl. Chem. 2023, 185, 213–220. [Google Scholar]
- Andersen, R.A.; Berges, J.A.; Harirson, P.J.; Watanabe, M.M. Appendix A—Recipes for Freshwater and Seawater Media. In Algal Culturing Techniques; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Duboc, P.; Marison, I.; von Stockar, U. Quantitative Calorimetry and Biochemical Engineering. In Handbook of Thermal Analysis and Calorimetry; Kemp, R.B., Ed.; Elsevier: Amsterdam, The Netherlands, 1999; Volume 4, pp. 287–309. [Google Scholar] [CrossRef]
- Smith, J.K.; Johnson, A.B.; Thompson, K.W.; Chen, H.; Lee, S. Impact of Ethylene Glycol-Bis (Β-Aminoethyl Ether)-N, N-Tetraacetic Acid (EGTA) on Methanogenic Granular Sludge: Stability and Activity Analysis. Environ. Sci. Technol. 2023, 45, 3210–3217. [Google Scholar]
- Bouarab, L.; Dauta, A.; Loudiki, M. Heterotrophic and mixotrophic growth of Micractinium pusillum Fresenius in the presence of acetate and glucose: Effect of light and acetate gradient concentration. Water Res. 2004, 38, 2706–2712. [Google Scholar] [CrossRef]
- Boelee, N.C.; Temmink, H.; Janssen, M.; Buisman, C.J.N.; Wijffels, R.H. Nitrogen and phosphorus removal from municipal wastewater effluent using microalgal biofilms. Water Res. 2011, 45, 5925–5933. [Google Scholar] [CrossRef] [PubMed]
- Huld, T.A.; Súri, M.; Dunlop, E.D.; Albuisson, M.; Wald, L. Integration of HELIOCLIM-1 Database Into PV-GIS to Estimate Solar Electricity Potential in Africa. In Proceedings of the 20th European Photovoltaic Solar Energy Conference and Exhibition, Barcelona, Spain, 6–10 June 2005. [Google Scholar]
- Karya, N.G.N.I.; van der Steen, N.P.; Lens, P.N.L. Photo-oxygenation to support nitrification in an algal–bacterial consortium treating artificial wastewater. Bioresour. Technol. 2013, 134, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Roeselers, G.; van Loosdrecht, M.C.M.; Muyzer, G. Phototrophic biofilms and their potential applications. J. Appl. Phycol. 2008, 20, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Raunkjær, K.; Nielsen, P.H.; Hvitved-Jacobsen, T. Acetate removal in sewer biofilms under aerobic conditions. Water Res. 1997, 31, 2727–2736. [Google Scholar] [CrossRef]
- McLean, B.M.; Baskaran, K.; Connor, M.A. The use of algal-bacterial biofilms to enhance nitrification rates in lagoons: Experience under laboratory and pilot-scale conditions. Water Sci. Technol. 2000, 42, 187–194. [Google Scholar] [CrossRef]
- Anderson, J.M.; Smith, R.L.; Thompson, K.W.; Chen, H.; Lee, S. Long-Term Persistence of Bacterial Denitrification Capacity under Aerobic Conditions. J. Environ. Microbiol. 2023, 25, 523–530. [Google Scholar]

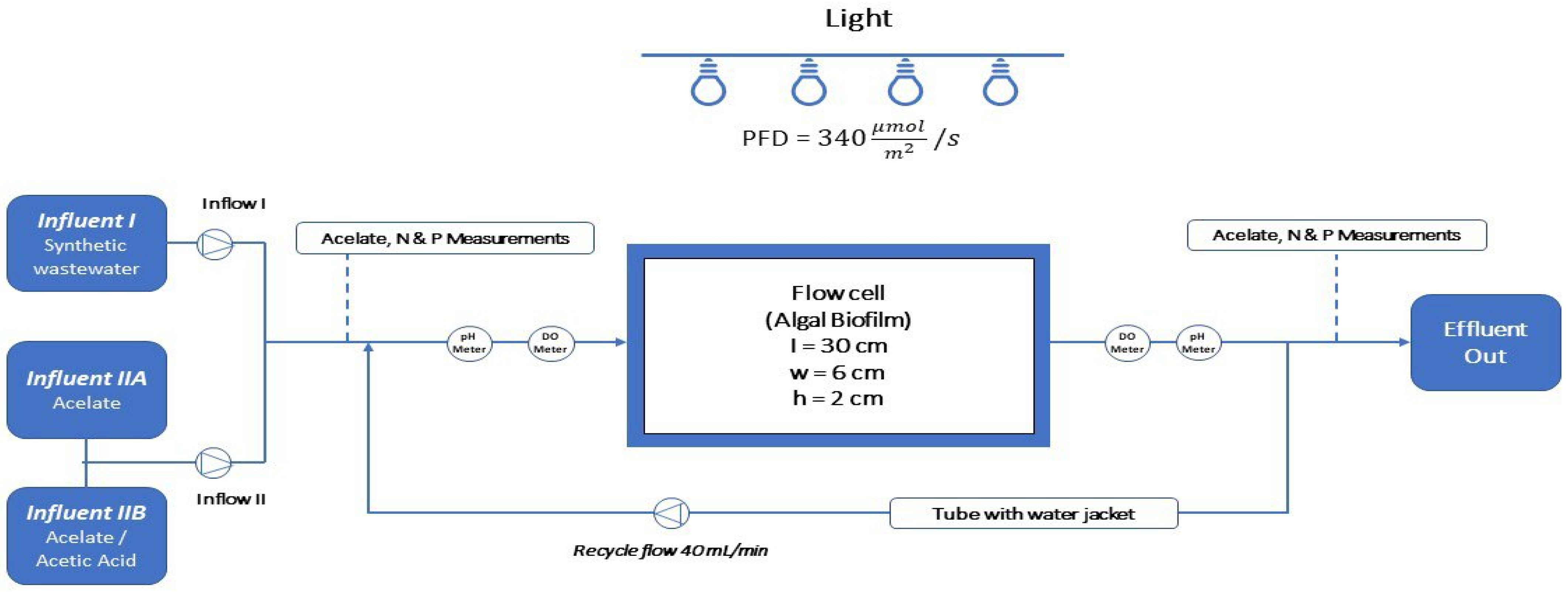


| Experiment | Influent I (mL/min) | Influent II (mL/min) | N in Synthetic Wastewater (mg/L) | N Loading Rate (g/m2/d) | HRT (h) | Extra HCO3− | Light Intensity (μmol/m2/s) |
|---|---|---|---|---|---|---|---|
| 1 | 2.7 | 0.7 | 50 | 14 | 2.6 | - | 615 |
| 2 | 1.6 | 0.4 | 50 | 8 | 4.5 | - | 340 |
| 3 | 1.6 | 0.4 | 50 | 8 | 4.5 | ✓ | 340 |
| 4 | 1.6 | 0.4 | 50 | 8 | 4.5 | ✓ | 340 |
| Test | Light | Acetate | Air | N Source | Experiment |
|---|---|---|---|---|---|
| A | X | ✓ | - | Ammonium | 1, 2, 3, 4 |
| B | X | ✓ | ✓ | Ammonium | 4 |
| C | X | ✓ | - | Nitrate | 4 |
| D | ✓ | X | - | Ammonium | 1, 2, 3, 4 |
| NH4+-N (mg/L) | PO43−-P (mg/L) | Acetate (mg/L) | |
|---|---|---|---|
| Influent | 50 | 10 | 323 |
| Effluent (calculated) | 27 | 5.7 | 0 |
| Experiment 1 Effluent | 43 | 7.4 | 179 |
| Experiment 2 Effluent | 30 | 7.9 | 166 |
| Experiment 3 Effluent | 30 | 5.7 | 39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sedghi, M.; Fagan, J.; Sedghi, S.; Küpper, F.C.; Amils, R. Harnessing Symbiotic Mixotrophic Microalgal–Bacterial Biofilms for N and P Elimination. Phycology 2023, 3, 459-471. https://doi.org/10.3390/phycology3040031
Sedghi M, Fagan J, Sedghi S, Küpper FC, Amils R. Harnessing Symbiotic Mixotrophic Microalgal–Bacterial Biofilms for N and P Elimination. Phycology. 2023; 3(4):459-471. https://doi.org/10.3390/phycology3040031
Chicago/Turabian StyleSedghi, Mahshid, John Fagan, Soheil Sedghi, Frithjof C. Küpper, and Ricardo Amils. 2023. "Harnessing Symbiotic Mixotrophic Microalgal–Bacterial Biofilms for N and P Elimination" Phycology 3, no. 4: 459-471. https://doi.org/10.3390/phycology3040031
APA StyleSedghi, M., Fagan, J., Sedghi, S., Küpper, F. C., & Amils, R. (2023). Harnessing Symbiotic Mixotrophic Microalgal–Bacterial Biofilms for N and P Elimination. Phycology, 3(4), 459-471. https://doi.org/10.3390/phycology3040031






