Chlorophyll Content and Photosynthetic Activity of Phytoplankton in Reservoirs of the Volga River (Russia)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Sampling
2.3. Chlorophyll
2.4. Photosynthetic Activity of Algae
2.5. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vinberg, G.G. Primary Production of the Basins, 1st ed.; Academic Press: Minsk, Belarus, 1960. [Google Scholar]
- Likens, J.E. Primary production of inland aquatic ecosystems. In Primary Productivity of the Biosphere; Leith, H., Whittaker, R.H., Eds.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1975; pp. 185–202. [Google Scholar]
- Reynolds, C.S. The Ecology of Phytoplankton, 1st ed.; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Behrenfeld, M.J.; Randerson, J.T.; McClain, C.R.; Feldman, G.C.; Los, S.O.; Tucker, C.J.; Falkowsli, P.G.; Field, C.B.; Frouin, R.; Esaias, W.E.; et al. Biospheric primary production during an ENSO transition. Science 2001, 291, 2594–2597. [Google Scholar] [CrossRef] [Green Version]
- OECD. Eutrophication of Waters: Monitoring, Assessment and Control; OECD: Paris, France, 1982. [Google Scholar]
- Goodwin, T.W. Chemistry and Biochemistry of Plant Pigments, 1st ed.; Goodwin, T.W., Ed.; Academic Press: London, UK; New York, NY, USA, 1965. [Google Scholar]
- Romanenko, V.I. Microbiological Processes of Production and Destruction of Organic Matter in Inland Waters, 1st ed.; Nauka Press: Leningrad, Russia, 1985. [Google Scholar]
- Mineeva, N.M. Phytoplankton Primary Production in the Volga River Reservoirs, 1st ed.; Print House: Yaroslavl, Russia, 2009. [Google Scholar]
- Pyrina, I.L. Primary production of phytoplankton in Ivankovo, Rybinsk, and Kuibyshev reservoirs in dependence on some factors. In Production and Cycle of Organic Matter in Inland Waters; Nauka Press: Moscow, Russia, 1966; pp. 249–270. [Google Scholar]
- Mineeva, N.M. Plant Pigments in the Waters of the Volga River Reservoirs, 1st ed.; Nauka Press: Moscow, Russia, 2004. [Google Scholar]
- Mineeva, N.M.; Makarova, O.S. Chlorophyll content as an indicator of the modern (2015–2016) trophic state of Volga River Reservoirs. Inland Water Biol. 2018, 11, 386–389. [Google Scholar] [CrossRef]
- Mineeva, N.M.; Semadeni, I.V.; Makarova, O.S. Chlorophyll content and the modern trophic state of the Volga River reservoirs (2017–2018). Inland Water Biol. 2020, 13, 327–330. [Google Scholar] [CrossRef]
- Mineeva, N.M. Chlorophyll and its role in freshwater ecosystem on the example of the Volga River reservoirs. In Chlorophylls; Ameen, S., Akhtar, M.S., Shin, H., Eds.; IntechOpen: London, UK, 2022; Available online: https://www.intechopen.com/books/11324 (accessed on 13 June 2023).
- SCOR-UNESCO Working Group 17. Determination of photosynthetic pigments. In Determination of Photosynthetic Pigments in Sea Water; UNESCO: Montreux, France, 1966; pp. 9–18. [Google Scholar]
- Mineeva, N.M. Composition and content of photosynthetic pigments in plankton of the Volga River reservoirs (2015–2016). Trans. Papanin Inst. Biol. Inland Waters RAS Russ. 2018, 81, 85–96. [Google Scholar] [CrossRef]
- Belous, O.; Klemeshova, K.; Panchenko, O. Comparative analysis of photosynthetic indicators in freesia hybrids on the Black Sea coast of Krasnodar region. Hortic. Sci. 2017, 44, 99–104. [Google Scholar] [CrossRef] [Green Version]
- Hartig, P.; Wolfstein, K.; Lippemeier, S.; Colijn, F. Photosynthetic activity of natural microphytobenthos populations measured by fluorescence (PAM) and 14C-tracer methods: A comparison. Mar. Ecol. Prog. Ser. 1998, 166, 53–62. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 345, 659–668. [Google Scholar] [CrossRef]
- Loll, B.; Kern, J.; Saenger, W.; Zouni, A.; Biesiadka, J. Towards complete cofactor arrangement in the 3.0 Å resolution structure of photosystem II. Nature 2005, 438, 1040–1044. [Google Scholar] [CrossRef]
- Zavaruev, V.V.; Aponasenko, A.D.; Lopatin, V.N.; Kachin, S.V. Research of correlation dependencies of the phytoplankton physiological state with a fluorescent response based on the use of it maximum and stationary parameters. J. Min. Inst. Russ. 2001, 149, 71–74. [Google Scholar]
- Braslavsky, S.E. Glossary of terms used in photochemistry. Pure Appl. Chem. 2007, 79, 293–465. [Google Scholar] [CrossRef]
- Matorin, D.N.; Rubin, A.B. Fluorescence of Chlorophyll in Higher Plants and Algae, 1st ed.; Institute for Computer Research: Moscow, Russia, 2012. [Google Scholar]
- Geider, R.J.; Greene, R.M.; Kolber, Z.; Maclntyre, H.L.; Falkowski, P.G. Fluorescence assessment of the maximum quantum efficiency of photosynthesis in the western North Atlantic. Deep. Sea Res. Part I Oceanogr. Res. Pap. 1993, 40, 1205–1224. [Google Scholar] [CrossRef]
- Gorbunov, M.Y.; Falkowski, P.G. Using chlorophyll fluorescence to determine the fate of photons absorbed by phytoplankton in the world’s oceans. Annu. Rev. Mar. Sci. 2022, 14, 213–238. [Google Scholar] [CrossRef]
- Semadeni, I.V. Assessment of phytoplankton photosynthetic activity in the Upper Volga reservoirs via fluorescence diagnosis. Issues Mod. Algol. 2021, 27, 18–25. [Google Scholar] [CrossRef]
- Semadeni, I.V. Chlorophyll Content and Photosynthetic Activity of Phytoplankton in the Rybinsk Reservoir in Years with Different hydroclimatic Conditions. Ph.D. Dissertation, Institute for Biology of Inland Waters RAS, Borok, Russia, 30 May 2023. [Google Scholar]
- Straškraba, M. Reservoirs and other artificial water bodies. In Lake Handbook, Vol. 2. Lake Restoration and Rehabilitation, 1st ed.; O’Sullivan, P.E., Reynolds, C.S., Eds.; Blackwell Publishing: Malden, MA, USA; Oxford, UK; Carlton, Australia, 2005; pp. 300–330. [Google Scholar]
- Devercelli, M.; Zalocar de Domitrovic, Y.; Forastier, M.E.; Meichtry de Zaburlín, N. Phytoplankton of the Paraná River Basin. Advanc. Limnol. 2014, 65, 39–65. [Google Scholar] [CrossRef] [Green Version]
- Dodrill, T.N.; Pan, Y.; Peterson, T.D. River discharge mediates extent of phytoplankton and harmful algal bloom habitat in the Columbia River Estuary (USA) during North Pacific marine heat waves. Estuaries Coasts 2023, 46, 166–181. [Google Scholar] [CrossRef]
- Li, J.; Gao, Y.; Bao, Y.; Gao, X.; Glibert, P.M. Summer phytoplankton photosynthetic characteristics in the Changjiang River Estuary and the adjacent East China Sea. Front. Mar. Sci. 2023, 10, 1111557. [Google Scholar] [CrossRef]
- Gitelzon, I.I. Comprehensive Research of Ecosystems of the Yenisei River Basin, 1st ed.; Gitelzon, I.I., Ed.; Krasnoyarsk State University Press: Krasnoyarsk, Russia, 1985. [Google Scholar]
- Kozhova, O.M. Primary Production in the Bratsk Reservoir, 1st ed.; Kozhova, O.M., Ed.; Nauka Press: Moscow, Russia, 1983. [Google Scholar]
- Priymachenko, A.D. Phytoplankton and Primary Production in the Dnieper and the Dnieper Reservoirs, 1st ed.; Nauk: Dumka, India; Kyiv, Ukraine, 1981. [Google Scholar]
- Beaver, J.R.; Jensen, D.E.; Casamatta, D.A.; Tausz, C.E.; Scotese, K.C.; Buccier, K.M.; Teacher, C.E.; Rosati, T.C.; Minerovic, A.D.; Renicker, T.R. Response of phytoplankton and zooplankton communities in six reservoirs of the middle Missouri River (USA) to drought conditions and a major flood event. Hydrobiologia 2013, 705, 173–189. [Google Scholar] [CrossRef]
- Butorin, N.V. The River Volga and Its Life, 1st ed.; Butorin, N.V., Mordukhai-Boltovskoy, P.D., Eds.; Dr W. Junk B. V. Publishers: Hague, The Netherlands; Boston, MA, USA; London, UK, 1979. [Google Scholar]
- Tockner, K.; Zarfl, C.; Robinson, C. Rivers of Europe, 2nd ed.; Tockner, K., Zarfl, C., Robinson, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Gol’d, V.M.; Gaevsky, N.A.; Grigoriev, Y.S.; Gekhman, A.V.; Popelʹnitsky, V.A. Theoretical Bases and Methods of Study Chlorophyll Fluorescence; Krasnoyarsk State University Press: Krasnoyarsk, Russia, 1984. [Google Scholar]
- Golʹd, V.M.; Gaevsky, N.A.; Shatrov, I.Y.; Popelʹnitsky, V.A.; Rybtsov, S.A. Experience of using fluorescence for differential evaluation of chlorophyll contents in planktonic algae. Hydrobiol. J. 1986, 22, 80–85. [Google Scholar]
- Gaevsky, N.A.; Kolmakov, V.I.; Anishchenko, O.V.; Gorbaneva, T.B. Using DCMU-fluorescence method for the identification of dominant phytoplankton groups. J. Appl. Phycol. 2005, 17, 483–494. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Reeves, S.; McMinn, A.; Martin, A. The effect of prolonged darkness on the growth, recovery and survival of Antarctic sea ice diatoms. Polar. Biol. 2011, 34, 1019–1032. [Google Scholar] [CrossRef] [Green Version]
- Franklin, D.J.; Choi, C.J.; Hughes, C.; Malin, G.; Berges, J.A. Effect of dead phytoplankton cells on the apparent efficiency of photosystem II. Mar. Ecol. Prog. Ser. 2009, 382, 35–40. [Google Scholar] [CrossRef]
- Ilyash, L.V.; Kurochkina, V.A.; Belevich, T.A.; Pogosyan, S.I. Fluorescence of individual cells of the algae Conticribra weissflogii under hyperosmotic stress. Issues Mod. Algol. 2012, 2. Available online: http://algology.ru/131 (accessed on 13 June 2023).
- Todorenko, D.A. Characteristics of Light Reactions of Photosynthesis When Exposed to Toxic Substances. Ph.D. Dissertation, Lomonosov Moscow State University, Moscow, Russia, 30 March 2016. [Google Scholar]
- Federal Service for Hydrometeorology and Environmental Monitoring (Roshydromet). Report on Climate Features on the Territory of the Russian Federation in 2017; Roshydromet: Moscow, Russia, 2018. [Google Scholar]
- Hardenbicker, P.; Weitere, M.; Ritz, S.; Schöll, F.; Fischer, H. Longitudinal Plankton Dynamics in the Rivers Rhine and Elbe. River Res. Appl. 2016, 32, 1264–1278. [Google Scholar] [CrossRef]
- Mineeva, N.M.; Shchure, L.A. Chlorophyll content in phytoplankton biomass. Review. Algology 2012, 22, 423–435. [Google Scholar]
- Korneva, L.G. Phytoplankton of Volga River Basin Reservoirs; Dom Pechati: Kostroma, Russia, 2015. [Google Scholar]
- Moreno-Ostos, E.; Cruz-Pizarro, L.; Basanta, A.; George, D.G. The spatial distribution of different phytoplankton functional groups in a Mediterranean reservoir. Aquat. Ecol. 2008, 42, 115–128. [Google Scholar] [CrossRef]
- Gaevsky, N.A.; Zotina, T.A.; Gorbaneva, T.B. Vertical structure and photosynthetic activity of Lake Shira. Aquat. Ecol. 2002, 36, 165–178. [Google Scholar] [CrossRef]
- Jeppesen, E.; Søndergaard, M.; Jensen, J.P.; Havens, K.; Anneville, O.; Carvalho, L.; Coveney, M.; Deneke, R.; Dokulil, M.; Foy, B.; et al. Lake responses to reduced nutrient loading—An analysis of contemporary long-term data from 35 case studies. Freshw. Biol. 2005, 50, 1747–1771. [Google Scholar] [CrossRef]
- Chu, Z.; Jin, X.; Iwami, N.; Inamori, Y. The effect of temperature on growth characteristics and competitions of Microcystis aeruginosa and Oscillatoria mougeotii in a shallow, eutrophic lake simulator system. Hydrobiologia 2007, 581, 217–223. [Google Scholar] [CrossRef]
- Winder, M.; Hunter, D.A. Temporal organization of phytoplankton communities linked to physical forcing. Oecologia 2008, 156, 179–192. [Google Scholar] [CrossRef]
- Bowes, M.; Gozzard, E.; Johnson, A.; Scarlett, P.; Roberts, C.; Read, D.; Armstrong, L.; Harman, S.; Wickham, H. Spatial and temporal changes in chlorophyll-a concentrations in the River Thames basin, UK: Are phosphorus concentrations beginning to limit phytoplankton biomass? Sci. Total Environ. 2012, 426, 45–55. [Google Scholar] [CrossRef] [Green Version]
- Duan, S.; Bianchi, T.S. Seasonal changes in the abundance and composition of plant pigments in particulate organic carbon in the Lower Mississippi and Pearl Rivers. Estuaries Coast 2006, 29, 427–442. Available online: www.jstor.org/stable/3809762 (accessed on 13 June 2023).
- Pautova, V.N.; Nomokonova, V.I. Phytoplankton Production of the Kuibyshev Reservoir, 1st ed.; Institute of Ecology of the Volga Basin RAS: Tolyatti, Russia, 1994. [Google Scholar]
- Mineeva, N.M.; Stepanova, I.E.; Semadeni, I.V. Biogenic elements and their significance in the development of phytoplankton in reservoirs of the Upper Volga. Inland Water Biol. 2021, 14, 32–42. [Google Scholar] [CrossRef]
- Mineeva, N.M.; Poddubny, S.A.; Stepanova, I.E.; Tsvetkov, A.I. Abiotic factors and their role in phytoplankton development in reservoirs of the Middle Volga River, Russia. Inland Water Biol. 2022, 15, 729–739. [Google Scholar] [CrossRef]
- Mineeva, N.M.; Poddubny, S.A.; Stepanova, I.E.; Tsvetkov, A.I. Abiotic factors and their role in phytoplankton development in reservoirs of the Lower Volga River, Russia. Inland Water Biol. 2023, 16, 70–80. [Google Scholar] [CrossRef]


| Parameters | Upper Volga | Middle Volga | Lower Volga | ||||
|---|---|---|---|---|---|---|---|
| Ivankovo | Uglich | Gorky | Cheboksary | Kuibyshev | Saratov | Volgograd | |
| Total water input, km3 per year | 10.07 | 11.46 | 49.53 | 118.89 | 244.3 | 248.3 | 259.2 |
| Surface area, km2 | 327 | 249 | 1591 | 1080 | 6150 | 1831 | 3117 |
| Length, km | 120 | 143 | 430 | 321 | 484 | 348 | 546 |
| Mean depth, m | 3.4 | 5.0 | 6.1 | 4.2 | 8.9 | 7.3 | 10.1 |
| Total storage, km3 | 1.12 | 1.25 | 8.82 | 4.60 | 57.30 | 12.87 | 31.45 |
| Water exchange, year−1 | 10.6 | 10.1 | 6.1 | 20.9 | 4.2 | 19.1 | 8.0 |
| Transparency, m | 0.8 | 0.8 | 1.2 | 1.2 | 1.5 | 2.2 | 2.0 |
| Water color, Cr-Co degree | 53 | 51 | 53 | 42 | 38 | 36 | 34 |
| Conductivity, μSim cm−1 | 240 | 250 | 206 | 355 | 315 | 345 | 424 |
| Total nitrogen, mg L−1 | 1.34 | 1.27 | 1.09 | 1.14 | 1.08 | 0.99 | 0.98 |
| Total phosphorus, μg L−1 | 90 | 93 | 68 | 124 | 145 | 127 | 134 |
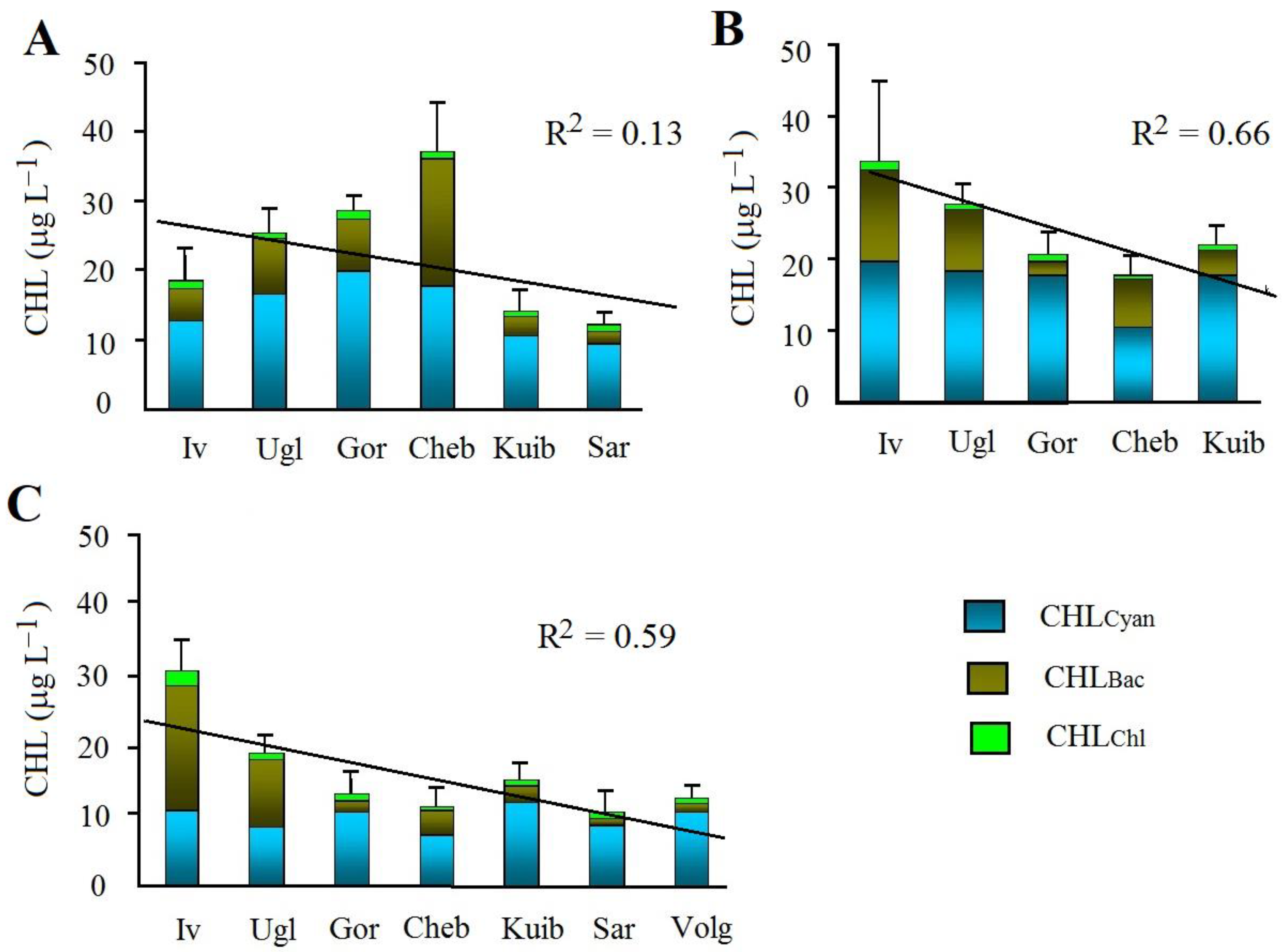
| Reservoir | Year | CHLCyan | CHLBac | CHLChlor | ∑CHL | |||
|---|---|---|---|---|---|---|---|---|
| μg L−1 | % | μg L−1 | % | μg L−1 | % | μg L−1 | ||
| Ivankovo | 2015 | 3.0–42.0 | 26–86 | 0.8–11.7 | 8–58 | 0.3–3.9 | 2–18 | 4.3–49.0 |
| 13.5 ± 3.6 | 64 ± 6 | 4.7 ± 1.0 | 29 ± 5 | 1.1 ± 0.3 | 7 ± 1 | 19.4 ± 4.0 | ||
| 2016 | 4.8–49.1 | 37–87 | 1.7–12.6 | 12–61 | 0.5–3.0 | 1–11 | 8.4–56.2 | |
| 19.7 ± 5.4 | 62 ± 4 | 6.7 ± 1.8 | 32 ± 4.2 | 1.2 ± 0.3 | 5 ± 1 | 33.7 ± 11.1 | ||
| 2017 | 1.3–29.1 | 11–58 | 1.2–31.8 | 36–85 | 0.2–3.7 | 3–18 | 3.2–45.7 | |
| 6.8 ± 2.1 | 31 ± 4 | 14.8 ± 3.2 | 62 ± 4 | 1.5 ± 0.3 | 7 ± 1 | 23.1 ± 4.9 | ||
| Uglich | 2015 | 2.2–28.5 | 20–84 | 3.2–14.2 | 12–74 | 0.3–1.7 | 2–7 | 17.1–35.2 |
| 16.6 ± 3.0 | 62 ± 6 | 8.0 ± 1.2 | 35 ± 6 | 0.8 ± 0.1 | 3 ± 1 | 25.3 ± 3.6 | ||
| 2016 | 10.9–27.1 | 40–85 | 3.9–26.1 | 14–57 | 0.3–1.6 | 1–5 | 16.7–45.9 | |
| 18.2 ± 1.5 | 68 ± 4 | 8.6 ± 2.2 | 29 ± 4.0 | 0.8 ± 0.2 | 3 ± 1 | 27.6 ± 2.8 | ||
| 2017 | 2.3–16.3 | 23–52 | 7.1–13.9 | 44–73 | 0.4–1.4 | 2–7 | 10.4–31.3 | |
| 7.9 ± 1.5 | 40 ± 3 | 10.2 ± 0.9 | 55 ± 3 | 0.9 ± 0.1 | 5 ± 1 | 19.0 ± 2.0 | ||
| Gorky | 2015 | 7.2–27.8 | 46–91 | 2.6–14.0 | 9–53 | 0.0–2.1 | 0.1–6 | 15.6–36.1 |
| 19.9 ± 1.6 | 71 ± 4 | 7.6 ± 1.0 | 28 ± 4 | 0.3 ± 0.2 | 1 ± 0.4 | 27.8 ± 1.5 | ||
| 2016 | 6.4–36.6 | 76–98 | 0.3–6.4 | 2.2–22 | 0.0–1.1 | 0.1–2 | 8.5–44.1 | |
| 17.7 ± 2.3 | 90 ± 2 | 1.8 ± 0.5 | 9 ± 2 | 0.1 ± 0.1 | 1 ± 0.3 | 19.6 ± 2.7 | ||
| 2017 | 0.7–12.2 | 55–98 | 0.2–1.6 | 1–35 | 0.0–0.5 | 1–9 | 1.2–13.1 | |
| 6.1 ± 1.0 | 79 ± 3 | 1.1 ± 0.1 | 18 ± 3 | 0.2 ± 0.0 | 3 ± 1 | 7.4 ± 1.0 | ||
| Cheboksary | 2015 | 0.5–26.1 | 2–89 | 1.6–33.0 | 10–97 | 0.0–0.4 | 0.1–1 | 16.2–52.1 |
| 17.8 ± 2.6 | 60 ± 10 | 18.3 ± 6.8 | 40 ± 10 | 0.2 ± 0.0 | 0.5 ± 0.1 | 36.3 ± 6.9 | ||
| 2016 | 3.8–18.7 | 14–97 | 0.5–31.4 | 3–82 | 0.0–1.3 | 0.1–7 | 5.2–38.1 | |
| 11.0 ± 1.9 | 66 ± 6 | 3.6 ± 1.2 | 30 ± 6 | 0.6 ± 0.1 | 4 ± 1 | 15.1 ± 2.2 | ||
| 2017 | 0.4–17.4 | 1–98 | 0.4–27.5 | 2–86 | 0.0–3.9 | 1–12 | 3.5–31.8 | |
| 5.0 ± 1.9 | 61 ± 11 | 4.7 ± 3.4 | 32 ± 9 | 0.8 ± 0.5 | 7 ± 2 | 10.5 ± 3.6 | ||
| Kuibyshev | 2015 | 1.6–22.8 | 59–93 | 0.6–13.6 | 7–35 | 0.0–2.5 | 0.1–6 | 2.2–38.9 |
| 10.6 ± 2.0 | 78 ± 3 | 2.7 ± 0.9 | 21 ± 3 | 0.3 ± 0.2 | 1 ± 0.5 | 13.6 ± 2.8 | ||
| 2016 | 6.7–47.2 | 44–95 | 0.6–11.9 | 5–46 | 0.1–3.0 | 0.2–12 | 7.9–49.9 | |
| 17.6 ± 2.0 | 79 ± 2.9 | 3.5 ± 0.6 | 17 ± 2 | 0.8 ± 0.2 | 4 ± 1 | 21.9 ± 2.0 | ||
| 2017 | 0.7–13.3 | 37–98 | 0.1–6.5 | 1–62 | 0.1–0.8 | 1–15 | 1.3–15.0 | |
| 6.2 ± 1.0 | 72 ± 5 | 1.9 ± 0.6 | 22 ± 5 | 0.4 ± 0.1 | 6 ± 1 | 8.5 ± 1.1 | ||
| Saratov | 2015 | 4.0–18.4 | 74–88 | 1.0–2.6 | 10–26 | 0.0–0.3 | 0.5–2 | 5.1–20.8 |
| 9.5 ± 1.3 | 82 ± 1 | 1.7 ± 0.2 | 17 ± 1 | 0.1 ± 0.0 | 1 ± 0.1 | 11.3 ± 1.4 | ||
| 2017 | 1.5–10.1 | 78–96 | 0.3–0.8 | 2–25 | 0.1–0.2 | 1–6 | 2.0–10.7 | |
| 4.6 ± 1.7 | 83 ± 5 | 0.5 ± 0.1 | 14 ± 4 | 0.1 ± 0.0 | 3 ± 1 | 5.2 ± 1.5 | ||
| Volgograd | 2017 | 0.6–14.2 | 28–93 | 0.1–2.2 | 2–63 | 0.2–0.4 | 1–9 | 2.1–15.8 |
| 6.6 ± 1.2 | 78 ± 6 | 1.1 ± 0.2 | 18 ± 6 | 0.3 ± 0.1 | 4 ± 1 | 8.0 ± 1.1 | ||
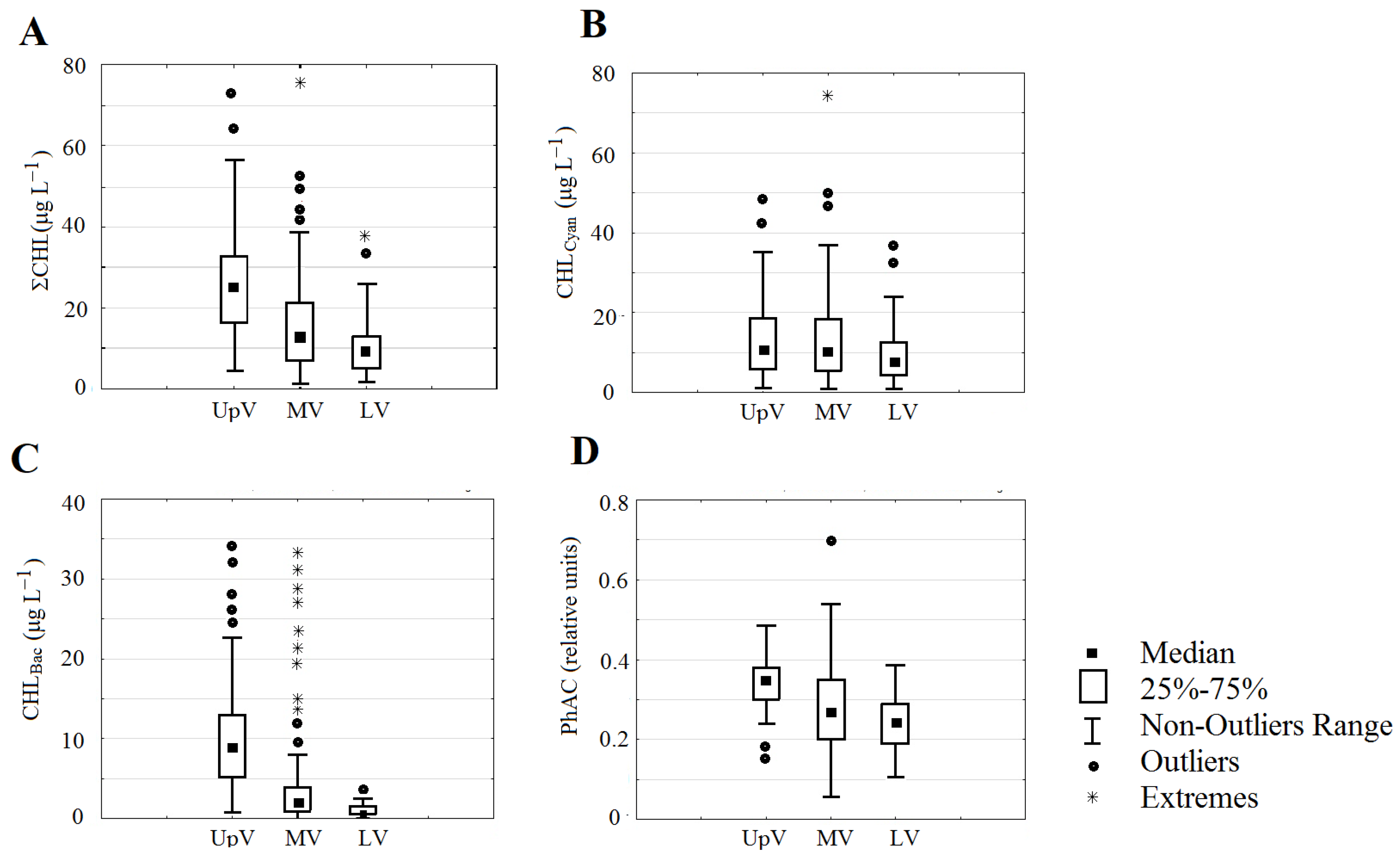
| Parameter | Source of Variation | SS | df | MS | F | P | Fcr |
|---|---|---|---|---|---|---|---|
| ∑CHL | Between groups | 7962 | 2 | 3981 | 27.1 | 0.00 | 3.03 |
| Within groups | 38,526 | 262 | 147 | ||||
| CHLCyan | Between groups | 454 | 2 | 227 | 2.28 | 0.10 | 3.03 |
| Within groups | 26,134 | 262 | 99 | ||||
| CHLBac | Between groups | 3408 | 2 | 1704 | 43.5 | 0.00 | 3.02 |
| Within groups | 10,262 | 262 | 39.2 | ||||
| PhAC | Between groups | 0.251 | 2 | 0.125 | 15.1 | 0.00 | 3.04 |
| Within groups | 1.588 | 191 | 0.008 |
| Reservoir | 2015 | 2016 | 2017 | |||
|---|---|---|---|---|---|---|
| Min–Max | X ± SE | Min–Max | X ± SE | Min–Max | X ± SE | |
| Ivankovo | 0.17–0.38 | 0.35 ± 0.01 | 0.37–0.59 | 0.38 ± 0.03 | 0.27–0.43 | 0.35 ± 0.02 |
| Uglich | 0.27–0.4 | 0.31 ± 0.02 | 0.18–0.57 | 0.31 ± 0.03 | 0.27–0.44 | 0.36 ± 0.02 |
| Gorky | 0.24–0.70 | 0.38 ± 0.03 | 0.14–0.44 | 0.26 ± 0.02 | 0.09–0.26 | 0.16 ± 0.01 |
| Cheboksary | 0.18–0.52 | 0.35 ± 0.03 | 0.18–0.44 | 0.33 ± 0.02 | 0.08–0.4 | 0.22 ± 0.04 |
| Kuibyshev | 0.12–0.39 | 0.26 ± 0.02 | 0.2–0.39 | 0.29 ± 0.02 | 0.06–0.36 | 0.24 ± 0.03 |
| Saratov | 0.19–0.38 | 0.28 ± 0.03 | – | – | 0.10–0.25 | 0.17 ± 0.02 |
| Volgograd | – | – | – | – | 0.12–0.38 | 0.25 ± 0.02 |
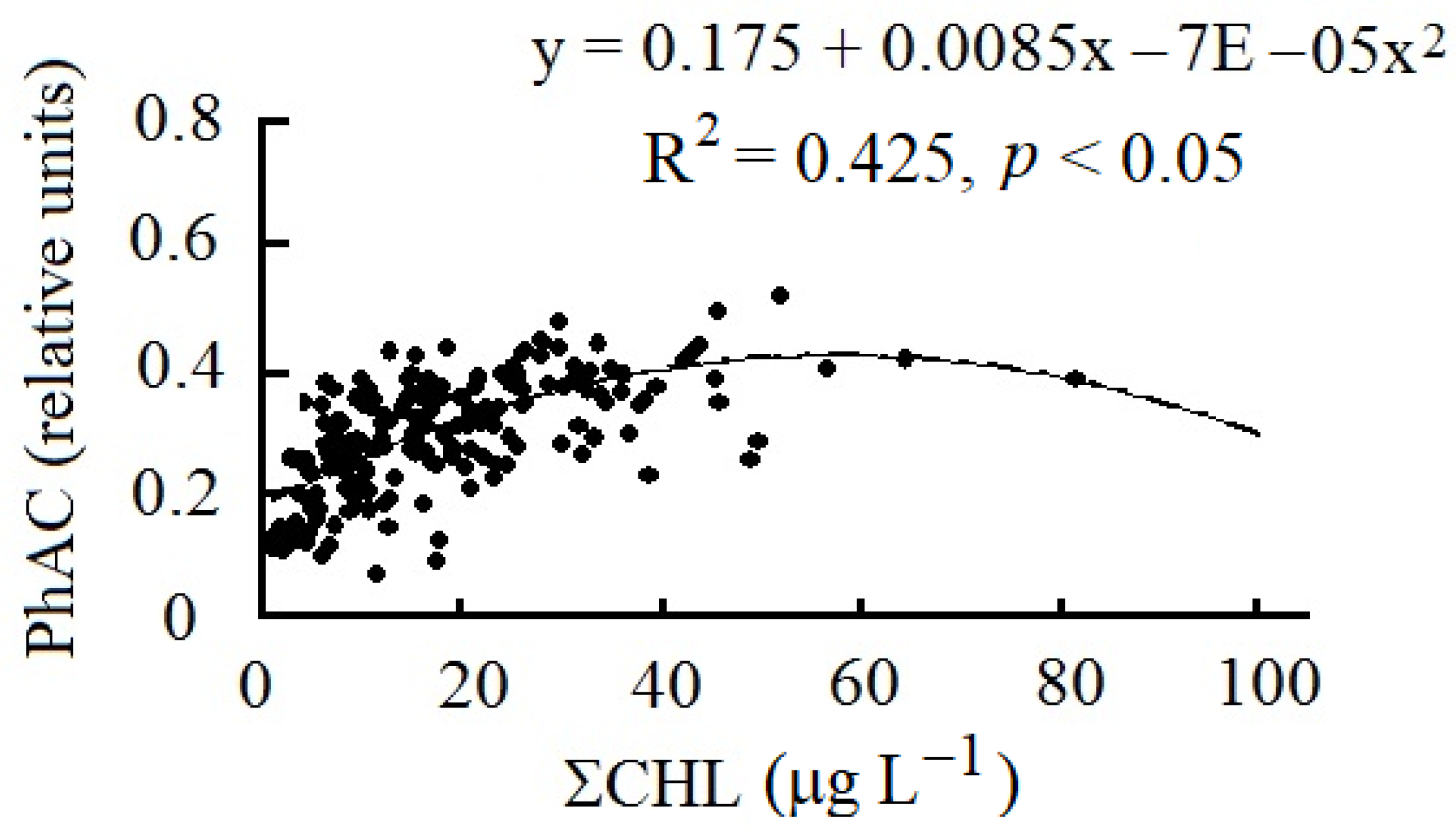
| Trophic State | ∑CHL, μg L−1 | PhAC, Relative Units |
|---|---|---|
| Oligotrophic | <3 | 0.12 ± 0.01 |
| Mesotrophic | 3–10 | 0.20 ± 0.01 |
| Moderate eutrophic | 10–15 | 0.27 ± 0.01 |
| Eutrophic | 15–30 | 0.32 ± 0.01 |
| Hypertrophic | >30 | 0.38 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mineeva, N.; Semadeni, I. Chlorophyll Content and Photosynthetic Activity of Phytoplankton in Reservoirs of the Volga River (Russia). Phycology 2023, 3, 368-381. https://doi.org/10.3390/phycology3030024
Mineeva N, Semadeni I. Chlorophyll Content and Photosynthetic Activity of Phytoplankton in Reservoirs of the Volga River (Russia). Phycology. 2023; 3(3):368-381. https://doi.org/10.3390/phycology3030024
Chicago/Turabian StyleMineeva, Natalya, and Ivan Semadeni. 2023. "Chlorophyll Content and Photosynthetic Activity of Phytoplankton in Reservoirs of the Volga River (Russia)" Phycology 3, no. 3: 368-381. https://doi.org/10.3390/phycology3030024
APA StyleMineeva, N., & Semadeni, I. (2023). Chlorophyll Content and Photosynthetic Activity of Phytoplankton in Reservoirs of the Volga River (Russia). Phycology, 3(3), 368-381. https://doi.org/10.3390/phycology3030024







