Are Microalgae New Players in Nitrous Oxide Emissions from Eutrophic Aquatic Environments?
Abstract
:1. Introduction
2. N2O Inventory for Aquatic Environments
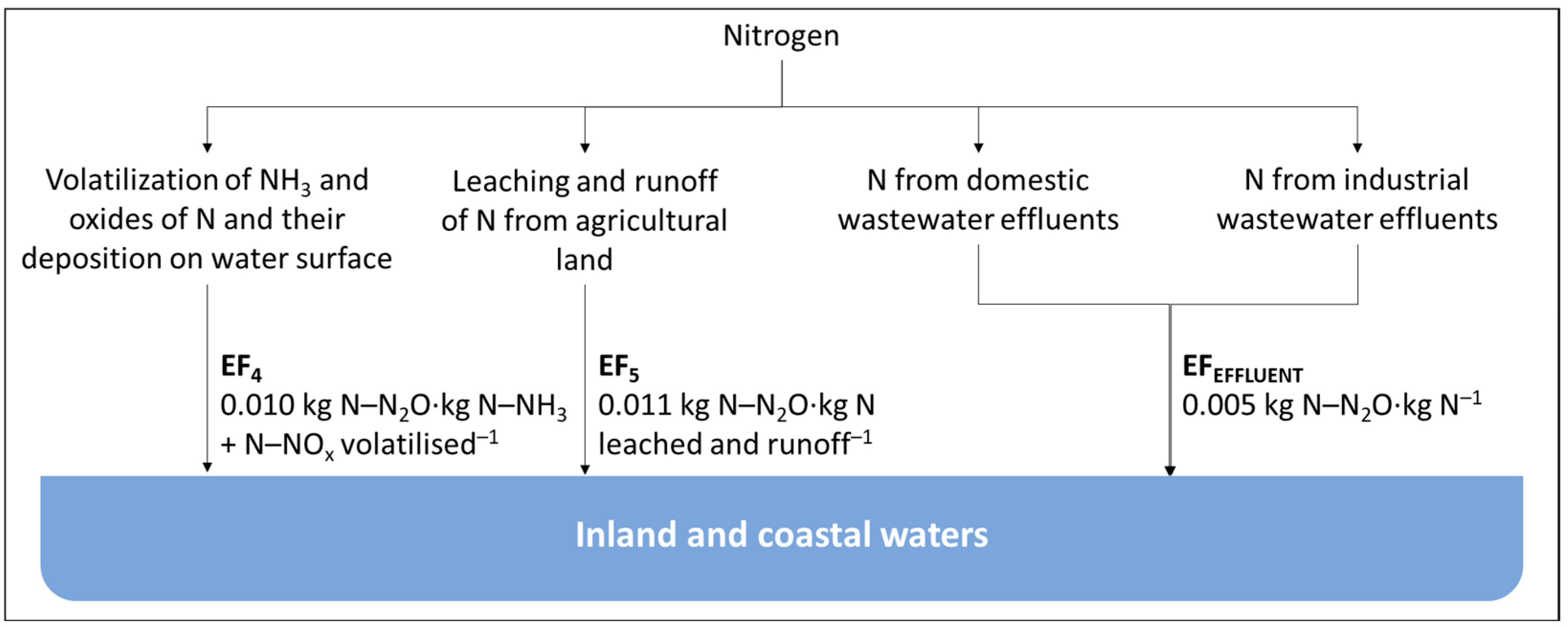
2.1. Current Methodology
2.2. Limitations to the Current Approach
3. Potential Significance of Microalgal N2O Synthesis
3.1. A case for Microalgal N2O Emissions
3.2. N2O Emissions from Microalgae Ecosystems
| N2O Emissions from Laboratory culturess and Engineered Systems | ||||
|---|---|---|---|---|
| Alga Division | Algal Species | Ecosystem | N2O Flux | Reference |
| Green microalgae | Chlorella vulgaris | Laboratory assays | 109–1480 nmole·h−1·g DW−1 | [16] |
| Photobioreactor | 563–4134 nmole·h−1·g DW−1 | [16] | ||
| Photobioreactor | 9.60–38,000 nmole·m−2·h−1 | [56] | ||
| Raceway pond | 2–5685 nmole·h−1·g DW−1 | [62] | ||
| C. rubescens | Laboratory assays | 1200–2500 nmole·h−1·g DW−1 | [14] | |
| C. variabilis | Laboratory assays | 300 µmole·L−1·h−1 | [17] | |
| Coelastrum sp. | Laboratory assays | 560–1100 nmole·h−1·g DW−1 | [14] | |
| Chlorococcum vacuolarum | Laboratory assays | 150–290 nmole·h−1·g DW−1 | [14] | |
| Neochloris sp. | Photobioreactor | 50–14,200 nmole·m−2·h−1 | [56] | |
| Scenedesmus dimorphus | Laboratory assays | 6–73 nmole·h−1·g DW−1 | [63] | |
| S. obliquus | Laboratory assays | 0–1000 nmole·h−1·g DW−1 | [14] | |
| Chlamydomonas reinhardtii | Laboratory assays | 7.5–74 nmole·h−1·g DW−1 | [13] | |
| Laboratory assays | 54 µmole·L−1·h−1 | [17] | ||
| Coccomyxa subellipsoidea | Laboratory assays | 225 µmole·L−1·h−1 | [17] | |
| Tetraselmis subcordiformis | Laboratory assays | 188 µmole·L−1·h−1 | [17] | |
| Eustigmatophyceae | Nannochloropsis oculata | Laboratory assays | 0.98 nmole·L−1·h−1 | [64] |
| Diatoms | Skeletonema marinoi | Laboratory assays | 0.039–0.31 nmole·h−1·aggregate−1 | [65] |
| Thalassiosira weissflogii | Laboratory assays | 0.087–0.3 nmole·L−1·h−1 | [66] | |
| Staurosira sp. | Raceway pond | −212.5–316.7 nmole·m−2·h−1 | [67] | |
| Cyanobacteria | Aphanocapsa 6308 | Laboratory assays | 0–1500 nmole·h−1·g DW−1 | [15] |
| Aphanocapsa 6714 | Laboratory assays | 0–5700 nmole·h−1·g DW−1 | [15] | |
| Nostoc sp. | Laboratory assays | 0–1500 nmole·h−1·g DW−1 | [15] | |
| Microcystis aeruginosa | Laboratory assays | 0–198.9 nmole·h−1·g DW−1 | [18] | |
| N2O Emissions from Aquatic Ecosystems | |||
|---|---|---|---|
| Ecosystem | N2O Flux | O2 Conditions 1 | Reference |
| Ocean | 115 nmole·m−2·h−1 | Normoxic | [68] |
| Ocean | 409 nmole·m−2·h−1 | Hypoxic | [69] |
| Coastal wetland | 125–228 nmole·m−2·h−1 | Anoxic and hypoxic | [70] |
| Ocean | 123–132% saturation | Normoxic | [71] |
| Lake (including eutrophic ones) | 300–700 nmole·m−2·h−1 | From anoxic to normoxic | [72] |
| Ocean | 88 nmole·m−2·h−1 | Not specified | [73] |
| Lake (eutrophic) | 357–2450 nmole·m−2·h−1 | Not specified | [74] |
| Lake | 0–10,057 nmole·m−2·h−1 | Oxic | [75] |
| Lake (eutrophic) | 46–230 nmole·m−2·h−1 | From anoxic to hypoxic | [54] |
| Lake | 12.5–2233 nmole·m−2·h−1 | Normoxic and Hyperoxic | [45] |
4. Nitrogen, the Perfect Culprit for N2O Emissions from Eutrophic Environments?
4.1. N2O Emissions under Oxia
4.2. Possible Impact of Phosphorus Inputs on N2O Emissions in Eutrophic Ecosystems
4.3. Possible Impact of Micronutrients Inputs and Microbial Interactions in Eutrophic Ecosystems
4.4. Are Microalgae New Players in Nitrous Oxide Emissions from Eutrophic Aquatic Environments?
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, X.; Wu, X.; Hao, H.; He, Z. Mechanisms and Assessment of Water Eutrophication. J. Zhejiang Univ. Sci. B 2008, 9, 197–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DelSontro, T.; Beaulieu, J.J.; Downing, J.A. Greenhouse Gas Emissions from Lakes and Impoundments: Upscaling in the Face of Global Change. Limnol. Oceanogr. Lett. 2018, 3, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Otten, T.G.; Kudela, R. Mitigating the Expansion of Harmful Algal Blooms Across the Freshwater-to-Marine Continuum. Environ. Sci. Technol. 2018, 52, 5519–5529. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Paerl, H.W.; Qin, B.; Zhu, G.; Gaoa, G. Nitrogen and Phosphorus Inputs Control Phytoplankton Growth in Eutrophic Lake Taihu, China. Limnol. Oceanogr. 2010, 55, 420–432. [Google Scholar] [CrossRef] [Green Version]
- Aubriot, L.; Zabaleta, B.; Bordet, F.; Sienra, D.; Risso, J.; Achkar, M.; Somma, A. Assessing the Origin of a Massive Cyanobacterial Bloom in the Río de La Plata (2019): Towards an Early Warning System. Water Res. 2020, 181, 115944. [Google Scholar] [CrossRef]
- Huang, F.; Pan, L.; He, Z.; Zhang, M.; Zhang, M. Identification, Interactions, Nitrogen Removal Pathways and Performances of Culturable Heterotrophic Nitrification-Aerobic Denitrification Bacteria from Mariculture Water by Using Cell Culture and Metagenomics. Sci. Total Environ. 2020, 732, 139268. [Google Scholar] [CrossRef]
- Rabalais, N.N.; Cal, W.-J.; Carstensen, J.; Conley, D.J.; Fry, B.; Hu, X.; Quinones-Rivera, Z.; Rosenberg, R.; Slomp, C.P.; Turner, R.E.; et al. Eutrophication-Driven Deoxygenation in the Coastal Ocean. Oceanography 2014, 27, 172–183. [Google Scholar] [CrossRef] [Green Version]
- Jenny, J.-P.; Francus, P.; Normandeau, A.; Lapointe, F.; Perga, M.-E.; Ojala, A.; Schimmelmann, A.; Zolitschka, B. Global Spread of Hypoxia in Freshwater Ecosystems during the Last Three Centuries Is Caused by Rising Local Human Pressure. Glob. Chang. Biol. 2016, 22, 1481–1489. [Google Scholar] [CrossRef]
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous Oxide (N2O): The Dominant Ozone-Depleting Substance Emitted in the 21st Century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef] [Green Version]
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.L.; Péan, C.; Berger, S.; Caud, N.; Chen, Y.; Goldfarb, L.; Gomis, M.I.; et al. IPCC, 2021: Summary for Policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021. [Google Scholar]
- Tian, H.; Xu, R.; Canadell, J.G.; Thompson, R.L.; Winiwarter, W.; Suntharalingam, P.; Davidson, E.A.; Ciais, P.; Jackson, R.B.; Janssens-Maenhout, G.; et al. A Comprehensive Quantification of Global Nitrous Oxide Sources and Sinks. Nature 2020, 586, 248–256. [Google Scholar] [CrossRef]
- Plouviez, M.; Shilton, A.; Packer, M.A.; Guieysse, B. Nitrous Oxide Emissions from Microalgae: Potential Pathways and Significance. J. Appl. Phycol. 2019, 31, 1–8. [Google Scholar] [CrossRef]
- Plouviez, M.; Wheeler, D.; Shilton, A.; Packer, M.A.; McLenachan, P.A.; Sanz-Luque, E.; Ocaña-Calahorro, F.; Fernández, E.; Guieysse, B. The Biosynthesis of Nitrous Oxide in the Green Alga Chlamydomonas reinhardtii. Plant J. 2017, 91, 45–56. [Google Scholar] [CrossRef] [Green Version]
- Weathers, P. N2O Evolution by Green Algae. Appl. Environ. Microbiol. 1984, 48, 1251–1253. [Google Scholar] [CrossRef] [Green Version]
- Weathers, P.J.; Niedzielski, J.J. Nitrous Oxide Production by Cyanobacteria. Arch. Microbiol. 1986, 146, 204–206. [Google Scholar] [CrossRef]
- Guieysse, B.; Plouviez, M.; Coilhac, M.; Cazali, L. Nitrous Oxide (N2O) Production in Axenic Chlorella vulgaris Microalgae Cultures: Evidence, Putative Pathways, and Potential Environmental Impacts. Biogeosciences 2013, 10, 6737–6746. [Google Scholar] [CrossRef] [Green Version]
- Burlacot, A.; Richaud, P.; Gosset, A.; Li-Beisson, Y.; Peltier, G. Algal Photosynthesis Converts Nitric Oxide into Nitrous Oxide. Proc. Natl. Acad. Sci. USA 2020, 117, 2704–2709. [Google Scholar] [CrossRef] [Green Version]
- Fabisik, F.; Guieysse, B.; Procter, J.; Plouviez, M. Nitrous Oxide (N2O) Synthesis by the Freshwater Cyanobacterium Microcystis aeruginosa. Biogeosciences 2023, 20, 687–693. [Google Scholar] [CrossRef]
- Plouviez, M.; Guieysse, B. Nitrous Oxide Emissions during Microalgae-Based Wastewater Treatment: Current State of the Art and Implication for Greenhouse Gases Budgeting. Water Sci. Technol. 2020, 82, 1025–1030. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.-H.; Zhang, J.-T.; Chi, Z.-Y.; Kong, F.-T.; Zhang, Q. The Long Overlooked Microalgal Nitrous Oxide Emission: Characteristics, Mechanisms, and Influencing Factors in Microalgae-Based Wastewater Treatment Scenarios. Sci. Total Environ. 2023, 856, 159153. [Google Scholar] [CrossRef]
- Hergoualc’h, K.; Akiyama, H.; Bernoux, M.; Chirinda, N.; del Prado, A.; Kasimir, Å.; MacDonald, J.D.; Ogle, S.M.; Regina, K.; van der Weerden, T.J. 2019 Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories—Volume 4 Agriculture, Forestry and Other Land Use—Chapter 11: N2O Emissions from Managed Soils, and CO2 Emissions from Lime and Urea Application; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2019. [Google Scholar]
- Bartram, D.; Short, M.D.; Ebie, Y. 2019 Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories—Volume 5 Waste—Chapter 6: Wastewater Treatment and Discharge; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2019. [Google Scholar]
- Tian, L.; Cai, Y.; Akiyama, H. A Review of Indirect N2O Emission Factors from Agricultural Nitrogen Leaching and Runoff to Update of the Default IPCC Values. Environ. Pollut. 2019, 245, 300–306. [Google Scholar] [CrossRef]
- Hahn, J.; Junge, C. Atmospheric Nitrous Oxide: A Critical Review. Z. Naturforsch. 1977, 32, 190–214. [Google Scholar] [CrossRef]
- Hallin, S.; Philippot, L.; Löffler, F.E.; Sanford, R.A.; Jones, C.M. Genomics and Ecology of Novel N2O-Reducing Microorganisms. Trends Microbiol. 2018, 26, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.-Y.; Gwak, J.-H.; Rohe, L.; Giesemann, A.; Kim, J.-G.; Well, R.; Madsen, E.L.; Herbold, C.W.; Wagner, M.; Rhee, S.-K. Indications for Enzymatic Denitrification to N2O at Low PH in an Ammonia-Oxidizing Archaeon. ISME J. 2019, 13, 2633–2638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellido-Pedraza, C.M.; Calatrava, V.; Sanz-Luque, E.; Tejada-Jiménez, M.; Llamas, Á.; Plouviez, M.; Guieysse, B.; Fernández, E.; Galván, A. Chlamydomonas reinhardtii, an Algal Model in the Nitrogen Cycle. Plants 2020, 9, 903. [Google Scholar] [CrossRef]
- Wrage, N.; Velthof, G.L.; van Beusichem, M.L.; Oenema, O. Role of Nitrifier Denitrification in the Production of Nitrous Oxide. Soil Biol. 2001, 33, 1723–1732. [Google Scholar] [CrossRef]
- Piña-Ochoa, E.; Álvarez-Cobelas, M. Denitrification in Aquatic Environments: A Cross-System Analysis. Biogeochemistry 2006, 81, 111–130. [Google Scholar] [CrossRef]
- Hayatsu, M.; Tago, K.; Saito, M. Various Players in the Nitrogen Cycle: Diversity and Functions of the Microorganisms Involved in Nitrification and Denitrification. Soil Sci. Plant Nutr. 2008, 54, 33–45. [Google Scholar] [CrossRef]
- Ward, B.B.; Arp, D.J.; Klotz, M.G. (Eds.) Nitrification; American Society for Microbiology, ASM Press: Washington, DC, USA, 2011; ISBN 978-1-55581-481-6. [Google Scholar]
- Zhu-Barker, X.; Cavazos, A.R.; Ostrom, N.E.; Horwath, W.R.; Glass, J.B. The Importance of Abiotic Reactions for Nitrous Oxide Production. Biogeochemistry 2015, 126, 251–267. [Google Scholar] [CrossRef]
- Ren, Y.; Ngo, H.H.; Guo, W.; Ni, B.-J.; Liu, Y. Linking the Nitrous Oxide Production and Mitigation with the Microbial Community in Wastewater Treatment: A Review. Bioresour. Technol. Rep. 2019, 7, 100191. [Google Scholar] [CrossRef]
- Shoun, H.; Fushinobu, S.; Jiang, L.; Kim, S.-W.; Wakagi, T. Fungal Denitrification and Nitric Oxide Reductase Cytochrome P450nor. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 1186–1194. [Google Scholar] [CrossRef] [Green Version]
- Higgins, S.A.; Welsh, A.; Orellana, L.H.; Konstantinidis, K.T.; Chee-Sanford, J.C.; Sanford, R.A.; Schadt, C.W.; Löffler, F.E. Detection and Diversity of Fungal Nitric Oxide Reductase Genes (P450nor) in Agricultural Soils. Appl. Environ. Microbiol. 2016, 82, 2919–2928. [Google Scholar] [CrossRef] [Green Version]
- Santoro, A.E.; Buchwald, C.; McIlvin, M.R.; Casciotti, K.L. Isotopic Signature of N2O Produced by Marine Ammonia-Oxidizing Archaea. Science 2011, 333, 1282–1285. [Google Scholar] [CrossRef]
- Löscher, C.R.; Kock, A.; Könneke, M.; LaRoche, J.; Bange, H.W.; Schmitz, R.A. Production of Oceanic Nitrous Oxide by Ammonia-Oxidizing Archaea. Biogeosciences 2012, 9, 2419–2429. [Google Scholar] [CrossRef] [Green Version]
- Qin, W.; Meinhardt, K.A.; Moffett, J.W.; Devol, A.H.; Virginia Armbrust, E.; Ingalls, A.E.; Stahl, D.A. Influence of Oxygen Availability on the Activities of Ammonia-Oxidizing Archaea: Influence of Oxygen Availability. Environ. Microbiol. Rep. 2017, 9, 250–256. [Google Scholar] [CrossRef]
- Webb, J.R.; Clough, T.J.; Quayle, W.C. A Review of Indirect N2O Emission Factors from Artificial Agricultural Waters. Environ. Res. Lett. 2021, 16, e043005. [Google Scholar] [CrossRef]
- Marzadri, A.; Dee, M.M.; Tonina, D.; Bellin, A.; Tank, J.L. Role of Surface and Subsurface Processes in Scaling N2O Emissions along Riverine Networks. Proc. Natl. Acad. Sci. USA 2017, 114, 4330–4335. [Google Scholar] [CrossRef]
- Wetzel, R.G. Rivers and Lakes—Their distribution, origins and forms. In Limnology; Elsevier: Amsterdam, The Netherlands, 2001; pp. 15–42. ISBN 978-0-12-744760-5. [Google Scholar]
- Pinckney, J.L.; Paerl, H.W.; Tester, P.; Richardson, T.L. The Role of Nutrient Loading and Eutrophication in Estuarine Ecology. Environ. Health Perspect. 2001, 109, 699–706. [Google Scholar]
- Yang, S.; Chang, B.X.; Warner, M.J.; Weber, T.S.; Bourbonnais, A.M.; Santoro, A.E.; Kock, A.; Sonnerup, R.E.; Bullister, J.L.; Wilson, S.T.; et al. Global Reconstruction Reduces the Uncertainty of Oceanic Nitrous Oxide Emissions and Reveals a Vigorous Seasonal Cycle. Proc. Natl. Acad. Sci. USA 2020, 117, 11954–11960. [Google Scholar] [CrossRef]
- Pinay, G.; Gascuel, C.; Ménesguen, A.; Souchon, Y.; Le Moal, M.; Levain, A.; Etrillard, C.; Moata, F.; Pannard, A.; Souchu, P. L’Eutrophisation: Manifestations, Causes, Conséquences et Prédictibilité; Synthèse de l’Expertise scientifique collective CNRS-Ifremer-INRA-Irstea: Paris, France, 2017; ISBN 978-2-7592-2757-0. [Google Scholar]
- Miao, Y.; Huang, J.; Duan, H.; Meng, H.; Wang, Z.; Qi, T.; Wu, Q.L. Spatial and Seasonal Variability of Nitrous Oxide in a Large Freshwater Lake in the Lower Reaches of the Yangtze River, China. Sci. Total Environ. 2020, 721, 137716. [Google Scholar] [CrossRef]
- Venkiteswaran, J.J.; Rosamond, M.S.; Schiff, S.L. Nonlinear Response of Riverine N2O Fluxes to Oxygen and Temperature. Environ. Sci. Technol. 2014, 48, 1566–1573. [Google Scholar] [CrossRef]
- Maavara, T.; Lauerwald, R.; Laruelle, G.G.; Akbarzadeh, Z.; Bouskill, N.J.; Van Cappellen, P.; Regnier, P. Nitrous Oxide Emissions from Inland Waters: Are IPCC Estimates Too High? Glob. Chang. Biol. 2019, 25, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Mulholland, P.J.; Helton, A.M.; Poole, G.C.; Hall, R.O.; Hamilton, S.K.; Peterson, B.J.; Tank, J.L.; Ashkenas, L.R.; Cooper, L.W.; Dahm, C.N.; et al. Stream Denitrification across Biomes and Its Response to Anthropogenic Nitrate Loading. Nature 2008, 452, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Marzadri, A.; Amatulli, G.; Tonina, D.; Bellin, A.; Shen, L.Q.; Allen, G.H.; Raymond, P.A. Global Riverine Nitrous Oxide Emissions: The Role of Small Streams and Large Rivers. Sci. Total Environ. 2021, 776, 145148. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhong, J.; Zheng, X.; Fan, C.; Yu, J.; Zhong, W. N2O Fluxes and Rates of Nitrification and Denitrification at the Sediment–Water Interface in Taihu Lake, China. Water 2018, 10, 911. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Wu, Z.; Liang, Z.; Liu, Y.; Wang, Y. Denitrification and the Controlling Factors in Yunnan Plateau Lakes (China): Exploring the Role of Enhanced Internal Nitrogen Cycling by Algal Blooms. J. Environ. Sci. 2019, 76, 349–358. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, X.; Song, K.; Yeerken, S.; Deng, M.; Li, L.; Riya, S.; Wang, Q.; Terada, A. Nonlinear Pattern and Algal Dual-Impact in N2O Emission with Increasing Trophic Levels in Shallow Lakes. Water Res. 2021, 203, e117489. [Google Scholar] [CrossRef]
- Harrison, J.; Matson, P. Patterns and Controls of Nitrous Oxide Emissions from Waters Draining a Subtropical Agricultural Valley. Glob. Biogeochem. Cycles 2003, 17, 1080. [Google Scholar] [CrossRef]
- Xiao, Q.; Xu, X.; Zhang, M.; Duan, H.; Hu, Z.; Wang, W.; Xiao, W.; Lee, X. Coregulation of Nitrous Oxide Emissions by Nitrogen and Temperature in China’s Third Largest Freshwater Lake (Lake Taihu). Limnol. Oceanogr. 2019, 64, 1070–1086. [Google Scholar] [CrossRef]
- Teuma, L. N2O Emissions from Eutrophic Lakes: Sources and Significance; Massey University: Palmerston North, New Zealand, 2022. [Google Scholar]
- Plouviez, M.; Shilton, A.; Packer, M.A.; Guieysse, B. N2O Emissions during Microalgae Outdoor Cultivation in 50 L Column Photobioreactors. Algal Res. 2017, 26, 348–353. [Google Scholar] [CrossRef]
- Paerl, H.W.; Fulton, R.S.; Moisander, P.H.; Dyble, J. Harmful Freshwater Algal Blooms, With an Emphasis on Cyanobacteria. Sci. World J. 2001, 1, 76–113. [Google Scholar] [CrossRef] [Green Version]
- Hale, S.S.; Cicchetti, G.; Deacutis, C.F. Eutrophication and Hypoxia Diminish Ecosystem Functions of Benthic Communities in a New England Estuary. Front. Mar. Sci. 2016, 3, 249. [Google Scholar] [CrossRef] [Green Version]
- Mosley, L.M.; Priestley, S.; Brookes, J.; Dittmann, S.; Farkaš, J.; Farrell, M.; Ferguson, A.J.; Gibbs, M.; Hipsey, M.; Huang, J.; et al. Extreme Eutrophication and Salinisation in the Coorong Estuarine-Lagoon Ecosystem of Australia’s Largest River Basin (Murray-Darling). Mar. Pollut. Bull. 2023, 188, 114648. [Google Scholar] [CrossRef]
- Malone, T.C.; Newton, A. The Globalization of Cultural Eutrophication in the Coastal Ocean: Causes and Consequences. Front. Mar. Sci. 2020, 7, 670. [Google Scholar] [CrossRef]
- Ulloa, M.J.; Álvarez-Torres, P.; Horak-Romo, K.P.; Ortega-Izaguirre, R. Harmful Algal Blooms and Eutrophication along the Mexican Coast of the Gulf of Mexico Large Marine Ecosystem. Environ. Dev. 2017, 22, 120–128. [Google Scholar] [CrossRef]
- Alcántara, C.; Muñoz, R.; Norvill, Z.; Plouviez, M.; Guieysse, B. Nitrous Oxide Emissions from High Rate Algal Ponds Treating Domestic Wastewater. Bioresour. Technol. 2015, 177, 110–117. [Google Scholar] [CrossRef]
- Bauer, S.K.; Grotz, L.S.; Connelly, E.B.; Colosi, L.M. Reevaluation of the Global Warming Impacts of Algae-Derived Biofuels to Account for Possible Contributions of Nitrous Oxide. Bioresour. Technol. 2016, 218, 196–201. [Google Scholar] [CrossRef]
- McLeod, A.R.; Brand, T.; Campbell, C.N.; Davidson, K.; Hatton, A.D. Ultraviolet Radiation Drives Emission of Climate-Relevant Gases from Marine Phytoplankton. J. Geophys. Res. Biogeosci. 2021, 126, e2021JG006345. [Google Scholar] [CrossRef]
- Stief, P.; Kamp, A.; Thamdrup, B.; Glud, R.N. Anaerobic Nitrogen Turnover by Sinking Diatom Aggregates at Varying Ambient Oxygen Levels. Front. Microbiol. 2016, 7, 98. [Google Scholar] [CrossRef] [Green Version]
- Kamp, A.; Stief, P.; Knappe, J.; de Beer, D. Response of the Ubiquitous Pelagic Diatom Thalassiosira weissflogii to Darkness and Anoxia. PLoS ONE 2013, 8, e82605. [Google Scholar] [CrossRef]
- Ferrón, S.; Ho, D.T.; Johnson, Z.I.; Huntley, M.E. Air–Water Fluxes of N2O and CH4 during Microalgae (Staurosira sp.) Cultivation in an Open Raceway Pond. Environ. Sci. Technol. 2012, 46, 10842–10848. [Google Scholar] [CrossRef]
- Cohen, Y.; Gordon, L.I. Nitrous Oxide in the Oxygen Minimum of the Eastern Tropical North Pacific: Evidence for Its Consumption during Denitrification and Possible Mechanisms for Its Production. Deep Sea Res. 1978, 25, 509–524. [Google Scholar] [CrossRef]
- Pierotti, D.; Rasmussen, R.A. Nitrous Oxide Measurements in the Eastern Tropical Pacific Ocean. Tellus 1980, 32, 56–72. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.J.; DeLaune, R.D.; Patrick, W.H. Nitrous Oxide Emission from Gulf Coast Wetlands. Geochim. Cosmochim. Acta 1983, 47, 1805–1814. [Google Scholar] [CrossRef]
- Oudot, C.; Andrie, C.; Montel, Y. Nitrous Oxide Production in the Tropical Atlantic Ocean. Deep-Sea Res. 1990, 37, 183–202. [Google Scholar] [CrossRef]
- Mengis, M.; Gächter, R.; Wehrli, B. Sources and Sinks of Nitrous Oxide (N2O) in Deep Lakes. Biogeochemistry 1997, 38, 281–301. [Google Scholar] [CrossRef]
- Morell, J.M.; Capella, J.; Mercado, A.; Bauzá, J.; Corredor, J.E. Nitrous Oxide Fluxes in Caribbean and Tropical Atlantic Waters: Evidence for near Surface Production. Mar. Chem. 2001, 74, 131–143. [Google Scholar] [CrossRef]
- Wang, H.; Wang, W.; Yin, C.; Wang, Y.; Lu, J. Littoral Zones as the “Hotspots” of Nitrous Oxide (N2O) Emission in a Hyper-Eutrophic Lake in China. Atmos. Environ. 2006, 40, 5522–5527. [Google Scholar] [CrossRef]
- McCrackin, M.L.; Elser, J.J. Greenhouse Gas Dynamics in Lakes Receiving Atmospheric Nitrogen Deposition. Glob. Biogeochem. Cycles 2011, 25, GB4005. [Google Scholar] [CrossRef] [Green Version]
- Diaz, R.J. Anoxia, Hypoxia, And Dead Zones. In Encyclopedia of Estuaries; Diaz, R.J., Ed.; Encyclopedia of Earth Sciences Series; Springer: Dordrecht, The Netherlands, 2016; pp. 19–26. ISBN 978-94-017-8800-7. [Google Scholar]
- Sakihama, Y.; Nakamura, S.; Yamasaki, H. Nitric Oxide Production Mediated by Nitrate Reductase in the Green Alga Chlamydomonas reinhardtii: An Alternative NO Production Pathway in Photosynthetic Organisms. Plant Cell Physiol. 2002, 43, 290–297. [Google Scholar] [CrossRef]
- Sanz-Luque, E.; Chamizo-Ampudia, A.; Llamas, A.; Galvan, A.; Fernandez, E. Understanding Nitrate Assimilation and Its Regulation in Microalgae. Front. Plant Sci. 2015, 6, 899. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Castellano, I.; Patti, F.P.; Palumbo, A.; Buia, M.C. Nitric Oxide in Marine Photosynthetic Organisms. Nitric Oxide 2015, 47, 34–39. [Google Scholar] [CrossRef]
- Chaux, F.; Burlacot, A.; Mekhalfi, M.; Auroy, P.; Blangy, S.; Richaud, P.; Peltier, G. Flavodiiron Proteins Promote Fast and Transient O2 Photoreduction in Chlamydomonas. Plant Physiol. 2017, 174, 1825–1836. [Google Scholar] [CrossRef] [Green Version]
- Almeida, C.C.; Romão, C.V.; Lindley, P.F.; Teixeira, M.; Saraiva, L.M. The Role of the Hybrid Cluster Protein in Oxidative Stress Defense. J. Biol. Chem. 2006, 281, 32445–32450. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Vine, C.E.; Balasiny, B.K.; Rizk, J.; Bradley, C.L.; Tinajero-Trejo, M.; Poole, R.K.; Bergaust, L.L.; Bakken, L.R.; Cole, J.A. The Roles of the Hybrid Cluster Protein, Hcp and Its Reductase, Hcr, in High Affinity Nitric Oxide Reduction That Protects Anaerobic Cultures of Escherichia coli against Nitrosative Stress: NO Reduction by the E. coli Hybrid Cluster Protein. Mol. Microbiol. 2016, 100, 877–892. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-W.; Fushinobu, S.; Zhou, S.; Wakagi, T.; Shoun, H. Eukaryotic NirK Genes Encoding Copper-Containing Nitrite Reductase: Originating from the Protomitochondrion? Appl. Environ. Microbiol. 2009, 75, 2652–2658. [Google Scholar] [CrossRef] [Green Version]
- Mori, T.; Ohta, S.; Ishizuka, S.; Konda, R.; Wicaksono, A.; Heriyanto, J.; Hardjono, A. Effects of Phosphorus Addition on N2O and NO Emissions from Soils of an Acacia mangium Plantation. Soil Sci. Plant Nutr. 2010, 56, 782–788. [Google Scholar] [CrossRef] [Green Version]
- Mehnaz, K.R.; Dijkstra, F.A. Denitrification and Associated N2O Emissions Are Limited by Phosphorus Availability in a Grassland Soil. Geoderma 2016, 284, 34–41. [Google Scholar] [CrossRef]
- Shen, Y.; Zhu, B. Effects of Nitrogen and Phosphorus Enrichment on Soil N2O Emission from Natural Ecosystems: A Global Meta-Analysis. Environ. Pollut. 2022, 301, 118993. [Google Scholar] [CrossRef]
- Gebremichael, A.W.; Wall, D.P.; O’Neill, R.M.; Krol, D.J.; Brennan, F.; Lanigan, G.; Richards, K.G. Effect of Contrasting Phosphorus Levels on Nitrous Oxide and Carbon Dioxide Emissions from Temperate Grassland Soils. Sci. Rep. 2022, 12, 2602. [Google Scholar] [CrossRef]
- Kalff, J. Phosphorus Limitation in Some Tropical African Lakes. Hydrobiologia 1983, 100, 101–112. [Google Scholar] [CrossRef]
- Nalewajko, C.; Murphy, T.P. Effects of Temperature, and Availability of Nitrogen and Phosphorus on the Abundance of Anabaena and Microcystis in Lake Biwa, Japan: An Experimental Approach. Limnology 2001, 2, 45–48. [Google Scholar] [CrossRef]
- Elser, J.J.; Bracken, M.E.S.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global Analysis of Nitrogen and Phosphorus Limitation of Primary Producers in Freshwater, Marine and Terrestrial Ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abell, J.M.; Özkundakci, D.; Hamilton, D.P. Nitrogen and Phosphorus Limitation of Phytoplankton Growth in New Zealand Lakes: Implications for Eutrophication Control. Ecosystems 2010, 13, 966–977. [Google Scholar] [CrossRef] [Green Version]
- Paerl, H.W.; Scott, J.T.; McCarthy, M.J.; Newell, S.E.; Gardner, W.S.; Havens, K.E.; Hoffman, D.K.; Wilhelm, S.W.; Wurtsbaugh, W.A. It Takes Two to Tango: When and Where Dual Nutrient (N&P) Reductions Are Needed to Protect Lakes and Downstream Ecosystems. Environ. Sci. Technol. 2016, 50, 10805–10813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smolders, A.J.P.; Lamers, L.P.M.; Lucassen, E.C.H.E.T.; Van Der Velde, G.; Roelofs, J.G.M. Internal Eutrophication: How It Works and What to Do about It—A Review. Chem. Ecol. 2006, 22, 93–111. [Google Scholar] [CrossRef]
- Paerl, H.W.; Havens, K.E.; Xu, H.; Zhu, G.; McCarthy, M.J.; Newell, S.E.; Scott, J.T.; Hall, N.S.; Otten, T.G.; Qin, B. Mitigating Eutrophication and Toxic Cyanobacterial Blooms in Large Lakes: The Evolution of a Dual Nutrient (N and P) Reduction Paradigm. Hydrobiologia 2020, 847, 4359–4375. [Google Scholar] [CrossRef]
- Glibert, P.M. Eutrophication, Harmful Algae and Biodiversity—Challenging Paradigms in a World of Complex Nutrient Changes. Mar. Pollut. Bull. 2017, 124, 591–606. [Google Scholar] [CrossRef]
- Andersen, I.M.; Williamson, T.J.; González, M.J.; Vanni, M.J. Nitrate, Ammonium, and Phosphorus Drive Seasonal Nutrient Limitation of Chlorophytes, Cyanobacteria, and Diatoms in a Hyper-eutrophic Reservoir. Limnol. Oceanogr. 2020, 65, 962–978. [Google Scholar] [CrossRef]
- Brookfield, A.E.; Hansen, A.T.; Sullivan, P.L.; Czuba, J.A.; Kirk, M.F.; Li, L.; Newcomer, M.E.; Wilkinson, G. Predicting Algal Blooms: Are We Overlooking Groundwater? Sci. Total Environ. 2021, 769, 144442. [Google Scholar] [CrossRef]
- Glibert, P.M.; Maranger, R.; Sobota, D.J.; Bouwman, L. The Haber Bosch–Harmful Algal Bloom (HB–HAB) Link. Environ. Res. Lett. 2014, 9, 105001. [Google Scholar] [CrossRef] [Green Version]
- Esteves, S.M.; Jadoul, A.; Iacono, F.; Schloesser, M.; Bosman, B.; Carnol, M.; Druet, T.; Cardol, P.; Hanikenne, M. Natural Variation of Nutrient Homeostasis among Laboratory and Field Strains of Chlamydomonas reinhardtii. J. Exp. Bot. 2023, 1, erad194. [Google Scholar] [CrossRef]
- Zhang, X.; Li, B.; Xu, H.; Wells, M.; Tefsen, B.; Qin, B. Effect of Micronutrients on Algae in Different Regions of Taihu, a Large, Spatially Diverse, Hypereutrophic Lake. Water Res. 2019, 151, 500–514. [Google Scholar] [CrossRef]
- Hassler, C.S.; Sinoir, M.; Clementson, L.A.; Butler, E.C.V. Exploring the Link between Micronutrients and Phytoplankton in the Southern Ocean during the 2007 Austral Summer. Front. Microbiol. 2012, 3, 202. [Google Scholar] [CrossRef] [Green Version]
- Ito, A.; Ye, Y.; Baldo, C.; Shi, Z. Ocean Fertilization by Pyrogenic Aerosol Iron. Npj Clim. Atmos. Sci. 2021, 4, 30. [Google Scholar] [CrossRef]
- Black, A.; Hsu, P.L.; Hamonts, K.E.; Clough, T.J.; Condron, L.M. Influence of Copper on Expression of NirS, NorB and NosZ and the Transcription and Activity of NIR, NOR and N2 or in the Denitrifying Soil Bacteria Pseudomonas stutzeri. Microb. Biotechnol. 2016, 9, 381–388. [Google Scholar] [CrossRef] [Green Version]
- Cirri, E.; Pohnert, G. Algae−bacteria Interactions That Balance the Planktonic Microbiome. New Phytol. 2019, 223, 100–106. [Google Scholar] [CrossRef] [Green Version]
- Calatrava, V.; Tejada-Jimenez, M.; Sanz-Luque, E.; Fernandez, E.; Galvan, A.; Llamas, A. Chlamydomonas reinhardtii, a Reference Organism to Study Algal–Microbial Interactions: Why Can’t They Be Friends? Plants 2023, 12, 788. [Google Scholar] [CrossRef]
- Arévalo-Martínez, D.L.; Kock, A.; Löscher, C.R.; Schmitz, R.A.; Bange, H.W. Massive Nitrous Oxide Emissions from the Tropical South Pacific Ocean. Nat. Geosci. 2015, 8, 530–533. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teuma, L.; Sanz-Luque, E.; Guieysse, B.; Plouviez, M. Are Microalgae New Players in Nitrous Oxide Emissions from Eutrophic Aquatic Environments? Phycology 2023, 3, 356-367. https://doi.org/10.3390/phycology3030023
Teuma L, Sanz-Luque E, Guieysse B, Plouviez M. Are Microalgae New Players in Nitrous Oxide Emissions from Eutrophic Aquatic Environments? Phycology. 2023; 3(3):356-367. https://doi.org/10.3390/phycology3030023
Chicago/Turabian StyleTeuma, Laura, Emanuel Sanz-Luque, Benoit Guieysse, and Maxence Plouviez. 2023. "Are Microalgae New Players in Nitrous Oxide Emissions from Eutrophic Aquatic Environments?" Phycology 3, no. 3: 356-367. https://doi.org/10.3390/phycology3030023
APA StyleTeuma, L., Sanz-Luque, E., Guieysse, B., & Plouviez, M. (2023). Are Microalgae New Players in Nitrous Oxide Emissions from Eutrophic Aquatic Environments? Phycology, 3(3), 356-367. https://doi.org/10.3390/phycology3030023






