Cultivation of Microalgae Chlorella vulgaris in Open Reactor for Bioethanol Production
Abstract
1. Introduction
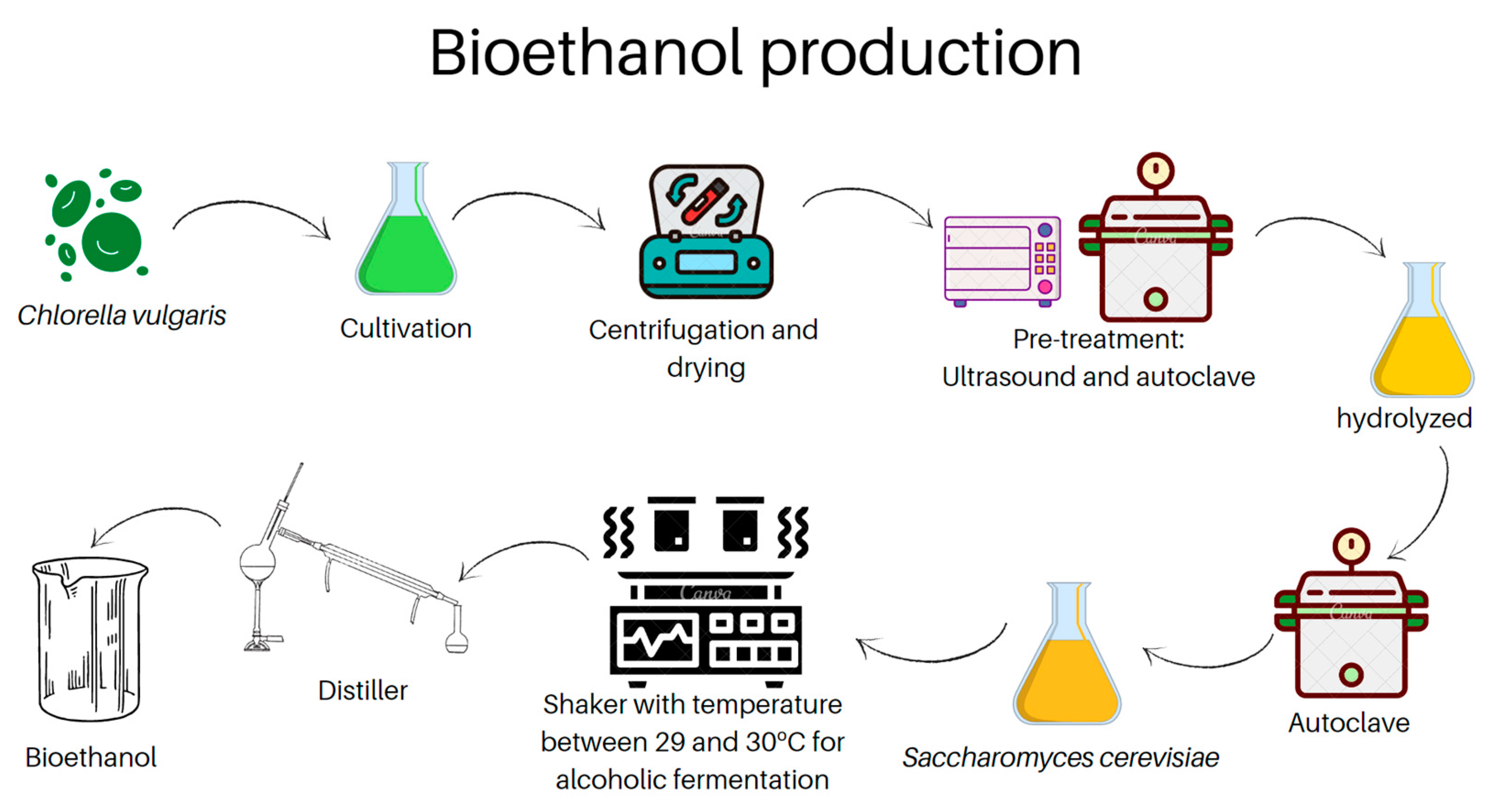
2. Materials and Methods
2.1. Microalgae Chlorella Vulgaris: Cultivation and Culture Medium
2.2. Alcoholic Fermentation
2.3. Carbohydrate Extraction (Pre-Treatment)
2.4. Bioethanol Characterization
2.5. Simple Distillation for Obtaining Bioethanol
3. Results
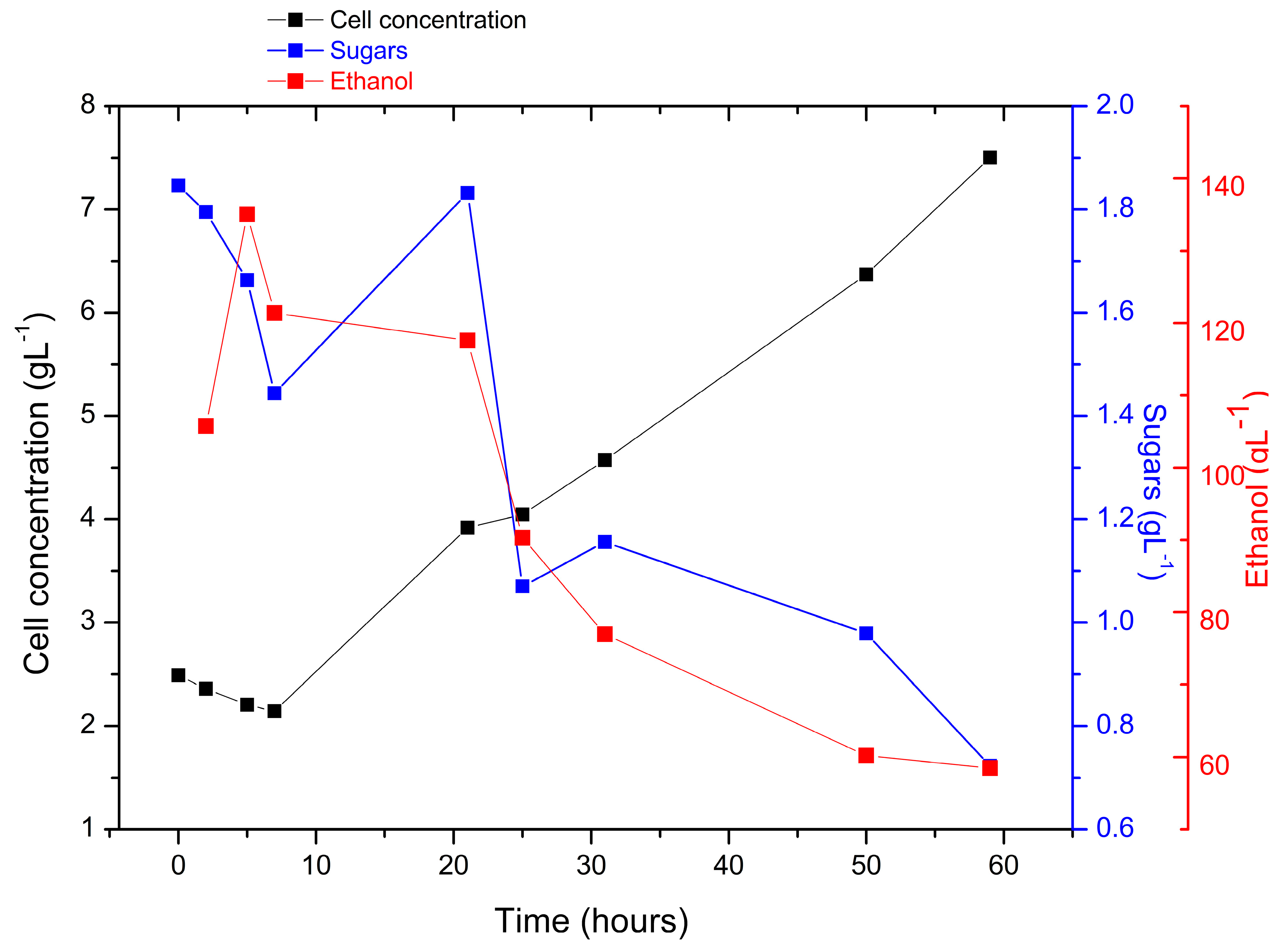
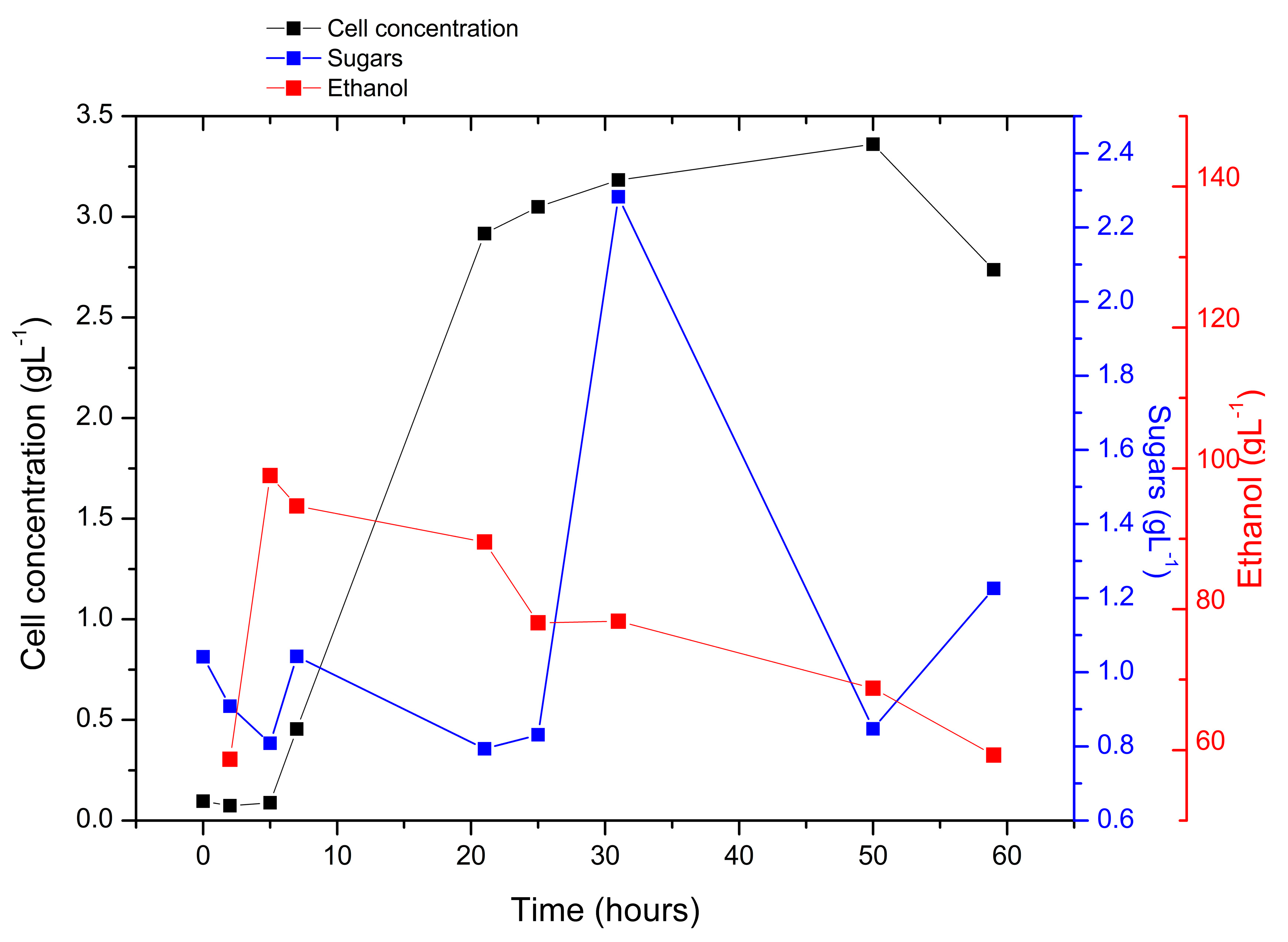
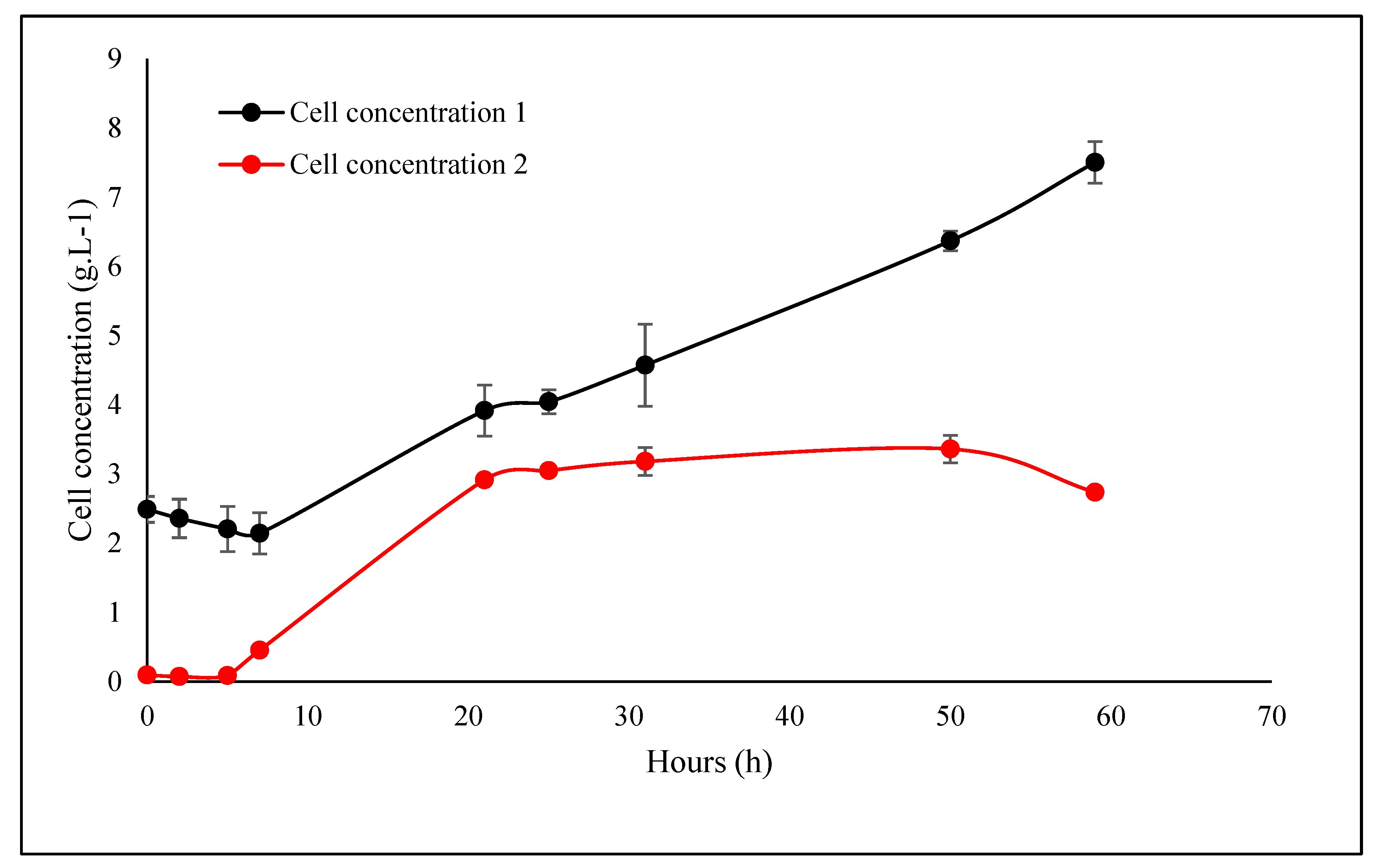
3.1. Biomass Yield
3.2. Pre-Treatment Evaluation
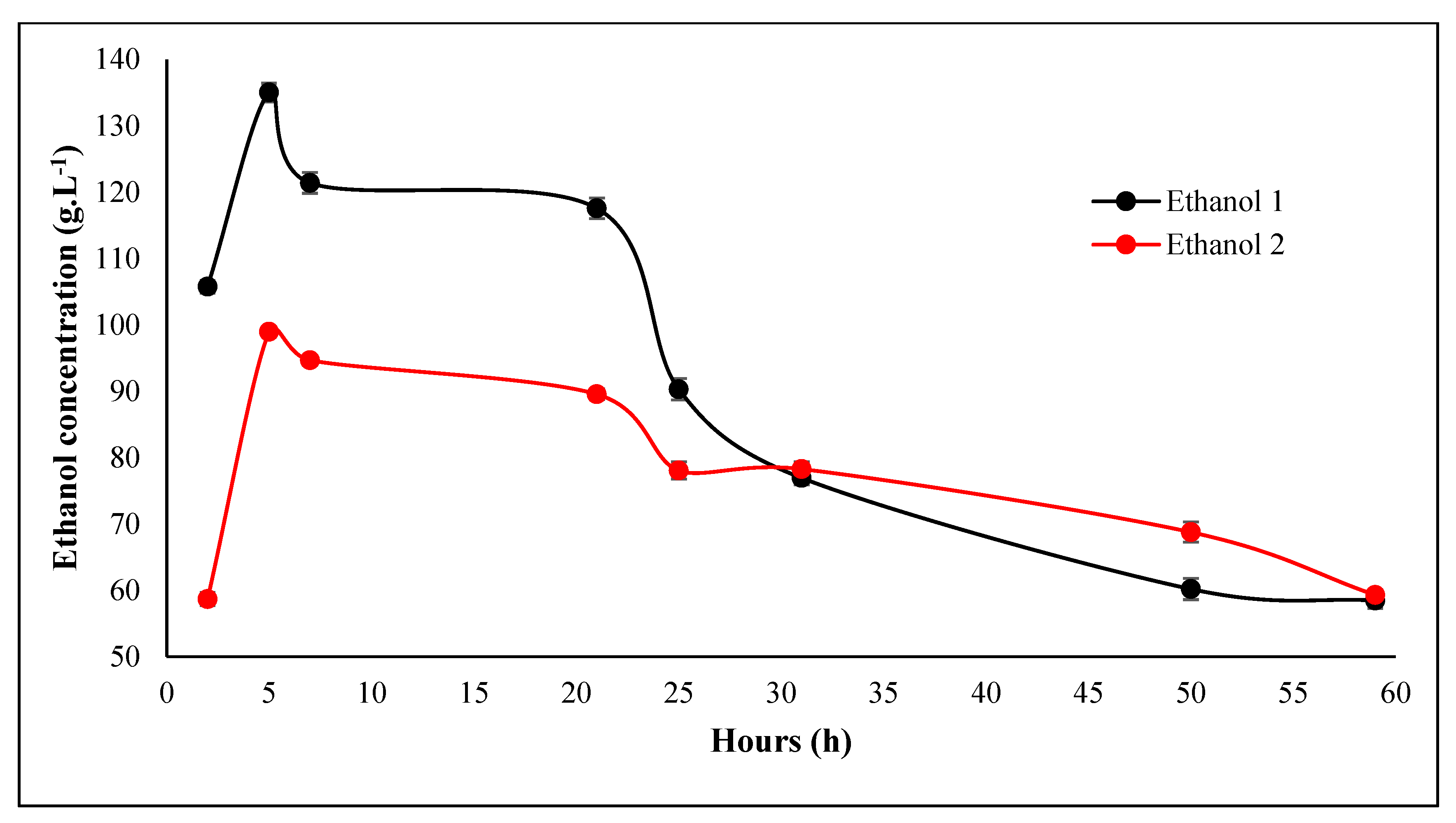
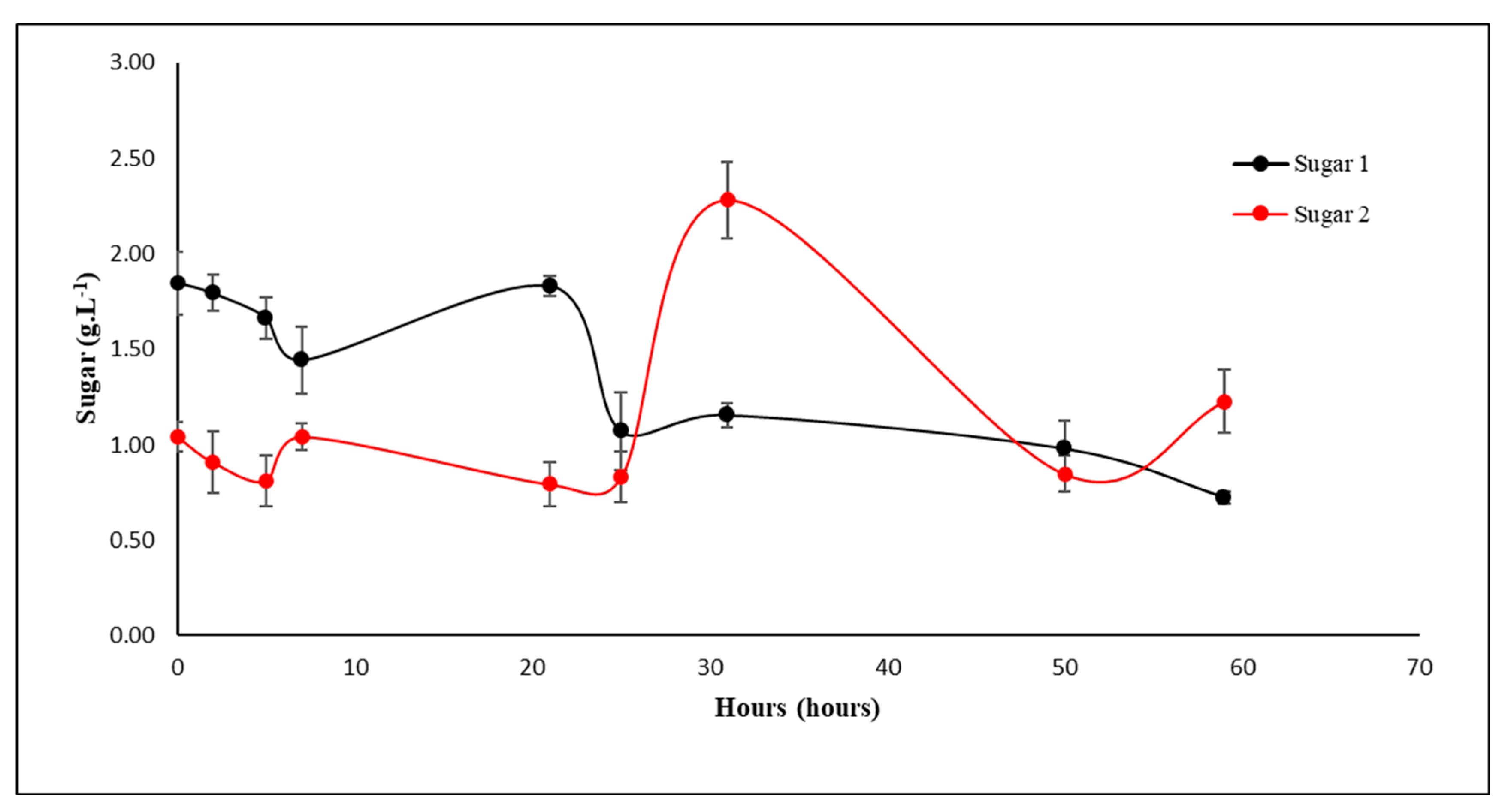
3.3. Fermentation of the Hydrolyzate
3.4. Alcoholic Content
4. Discussion
4.1. Biomass Yield
4.2. Pre-Treatment Evaluation
4.3. Fermentation of the Hydrolysate
4.4. Alcohol Content
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pérez, L.; Salgueiro, J.L.; González, J.; Parralejo, A.I.; Maceiras, R.; Cancela, Á. Scaled up from indoor to outdoor cultures of Chaetoceros gracilis and Skeletonema costatum microalgae for biomass and oil production. Biochem. Eng. J. 2017, 127, 180–187. [Google Scholar] [CrossRef]
- Gui, L.; Xu, L.; Liu, Z.-y.; Zhou, Z.-g.; Sun, Z. Carotenoid-rich microalgae promote growth and health conditions of Artemia nauplii. Aquaculture 2022, 546, 737289. [Google Scholar] [CrossRef]
- Mota, G.F.; de Sousa, I.G.; de Oliveira, A.L.B.; Cavalcante, A.L.G.; da Silva Moreira, K.; Cavalcante, F.T.T.; da Silva Souza, J.E.; de Aguiar Falcão, Í.R.; Rocha, T.G.; Valério, R.B.R. Biodiesel production from microalgae using lipase-based catalysts: Current challenges and prospects. Algal Res. 2022, 62, 102616. [Google Scholar] [CrossRef]
- Maia, J.L.d.; Cardoso, J.S.; da Silveira Mastrantonio, D.J.; Bierhals, C.K.; Moreira, J.B.; Costa, J.A.V.; de Morais, M.G. Microalgae starch: A promising raw material for the bioethanol production. Int. J. Biol. Macromol. 2020, 165, 2739–2749. [Google Scholar] [CrossRef]
- Kim, K.H.; Choi, I.S.; Kim, H.M.; Wi, S.G.; Bae, H.-J. Bioethanol production from the nutrient stress-induced microalga Chlorella vulgaris by enzymatic hydrolysis and immobilized yeast fermentation. Bioresour. Technol. 2014, 153, 47–54. [Google Scholar] [CrossRef]
- Koyande, A.K.; Show, P.-L.; Guo, R.; Tang, B.; Ogino, C.; Chang, J.-S. Bio-processing of algal bio-refinery: A review on current advances and future perspectives. Bioengineered 2019, 10, 574–592. [Google Scholar] [CrossRef]
- Rétfalvi, T.; Szabó, P.; Hájos, A.-T.; Albert, L.; Kovács, A.; Milics, G.; Neményi, M.; Lakatos, E.; Ördög, V. Effect of co-substrate feeding on methane yield of anaerobic digestion of Chlorella vulgaris. J. Appl. Phycol. 2016, 28, 2741–2752. [Google Scholar] [CrossRef]
- Khan, S.; Naushad, M.; Iqbal, J.; Bathula, C.; Sharma, G. Production and harvesting of microalgae and an efficient operational approach to biofuel production for a sustainable environment. Fuel 2022, 311, 122543. [Google Scholar] [CrossRef]
- Ray, A.; Nayak, M.; Ghosh, A. A review on co-culturing of microalgae: A greener strategy towards sustainable biofuels production. Sci. Total Environ. 2022, 802, 149765. [Google Scholar] [CrossRef]
- Khoshkho, S.M.; Mahdavian, M.; Karimi, F.; Karimi-Maleh, H.; Razaghi, P. Production of bioethanol from carrot pulp in the presence of Saccharomyces cerevisiae and beet molasses inoculum; a biomass based investigation. Chemosphere 2022, 286, 131688. [Google Scholar] [CrossRef]
- Dempfle, D.; Kröcher, O.; Studer, M.H.-P. Techno-economic assessment of bioethanol production from lignocellulose by consortium-based consolidated bioprocessing at industrial scale. New Biotechnol. 2021, 65, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Silvello, M.A.d.C.; Gonçalves, I.S.; Azambuja, S.P.H.; Costa, S.S.; Silva, P.G.P.; Santos, L.O.; Goldbeck, R. Microalgae-based carbohydrates: A green innovative source of bioenergy. Bioresour. Technol. 2022, 344, 126304. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Zhao, X.-Q.; Yen, H.-W.; Ho, S.-H.; Cheng, C.-L.; Lee, D.-J.; Bai, F.-W.; Chang, J.-S. Microalgae-based carbohydrates for biofuel production. Biochem. Eng. J. 2013, 78, 1–10. [Google Scholar] [CrossRef]
- Acebu, P.I.G.; de Luna, M.D.G.; Chen, C.-Y.; Abarca, R.R.M.; Chen, J.-H.; Chang, J.-S. Bioethanol production from Chlorella vulgaris ESP-31 grown in unsterilized swine wastewater. Bioresour. Technol. 2022, 352, 127086. [Google Scholar] [CrossRef]
- Pancha, I.; Chokshi, K.; Ghosh, T.; Paliwal, C.; Maurya, R.; Mishra, S. Bicarbonate supplementation enhanced biofuel production potential as well as nutritional stress mitigation in the microalgae Scenedesmus sp. CCNM 1077. Bioresour. Technol. 2015, 193, 315–323. [Google Scholar] [CrossRef]
- Singh, J.; Saxena, R. Handbook of marine microalgae. In Biotechnology Advances; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Crawford, B.; Kasmidi, M.; Korompis, F.; Pollnac, R.B. Factors influencing progress in establishing community-based marine protected areas in Indonesia. Coast. Manag. 2006, 34, 39–64. [Google Scholar] [CrossRef]
- Jacob-Lopes, E.; Maroneze, M.M.; Queiroz, M.I.; Zepka, L.Q. Handbook of Microalgae-Based Processes and Products: Fundamentals and Advances in Energy, Food, Feed, Fertilizer, and Bioactive Compounds; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology 1979, 111, 1–61. [Google Scholar] [CrossRef]
- Raillani, B.; Mezrhab, A.; Amraqui, S.; Moussaoui, M.A.; Mezrhab, A. Regression-based spatial GIS analysis for an accurate assessment of renewable energy potential. Energy Sustain. Dev. 2022, 69, 118–133. [Google Scholar] [CrossRef]
- Miranda, J.; Passarinho, P.C.; Gouveia, L. Pre-treatment optimization of Scenedesmus obliquus microalga for bioethanol production. Bioresour. Technol. 2012, 104, 342–348. [Google Scholar] [CrossRef]
- Ferreira, A.F.; Dias, A.P.S.; Silva, C.M.; Costa, M. Effect of low frequency ultrasound on microalgae solvent extraction: Analysis of products, energy consumption and emissions. Algal Res. 2016, 14, 9–16. [Google Scholar] [CrossRef]
- Lee, S.; Oh, Y.; Kim, D.; Kwon, D.; Lee, C.; Lee, J. Converting carbohydrates extracted from marine algae into ethanol using various ethanolic Escherichia coli strains. Appl. Biochem. Biotechnol. 2011, 164, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Cabral, M.B. Caracterização Físico-Química de Aguardentes de Cana-de-Açúcar Produzidas na Região do Brejo Paraibano; Universidade Federal de Campina Grande: Campina Grande, Brazil, 2019. [Google Scholar]
- Miller, G. Modified DNS method for reducing sugars. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Sakarika, M.; Kornaros, M. Chlorella vulgaris as a green biofuel factory: Comparison between biodiesel, biogas and combustible biomass production. Bioresour. Technol. 2019, 273, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Ngamsirisomsakul, M.; Reungsang, A.; Liao, Q.; Kongkeitkajorn, M.B. Enhanced bio-ethanol production from Chlorella sp. biomass by hydrothermal pretreatment and enzymatic hydrolysis. Renew. Energy 2019, 141, 482–492. [Google Scholar] [CrossRef]
- Sakarika, M.; Kornaros, M. Effect of pH on growth and lipid accumulation kinetics of the microalga Chlorella vulgaris grown heterotrophically under sulfur limitation. Bioresour. Technol. 2016, 219, 694–701. [Google Scholar] [CrossRef]

| Sample | Concentration Sulfuric Acid (%) | °Brix | ART (g·L−1) | ||
|---|---|---|---|---|---|
| Before | After | Before | After | ||
| 1 | 1.5 | 3 | 15.5 | 0.69 | 1.80 |
| 2 | 3 | 3 | 9 | 0.51 | 1.01 |
| Sample | Alcohol Concentration (g·L−1) | Time (h) | Ethanol Content (°GL) |
|---|---|---|---|
| 1 | 136.60 | 5 | 68 |
| 2 | 98.17 | 5 | 49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, G.; Cerqueira, K.; Rodrigues, J.; Silva, K.; Coelho, D.; Souza, R. Cultivation of Microalgae Chlorella vulgaris in Open Reactor for Bioethanol Production. Phycology 2023, 3, 325-336. https://doi.org/10.3390/phycology3020021
Silva G, Cerqueira K, Rodrigues J, Silva K, Coelho D, Souza R. Cultivation of Microalgae Chlorella vulgaris in Open Reactor for Bioethanol Production. Phycology. 2023; 3(2):325-336. https://doi.org/10.3390/phycology3020021
Chicago/Turabian StyleSilva, Graziella, Keilla Cerqueira, Jacqueline Rodrigues, Karollyna Silva, Diego Coelho, and Roberto Souza. 2023. "Cultivation of Microalgae Chlorella vulgaris in Open Reactor for Bioethanol Production" Phycology 3, no. 2: 325-336. https://doi.org/10.3390/phycology3020021
APA StyleSilva, G., Cerqueira, K., Rodrigues, J., Silva, K., Coelho, D., & Souza, R. (2023). Cultivation of Microalgae Chlorella vulgaris in Open Reactor for Bioethanol Production. Phycology, 3(2), 325-336. https://doi.org/10.3390/phycology3020021






