Growth of the Macroalgae Ulva lactuca Cultivated at Different Depths in a Biofloc Integrated System with Shrimp and Fish
Abstract
1. Introduction
2. Materials and Methods
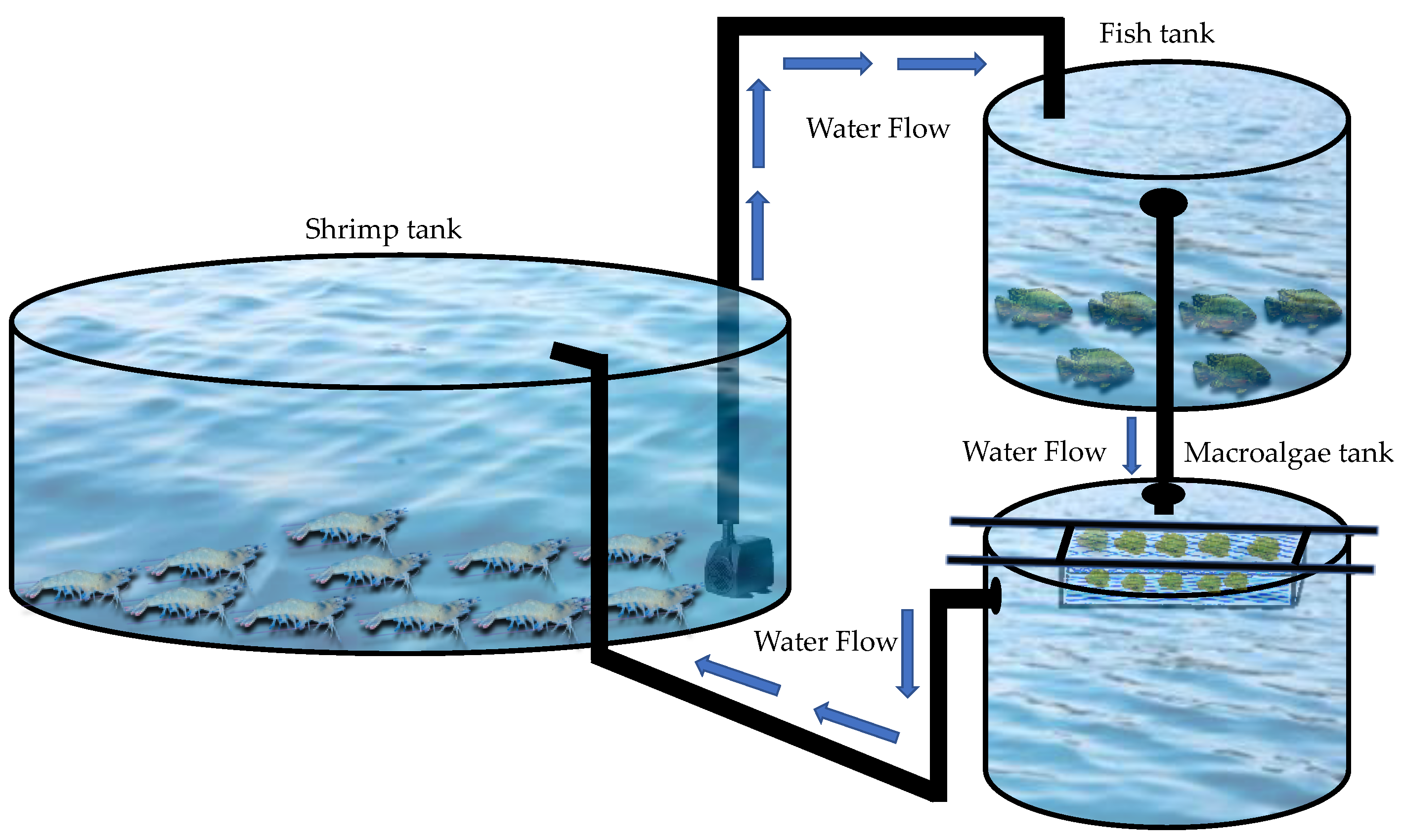
2.1. Experimental Design and Facilities
2.2. Physical and Chemical Parameters
2.3. Performance of Macroalgae
2.4. Shrimp and Fish Performance
- Final average weight (g): final biomass of live animals (g)/total number of animals;
- Specific growth rate (g week−1): weight gain (g)/number of weeks;
- Final biomass yield (g): sum of final weight of all live animals (g);
- Feed conversion rate (FCR) = feed offered (g)/(final biomass (g) − initial biomass (g));
- Productivity (kg m−3): [(final biomass (kg) − initial biomass (kg)) × 100]/tank volume (m−3).
- Survival (%) = (final number of animals/initial number of animals) × 100;
2.5. Statistical Analysis
3. Results
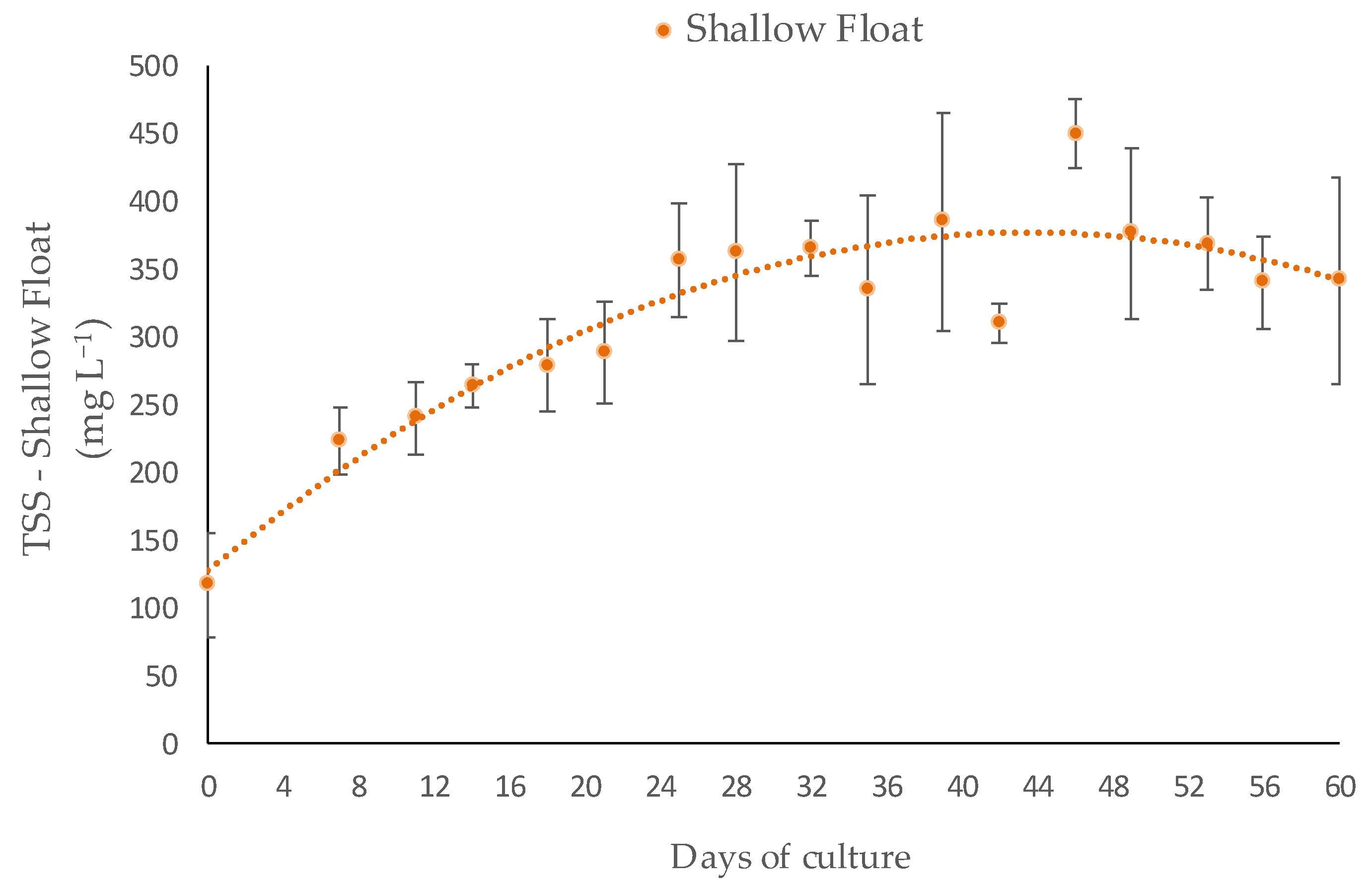
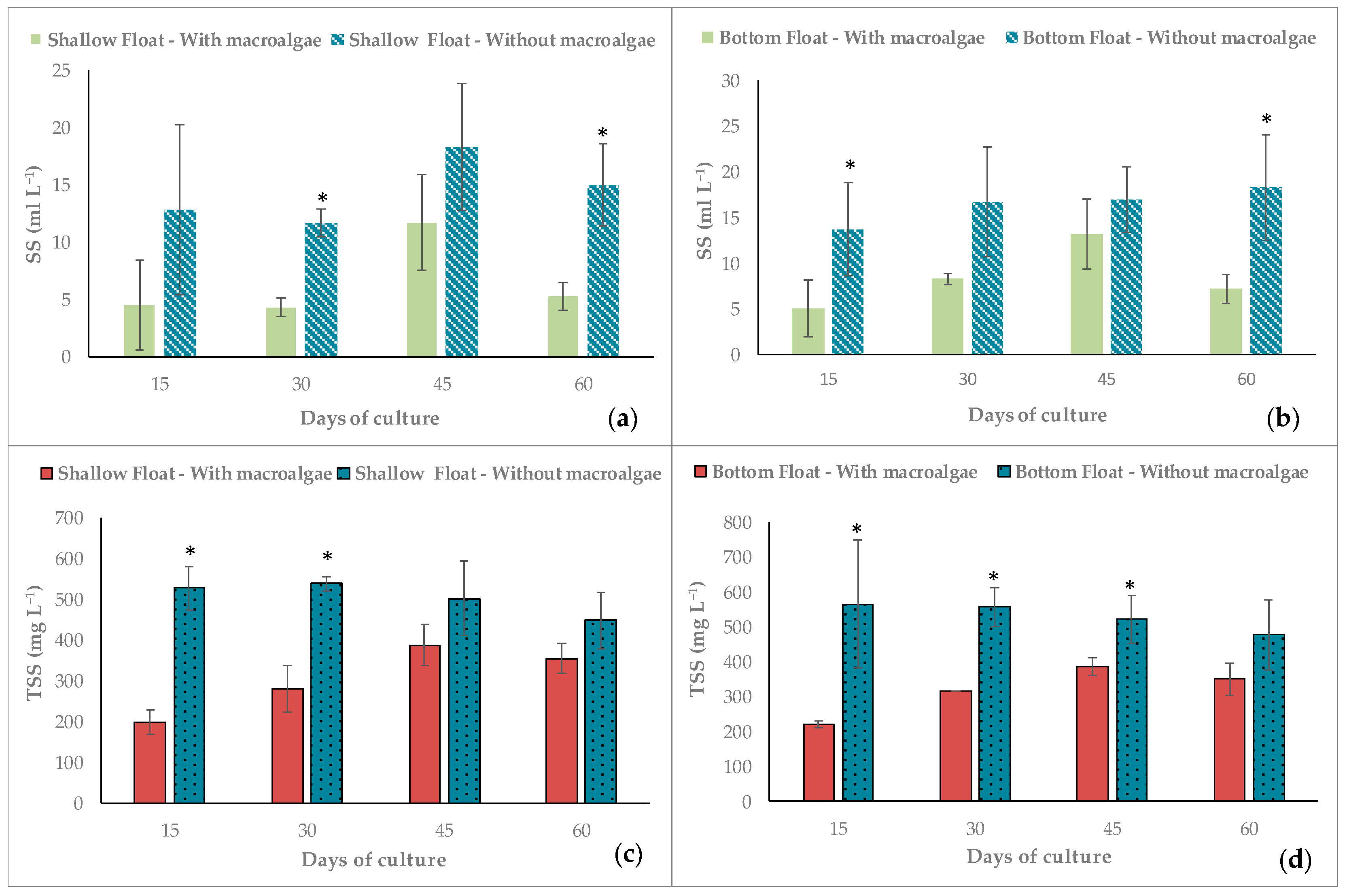
3.1. Water Quality
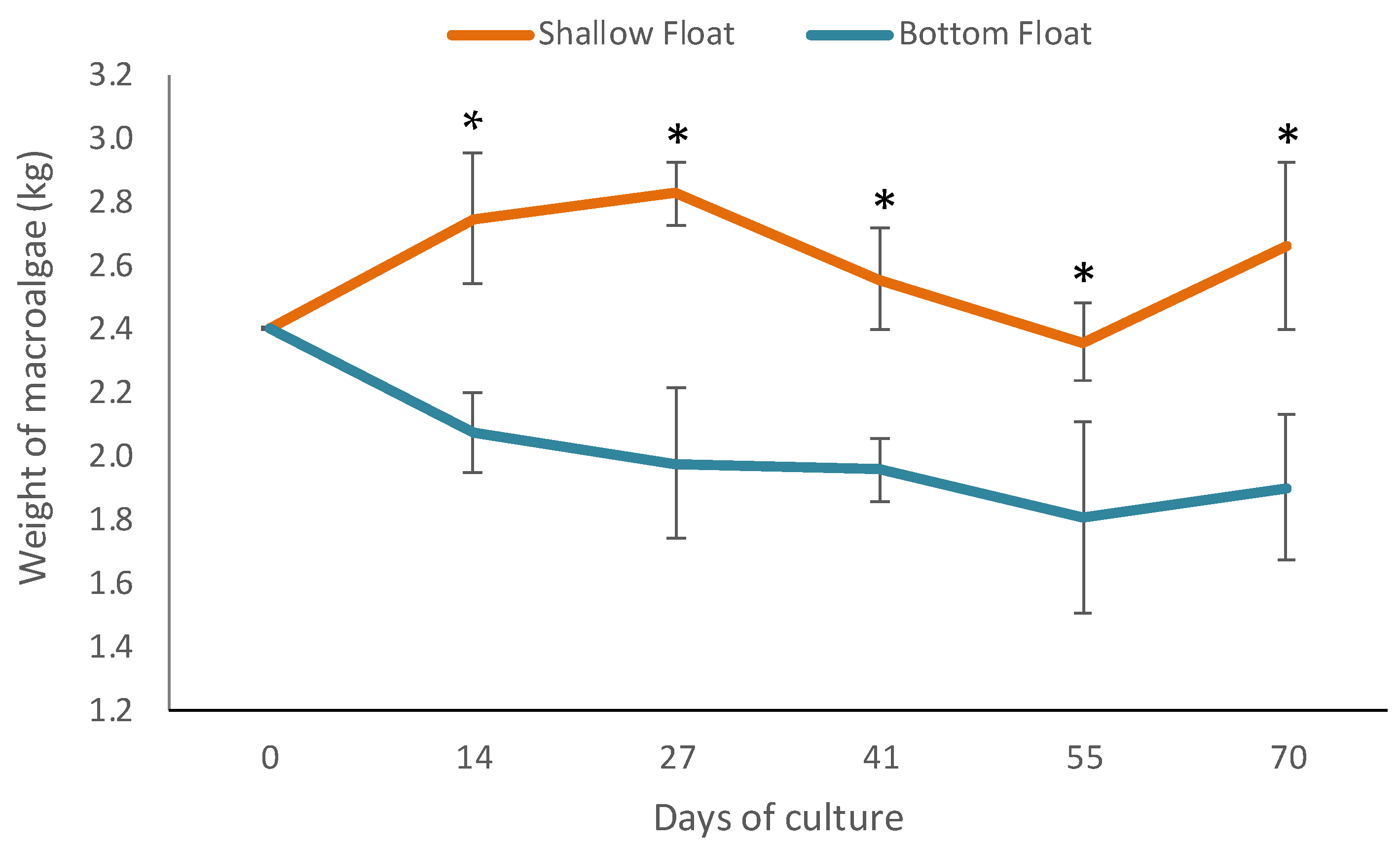
3.2. Performance of Macroalgae
3.3. Shrimp Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of the World Fisheries and Aquaculture—2022 (SOFIA); FAO: Rome, Italy, 2022; ISBN 9789251072257. [Google Scholar]
- Francavilla, M.; Pineda, A.; Lin, C.S.K.; Franchi, M.; Trotta, P.; Romero, A.A.; Luque, R. Natural Porous Agar Materials from Macroalgae. Carbohydr. Polym. 2013, 92, 1555–1560. [Google Scholar] [CrossRef] [PubMed]
- Poli, M.A.; Legarda, E.C.; de Lorenzo, M.A.; Pinheiro, I.; Martins, M.A.; Seiffert, W.Q.; do Nascimento Vieira, F. Integrated Multitrophic Aquaculture Applied to Shrimp Rearing in a Biofloc System. Aquaculture 2019, 511, 734274. [Google Scholar] [CrossRef]
- Da Silva, K.R.; Wasielesky, W.; Abreu, P.C. Nitrogen and Phosphorus Dynamics in the Biofloc Production of the Pacific White Shrimp, Litopenaeus vannamei. J. World Aquac. Soc. 2013, 44, 30–41. [Google Scholar] [CrossRef]
- Chopin, T. Marine Aquaculture in Canada: Well-Established Monocultures of Finfish and Shellfish and an Emerging Integrated Multi-Trophic Aquaculture (IMTA) Approach Including Seaweeds, Other Invertebrates, and Microbial Communities. Fisheries 2015, 40, 28–31. [Google Scholar] [CrossRef]
- Troell, M.; Joyce, A.; Chopin, T.; Neori, A.; Buschmann, A.H.; Fang, J.G. Ecological Engineering in Aquaculture—Potential for Integrated Multi-Trophic Aquaculture (IMTA) in Marine Offshore Systems. Aquaculture 2009, 297, 1–9. [Google Scholar] [CrossRef]
- Chopin, T.; Buschmann, A.H.; Halling, C.; Troell, M.; Kautsky, N.; Neori, A.; Kraemer, G.P.; Zertuche-González, J.A.; Yarish, C.; Neefus, C. Integrating Seaweeds into Marine Aquaculture Systems: A Key toward Sustainability. J. Phycol. 2001, 37, 975–986. [Google Scholar] [CrossRef]
- Nardelli, A.E.; Chiozzini, V.G.; Braga, E.S.; Chow, F. Integrated Multi-Trophic Farming System between the Green Seaweed Ulva lactuca, Mussel, and Fish: A Production and Bioremediation Solution. J. Appl. Phycol. 2019, 31, 847–856. [Google Scholar] [CrossRef]
- Turan, G.; Tekogul, H. The Turkish Mezzes Formulated with Protein-Rich Green Sea Vegetable (Chlorophyta), Ulva rigida, Cultured in Onshore Tank System. J. Aquat. Food Prod. Technol. 2014, 23, 447–452. [Google Scholar] [CrossRef]
- Wasielesky, W.; Krummenauer, D.; Lara, G.; Fóes, G. Cultivo de Camarões Em Sistema de Bioflocos: Realidades e Perspectivas; Revista ABCC: Natal, Brazil, 2013; pp. 30–36. [Google Scholar]
- Ebeling, J.M.; Timmons, M.B.; Bisogni, J.J. Engineering Analysis of the Stoichiometry of Photoautotrophic, Autotrophic, and Heterotrophic Removal of Ammonia-Nitrogen in Aquaculture Systems. Aquaculture 2006, 257, 346–358. [Google Scholar] [CrossRef]
- Furtado, P.S.; Campos, B.R.; Serra, F.P.; Klosterhoff, M.; Romano, L.A.; Wasielesky, W. Effects of Nitrate Toxicity in the Pacific White Shrimp, Litopenaeus vannamei, Reared with Biofloc Technology (BFT). Aquac. Int. 2015, 23, 315–327. [Google Scholar] [CrossRef]
- Gaona, C.A.P.; de Almeida, M.S.; Viau, V.; Poersch, L.H.; Wasielesky, W. Effect of Different Total Suspended Solids Levels on a Litopenaeus vannamei (Boone, 1931) BFT Culture System during Biofloc Formation. Aquac. Res. 2017, 48, 1070–1079. [Google Scholar] [CrossRef]
- Holanda, M.; Santana, G.; Furtado, P.; Rodrigues, R.V.; Cerqueira, V.R.; Sampaio, L.A.; Wasielesky, W.; Poersch, L.H. Evidence of Total Suspended Solids Control by Mugil liza Reared in an Integrated System with Pacific White Shrimp Litopenaeus vannamei Using Biofloc Technology. Aquac. Rep. 2020, 18, 100479. [Google Scholar] [CrossRef]
- Azim, M.E.; Little, D.C. The Biofloc Technology (BFT) in Indoor Tanks: Water Quality, Biofloc Composition, and Growth and Welfare of Nile Tilapia (Oreochromis niloticus). Aquaculture 2008, 283, 29–35. [Google Scholar] [CrossRef]
- Brito, L.O.; Arantes, R.; Magnotti, C.; Derner, R.; Pchara, F.; Olivera, A.; Vinatea, L. Water Quality and Growth of Pacific White Shrimp Litopenaeus vannamei (Boone) in Co-Culture with Green Seaweed Ulva lactuca (Linaeus) in Intensive System. Aquac. Int. 2014, 22, 497–508. [Google Scholar] [CrossRef]
- Reis, W.G.; Wasielesky, W.; Abreu, P.C.; Brandão, H.; Krummenauer, D. Rearing of the Pacific White Shrimp Litopenaeus vannamei (Boone, 1931) in BFT System with Different Photoperiods: Effects on the Microbial Community, Water Quality and Zootechnical Performance. Aquaculture 2019, 508, 19–29. [Google Scholar] [CrossRef]
- de Alencar, J.R.; Junior, P.A.H.; Celino, J.J. Cultivo de Camarão Branco Litopenaeus vannamei (Boone, 1931) Com a Macroalga Ulva lactuca Linneaus (Chlorophyta) No Tratamento de Efluentes Em Sistema Fechado de Recirculação. Rev. Biol. Ciências Terra 2010, 10, 117–137. [Google Scholar]
- Copertino, M.D.S.; Tormena, T.; Seeliger, U. Biofiltering Efficiency, Uptake and Assimilation Rates of Ulva clathrata (Roth) J. Agardh (Clorophyceae) Cultivated in Shrimp Aquaculture Waste Water. J. Appl. Phycol. 2009, 21, 31–45. [Google Scholar] [CrossRef]
- Liu, G.; Zhu, S.; Liu, D.; Guo, X.; Ye, Z. Effects of Stocking Density of the White Shrimp Litopenaeus vannamei (Boone) on Immunities, Antioxidant Status, and Resistance against Vibrio Harveyi in a Biofloc System. Fish Shellfish Immunol. 2017, 67, 19–26. [Google Scholar] [CrossRef]
- Gaona, C.A.P.; Poersch, L.H.; Krummenauer, D.; Foes, G.K.; Wasielesky, W.J. The effect of solids removal on water quality, growth and survival of Litopenaeus vannamei in a biofloc technology culture system. Shrimp Int. J. Recirc. Aquac. 2011, 12, 54–73. [Google Scholar] [CrossRef]
- Jory, D.E.; Cabrera, T.R.; Dugger, D.M.; Fegan, D.; Lee, P.G.; Lawrence, L.; Jackson, C.J.; Mcintosh, R.P.; Castañeda, J.I.; Al, B.; et al. A Global Review of Shrimp Feed Management: Status and Perspectives. Aquaculture 2011, 318, 104–152. [Google Scholar]
- American Water Works Association, Water Pollution Control Association, APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Furtado, P.S.; Poersch, L.H.; Wasielesky, W. Effect of Calcium Hydroxide, Carbonate and Sodium Bicarbonate on Water Quality and Zootechnical Performance of Shrimp Litopenaeus vannamei Reared in Biofloc Technology (BFT) Systems. Aquaculture 2011, 321, 130–135. [Google Scholar] [CrossRef]
- Unesco. Chemical Methods for Use in Marine Environmental Monitoring; Intergovernmental Oceanographic Commission: Paris, France, 1983. [Google Scholar]
- Bendschneider, K.; Robinson, R.J. New Spectrophotometric Method for the Determination of Nitrite in Water. Fresenius Environ. Bull. 2001, 10, 781–785. [Google Scholar]
- Aminot, A.; Chaussepied, M. Manuel des Analyses Chimiques en Milieu Marin; Centre National Pour L’exploitation des Océans: Brest, France, 1983. [Google Scholar]
- Baumgarten, M.D.G.Z.; De Barros Rocha, J.M.; Niencheski, L.F.H. Manual de Análises em Oceanografia Química; FURG: Rio Grande, Brazil, 1996. [Google Scholar]
- Loureiro, R.R.; Reis, R.P.; Critchley, A.T. In Vitro Cultivation of Three Kappaphycus alvarezii (Rhodophyta, Areschougiaceae) Variants (Green, Red and Brown) Exposed to a Commercial Extract of the Brown Alga Ascophyllum nodosum (Fucaceae, Ochrophyta). J. Appl. Phycol. 2010, 22, 101–104. [Google Scholar] [CrossRef]
- De Miranda Cabral Gontijo, Á.M.; Barreto, R.E.; Speit, G.; Valenzuela Reyes, V.A.; Volpato, G.L.; Favero Salvadori, D.M. Anesthesia of Fish with Benzocaine Does Not Interfere with Comet Assay Results. Mutat. Res.-Genet. Toxicol. Environ. Mutagen. 2003, 534, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.S.; Santos, D.; Schmachtl, F.; Machado, C.; Fernandes, V.; Bögner, M.; Schleder, D.D.; Seiffert, W.Q.; Vieira, F.N. Heterotrophic, Chemoautotrophic and Mature Approaches in Biofloc System for Pacific White Shrimp. Aquaculture 2021, 533, 736099. [Google Scholar] [CrossRef]
- Liang, W.; Luo, G.; Tan, H.; Ma, N.; Zhang, N.; Li, L. Efficiency of Biofloc Technology in Suspended Growth Reactors Treating Aquacultural Solid under Intermittent Aeration. Aquac. Eng. 2014, 59, 41–47. [Google Scholar] [CrossRef]
- Carvalho, A.; Costa, L.C.D.O.C.d.O.; Holanda, M.; Poersch, L.H.; Turan, G. Influence of Total Suspended Solids on the Growth of the Sea Lettuce Ulva lactuca Integrated with the Pacific White Shrimp Litopenaeus vannamei in a Biofloc System. Fishes 2023, 8, 163. [Google Scholar] [CrossRef]
- Luo, M.B.; Liu, F.; Xu, Z.L. Growth and Nutrient Uptake Capacity of Two Co-Occurring Species, Ulva prolifera and Ulva linza. Aquat. Bot. 2012, 100, 18–24. [Google Scholar] [CrossRef]
- Legarda, E.C.; da Silva, D.; Miranda, C.S.; Pereira, P.K.M.; Martins, M.A.; Machado, C.; de Lorenzo, M.A.; Hayashi, L.; do Nascimento Vieira, F. Sea Lettuce Integrated with Pacific White Shrimp and Mullet Cultivation in Biofloc Impact System Performance and the Sea Lettuce Nutritional Composition. Aquaculture 2021, 534, 736265. [Google Scholar] [CrossRef]
- Resende, L.; Flores, J.; Moreira, C.; Pacheco, D.; Baeta, A.; Garcia, A.C.; Rocha, A.C.S. Effective and Low-Maintenance IMTA System as Effluent Treatment Unit for Promoting Sustainability in Coastal Aquaculture. Appl. Sci. 2022, 12, 398. [Google Scholar] [CrossRef]
- Li, E.; Chen, L.; Zeng, C.; Chen, X.; Yu, N.; Lai, Q.; Qin, J.G. Growth, Body Composition, Respiration and Ambient Ammonia Nitrogen Tolerance of the Juvenile White Shrimp, Litopenaeus vannamei, at Different Salinities. Aquaculture 2007, 265, 385–390. [Google Scholar] [CrossRef]
- Bews, E.; Booher, L.; Polizzi, T.; Long, C.; Kim, J.H.; Edwards, M.S. Effects of Salinity and Nutrients on Metabolism and Growth of Ulva lactuca: Implications for Bioremediation of Coastal Watersheds. Mar. Pollut. Bull. 2021, 166, 112199. [Google Scholar] [CrossRef]
- Mantri, V.A.; Singh, R.P.; Bijo, A.J.; Kumari, P.; Reddy, C.R.K.; Jha, B. Differential Response of Varying Salinity and Temperature on Zoospore Induction, Regeneration and Daily Growth Rate in Ulva fasciata (Chlorophyta, Ulvales). J. Appl. Phycol. 2011, 23, 243–250. [Google Scholar] [CrossRef]
- Fernand, F.; Israel, A.; Skjermo, J.; Wichard, T.; Timmermans, K.R.; Golberg, A. Offshore Macroalgae Biomass for Bioenergy Production: Environmental Aspects, Technological Achievements and Challenges. Renew. Sustain. Energy Rev. 2017, 75, 35–45. [Google Scholar] [CrossRef]
- de Souza, R.L.; de Lima, E.C.R.; de Melo, F.P.; Ferreira, M.G.P.; de Souza Correia, E. The Culture of Nile Tilapia at Different Salinities Using a Biofloc System. Rev. Cienc. Agron. 2019, 50, 267–275. [Google Scholar] [CrossRef]
- Ren, X.; Wang, Q.; Shao, H.; Xu, Y.; Liu, P.; Li, J. Effects of Low Temperature on Shrimp and Crab Physiology, Behavior, and Growth: A Review. Front. Mar. Sci. 2021, 8, 746177. [Google Scholar] [CrossRef]
- Nobrega, R.O.; Banze, J.F.; Batista, R.O.; Fracalossi, D.M. Improving Winter Production of Nile Tilapia: What Can Be Done? Aquac. Rep. 2020, 18, 100453. [Google Scholar] [CrossRef]
- Holanda, M.; Wasielesky, W.; de Lara, G.R.; Poersch, L.H. Production of Marine Shrimp Integrated with Tilapia at High Densities and in a Biofloc System: Choosing the Best Spatial Configuration. Fishes 2022, 7, 283. [Google Scholar] [CrossRef]

| Treatments | ||
|---|---|---|
| Parameters | Shallow Float | Bottom Float |
| Temperature (°C) | 22.30 ± 1.59 | 22.28 ± 1.58 |
| DO (mg L−1) | 6.70 ± 0.56 | 6.72 ± 0.59 |
| pH | 8.00 ± 0.21 | 8.06 ± 0.20 |
| Salinity (‰) | 19.92 ± 0.98 | 19.00 ± 1.55 |
| Alkalinity (mgCaCO3 L−1) | 218.14 ± 20.06 | 219.90 ± 22.30 |
| TAN (mg L−1) | 0.18 ± 0.20 | 0.15 ± 0.19 |
| Nitrite (mg L−1) | 2.16 ± 2.38 | 1.29 ± 1.30 |
| Nitrate (mg L−1) | 64.40 ± 28.39 | 61.71 ± 24.17 |
| Phosphate (mg L−1) | 5.99 ± 4.24 | 5.43 ± 3.78 |
| Turbidity (NTU) | 219.49 ± 86.66 | 196.02 ± 68.72 |
| SS (ml L−1) | 3.88 ± 2.09 | 5.01 ± 2.27 |
| TSS (mg L−1) | 307.92 ± 87.48 | 303.43 ± 91.60 |
| Treatments | ||||
|---|---|---|---|---|
| Shallow Float | Bottom Float | |||
| Parameters | with Macroalgae | without Macroalgae | with Macroalgae | without Macroalgae |
| SS (ml L−1) | 6.50 ± 3.50 a | 14.50 ± 2.90 b | 8.40 ± 3.50 a | 16.40 ± 1.90 b |
| TSS (mg L−1) | 305.10 ± 84.10 a | 503.80 ± 40.10 b | 317.00 ± 71.30 a | 530.30 ± 40.10 b |
| Treatments | ||
|---|---|---|
| Shallow Float | Bottom Float | |
| SGR (% day−1) | ||
| 14 day | 0.95 ± 0.54 a | −1.06 ± 0.43 b |
| 27 day | 0.23 ± 0.78 | −0.39 ± 0.48 |
| 41 day | −0.72 ± 0.28 | −0.05 ± 0.48 |
| 55 day | −0.58 ± 0.19 | −0.63 ± 0.84 |
| 70 day | 0.85 ± 0.58 | 0.38 ± 0.47 |
| Initial mean weight (kg—FW) | 2.40 ± 1.64 | 2.40 ± 0.88 |
| Final mean weight (kg—FW) | 2.63 ± 0.23 a | 1.94 ± 0.20 b |
| RGR (% day−1) | 0.14 ± 0.14 a | −0.35 ± 0.17 b |
| Biomass gain (kg) | 0.26 ± 0.27 a | −0.50 ± 0.23 b |
| Treatments | ||
|---|---|---|
| Shallow Float | Bottom Float | |
| Shrimp | ||
| Final mean weight (g) | 8.05 ± 0.52 | 8.92 ± 0.36 |
| WWG (g week−1) ## | 0.38 ± 0.06 | 0.48 ± 0.04 |
| Final biomass (kg) | 27.90 ± 4.95 | 27.80 ± 2.94 |
| FCR # | 2.63 ± 0.47 | 2.99 ± 0.86 |
| Yield (kg m−3) | 1.33 ± 0.24 | 1.32 ± 0.24 |
| Survival (%) | 85.64 ± 19.84 | 76.24 ± 7.53 |
| Fish | ||
| Final mean weight (g) | 289.74 ± 30.09 | 227.15 ± 48.97 |
| WWG (g week−1) ## | 9.93 ± 1.90 | 8.02 ± 1.75 |
| Final biomass (kg) | 26.73 ± 1.73 | 16.94 ± 8.05 |
| FCR # | 0.39 ± 0.09 | 0.49 ± 0.16 |
| Yield (kg m−3) | 1.27 ± 0.08 | 0.81 ± 0.38 |
| Survival (%) | 88.77 ± 8.04 | 89.05 ± 6.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, A.; Costa, L.C.d.O.; Holanda, M.; Gonçalves, M.; Santos, J.; Costa, C.S.B.; Turan, G.; Poersch, L.H. Growth of the Macroalgae Ulva lactuca Cultivated at Different Depths in a Biofloc Integrated System with Shrimp and Fish. Phycology 2023, 3, 280-293. https://doi.org/10.3390/phycology3020018
Carvalho A, Costa LCdO, Holanda M, Gonçalves M, Santos J, Costa CSB, Turan G, Poersch LH. Growth of the Macroalgae Ulva lactuca Cultivated at Different Depths in a Biofloc Integrated System with Shrimp and Fish. Phycology. 2023; 3(2):280-293. https://doi.org/10.3390/phycology3020018
Chicago/Turabian StyleCarvalho, Andrezza, Léa Carolina de Oliveira Costa, Mariana Holanda, Mayra Gonçalves, Jorge Santos, César S. B. Costa, Gamze Turan, and Luís H. Poersch. 2023. "Growth of the Macroalgae Ulva lactuca Cultivated at Different Depths in a Biofloc Integrated System with Shrimp and Fish" Phycology 3, no. 2: 280-293. https://doi.org/10.3390/phycology3020018
APA StyleCarvalho, A., Costa, L. C. d. O., Holanda, M., Gonçalves, M., Santos, J., Costa, C. S. B., Turan, G., & Poersch, L. H. (2023). Growth of the Macroalgae Ulva lactuca Cultivated at Different Depths in a Biofloc Integrated System with Shrimp and Fish. Phycology, 3(2), 280-293. https://doi.org/10.3390/phycology3020018








