Thinning of Botryococcus braunii Colony Sheath by Pretreatment Enhances Solvent-Based Hydrocarbon Recovery
Abstract
1. Introduction
2. Materials and Methods
2.1. Algal Strain and Culture
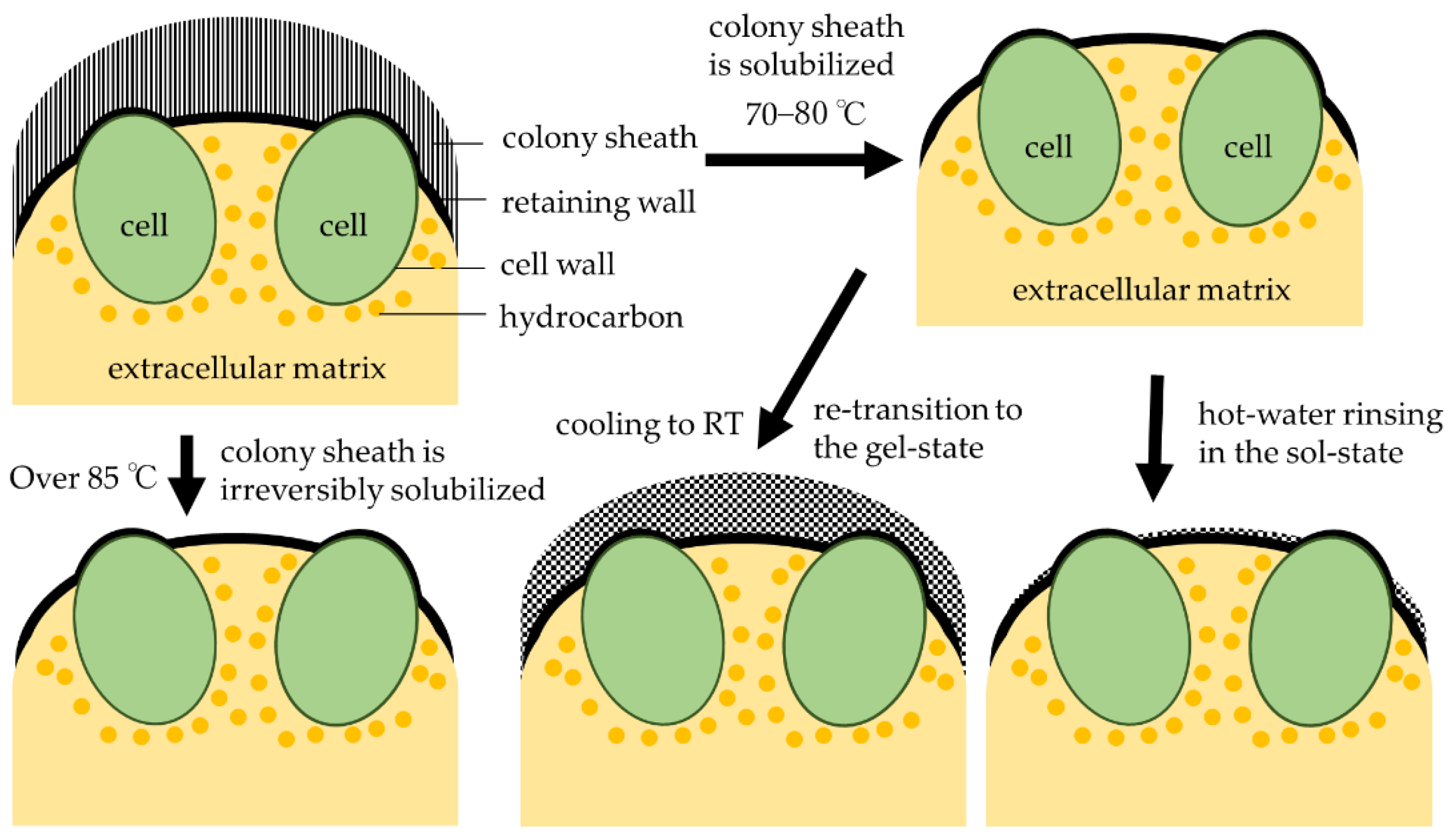
2.2. Observation of Colony Sheath Using India Ink
2.3. Hot-Water Rinsing Process
2.4. Hydrocarbon Recovery Rate by n-Hexane
3. Results
3.1. Growth Rates of Yamanaka Strain by Salt-Water Culture
3.2. Changes in Hydrocarbon Recovery Rate and Colony Sheath of Yamanaka Strain
3.3. Hot-Water Rinsing for Showa Strain
4. Discussion
4.1. Relationship between Colony Sheath and Hydrocarbon Recovery in Race A
4.2. Rinsing of Colony Sheath in Race B at Lower Temperatures
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bwapwa, J.K.; Anandraj, A.; Trois, C. Possibilities for conversion of microalgae oil into aviation fuel: A review. Renew. Sustain. Energy Rev. 2017, 80, 1345–1354. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M.; Świca, I.; Kazimierowicz, J. Algae Biomass as a Potential Source of Liquid Fuels. Phycology 2021, 1, 105–118. [Google Scholar] [CrossRef]
- Shen, Y.; Pei, Z.; Yuan, W.; Mao, E. Effect of nitrogen and extraction method on algae lipid yield. Int. J. Agric. Biol. Eng. 2009, 2, 51–57. [Google Scholar] [CrossRef]
- Mercer, P.; Armenta, R.E. Developments in oil extraction from microalgae. Eur. J. Lipid Sci. Technol. 2011, 113, 539–547. [Google Scholar] [CrossRef]
- Lee, J.; Yoo, C.; Jun, S.; Ahn, C.; Oh, H. Comparison of several methods for effective lipid extraction from microalgae. Bioresour. Technol. 2010, 101, S75–S77. [Google Scholar] [CrossRef]
- Kanda, H.; Li, P.; Ikehara, T.; Yasumoto-Hirose, M. Lipids extracted from several species of natural blue–green microalgae by dimethyl ether: Extraction yield and properties. Fuel 2012, 95, 88–92. [Google Scholar] [CrossRef]
- Andrich, G.; Nesti, U.; Venturi, F.; Zinnai, A.; Fiorentini, R. Supercritical fluid extraction of bioactive lipids from the microalga Nannochloropsis sp. Eur. J. Lipid Sci. Technol. 2005, 107, 381–386. [Google Scholar] [CrossRef]
- Kim, Y.; Choi, Y.; Park, J.; Lee, S.; Yang, Y.; Kim, H.J.; Park, T.; Hwan Kim, Y.; Lee, S.H. Ionic liquid-mediated extraction of lipids from algal biomass. Bioresour. Technol. 2012, 109, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Sharma, R.; Chisti, Y.; Banerjee, U.C. Botryococcus braunii: A Renewable Source of Hydrocarbons and Other Chemicals. Crit. Rev. Biotechnol. 2002, 22, 245–279. [Google Scholar] [CrossRef]
- Largeau, C.; Casadevall, E.; Berkaloff, C.; Dhamelincourt, P. Sites of accumulation of and composition of hydrocarbons in Botryococcus braunii. Phytochemistry 1980, 19, 1043–1051. [Google Scholar] [CrossRef]
- Jin, J.; Dupré, D.; Legrand, J.; Grizeau, D. Extracellular hydrocarbon and intracellular lipid accumulation are related to nutrient-sufficient conditions in pH-controlled chemostat cultures of the microalga Botryococcus braunii SAG 30.81. Algal Res. 2016, 17, 244–252. [Google Scholar] [CrossRef]
- Jin, J.; Dupré, D.; Watanabe, M.M.; Legrand, J.; Grizeau, D. Characteristics of extracellular hydrocarbon-rich microalga Botryococcus braunii for biofuels production: Recent advances and opportunities. Process Biochem. 2016, 51, 1866–1875. [Google Scholar] [CrossRef]
- Frenz, J.; Largeau, C.; Casadevall, E.; Kollerup, F.; Daugulis, A.J. Hydrocarbon recovery and biocompatibility of solvents for extraction from cultures of Botryococcus braunii. Biotechnol. Bioeng. 1989, 34, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.L.; Nobre, B.P.; Cardoso, M.T.; Pereira, A.P.; Palavra, A.F. Supercritical carbon dioxide extraction of compounds with pharmaceutical importance from microalgae. Inorg. Chim. Acta 2003, 356, 328–334. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, S.; Xu, L.; Wang, F.; Guo, C. Algal oil extraction from wet biomass of Botryococcus braunii by 1,2-dimethoxyethane. Appl. Energy 2013, 102, 971–974. [Google Scholar] [CrossRef]
- Weiss, T.L.; Roth, R.; Goodson, C.; Vitha, S.; Black, I.; Azadi, P.; Rusch, J.; Holzenburg, A.; Devarenne, T.P.; Goodenough, U. Colony organization in the green alga Botryococcus braunii (Race B) is specified by a complex extracellular matrix. Eukaryot. Cell 2012, 11, 1424–1440. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, S.; Saito, Y.; Matsushita, Y.; Aoki, H. Effect of mechanical pretreatment on hydrocarbon extraction from concentrated wet hydrocarbon-rich microalga, Botryococcus braunii. Energy Fuels 2018, 32, 1761–1770. [Google Scholar] [CrossRef]
- Magota, A.; Saga, K.; Okada, S.; Atobe, S.; Imou, K. Effect of thermal pretreatments on hydrocarbon recovery from Botryococcus braunii. Bioresour. Technol. 2012, 123, 195–198. [Google Scholar] [CrossRef]
- Furuhashi, K.; Noguchi, T.; Okada, S.; Hasegawa, F.; Kaizu, Y.; Imou, K. The surface structure of Botryococcus braunii colony prevents the entry of extraction solvents into the colony interior. Algal Res. 2016, 16, 160–166. [Google Scholar] [CrossRef]
- Metzger, P.; Largeau, C. Botryococcus braunii: A rich source for hydrocarbons and related ether lipids. Appl. Microbiol. Biotechnol. 2005, 66, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Hirose, M.; Mukaida, F.; Okada, S.; Noguchi, T. Active Hydrocarbon Biosynthesis and Accumulation in a Green Alga, Botryococcus braunii (Race A). Eukaryot. Cell 2013, 12, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Overmans, S.; Ignacz, G.; Beke, A.K.; Xu, J.; Saikaly, P.E.; Szekely, G.; Lauersen, K.J. Continuous extraction and concentration of secreted metabolites from engineered microbes using membrane technology. Green Chem. 2022, 24, 5479–5486. [Google Scholar] [CrossRef]
- Jackson, B.A.; Bahri, P.A.; Moheimani, N.R. Repetitive non-destructive milking of hydrocarbons from Botryococcus braunii. Renew. Sustain. Energy Rev. 2017, 79, 1229–1240. [Google Scholar] [CrossRef]
- Kleinert, C.; Griehl, C. In situ extraction (milking) of the two promising Botryococcus braunii strains Showa and Bot22 under optimized extraction time. J. Appl. Phycol. 2022, 34, 269–283. [Google Scholar] [CrossRef]
- Atobe, S.; Saga, K.; Hasegawa, F.; Furuhashi, K.; Tashiro, Y.; Suzuki, T.; Okada, S.; Imou, K. Effect of amphiphilic polysaccharides released from Botryococcus braunii Showa on hydrocarbon recovery. Algal Res. 2015, 10, 172–176. [Google Scholar] [CrossRef]
- Atobe, S.; Saga, K.; Furuhashi, K.; Okada, S.; Suzuki, T.; Imou, K. The effect of the water-soluble polymer released from Botryococcus braunii Showa strain on solvent extraction of hydrocarbon. J. Appl. Phycol. 2015, 27, 755–761. [Google Scholar] [CrossRef]
- Saga, K.; Hasegawa, F.; Miyagi, S.; Atobe, S.; Okada, S.; Imou, K.; Osaka, N.; Yamagishi, T. Comparative evaluation of wet and dry processes for recovering hydrocarbon from Botryococcus Braunii. Appl. Energy 2015, 141, 90–95. [Google Scholar] [CrossRef]
- Okada, S.; Murakami, M.; Yamaguchi, K. Hydrocarbon composition of newly isolated strains of the green microalga Botryococcus braunii. J. Appl. Phycol. 1995, 7, 555–559. [Google Scholar] [CrossRef]
- Nonomura, A.M. Botryococcus braunii var. showa(Chlorophyceae) from Berkeley, California, United States of America. Jpn. J. Phycol. 1988, 36, 285–291. [Google Scholar]
- Furuhashi, K.; Hasegawa, F.; Saga, K.; Kudou, S.; Okada, S.; Kaizu, Y.; Imou, K. Effects of culture medium salinity on the hydrocarbon extractability, growth and morphology of Botryococcus braunii. Biomass Bioenergy 2016, 91, 83–90. [Google Scholar] [CrossRef]
- Uno, Y.; Nishii, I.; Kagiwada, S.; Noguchi, T. Colony sheath formation is accompanied by shell formation and release in the green alga Botryococcus braunii (race B). Algal Res. 2015, 8, 214–223. [Google Scholar] [CrossRef]
- Furuhashi, K.; Hasegawa, F.; Yamauchi, M.; Kaizu, Y.; Imou, K. Improving the energy balance of hydrocarbon production using an inclined solid–liquid separator with a wedge-wire screen and easy hydrocarbon recovery from Botryococcus braunii. Energies 2020, 13, 4139. [Google Scholar] [CrossRef]
- Khatri, W.; Hendrix, R.; Niehaus, T.; Chappell, J.; Curtis, W.R. Hydrocarbon production in high density Botryococcus braunii race B continuous culture. Biotechnol. Bioeng. 2014, 111, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Tanoi, T.; Kawachi, M.; Watanabe, M.M. Iron and glucose effects on the morphology of Botryococcus braunii with assumption on the colony formation variability. J. Appl. Phycol. 2014, 26, 933–945. [Google Scholar] [CrossRef]
- Zhang, K.; Kojima, E. Effect of light intensity on colony size of microalga Botryococcus braunii in bubble column photobioreactors. J. Ferment. Bioeng. 1998, 86, 573–576. [Google Scholar] [CrossRef]
- James, S.C.; Boriah, V. Modeling algae growth in an open-channel raceway. J. Comput. Biol. 2010, 17, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Sivaramakrishnan, R.; Incharoensakdi, A. Production of methyl ester from two microalgae by two-step transesterification and direct transesterification. Environ. Sci. Pollut. Res. 2017, 24, 4950–4963. [Google Scholar] [CrossRef]
- Jouhara, H.; Khordehgah, N.; Almahmoud, S.; Delpech, B.; Chauhan, A.; Tassou, S.A. Waste heat recovery technologies and applications. Therm. Sci. Eng. Prog. 2018, 6, 268–289. [Google Scholar] [CrossRef]
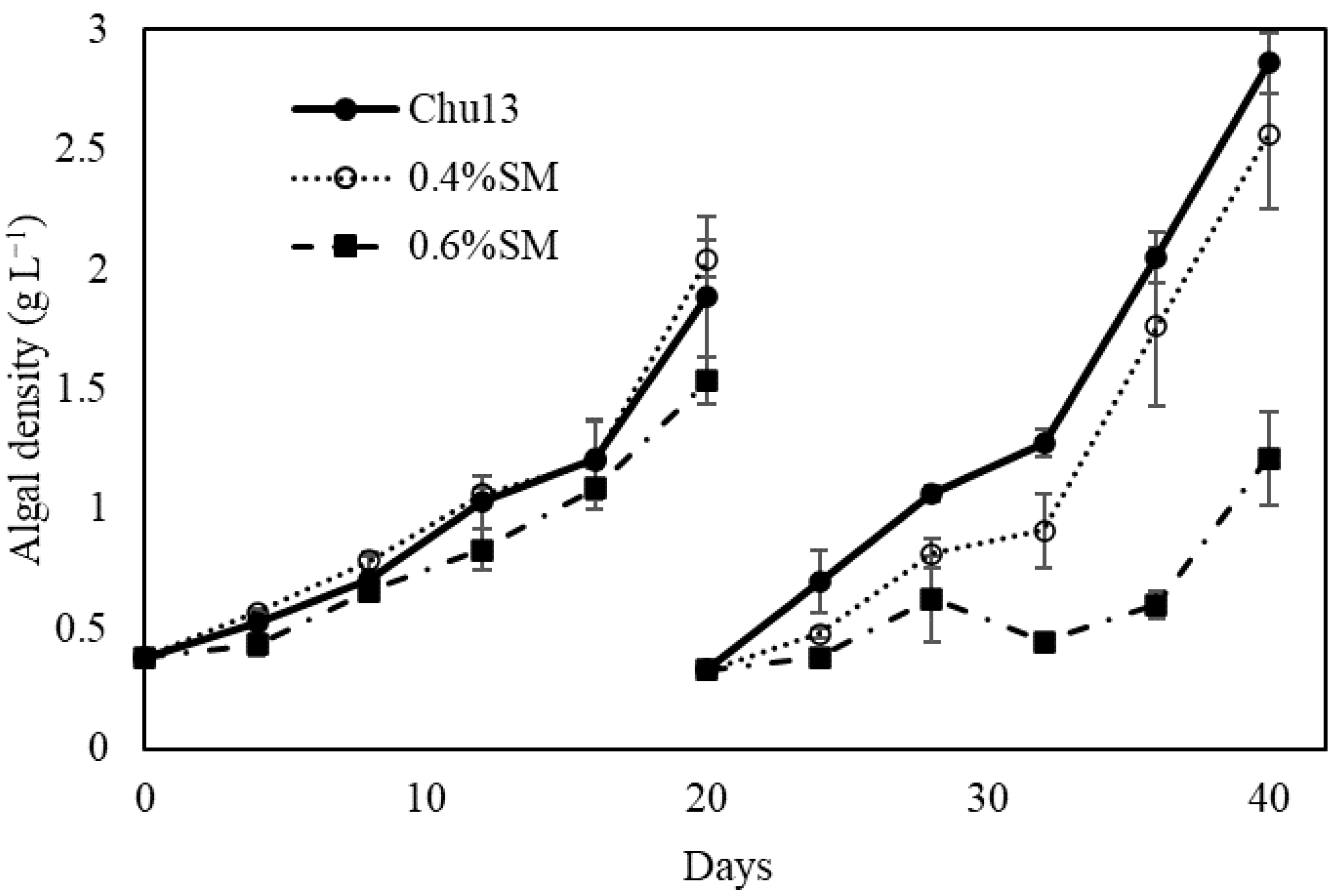
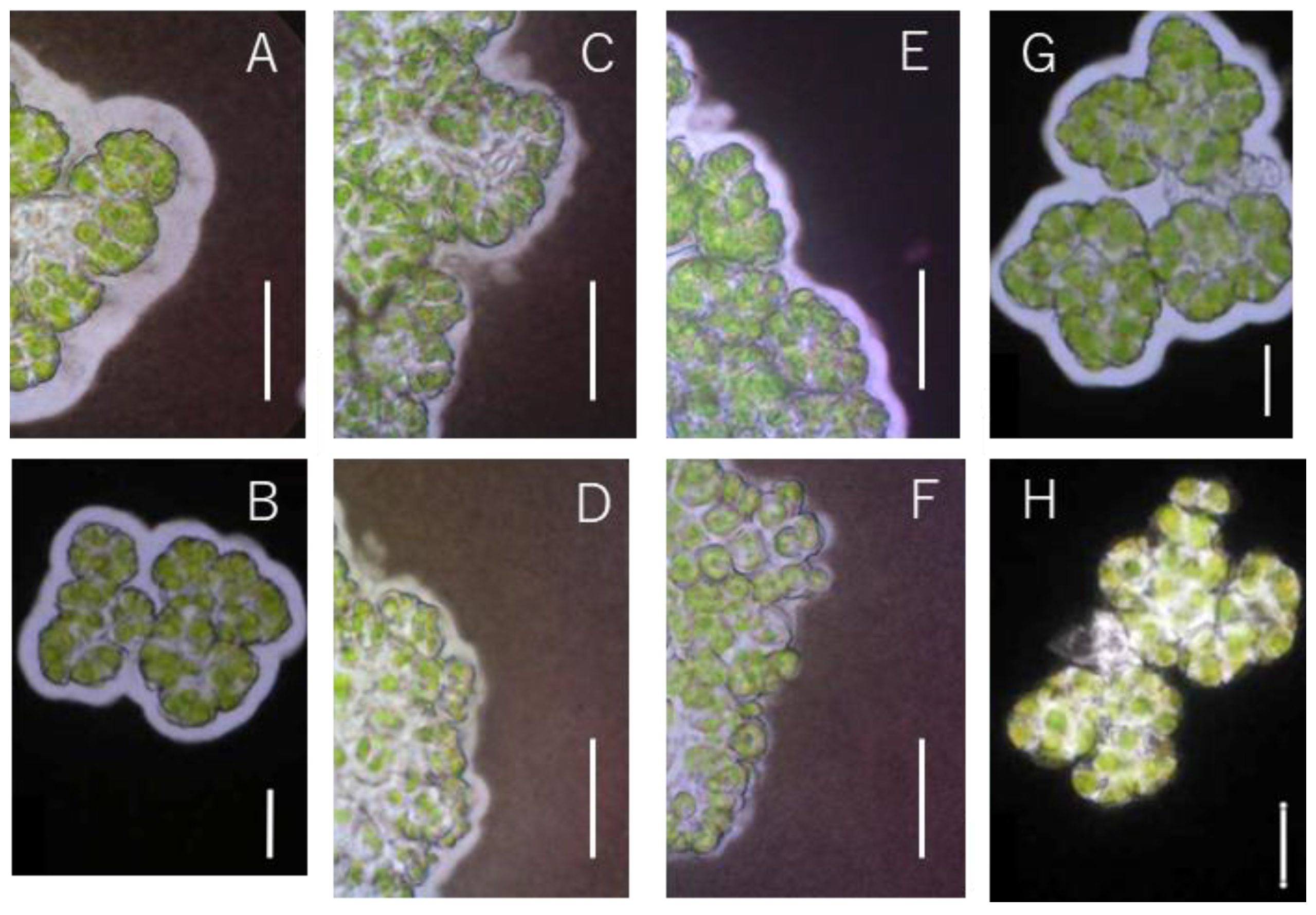
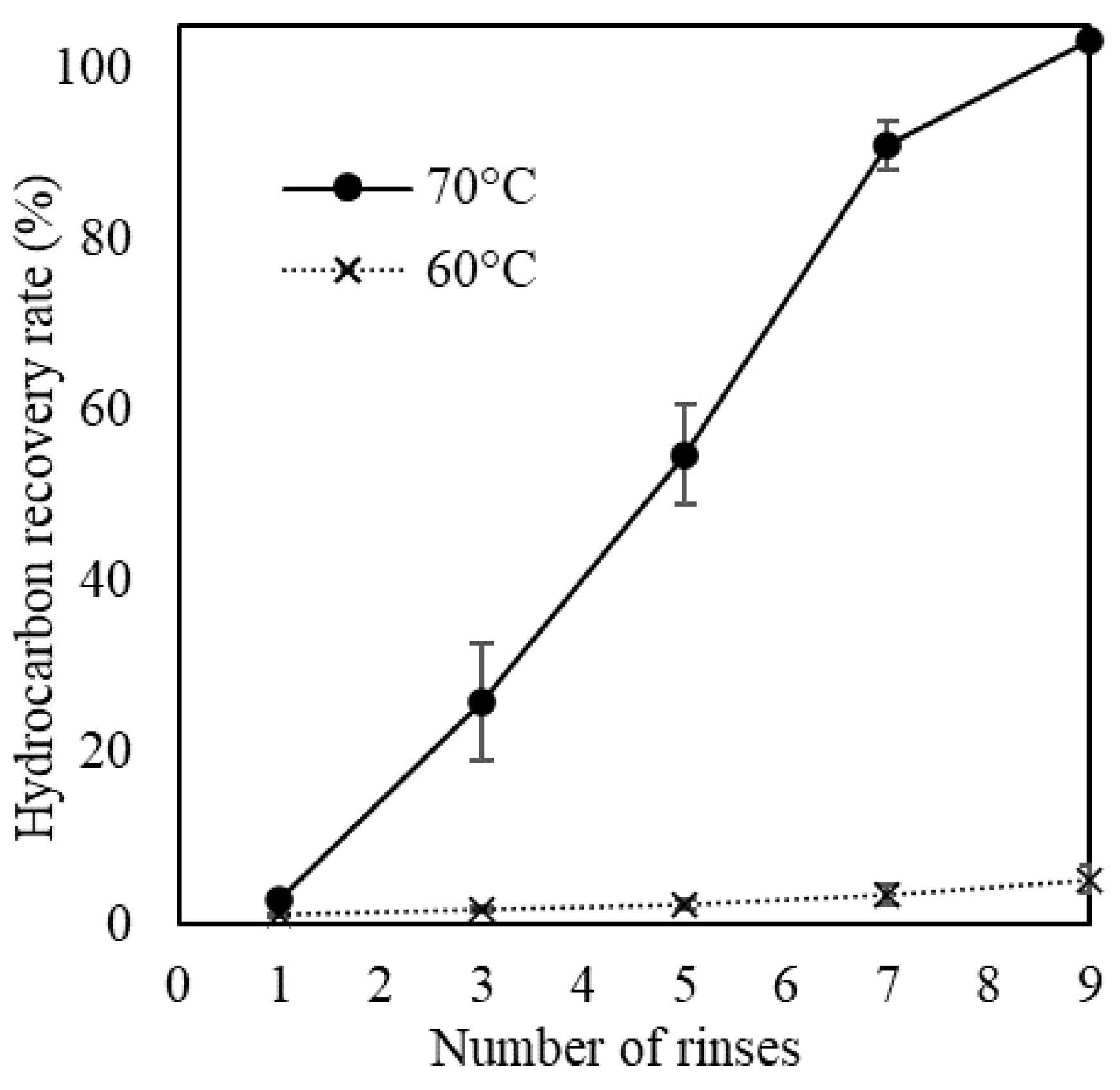
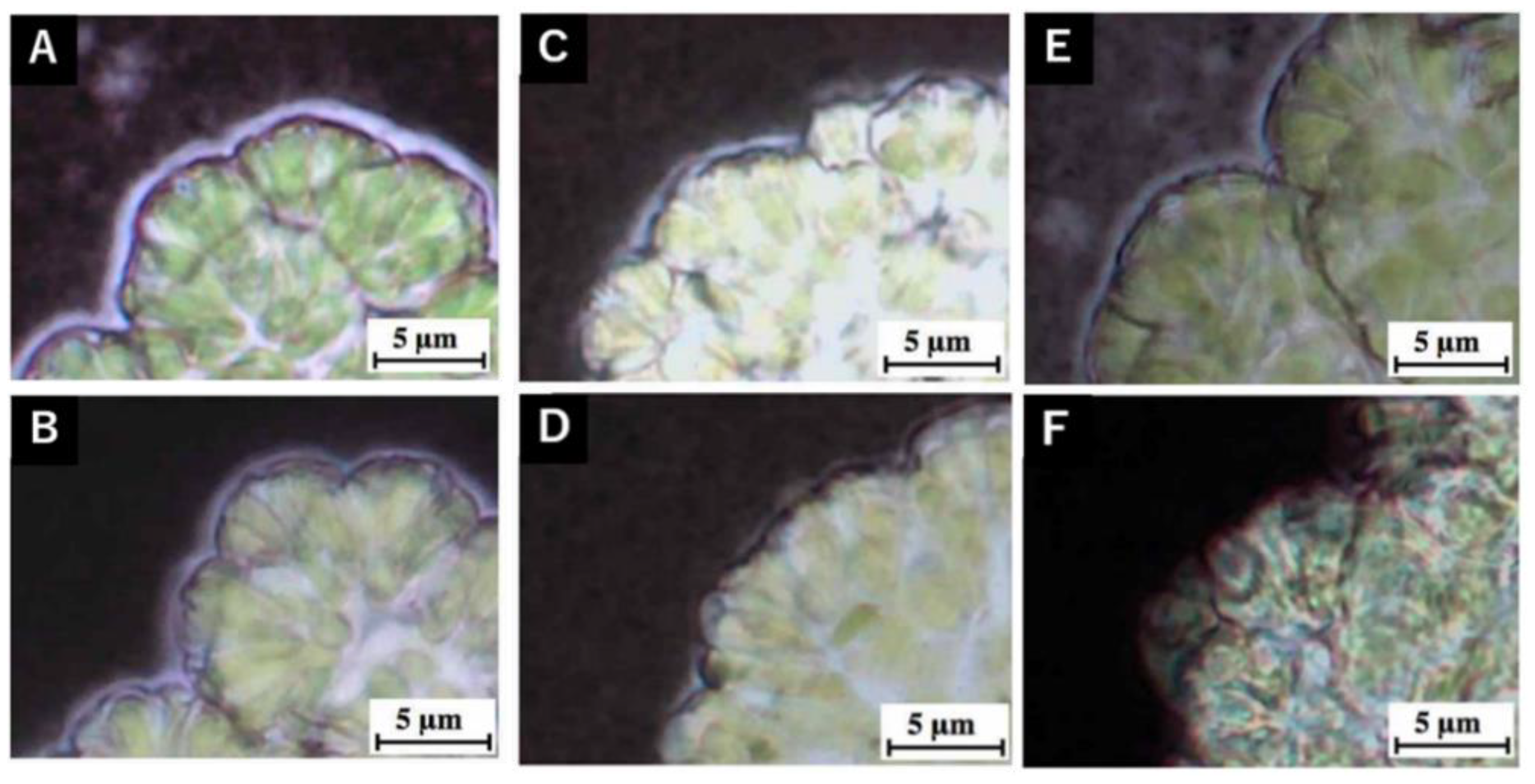
| Strain | Race | Pretreatment |
|---|---|---|
| Yamanaka | A race | Salt-water culture (0.4, 0.6%) |
| Showa | B race | Hot-water rinsing (60, 70 °C) |
| Medium | Hydrocarbon Recovery Rate (%) | Thickness (µm) | |
|---|---|---|---|
| 20 d | 40 d | 20 d | |
| Chu13 | 3.9 ± 1.6 | 1.8 ± 0.6 | 2.00 ± 0.15 |
| 0.4%SM | 12.0 ± 3.6 | 13.3 ± 3.6 | 1.21 ± 0.18 |
| 0.6%SM | 54.0 ± 4.2 | 66.1 ± 6.6 | 0.42 ± 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furuhashi, K.; Magota, A.; Liu, Y.; Hasegawa, F.; Okada, S.; Kaizu, Y.; Imou, K. Thinning of Botryococcus braunii Colony Sheath by Pretreatment Enhances Solvent-Based Hydrocarbon Recovery. Phycology 2022, 2, 363-373. https://doi.org/10.3390/phycology2040020
Furuhashi K, Magota A, Liu Y, Hasegawa F, Okada S, Kaizu Y, Imou K. Thinning of Botryococcus braunii Colony Sheath by Pretreatment Enhances Solvent-Based Hydrocarbon Recovery. Phycology. 2022; 2(4):363-373. https://doi.org/10.3390/phycology2040020
Chicago/Turabian StyleFuruhashi, Kenichi, Akinari Magota, Yifan Liu, Fumio Hasegawa, Shigeru Okada, Yutaka Kaizu, and Kenji Imou. 2022. "Thinning of Botryococcus braunii Colony Sheath by Pretreatment Enhances Solvent-Based Hydrocarbon Recovery" Phycology 2, no. 4: 363-373. https://doi.org/10.3390/phycology2040020
APA StyleFuruhashi, K., Magota, A., Liu, Y., Hasegawa, F., Okada, S., Kaizu, Y., & Imou, K. (2022). Thinning of Botryococcus braunii Colony Sheath by Pretreatment Enhances Solvent-Based Hydrocarbon Recovery. Phycology, 2(4), 363-373. https://doi.org/10.3390/phycology2040020







