Seaweeds as a Source of Functional Proteins
Abstract
:1. Introduction
2. Protein Content of Seaweeds
3. Quality of Protein in Seaweeds
3.1. Amino Acid Composition
3.2. Amino Acid Ratio
3.3. Amino Acid Score
3.4. Digestibility and Bioavailability
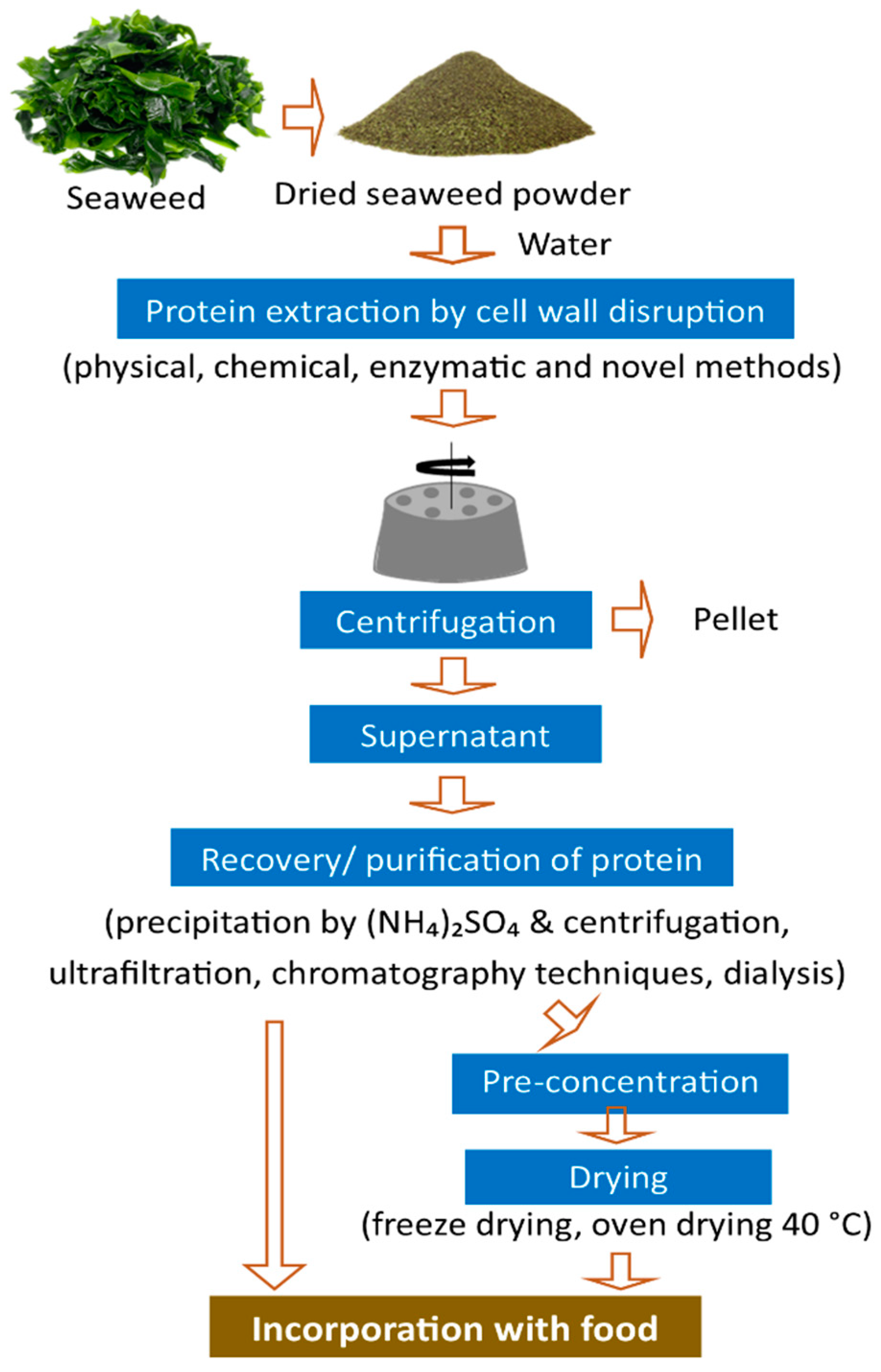
4. Protein Extraction Methods
5. Functional Properties of Seaweed Proteins and Their Role in Health
5.1. Amino Acids
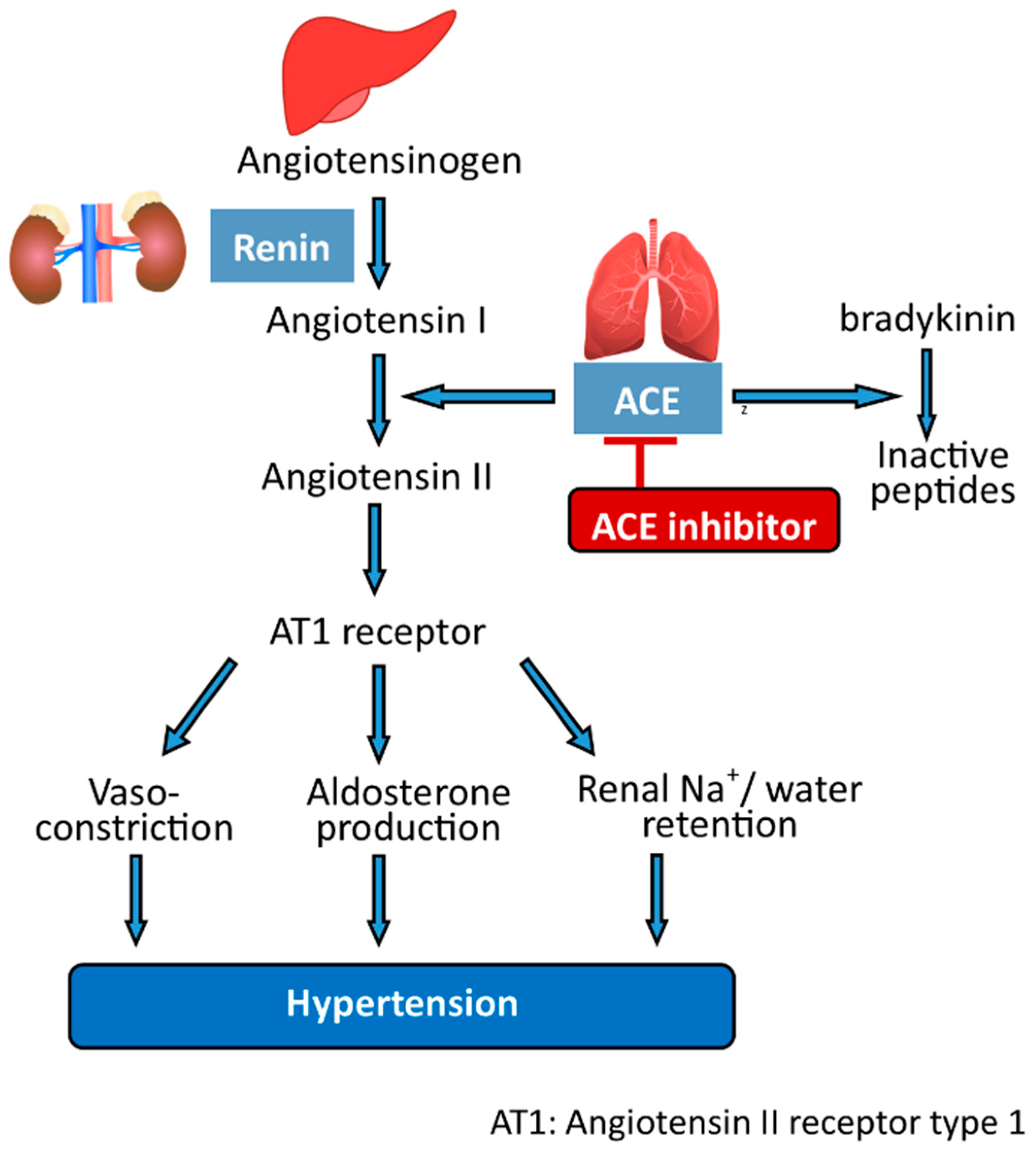
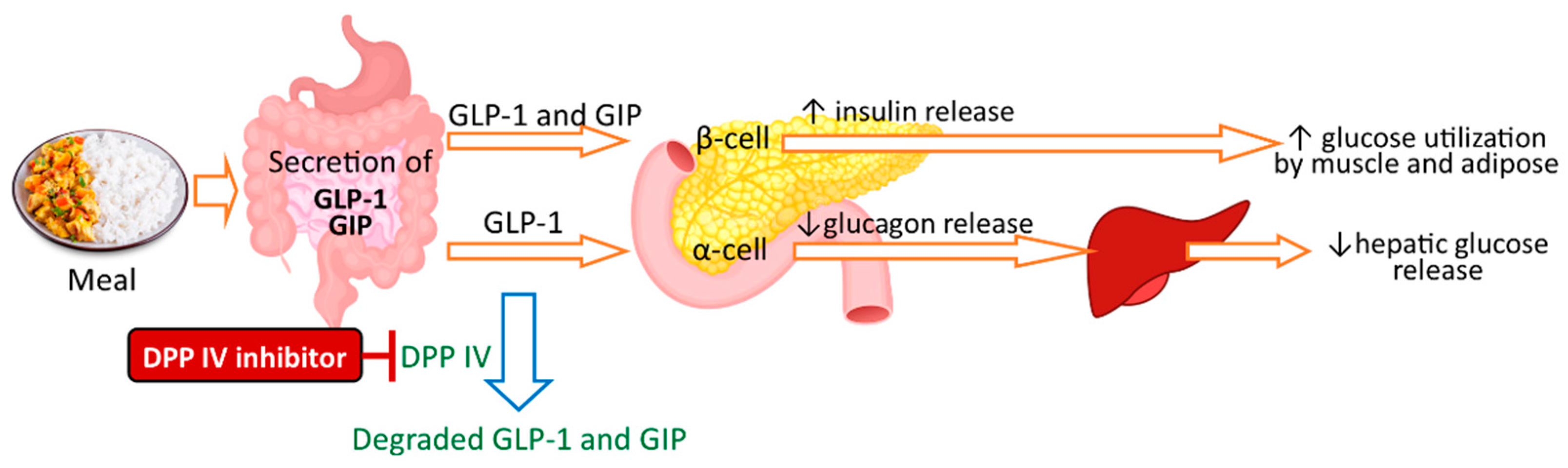
5.2. Peptides
5.3. Lectins
5.4. Phycobilliproteins
5.5. Free Amino Acids
6. Applications
7. Safety
8. Future Perspectives
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | Amino acid(s) |
| ACE | Angiotensin-I-converting enzyme |
| Ang II | Angiotensin II |
| DPP-IV | Dipeptidyl peptidase IV |
| EAA | Essential amino acid(s) |
| FAO | Food and Agriculture Organization of the United Nations |
| GIP | Glucose-dependent insulinotropic poly-peptide |
| GLP-1 | Glucagon-like peptide-1 |
| MERS-CoV | Middle East respiratory syndrome coronavirus |
| NEAA | Non-essential amino acid(s) |
| PAF-AH | Platelet-activating factor acetylhydrolase |
| SARS-CoV | Severe acute respiratory syndrome coronavirus |
| T2DM | Type 2 diabetes mellitus |
| WHO | World Health Organization |
References
- Bleakley, S.; Hayes, M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods 2017, 6, 33. [Google Scholar] [CrossRef] [Green Version]
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future Protein Supply and Demand: Strategies and Factors Influencing a Sustainable Equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef] [Green Version]
- Wijers, T.; Hylkema, A.; Visser, T.; Timmermans, K. Effects of Preservation on Protein Extraction in Four Seaweed Species. J. Appl. Phycol. 2020, 32, 3401–3409. [Google Scholar] [CrossRef]
- Ferrara, L. Seaweeds: A Food for Our Future. J. Food Chem. Nanotechnol. 2020, 6, 56–64. [Google Scholar] [CrossRef]
- MacArtain, P.; Gill, C.I.R.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional Value of Edible Seaweeds. Nutr. Rev. 2007, 65, 535–543. [Google Scholar] [CrossRef]
- Rajauria, G.; Cornish, L.; Ometto, F.; Msuya, F.E.; Villa, R. Chapter 12—Identification and Selection of Algae for Food, Feed, and Fuel Applications. In Seaweed Sustainability; Tiwari, B.K., Troy, D.J., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 315–345. ISBN 978-0-12-418697-2. [Google Scholar]
- Baweja, P.; Kumar, S.; Sahoo, D.; Levine, I. Chapter 3—Biology of Seaweeds. In Seaweed in Health and Disease Prevention; Fleurence, J., Levine, I., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 41–106. ISBN 978-0-12-802772-1. [Google Scholar]
- Mahadevan, K. Chapter 13—Seaweeds: A Sustainable Food Source. In Seaweed Sustainability; Tiwari, B.K., Troy, D.J., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 347–364. ISBN 978-0-12-418697-2. [Google Scholar]
- Pandey, A.K.; Chauhan, O.P.; Semwal, A.D. Seaweeds. Def. Life Sci. J. 2020, 5, 315–322. [Google Scholar] [CrossRef]
- Fleurence, J.; Morançais, M.; Dumay, J. 9—Seaweed Proteins. In Proteins in Food Processing, 2nd ed.; Yada, R.Y., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2018; pp. 245–262. ISBN 978-0-08-100722-8. [Google Scholar]
- Shannon, E.; Abu-Ghannam, N. Seaweeds as Nutraceuticals for Health and Nutrition. Phycologia 2019, 58, 563–577. [Google Scholar] [CrossRef] [Green Version]
- Lamont, T.; McSweeney, M. Consumer Acceptability and Chemical Composition of Whole-Wheat Breads Incorporated with Brown Seaweed (Ascophyllum Nodosum) or Red Seaweed (Chondrus Crispus). J. Sci. Food Agric. 2021, 101, 1507–1514. [Google Scholar] [CrossRef]
- Lomartire, S.; Marques, J.C.; Gonçalves, A.M.M. An Overview to the Health Benefits of Seaweeds Consumption. Mar. Drugs 2021, 19, 341. [Google Scholar] [CrossRef]
- Sabeena Farvin, K.H.; Jacobsen, C. Phenolic Compounds and Antioxidant Activities of Selected Species of Seaweeds from Danish Coast. Food Chem. 2013, 138, 1670–1681. [Google Scholar] [CrossRef]
- Fernández-Segovia, I.; Lerma-García, M.J.; Fuentes, A.; Barat, J.M. Characterization of Spanish Powdered Seaweeds: Composition, Antioxidant Capacity and Technological Properties. Food Res. Int. 2018, 111, 212–219. [Google Scholar] [CrossRef] [PubMed]
- FAO. FAO Yearbook. Fishery and Aquaculture Statistics 2018/FAO Annuaire. Statistiques des Pêches et de L’aquaculture 2018/FAO anuario. Estadísticas de Pesca y Acuicultura 2018; FAO Yearbook of Fishery and Aquaculture Statistics; FAO: Rome, Italy, 2020; ISBN 978-92-5-133371-6. [Google Scholar]
- Kraan, S. Chapter 3—Seaweed Resources, Collection, and Cultivation with Respect to Sustainability. In Sustainable Seaweed Technologies; Torres, M.D., Kraan, S., Dominguez, H., Eds.; Advances in Green and Sustainable Chemistry; Elsevier: Amsterdam, The Netherlands, 2020; pp. 89–102. ISBN 978-0-12-817943-7. [Google Scholar]
- Buschmann, A.H.; Camus, C.; Infante, J.; Neori, A.; Israel, Á.; Hernández-González, M.C.; Pereda, S.V.; Gomez-Pinchetti, J.L.; Golberg, A.; Tadmor-Shalev, N.; et al. Seaweed Production: Overview of the Global State of Exploitation, Farming and Emerging Research Activity. Eur. J. Phycol. 2017, 52, 391–406. [Google Scholar] [CrossRef]
- Geada, P.; Moreira, C.; Silva, M.; Nunes, R.; Madureira, L.; Rocha, C.M.R.; Pereira, R.N.; Vicente, A.A.; Teixeira, J.A. Algal Proteins: Production Strategies and Nutritional and Functional Properties. Bioresour. Technol. 2021, 332, 125125. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.; Hashim, S.N.; Rahman, H.A. Seaweeds: A Sustainable Functional Food for Complementary and Alternative Therapy. Trends Food Sci. Technol. 2012, 23, 83–96. [Google Scholar] [CrossRef]
- Nagarajan, M.; Rajesh Kumar, R.; Meenakshi Sundaram, K.; Sundararaman, M. Marine Biotechnology: Potentials of Marine Microbes and Algae with Reference to Pharmacological and Commercial Values. In Plant Biology and Biotechnology: Volume II: Plant Genomics and Biotechnology; Bahadur, B., Venkat Rajam, M., Sahijram, L., Krishnamurthy, K.V., Eds.; Springer India: New Delhi, India, 2015; pp. 685–723. ISBN 978-81-322-2283-5. [Google Scholar]
- López-Hortas, L.; Flórez-Fernández, N.; Torres, M.D.; Ferreira-Anta, T.; Casas, M.P.; Balboa, E.M.; Falqué, E.; Domínguez, H. Applying Seaweed Compounds in Cosmetics, Cosmeceuticals and Nutricosmetics. Mar. Drugs 2021, 19, 552. [Google Scholar] [CrossRef]
- Cassani, L.; Lourenço-Lopes, C.; Barral-Martinez, M.; Chamorro, F.; Garcia-Perez, P.; Simal-Gandara, J.; Prieto, M.A. Thermochemical Characterization of Eight Seaweed Species and Evaluation of Their Potential Use as an Alternative for Biofuel Production and Source of Bioactive Compounds. Int. J. Mol. Sci. 2022, 23, 2355. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, D.; Luo, G.; Zhang, S.; Chen, J. Macroalgae for Biofuels Production: Progress and Perspectives. Renew. Sustain. Energy Rev. 2015, 47, 427–437. [Google Scholar] [CrossRef]
- Nisizawa, K.; Noda, H.; Kikuchi, R.; Watanabe, T. The Main Seaweed Foods in Japan. Hydrobiologia 1987, 151, 5–29. [Google Scholar] [CrossRef]
- Ganesan, A.R.; Tiwari, U.; Rajauria, G. Seaweed Nutraceuticals and Their Therapeutic Role in Disease Prevention. Food Sci. Hum. Wellness 2019, 8, 252–263. [Google Scholar] [CrossRef]
- Admassu, H.; Gasmalla, M.A.A.; Yang, R.; Zhao, W. Bioactive Peptides Derived from Seaweed Protein and Their Health Benefits: Antihypertensive, Antioxidant, and Antidiabetic Properties. J. Food Sci. 2018, 83, 6–16. [Google Scholar] [CrossRef] [Green Version]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as Nutritional and Functional Food Sources: Revisiting Our Understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.; Machado, S.; Pimentel, F.B.; Freitas, V.; Alves, R.C.; Oliveira, M.B.P.P. Amino Acid Profile and Protein Quality Assessment of Macroalgae Produced in an Integrated Multi-Trophic Aquaculture System. Foods 2020, 9, 1382. [Google Scholar] [CrossRef] [PubMed]
- Pliego-Cortés, H.; Wijesekara, I.; Lang, M.; Bourgougnon, N.; Bedoux, G. Chapter Nine—Current Knowledge and Challenges in Extraction, Characterization and Bioactivity of Seaweed Protein and Seaweed-Derived Proteins. Adv. Bot. Res. 2020, 95, 289–326. [Google Scholar] [CrossRef]
- Naseri, A.; Marinho, G.S.; Holdt, S.L.; Bartela, J.M.; Jacobsen, C. Enzyme-Assisted Extraction and Characterization of Protein from Red Seaweed Palmaria palmata. Algal Res. 2020, 47, 101849. [Google Scholar] [CrossRef]
- Peñalver, R.; Lorenzo, J.M.; Ros, G.; Amarowicz, R.; Pateiro, M.; Nieto, G. Seaweeds as a Functional Ingredient for a Healthy Diet. Mar. Drugs 2020, 18, 301. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, J.; Seca, A.M.L.; Pinto, D.C.G.A.; Michalak, I.; Trincone, A.; Mishra, A.P.; Nigam, M.; Zam, W.; Martins, N. Current Trends on Seaweeds: Looking at Chemical Composition, Phytopharmacology, and Cosmetic Applications. Molecules 2019, 24, 4182. [Google Scholar] [CrossRef] [Green Version]
- Pereira, L. A Review of the Nutrient Composition of Selected Edible Seaweeds. In Seaweed: Ecology, Nutrient Composition and Medicinal Uses; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2011; pp. 15–47. ISBN 978-1-61470-878-0. [Google Scholar]
- Marsham, S.; Scott, G.W.; Tobin, M.L. Comparison of Nutritive Chemistry of a Range of Temperate Seaweeds. Food Chem. 2007, 100, 1331–1336. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Agregán, R.; Munekata, P.E.S.; Franco, D.; Carballo, J.; Şahin, S.; Lacomba, R.; Barba, F.J. Proximate Composition and Nutritional Value of Three Macroalgae: Ascophyllum Nodosum, Fucus Vesiculosus and Bifurcaria Bifurcata. Mar. Drugs 2017, 15, 360. [Google Scholar] [CrossRef] [Green Version]
- Fleurence, J. Seaweed Proteins: Biochemical, Nutritional Aspects and Potential Uses. Trends Food Sci. Technol. 1999, 10, 25–28. [Google Scholar] [CrossRef]
- Harnedy, P.A.; FitzGerald, R.J. Bioactive Proteins, Peptides, and Amino Acids from Macroalgae. J. Phycol. 2011, 47, 218–232. [Google Scholar] [CrossRef]
- Dickson, R.; Ryu, J.-H.; Liu, J.J. Optimal Plant Design for Integrated Biorefinery Producing Bioethanol and Protein from Saccharina Japonica: A Superstructure-Based Approach. Energy 2018, 164, 1257–1270. [Google Scholar] [CrossRef]
- Dawczynski, C.; Schubert, R.; Jahreis, G. Amino Acids, Fatty Acids, and Dietary Fibre in Edible Seaweed Products. Food Chem. 2007, 103, 891–899. [Google Scholar] [CrossRef]
- Nagappan, T.; Vairappan, C.S. Nutritional and Bioactive Properties of Three Edible Species of Green Algae, Genus Caulerpa (Caulerpaceae). J. Appl. Phycol. 2014, 26, 1019–1027. [Google Scholar] [CrossRef]
- Dumay, J.; Morançais, M. Chapter 9—Proteins and Pigments. In Seaweed in Health and Disease Prevention; Fleurence, J., Levine, I., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 275–318. ISBN 978-0-12-802772-1. [Google Scholar]
- Afonso, C.; Correia, A.P.; Freitas, M.V.; Baptista, T.; Neves, M.; Mouga, T. Seasonal Changes in the Nutritional Composition of Agarophyton vermiculophyllum (Rhodophyta, Gracilariales) from the Center of Portugal. Foods 2021, 10, 1145. [Google Scholar] [CrossRef]
- Kulshreshtha, G.; Burlot, A.-S.; Marty, C.; Critchley, A.; Hafting, J.; Bedoux, G.; Bourgougnon, N.; Prithiviraj, B. Enzyme-Assisted Extraction of Bioactive Material from Chondrus Crispus and Codium Fragile and Its Effect on Herpes Simplex Virus (HSV-1). Mar. Drugs 2015, 13, 558–580. [Google Scholar] [CrossRef] [Green Version]
- Tabarsa, M.; Rezaei, M.; Ramezanpour, Z.; Waaland, J.R. Chemical Compositions of the Marine Algae Gracilaria Salicornia (Rhodophyta) and Ulva Lactuca (Chlorophyta) as a Potential Food Source. J. Sci. Food Agric. 2012, 92, 2500–2506. [Google Scholar] [CrossRef]
- Rosemary, T.; Arulkumar, A.; Paramasivam, S.; Mondragon-Portocarrero, A.; Miranda, J.M. Biochemical, Micronutrient and Physicochemical Properties of the Dried Red Seaweeds Gracilaria Edulis and Gracilaria Corticata. Molecules 2019, 24, 2225. [Google Scholar] [CrossRef] [Green Version]
- Francavilla, M.; Manara, P.; Kamaterou, P.; Monteleone, M.; Zabaniotou, A. Cascade Approach of Red Macroalgae Gracilaria Gracilis Sustainable Valorization by Extraction of Phycobiliproteins and Pyrolysis of Residue. Bioresour. Technol. 2015, 184, 305–313. [Google Scholar] [CrossRef]
- Patarra, R.F.; Paiva, L.; Neto, A.I.; Lima, E.; Baptista, J. Nutritional Value of Selected Macroalgae. J. Appl. Phycol. 2011, 23, 205–208. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Jónsdóttir, R.; Kristinsson, H.G.; Hreggvidsson, G.O.; Jónsson, J.Ó.; Thorkelsson, G.; Ólafsdóttir, G. Enzyme-Enhanced Extraction of Antioxidant Ingredients from Red Algae Palmaria palmata. LWT—Food Sci. Technol. 2010, 43, 1387–1393. [Google Scholar] [CrossRef]
- Cian, R.E.; Fajardo, M.A.; Alaiz, M.; Vioque, J.; González, R.J.; Drago, S.R. Chemical Composition, Nutritional and Antioxidant Properties of the Red Edible Seaweed Porphyra Columbina. Int. J. Food Sci. Nutr. 2014, 65, 299–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Sahoo, D.; Levine, I. Assessment of Nutritional Value in a Brown Seaweed Sargassum Wightii and Their Seasonal Variations. Algal Res. 2015, 9, 117–125. [Google Scholar] [CrossRef]
- O’ Connor, J.; Meaney, S.; Williams, G.A.; Hayes, M. Extraction of Protein from Four Different Seaweeds Using Three Different Physical Pre-Treatment Strategies. Molecules 2020, 25, 2005. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.Y.; Robertson, J.; Hamid, N.; Ma, Q.; Lu, J. Changes in Total Nitrogen and Amino Acid Composition of New Zealand Undaria pinnatifida with Growth, Location and Plant Parts. Food Chem. 2015, 186, 319–325. [Google Scholar] [CrossRef]
- Biancarosa, I.; Espe, M.; Bruckner, C.G.; Heesch, S.; Liland, N.; Waagbø, R.; Torstensen, B.; Lock, E.J. Amino Acid Composition, Protein Content, and Nitrogen-to-Protein Conversion Factors of 21 Seaweed Species from Norwegian Waters. J. Appl. Phycol. 2017, 29, 1001–1009. [Google Scholar] [CrossRef]
- Bjarnadóttir, M.; Aðalbjörnsson, B.V.; Nilsson, A.; Slizyte, R.; Roleda, M.Y.; Hreggviðsson, G.Ó.; Friðjónsson, Ó.H.; Jónsdóttir, R. Palmaria palmata as an Alternative Protein Source: Enzymatic Protein Extraction, Amino Acid Composition, and Nitrogen-to-Protein Conversion Factor. J. Appl. Phycol. 2018, 30, 2061–2070. [Google Scholar] [CrossRef]
- Lourenço, S.O.; Barbarino, E.; De-Paula, J.C.; Pereira, L.O.D.S.; Marquez, U.M.L. Amino Acid Composition, Protein Content and Calculation of Nitrogen-to-Protein Conversion Factors for 19 Tropical Seaweeds. Phycol. Res. 2002, 50, 233–241. [Google Scholar] [CrossRef]
- Shuuluka, D.; Bolton, J.J.; Anderson, R.J. Protein Content, Amino Acid Composition and Nitrogen-to-Protein Conversion Factors of Ulva Rigida and Ulva Capensis from Natural Populations and Ulva Lactuca from an Aquaculture System, in South Africa. J. Appl. Phycol. 2013, 25, 677–685. [Google Scholar] [CrossRef]
- Černá, M. Chapter 24—Seaweed Proteins and Amino Acids as Nutraceuticals. Adv. Food Nutr. Res. 2011, 64, 297–312. [Google Scholar] [CrossRef]
- Cherry, P.; O’Hara, C.; Magee, P.J.; McSorley, E.M.; Allsopp, P.J. Risks and Benefits of Consuming Edible Seaweeds. Nutr. Rev. 2019, 77, 307–329. [Google Scholar] [CrossRef] [Green Version]
- Pangestuti, R.; Kim, S.-K. Chapter 6—Seaweed Proteins, Peptides, and Amino Acids. In Seaweed Sustainability; Tiwari, B.K., Troy, D.J., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 125–140. ISBN 978-0-12-418697-2. [Google Scholar]
- Joint FAO/WHO/UNU Protein and Amino Acid Requirements in Human Nutrition; World Health Organization: Geneva, Switzerland, 2007; ISBN 978-92-4-120935-9.
- Cofrades, S.; López-Lopez, I.; Bravo, L.; Ruiz-Capillas, C.; Bastida, S.; Larrea, M.T.; Jiménez-Colmenero, F. Nutritional and Antioxidant Properties of Different Brown and Red Spanish Edible Seaweeds. Food Sci. Technol. Int. 2010, 16, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Vieira, E.F.; Soares, C.; Machado, S.; Correia, M.; Ramalhosa, M.J.; Oliva-teles, M.T.; Paula Carvalho, A.; Domingues, V.F.; Antunes, F.; Oliveira, T.A.C.; et al. Seaweeds from the Portuguese Coast as a Source of Proteinaceous Material: Total and Free Amino Acid Composition Profile. Food Chem. 2018, 269, 264–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benjama, O.; Masniyom, P. Nutritional Composition and Physicochemical Properties of Two Green Seaweeds (Ulva Pertusa and U. Intestinalis) from the Pattani Bay in Southern Thailand. Songklanakarin J. Sci. Technol. 2011, 33, 575–583. [Google Scholar]
- Wong, K.H.; Cheung, P.C.K. Nutritional Evaluation of Some Subtropical Red and Green Seaweeds Part II. In Vitro Protein Digestibility and Amino Acid Profiles of Protein Concentrates. Food Chem. 2001, 72, 11–17. [Google Scholar] [CrossRef]
- Chan, P.T.; Matanjun, P. Chemical Composition and Physicochemical Properties of Tropical Red Seaweed, Gracilaria Changii. Food Chem. 2017, 221, 302–310. [Google Scholar] [CrossRef]
- Mohammed, H.O.; O’Grady, M.N.; O’Sullivan, M.G.; Hamill, R.M.; Kilcawley, K.N.; Kerry, J.P. An Assessment of Selected Nutritional, Bioactive, Thermal and Technological Properties of Brown and Red Irish Seaweed Species. Foods 2021, 10, 2784. [Google Scholar] [CrossRef]
- De Bhowmick, G.; Hayes, M. In Vitro Protein Digestibility of Selected Seaweeds. Foods 2022, 11, 289. [Google Scholar] [CrossRef]
- Astorga-España, M.S.; Rodríguez-Galdón, B.; Rodríguez-Rodríguez, E.M.; Díaz-Romero, C. Amino Acid Content in Seaweeds from the Magellan Straits (Chile). J. Food Compos. Anal. 2016, 53, 77–84. [Google Scholar] [CrossRef]
- Bikker, P.; Stokvis, L.; Van Krimpen, M.M.; Van Wikselaar, P.G.; Cone, J.W. Evaluation of Seaweeds from Marine Waters in Northwestern Europe for Application in Animal Nutrition. Anim. Feed. Sci. Technol. 2020, 263, 114460. [Google Scholar] [CrossRef]
- Kadam, S.U.; Álvarez, C.; Tiwari, B.K.; O’Donnell, C.P. Extraction and Characterization of Protein from Irish Brown Seaweed Ascophyllum Nodosum. Food Res. Int. 2017, 99, 1021–1027. [Google Scholar] [CrossRef]
- Kazir, M.; Abuhassira, Y.; Robin, A.; Nahor, O.; Luo, J.; Israel, A.; Golberg, A.; Livney, Y.D. Extraction of Proteins from Two Marine Macroalgae, Ulva sp. and Gracilaria sp., for Food Application, and Evaluating Digestibility, Amino Acid Composition and Antioxidant Properties of the Protein Concentrates. Food Hydrocoll. 2019, 87, 194–203. [Google Scholar] [CrossRef]
- Bak, U.G.; Nielsen, C.W.; Marinho, G.S.; Gregersen, Ó.; Jónsdóttir, R.; Holdt, S.L. The Seasonal Variation in Nitrogen, Amino Acid, Protein and Nitrogen-to-Protein Conversion Factors of Commercially Cultivated Faroese Saccharina latissima. Algal Res. 2019, 42, 101576. [Google Scholar] [CrossRef]
- Uribe, E.; Vega-Gálvez, A.; García, V.; Pastén, A.; López, J.; Goñi, G. Effect of Different Drying Methods on Phytochemical Content and Amino Acid and Fatty Acid Profiles of the Green Seaweed, Ulva spp. J. Appl. Phycol. 2019, 31, 1967–1979. [Google Scholar] [CrossRef]
- Ramu Ganesan, A.; Subramani, K.; Shanmugam, M.; Seedevi, P.; Park, S.; Alfarhan, A.H.; Rajagopal, R.; Balasubramanian, B. A Comparison of Nutritional Value of Underexploited Edible Seaweeds with Recommended Dietary Allowances. J. King Saud Univ.—Sci. 2020, 32, 1206–1211. [Google Scholar] [CrossRef]
- Wong, K.H.; Cheung, P.C.K. Nutritional Evaluation of Some Subtropical Red and Green Seaweeds: Part I —Proximate Composition, Amino Acid Profiles and Some Physico-Chemical Properties. Food Chem. 2000, 71, 475–482. [Google Scholar] [CrossRef]
- Norziah, M.H.; Ching, C.Y. Nutritional Composition of Edible Seaweed Gracilaria Changgi. Food Chem. 2000, 68, 69–76. [Google Scholar] [CrossRef]
- Nazarudin, M.F.; Alias, N.H.; Balakrishnan, S.; Wan Hasnan, W.N.I.; Noor Mazli, N.A.I.; Ahmad, M.I.; Md Yasin, I.-S.; Isha, A.; Aliyu-Paiko, M. Chemical, Nutrient and Physicochemical Properties of Brown Seaweed, Sargassum Polycystum C. Agardh (Phaeophyceae) Collected from Port Dickson, Peninsular Malaysia. Molecules 2021, 26, 5216. [Google Scholar] [CrossRef]
- Conde, E.; Balboa, E.M.; Parada, M.; Falqué, E. 4—Algal Proteins, Peptides and Amino Acids. In Functional Ingredients from Algae for Foods and Nutraceuticals; Domínguez, H., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2013; pp. 135–180. ISBN 978-0-85709-512-1. [Google Scholar]
- Galland-Irmouli, A.-V.; Fleurence, J.; Lamghari, R.; Luçon, M.; Rouxel, C.; Barbaroux, O.; Bronowicki, J.-P.; Villaume, C.; Guéant, J.-L. Nutritional Value of Proteins from Edible Seaweed Palmaria Palmata (Dulse). J. Nutr. Biochem. 1999, 10, 353–359. [Google Scholar] [CrossRef]
- Bejosano, F.P.; Corke, H. Protein Quality Evaluation of Amaranthus Wholemeal Flours and Protein Concentrates. J. Sci. Food Agric. 1998, 76, 100–106. [Google Scholar] [CrossRef]
- Kies, C. Bioavailability: A Factor in Protein Quality. J. Agric. Food Chem. 1981, 29, 435–440. [Google Scholar] [CrossRef]
- Swaisgood, H.E.; Catignani, G.L. Protein Digestibility: In Vitro Methods of Assessment. In Advances in Food and Nutrition Research; Kinsella, J.E., Ed.; Academic Press: Cambridge, MA, USA, 1991; Volume 35, pp. 185–236. [Google Scholar]
- Marrion, O.; Fleurence, J.; Schwertz, A.; Guéant, J.-L.; Mamelouk, L.; Ksouri, J.; Villaume, C. Evaluation of Protein in Vitro Digestibility of Palmaria Palmata and Gracilaria Verrucosa. J. Appl. Phycol. 2005, 17, 99–102. [Google Scholar] [CrossRef]
- Tibbetts, S.M.; Milley, J.E.; Lall, S.P. Nutritional Quality of Some Wild and Cultivated Seaweeds: Nutrient Composition, Total Phenolic Content and in Vitro Digestibility. J. Appl. Phycol. 2016, 28, 3575–3585. [Google Scholar] [CrossRef]
- Mišurcová, L.; Kráčmar, S.; Klejdus, B.; Vacek, J. Nitrogen Content, Dietary Fiber, and Digestibility in Algal Food Products. Czech. J. Food Sci. 2010, 28, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Machů, L.; Mišurcová, L.; Samek, D.; Hrabě, J.; Fišera, M. In Vitro Digestibility of Different Commercial Edible Algae Products. J. Aquat. Food Prod. Technol. 2014, 23, 423–435. [Google Scholar] [CrossRef]
- Fujiwara-Arasaki, T.; Mino, N.; Kuroda, M. The Protein Value in Human Nutrition of Edible Marine Algae in Japan. In Proceedings of the Eleventh International Seaweed Symposium, Qingdao, China, 19–25 June 1983; Bird, C.J., Ragan, M.A., Eds.; Springer: Dordrecht, The Netherlands, 1984; pp. 513–516. [Google Scholar]
- Echave, J.; Fraga-Corral, M.; Garcia-Perez, P.; Popović-Djordjević, J.; Avdović, E.H.; Radulović, M.; Xiao, J.; Prieto, M.A.; Simal-Gandara, J. Seaweed Protein Hydrolysates and Bioactive Peptides: Extraction, Purification, and Applications. Mar. Drugs 2021, 19, 500. [Google Scholar] [CrossRef] [PubMed]
- Gajaria, T.K.; Suthar, P.; Baghel, R.S.; Balar, N.B.; Sharnagat, P.; Mantri, V.A.; Reddy, C.R.K. Integration of Protein Extraction with a Stream of Byproducts from Marine Macroalgae: A Model Forms the Basis for Marine Bioeconomy. Bioresour. Technol. 2017, 243, 867–873. [Google Scholar] [CrossRef]
- Vásquez, V.; Martínez, R.; Bernal, C. Enzyme-Assisted Extraction of Proteins from the Seaweeds Macrocystis pyrifera and Chondracanthus chamissoi: Characterization of the Extracts and Their Bioactive Potential. J. Appl. Phycol. 2019, 31, 1999–2010. [Google Scholar] [CrossRef]
- Amorim, M.L.; Soares, J.; Coimbra, J.S.D.R.; De Oliviera Leite, M.; Albino, L.F.T.; Martins, M.A. Microalgae Proteins: Production, Separation, Isolation, Quantification, and Application in Food and Feed. Crit. Rev. Food Sci. Nutr. 2021, 61, 1976–2002. [Google Scholar] [CrossRef]
- Naseri, A.; Jacobsen, C.; Sejberg, J.J.P.; Pedersen, T.E.; Larsen, J.; Hansen, K.M.; Holdt, S.L. Multi-Extraction and Quality of Protein and Carrageenan from Commercial Spinosum (Eucheuma denticulatum). Foods 2020, 9, 1072. [Google Scholar] [CrossRef]
- Nadeeshani, H.; Hassouna, A.; Lu, J. Proteins Extracted from Seaweed Undaria pinnatifida and Their Potential Uses as Foods and Nutraceuticals. Crit. Rev. Food Sci. Nutr. 2021, 1–17. [Google Scholar] [CrossRef]
- Tremblay, A.; Beaulieu, L. Extraction Technologies for Proteins and Peptides. In Recent Advances in Micro and Macroalgal Processing; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2021; pp. 141–162. ISBN 978-1-119-54265-0. [Google Scholar]
- Abdollahi, M.; Axelsson, J.; Carlsson, N.-G.; Nylund, G.M.; Albers, E.; Undeland, I. Effect of Stabilization Method and Freeze/Thaw-Aided Precipitation on Structural and Functional Properties of Proteins Recovered from Brown Seaweed (Saccharina latissima). Food Hydrocoll. 2019, 96, 140–150. [Google Scholar] [CrossRef]
- Cermeño, M.; Kleekayai, T.; Amigo-Benavent, M.; Harnedy-Rothwell, P.; FitzGerald, R.J. Current Knowledge on the Extraction, Purification, Identification, and Validation of Bioactive Peptides from Seaweed. Electrophoresis 2020, 41, 1694–1717. [Google Scholar] [CrossRef]
- Badmus, U.O.; Taggart, M.A.; Boyd, K.G. The Effect of Different Drying Methods on Certain Nutritionally Important Chemical Constituents in Edible Brown Seaweeds. J. Appl. Phycol. 2019, 31, 3883–3897. [Google Scholar] [CrossRef] [Green Version]
- Harrysson, H.; Hayes, M.; Eimer, F.; Carlsson, N.-G.; Toth, G.B.; Undeland, I. Production of Protein Extracts from Swedish Red, Green, and Brown Seaweeds, Porphyra umbilicalis Kützing, Ulva Lactuca Linnaeus, and Saccharina latissima (Linnaeus) J. V. Lamouroux Using Three Different Methods. J. Appl. Phycol. 2018, 30, 3565–3580. [Google Scholar] [CrossRef] [Green Version]
- Tanna, B.; Mishra, A. Metabolites Unravel Nutraceutical Potential of Edible Seaweeds: An Emerging Source of Functional Food. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1613–1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, E.M.; Allsopp, P.J.; Magee, P.J.; Gill, C.I.; Nitecki, S.; Strain, C.R.; McSorley, E.M. Seaweed and Human Health. Nutr. Rev. 2014, 72, 205–216. [Google Scholar] [CrossRef]
- Saikia, S.; Mahnot, N.K.; Sahu, R.K.; Kalita, J. Edible Seaweeds as Potential Source of Nutraceuticals. In Marine Niche: Applications in Pharmaceutical Sciences: Translational Research; Nathani, N.M., Mootapally, C., Gadhvi, I.R., Maitreya, B., Joshi, C.G., Eds.; Springer: Singapore, 2020; pp. 183–201. ISBN 9789811550171. [Google Scholar]
- Flórez-Fernández, N.; Torres, M.D.; Braz, L.; Grenha, A.; Loret, E.P.; Domínguez, H. Seaweed and Sea Anemones Proteins as a Source of New Pharmaceutical Active Principles. In Marine Niche: Applications in Pharmaceutical Sciences: Translational Research; Nathani, N.M., Mootapally, C., Gadhvi, I.R., Maitreya, B., Joshi, C.G., Eds.; Springer: Singapore, 2020; pp. 203–219. ISBN 9789811550171. [Google Scholar]
- Dmitrenok, A.; Iwashita, T.; Nakajima, T.; Sakamoto, B.; Namikoshi, M.; Nagai, H. New Cyclic Depsipeptides from the Green Alga Bryopsis Species; Application of a Carboxypeptidase Hydrolysis Reaction to the Structure Determination. Tetrahedron 2006, 62, 1301–1308. [Google Scholar] [CrossRef]
- Deng, Z.; Liu, Y.; Wang, J.; Wu, S.; Geng, L.; Sui, Z.; Zhang, Q. Antihypertensive Effects of Two Novel Angiotensin I-Converting Enzyme (ACE) Inhibitory Peptides from Gracilariopsis lemaneiformis (Rhodophyta) in Spontaneously Hypertensive Rats (SHRs). Mar. Drugs 2018, 16, 299. [Google Scholar] [CrossRef] [Green Version]
- Kumagai, Y.; Kitade, Y.; Kobayashi, M.; Watanabe, K.; Kurita, H.; Takeda, H.; Yasui, H.; Kishimura, H. Identification of ACE Inhibitory Peptides from Red Alga Mazzaella japonica. Eur. Food Res. Technol. 2020, 246, 2225–2231. [Google Scholar] [CrossRef]
- Kitade, Y.; Miyabe, Y.; Yamamoto, Y.; Takeda, H.; Shimizu, T.; Yasui, H.; Kishimura, H. Structural Characteristics of Phycobiliproteins from Red Alga Mazzaella japonica. J. Food Biochem. 2018, 42, e12436. [Google Scholar] [CrossRef] [Green Version]
- Qu, W.; Ma, H.; Pan, Z.; Luo, L.; Wang, Z.; He, R. Preparation and Antihypertensive Activity of Peptides from Porphyra Yezoensis. Food Chem. 2010, 123, 14–20. [Google Scholar] [CrossRef]
- Jiao, K.; Gao, J.; Zhou, T.; Yu, J.; Song, H.; Wei, Y.; Gao, X. Isolation and Purification of a Novel Antimicrobial Peptide from Porphyra Yezoensis. J. Food Biochem. 2019, 43, e12864. [Google Scholar] [CrossRef] [PubMed]
- Furuta, T.; Miyabe, Y.; Yasui, H.; Kinoshita, Y.; Kishimura, H. Angiotensin I Converting Enzyme Inhibitory Peptides Derived from Phycobiliproteins of Dulse Palmaria palmata. Mar. Drugs 2016, 14, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLaughlin, C.M.; Harnedy-Rothwell, P.A.; Lafferty, R.A.; Sharkey, S.; Parthsarathy, V.; Allsopp, P.J.; McSorley, E.M.; FitzGerald, R.J.; O’Harte, F.P.M. Macroalgal Protein Hydrolysates from Palmaria palmata Influence the ‘Incretin Effect’ in Vitro via DPP-4 Inhibition and Upregulation of Insulin, GLP-1 and GIP Secretion. Eur. J. Nutr. 2021, 60, 4439–4452. [Google Scholar] [CrossRef]
- McLaughlin, C.M.; Sharkey, S.J.; Harnedy-Rothwell, P.; Parthsarathy, V.; Allsopp, P.J.; McSorley, E.M.; FitzGerald, R.J.; O’Harte, F.P.M. Twice Daily Oral Administration of Palmaria palmata Protein Hydrolysate Reduces Food Intake in Streptozotocin Induced Diabetic Mice, Improving Glycaemic Control and Lipid Profiles. J. Funct. Foods 2020, 73, 104101. [Google Scholar] [CrossRef]
- Harnedy, P.A.; O’Keeffe, M.B.; FitzGerald, R.J. Purification and Identification of Dipeptidyl Peptidase (DPP) IV Inhibitory Peptides from the Macroalga Palmaria palmata. Food Chem. 2015, 172, 400–406. [Google Scholar] [CrossRef]
- Fitzgerald, C.; Gallagher, E.; O’Connor, P.; Prieto, J.; Mora-Soler, L.; Grealy, M.; Hayes, M. Development of a Seaweed Derived Platelet Activating Factor Acetylhydrolase (PAF-AH) Inhibitory Hydrolysate, Synthesis of Inhibitory Peptides and Assessment of Their Toxicity Using the Zebrafish Larvae Assay. Peptides 2013, 50, 119–124. [Google Scholar] [CrossRef]
- Fitzgerald, C.; Mora-Soler, L.; Gallagher, E.; O’Connor, P.; Prieto, J.; Soler-Vila, A.; Hayes, M. Isolation and Characterization of Bioactive Pro-Peptides with in Vitro Renin Inhibitory Activities from the Macroalga Palmaria palmata. J. Agric. Food Chem. 2012, 60, 7421–7427. [Google Scholar] [CrossRef]
- Harnedy, P.A.; O’Keeffe, M.B.; FitzGerald, R.J. Fractionation and Identification of Antioxidant Peptides from an Enzymatically Hydrolysed Palmaria palmata Protein Isolate. Food Res. Int. 2017, 100, 416–422. [Google Scholar] [CrossRef] [Green Version]
- Cermeño, M.; Stack, J.; Tobin, P.R.; O’Keeffe, M.B.; Harnedy, P.A.; Stengel, D.B.; FitzGerald, R.J. Peptide Identification from a Porphyra Dioica Protein Hydrolysate with Antioxidant, Angiotensin Converting Enzyme and Dipeptidyl Peptidase IV Inhibitory Activities. Food Funct. 2019, 10, 3421–3429. [Google Scholar] [CrossRef]
- Cian, R.E.; Martínez-Augustin, O.; Drago, S.R. Bioactive Properties of Peptides Obtained by Enzymatic Hydrolysis from Protein Byproducts of Porphyra Columbina. Food Res. Int. 2012, 49, 364–372. [Google Scholar] [CrossRef]
- Beaulieu, L.; Bondu, S.; Doiron, K.; Rioux, L.-E.; Turgeon, S.L. Characterization of Antibacterial Activity from Protein Hydrolysates of the Macroalga Saccharina Longicruris and Identification of Peptides Implied in Bioactivity. J. Funct. Foods 2015, 17, 685–697. [Google Scholar] [CrossRef]
- Liu, X.; Wang, C.-Y.; Shao, C.-L.; Wei, Y.-X.; Wang, B.-G.; Sun, L.-L.; Zheng, C.-J.; Guan, H.-S. Chemical Constituents from Sargassum Pallidum (Turn.) C. Agardh. Biochem. Syst. Ecol. 2009, 37, 127–129. [Google Scholar] [CrossRef]
- Paiva, L.; Lima, E.; Neto, A.I.; Baptista, J. Isolation and Characterization of Angiotensin I-Converting Enzyme (ACE) Inhibitory Peptides from Ulva Rigida, C. Agardh Protein Hydrolysate. J. Funct. Foods 2016, 26, 65–76. [Google Scholar] [CrossRef]
- Suetsuna, K.; Nakano, T. Identification of an Antihypertensive Peptide from Peptic Digest of Wakame (Undaria pinnatifida). J. Nutr. Biochem. 2000, 11, 450–454. [Google Scholar] [CrossRef]
- Sato, M.; Hosokawa, T.; Yamaguchi, T.; Nakano, T.; Muramoto, K.; Kahara, T.; Funayama, K.; Kobayashi, A.; Nakano, T. Angiotensin I-Converting Enzyme Inhibitory Peptides Derived from Wakame (Undaria pinnatifida) and Their Antihypertensive Effect in Spontaneously Hypertensive Rats. J. Agric. Food Chem. 2002, 50, 6245–6252. [Google Scholar] [CrossRef]
- Barre, A.; Van Damme, E.J.M.; Simplicien, M.; Le Poder, S.; Klonjkowski, B.; Benoist, H.; Peyrade, D.; Rougé, P. Man-Specific Lectins from Plants, Fungi, Algae and Cyanobacteria, as Potential Blockers for SARS-CoV, MERS-CoV and SARS-CoV-2 (COVID-19) Coronaviruses: Biomedical Perspectives. Cells 2021, 10, 1619. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Wong, J.H.; Pan, W.; Chan, Y.S.; Yin, C.; Dan, X.; Ng, T.B. Marine Lectins and Their Medicinal Applications. Appl. Microbiol. Biotechnol. 2015, 99, 3755–3773. [Google Scholar] [CrossRef]
- Vanderlei, E.S.O.; Patoilo, K.K.N.R.; Lima, N.A.; Lima, A.P.S.; Rodrigues, J.A.G.; Silva, L.M.C.M.; Lima, M.E.P.; Lima, V.; Benevides, N.M.B. Antinociceptive and Anti-Inflammatory Activities of Lectin from the Marine Green Alga Caulerpa cupressoides. Int. Immunopharmacol. 2010, 10, 1113–1118. [Google Scholar] [CrossRef]
- Smit, A.J. Medicinal and Pharmaceutical Uses of Seaweed Natural Products: A Review. J. Appl. Phycol. 2004, 16, 245–262. [Google Scholar] [CrossRef]
- Mendis, E.; Kim, S.-K. Chapter 1—Present and Future Prospects of Seaweeds in Developing Functional Foods. In Advances in Food and Nutrition Research; Kim, S.-K., Ed.; Marine Medicinal Foods; Academic Press: Cambridge, MA, USA, 2011; Volume 64, pp. 1–15. [Google Scholar]
- Wang, F.; Guo, X.-Y.; Zhang, D.-N.; Wu, Y.; Wu, T.; Chen, Z.-G. Ultrasound-Assisted Extraction and Purification of Taurine from the Red Algae Porphyra yezoensis. Ultrason. Sonochem. 2015, 24, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.D.; Flórez-Fernández, N.; Domínguez, H. Integral Utilization of Red Seaweed for Bioactive Production. Mar. Drugs 2019, 17, 314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holdt, S.L.; Kraan, S. Bioactive Compounds in Seaweed: Functional Food Applications and Legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Mittal, R.; Tavanandi, H.A.; Mantri, V.A.; Raghavarao, K.S.M.S. Ultrasound Assisted Methods for Enhanced Extraction of Phycobiliproteins from Marine Macro-Algae, Gelidium pusillum (Rhodophyta). Ultrason. Sonochem. 2017, 38, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.H. A Short Guide to Abbreviations and Their Use in Peptide Science. J. Pept. Sci. 1999, 5, 465–471. [Google Scholar] [CrossRef]
- Wu, G. Functional Amino Acids in Nutrition and Health. Amino Acids 2013, 45, 407–411. [Google Scholar] [CrossRef] [Green Version]
- Samarakoon, K.; Jeon, Y.-J. Bio-Functionalities of Proteins Derived from Marine Algae—A Review. Food Res. Int. 2012, 48, 948–960. [Google Scholar] [CrossRef]
- Pimentel, F.B.; Alves, R.C.; Harnedy, P.A.; FitzGerald, R.J.; Oliveira, M.B.P.P. Macroalgal-Derived Protein Hydrolysates and Bioactive Peptides: Enzymatic Release and Potential Health Enhancing Properties. Trends Food Sci. Technol. 2019, 93, 106–124. [Google Scholar] [CrossRef]
- Hayes, M. Chapter 14—Seaweeds: A Nutraceutical and Health Food. In Seaweed Sustainability; Tiwari, B.K., Troy, D.J., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 365–387. ISBN 978-0-12-418697-2. [Google Scholar]
- Turner, J.M.; Kodali, R. Should Angiotensin-Converting Enzyme Inhibitors Ever Be Used for the Management of Hypertension? Curr. Cardiol. Rep. 2020, 22, 95. [Google Scholar] [CrossRef]
- Sun, S.; Xu, X.; Sun, X.; Zhang, X.; Chen, X.; Xu, N. Preparation and Identification of ACE Inhibitory Peptides from the Marine Macroalga Ulva Intestinalis. Mar. Drugs 2019, 17, 179. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef] [PubMed]
- Tamama, K. Potential Benefits of Dietary Seaweeds as Protection against COVID-19. Nutr. Rev. 2021, 79, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Admassu, H.; Gasmalla, M.A.A.; Yang, R.; Zhao, W. Identification of Bioactive Peptides with α-Amylase Inhibitory Potential from Enzymatic Protein Hydrolysates of Red Seaweed (Porphyra spp). J. Agric. Food Chem. 2018, 66, 4872–4882. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.-K.; Jatwa, R.; Purohit, A.; Ram, H. Synthetic and Phytocompounds Based Dipeptidyl Peptidase-IV (DPP-IV) Inhibitors for Therapeutics of Diabetes. J. Asian Nat. Prod. Res. 2017, 19, 1036–1045. [Google Scholar] [CrossRef] [PubMed]
- Harnedy, P.A.; FitzGerald, R.J. In Vitro Assessment of the Cardioprotective, Anti-Diabetic and Antioxidant Potential of Palmaria palmata Protein Hydrolysates. J. Appl. Phycol. 2013, 25, 1793–1803. [Google Scholar] [CrossRef]
- Kim, E.-Y.; Choi, Y.H.; Nam, T.-J. Identification and Antioxidant Activity of Synthetic Peptides from Phycobiliproteins of Pyropia Yezoensis. Int. J. Mol. Med. 2018, 42, 789–798. [Google Scholar] [CrossRef] [Green Version]
- Lafarga, T.; Acién-Fernández, F.G.; Garcia-Vaquero, M. Bioactive Peptides and Carbohydrates from Seaweed for Food Applications: Natural Occurrence, Isolation, Purification, and Identification. Algal Res. 2020, 48, 101909. [Google Scholar] [CrossRef]
- Ahn, C.-B.; Jeon, Y.-J.; Kang, D.-S.; Shin, T.-S.; Jung, B.-M. Free Radical Scavenging Activity of Enzymatic Extracts from a Brown Seaweed Scytosiphon Lomentaria by Electron Spin Resonance Spectrometry. Food Res. Int. 2004, 37, 253–258. [Google Scholar] [CrossRef]
- Heo, S.-J.; Park, E.-J.; Lee, K.-W.; Jeon, Y.-J. Antioxidant Activities of Enzymatic Extracts from Brown Seaweeds. Bioresour. Technol. 2005, 96, 1613–1623. [Google Scholar] [CrossRef]
- Lakmal, H.C.; Samarakoon, K.W.; Lee, W.; Lee, J.-H.; Abeytunga, D.T.U.; Lee, H.-S.; Jeon, Y.-J. Anticancer and Antioxidant Effects of Selected Sri Lankan Marine Algae. J. Natl. Sci. Found. Sri Lanka 2014, 42, 315–323. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.; Silva, S.A.; Carpena, M.; Garcia-Oliveira, P.; Gullón, P.; Barroso, M.F.; Prieto, M.A.; Simal-Gandara, J. Macroalgae as a Source of Valuable Antimicrobial Compounds: Extraction and Applications. Antibiotics 2020, 9, 642. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Walia, A.K. Lectins from Red Algae and Their Biomedical Potential. J. Appl. Phycol. 2018, 30, 1833–1858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontenelle, T.P.C.; Lima, G.C.; Mesquita, J.X.; De Souza Lopes, J.L.; De Brito, T.V.; Das Chagas Vieira Júnior, F.; Sales, A.B.; Aragão, K.S.; Souza, M.H.L.P.; Dos Reis Barbosa, A.L.; et al. Lectin Obtained from the Red Seaweed Bryothamnion Triquetrum: Secondary Structure and Anti-Inflammatory Activity in Mice. Int. J. Biol. Macromol. 2018, 112, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, M.A.; Arruda, F.V.S.; Carneiro, V.A.; Silva, H.C.; Nascimento, K.S.; Sampaio, A.H.; Cavada, B.; Teixeira, E.H.; Henriques, M.; Pereira, M.O. Effect of Algae and Plant Lectins on Planktonic Growth and Biofilm Formation in Clinically Relevant Bacteria and Yeasts. BioMed Res. Int. 2014, 2014, e365272. [Google Scholar] [CrossRef] [Green Version]
- Gonzaga do Nascimento-Neto, L.; Carneiro, R.F.; Da Silva, S.R.; Da Silva, B.R.; Arruda, F.V.S.; Carneiro, V.A.; Do Nascimento, K.S.; Saker-Sampaio, S.; Da Silva, V.A.; Porto, A.L.F.; et al. Characterization of Isoforms of the Lectin Isolated from the Red Algae Bryothamnion Seaforthii and Its Pro-Healing Effect. Mar. Drugs 2012, 10, 1936–1954. [Google Scholar] [CrossRef] [Green Version]
- Mori, T.; O’Keefe, B.R.; Sowder, R.C.; Bringans, S.; Gardella, R.; Berg, S.; Cochran, P.; Turpin, J.A.; Buckheit, R.W.; McMahon, J.B.; et al. Isolation and Characterization of Griffithsin, a Novel HIV-Inactivating Protein, from the Red Alga Griffithsia sp. J. Biol. Chem. 2005, 280, 9345–9353. [Google Scholar] [CrossRef] [Green Version]
- Lusvarghi, S.; Bewley, C.A. Griffithsin: An Antiviral Lectin with Outstanding Therapeutic Potential. Viruses 2016, 8, 296. [Google Scholar] [CrossRef]
- Nascimento da Silva, L.C.; Mendonça, J.S.P.; de Oliveira, W.F.; Batista, K.L.R.; Zagmignan, A.; Viana, I.F.T.; dos Santos Correia, M.T. Exploring Lectin–Glycan Interactions to Combat COVID-19: Lessons Acquired from Other Enveloped Viruses. Glycobiology 2021, 31, 358–371. [Google Scholar] [CrossRef]
- O’Keefe, B.R.; Giomarelli, B.; Barnard, D.L.; Shenoy, S.R.; Chan, P.K.S.; McMahon, J.B.; Palmer, K.E.; Barnett, B.W.; Meyerholz, D.K.; Wohlford-Lenane, C.L.; et al. Broad-Spectrum in Vitro Activity and in Vivo Efficacy of the Antiviral Protein Griffithsin against Emerging Viruses of the Family Coronaviridae. J. Virol. 2010, 84, 2511–2521. [Google Scholar] [CrossRef] [Green Version]
- Dumay, J.; Morançais, M.; Munier, M.; Le Guillard, C.; Fleurence, J. Chapter Eleven—Phycoerythrins: Valuable Proteinic Pigments in Red Seaweeds. Adv. Bot. Res. 2014, 71, 321–343. [Google Scholar] [CrossRef]
- Osório, C.; Machado, S.; Peixoto, J.; Bessada, S.; Pimentel, F.B.; Alves, R.C.; Oliveira, M.B.P.P. Pigments Content (Chlorophylls, Fucoxanthin and Phycobiliproteins) of Different Commercial Dried Algae. Separations 2020, 7, 33. [Google Scholar] [CrossRef]
- Sudhakar, M.P.; Jagatheesan, A.; Perumal, K.; Arunkumar, K. Methods of Phycobiliprotein Extraction from Gracilaria Crassa and Its Applications in Food Colourants. Algal Res. 2015, 8, 115–120. [Google Scholar] [CrossRef]
- Lee, D.; Nishizawa, M.; Shimizu, Y.; Saeki, H. Anti-Inflammatory Effects of Dulse (Palmaria Palmata) Resulting from the Simultaneous Water-Extraction of Phycobiliproteins and Chlorophyll a. Food Res. Int. 2017, 100, 514–521. [Google Scholar] [CrossRef]
- Nguyen, H.P.T.; Morançais, M.; Déléris, P.; Fleurence, J.; Nguyen-Le, C.T.; Vo, K.H.; Dumay, J. Purification of R-Phycoerythrin from a Marine Macroalga Gracilaria Gracilis by Anion-Exchange Chromatography. J. Appl. Phycol. 2020, 32, 553–561. [Google Scholar] [CrossRef]
- Le Guillard, C.; Dumay, J.; Donnay-Moreno, C.; Bruzac, S.; Ragon, J.-Y.; Fleurence, J.; Bergé, J.-P. Ultrasound-Assisted Extraction of R-Phycoerythrin from Grateloupia Turuturu with and without Enzyme Addition. Algal Res. 2015, 12, 522–528. [Google Scholar] [CrossRef] [Green Version]
- Cotas, J.; Leandro, A.; Pacheco, D.; Gonçalves, A.M.M.; Pereira, L. A Comprehensive Review of the Nutraceutical and Therapeutic Applications of Red Seaweeds (Rhodophyta). Life 2020, 10, 19. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.-H.; Chen, W.-C.; Gao, Y.-H.; Chen, G.-W.; Lin, H.-T.V.; Pan, C.-L. Enzyme-Assisted Method for Phycobiliproteins Extraction from Porphyra and Evaluation of Their Bioactivity. Processes 2021, 9, 560. [Google Scholar] [CrossRef]
- Kawasaki, A.; Ono, A.; Mizuta, S.; Kamiya, M.; Takenaga, T.; Murakami, S. The Taurine Content of Japanese Seaweed. Adv. Exp. Med. Biol. 2017, 975 Pt 2, 1105–1112. [Google Scholar] [CrossRef]
- Hwang, E.-S.; Ki, K.-N.; Chung, H.-Y. Proximate Composition, Amino Acid, Mineral, and Heavy Metal Content of Dried Laver. Prev. Nutr. Food Sci. 2013, 18, 139–144. [Google Scholar] [CrossRef] [Green Version]
- Bito, T.; Teng, F.; Watanabe, F. Bioactive Compounds of Edible Purple Laver Porphyra sp. (Nori). J. Agric. Food Chem. 2017, 65, 10685–10692. [Google Scholar] [CrossRef]
- Mochizuki, H.; Takido, J.; Oda, H.; Yokogoshi, H. Improving Effect of Dietary Taurine on Marked Hypercholesterolemia Induced by a High-Cholesterol Diet in Streptozotocin-Induced Diabetic Rats. Biosci. Biotechnol. Biochem. 1999, 63, 1984–1987. [Google Scholar] [CrossRef] [PubMed]
- Roberfroid, M.B. Global View on Functional Foods: European Perspectives. Br. J. Nutr. 2002, 88, S133–S138. [Google Scholar] [CrossRef] [PubMed]
- Siró, I.; Kápolna, E.; Kápolna, B.; Lugasi, A. Functional Food. Product Development, Marketing and Consumer Acceptance—A Review. Appetite 2008, 51, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Spence, J.T. Challenges Related to the Composition of Functional Foods. J. Food Compos. Anal. 2006, 19, S4–S6. [Google Scholar] [CrossRef]
- Kumar, Y.; Tarafdar, A.; Badgujar, P.C. Seaweed as a Source of Natural Antioxidants: Therapeutic Activity and Food Applications. J. Food Qual. 2021, 2021, 5753391. [Google Scholar] [CrossRef]
- Siddiqi, R.A.; Singh, T.P.; Rani, M.; Sogi, D.S.; Bhat, M.A. Diversity in Grain, Flour, Amino Acid Composition, Protein Profiling, and Proportion of Total Flour Proteins of Different Wheat Cultivars of North India. Front. Nutr. 2020, 7, 141. [Google Scholar] [CrossRef]
- Prabhasankar, P.; Ganesan, P.; Bhaskar, N.; Hirose, A.; Stephen, N.; Gowda, L.R.; Hosokawa, M.; Miyashita, K. Edible Japanese Seaweed, Wakame (Undaria pinnatifida) as an Ingredient in Pasta: Chemical, Functional and Structural Evaluation. Food Chem. 2009, 115, 501–508. [Google Scholar] [CrossRef]
- Qin, Y. 6—Applications of Bioactive Seaweed Substances in Functional Food Products. In Bioactive Seaweeds for Food Applications; Qin, Y., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 111–134. ISBN 978-0-12-813312-5. [Google Scholar]
- Mouritsen, O.G.; Duelund, L.; Petersen, M.A.; Hartmann, A.L.; Frøst, M.B. Umami Taste, Free Amino Acid Composition, and Volatile Compounds of Brown Seaweeds. J. Appl. Phycol. 2019, 31, 1213–1232. [Google Scholar] [CrossRef]
- Laohakunjit, N.; Selamassakul, O.; Kerdchoechuen, O. Seafood-like Flavour Obtained from the Enzymatic Hydrolysis of the Protein by-Products of Seaweed (Gracilaria sp.). Food Chem. 2014, 158, 162–170. [Google Scholar] [CrossRef]
- Cardoso, S.M.; Pereira, O.R.; Seca, A.M.L.; Pinto, D.C.G.A.; Silva, A.M.S. Seaweeds as Preventive Agents for Cardiovascular Diseases: From Nutrients to Functional Foods. Mar. Drugs 2015, 13, 6838–6865. [Google Scholar] [CrossRef] [Green Version]
- Suganya, T.; Varman, M.; Masjuki, H.H.; Renganathan, S. Macroalgae and Microalgae as a Potential Source for Commercial Applications along with Biofuels Production: A Biorefinery Approach. Renew. Sustain. Energy Rev. 2016, 55, 909–941. [Google Scholar] [CrossRef]
- Griffiths, M.; Harrison, S.T.L.; Smit, M.; Maharajh, D. Major Commercial Products from Micro- and Macroalgae. In Algae Biotechnology: Products and Processes; Bux, F., Chisti, Y., Eds.; Green Energy and Technology; Springer International Publishing: Cham, Switzerland, 2016; pp. 269–300. ISBN 978-3-319-12334-9. [Google Scholar]
- Sekar, S.; Chandramohan, M. Phycobiliproteins as a Commodity: Trends in Applied Research, Patents and Commercialization. J. Appl. Phycol. 2008, 20, 113–136. [Google Scholar] [CrossRef]
- Jayasinghe, P.S.; Pahalawattaarachchi, V.; Ranaweera, K.K.D.S. Seaweed Extract as Natural Food Colouring Agents in Jelly Dessert on Chemical Microbiological and Sensory Quality. In Proceedings of the National Aquatic Resources Research and Development Agency (NARA), Colombo, Sri Lanka, 29 March 2016; pp. 121–124. [Google Scholar]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial Applications of Microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Den Banach, J.L.; Hil, E.F.H.; Van der Fels-Klerx, H.J. Food Safety Hazards in the European Seaweed Chain. Compr. Rev. Food Sci. Food Saf. 2020, 19, 332–364. [Google Scholar] [CrossRef]
- Arulkumar, A.; Nigariga, P.; Paramasivam, S.; Rajaram, R. Metals Accumulation in Edible Marine Algae Collected from Thondi Coast of Palk Bay, Southeastern India. Chemosphere 2019, 221, 856–862. [Google Scholar] [CrossRef]
- Chen, Q.; Pan, X.-D.; Huang, B.-F.; Han, J.-L. Distribution of Metals and Metalloids in Dried Seaweeds and Health Risk to Population in Southeastern China. Sci. Rep. 2018, 8, 3578. [Google Scholar] [CrossRef] [Green Version]
- Taylor, V.F.; Li, Z.; Sayarath, V.; Palys, T.J.; Morse, K.R.; Scholz-Bright, R.A.; Karagas, M.R. Distinct Arsenic Metabolites Following Seaweed Consumption in Humans. Sci. Rep. 2017, 7, 3920. [Google Scholar] [CrossRef]
- Seng, N.; Lai, S.; Fong, J.; Saleh, M.F.; Cheng, C.; Cheok, Z.Y.; Todd, P.A.; Seng, N.; Lai, S.; Fong, J.; et al. Early Evidence of Microplastics on Seagrass and Macroalgae. Mar. Freshw. Res. 2020, 71, 922–928. [Google Scholar] [CrossRef]
- Li, Q.; Feng, Z.; Zhang, T.; Ma, C.; Shi, H. Microplastics in the Commercial Seaweed Nori. J. Hazard. Mater. 2020, 388, 122060. [Google Scholar] [CrossRef]
- Naidu, K.A.; Tewari, A.; Joshi, H.V.; Viswanath, S.; Ramesh, H.P.; Rao, S.V. Evaluation of Nutritional Quality and Food Safety of Seaweeds of India. J. Food Saf. 1993, 13, 77–90. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Mora, L.; Hayes, M. In Vitro and in Silico Approaches to Generating and Identifying Angiotensin-Converting Enzyme I Inhibitory Peptides from Green Macroalga Ulva Lactuca. Mar. Drugs 2019, 17, 204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motoyama, K.; Hamada, Y.; Nagashima, Y.; Shiomi, K. Allergenicity and Allergens of Amphipods Found in Nori (Dried Laver). Food Addit. Contam. 2007, 24, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Polikovsky, M.; Fernand, F.; Sack, M.; Frey, W.; Müller, G.; Golberg, A. In Silico Food Allergenic Risk Evaluation of Proteins Extracted from Macroalgae Ulva sp. with Pulsed Electric Fields. Food Chem. 2019, 276, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Cho, T.J.; Rhee, M.S. Health Functionality and Quality Control of Laver (Porphyra, Pyropia): Current Issues and Future Perspectives as an Edible Seaweed. Mar. Drugs 2020, 18, 14. [Google Scholar] [CrossRef] [Green Version]
- Chapman, R.L. Algae: The World’s Most Important “Plants”—An Introduction. Mitig. Adapt. Strateg. Glob. Chang. 2013, 18, 5–12. [Google Scholar] [CrossRef] [Green Version]

| Green Algae | Red Algae | Brown Algae |
|---|---|---|
| Caulerpa spp. | Champia compressa | Alaria esculenta |
| Codium spp. | Chondrus crispus | Ascophyllum nodosum |
| Enteromorpha spp. | Eucheuma denticulatum (formerly Eucheuma spinosum) | Durvillaea antarctica |
| Monostroma spp. | Gelidiella acerosa | Eisenia bicyclis |
| Ulva spp. (formerly Enteromorpha spp.) | Gracilaria corticata | Fucus serratus |
| Ulva lactuca (formerly Ulva fasciata) (sea lettuce) | Gracilaria edulis | Fucus vesiculosus |
| Ulva australis (formerly Ulva pertusa) | Gracilariopsis longissima (formerly Gracilaria verrucosa) | Himanthalia elongata |
| Mastocarpus stellatus | Laminaria digitata | |
| Osmundea pinnatifida | Laminaria hyperborea | |
| Palmaria palmata (dulse) | Postelsia palmiformis | |
| Porphyra spp. (nori) | Saccharina japonica (formerly Laminaria japonica) | |
| Porphyra laciniata | Padina spp. | |
| Porphyra umbilicalis | Sargassum fusiforme | |
| Pyropia columbina (formerly Porphyra columbina) | Sargassum muticum | |
| Solieria robusta | Sargassum swartzii | |
| Sargassum vulgare | ||
| Stoechospermum marginatum | ||
| Undaria pinnatifida | ||
| Undaria undarioides |
| Seaweed Species or Genus | Protein (% of Dry Mass) | Reference |
|---|---|---|
| Phaeophyceae (Brown Algae) | ||
| A. nodosum | 3–15 | [10] |
| A. esculenta | 9–20 | [34] |
| F. serratus | 3–11 | [35] |
| F. vesiculosus | 12.9 | [36] |
| Fucus spp. | 3–11 | [37] |
| H. elongata | 6–11 | [38] |
| L. digitata | 8–15 | [10,35] |
| S. japonica (formerly L. japonica) (kombu) | 12 | [39] |
| U. pinnatifida (wakame) | 11–24 | [10,40] |
| Chlorophyta (Green Algae) | ||
| Caulerpa lentillifera | 19.38 | [41] |
| Cladophora rupestris | 29.8 | [35] |
| Ulva intestinalis (formerly Enteromorpha intestinalis) | 10–18 | [38] |
| U. lactuca (formerly U. fasciata) (sea lettuce) | 8.7–32.7 | [10,35] |
| U. australis (formerly U. pertusa) | 17.5–26.0 | [10] |
| Ulva rigida | 15–25 | [38] |
| Ulva rotundata (formerly Ulva pseudorotundata) | 10.0 | [42] |
| Rhodophyta (Red Algae) | ||
| Agarophyton vermiculophyllum (previously Gracilaria vermiculophylla) | 17.0% | [43] |
| C. crispus | 21–27 | [10,44] |
| Gracilaria spp. | 7–13 | [45] |
| G. corticata | 22.8 | [46] |
| G. edulis | 25.3 | [46] |
| Gracilaria salicornia | 9.58 | [45] |
| Gracilaria gracilis | 31–45 | [42,47] |
| O. pinnatifida | 20.6–27.3 | [35,48] |
| P. palmata (dulse) | 8–35 | [10,49] |
| Porphyra spp. (nori or purple laver) | 33–50 | [35] |
| P. columbina (formerly P. columbina) | 25 | [50] |
| N. tenera (formerly P. tenera) (nori) | 33–47 | [10] |
| P. umbilicalis (nori) | 15–37 | [38] |
| Protein (% dw) | His | Ile | Leu | Lys | Met + Cys 2 | Met | Cys | Phe + Tyr 3 | Phe | Tyr | Thr | Trp | Val | EAA/ NEAA | EAA% | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FAO/WHO/UNU, 2007 AA Scoring Pattern 1 [61] | 15 | 30 | 59 | 45 | 22 | 16 | 6 | 38 | 23 | 6 | 39 | |||||
| Brown Seaweeds | ||||||||||||||||
| U. Pinnatifida [53] | 13.1 | 68.2 | 50.8 | 78.5 | 69.6 | NR | 30.9 | NR | 93.2 | 47.1 | 46.1 | 42.7 | NR | 35 | NR | NR |
| U. pinnatifida [62] | 12.5 | 21.6 | 47.3 | 89 | 58 | 73.1 | 7.3 | 65.8 | 110.4 | 49.4 | 61 | 53.6 | NR | 31.1 | NR | 48.4 |
| H. elongata [62] | 5 | 20.2 | 43.6 | 79.3 | 60.9 | 68.8 | 4.3 | 64.5 | 113.7 | 55.9 | 57.8 | 54.8 | NR | 28.2 | NR | 47 |
| A. nodosum [63] a | 7.6 | 49.8 | 48 | 58.8 | 14.3 | 11.2 | NR | NR | 46.6 | 30.7 | 15.9 | 62.4 | 5.8 | 54 | 1 | 37.7 |
| Green Seaweeds | ||||||||||||||||
| U. australis (formerly U. pertusa) [64] | 15.4 | 8.6 | 25.9 | 52 | 30.1 | NR | NR | NR | 59.6 | 36.7 | 22.9 | 34.8 | NR | 39.1 | 0.72 | 42 |
| U. intestinalis (formerly E. intestinalis) [64] | 17.9 | 7.4 | 25.3 | 49.7 | 19.6 | NR | NR | NR | 52.1 | 35.9 | 16.2 | 41.7 | NR | 40.5 | 0.67 | 40 |
| U. rigida [29] | 10.2 | 30.7 | 46.1 | 82 | 49.4 | 19.9 | NR | NR | 93.9 | NR | NR | 50.8 | 8.8 | 60.1 | 0.69 | 40.8 |
| U. lactuca (formerly U. fasciata) [65] | 7.1 | 13.1 | 40 | 72.6 | 46.4 | 6.1 | 6.1 | 0 | 93.4 | 57.1 | 36.3 | 62 | NR | 70.1 | NR | NR |
| Red Seaweeds | ||||||||||||||||
| P. umbilicalis [62] | 39 | 15.7 | 36.5 | 76.8 | 56.1 | 75.9 | 8.7 | 67.2 | 93 | 46.8 | 46.2 | 57.8 | NR | 12.3 | NR | 42.4 |
| P. palmata [31] | 15.2 | 18.5 | 65 | 81 | 107.8 | 34.7 | 28 | 6.7 | 93.3 | NR | NR | 47.4 | NR | 143.6 | 0.89 | 47 |
| P. columbina (formerly P. columbina) [50] a | 24.6 | 12.6 | 27.1 | 73.8 | 60.1 | 35.7 | 16.8 | 18.9 | 62.5 | 37 | 25.5 | 59.1 | 6.3 | 58.5 | 0.65 | NR |
| Gracilaria changii [66] | 12.6 | NR | 42.3 | 53.4 | 48.7 | 16.1 | NR | NR | 63.5 | NR | NR | 49.3 | NR | 39.1 | 1.61 | NR |
| A. vermiculophyllum (formerly G. vermiculophylla) [29] | 13.4 | 10.7 | 54.9 | 84.5 | 54.4 | 12.9 | NR | NR | 90.8 | NR | NR | 58.2 | 4.0 | 64.1 | 0.67 | 40.1 |
| Seaweeds | Digestibility (%) 1 |
|---|---|
| Red Seaweed | |
| H. charoides [65] | 88.7 |
| H. japonica [65] | 88.9 |
| P. palmata [85] | 85.8 |
| C. crispus [85] | 84.2 |
| Sarcodiotheca gaudichaudii [85] | 86.7 |
| Green Seaweed | |
| U. lactuca (formerly U. fasciata) [65] (green seaweed) | 85.7 |
| Brown Seaweed | |
| A. nodosum [85] | 78.7 |
| F. vesiculosus [85] | 78.8 |
| A. esculenta [85] | 79.2 |
| Seaweeds | Digestibility (%) 1 | Reference | |||
|---|---|---|---|---|---|
| Pepsin | Pancreatin | Pepsin+ Pancreatin | Pronase | ||
| U. australis (formerly U. pertusa) (green) | 17.0 | 66.6 | - | 94.8 | [37,88] a |
| U. pinnatifida (brown) | 23.9 | 48.1 | - | 87.2 | |
| N. tenera (formerly P. tenera) (red) | 56.7 | 56.1 | - | 78.4 | |
| S. japonica (formerly L. japonica) (brown) | 39.0 | 54.0 | - | 83.9 | |
| P. palmata (red) | - | 56.0 | - | - | |
| P. columbina (formerly P. columbina) (red) | - | - | 74.3 | - | [50] b |
| P. palmata (red) | 87.4 | 84.9 | 87.3 | - | [86] c |
| N. tenera (formerly P. tenera) (red) | 73.2 | 65.9 | 70.2 | - | |
| E. bicyclis (brown) | 57.6 | 73.2 | 57.1 | - | |
| Sargassum fusiforme (formerly Hizikia fusiformis) (brown) | 51.8 | 65.8 | 51.8 | - | |
| S. japonica (formerly L. japonica) (brown) | 70.2 | 76.1 | 72.1 | - | |
| U. pinnatifida (brown) | 69.1 | 87.5 | 68.6 | - | |
| Seaweed | Bioactive Compounds | Properties | References |
|---|---|---|---|
| Bryopsis spp. (green) | Cyclic depsipeptide | Antimicrobial activity against Mycobacterium tuberculosis | [104] |
| Gracilariopsis lemaneiformis (red) | TGAPCR, FQIN [M(O)] CILR | Angiotensin-I-converting enzyme (ACE) inhibitory activity | [105] |
| Mazzaella japonica (red) | YRD, VSEGLD, TIMPHPR, GGPAT, SSNDYPI, SRIYNVKSNG, VDAHY, CPYDWV, YGDPDHY, NLGN, DFGVPGHEP | ACE inhibitory activity | [106] |
| YRD, LDY, LRY, VY, LF, FY | ACE inhibitory activity | [107] | |
| Neopyropia yezoensis (formerly Porphyra yezoensis) (red) | Di- and tripeptides TPDSEAL | ACE inhibition/antihypertensive activity Antimicrobial activity against Staphylococcus aureus | [108] [109] |
| P. palmata (dulse) (red) | Peptides derived from phycobiliproteins: YRD, AGGEY, VYRT, VDHY, IKGHY, LKNPG, LDY, LRY, FEQDWAS | ACE inhibition, antioxidant, | [110] |
| Alcalase, bromelain, and Promod-derived hydrolysates | dipeptidyl peptidase IV (DPP-IV) inhibitory activities, | [111] | |
| Alcalase/Flavourzyme hydrolysates | Antihyperglycemic/antidiabetic potential | [112] | |
| Peptides: ILAP, LLAP, MAGVDHI | DPP-IV inhibitory activities | [113] | |
| Papain hydrolysates: NIGK | Platelet-activating factor acetyl-hydrolase (PAF-AH) inhibitory peptides | [114] | |
| IRLIIVLMPILMA | Renin inhibitory activity | [115] | |
| SDITRPGGQM | Antioxidant | [116] | |
| P. dioica (red) | Peptides DYYKR, YLVA | Antioxidant, ACE inhibition, DPP-IV inhibitory activities | [117] |
| P. columbina (formerly P. columbina) (red) | Peptides | ACE inhibitory, immunosuppressive, antioxidant properties | [118] |
| Saccharina longicruris (formerly Laminaria longicruris) (brown) | TITLDVEPSDTIDGVK, ISGLIYEETR, MALSSLPR, ILVLQSNQIR, ISAILPSR, IGNGGELPR, LPDAALNR, EAESSLTGGNGCAK, QVHPDTGISK | Antibacterial activities | [119] |
| Sargassum pallidum (brown) | Dipeptides (aurantiamide, aurantiamideacetate, dia-aurantiamide) | Antibiotic activity in vitro against S. aureus, Staphylococcus epidermidis, and Pseudomonas aeruginosa | [120] |
| Sargassum thunbergia (brown) | Iodo-amino acids | Possibly helps in human thyroid metabolism | [79] |
| U. rigida (green) | Peptides | ACE inhibition | [121] |
| U. pinnatifida (brown) | Di-, tri-, and tetrapeptides VYIY, AWFY, VW, IW, LW, YNKLKFYG, YKYY | ACE inhibition/ antihypertensive activity, antioxidant | [122,123] |
| Boodlea coacta (green), Griffithsia spp. (red) | Lectins griffithsin | Antiviral effects against human immunodeficiency virus (HIV), Hepatitis C virus and SARS-CoV/ e SARS-CoV-2 by preventing the entry into the host cells | [124,125] |
| Caulerpa cupressoides (green) | Lectins | Antinociceptive and anti-inflammatory activities | [126] |
| C. fragile (green), Eucheuma serra (red) | Lectins | Mitogenic activities, lipogenic activity | [127] |
| Mimica amakusaensis (formerly Eucheuma amakusaense) (red), Ulva spp. (formerly Enteromorpha spp.) (green) | Lectins | Induce apoptosis, metastasis, and cell differentiation in cancer cells, antibiotic, anti-inflammatory, anti-HIV activity, and human platelet aggregation inhibition | [128] |
| N. yezoensis (formerly P. yezoensis) (red) | Taurine | Antioxidant | [129,130] |
| Saccharina angustata (formerly Laminaria angustata) (brown), Chondria armata (red) | Laminine | Hypertensive effect, depress contraction of smooth muscles | [131] |
| C. crispus, Gelidium pusillum, Dasysiphonia japonica (formerly Heterosiphonia japonica), P. palmata (red) | Phycobiliproteins | Antioxidant, antidiabetic, antitumor, anti-inflammatory, neuro-protective, and hepato-protective properties | [132] |
| Gracilaria tikvahiae, P. palmata (red) | Phycobilliproteins (phycocyanins and allphycocyanins) | Anti-inflammatory, liver-protecting, antiviral, antitumor, antiatherosclerosis, lipase activity inhibitor, serum lipid reducing agent, and antioxidant | [128] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thiviya, P.; Gamage, A.; Gama-Arachchige, N.S.; Merah, O.; Madhujith, T. Seaweeds as a Source of Functional Proteins. Phycology 2022, 2, 216-243. https://doi.org/10.3390/phycology2020012
Thiviya P, Gamage A, Gama-Arachchige NS, Merah O, Madhujith T. Seaweeds as a Source of Functional Proteins. Phycology. 2022; 2(2):216-243. https://doi.org/10.3390/phycology2020012
Chicago/Turabian StyleThiviya, Punniamoorthy, Ashoka Gamage, Nalin Suranjith Gama-Arachchige, Othmane Merah, and Terrence Madhujith. 2022. "Seaweeds as a Source of Functional Proteins" Phycology 2, no. 2: 216-243. https://doi.org/10.3390/phycology2020012
APA StyleThiviya, P., Gamage, A., Gama-Arachchige, N. S., Merah, O., & Madhujith, T. (2022). Seaweeds as a Source of Functional Proteins. Phycology, 2(2), 216-243. https://doi.org/10.3390/phycology2020012









