Perspectives on the Use of Algae in Agriculture and Animal Production
Abstract
:Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Craigie, J.S. Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 2011, 23, 371–393. [Google Scholar] [CrossRef]
- Fleurence, J. Biostimulant Potential of Seaweed Extracts Derived from Laminaria and Ascophyllum nodosum. In Biostimulants: Exploring Sources and Applications; Ramawat, N., Bhardwaj, V., Eds.; Springer Nature: Singapore, 2022; in press. [Google Scholar] [CrossRef]
- Sauvageau, C. Utilisation des Algues Marines; Doin, G., Ed.; Librairie Octave Dion: Paris, France, 1920; pp. 116–161. [Google Scholar]
- Pérez, R. Ces Algues Qui Nous Entourent: Conception Actuelle, Rôle Dans la Biosphère, Utilisations, Culture; IFREMER, Ed.; Editions Quae: Plouzané, France, 1997; pp. 169–171. [Google Scholar]
- Battacharyya, D.; Bagohari, M.B.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Arioli, T.; Mattner, S.W.; Winberg, P.C. Application of seaweed extracts in Australian agriculture: Past, present and future. J. Appl. Phycol. 2015, 27, 2007–2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, H.S.S.; Fleming, C.; Selby, C.; Rao, J.R.; Martin, T. Plants biostimulants: A review on processing of macroalgae and use of extracts for crop management to reduce abiotic and biotic stresses. J. Appl. Phycol. 2014, 26, 465–490. [Google Scholar] [CrossRef]
- Delaney, A.; Frangoudes, K.; Li, S.A. Society and Seaweed: Understanding the Past and Present. In Seaweed: In Health and Disease Prevention; Fleurence, J., Levine, I., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 7–38. [Google Scholar]
- Garcia-Bueno, N.; Dumay, J.; Guérin, T.; Turpin, V.; Paillard, C.; Stiger-Pouvreau, V.; Pouchus, Y.F.; Marin-Atucha, A.A.; Decottignies, P.; Fleurence, J. Seasonal variation in the antivibrio activity from two red seaweed: Palmaria palmata and the introduced Grateloupia turuturu against the abalone pathogen Vibrio harveyi. Aquat. Living Resour. 2015, 28, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Fleurence, J.; Morançais, M.; Dumay, J.; Decottignies, P.; Turpin, V.; Munier, M.; Garcia-Bueno, N.; Jaouen, P. What are the prospects for using seaweed in human nutrition and for marine animals raised through aquaculture? Trends Food Sci. Technol. 2012, 27, 57–61. [Google Scholar] [CrossRef]
- Fleurence, J. Microalgae: From Future Food to Cellular Factory; Wiley-ISTE: London, UK, 2021; pp. 1–178. [Google Scholar]
- Roque, B.M.; Venegas, M.; Kinley, R.D.; de Nys, R.; Duarte, T.L.; Yang, X.; Kebreab, E. Red seaweed (Asparagopsis taxiformis) supplementation reduces enteric methane by over 80 percent in beef steers. PLoS ONE 2021, 16, e0247820. [Google Scholar] [CrossRef] [PubMed]
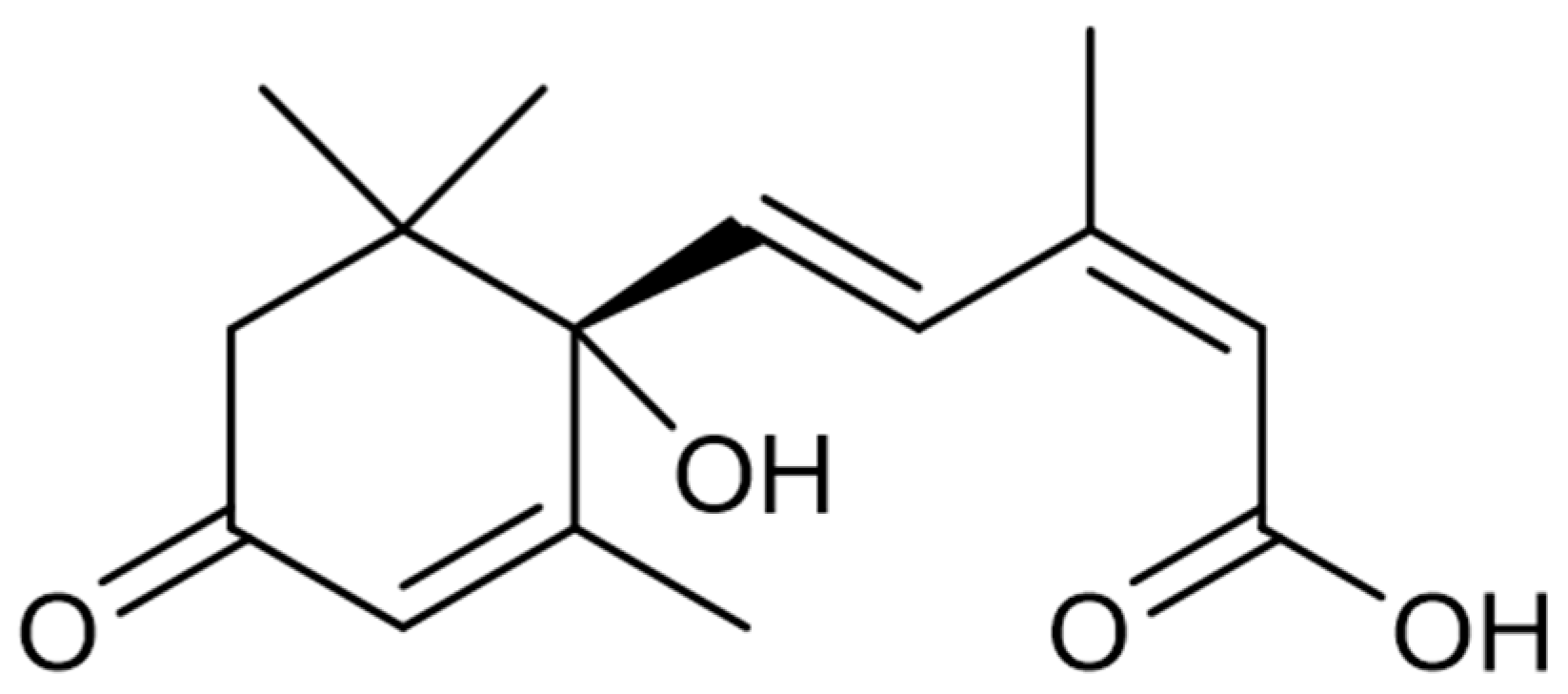
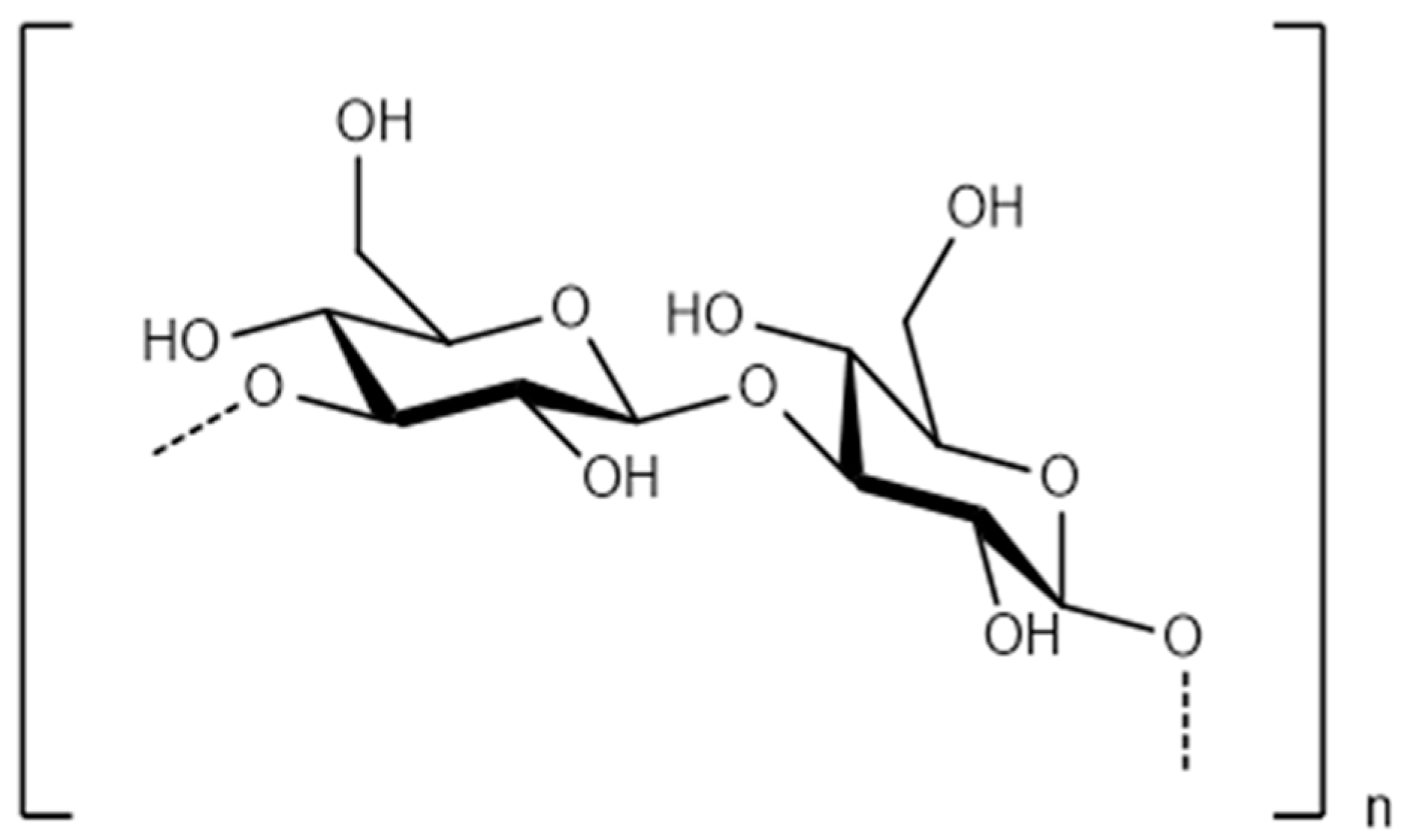
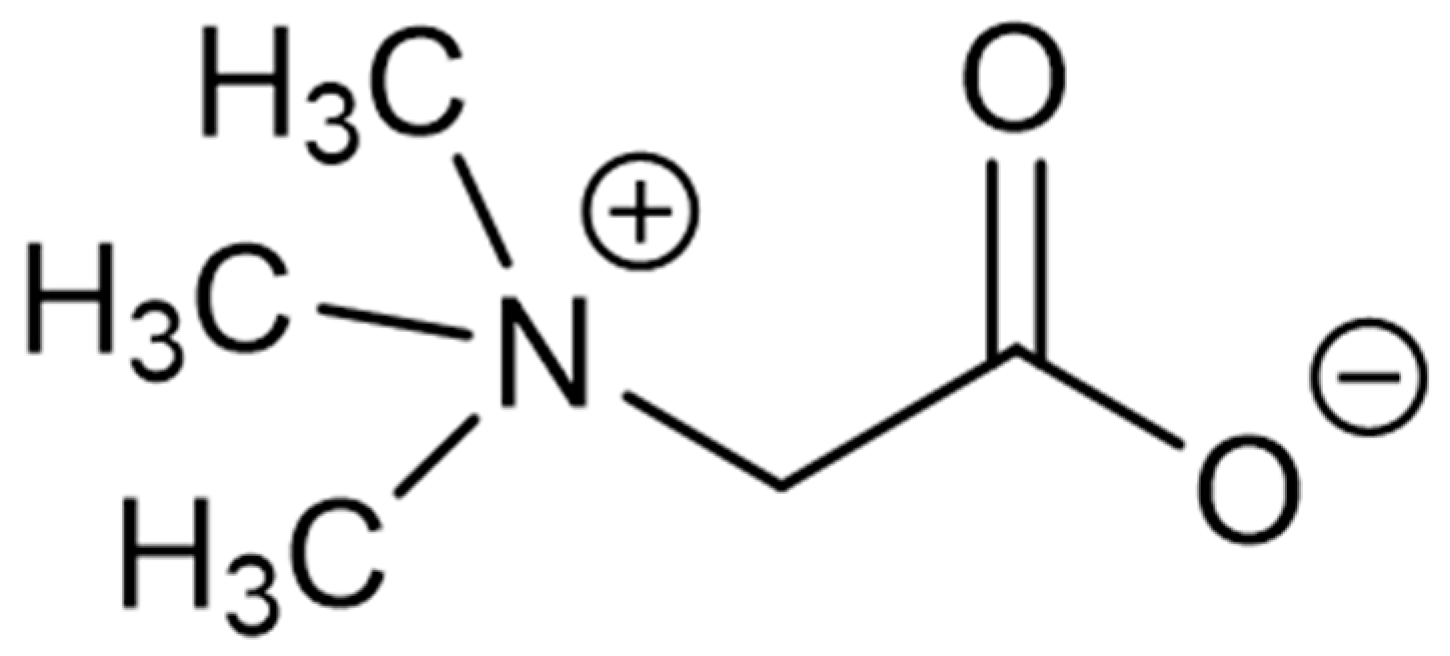
| Seaweed | Products | Molecules | Company | Country |
|---|---|---|---|---|
| Ecklonia maxima | Kelpak | Abscisic acid, gibberellins, cytokinins | Kelp Products | South Africa |
| Durvillaea potatorum | Seasol | Cytokinins (i.e., Zeatin) | Seasol International | Australia |
| Durvillaea antartica | Profert | - | BASF | Chile |
| Ascophyllum nodosum | Goëmar | Abscisic acid, auxins, cytokinins | Goëmar laboratories | France |
| Gofar | Gofar Agro | China | ||
| Laminaria digitata | Agrocean | Abscisic acid, auxins | Agrocean | France |
| Fucus sp. | Seaweed flakes | Gibberellins, auxins | Aquatic Chemical | India |
| Seaweed Molecule Used as Elicitor | Plant | Natural Defense Mechanisms |
|---|---|---|
| Alginate | Wheat | Stimulation of phenylpropanoid pathway (Phenyl ammonia lyase (PAL) induction) |
| Alginate | Tobacco | Increased PAL activity and activation of hypersensitivity reaction to Tobacco Mosaic Virus (TMV) |
| Alginate | Japanase horseradish | Activation of Chitinase |
| Laminarin | Tobacco | Increased of pathogenesis proteins (PR) and PAL induction |
| Laminarin | Grapevine | Increased PR proteins and phytoalexin production |
| Laminarin | Rice | Induction of enzymes such as PAL and Chitinase |
| Fucoidan | Tobacco | Increased PR proteins |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fleurence, J. Perspectives on the Use of Algae in Agriculture and Animal Production. Phycology 2021, 1, 79-82. https://doi.org/10.3390/phycology1020006
Fleurence J. Perspectives on the Use of Algae in Agriculture and Animal Production. Phycology. 2021; 1(2):79-82. https://doi.org/10.3390/phycology1020006
Chicago/Turabian StyleFleurence, Joël. 2021. "Perspectives on the Use of Algae in Agriculture and Animal Production" Phycology 1, no. 2: 79-82. https://doi.org/10.3390/phycology1020006
APA StyleFleurence, J. (2021). Perspectives on the Use of Algae in Agriculture and Animal Production. Phycology, 1(2), 79-82. https://doi.org/10.3390/phycology1020006





