Effects of Varying Levels of Baobab Oilseed Cake Combined with Fossil Shell Flour Diets on Nutritional Status Indicators and Associated Blood Metabolites of Angora Goats
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Goats Management, Feeding, and Experimental Design
2.3. Feed Analytical Procedure
Blood Collection Procedure
2.4. Measurements
2.4.1. Body Weight Gain
2.4.2. Body Condition Score
2.4.3. Blood Metabolites
2.5. Statistical Analysis
- Yijk = Observation (BSC, BWG, Blood metabolites);
- U = Overall means common to all observations;
- Mi = Effect of BOSC inclusion level;
- Wk = Effect of feed weeks;
- (M × W)ijk = Interaction between treatment and week;
- Eijk = Random error for i and j = 1, 2, 3, 4, and K = 1.
3. Results
4. Discussion
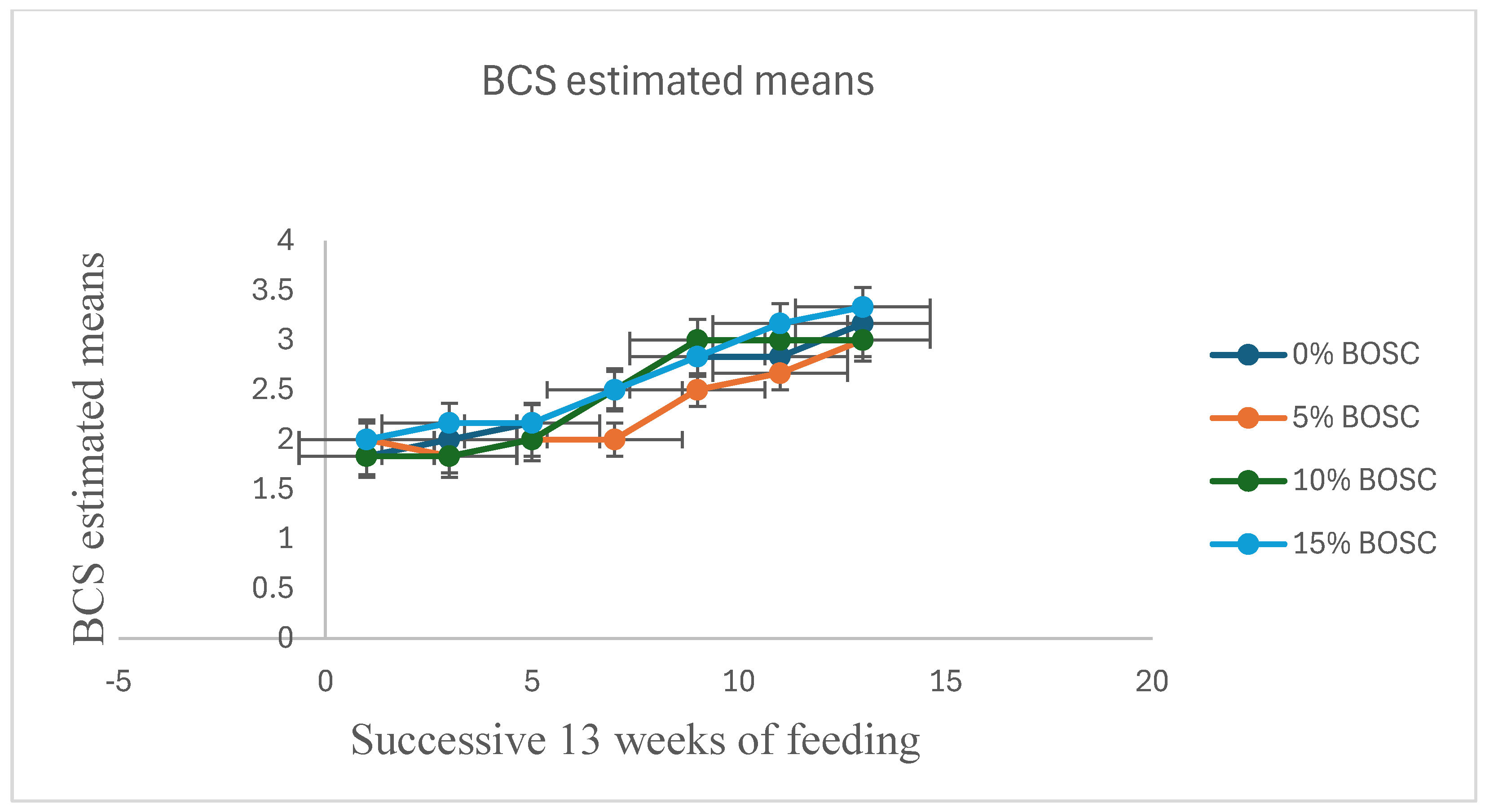
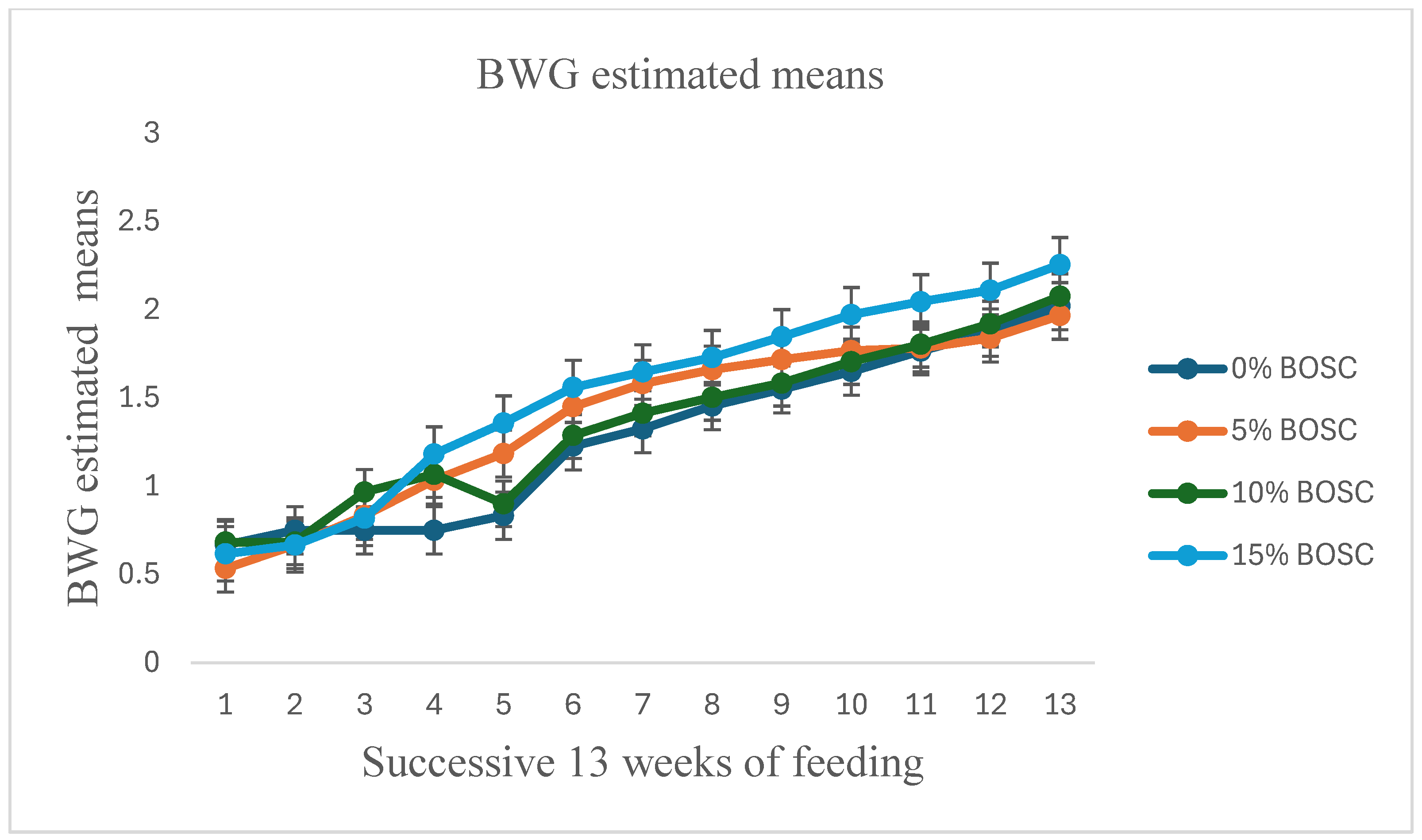
4.1. Body Weight Gain and Body Condition Score
4.2. Glucose
4.3. Albumin
4.4. Urea and Creatinine
4.5. Total Protein
4.6. Calcium
4.7. Magnesium
4.8. Phosphorus
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BOSC | Baobab oilseed cake |
| FSF | Fossil shell flour |
| BCS | Body condition score |
| SEM | Standard Error Mean |
| Sign | Significance |
| BWG | Body Weight Gain |
| NS | Not significant |
References
- Yadav, S.; Singh, A.; Singh, Y. (Eds.) Advances in Sheep and Goat Production and Management. 2016. Available online: https://www.researchgate.net/publication/305323919 (accessed on 26 June 2016).
- Sariçiçek, B.Z. The effect of rangeland quality on the mohair quality of Angora goats fed on the natural rangelands. Turkish J. Vet. Anim. Sci. 2021, 45, 678–690. [Google Scholar] [CrossRef]
- Cannas, A.; Tedeschi, L.O.; Atzori, A.S.; Lunesu, M.F. How can nutrition models increase the production efficiency of sheep and goat operations? Anim. Front. 2019, 9, 33–44. [Google Scholar] [CrossRef] [PubMed]
- McGregor, B.A.A. Development and growth of mohair fleeces from birth and relationships between skin follicle populations, mohair physical properties, animal size, and fleece value. Small Rumin. Res. 2020, 189, 106142. [Google Scholar] [CrossRef]
- Snyman, M.A. Genetic analysis of reproduction, body weight, and mohair production in South African Angora goats. Small Rumin. Res. 2020, 192, 106183. [Google Scholar] [CrossRef]
- Mtenjwa, B.; Ikusika, O.; Mpendulo, C.T.; Gajana, S.C. Angora Goats and Mohair Production in South Africa: A Review. Online J. Anim. Feed. Res. 2024, 14, 347–357. [Google Scholar] [CrossRef]
- Visser, C.; Lashmar, S.F.; Marle-köster, E.; Van Poli, M.A. Genetic Diversity and Population Structure in South African, French, and Argentinian Angora Goats from Genome-Wide SNP Data. PLoS ONE 2016, 11, e0154353. [Google Scholar] [CrossRef] [PubMed]
- Marius, L.N.; Shipandeni, M.N.T.; Togarepi, C. Review on the status of goat production, marketing, challenges, and opportunities in Namibia. Trop. Anim. Health Prod. 2021, 53, 30. [Google Scholar] [CrossRef]
- Mpyana, B.M. The Socio-Economic Benefits of Mohair Trust-LED Agrarian Transformation Projects: A Case Study of Smallholder Farmers in the Eastern Cape Province of South Africa. Ph.D. Dissertation, North-West University (South Africa), Potchefstroom, South Africa, 2019. Available online: https://repository.nwu.ac.za/handle/10394/33889 (accessed on 2 June 2023).
- Nipane, S.F.; Roupesh, G.; Kawitkar, S.B.; Dhok, A.P.; Jawale, M.R. Chapter 18 Nutritional Strategy in Goat. Indian J. Livest. Vet. Res. 2023, 3, 144–157. [Google Scholar]
- Kumar, R. Nutrition and Management of Goats. In Principles of Goat Disease and Prevention; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2023. [Google Scholar] [CrossRef]
- Wu, G. Management of metabolic disorders (including diseases) in ruminant and nonruminant animals. In Animal Agriculture; Academic Press: Cambridge, MA, USA, 2020; pp. 471–491. [Google Scholar] [CrossRef]
- Maurya, S.K. Assessment of blood biochemical profile and nutritional status of buffaloes under field conditions. Buffalo Bull. 2015, 34, 161–167. Available online: https://www.researchgate.net/publication/280540625 (accessed on 29 July 2015).
- Aliarabi, H.; Fadayifar, A.; Tabatabaei, M.M.; Zamani, P.; Bahari, A.; Farahavar, A.; Dezfoulian, A.H. Effect of Zinc Source on Haematological, Metabolic Parameters and Mineral Balance in Lambs. Biol. Trace Elem. Res. 2015, 168, 82–90. [Google Scholar] [CrossRef]
- Mpendulo, C.T.; Akinmoladun, O.F.; Ikusika, O.O.; Chimonyo, M. Effect of hydric stress on Nguni goats’ intake, growth performance, and nutritional status. Ital. J. Anim. Sci. 2020, 19, 1071–1078. [Google Scholar] [CrossRef]
- Gonzalez, L.A.; Kyriazakis, I.; Tedeschi, L.O. Review: Precision nutrition of ruminants: Approaches, challenges and potential gains. Animal 2018, 12, S246–S261. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.D.G.; Loiola, M.V.G.; Filho, A.L.R.; Cotrim, D.C.; dos Santos Rekowsky, B.S.; Lopes, I.M.S.; Bulcão, L.F.d.A.; de Araújo, M.L.G.M.L.; Pina, D.d.S.; de Carvalho, G.G.P.; et al. Effect of the racial group and body condition score at calving on buffaloes’ production performance and metabolic profile during the transition period. Trop. Anim. Health Prod. 2023, 55, 261. [Google Scholar] [CrossRef]
- Adedokun, S.A.; Olojede, O.C. Optimizing gastrointestinal integrity in poultry: The role of nutrients and feed additives. Front. Vet. Sci. 2019, 5, 348. [Google Scholar] [CrossRef] [PubMed]
- Gorniak, W.; Cholewińska, P.; Konkol, D. Feed additives produced based on organic forms of micronutrients as a means of biofortification of food of animal origin. J. Chem. 2018, 2018, 8084127. [Google Scholar] [CrossRef]
- McGregor, B.A. Effects of supplementary feeding lucerne hay and barley grain to Angora during the last third of pregnancy and lactation, and litter size on pasture, birth weight, live weight, parasitism, milk production, milk composition, and mohair production. Small Rumin. Res. 2021, 195, 106303. [Google Scholar] [CrossRef]
- Mantovani, A.; Aquilina, G.; Cubadda, F.; Marcon, F. Risk-Benefit Assessment of Feed Additives in the One Health Perspective. Front. Nutr. 2022, 9, 843124. [Google Scholar] [CrossRef]
- Untea, A.E.; Saracila, M.; Vlaicu, P.A. Feeding Strategies and Nutritional Quality of Animal Products. Agriculture 2023, 13, 1788. [Google Scholar] [CrossRef]
- Ikusika, O.O.; Mpendulo, C.T.; Zindove, T.J.; Okoh, A.I. Fossil Shell Flour in Livestock Production: A Review. Animals 2019, 9, 70. [Google Scholar] [CrossRef]
- Ikusika, O.O. Effect of Increasing Fossil Shell Flour Levels on Digestive and Metabolic Utilization, Health, Body Weight Change, and Wool Production and Quality in Dohne-Merino Wethers. Ph.D. Thesis, University of Fort Hare, Dikeni, South Africa, February 2020. [Google Scholar]
- Saho, S.; Mupangwa, J.; Moyo, D. Influence of graded levels of baobab oilseed cake on growth performance and enteric methane emissions in Savannah × Boer crossbreed yearling goats. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Ikusika, O.O.; Mpendulo, C.T. Effect of Dietary Supplementation with Fossil Shell Flour on Enteric Methane Output and Position-Dependent Variations in Dohne-Merino Wethers. Sib. J. Life Sci. Agric. 2023, 15, 163–177. [Google Scholar] [CrossRef]
- Mapiye, C.; Chimonyo, M.; Dzama, K.; Strydom, P.E.; Muchenje, V.; Marufu, M.C. Nutritional status, growth performance, and carcass characteristics of Nguni steers supplemented with Acacia karroo leaf meal. Livest. Sci. 2009, 126, 206–214. [Google Scholar] [CrossRef]
- Qokweni, L.; Chimonyo, M.; Marufu, M.C. Gastrointestinal Nematode Infestation, Goat Performance, and Nutritionally-Related Blood Metabolites of Xhosa Lop-Eared do Foraging in Grasslands and Forestland Vegetation Types. Ph.D. Thesis, University of KwaZulu-Natal, Pietermaritzburg, South Africa, 2022. Available online: https://researchspace.ukzn.ac.za/handle/10413/20948 (accessed on 23 May 2023).
- Hlatini, V.A.; Chimonyo, M. Influence of Polyethene Glycol Inclusion on Growth Performance and Serum Biochemistry of Growing Pigs Fed on Acacia Tortilis Leaf Meal. Master’s Thesis, University of KwaZulu-Natal, Pietermaritzburg, South Africa, 2015. Available online: https://researchspace.ukzn.ac.za/handle/10413/13330 (accessed on 9 September 2016).
- Ghosh, C.P.; Mandal, D.; Roy, D.C.; Datta, S.; Das, A.K.; Roy, A.; Tudu, N.K. Body condition scoring in goat: Impact and significance. J. Entomol. Zool. Stud. 2019, 7, 554–560. [Google Scholar]
- Yaseen, A.; Hussain, T.; Hameed, A.; Shahzad, M.; Mazhar, M.U.; Chughtai, M.I. Flavonoid-enriched supplementation mitigates prenatal stress and improves the performance of goat kids reared in a subtropical environment. Res. Vet. Sci. 2022, 146, 70–79. [Google Scholar] [CrossRef]
- Sacchero, D.; Gonzalez, E.B.; Maurino, J.; Lopez, M.; Cortes, M.V.; Alvarez, R.; Bidinost, F. Performance of Angora goats, mohair production, and farmer income in extensive livestock systems of north Patagonia, Argentina. Front. Anim. Sci. 2023, 4, 1208778. [Google Scholar] [CrossRef]
- Wachiebene, S.K. Assessment of Feed Resources for Ruminant Production in the Northern Region of Ghana. Ph.D. Dissertation, University of Development Studies, Tamale, Ghana, 2021. Available online: https://hdl.handle.net/10568/115599 (accessed on 21 October 2021).
- Obeidat, B.S.; Thomas, M.G. Growth Performance, Blood Metabolites, and Carcass Characteristics of Black Goat Kids Fed Diets Containing Olive Cake. Animals 2024, 14, 272. [Google Scholar] [CrossRef]
- Cériac, S.; Jayles, C.; Arquet, R.; Feuillet, D.; Félicité, Y.; Archimède, H.; Bambou, J.C. The nutritional status affects the complete blood count of goats experimentally infected with Haemonchus contortus. BMC Vet. Res. 2017, 13, 326. [Google Scholar] [CrossRef]
- Widiyono, I.; Sarmin; Putro, P.P. Influence of feed intake on blood chemistry parameters in Kacang goats. AIP Conf. Proc. 2016, 1755, 140011. [Google Scholar] [CrossRef]
- Gao, J.; Yang, D.; Sun, Z.; Niu, J.; Bao, Y.; Liu, S.; Tan, Z.; Hao, L.; Cheng, Y.; Liu, S. Changes in Blood Metabolic Profiles Reveal the Dietary Deficiencies of Specific Nutrients and Physiological Status of Grazing Yaks during the Cold Season in Qinghai Province of China. Metabolites 2022, 12, 738. [Google Scholar] [CrossRef] [PubMed]
- Tahuk, P.K.; Bira, G.F. Blood Glucose and Blood Urea Levels from Castrated, Non-Castrated Male, and Female Domestic Goats that were Fed Complete Feed. In Proceedings of the International Conference on Improving Tropical Animal Production for Food Security (ITAPS 2021), Kendari, Indonesia, 20–21 November 2021; Atlantis Press: Paris, France, 2022; Volume 20, pp. 191–196. [Google Scholar] [CrossRef]
- Samira, A.M.; Mohammed, A.R.; Anaam, E.O.; Sheeba, A.; Waleed, M.A.G. Biochemical and haematological profile of different goat breeds maintained under an intensive production system. Afr. J. Biotechnol. 2016, 15, 1253–1257. [Google Scholar] [CrossRef]
- Kholif, A.E. A Review of the Effect of Saponins on Ruminal Fermentation, Health, and Performance of Ruminants. Vet. Sci. 2023, 10, 450. [Google Scholar] [CrossRef]
- Aktaş, R.; Pehlivan, E. A comparative profile of certain biochemical and haematological parameters in Angora and Akkeçi goats during the transition period. Turk. J. Vet. Anim. Sci. 2023, 47, 80–90. [Google Scholar] [CrossRef]
- Allaoua, S.A.; Mahdi, D. Plasma biochemical and mineral parameters in arbia goats of a semi-arid region of North-Eastern Algeria during different stages of production. Vet. Arh. 2018, 88, 643–660. [Google Scholar] [CrossRef]
- Smuts, M.P.; De Bruyn, S.; Thompson, P.N.; Holm, D.E. Serum albumin concentration of donor cows as an indicator of developmental competence of oocytes. Theriogenology 2019, 125, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Ilori, H.B.; Salami, S.A.; Majoka, M.A.; Okunlola, D. Acceptability and Nutrient Digestibility of West African Dwarf Goat Fed Different Dietary Inclusions of Baobab (Adansonia digitata). J. Agric. Vet. Sci. 2013, 6, 22–26. [Google Scholar]
- Chanjula, P.; Wungsintaweekul, J.; Chiarawipa, R.; Phesatcha, K.; Suntara, C.; Prachumchai, R.; Cherdthong, A. Effects of supplementing finishing goats with Mitragyna speciosa (Korth) Havil leaves powder on growth performance, hematological parameters, carcass composition, and meat quality. Animals 2022, 12, 1637. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Xu, W.; Wei, C.; Zhang, Z.; Jiang, C.; Chen, X. Effects of decreasing dietary crude protein level on growth performance, nutrient digestion, serum metabolites, and nitrogen utilization in growing goat kids (Capra hircus). Animals 2020, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Kuru, M.; Akyüz, E.; Makav, M. Some Metabolic Profile Markers in Goats. Turk. J. Vet. Intern. Med. 2022, 1, 32–39. [Google Scholar] [CrossRef]
- Ikusika, O.O.; Mpendulo, C.T.; Zindove, T.J.; Okoh, A.I. Effect of Varying Inclusion Levels of Fossil Shell Flour on Growth Performance, Water Intake, Digestibility, and N Retention in Dohne-Merino Wethers. Animals 2019, 9, 565. [Google Scholar] [CrossRef]
- Cameron, J.M.; Bruno, C.; Parachalil, D.R.; Baker, M.J.; Bonnier, F.; Butler, H.J.; Byrne, H.J. Vibrational spectroscopic analysis and quantification of proteins in human blood plasma and serum. In Vibrational Spectroscopy in Protein Research; Academic Press: Cambridge, MA, USA, 2020; pp. 269–314. [Google Scholar] [CrossRef]
- David, L.S.; Anwar, M.N.; Abdollahi, M.R.; Bedford, M.R.; Ravindran, V. Calcium Nutrition of Broilers: Current Perspectives and Challenges. Animals 2023, 13, 1590. [Google Scholar] [CrossRef]
- Karaşahin, T.; Aksoy, N.H.; Dursun, Ş.; Bulut, G.; Haydardedeoğlu, A.E.; Çamkerten, G.; Çamkerten, İ.; İlgün, R. Effects of age and sex on some haematological and biochemical parameters in Hair goats. Vet. Res. Forum 2022, 13, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Sindhura, A.; Sarma, M.; MonoshreeSarma, C. Significance of Goat Meat and Meat Products in Human Nutrition. Spec. Issue 2023, 3, 1. [Google Scholar]
- Froghi, T.; Hosaini, A. Nutrition and feeding: The type of nutrients required by the Markhoz (Iranian Angora) goat Tofiq. Life Sci. J. 2012, 9, 32. Available online: http://www.lifesciencesite.com (accessed on 15 September 2012).
- Raja, S.; Murugan, M.; Lakshmikantan, U.; Vasanthakumar, P.; Selvaraju, M. Importance of minerals in animal nutrition. Vet. Digit. 2021, 8, 542–549. Available online: https://www.veterinariadigital.com/en/articulos/importance-of-minerals-in-animal-nutrition/ (accessed on 15 December 2021).
| Items | Content |
|---|---|
| Dry Matter % | 93.00 |
| % Calcium | 0.40 |
| % CaO (calculated from %Ca) | 0.55 |
| % Magnesium | 0.21 |
| %MgO (calculated from %Mg) | 0.34 |
| Potassium% | 0.16 |
| Copper (mg/kg) | 30.00 |
| Sodium (mg/kg) | 923.00 |
| Zinc (mg/kg) | 118.00 |
| Iron (mg/kg) | 7944.00 |
| Manganese (mg/kg) | 69.00 |
| Phosphorus (as P2O5) | 0.04 |
| Sulfate Sulfur (S)% | 0.06 |
| Aluminium (Al) % | 0.07 |
| Vanadium (V) % | 0.00438 |
| Boron (B) % | 0.0023 |
| Nutrient | Content |
|---|---|
| Dry Matter (%) | 90.47 |
| Moisture (%) | 9.53 |
| Organic Matter (%) | 83.75 |
| Crude Protein (%) | 25.28 |
| Crude Fiber (%) | 12.88 |
| Ether Extract (%) | 5.92 |
| Ash (%) | 6.72 |
| ADF (%) | 25.23 |
| NDF (%) | 43.53 |
| Total Nitrogen (%) | 4.05 |
| Starch (%) | 16.42 |
| Phosphorus (%) | 0.07 |
| Calcium (%) | 0.79 |
| Magnesium (%) | 0.79 |
| Sodium (mg/kg) | 8.00 |
| Copper (mg/kg) | 12.00 |
| Iron (mg/kg) | 65.00 |
| Manganese (mg/kg) | 7.00 |
| Dry Matter (%) | 90.47 |
| Ingredients | Experimental Diets | |||
|---|---|---|---|---|
| 0% BOSC | 5% BOSC | 10% BOSC | 15% BOSC | |
| Dry Matter (g/kg) | 88.96 | 88.72 | 88.73 | 88.83 |
| Organic Matter | 79.68 | 79.88 | 79.95 | 79.52 |
| Moisture | 11.04 | 11.28 | 11.27 | 11.17 |
| Crude Protein | 13.3 | 12.91 | 12.67 | 11.57 |
| Ether Extract | 1.79 | 1.82 | 2.04 | 1.98 |
| Ash | 9.32 | 8.84 | 8.78 | 9.31 |
| ADF | 17.47 | 21.34 | 31.08 | 41.70 |
| NDF | 39.11 | 51.09 | 51.22 | 59.10 |
| Nitrogen | 2.13 | 2.07 | 2.03 | 1.85 |
| Starch | 10.68 | 10.02 | 10.62 | 9.79 |
| Parameter | Diets (%BOSC) | Weeks of the Trial | SEM | Contrasts | Sign | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 5 | 7 | 9 | 11 | 13 | Linear | Quadratic | ||||
| BCS | 0 | 1.833 | 2.000 | 2.167 | 2.500 | 2.833 | 2.833 | 3.167 | 0.191 | 0.0002 | 0.7897 | ** |
| 5 | 2.000 | 1.833 | 2.000 | 2.000 | 2.500 | 2.667 | 3.000 | 0.191 | 0.0115 | 0.0517 | * | |
| 10 | 1.833 | 1.833 | 2.000 | 2.500 | 3.000 | 3.000 | 3.000 | 0.191 | 0.0046 | 0.8647 | * | |
| 15 | 2.000 | 2.167 | 2.167 | 2.500 | 2.833 | 3.167 | 3.333 | 0.191 | 0.0005 | 0.3690 | ** | |
| Parameter | Diets(%BOSC) | Successive Weeks of Feeding | SEM | Contrasts | Sign | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | Linear | Quadratic | ||||
| BWG (kg) | 0 | 0.667 | 0.750 | 0.750 | 0.750 | 0.833 | 1.227 | 1.324 | 1.454 | 1.549 | 1.650 | 1.765 | 1.870 | 2.020 | 0.138 | <0.0001 | 0.6628 | ** |
| 5 | 0.533 | 0.667 | 0.833 | 1.033 | 1.185 | 1.450 | 1.581 | 1.660 | 1.717 | 1.768 | 1.783 | 1.838 | 1.967 | 0.138 | <0.0001 | 0.0038 | ** | |
| 10 | 0.683 | 0.683 | 0.967 | 1.067 | 0.900 | 1.287 | 1.413 | 1.503 | 1.583 | 1.705 | 1.804 | 1.919 | 2.076 | 0.138 | <0.0001 | 0.3842 | ** | |
| 15 | 0.617 | 0.667 | 0.819 | 1.182 | 1.358 | 1.559 | 1.647 | 1.729 | 1.846 | 1.972 | 2.045 | 2.110 | 2.255 | 0.138 | <0.0001 | 0.0001 | ** | |
| Parameters | Diets | SEM | Contrasts | Sign | ||||
|---|---|---|---|---|---|---|---|---|
| 0%BOSC | 5%BOSC | 10%BOSC | 15%BOSC | Linear | Quadratic | |||
| Glucose (mmol/L) | 3.05 | 4.25 | 4.55 | 3.60 | 0.521 | 0.692 | 0.560 | NS |
| Albumin (g/L) | 13.50 | 14.50 | 14.00 | 14.50 | 0.968 | 0.480 | 0.655 | NS |
| Creatinine (umol/L) | 50.00 | 48.50 | 46.50 | 33.50 | 6.359 | 0.013 | 0.026 | * |
| Total protein (g/L) | 69.00 | 60.50 | 61.00 | 74.50 | 4.168 | 0.573 | 0.286 | NS |
| Urea (mmol/L) H | 10.65 | 9.95 | 9.35 | 8.80 | 0.646 | 0.002 | 0.044 | ** |
| Calcium (mg/dL) | 10.60 | 9.19 | 9.50 | 11.25 | 0.504 | 0.736 | 0.516 | NS |
| Magnesium (mg/dL) | 2.30 | 1.79 | 2.10 | 2.42 | 0.193 | 0.790 | 0.829 | NS |
| Phosphorus (mg/dL) | 6.35 | 5.70 | 5.61 | 6.69 | 0.450 | 0.074 | 0.017 | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mtenjwa, B.; Ikusika, O.O.; Mpendulo, C.T. Effects of Varying Levels of Baobab Oilseed Cake Combined with Fossil Shell Flour Diets on Nutritional Status Indicators and Associated Blood Metabolites of Angora Goats. Ruminants 2025, 5, 56. https://doi.org/10.3390/ruminants5040056
Mtenjwa B, Ikusika OO, Mpendulo CT. Effects of Varying Levels of Baobab Oilseed Cake Combined with Fossil Shell Flour Diets on Nutritional Status Indicators and Associated Blood Metabolites of Angora Goats. Ruminants. 2025; 5(4):56. https://doi.org/10.3390/ruminants5040056
Chicago/Turabian StyleMtenjwa, Bukeka, Olusegun Oyebade Ikusika, and Conference Thando Mpendulo. 2025. "Effects of Varying Levels of Baobab Oilseed Cake Combined with Fossil Shell Flour Diets on Nutritional Status Indicators and Associated Blood Metabolites of Angora Goats" Ruminants 5, no. 4: 56. https://doi.org/10.3390/ruminants5040056
APA StyleMtenjwa, B., Ikusika, O. O., & Mpendulo, C. T. (2025). Effects of Varying Levels of Baobab Oilseed Cake Combined with Fossil Shell Flour Diets on Nutritional Status Indicators and Associated Blood Metabolites of Angora Goats. Ruminants, 5(4), 56. https://doi.org/10.3390/ruminants5040056





