1. Introduction
Animal vocalizations play a crucial role not only in survival but also in enabling communication and individual recognition within the same species. These acoustic signals can transmit multifaceted information about the sender, either static or more dynamic, such as age, body size, and sex, as well as emotional state and social status, respectively [
1,
2]. Unlike visual or olfactory-based signals, they are less susceptible to attenuation by distance or physical obstructions [
3]. Depending on their frequency, sound waves are capable of traversing barriers more effectively than visual or chemical (olfactory) signals, thereby allowing auditory communication to occur over extended ranges. Consequently, individuals do not need to be in proximity to detect and interpret vocal information from conspecifics [
4]. Furthermore, vocalizations can encode individual identity. Species that inhabit larger social groups tend to exhibit a greater degree of individual-specific acoustic variation than those residing in smaller social groups [
2,
4].
The source-filter theory has significantly advanced the analysis of vocal characteristics by offering a comprehensive framework for investigating vocal signal production in both human and non-human mammals [
5,
6,
7,
8]. The theory distinguishes two processes: sound generation via vocal fold vibrations (which constitutes the “source”) and sound shaping by the supra-laryngeal vocal tract (acting as the “filter”). The vocal folds determine the fundamental frequency (F0), while the vocal tract modifies the signal to produce formants (F1, F2, etc.) and shape energy distribution (amplification or attenuation of the signal). The theory has been widely applied to a range of mammalian species, including domesticated animals, uncovering links between vocal output and the anatomical or physiological traits of the emitter [
8,
9,
10].
Discriminative acoustic signals play a pivotal role in species that exhibit communal living or complex social structures, since they can mediate social interactions among animals, such as mate choice, parental investment, and coordinated group behaviors. They can also be noticed in challenging farming stimuli like feed frustration or anticipation, mating signs (estrus), physical and/or visual isolation [
11]. Individual-specific vocal responses are most evident when intra-individual variability is minimal and inter-individual variability is pronounced [
12,
13]. Numerous studies have documented the existence of distinctive vocal signatures in mammals [
11,
14,
15,
16,
17,
18,
19], characterized by unique spectral and temporal features. These acoustic parameters could serve as reliable indicators of individual identity, facilitating recognition and social differentiation within populations [
17,
18,
19,
20].
Sheep are highly gregarious [
21], with their vocal repertoire comprising two distinct types of bleats: high-pitched and low-pitched [
21]. High-pitched bleats are typically emitted in contexts associated with stress or negative affective states, such as social isolation or maternal separation, and are characterized by elevated frequency and amplitude, often produced with an open-mouth posture. In contrast, low-pitched bleats are generated with the mouth closed and have a lower frequency and amplitude. They predominantly occur during early postnatal interactions between the ewe and her lamb(s), serving to strengthen the maternal–offspring bond [
19,
21]. Contrary to ewes, which are more vocally expressive in affiliative and caregiving contexts (i.e., ewes–lambs), rams’ vocalizations are more situational and less frequent—primarily linked to mating and dominance [
21,
22,
23].
Generally, sheep demonstrate vocal distinctiveness, with acoustic signals varying across individuals in ways that support recognition and context differentiation [
3,
24,
25,
26]. Previous research [
19,
25] has predominantly examined vocal individuality within mother–offspring pairs of meat- or wool-type sheep breeds during the early postnatal period (2–15 days of age), a stage characterized by close physical proximity between them. A recent study [
27] indicated that comparable levels of vocal individuality between dairy ewes and lambs persist at a later postnatal stage (40 days postpartum); however, these levels were lower than those observed during an early postnatal period [
19,
25]. Vocal individuality was also evident among lamb siblings; however, fewer acoustic parameters contributed to their vocal distinctiveness compared to those differentiating vocal cues between ewes and their lambs. Notably, dairy ewes exhibited vocal individuality not only during the suckling period but also at a distinct time point, that of the dry season, suggesting that individualized vocal signals may serve functions beyond immediate maternal–offspring recognition [
26,
27]. However, to the best of our knowledge, data on vocal individuality in rams remains limited, especially regarding its expression across various contexts.
Consequently, the aim of the present study was to investigate individuality coding in rams’ vocalizations within and across different contexts. Specifically, it aimed at investigating whether ram vocalizations are individually distinctive, their consistency across different context situations, and the variation in informational content. Additionally, we assessed which acoustic parameters most effectively encode individual identity both within and across contexts. We hypothesized that rams exhibit vocal individuality, which may vary in terms of distinctiveness and informational content depending on the context. Finally, we predicted that individual identity under different contexts would be conveyed through diverse acoustic parameters.
3. Results
The mean values of the determined acoustic parameters in each context (Contexts 1–5) are presented in
Table A3 (
Appendix A). Regarding PIC values, most of the estimated acoustic parameters showed considerable informational content (PIC > 1), indicating that they could potentially encode an individual vocal signature (
Table 2). Depending on the context, the highest PIC values being the most promising across vocal signals were observed for (a) AMExtent and AmpVar (Contexts: 1, 2, and 5; (b) F0mean and AmpVar (Context 2); and (c) F3mean and F4mean (Context 4).
The 2
Hs information value was also computed for each call within each context as an estimate of how many individuals could potentially be classified for each context. According to
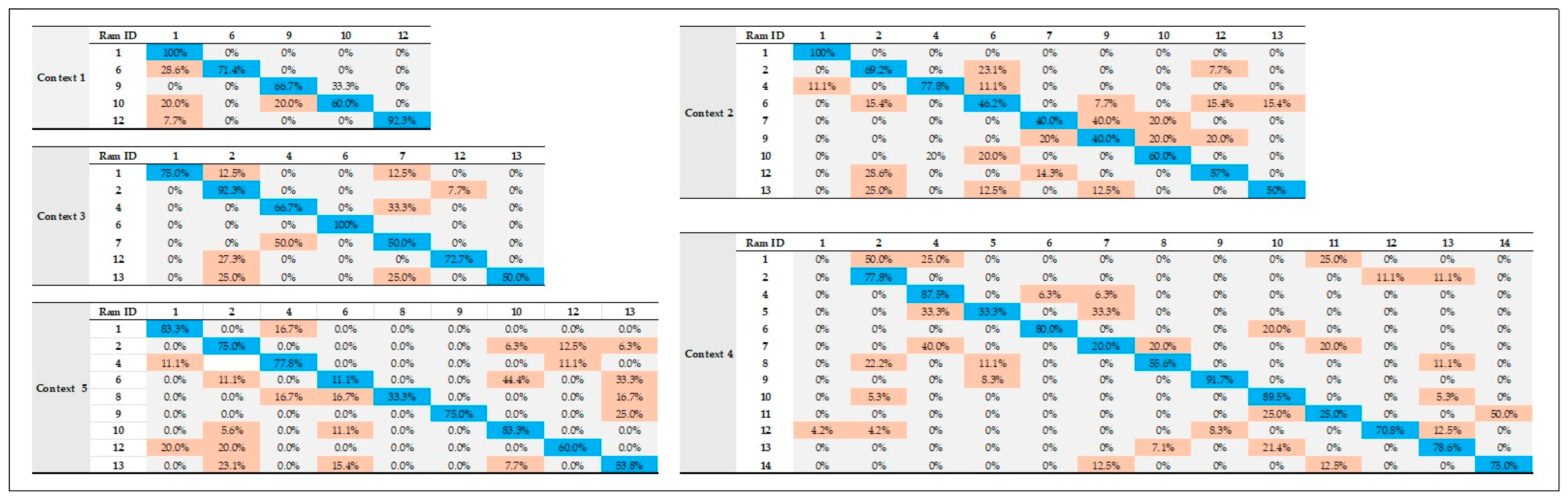
Table 3, calls in each context contained low information capacity since they could assist in distinguishing at least two individuals, with the exception of calls in Context 4, where the highest number of bits was achieved (2.61; distinguishing across the call at least six individuals).
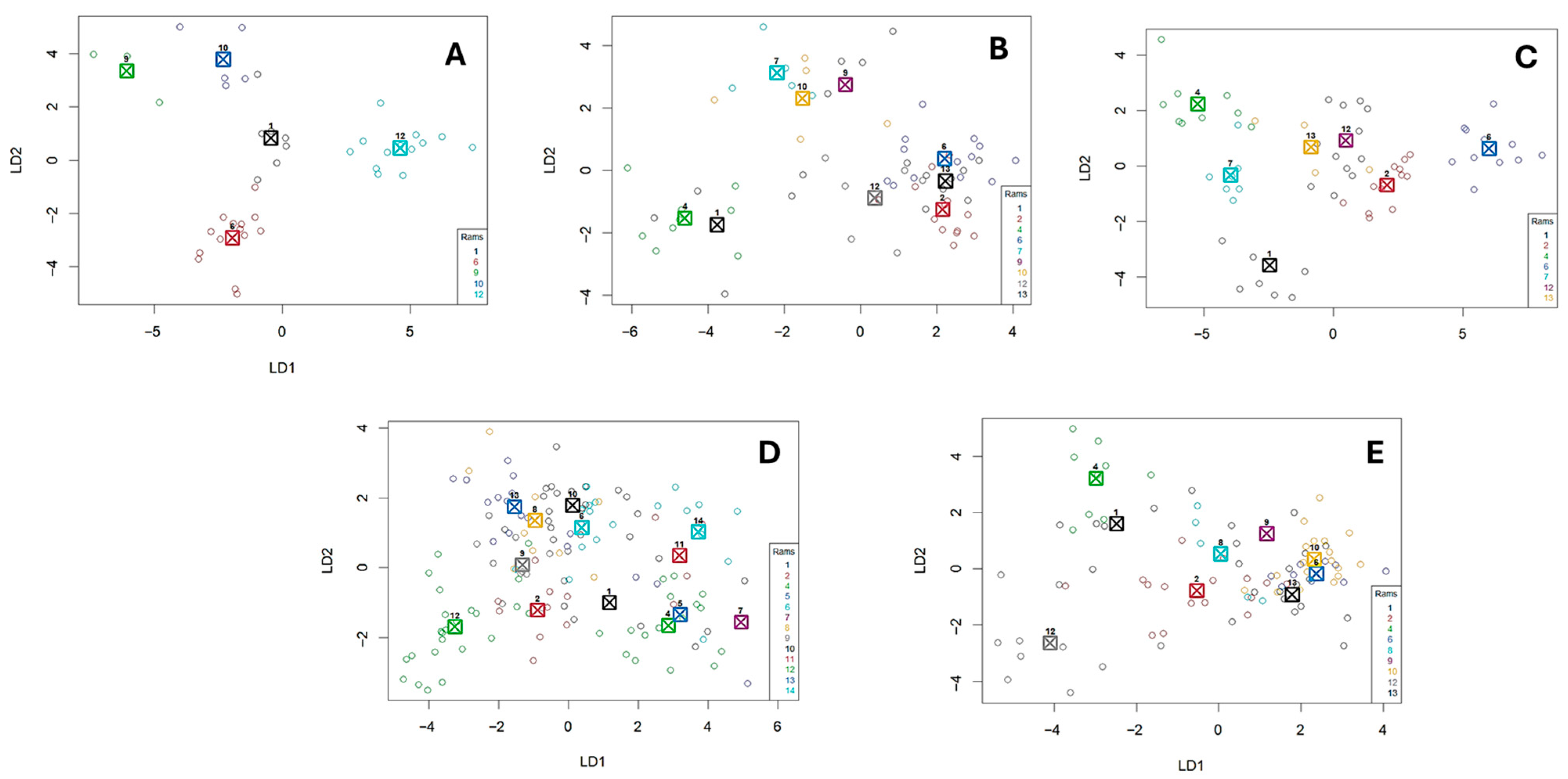
The results of the DFA concerning the correct classification of rams’ calls in each examined context are shown in
Figure 1. According to the results, vocalizations could be correctly classified by an individual within each context better than by chance (DFA; relative cross-classification level > 3.2,
p < 0.0001 in all contexts examined;
Table 4;
Figure 1). The correct percentage classification was 80.95%, 59.42%, 76.19%, 71.74%, and 64.44% for each of the examined contexts, respectively (Contexts 1–5). In
Figure A1 (
Appendix A) are also presented the percentages (%) of correct classification of each ram to itself based on their vocalizations within each context (Contexts 1–5). Furthermore, parameters related mainly to amplitude and F0 contour and higher formants were mostly correlated with the first two (LD1, LD1) discriminating roots (
Appendix A:
Table A4).
Regarding the correct classification of individuals across all vocalizations, the percentage of individuals correctly classified across all contexts was 29.76% (pDFA; relative cross-classification level = 3.59,
p = 0.001,
Table 5).
Figure 2 presents examples of vocal individuality variation in the examined contexts. Further, according to
Table 5, 38.79% of the cross-validated rams’ calls could be classified in the correct context by the pDFA above chance levels (
p < 0.001).
The VTLmax did not significantly differentiate between the contexts (F = 0.61,
p > 0.05; Context 1: 25.57 ± 0.91 cm; Context 2: 24.79 ± 0.81 cm; Context 3: 24.50 ± 0.83 cm; Context 4: 24.61 ± 0.74 cm; Context E: 24.61 ± 0.79 cm). Since VTLmax did not vary significantly between contexts, it was not included as a control factor in the LMMs that were conducted for the acoustic parameters. Consequently, the F0mean was not found to differ significantly between contexts (F = 1.61,
p > 0.05; LLM log-transformed scale: Context 1: 2.14 ± 0.03; Context 2: 2.17 ± 0.03 Hz; Context 3: 2.16 ± 0.03 Hz; Context 4: 2.16 ± 0.02 Hz; Context 5: 2.19 ± 0.03; for interpretability purposes the corresponding back-transformed values were: Context 1 = 143.31 ± 12.85 Hz, Context 2 = 163.26 ± 11.02 Hz, Context 3 = 153.15 ± 11.37 Hz, Context 4 = 155.31 ± 9.38 Hz, and Context 5 = 169.64 ± 10.46 Hz). Contrary to this, formant dispersion (Fd) was found to significantly differ between contexts (F = 6.88,
p < 0.001,
Figure 3a). Vocalizations in Context 5 had a higher formant dispersion (970.71 ± 12.71 Hz) compared to rams’ vocalizations in Context 2 (
p < 0.05; 948.78 ± 12.64 Hz), Context 3 (
p < 0.05; 946.41 ± 12.84 Hz), and Context 4 (
p < 0.001; 936.28 ± 11.74 Hz). Regarding AmpVar, it was significantly different between contexts (F = 8.76,
p < 0.001,
Figure 3b).
Specifically, rams’ vocalizations in Context 1 had significantly (p < 0.01) higher AmpVar values (111.55 ± 6.93 dB/s) compared to those of Context 4 (91.87 ± 5.70 dB/s) and Context 5 (93.79 ± 6.01 dB/s). The same was noticed when the respective values of Context 3 (113.39 ± 6.40 dB/s) were compared to those of Context 4 (91.87 ± 5.70 dB/s; p < 0.001) and Context 5 (93.79 ± 6.01dB/s; p < 0.001).
4. Discussion
This study examined whether high-frequency vocalizations produced by rams in different contexts (positive or negative) convey individual-specific acoustic signatures. Analysis revealed that multiple vocal parameters contribute to the acoustic distinctiveness among individuals. Furthermore, the degree of vocal individuality exhibited was found to be context dependent. To our knowledge, this is the first evidence demonstrating that male sheep preserve vocal identity markers across diverse husbandry conditions. These findings advance the current understanding of vocal communication in Ovis aries and suggest further exploration for potential applications of non-invasive welfare monitoring in farming environments.
All the examined acoustic variables showed the potential to effectively convey in rams’ vocalizations information about the context in which they find themselves (PIC > 1), likely reflecting distinct affective valences. Higher individuality was noted mainly in parameters related to source or amplitude characteristics. These findings align with previous studies indicating that fundamental frequency (F0) and amplitude parameters are key contributors to vocal distinctiveness in lambs and ewes [
25,
26,
27]. Moreover, the observed expression patterns are consistent with vocal individuality levels observed across mammals. Source-related acoustic parameters (e.g., fundamental frequency, F0) and spectral-related features have been widely recognized as reliable cues of individual identity in various mammalian species, including deer [
9,
10,
45], giant pandas [
14,
46], sows [
43,
47], and ruminants [
11,
24,
25,
26,
27,
48]. Interestingly, the higher formant frequencies (F3 and F4) revealed higher encoded information related to individual identity in rams under the positive context of feed anticipation, which, to the best of our knowledge, has not previously been documented in ungulates. High values of the higher formants have been reported previously under positive contexts compared to negative grunts in pigs [
43]. In heifers experiencing similar feed anticipation contexts, vocal individuality was primarily linked to amplitude-related parameters [
11]. In our case, the enhanced individuality conveyed through F3 and F4 may be functionally significant in rams, particularly in relation to direct access to feed resources. This could reflect an adaptive need to communicate individual identity over longer distances. Higher frequencies, such as those in F3 and F4, are known to propagate more effectively in open environments, which may be advantageous in natural settings, where searching and locating food is a competitive and spatially challenging task.
We further examined the information content of vocalizations using Beecher’s information statistic (Hs), considered one of the most robust and reliable indices for vocal individuality [
36]. The Hs index reflects the entropy embedded in a vocal cue and estimates the number of bits of information conveyed by a signal, that is, how many individuals can be reliably discriminated based on their acoustic features [
12]. Species that live in large social groups are generally expected to produce vocalizations with high information content, resulting in elevated Hs values [
2]. However, this expectation was not met in our study, where Hs values ranged from 0.68 to 2.61 across the vocal cues analyzed. Accordingly, the noted values are comparable to those reported for
Myiopsitta monachus (Hs up to 2.77; [
49]), but notably lower than those observed in various sciurid rodent species (Hs = 4.89–7.76; [
2]),
Alle alle (Hs = 3.58–5.39; [
34]), and
Tursiops truncatus (Hs = 13.7; [
50]). A profound explanation for the relatively low Hs values in our data set could be the limited vocal repertoire of sheep, which predominantly consists of two types of bleats—low-pitched and high-pitched. Further, a study on zebra finches demonstrated that vocalizations used in specific contexts, like feed anticipation, showed reduced acoustic variability, supporting the notion that emotionally uniform contexts can lead to simplified vocal patterns [
51]. Therefore, when vocalizations are produced in uniform contexts such as feed anticipation, individuals may rely on a restricted set of acoustic patterns, further reducing entropy and overall information content.
The analyzed vocalizations exhibited, also, individual-specific acoustic features, allowing for accurate classification of signals to specific individuals within each context. The noted variation in the accuracy of classification (59–80%) can be attributed to the context, reflecting the need for higher individual distinctiveness, mainly under negative contexts. Importantly, we observed that vocal distinctiveness was retained across different contexts, indicating a degree of acoustic stability. This suggests that identity cues are not merely context-dependent but may reflect intrinsic vocal characteristics. Previous studies have also reported vocal individuality in lambs at different postpartum time points, suggesting that even young animals exhibit distinct acoustic signatures early in life [
24,
25,
27]. Additionally, ewes have been shown to modulate their vocalizations depending on social and environmental contexts, i.e., dry season or weaning period [
26,
27], further supporting the notion that vocal cues encode both identity and situational information. However, when call types from all contexts were pooled, classification accuracy declined (~30%). This reduction likely reflects increased acoustic variability due to different contexts, which can obscure individual-specific features and lead to overlap between individuals. Maintaining vocal individuality across different signal types is not a universal trait among animals. Among mammals, vocal individuality has been documented in species such as bottlenose dolphins (
Tursiops truncatus), whose signature whistles are highly distinctive and stable over time [
50], and in primates like baboons and macaques, where vocal cues convey identity and social status [
52]. Ungulates also demonstrate vocal individuality, though it can be influenced by emotional context. Domestic goats (
Capra hircus) and goitred gazelles (
Gazella subgutturosa) show increasing vocal distinctiveness with age and social experience [
8,
53]. However, individuality in vocalizations may diminish under high arousal or negative emotional states, as shown across ungulate species including cattle, pigs, horses, and wild boars [
11,
43,
54,
55]. Future studies should explore the extent to which these identity cues are perceptible to conspecifics and whether they influence social interactions or group dynamics. Additionally, investigating the neural or physiological mechanisms underlying vocal consistency across contexts could provide deeper insight into the evolution of individual vocal signatures.
In addition to individual identity, the permutated discrimination analysis revealed a moderate classification accuracy (39%; 1.65 times better than random classification) of rams’ vocalizations, indicating that the discriminant functions captured meaningful structure in the data beyond chance expectations and further that rams’ vocal cues could encode some information about their affective state. This is in accordance with previous studies indicating context-specific vocal modulation in other species, including cattle [
48], pigs [
43,
55], and primates [
52]. In addition, our results support the notion that animal vocalizations are not solely identity markers but can simultaneously convey emotional states or motivational contexts. The ability to discriminate between contexts based on acoustic structure suggests that rams modulate their vocal output in response to situational factors, such as feed anticipation or social isolation. Understanding the dual encoding of identity and emotion in vocal signals may offer deeper insights into the evolution of complex communication systems in social mammals.
The comparative analysis of acoustic parameters across contexts revealed that rams’ vocalizations under negative emotional states—such as feed frustration and nocturnal isolation—were characterized by greater formant dispersion and increased amplitude variation compared to those produced during positive contexts (e.g., feed anticipation). These findings suggest that negative affective states lead to deeper and more unstable calls. Formant dispersion may reflect physiological changes in vocal tract configuration due to heightened arousal, while amplitude variability could indicate reduced vocal control or urgency in signal transmission. Although the estimated values of VTLmax did not differ significantly between the examined contexts, similar values (e.g., 21.3 ± 0.2 cm) have been reported in adult goats, a species closely related to sheep, suggesting changes in tract configuration under different contexts [
56]. In sheep, craniometric measurements of basal skull length in Romanov male rams [
57] imply a lower respective vocal tract length (17.6 ± 1.88 cm) in the resting (static) position or higher (24.6 ± 2.1 cm) in Barbados Black Belly sheep [
58]. To the best of our knowledge, direct anatomic evidence of laryngeal retraction (i.e., video validation to assess dynamic vocal tract adjustments) is currently lacking. Therefore, any potential implication of tract elongation during vocalization requires further investigation to assess its plausibility in relation to breed and formant dispersion. However, physiological changes in vocal tract configuration have been observed in other ruminants [
9,
45,
53] and other species, including pigs and cattle, where vocal cues shift in response to emotional intensity [
43,
48]. In primates, emotional arousal has been linked to changes in pitch and temporal dynamics [
52]. However, previous studies in sheep revealed that amplitude modulation and jitter were the key acoustic parameters contributing to individual vocal identity in ewes and lambs during a short separation among them in an early or later postpartum period [
25,
27]. Sibling pairs also demonstrated unique vocal signatures, particularly through variations in fundamental frequency (F0) contour parameters. Notably, the individuality of ewes’ vocalizations was not limited to the suckling phase; it was also observed during the dry season, indicating that vocal distinctiveness persists across different reproductive stages [
27]. In rams, the observed acoustic modulations may serve adaptive functions—such as signaling distress or soliciting social support. Considering the presence of individuality, they may also reduce the clarity of individual vocal signatures, as emotional modulation can mask identity-related features. This dual encoding of affect and identity presents a complex dynamic in vocal communication, opening avenues for further investigation into how conspecifics interpret and respond to such signals. For example, beyond individual recognition, these vocal cues may play a pivotal role in shaping group cohesion and social structure. In highly social species such as sheep, the ability to discern emotional states and individual identities through vocalizations could influence affiliative behaviors, stress responses, and collective decision-making. For instance, the vocal expression of distress or contentment by a single member may elicit corresponding behavioral adjustments in nearby individuals, potentially amplifying emotional states across the flock. This, potentially, raises questions about the extent to which vocal signals contribute to emergent group-level phenomena such as synchronization, leadership, and conflict resolution. Investigating these dynamics could provide deeper insight into the mechanisms of social modulation in animal groups and inform welfare practices by identifying vocal markers of group stability or disruption.
While vocalizations often contain a range of acoustic parameters, not all of these are necessarily meaningful or detectable to receivers in social species [
59]. Depending on the context and species, certain parameters may contribute more significantly to vocal distinctiveness and/or transmission of context information. To determine which features truly function as markers of individual identity or contextual situations, playback experiments using bleats with altered acoustic structures would be essential. Such experiments could help clarify whether specific parameters are perceived and utilized by the receiver, as the presence of encoded information does not a priori guarantee their vocal signature.