Seroprevalence of Anti-Neospora caninum and Anti-Toxoplasma gondii Antibodies in Cattle Intended for Human Consumption in the State of Paraíba, Brazil
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
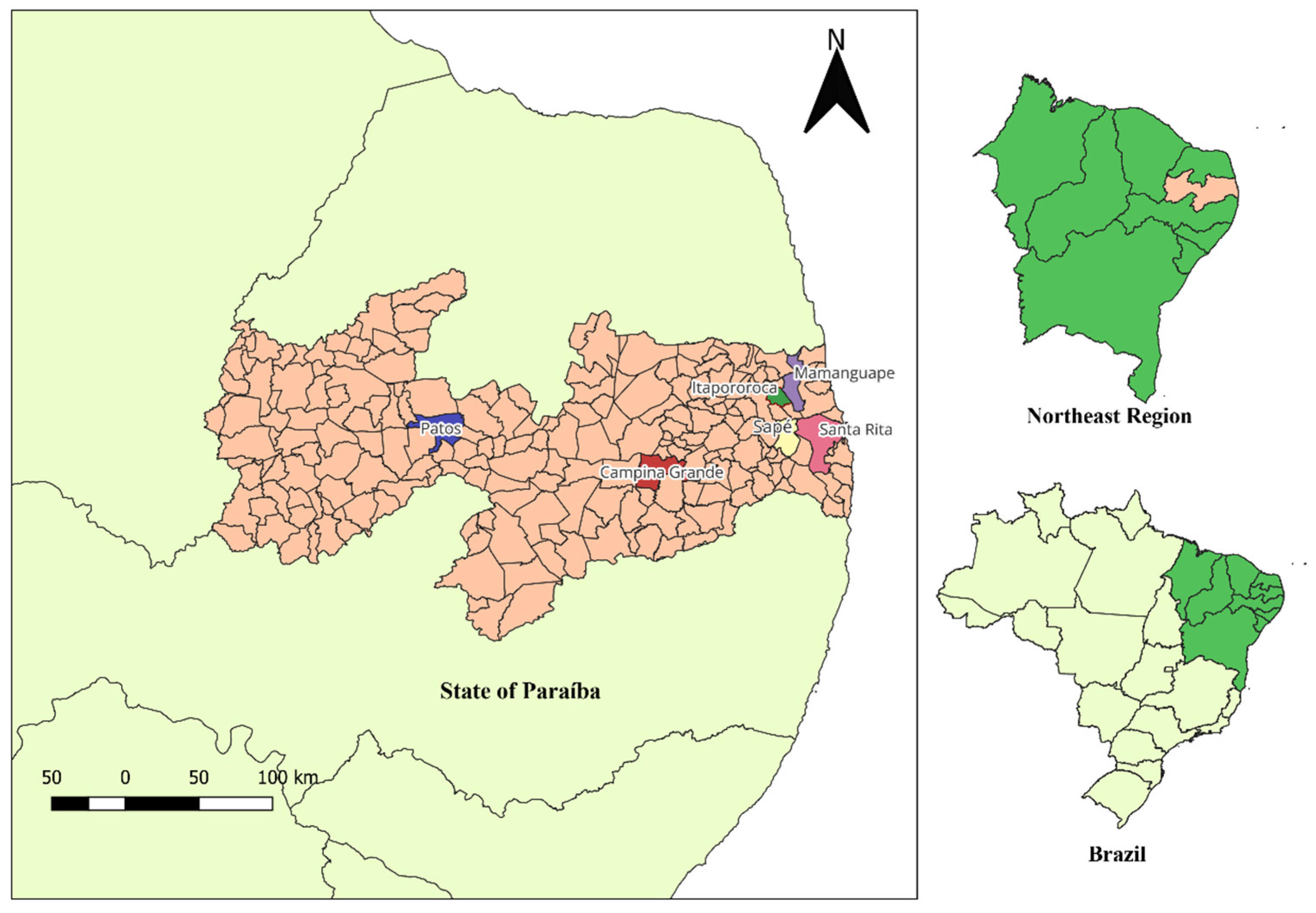
Study Area and Sampling
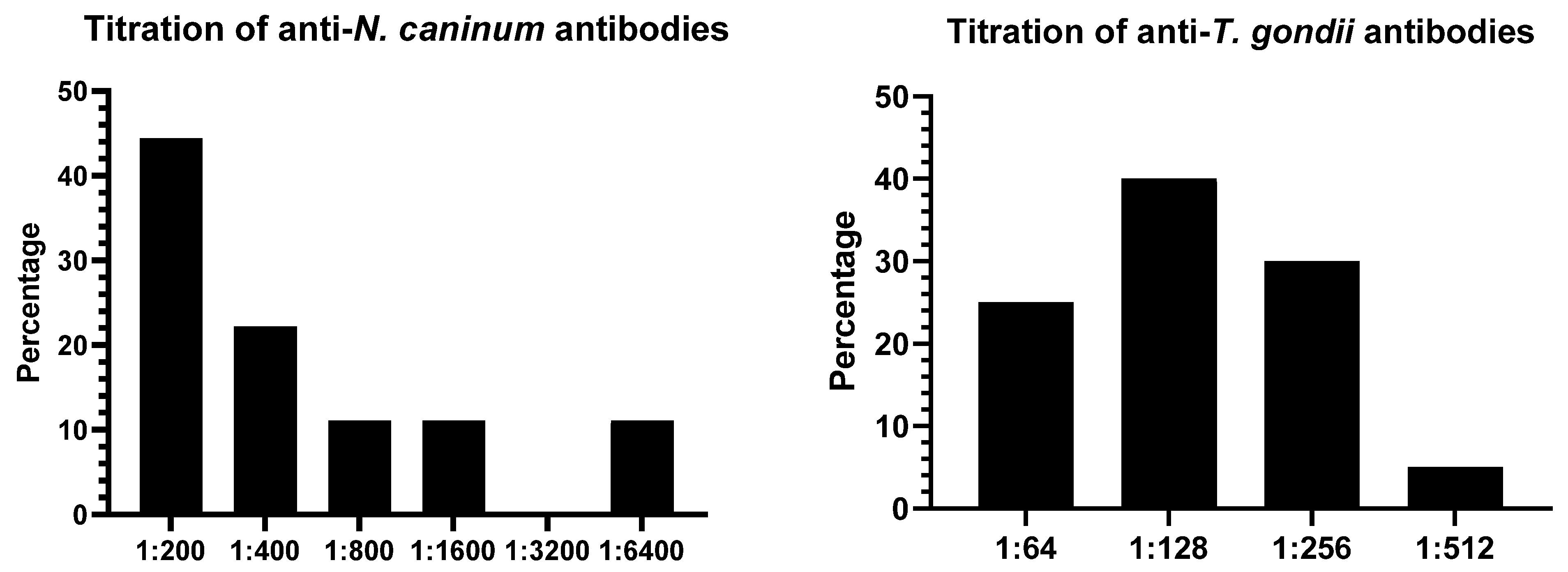
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dubey, J.P.; Schares, G. Neosporosis in animals—The last five years. Vet. Parasitol. 2011, 180, 90–108. [Google Scholar] [CrossRef] [PubMed]
- Vilela, V.L.R.; Feitosa, T.F. Recent Advances in Toxoplasma gondii Infection and Toxoplasmosis. Trop. Med. Infect. Dis. 2024, 9, 160. [Google Scholar] [CrossRef]
- Reichel, M.P.; Wahl, L.C.; Ellis, J.T. Research into Neospora caninum—What Have We Learnt in the Last Thirty Years? Pathogens 2020, 9, 505. [Google Scholar] [CrossRef]
- Guedes, M.H.P.; Guimarães, A.M.; Rocha, C.M.B.M.; Hirsch, C. Frequência de anticorpos anti-Neospora caninum em vacas e fetos provenientes de municípios do sul de Minas Gerais. Rev. Bras. Parasitol. Vet. 2008, 17, 189–194. [Google Scholar] [PubMed]
- Maia, A.R.A.; de Melo, R.P.B.; Mota, R.A.M.; Clementino, I.J.; Alves, C.J.; de Santos, C.S.A.B.; Fernandes, L.G.; de Azevedo, S.S. Herd and animal level prevalences and risk factors for Neospora caninum infection in cattle in the state of Paraíba, northeastern Brazil. Vet. Parasitol. 2023, 40, 100866. [Google Scholar] [CrossRef]
- Reichel, M.P.; Ayanegui-Alcérreca, M.; Gondim, L.F.; Ellis, J.T. What is the global economic impact of Neospora caninum in cattle—The billion dollar question. Int. J. Parasitol. 2013, 43, 133–142. [Google Scholar] [CrossRef]
- Duarte, P.O.; Oshiro, L.M.; Zimmermann, N.P.; Csorda, B.G.; Dourado, D.M.; Barros, J.C.; Andreott, R. Serological and molecular detection of Neospora caninum and Toxoplasma gondii in human umbilical cord blood and placental tissue samples. Sci. Rep. 2020, 10, 9043. [Google Scholar] [CrossRef]
- Duarte, P.O.; Csordas, B.G.; Oshiro, L.M.; de Higa, L.O.S.; Zimmermann, N.P.; Martins, K.R.; Barros, J.C.; Andreotti, R. Serological evaluation of Neospora caninum in pregnant women treated at referral center for prenatal screening in Mato Grosso do Sul, Brazil. Rev. Bras. Parasitol. Vet. 2020, 29, 4. [Google Scholar] [CrossRef] [PubMed]
- Abbas, I.E.; Villena, I.; Dubey, J.P. A review on toxoplasmosis in humans and animals from Egypt. Parasitology 2020, 147, 135–159. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Jones, J.L. Toxoplasma gondii infection in humans and animals in the United States. Int. J. Parasitol. 2008, 38, 1257–1278. [Google Scholar] [CrossRef]
- World Organization for Animal Health—OIE. Terrestrial Manual: Toxoplasmosis. Available online: https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.09.09_TOXO.pdf (accessed on 6 October 2025).
- Gomes, D.F.C.; da Krawczak, F.S.; de Oliveira, C.H.S.; Ferreira Júnior, Á.; Fernandes, E.K.K.; Lopes, W.D.Z.; da Sevá, A.P.; Gennari, S.M. Toxoplasma gondii in cattle in Brazil: A review. Rev. Bras. Parasitol. Vet. 2020, 29, e015719. [Google Scholar] [CrossRef]
- Silva, J.O.; Batista, S.P.; Matos, T.S.; Bison, I.; Parentoni, R.N.; Santos, J.R.S.; Brasil, A.W.L.; Feitosa, T.F.; Vilela, V.L.R. Elevated anti-Toxoplasma gondii antibody levels among slaughterhouse workers in Paraíba, Brazil: Implications for occupational health. Acta Trop. 2025, 270, 107773. [Google Scholar] [CrossRef]
- Kean, B.H.; Kimball, A.C.; Christenson, W.N. An epidemic of acute toxoplasmosis. J. Am. Med. Assoc. 1969, 208, 1002–1004. [Google Scholar]
- Dubey, J.P. Outbreaks of clinical toxoplasmosis in humans: Five decades of personal experience, perspectives and lessons learned. Parasites Vectors 2021, 14, 263. [Google Scholar] [CrossRef] [PubMed]
- Thrusfield, M. Veterinary Epidemiology, 3rd ed.; Blackwell Science: Oxford, UK, 2007; pp. 1–610. [Google Scholar]
- Maia, A.R.A.; Bezerra, R.A.; Silva, S.S.; Álvares, F.B.V.; de Santos, C.S.A.B.; Alves, C.J.; Clementino, I.J.; Feitosa, T.F.; Vilela, V.L.R.; de Azevedo, S.S. Herd-level based seroprevalence and associated factors for Toxoplasma gondii in cows in the state of Paraíba, Northeastern Brazil. Braz. J. Vet. Parasitol. 2023, 32, e017222. [Google Scholar] [CrossRef]
- Camargo, M.E. Improvised technique of indirect immunofluorescence for serological diagnosis of toxoplasmosis. Rev. Inst. Med. Trop. 1964, 6, 117–118. [Google Scholar]
- Costa, F.R.T.; Nogueira, D.B.; Oliveira, M.A.G.; Silva, S.S.; Silva, R.F.; Sarmento, W.F.; Azevedo, S.S.; Gennari, S.M.; Pena, H.F.J.; Brasil, A.W.L.; et al. Vertical transmission of Toxoplasma gondii in naturally infected ewes in the semiarid region of Brazil. Comp. Immunol. Microbiol. Infect. Dis. 2021, 74, 101595. [Google Scholar] [CrossRef]
- Gondim, L.F.P.; Sartor, I.F.; Hasegawa, M.; Yamane, I. Seroprevalence of Neospora caninum in dairy cattle in Bahia, Brazil. Vet. Parasitol. 1999, 86, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Amaral, R.L.G.; Silva, L.B.G.; Pinheiro Júnior, J.W.; Souza Neto, O.L.; Leal, C.A.S.; Porto, W.J.N.; Mota, R.A. Neospora caninum em bovinos em matadouros de Pernambuco e Alagoas. Pesq. Vet. Bras. 2012, 32, 963–966. [Google Scholar] [CrossRef]
- Batista, S.P.; Silva, J.O.; Ferraz, C.M.; de Assis, J.P.B.; Rossi, G.A.M.; Daleprani, L.G.; Tobias, F.L.; Feitosa, T.F.; Braga, F.R.; Vilela, V.L.R. Anti-Neospora caninum and anti-Toxoplasma gondii antibodies in cattle intended for human consumption in the State of Espírito Santo, Brazil: Prevalence and associated factors. Braz. J. Vet. Parasitol. 2025, 34, e001225. [Google Scholar]
- Holec-Gąsior, L.; Sołowińska, K. IgG Avidity Test as a Tool for Discrimination Between Recent and Distant Toxoplasma gondii Infection—Current Status of Studies. Antibodies 2022, 11, 52. [Google Scholar] [CrossRef]
- Webster, J. Toxoplasmosis: A review of the disease and its public health significance. J. Am. Vet. Med. Assoc. 2010, 236, 15–22. [Google Scholar]
- Guerra, J.L.; Okano, W.; Bogado, A.L.G.; Nino, B.S.L.; Martins, F.D.C.; Cardim, S.T.; de Barros, L.D.; Garcia, J.L. Anti-Neospora caninum antibodies in beef cattle from the northern region of Paraná state, Brazil. Cienc. Rural 2019, 49, e20180869. [Google Scholar] [CrossRef]
- Dubey, J.P.; Schares, G.; Ortega-Mora, L.M. Epidemiology and control of neosporosis and Neospora caninum. Clin. Microbiol. Rev. 2007, 20, 323–367. [Google Scholar] [CrossRef] [PubMed]
- Roman, I.J.; Tagarra, L.G.; Rodrigues, F.S.; Cargnelutti, J.F.; Sangioni, L.A.; Vogel, F.S.F. Sarcocystis neurona, Toxoplasma gondii and Neospora caninum infection in bovine fetuses from a slaughterhouse in southern Brazil. Pesq. Vet. Bras. 2025, 45, e07504. [Google Scholar]
- Sartor, I.F.; Garcia Filho, A.; Vianna, L.C.; Pituco, E.M.; Dal Pai, V.; Sartor, R. Occurrence of antibodies anti-Neospora caninum in dairy and beef cattle in the region of Presidente Prudente, SP, Brazil. Arq. Inst. Biol. 2005, 72, 4. [Google Scholar]
- Spagnol, R.; Paranhos, E.B.; Oliveira, L.L.S.; Medeiros, S.M.; Lopes, C.W.G.; Albuquerque, G.R. Prevalência de anticorpos anti-Toxoplasma gondii em bovinos abatidos em matadouros do estado da Bahia, Brasil. Rev. Bras. Parasitol. Vet. 2009, 18, 42–45. [Google Scholar]
- Tenter, A.M.; Heckeroth, A.R.; Weiss, L.M. Toxoplasma gondii: From animals to humans. Int. J. Parasitol. 2000, 30, 1217–1258. [Google Scholar] [CrossRef]
- Santos, S.L.; de Souza Costa, K.; Gondim, L.Q.; da Silva, M.S.A.; Uzêda, R.S.; Gondim, L.F.P. Investigation of Neospora caninum, Hammondia sp., and Toxoplasma gondii in tissues from slaughtered beef cattle in Bahia, Brazil. Parasitol. Res. 2010, 106, 457–461. [Google Scholar] [CrossRef]
- Silva, A.L.P.; Lima, B.A.; Formiga, V.H.A.S.; Lima, E.F.; Silva Filho, G.M.; Silva, W.I.; Silva, J.O.; Alvares, F.B.V.; Vilela, V.L.R.; Feitosa, T.F. Survival and viability of Toxoplasma gondii oocysts under natural dry season conditions in the Brazilian semi-arid region. Vet. Res. Commun. 2025, 49, 191. [Google Scholar] [CrossRef]
- Magalhães, F.J.R.; Ribeiro-Andrade, M.; Alcântara, A.M.; Pinheiro Júnior, J.W.; Sena, M.J.; Porto, W.J.N.; Vieira, R.F.D.C.; Mota, R.A. Risk factors for Toxoplasma gondii infection in sheep and cattle from Fernando de Noronha Island, Brazil. Braz. J. Vet. Parasitol. 2016, 25, 511–515. [Google Scholar] [CrossRef]
- Snak, A.; Garcia, F.G.; Lara, A.A.; Pena, H.F.J.; Osaki, S.C. Neospora caninum in properties in the west region of Paraná, Brazil: Prevalence and risk factors. Rev. Bras. Parasitol. Vet. 2018, 27, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P. Review of Neospora caninum and neosporosis in animals. Korean J. Parasitol. 2003, 41, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.C.; McAllister, A.J.; de Queiroz, S.A. Efeito das taxas de descarte sobre medidas econômicas de vacas leiteiras em Kentucky. Rev. Bras. Zootec. 2003, 32, 1737–1746. [Google Scholar] [CrossRef]
- Hill, D.E.; Dubey, J.P. Toxoplasma gondii prevalence in farm animals in the United States. Int. J. Parasitol. 2013, 43, 107–113. [Google Scholar] [CrossRef]
- Da Fonseca, F.M.; Sato, A.P.; Becker, A.P.B.B.; da Pinto, G.O.P.A.; de Souza, G.S.; Perotta, J.H.; de Filho, I.R.B.; Rodriguez, M.C.; Locatelli-Dittrich, R. Detection of Toxoplasma gondii DNA in milk of dairy cows from southern Brazil. Parasitol. Int. 2023, 95, 102750. [Google Scholar] [CrossRef]
- Khan, S.; Rafiq, K.; Khabir, M.N.; Khan, M.B.; Khan, S.N.; Khattak, A.; Attaullah, S. Toxoplasma gondii in lactating animals: Potential risk to milk consuming population in Khyber Pakhtunkhwa. Braz. J. Biol. 2023, 83, e267369. [Google Scholar] [CrossRef]
| Variables | N. caninum | T. gondii | |||
|---|---|---|---|---|---|
| Total | Positive (%) | p | Positive (%) | p | |
| Sex | |||||
| Male | 53 | 0 (0) | 0.003 * | 14 (26.4) | 0.046 * |
| Female | 57 | 9 (15.8) | 6 (10.5) | ||
| Age | |||||
| ≤24 months | 38 | 2 (5.3) | 0.7161 | 4 (10.5) | 0.193 |
| >24 months | 72 | 7 (9.7) | 16 (22.2) | ||
| Breed | |||||
| Pure | 19 | 1 (5.3) | >0.999 | 3 (15.8) | >0.999 |
| Crossbred | 91 | 8 (8.8) | 17 (18.7) | ||
| History of abortion in the last 12 months | |||||
| Yes | 68 | 6 (8.8) | >0.999 | 16 (23.5) | 0.078 |
| No | 42 | 3 (7.1) | 4 (9.5) | ||
| Contact with cats | |||||
| Yes | 74 | 6 (8.3) | >0.999 | 14 (18.9) | >0.999 |
| No | 36 | 3 (8.1) | 6 (16.7) | ||
| Contact with dogs | |||||
| Yes | 83 | 7 (8.4) | >0.999 | 13 (15.6) | 0.256 |
| No | 27 | 2 (7.4) | 7 (25.9) | ||
| Provision of treated water | |||||
| Yes | 34 | 4 (11.8) | 0.454 | 5 (14.7) | 0.603 |
| No | 76 | 5 (6.6) | 15 (19.7) | ||
| Frequent purchase of animals | |||||
| Yes | 20 | 5 (10.9) | 0.487 | 14 (30.4) | 0.006 * |
| No | 90 | 4 (6.2) | 6 (9.4) | ||
| Separation of young and adult animals | |||||
| Yes | 70 | 5 (7.1) | 0.721 | 7 (10) | 0.005 * |
| No | 40 | 4 (10) | 13 (32.5) | ||
| Farming system | |||||
| Extensive | 39 | 7 (18) | 0.009 * | 16 (41) | <0.001 * |
| Semi-intensive | 71 | 2 (2.8) | 4 (5.6) | ||
| Veterinary assistance | |||||
| Yes | 32 | 3 (9.4) | 0.718 | 6 (18.7) | >0.999 |
| No | 78 | 6 (7.7) | 14 (18) | ||
| Presence of maternity pens | |||||
| Yes | 59 | 2 (3.9) | 0.172 | 5 (9.8) | 0.047 * |
| No | 51 | 7 (11.9) | 15 (25.4) | ||
| Variables | Prevalence Ratio | IC 95% | p |
|---|---|---|---|
| anti-N. caninum antibodies | |||
| Sex (Female) | 4.8 | 1.2–19.1 | 0.025 * |
| Farming system (Extensive) | 5.1 | 1.5–17.3 | 0.009 * |
| anti-T. gondii antibodies | |||
| Farming system (Extensive) | 4.3 | 1.4–13.2 | 0.011 * |
| Frequent purchase of animals (Yes) | 3.9 | 1.2–12.8 | 0.023 * |
| Separation of young/adult animals (No) | 3.1 | 1.1–8.7 | 0.032 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva Filho, G.M.; Silva, J.O.; Costa Filho, A.A.; Parentoni, R.N.; Brasil, A.W.L.; Feitosa, T.F.; Vilela, V.L.R. Seroprevalence of Anti-Neospora caninum and Anti-Toxoplasma gondii Antibodies in Cattle Intended for Human Consumption in the State of Paraíba, Brazil. Ruminants 2025, 5, 48. https://doi.org/10.3390/ruminants5040048
Silva Filho GM, Silva JO, Costa Filho AA, Parentoni RN, Brasil AWL, Feitosa TF, Vilela VLR. Seroprevalence of Anti-Neospora caninum and Anti-Toxoplasma gondii Antibodies in Cattle Intended for Human Consumption in the State of Paraíba, Brazil. Ruminants. 2025; 5(4):48. https://doi.org/10.3390/ruminants5040048
Chicago/Turabian StyleSilva Filho, Geraldo Moreira, Jordania Oliveira Silva, Audisio Alves Costa Filho, Roberta Nunes Parentoni, Arthur Willian Lima Brasil, Thais Ferreira Feitosa, and Vinícius Longo Ribeiro Vilela. 2025. "Seroprevalence of Anti-Neospora caninum and Anti-Toxoplasma gondii Antibodies in Cattle Intended for Human Consumption in the State of Paraíba, Brazil" Ruminants 5, no. 4: 48. https://doi.org/10.3390/ruminants5040048
APA StyleSilva Filho, G. M., Silva, J. O., Costa Filho, A. A., Parentoni, R. N., Brasil, A. W. L., Feitosa, T. F., & Vilela, V. L. R. (2025). Seroprevalence of Anti-Neospora caninum and Anti-Toxoplasma gondii Antibodies in Cattle Intended for Human Consumption in the State of Paraíba, Brazil. Ruminants, 5(4), 48. https://doi.org/10.3390/ruminants5040048









