Effect of Polyphenol Supplementation on Milk Composition and Fatty Acid of Dairy Animal: A Systematic Review
Simple Summary
Abstract
1. Introduction
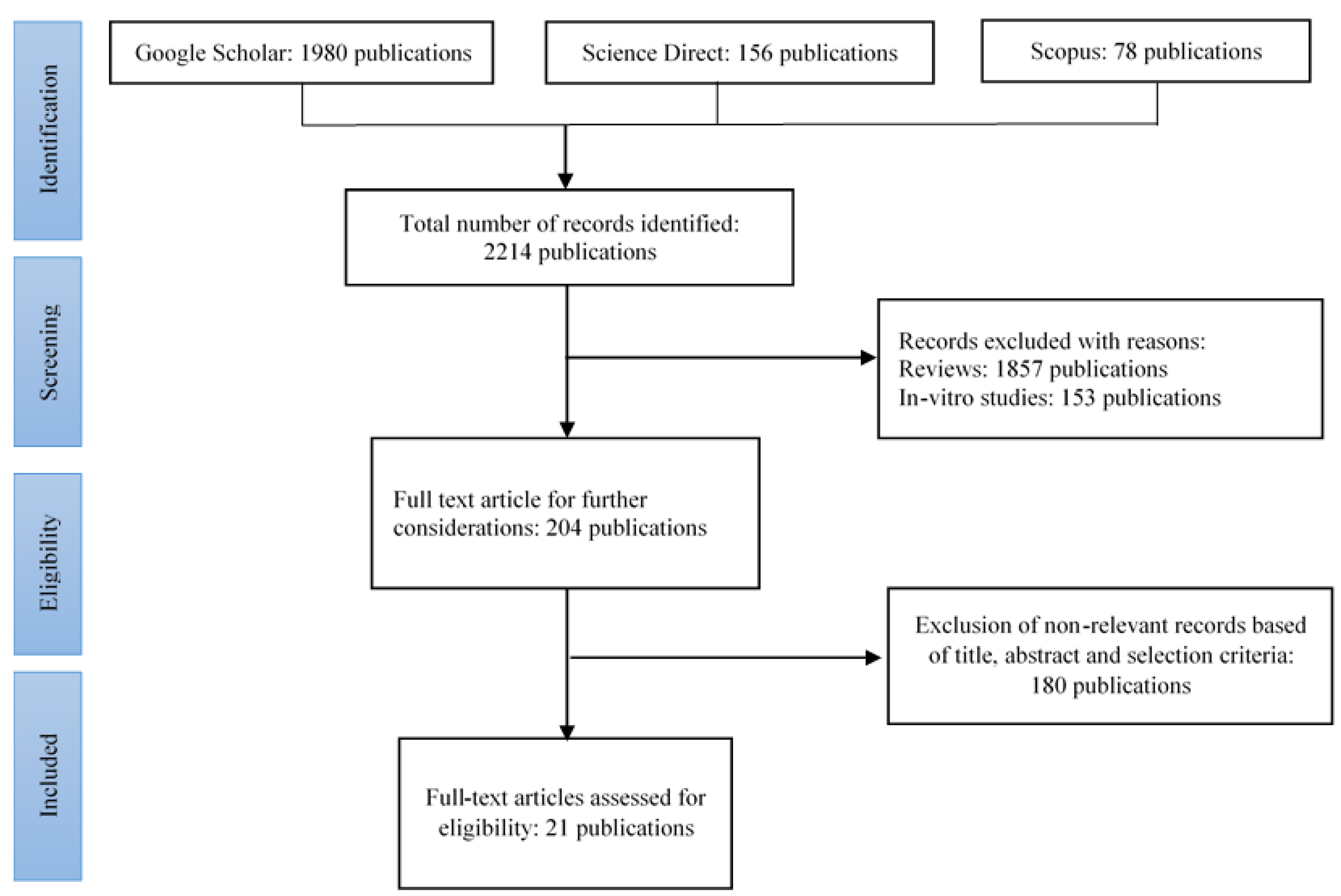
2. Materials and Methods
2.1. Effect of Polyphenol Supplementation on Milk Fat Content
2.2. Effect of Polyphenol Supplementation on Milk Protein Content
2.3. Effect of Polyphenol Supplementation on Milk Lactose Content
2.4. Effect of Polyphenol Supplementation on Milk Saturated Fatty Acid Content
2.5. Effect of Polyphenol Supplementation on Milk Medium-Chained Fatty Acid Content
3. Results
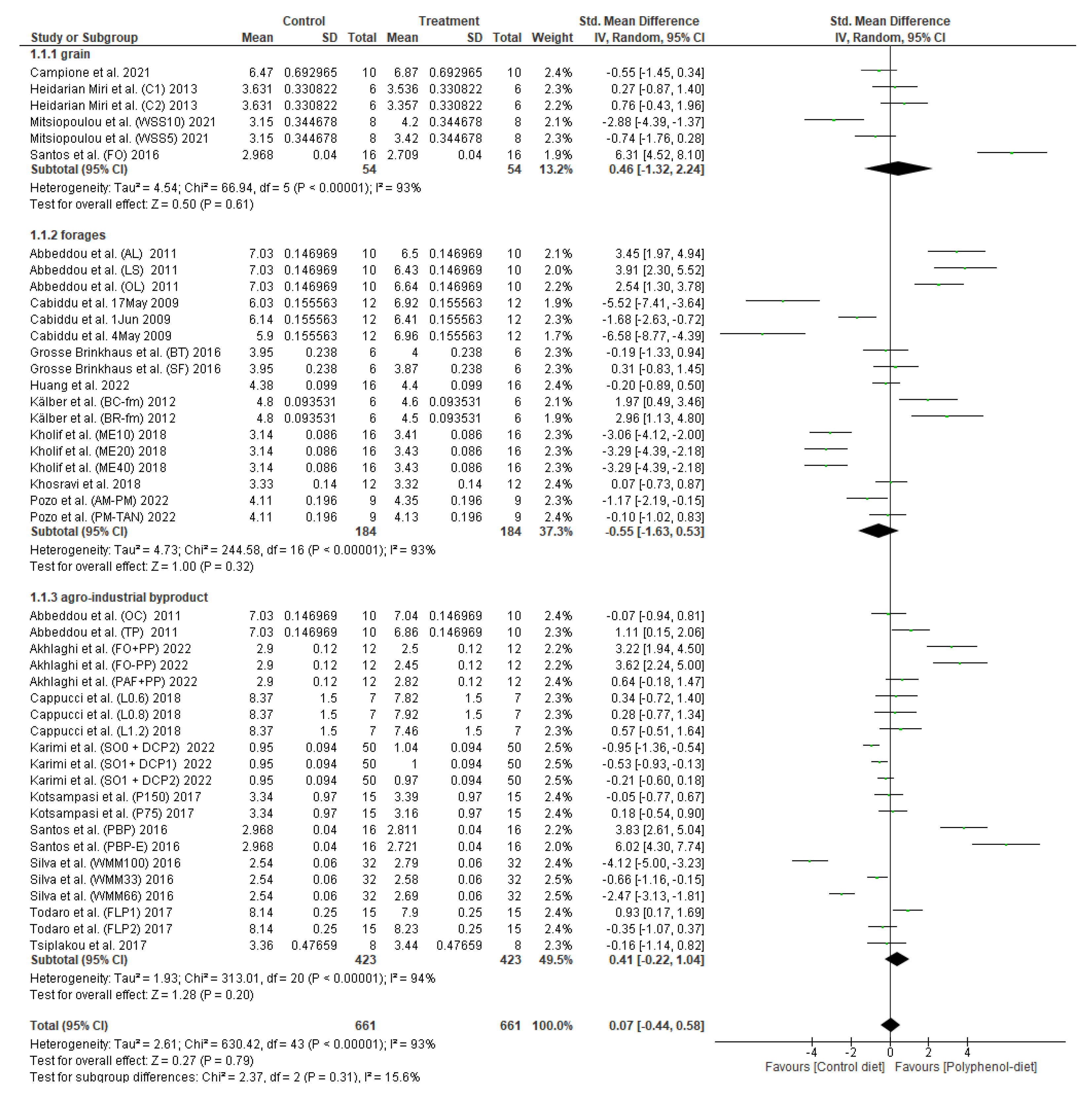
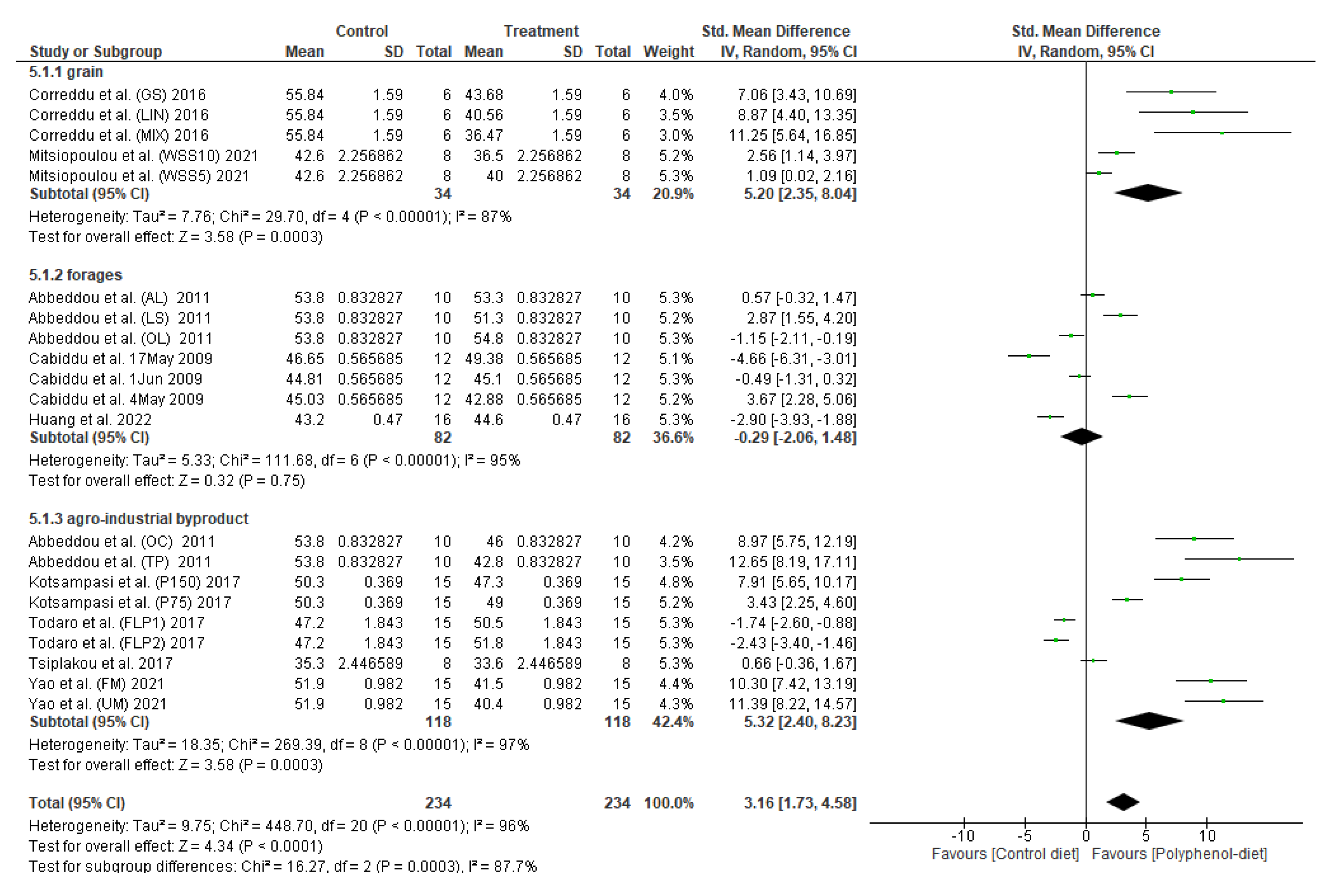
3.1. Effect of Polyphenol Supplementation on Milk Fat Content
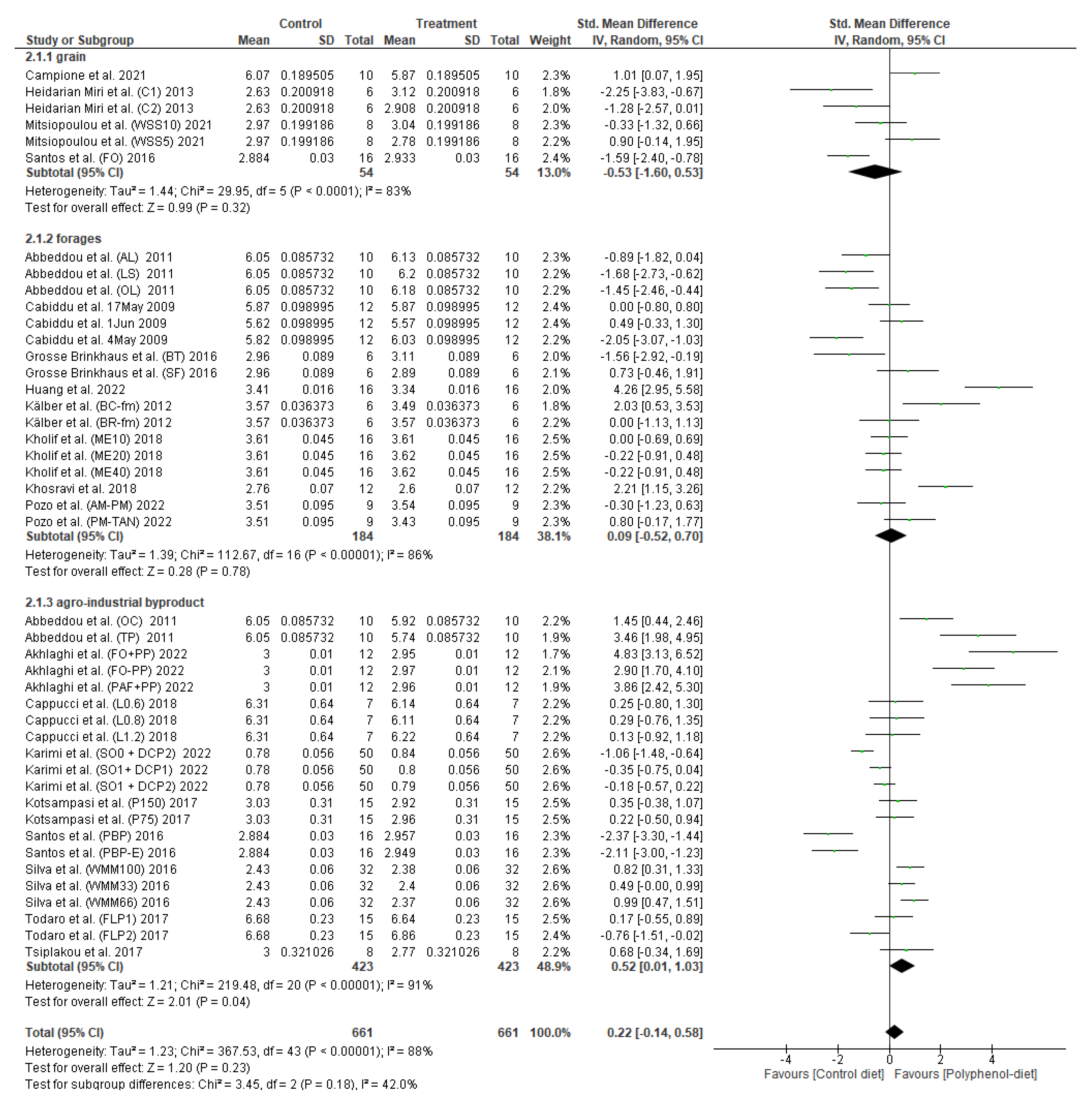
3.2. Effect of Polyphenol Supplementation on Milk Protein Content
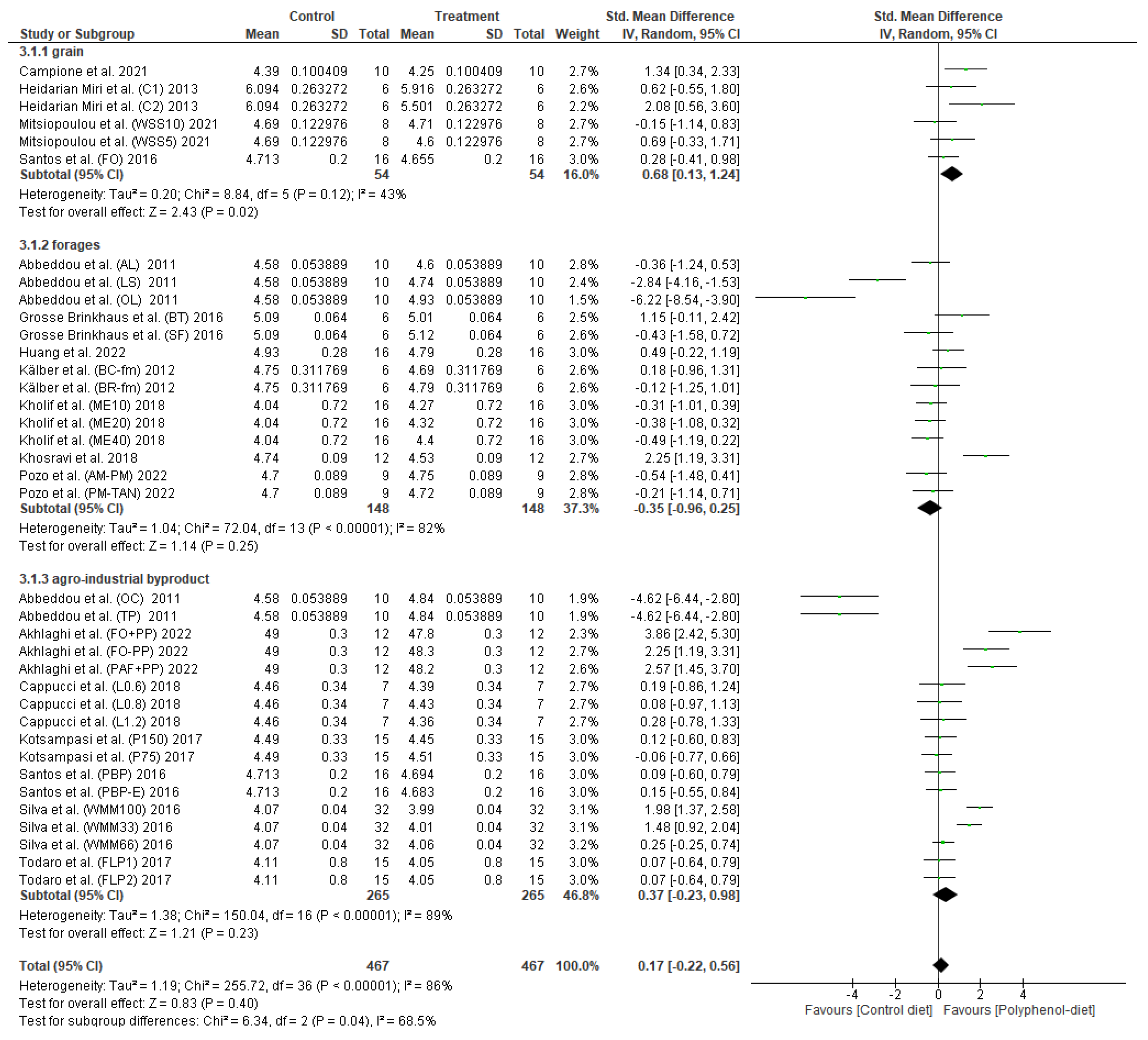
3.3. Effect of Polyphenol Supplementation on Milk Lactose Content
3.4. Effect of Polyphenol Supplementation on Milk SFA Content
3.5. Effect of Polyphenol Supplementation on Milk MCFA Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| SFA | Saturated Fatty Acid |
| MCFA | Medium-Chain Fatty Acid |
| SMD | Standard Mean Difference |
| CI | Confidence Intervals |
| F:C | Forage to Concentrate Ratio |
| TMR | Total Mixed Ration |
| PMR | Partial Mixed Ration |
| UM | Unfermented |
| FM | Fermented |
| ME10 | 10 mL M. oleifera leaves extract |
| ME20 | 20 mL M. oleifera leaves extract |
| ME40 | 40 mL M. oleifera leaves extract |
| WSS5 | 5% of whole sesame seed |
| WSS10 | 10% of whole sesame seed |
| LS | Lentil straw |
| AL | Atriplex leaves |
| OL | Olive leaves |
| OC | Olive cake |
| TP | Tomato pomace |
| GS | Grape seed |
| LIN | Extruded linseed |
| MIXED | Mixed of grape seed and extruded linseed |
| FO + PP | 1.5% Ca salts of Fish Oil with 8.7% Pomegranate Peel |
| PAF-PP | 1.5% of Palmitic Acid-enriched Fat without Pomegranate Peel |
| FO-PP | 1.5% Ca salts of Fish Oil without Pomegranate Peel |
| PPS | Pomegranate pulp silage |
| P75 | 7.5% of Pomegranate pulp silage |
| P150 | 15% of Pomegranate pulp silage |
| FLP | Fresh lemon pulp |
| FLP1 | 9.01% of Fresh lemon pulp |
| FLP2 | 15.7% of Fresh lemon pulp |
| OCPC | Olive Crude Phenolic Concentrates |
| L0.6 | 0.6% of Olive Crude Phenolic Concentrates |
| L0.8 | 0.8% of Olive Crude Phenolic Concentrates |
| L1.2 | 1.2% of Olive Crude Phenolic Concentrates |
| AL | Alfalfa |
| SF | Sainfoin |
| BT | Birdsfoot Trefoil |
| CSE | Cumin Seed Extract |
| C1 | 1.27% Cumin Seed Extract |
| C2 | 2.53% Cumin Seed Extract |
| SO0 | Without Soybean Oil |
| DCP | Dried Citrus Pulp |
| SO0 + DCP2 | SO0 + 17.3% of DCP |
| SO1 + DCP1 | 17.3 g of soybean oil + 8.65% of DCP |
| SO1 + DCP2 | 17.3 g of soybean oil + 17.3% of DCP |
| DM | Dry matter |
| AM | Morning |
| PM | Afternoon |
| TAN | Tannin |
| FO | Flaxseed Oil |
| PBP | Propolis-Based Product |
| PBP-E | Propolis-Based Product and Vitamin E |
| BC-fm | Chicory diet |
| BR-fm | Ryegrass diet |
| WMM | Whole Mango Meal |
| WMM33 | 33% Whole Mango Meal |
| WMM66 | 66% Whole Mango Meal |
| WMM100 | 100% Whole Mango Meal |
| MUFA | Monounsaturated fatty acid |
| PUFA | Polyunsaturated fatty acid |
References
- Robards, K.; Antolovich, M. Analytical chemistry of fruit bioflavonoids. A review. Analyst 1997, 122, 11R–34R. [Google Scholar] [CrossRef]
- Patra, A.K. Recent advances in measurement and dietary mitigation of enteric methane emissions in ruminants. Front. Vet. Sci. 2016, 3, 39. [Google Scholar]
- Patra, A.; Park, T.; Kim, M.; Yu, Z. Rumen methanogens and mitigation of methane emission by anti-methanogenic compounds and substances. J. Anim. Sci. Biotechnol. 2017, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K.; Saxena, J. A new perspective on the use of plant secondary metabolites to inhibit methanogenesis in the rumen. Phytochemistry 2010, 71, 1198–1222. [Google Scholar]
- Castillo, C.; Hernandez, J.; Bravo, A.; Lopez-Alonso, M.; Pereira, V.; Benedito, J.L. Oxidative status during late pregnancy and early lactation in dairy cows. Vet J. 2005, 169, 286–292. [Google Scholar]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2012, 13, 572–584. [Google Scholar]
- Winkler, A.; Gessner, D.K.; Koch, C.; Romberg, F.J.; Dusel, G.; Herzog, E.; Most, E.; Eder, K. Effects of a plant product consisting of green tea and curcuma extract on milk production and the expression of hepatic genes involved in endoplasmic stress response and inflammation in dairy cows. Arch. Anim. Nutr. 2015, 69, 425–441. [Google Scholar]
- Correddu, F.; Lunesu, M.F.; Buffa, G.; Atzori, A.S.; Nudda, A.; Battacone, G.; Pulina, G. Can Agro-Industrial By-Products Rich in Polyphenols be Advantageously Used in the Feeding and Nutrition of Dairy Small Ruminants? Animals 2020, 10, 131. [Google Scholar] [CrossRef]
- Tian, X.Z.; Xu, Y.Q.; Qin, J.X.; Wang, X.; Xie, S.L.; Chen, R.; Lu, Q.; Chen, X. Effects of Coix seed polyphenol extract on rumen fermentation, milk production, fatty acid profile, antioxidant activity, and polyphenol content in dairy goats. J. Dairy Sci. 2025, 108, 2407–2421. [Google Scholar]
- O’Callaghan, T.F.; Hennessy, D.; McAuliffe, S.; Kilcawley, K.N.; O’Donovan, M.; Dillon, P.; Ross, R.P.; Stanton, C. Effect of pasture versus indoor feeding systems on raw milk composition and quality over an entire lactation. J. Dairy Sci. 2016, 99, 9424–9440. [Google Scholar]
- Wallace, R.J.; McEwan, N.R.; McIntosh, F.M.; Teferedegne, B.; Newbold, C.J. Natural products as manipulators of rumen fermentation. Asian-Australas. J. Anim. Sci. 2002, 10, 1458–1468. [Google Scholar] [CrossRef]
- Calsamiglia, S.; Busquet, M.; Cardozo, P.W.; Castillejos, L.; Ferret, A. Invited review: EOs as modifiers of rumen microbial fermentation. J. Dairy Sci. 2007, 90, 2580–2595. [Google Scholar] [CrossRef]
- Hart, K.J.; Yáñez-Ruiz, D.R.; Duval, S.M.; McEwan, N.R.; Newbold, C.J. Plant extracts to manipulate rumen fermentation. Anim. Feed. Sci. Technol. 2008, 147, 8–35. [Google Scholar] [CrossRef]
- Kamra, D.N.; Patra, A.K.; Chatterjee, P.N.; Kumar, R.; Agarwal, N.; Chaudhary, L.C. Effect of plant extracts on methanogenesis and microbial profile of the rumen of buffalo: A brief overview. Aust. J. Exp. Agric. 2008, 48, 175–178. [Google Scholar] [CrossRef]
- Dai, X.; Faciola, A.P. Evaluating strategies to reduce ruminal protozoa and their impacts on nutrient utilization and animal performance in ruminants–a meta-analysis. Front. Microbiol. 2019, 10, 2648. [Google Scholar]
- Torres, R.N.S.; Moura, D.C.; Ghedini, C.P.; Ezequiel, J.M.B.; Almeida, M.T.C. Meta-analysis of the effects of essential oils on ruminal fermentation and performance of sheep. Small Rumin. Res. 2020, 189, 106148. [Google Scholar] [CrossRef]
- Khiaosa-Ard, R.; Zebeli, Q. Meta-analysis of the effects of essential oils and their bioactive compounds on rumen fermentation characteristics and feed efficiency in ruminants. J. Anim. Sci. 2013, 91, 1819–1830. [Google Scholar] [CrossRef]
- Gessner, D.K.; Koch, C.; Romberg, F.J.; Winkler, A.; Dusel, G.; Herzog, E.; Most, E.; Eder, K. The effect of grape seed and grape marc meal extract on milk performance and the expression of genes of endoplasmic reticulum stress and inflammation in the liver of dairy cows in early lactation. J. Dairy Sci. 2015, 98, 8856–8868. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; The Cochrane Collaboration: London, UK, 2011. [Google Scholar]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Yao, K.; Jiang, L.; Liu, J.; Wang, D.; Liu, H.; Ren, D. Effect of Yellow Wine Lees Supplementation on Milk Antioxidant Capacity and Hematological Parameters in Lactating Cows under Heat Stress. Animals 2021, 11, 2643. [Google Scholar] [CrossRef] [PubMed]
- Kholif, A.E.; Gouda, G.A.; Olafadehan, O.A.; Abdo, M.M. Effects of replacement of Moringa oleifera for berseem clover in the diets of Nubian goats on feed utilisation, and milk yield, composition and fatty acid profile. Animal 2018, 12, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, M.; Rouzbehan, Y.; Rezaei, M.; Rezaei, J. Total replacement of corn silage with sorghum silage improves milk fatty acid profile and antioxidant capacity of Holstein dairy cows. J. Dairy Sci. 2018, 101, 10953–10961. [Google Scholar] [PubMed]
- Mitsiopoulou, C.; Sotirakoglou, K.; Labrou, N.E.; Tsiplakou, E. The effect of whole sesame seeds on milk chemical composition, fatty acid profile and antioxidant status in goats. Livest. Sci. 2021, 245, 104452. [Google Scholar]
- Cabiddu, A.; Molle, G.; Decandia, M.; Spada, S.; Fiori, M.; Piredda, G.; Addis, M. Responses to condensed tannins of flowering sulla (Hedysarum coronarium L.) grazed by dairy sheep: Part 2: Effects on milk fatty acid profile. Livest. Sci. 2009, 123, 230–240. [Google Scholar]
- Abbeddou, S.; Rischkowsky, B.; Richter, E.K.; Hess, H.D.; Kreuzer, M. Modification of milk fatty acid composition by feeding forages and agro-industrial byproducts from dry areas to Awassi sheep. J. Dairy Sci. 2011, 94, 4657–4668. [Google Scholar] [CrossRef]
- Campione, A.; Pauselli, M.; Natalello, A.; Valenti, B.; Pomente, C.; Avondo, M.; Luciano, G.; Caccamo, M.; Morbidini, L. Inclusion of cocoa by-product in the diet of dairy sheep: Effect on the fatty acid profile of ruminal content and on the composition of milk and cheese. Animal 2021, 15, 100243. [Google Scholar] [CrossRef]
- Correddu, F.; Gaspa, G.; Pulina, G.; Nudda, A. Grape seed and linseed, alone and in combination, enhance unsaturated fatty acids in the milk of Sarda dairy sheep. J. Dairy Sci. 2016, 99, 1725–1735. [Google Scholar]
- Akhlaghi, B.; Ghasemi, E.; Alikhani, M.; Ghaffari, M.H.; Razzaghi, A. Effects of supplementing pomegranate peel with fatty acid sources on oxidative stress, blood metabolites, and milk production of dairy cows fed high-concentrate diets. Anim. Feed. Sci. Technol. 2022, 286, 115228. [Google Scholar] [CrossRef]
- Kotsampasi, Β.; Christodoulou, C.; Tsiplakou, E.; Mavrommatis, A.; Mitsiopoulou, C.; Karaiskou, C.; Dotas, V.; Robinson, P.H.; Bampidis, V.A.; Christodoulou, V.; et al. Effects of dietary pomegranate pulp silage supplementation on milk yield and composition, milk fatty acid profile and blood plasma antioxidant status of lactating dairy cows. Anim. Feed. Sci. Technol. 2017, 234, 228–236. [Google Scholar]
- Todaro, M.; Alabiso, M.; Scatassa, M.L.; Di Grigoli, A.; Mazza, F.; Maniaci, G.; Bonanno, A. Effect of the inclusion of fresh lemon pulp in the diet of lactating ewes on the properties of milk and cheese. Anim. Feed. Sci. Technol. 2017, 225, 213–223. [Google Scholar]
- Cappucci, A.; Alves, S.P.; Bessa, R.J.; Buccioni, A.; Mannelli, F.; Pauselli, M.; Viti, C.; Pastorelli, R.; Roscini, V.; Serra, A.; et al. Effect of increasing amounts of olive crude phenolic concentrate in the diet of dairy ewes on rumen liquor and milk fatty acid composition. J. Dairy Sci. 2018, 101, 4992–5005. [Google Scholar] [PubMed]
- Brinkhaus, A.G.; Bee, G.; Silacci, P.; Kreuzer, M.; Dohme-Meier, F. Effect of exchanging Onobrychis viciifolia and Lotus corniculatus for Medicago sativa on ruminal fermentation and nitrogen turnover in dairy cows. J. Dairy Sci. 2016, 99, 4384–4397. [Google Scholar] [CrossRef]
- Miri, V.H.; Tyagi, A.K.; Ebrahimi, S.H.; Mohini, M. Effect of cumin (Cuminum cyminum) seed extract on milk fatty acid profile and methane emission in lactating goat. Small Rumin. Res. 2013, 113, 66–72. [Google Scholar]
- Karimi, A.; Abarghuei, M.J.; Ghanatsaman, Z.A.; Agah, M.J.; Boostani, A. Dietary application of dried citrus pulp, with or without soybean oil, in lactating Holstein cow diet: Effects on feed intake, digestibility, performance, a milk fatty acid profile and total phenolics. Anim. Feed. Sci. Technol. 2022, 294, 115488. [Google Scholar]
- Pozo, C.A.; Kozloski, G.V.; Cuffia, M.; Repetto, J.L.; Cajarville, C. Changing the grazing session from morning to afternoon or including tannins in the diet was effective in decreasing the urinary nitrogen of dairy cows fed a total mixed ration and herbage. J. Dairy Sci. 2022, 105, 4987–5003. [Google Scholar]
- Santos, N.W.; Yoshimura, E.H.; Machado, E.; Matumoto-Pintro, P.T.; Montanher, P.F.; Visentainer, J.V.; dos Santos, G.T.; Zeoula, L.M. Antioxidant effects of a propolis extract and vitamin E in blood and milk of dairy cows fed diet containing flaxseed oil. Livest. Sci. 2016, 191, 132–138. [Google Scholar]
- Huang, H.; Lechniak, D.; Szumacher-Strabel, M.; Patra, A.K.; Kozłowska, M.; Kolodziejski, P.; Gao, M.; Ślusarczyk, S.; Petrič, D.; Cieslak, A. The effect of ensiled paulownia leaves in a high-forage diet on ruminal fermentation, methane production, fatty acid composition, and milk production performance of dairy cows. Anim. Feed. Sci. Technol. 2022, 13, 104. [Google Scholar]
- Kälber, T.; Kreuzer, M.; Leiber, F. Effect of feeding buckwheat and chicory silages on fatty acid profile and cheese-making properties of milk from dairy cows. J. Dairy Sci. 2013, 80, 81–88. [Google Scholar]
- Silva, J.D.L.; Guim, A.; de Carvalho, F.F.; Mattos, C.W.; Menezes, D.R.; Coelho, M.C.S.; Garcia, D.A.; Pereira Neto, J.D.; Soares, L.F. Replacement of corn with mango meal for dairy goats. Rev. Colomb. Cienc. Pecu. 2016, 29, 178–187. [Google Scholar]
- Tsiplakou, E.; Abdullah, M.A.; Alexandros, M.; Chatzikonstantinou, M.; Skliros, D.; Sotirakoglou, K.; Flemetakis, E.; Labrou, N.E.; Zervas, G. The effect of dietary Chlorella pyrenoidosa inclusion on goats milk chemical composition, fatty acids profile and enzymes activities related to oxidation. Livest. Sci. 2017, 197, 106–111. [Google Scholar] [CrossRef]
- Qin, S.; Hou, D.X. The biofunctions of phytochemicals and their applications in farm animals: The nrf2/keap1 system as a target. Engineering 2017, 3, 738–752. [Google Scholar]
- Xue, M.Y.; Sun, H.Z.; Wu, X.H.; Guan, L.L.; Liu, J.X. Assessment of rumen bacteria in dairy cows with varied milk protein yield. J. Dairy Sci. 2019, 102, 5031–5041. [Google Scholar]
- Butler, G.; Stergiadis, S. Organic milk: Does it confer health benefits? In Milk and Dairy Foods; Ians Givens, D., Ed.; Academic Press: London, UK, 2020; pp. 121–143. [Google Scholar]
- Mioč, B.; Prpić, Z.; Vnučec, I.; Barać, Z.; Samaržija, D.; Pavić, V. Factors affecting goat milk yield and composition. Mljekarstvo. 2008, 58, 305. [Google Scholar]
- Modaresi, J.; Nasri, M.F.; Rashidi, L.; Dayani, O.; Kebreab, E. Effects of supplementation with pomegranate seed pulp on concentrations of conjugated linoleic acid and punicic acid in goat milk. J. Dairy Sci. 2011, 94, 4075–4080. [Google Scholar]
- Caroprese, M.; Albenzio, M.; Bruno, A.; Fedele, V.; Santillo, A.; Sevi, A. Effect of solar radiation and flaxseed supplementation on milk production and fatty acid profile of lactating ewes under high ambient temperature. J. Dairy Sci. 2011, 94, 3856–3867. [Google Scholar]
- Ineichen, S.; Marquardt, S.; Wettstein, H.R.; Kreuzer, M.; Reidy, B. Milk fatty acid profile and nitrogen utilization of dairy cows fed ryegrass-red clover silage containing plantain (Plantago lanceolata L.). Livest. Sci. 2019, 221, 123–132. [Google Scholar]
- Di Meo, M.C.; Giacco, A.; Zarrelli, A.; Mandrone, V.M.; D’Angelo, L.; Silvestri, E.; De Girolamo, P.; Varricchio, E. Effects of Olea europaea L. Polyphenols on the Animal Welfare and Milk Quality in Dairy Cows. Animals 2023, 13, 3225. [Google Scholar] [CrossRef]
- Chiofalo, B.; Liotta, L.; Zumbo, A.; Chiofalo, V. Administration of olive cake for ewe feeding: Effect on milk yield and composition. Small Rumin. Res. 2004, 55, 169–176. [Google Scholar]
- Terramoccia, S.; Bartocci, S.; Taticchi, A.; Di Giovanni, S.; Pauselli, M.; Mourvaki, E.; Urbani, S.; Servili, M. Use of dried stoned olive pomace in the feeding of lactating buffaloes: Effect on the quantity and quality of the milk produced. Asian-Australas J Anim Sci. 2013, 26, 971. [Google Scholar] [CrossRef]
- Pieracci, Y.; Pistelli, L.; Cecchi, M.; Pistelli, L.; De Leo, M. Phytochemical characterization of citrus-based products supporting their antioxidant effect and sensory quality. Foods 2022, 11, 1550. [Google Scholar] [CrossRef]
- Maldonado-Celis, M.E.; Yahia, E.M.; Bedoya, R.; Landázuri, P.; Loango, N.; Aguillón, J.; Restrepo, B.; Guerrero Ospina, J.C. Chemical composition of mango (Mangifera indica L.) fruit: Nutritional and phytochemical compounds. Front. in Plant Sci. 2019, 10, 1073. [Google Scholar] [CrossRef]
- Chabi, I.B.; Zannou, O.; Dedehou, E.S.C.A.; Ayegnon, B.P.; Oscar Odouaro, O.B.; Maqsood, S.; Galanakis, C.M.; Pierre Polycarpe Kayodé, A. Tomato pomace as a source of valuable functional ingredients for improving physicochemical and sensory properties and extending the shelf life of foods: A review. Heliyon 2024, 10, e25261. [Google Scholar] [CrossRef]
- Hashmi, M.A.; Khan, A.; Hanif, M.; Farooq, U.; Perveen, S. Traditional uses, phytochemistry, and pharmacology of Olea europaea (olive). eCAM 2015, 1, 541591. [Google Scholar]

| No. Exp | References | ID | Species | Parity Status | Basal Feed | Polyphenol Sources | Type of Polyphenol Sources | Total Phenolic Content, % of DM | Adaptation Period/Long Treatment (day) | Milking (time/day) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | [22] | - | Cow (Holstein) | Multi- parous | TMR 2; 60:40 of forage to concentrate ratio (F:C) 1 | 18% Soybean Meal | Agro-industrial by-product | 0.202 | 15/5 | 2 |
| Yao et al. (UM) 2021 | 11% Unfermented yellow wine less | Agro-industrial by-product | 0.582 | |||||||
| Yao et al. (FM) 2021 | 11% Fermented yellow wine less | Agro-industrial by-product | 0.676 | |||||||
| 2 | [23] | - | Goat (Nubian) | Multi- parous | 40:60 (F:C) 1 Egyptian berseem clover and concentrates mixture. | Control | Forages | - | 15/7 | 2 |
| Kholif et al. (ME10) 2018 | 10 mL M. oleifera leaves extract | Forages | 0.6 | |||||||
| Kholif et al. (ME20) 2018 | 20 mL M. oleifera leaves extract | Forages | 1.2 | |||||||
| Kholif et al. (ME40) 2018 | 40 mL M. oleifera leaves extract | Forages | 2.4 | |||||||
| 3 | [24] | - | Cow (Holstein) | Multi-parous | TMR 2 | Corn silage | Forages | 0.053 | 21/7 | 3 |
| Khosravi et al. 2018 | Sorghum silage | Forages | 1.698 | |||||||
| 4 | [25] | - | Goat (Alpine x Local breed) | n/a | Component feeding; 50:50 (F:C) 1 | Control | Forages | 6.312 | 7/100 | 2 |
| Mitsiopoulou et al. WSS5 (2021) | 5% of whole sesame seed | Seed/grain | 9.43 | |||||||
| Mitsiopoulou et al. WSS10 (2021) | 10% of whole sesame seed | Seed/grain | 12.603 | |||||||
| 5 | [26] | Cabiddu et al. 4 May 2009 | Sheep (Sardinian) | Grazing Sulla-based pasture | Sulla pasture on 4th May | Forages | 3.21 | 14/14 | 2 | |
| Cabiddu et al. 17 May 2009 | Sulla pasture on 17th May | Forages | 3.55 | |||||||
| Cabiddu et al. 1 Jun 2009 | Sulla pasture on 1st Jun | Forages | 3.42 | |||||||
| 6 | [27] | - | Sheep (Awassi) | Multi-parous | 30:70 (F:C) 1 except Olive cake diet 20:80 (F:C) 1 | Control | Forages | 0.67 | 10/50 | 2 |
| Abbeddou et al. (LS) 2011 | Lentil straw | Forages | 1.32 | |||||||
| Abbeddou et al. (AL) 2011 | Atriplex leaves | Forages | 0.57 | |||||||
| Abbeddou et al. (OL) 2011 | Olive leaves | Forages | 2.25 | |||||||
| Abbeddou et al. (OC) 2011 | Olive cake | Agro-industrial by-product | 0.53 | |||||||
| Abbeddou et al. (TP) 2011 | Tomato pomace | Agro-industrial by-product | 0.60 | |||||||
| 7 | [28] | - | Sheep (Comisana) | Multi-parous | Concentrates with ad libitum hay | Conventional concentrate as control concentrate | Agro-industrial by-product | 0.194 | 14/21 | 2 |
| Campione et al. 2021 | Experimental concentrate: with Cocoa bean shell | Seed/grain | 0.52 | |||||||
| 8 | [29] | - | Sheep (Sarda) | Multi-parous | TMR 2 | Control (corn, soybean, pea) | Seed/grain | - | 14/56 | weekly |
| Coreddu et al. (GS) 2016 | Grape seed | Seed/grain | 0.037 | |||||||
| Coreddu et al. (LIN) 2016 | Extruded linseed | Seed/grain | - | |||||||
| Coreddu et al. (MIX) 2016 | Mixed of grape seed and extruded linseed | Seed/grain | 0.038 | |||||||
| 9 | [30] | - | Cow (Holstein) | Primi-parous | TMR 2; 32:68 (F:C) 1 | 1.5% of palmitic acid- enriched fat with 8.7% pomegranate peel | Agro-industrial by-product | 0.288 | 21/7 | 3 |
| Akhlaghi et al. (FO + PP) 2022 | 1.5% Ca salts of fish oil with 8.7% pomegranate peel | Agro-industrial by-product | 0.357 | |||||||
| Akhlaghi et al. (PAF + PP) 2022 | 1.5% of palmitic acid- enriched fat with 8.7% pomegranate peel | Agro-industrial by-product | 0.23 | |||||||
| Akhlaghi et al. (FO-PP) 2022 | 1.5% Ca salts of fish oil without pomegranate peel | Agro-industrial by-product | 0.22 | |||||||
| 10 | [31] | - | Cow (Holstein) | Multi-parous | TMR 2 | 0% of Pomegranate pulp silage (PPS) as control | Agro-industrial byproduct | 0.078 | 20/5 | 1 |
| Kotsampasi et al. (P75) 2017 | 7.5% of PPS | Agro-industrial byproduct | 0.226 | |||||||
| Kotsampasi et al. (P150) 2017 | 15% of PPS | Agro-industrial byproduct | 0.364 | |||||||
| 11 | [32] | - | Sheep (Valle del Belice) | Multi-parous | Component feeding; partial mixed ration (PMR) 3 of ad libitum hay with 600 g of concentrates daily with 1 kg of FLP replaced 200 g of concentrates | 0% of Fresh lemon pulp (FLP) as control | Forages | 0.84 | 14/7 | 1 |
| Todaro et al. (FLP1) 2017 | 9.01% of FLP | Agro-industrial by-product | 1.132 | |||||||
| Todaro et al. (FLP2) 2017 | 15.7% of FLP | Agro-industrial by-product | 1.322 | |||||||
| 12 | [33] | - | Sheep (Comisana) | Multi-parous | Component feeding; PMR 3 of ad libitum chopped lucerne hay with 100 g of rolled barley, and 800 g/animal daily | 0% of olive crude phenolic concentrates (OCPC) | - | - | 21/14 | 2 |
| Cappucci et al. (L0.6) (2018) | 0.6% of OCPC | Agro-industrial by-product | 0.064 | |||||||
| Cappucci et al. (L0.8) (2018) | 0.8% of OCPC | Agro-industrial by-product | 0.083 | |||||||
| Cappucci et al. (L1.2) (2018) | 1.2% of OCPC | Agro-industrial by-product | 0.118 | |||||||
| 13 | [34] | - | Cows (Holstein -Friesian) | Multi-parous | Component feeding; PMR 3 of grass hay, corn silage, and Extrulin 135 (contain 60% of extruded linseed oil and 40% of wheat bran) Experimental dietary variation generated by offering AL/SF/BT in amounts representing approximately 20% of the basal diet | Pelleted alfalfa (AL) (Medicago sativa L. ‘Sanditi’) | Forages | 0.97 | 21/7 | 2 |
| Grosse Brinkhaus et al. (SF) 2016 | Pelleted sainfoin (SF) (Onobrychis viciifolia L. ‘Perly’) | Forages | 3.241 | |||||||
| Grosse Brinkhaus et al. (BT) 2016 | Pelleted birdsfoot trefoil (BT) (Lotus corniculatus L. ‘Polom’) | Forages | 1.633 | |||||||
| 14 | [35] | - | Goat (Alpine × Beetal) | n/a | TMR 2; 50:50 (F:C) 1 berseem hay and concentrate | 0% of cumin seed extract (CSE) | - | - | 28/2 | 2 |
| Heidarian Miri et al. (C1) 2013 | 1.27% CSE | Seed/grain | 3.89 | |||||||
| Heidarian Miri et al. (C2) 2013 | 2.53% CSE | Seed/grain | 7.77 | |||||||
| 15 | [36] | - | Cow (Holstein) | n/a | TMR 2 | diet without soybean oil (SO0) and dried citrus pulp (DCP) | - | 0.25 | 21/7 | 2 |
| - | SO0 + 8.65% of DCP | Agro-industrial by-product | 0.71 | |||||||
| Karimi et al. (SO0 + DCP2) 2022 | SO0 + 17.3% of DCP | Agro-industrial by-product | 1.17 | |||||||
| Karimi et al. (SO1 + DCP1) 2022 | 17.3 g of soybean oil + 8.65% of DCP | Agro-industrial by-product | 0.71 | |||||||
| Karimi et al. (SO1 + DCP2) 2022 | 17.3 g of soybean oil + 17.3% of DCP | Agro-industrial by-product | 1.17 | |||||||
| 16 | [37] | - | Cow (Holstein) | Multi-parous | PMR 3 Acacia mearnsii bark (694 g per kg DM of total tannin) as tannin source | Morning grazing + afternoon PMR meal with 9.0 g of tannins/kg of PMR DM | - | - | 14/8 | 2 |
| Pozo et al. (AM-PM) 2022 | Morning PMR with 9.0 g of tannins/kg of PMR DM + afternoon grazing meal | - | - | |||||||
| Pozo et al. (PM-TAN) 2022 | Morning grazing + afternoon PMR with 15.0 g of tannins/kg of PMR DM | Agro-industrial by-product | 6.885 | |||||||
| 17 | [38] | - | Cannulated cow (Holstein) | n/a | 60:40 (F:C) 1 corn silage as forage and concentrate of soybean meal, ground corn grain, wheat bran, urea and mineral supplements | Control (basal diet) | Forages | 0.345 | 14/7 | 2 |
| Santos et al. (FO) 2016 | Diet with flaxseed oil (FO) | Seed/grain | 0.392 | |||||||
| Santos et al. (PBP) 2016 | Diet with FO + propolis-based Product (PBP) | Agro-industrial by-product | 2.827 | |||||||
| Santos et al. (PBP-E) 2016 | Diet with FO + PBP + vit E | Agro-industrial by-product | 2.923 | |||||||
| 18 | [39] | - | Cows (Holstein -Friesian) | Multi-parous | PMR of 76:24 (F:C) 1 | Control | Forages | - | 21/5 | 2 |
| Huang et al. 2022 | 6% of paulownia leaves silage diet replacing alfalfa silage | Forages | 0.36 | |||||||
| 19 | [40] | - | Cows (Holstein -Friesian) | n/a | Component feeding; partial mixed ration (PMR) 3 of ad libitum forage with 2 kg of concentrates daily | Buckwheat diet | Forages | 0.85 | 9/6 | 2 |
| Kälber et al. (BC-fm) 2012 | Chicory diet | Forages | 0.71 | |||||||
| Kälber et al. (BR-fm) 2012 | Ryegrass diet | Forages | 0.82 | |||||||
| 20 | [41] | - | Goats (crossbred Saanen) | n/a | TMR 2; 60:40 (F:C) 1 | 0% whole mango meal (WMM) as control | - | - | 14/5 | 2 |
| Silva et al. (WMM33) 2016 | 33% WMM | Agro-industrial by-product | 3.96 | |||||||
| Silva et al. (WMM66) 2016 | 66% WMM | Agro-industrial by-product | 7.91 | |||||||
| Silva et al. (WMM100) 2016 | 100% WMM | Agro-industrial by-product | 11.9 | |||||||
| 21 | [42] | - | Goat (cross- bred) | n/a | Component feeding; 50:50 (F:C) 1 | Control | - | - | 14/30 | 2 |
| Tsiplakou et al. 2017 | 1% of Chlorella pyrenoidosa | Marine microalgae | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harun, N.L.A.; Mohd Yusof, H.; Samsudin, A.A.; Sazili, A.Q.; Goh, Y.-M. Effect of Polyphenol Supplementation on Milk Composition and Fatty Acid of Dairy Animal: A Systematic Review. Ruminants 2025, 5, 15. https://doi.org/10.3390/ruminants5020015
Harun NLA, Mohd Yusof H, Samsudin AA, Sazili AQ, Goh Y-M. Effect of Polyphenol Supplementation on Milk Composition and Fatty Acid of Dairy Animal: A Systematic Review. Ruminants. 2025; 5(2):15. https://doi.org/10.3390/ruminants5020015
Chicago/Turabian StyleHarun, Nur Liyana Akmal, Hidayat Mohd Yusof, Anjas Asmara Samsudin, Awis Qurni Sazili, and Yong-Meng Goh. 2025. "Effect of Polyphenol Supplementation on Milk Composition and Fatty Acid of Dairy Animal: A Systematic Review" Ruminants 5, no. 2: 15. https://doi.org/10.3390/ruminants5020015
APA StyleHarun, N. L. A., Mohd Yusof, H., Samsudin, A. A., Sazili, A. Q., & Goh, Y.-M. (2025). Effect of Polyphenol Supplementation on Milk Composition and Fatty Acid of Dairy Animal: A Systematic Review. Ruminants, 5(2), 15. https://doi.org/10.3390/ruminants5020015








