Population Structure, Morphology, and Health Assessment of Philippine Swamp Buffalo (Bubalus kerabau, Fitzinger, 1860) in Calayan Island, Cagayan
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
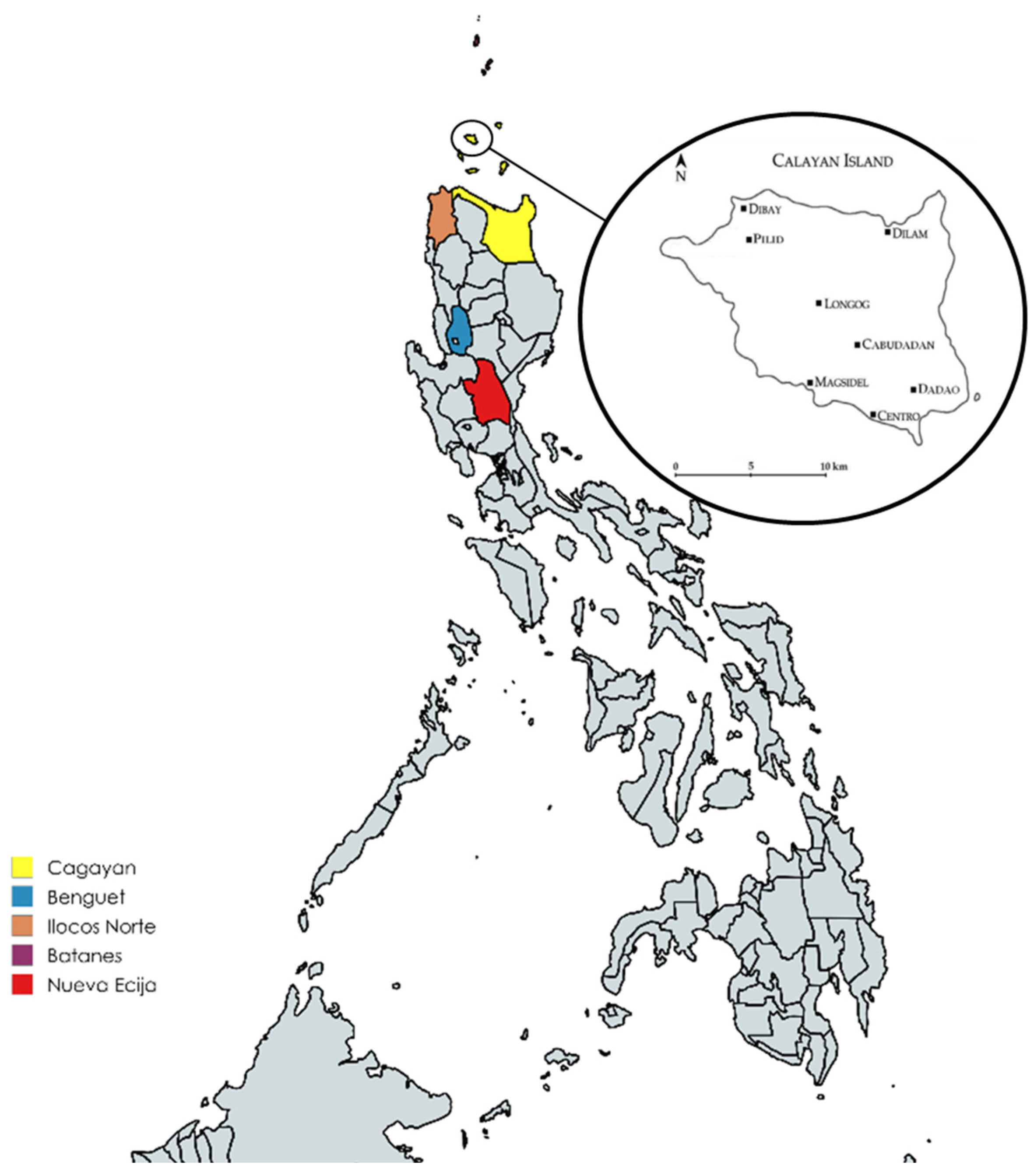
2.1. Sites and Sample Collection
2.2. Morphological Characteristics and Physical Features
- Yjkl = Individual observation;
- μ = Overall Mean;
- Sj = Effect of the jth sex;
- Ak = Effect of the kth age category of the buffalo;
- ejkl = Random error.
2.3. DNA Isolation and PCR Optimization
2.4. Microsatellite Analysis
2.5. Animal Health Screening
3. Results
3.1. Morphological Characteristics and Physical Features
3.2. Population Structure
3.3. Animal Health Screening
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cruz, L.C. Trends in Buffalo Production in Asia. Ital. J. Anim. Sci. 2007, 6 (Suppl. S2), 9–24. [Google Scholar] [CrossRef]
- Mingala, C.N.; Belotindos, L.P.; Abes, N.S.; Cruz, L.C. Genotyping and Molecular Characterization of NRAMP1/-2 Genes as Location of Markers for Resistance and/or Susceptibility to Mycobacterium Bovis in Swamp and Riverine Type Water Buffaloes. Buffalo Bull. 2013, 32, 730–733. [Google Scholar]
- Escuadro, A.J.D.; Villamor, L.P. Genotyping and Assessment of Microsatellite DNA Markers for Genetic Diversity and Potential Forensic Efficacy of Philippine Carabao (Bubalus bubalis) Swamp Buffalo. Sci. Eng. J. 2021, 14, 235–240. [Google Scholar]
- Blackburn, H.D. Development of National Animal Genetic Resource Programs. Reprod. Fertil. Dev. 2004, 16, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.C. Transforming Swamp Buffaloes to Producers of Milk and Meat through Crossbreeding and Backcrossing. Wartazoa 2010, 19, 103–116. [Google Scholar]
- Lacetera, N. Impact of Climate Change on Animal Health and Welfare. Anim. Front. 2019, 9, 26–31. [Google Scholar] [CrossRef]
- Chaidanya, K.; Shaji, S.; Abdul Niyas, P.A.; Sejian, V.; Bhatta, R.; Bagath, M.; Rao, G.S.L.H.V.P.; Kurien, E.K.; Girish, V. Climate Change and Livestock Nutrient Availability: Impact and Mitigation. J. Vet. Sci. Med. Diagn. 2015, 4. [Google Scholar] [CrossRef]
- Española, C.P.; Oliveros, C.H. Conservation of an Island Endemic: Calayan Rail (Gallirallus calayanensis); Final Report; Conservation Leadership Programme: Oxford, UK, 2005. [Google Scholar]
- Khan, M.; Rahim, I.; Rueff, H.; Jalali, S.; Saleem, M.; Maselli, D.; Muhammad, S.; Wiesmann, U. Morphological Characterization of the Azikheli Buffalo in Pakistan; Animal genetic resources; Cambridge University Press: Cambridge, UK, 2013; Volume 52, pp. 65–70. [Google Scholar] [CrossRef]
- Paraguas, A.M.; Cailipan, C.; Flores, E.B.; Villamor, L.P. Morphology and Phylogeny of Swamp Buffaloes (Bubalus bubalis) in Calayan Island, Cagayan. Philipp. J. Vet. Anim. Sci. 2018, 44, 59–67. [Google Scholar]
- Villamor, L.P.; Takahashi, Y.; Nomura, K.; Amano, T. Genetic Diversity of Philippine Carabao (Bubalus bubalis) Using Mitochondrial Dna d-Loop Variation: Implications to Conservation and Management. Philipp. J. Sci. 2021, 150, 837–846. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of Population Structure Using Multilocus Genotype Data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Earl, D.A.; vonHoldt, B.M. STRUCTURE HARVESTER: A Website and Program for Visualizing STRUCTURE Output and Implementing the Evanno Method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Barghash, S.M.; Darwish, A.M.; Abou-ElNaga, T.R. Molecular Characterization and Phylogenetic Analysis of Trypanosoma Evansi from Local and Imported Camels in Egypt. J. Phylogenetics Evol. Biol. 2016, 4, 3. [Google Scholar] [CrossRef]
- Marai, I.F.M.; Haeeb, A.A.M. Buffalo’s Biological Functions as Affected by Heat Stress—A Review. Livest. Sci. 2010, 127, 89–109. [Google Scholar] [CrossRef]
- Siddiquee, N.; Faruque, M.; Islam, F.; Mijan, M.; Habib, M. Morphometric Measurements, Productive and Reproductive Performance of Buffalo in Trishal and Companiganj Sub-Districts of Bangladesh. Int. J. Biol. Res. 2010, 1, 15–21. [Google Scholar]
- Rahman, M.; Islam, R.; Hossain, M.K.; Lucky, N.S.; Zahan, N. Full Length Research Paper Phenotypic Characterization of Indigenous Buffalo at Sylhet District. Int. J. Sci. Res. Agric. Sci. 2015, 2, 1–6. [Google Scholar]
- Gamarra, D.; Lopez-Oceja, A.; De Pancorbo, M.M. Genetic Characterization and Founder Effect Analysis of Recently Introduced Salers Cattle Breed Population. Animal 2017, 11, 24–32. [Google Scholar] [CrossRef]
- Winkler, L.R.; Bonman, J.M.; Chao, S.; Yimer, B.A.; Bockelman, H.; Klos, K.E. Population Structure and Genotype-Phenotype Associations in a Collection of Oat Landraces and Historic Cultivars. Front. Plant Sci. 2016, 7, 1077. [Google Scholar] [CrossRef]
- Liu, K.; Goodman, M.; Muse, S.; Smith, J.S.; Buckler, E.; Doebley, J. Genetic Structure and Diversity among Maize Inbred Lines as Inferred from DNA Microsatellites. Genetics 2003, 165, 2117–2128. [Google Scholar] [CrossRef]
- Liu, F.M.; Zhang, N.N.; Liu, X.J.; Yang, Z.J.; Jia, H.Y.; Xu, D.P. Genetic Diversity and Population Structure Analysis of Dalbergia Odorifera Germplasm and Development of a Core Collection Using Microsatellite Markers. Genes 2019, 10, 281. [Google Scholar] [CrossRef]
- Li, H.; Chappell, M.; Zhang, D. Assessing Genetic Diversity and Population Structure of Kalmia latifolia L. in the Eastern United States: An Essential Step towards Breeding for Adaptability to Southeastern Environmental Conditions. Sustainability 2020, 12, 8284. [Google Scholar] [CrossRef]
- Heaney, L.R. Zoogeographic Evidence for Middle and Late Pleistocene Land Bridges to the Philippine Islands. Mod. Quat. Res. Southeast Asia 1985, 9, 127–143. [Google Scholar]
- Dargantes, A.P. Epidemiology, Control and Potential Insect Vectors of Trypanosoma Evansi (Surra) in Village Livestock in Southern Philippines. Ph.D. Dissertation, Murdoch University, Perth, Australia, August 2010. [Google Scholar]
- Desquesnes, M.; Dargantes, A.; Lai, D.H.; Lun, Z.R.; Holzmuller, P.; Jittapalapong, S. Trypanosoma Evansi and Surra: A Review and Perspectives on Transmission, Epidemiology and Control, Impact, and Zoonotic Aspects. Biomed Res. Int. 2013, 2013, 1–20. [Google Scholar] [CrossRef]
- Manuel, M.F. Sporadic Outbreaks of Surra in the Philippines and Its Economic Impact. J. Protozool. Res. 1998, 8, 131–138. [Google Scholar]
- Perumal, P.; Kiran Kumar, T.; Srivastava, S.K. Infectious Causes of Infertility in Buffalo Bull (Bubalus bubalis). Buffalo Bull. 2013, 32, 71–82. [Google Scholar]
- Qureshi, K.A.; Parvez, A.; Fahmy, N.A.; Abdel Hady, B.H.; Kumar, S.; Ganguly, A.; Atiya, A.; Elhassan, G.O.; Alfadly, S.O.; Parkkila, S.; et al. Brucellosis: Epidemiology, Pathogenesis, Diagnosis and Treatment–a Comprehensive Review. Ann. Med. 2023, 55, 2295398. [Google Scholar] [CrossRef]
- Kim, J.; Álvarez-Rodríguez, A.; Li, Z.; Radwanska, M.; Magez, S. Recent Progress in the Detection of Surra, a Neglected Disease Caused by Trypanosoma Evansi with a One Health Impact in Large Parts of the Tropic and Sub-Tropic World. Microorganisms 2024, 12, 44. [Google Scholar] [CrossRef]


| Species Name | No. of Sample | Location |
|---|---|---|
| Swamp buffalo | 79 | Calayan Island |
| Swamp buffalo | 26 | Batanes |
| Swamp buffalo | 12 | Benguet |
| Crossbred buffalo | 16 | Ilocos Norte |
| Riverine buffalo | 5 | Nueva Ecija |
| Morphometric Traits | Calayan Island | Batanes | Benguet | |||
|---|---|---|---|---|---|---|
| LSMean (SE) | Range (cm) | LSMean (SE) | Range (cm) | LSMean (SE) | Range (cm) | |
| Body Length | 114.0 (1.5) | 62–147 | 137.4 (2.6) | 112–164 | 125.8 (2.1) | 102–144 |
| Heart Girth | 188.3 (1.5) | 106–225 | 184.3 (2.8) | 145–206 | 180.7 (2.3) | 155–208 |
| Height at withers | 135.9 (1.2) | 88–199 | 132.6 (2.2) | 119–160 | 127.5 (1.8) | 111–140 |
| Neck circumference | 97.2 (2.1) | 77–123 | 96.4 (2.10) | 73–149 | 95.4 (1.7) | 66–120 |
| Face Length | 52.6 (0.8) | 44–67 | 50.3 (0.78) | 43–56 | 49.4 (0.6) | 38–55 |
| Horn Length | ||||||
| Horn greater curvature | 59.5 (2.0) | 37–81 | 54.4 (1.4) | 22–82 | 49.5 (1.6) | 23–68 |
| Horn lesser curvature | 47.0 (1.5) | 29–64 | 43.9 (1.6) | 20–70 | 38.9 (1.2) | 14–53 |
| Width between horns | 16.5 (0.4) | 14–20 | 18.0 (0.4) | 14–24 | 19.4 (0.4) | 12–23 |
| Distance between horns | 54.1 (3.0) | 34–73 | 60.5 (3.0) | 24–114 | 53.7 (2.4) | 16–70 |
| Collection Site | Average Q Value | ||
|---|---|---|---|
| Cluster 1 | Cluster 2 | Cluster 3 | |
| Calayan Island | 0.998 | 0.002 | 0.000 |
| Batanes | 0.852 | 0.137 | 0.011 |
| Benguet | 0.878 | 0.120 | 0.002 |
| Ilocos Norte | 0.433 | 0.120 | 0.447 |
| Nueva Ecija | 0.003 | 0.060 | 0.937 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villamor, L.P.; Cuanang, A.J.E.; Cailipan, T.P.C.; Paraguas, A.M.; Flores, E.B.; Villanueva, M.A.; Balbin, M.M.; Belotindos, L.P.; Rellin, F.T. Population Structure, Morphology, and Health Assessment of Philippine Swamp Buffalo (Bubalus kerabau, Fitzinger, 1860) in Calayan Island, Cagayan. Ruminants 2024, 4, 556-564. https://doi.org/10.3390/ruminants4040039
Villamor LP, Cuanang AJE, Cailipan TPC, Paraguas AM, Flores EB, Villanueva MA, Balbin MM, Belotindos LP, Rellin FT. Population Structure, Morphology, and Health Assessment of Philippine Swamp Buffalo (Bubalus kerabau, Fitzinger, 1860) in Calayan Island, Cagayan. Ruminants. 2024; 4(4):556-564. https://doi.org/10.3390/ruminants4040039
Chicago/Turabian StyleVillamor, Lilian P., Aivhie Jhoy E. Cuanang, Therese Patricka C. Cailipan, Alexander M. Paraguas, Ester B. Flores, Marvin A. Villanueva, Michelle M. Balbin, Lawrence P. Belotindos, and Franklin T. Rellin. 2024. "Population Structure, Morphology, and Health Assessment of Philippine Swamp Buffalo (Bubalus kerabau, Fitzinger, 1860) in Calayan Island, Cagayan" Ruminants 4, no. 4: 556-564. https://doi.org/10.3390/ruminants4040039
APA StyleVillamor, L. P., Cuanang, A. J. E., Cailipan, T. P. C., Paraguas, A. M., Flores, E. B., Villanueva, M. A., Balbin, M. M., Belotindos, L. P., & Rellin, F. T. (2024). Population Structure, Morphology, and Health Assessment of Philippine Swamp Buffalo (Bubalus kerabau, Fitzinger, 1860) in Calayan Island, Cagayan. Ruminants, 4(4), 556-564. https://doi.org/10.3390/ruminants4040039





