Behavioral, Physiological and Hormonal Changes in Primiparous and Multiparous Goats and Their Kids During Peripartum
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Feeding and Housing Conditions
2.2. Experimental Process
2.2.1. Measurement of Prepartum Behavior
2.2.2. Measurement of Behavior During and Immediately After Parturition
2.2.3. Maternal Selectivity Test at 2 h Postpartum
2.2.4. Maternal Motivation Score
2.2.5. Determination of the Hormone Profile Before and After Birth
2.2.6. Physiological Parameters of Mother and Offspring
2.3. Statistical Analysis
3. Results
3.1. Prepartum Behaviors in Goats
3.2. Behavior of the Goat and Its Offspring During and Immediately After Parturition
3.3. Maternal Selectivity
3.4. Progesterone and Estradiol Concentrations of Goats Before and After Parturition
3.5. Physiological Parameters of the Goats and Their Kids
3.5.1. Goat Body Weight
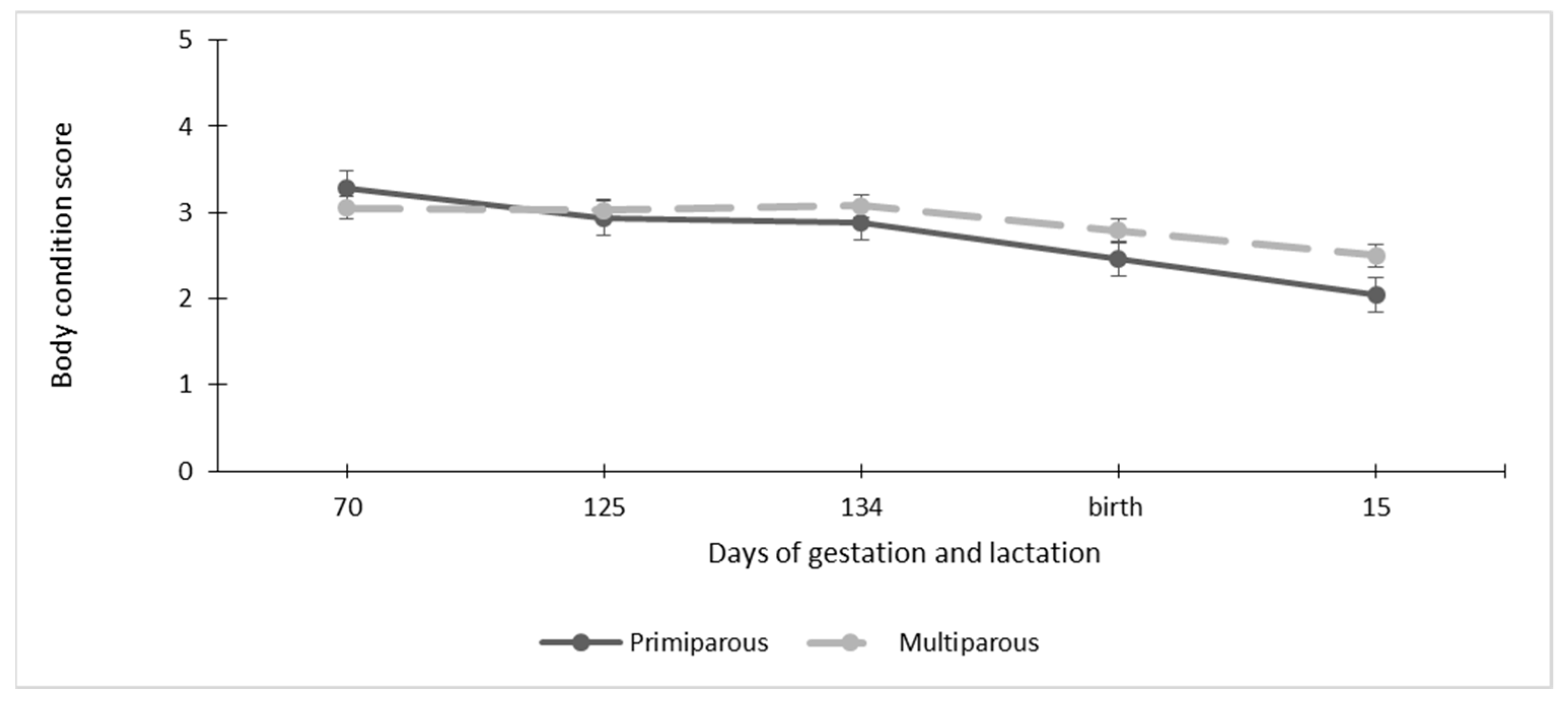
3.5.2. Goat Body Condition
3.5.3. Body Weight of Kids at Birth
3.5.4. Internal and External Temperature of the Kids at Birth
3.5.5. Kids’ Mortality Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hafez, E.S.E. Reproducción e Inseminación Artificial en Animales; Interamericana.McGraw-Hill.: Mexico City, Mexico, 2002. [Google Scholar]
- González-Stagnaro, C.; Madrid-Bury, N. El parto en cabras criollas. Rev. Cienti. Fac. Cien. Vet. 2004, 14, 124–133. [Google Scholar]
- Bickell, S.; Poindron, P.; Nowak, R.; Ferguson, D.; Blackberry, M.; Blache, D. Maternal behaviour and peripartum levels of oestradiol and progesterone show little difference in Merino ewes selected for calm or nervous temperament under indoor housing conditions. Animal 2011, 5, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, I.S.; Misra, S.S.; Kumar, A.; Gowane, G.R. Survival analysis of mortality in pre-weaning kids of Sirohi goat. Animal 2019, 13, 2896–2902. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, C.M. Maternal behaviour and lamb survival: From neuroendocrinology to practical application. Animal 2014, 8, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, C.M.; Conington, J.; Corbiere, F.; Holmøy, I.H.; Muri, K.; Nowak, R.; Rooke, J.; Vipond, J.; Gautier, J.M. Invited review: Improving neonatal survival in small ruminants: Science into practice. Animal 2016, 10, 449–459. [Google Scholar] [CrossRef]
- Mellor, D.J.; Stafford, K.J. Animal welfare implications of neonatal mortality and morbidity in farm animals. Vet. J. 2004, 168, 118–133. [Google Scholar] [CrossRef]
- Yitagesu, E.; Enyiew, A. Mortality rate of Boer, Central Highland goat and their crosses in Ethiopia: Nonparametric survival analysis and piecewise exponential model. Vet. Med. Sci. 2022, 8, 2183–2193. [Google Scholar] [CrossRef]
- Terrazas, A.; Hernandez, H.; Ramirez-Vera, S.; Fierros, A.; Rojas, S.; Serafin, N. Undernutrition during pregnancy in goats and sheep, their repercussion on mother-young relationship and behavioural development of the young. Trop. Subtrop. Agroecosystems 2012, 15, S161–S174. [Google Scholar]
- O’Connor, C.E.; Lawrence, A.B.; Wood-Gush, D.G.M. Influence of litter size and parity on maternal behaviour at parturition in Scottish Blackface sheep. Appl. Anim. Behav. Sci. 1992, 33, 345–355. [Google Scholar] [CrossRef]
- Dwyer, C.M.; Lawrence, A.B. Maternal behaviour in domestic sheep (Ovis aries): Constancy and change with maternal experience. Behaviour 2000, 137, 1391–1413. [Google Scholar] [CrossRef]
- Dwyer, C.M.; Smith, L.A. Parity effects on maternal behaviour are not related to circulating oestradiol concentrations in two breeds of sheep. Physiol. Behav. 2008, 93, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Damian, J.P.; Terrazas, A.; Cabrera, E.; Simonetti, S.; Aragunde, R.; Fila, D. Growth of foetal bones and metabolic profile during gestation in primiparous ewes and multiparous ewes. Reprod. Domest. Anim. 2020, 55, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Lickliter, R.E. Effects of a post-partum separation on maternal responsiveness in primiparous and multiparous domestic goats. Appl. Anim. Ethol. 1982, 8, 537–542. [Google Scholar] [CrossRef]
- Martínez, M.; Otal, J.; Ramírez, A.; Hevia, M.L.; Quiles, A. Variability in the behavior of kids born of primiparous goats during the first hour after parturition: Effect of the type of parturition, sex, duration of birth, and maternal behavior. J. Anim. Sci. 2009, 87, 1772–1777. [Google Scholar] [CrossRef] [PubMed]
- Baxter, E.M.; Mulligan, J.; Hall, S.A.; Donbavand, J.E.; Palme, R.; Aldujaili, E.; Zanella, A.J.; Dwyer, C.M. Positive and negative gestational handling influences placental traits and mother-offspring behavior in dairy goats. Physiol. Behav. 2016, 157, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.K.; Rai, B.; Sharma, N. Factors affecting survivability of Jamunapari kids under semi-intensive management system. Indian J. Anim. Sci. 2008, 78, 178–181. [Google Scholar]
- Lévy, F.; Porter, R.H.; Kendrick, K.M.; Keverne, E.B.; Romeyer, A. Physiological, sensory, and experiential factors of parental care in sheep. Adv. Stud. Behav. 1996, 25, 385–422. [Google Scholar] [CrossRef]
- Dwyer, C.M.; Lawrence, A.B. Does the behaviour of the neonate influence the expression of maternal behaviour in sheep? Behaviour 1999, 136, 367–389. [Google Scholar] [CrossRef]
- Rosenblatt, J.S.; Siegel, H.I. Factors governing the onset and maintenance of maternal behavior among nonprimate mammals: The role of hormonal and nonhormonal factors. Parent. Care Mamm. 1981, 13–76. [Google Scholar] [CrossRef]
- Kendrick, K.M.; Keverne, E.B. Importance of progesterone and estrogen priming for the induction of maternal behavior by vaginocervical stimulation in sheep: Effects of maternal experience. Physiol. Behav. 1991, 49, 745–750. [Google Scholar] [CrossRef]
- Soto, R.; Terrazas, A.; Poindron, P.; González-Mariscal, G. Regulation of maternal behavior, social isolation responses, and postpartum estrus by steroid hormones and vaginocervical stimulation in sheep. Horm. Behav. 2021, 136, 105061. [Google Scholar] [CrossRef] [PubMed]
- Broad, K.D.; Lévy, F.; Evans, G.; Kimura, T.; Keverne, E.B.; Kendrick, K.M. Previous maternal experience potentiates the effect of parturition on oxytocin receptor mRNA expression in the paraventricular nucleus. Eur. J. Neurosci. 1999, 11, 3725–3737. [Google Scholar] [CrossRef] [PubMed]
- Lévy, F.; Poindron, P. The importance of amniotic fluids for the establishment of maternal behaviour in experienced and inexperienced ewes. Anim. Behav. 1987, 35, 1188–1192. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids; The National Academies Press: Washington, DC, USA, 2007. [Google Scholar]
- Whitley, N.C.; Jackson, D.J. An update on estrus synchronization in goats: A minor species. J. Anim. Sci. 2004, 82 (Suppl. S13), E270–E276. [Google Scholar] [PubMed]
- Damián, J.P.; Hötzel, M.J.; Banchero, G.; Ungerfeld, R. Behavioural response of grazing lambs to changes associated with feeding and separation from their mothers at weaning. Res. Vet. Sci. 2013, 95, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Bateson, M.; Martin, P. Measuring Behaviour: An Introductory Guide, 4th ed.; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Lickliter, R.E. Behavior associated with parturition in the domestic goat. Appl. Anim. Behav. Sci. 1985, 13, 335–345. [Google Scholar] [CrossRef]
- Bosc, M.; Guillimin, P.; Bourgy, G.; Pignon, P. Hourly distribution of time of parturition in the domestic goat. Theriogenology 1988, 30, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Romano, J.E.; Piaggio, J. Time of parturition in Nubian goats. Small Rumin. Res. 1999, 33, 285–288. [Google Scholar] [CrossRef]
- Terrazas, A.; Cano, P.; Soto, R.; Zaragoza, J.; Ibarra, R.; Ayala, K.; Castillo, L.; Mercado, G.; Hernandez, H.; Ramirez, J. Characterization of goat’s parturition associated with time of the day of delivery. In Proceedings of the 1st European Symposium on Animal Reproductions, Nantes, France, 21–23 September 2023. [Google Scholar]
- El-Hamamy, E.; Arulkumaran, S. Poor progress of labour. Curr. Obstet. Gynaecol. 2005, 15, 1–8. [Google Scholar] [CrossRef]
- Rahim, A.T.; Arthur, G.H. Obstetrical conditions in goats. Cornell Vet. 1982, 72, 279–284. [Google Scholar] [PubMed]
- Poindron, P.; Otal, J.; Ferreira, G.; Keller, M.; Guesdon, V.; Nowak, R.; Lévy, F. Amniotic fluid is important for the maintenance of maternal responsiveness and the establishment of maternal selectivity in sheep. Animal 2010, 4, 2057–2064. [Google Scholar] [CrossRef] [PubMed]
- Everett-Hincks, J.M.; Lopez-Villalobos, N.; Blair, H.T.; Stafford, K.J. The effect of ewe maternal behaviour score on lamb and litter survival. Livest. Prod. Sci. 2004, 93, 51–61. [Google Scholar] [CrossRef]
- Walkden-Brown, S.W.; Restall, B.J.; Scaramuzzi, R.J.; Martin, G.B.; Blackberry, M.A. Seasonality in male Australian cashmere goats: Long term effects of castration and testosterone or oestradiol treatment on changes in LH, FSH and prolactin concentrations, and body growth. Small Rumin. Res. 1997, 26, 239–252. [Google Scholar] [CrossRef]
- Vicente-Pérez, R.; Avendaño-Reyes, L.; Correa-Calderón, A.; Mellado, M.; Meza-Herrera, C.A.; Montañez-Valdez, O.D.; Macías-Cruz, U. Relationships of body surface thermography with core temperature, birth weight and climatic variables in neonatal lambs born during early spring in an arid region. J. Therm. Biol. 2019, 82, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Vas, J.; Andersen, I.L. Density-Dependent Spacing Behaviour and Activity Budget in Pregnant, Domestic Goats (Capra hircus). PLoS ONE 2015, 10, e0144583. [Google Scholar] [CrossRef]
- O’Brien, P.H. Leavers and stayers: Maternal post-partum strategies in feral goats. Appl. Anim. Behav. Sci. 1984, 12, 233–243. [Google Scholar] [CrossRef]
- Mansour, N.; AlKhateeb, R.; Lamghari, F. Impact of parity on prepartum activities and behaviour in dromedary camels under farm conditions. Reprod. Domest. Anim. 2024, 59, e14572. [Google Scholar] [CrossRef] [PubMed]
- Barraclough, R.A.C.; Shaw, D.J.; Boyce, R.; Haskell, M.J.; Macrae, A.I. The behavior of dairy cattle in late gestation: Effects of parity and dystocia. J. Dairy Sci. 2020, 103, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, C.M. Genetic and physiological determinants of maternal behavior and lamb survival: Implications for low-input sheep management. J. Anim. Sci. 2008, 86 (Suppl. S14), E246–E258. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.M.; Redifer, C.A. The curse of the firstborn: Effects of dam primiparity on developmental programming in ruminant offspring. Anim. Reprod. Sci. 2024, 265, 107469. [Google Scholar] [CrossRef] [PubMed]
- McDonald, B.J.; O’rourke, P.K.; Connell, J.A.; Hoey, W.A. Prenatal growth and estimation of fetal age in the Australian feral goat. Aust. J. Agric. Res. 1988, 39, 729–734. [Google Scholar] [CrossRef]
- Lidfors, L.M.; Moran, D.; Jung, J.; Jensen, P.; Castren, H. Behaviour at calving and choice of calving place in cattle kept in different environments. Appl. Anim. Behav. Sci. 1994, 42, 11–28. [Google Scholar] [CrossRef]
- Terrazas, A.; Robledo, V.; Serafín, N.; Soto, R.; Hernández, H.; Poindron, P. Differential effects of undernutrition during pregnancy on the behaviour of does and their kids at parturition and on the establishment of mutual recognition. Animal 2009, 3, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Rahman, I.; Aguli, Z. Maternal and neonatal factors affecting neonatal behaviour in West African Dwarf goats. Trop. Anim. Health Prod. 2023, 56, 3. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Vera, S.; Terrazas, A.; Delgadillo, J.A.; Flores, J.A.; Serafín, N.; Vielma, J.; Duarte, G.; Fernández, G.; Fitz-Rodríguez, G.; Hernández, H. Inclusion of maize in the grazing diet of goats during the last 12 days of gestation reinforces the expression of maternal behaviour and selectivity during the sensitive period. Livest. Sci. 2012, 148, 52–59. [Google Scholar] [CrossRef]
- Romeyer, A.; Poindron, P. Early maternal discrimination of alien kids by post-parturient goats. Behav. Process. 1992, 26, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Lévy, F.; Gervais, R.; Kindermann, U.; Litterio, M.; Poindron, P.; Porter, R. Effects of early post-partum separation on maintenance of maternal responsiveness and selectivity in parturient ewes. Appl. Anim. Behav. Sci. 1991, 31, 101–110. [Google Scholar] [CrossRef]
- Lévy, F. The Onset of Maternal Behavior in Sheep and Goats: Endocrine, Sensory, Neural, and Experiential Mechanisms. Adv. Neurobiol. 2022, 27, 79–117. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.; Meurisse, M.; Poindron, P.; Nowak, R.; Ferreira, G.; Shayit, M.; Frédéric, L. Maternal experience influences the establishment of visual/auditory, but not olfactory recognition of the newborn lamb by ewes at parturition. Dev. Psychobiol. 2003, 43, 167–176. [Google Scholar] [CrossRef]
- Lv, S.J.; Yang, Y.; Li, F.K. Parity and litter size effects on maternal behavior of Small Tail Han sheep in China. Anim. Sci. J. 2016, 87, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Romeyer, A.; Porter, R.H.; Levy, F.; Nowak, R.; Orgeur, P.; Poindron, P. Maternal labelling is not necessary for the establishment of discrimination between kids by recently parturient goats. Anim. Behav. 1993, 46, 705–712. [Google Scholar] [CrossRef]
- García y González, E.; Cuellar, A.; Hernández, H.; Nandayapa, E.; Álvarez, L.; Tórtora, J.; Terrazas, A. Maternal experience in Romanov sheep impairs mother-lamb recognition during the first 24 hours postpartum. J. Vet. Behav. 2015, 10, 66–72. [Google Scholar] [CrossRef]
- Ramírez Martínez, M.G.; Soto González, R.; Poindron Massot, P.; Álvarez Ramírez, L.; Valencia Méndez, J.D.J.; González Díaz, F.R.; Terrazas García, A.M. Comportamiento maternal alrededor del parto y reconocimiento madre-cría en ovinos Pelibuey. Vet. Mex. 2011, 42, 11–25. [Google Scholar]
- Freitas-de-Melo, A.; Pérez-Clariget, R.; Terrazas, A.; Ungerfeld, R. Ewe-lamb bond of experienced and inexperienced mothers undernourished during gestation. Sci. Rep. 2021, 11, 4634. [Google Scholar] [CrossRef] [PubMed]
- Meurisse, M.; Gonzalez, A.; Delsol, G.; Caba, M.; Lévy, F.; Poindron, P. Estradiol receptor-α expression in hypothalamic and limbic regions of ewes is influenced by physiological state and maternal experience. Horm. Behav. 2005, 48, 34–43. [Google Scholar] [CrossRef]



| Conduct | Description |
|---|---|
| Walking | Locomotor activity and/or moved around the corral. |
| Eating | When the goat was ingesting feed or its head was in the feed troughs. |
| Isolation | When the goat moved more than 3 m away from the herd or the nearest animal. |
| Aggression | When the goat showed signs of aggression towards any other member of the herd. |
| Rumination | When the goat was chewing the regurgitated feed. |
| Inactivity | When the goat was not performing any of the above behaviors. |
| Conduct | Description |
|---|---|
| Time elapsed between amniotic sac and limb emergence | When the kid’s limbs were observed through the birth canal. |
| Time elapsed between limb emergence and expulsion of the offspring | When the kid was fully expelled through the birth canal. |
| Lick latency | When for the first time the female cleaned, by way of grooming, the kid after its expulsion. |
| Nursing latency | When for the first time the female allowed the kid to ingest colostrum and was considered only when the episode lasted more than 5 continuous seconds. |
| Vocalizations of the dam | Sounds emitted with the mouth open or closed. |
| Latency of the kid trying to stand up | When the kid made first attempt to stand up, either assisted with one or both limbs and with the head or with the trunk. |
| Latency of kid standing | When the kid is stand up, in four legs for the first time. |
| Vocalizations of the kid | Sounds emitted with the mouth open or closed. |
| Behavior | Primiparous N = 10 | Multiparous N = 13 | p-Value |
|---|---|---|---|
| Time elapsed between amniotic sac and limb emergence (s) | 300.0 ± 89.44 | 814.2 ± 194.74 | 0.127 |
| Time from limbs to expulsion of the kid (s) | 343.6 ± 99.44 | 324.5 ± 53.20 | 0.758 |
| Lick latency (s) | 365.6 ± 188.56 | 60.5 ± 24.09 | 0.015 |
| Number of vocalizations of the dam | 509.4 ± 242.38 | 153.3 ± 27.35 | 0.501 |
| Maternal motivation score | 2.0 ± 0.23 | 2.14 ± 0.16 | 0.584 |
| Latency of the kid trying to stand up (s) | 1704.0 ± 753.45 | 680.0 ± 87.92 | 0.384 |
| Latency of kid standing (s) | 3349.0 ± 775.15 | 2287.5 ± 397.9 | 0.206 |
| Nursing latency (s) | 6114.5 ± 585.2 | 5010.0 ± 454.49 | 0.175 |
| Number of vocalizations of the kid | 157.3 ± 36.30 | 123.12 ± 23.88 | 0.407 |
| Behavior | Primiparous (n = 9) | Multiparous (n = 16) | ||
|---|---|---|---|---|
| Own Kid | Foreign Kid | Own Kid | Foreign Kid | |
| Number of low bleats | 15.33 ± 3.83 a | 32.88 ± 7.35 b | 15.43 ± 4.87 a | 37.75 ± 6.98 b |
| Number of high bleats | 0.66 ± 0.37 a | 13.44 ± 7.05 b | 0.56 ± 0.40 A | 10.50 ± 5.74 B |
| Time near the udder (s) | 13.66 ± 8.9 A | 0 ± 0 B | 33.37 ± 12.2 a | 4.5 ± 3.19 b |
| Number of rejections of kid | 0 ± 0 A | 0.55 ± 0.24 B | 0 ± 0 | 0.37 ± 0.25 |
| Number of acceptances to the udder | 1.33 ± 0.60 A | 0 ± 0 B | 1.25 ± 0.23 a | 0.5 ± 0.24 b |
| Aggression | 0 ± 0 | 0.55 ± 0.44 | 0 ± 0 a | 0.62 ± 0.27 b |
| Multiparous n = 38 | Primiparous n = 15 | p-Value | |
|---|---|---|---|
| Weight at birth (kg) | 2.9 ± 0.12 | 3.2 ± 0.20 | 0.263 |
| Rectal temperature (°C) | 39.3 ± 0.10 | 38.8 ± 0.35 | 0.233 |
| Surface temperature (°C) | 29.1 ± 0.80 | 30.7 ± 0.97 | 0.211 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cano-Suarez, P.; Damian, J.P.; Soto, R.; Ayala, K.; Zaragoza, J.; Ibarra, R.; Ramírez-Espinosa, J.J.; Castillo, L.; Candanosa Aranda, I.E.; Terrazas, A. Behavioral, Physiological and Hormonal Changes in Primiparous and Multiparous Goats and Their Kids During Peripartum. Ruminants 2024, 4, 515-532. https://doi.org/10.3390/ruminants4040036
Cano-Suarez P, Damian JP, Soto R, Ayala K, Zaragoza J, Ibarra R, Ramírez-Espinosa JJ, Castillo L, Candanosa Aranda IE, Terrazas A. Behavioral, Physiological and Hormonal Changes in Primiparous and Multiparous Goats and Their Kids During Peripartum. Ruminants. 2024; 4(4):515-532. https://doi.org/10.3390/ruminants4040036
Chicago/Turabian StyleCano-Suarez, Paolo, Juan Pablo Damian, Rosalba Soto, Karen Ayala, Joob Zaragoza, Rocio Ibarra, Jesús Jonathan Ramírez-Espinosa, Laura Castillo, Irma Eugenia Candanosa Aranda, and Angélica Terrazas. 2024. "Behavioral, Physiological and Hormonal Changes in Primiparous and Multiparous Goats and Their Kids During Peripartum" Ruminants 4, no. 4: 515-532. https://doi.org/10.3390/ruminants4040036
APA StyleCano-Suarez, P., Damian, J. P., Soto, R., Ayala, K., Zaragoza, J., Ibarra, R., Ramírez-Espinosa, J. J., Castillo, L., Candanosa Aranda, I. E., & Terrazas, A. (2024). Behavioral, Physiological and Hormonal Changes in Primiparous and Multiparous Goats and Their Kids During Peripartum. Ruminants, 4(4), 515-532. https://doi.org/10.3390/ruminants4040036





