Detection of Bovine Respiratory Syncytial Virus in Cattle: A Systematic Review and Meta-Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Data Extraction and Quality Assessment
2.3. Qualitative Data Selection and Analysis
2.4. Statistical and Meta-Analysis
2.5. Assessment of Certainty of Evidence
3. Results
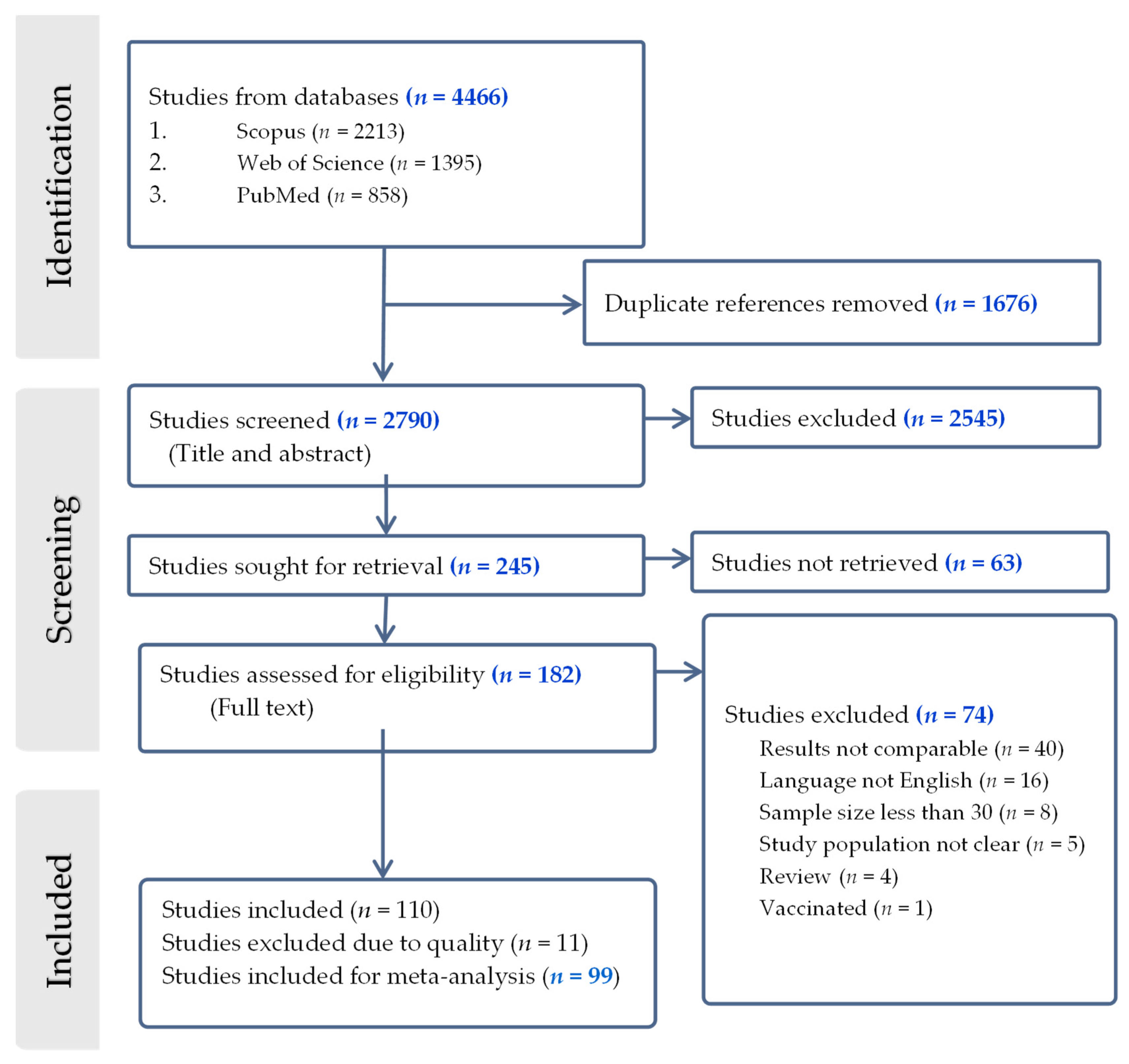
3.1. Study Selection and Characteristics
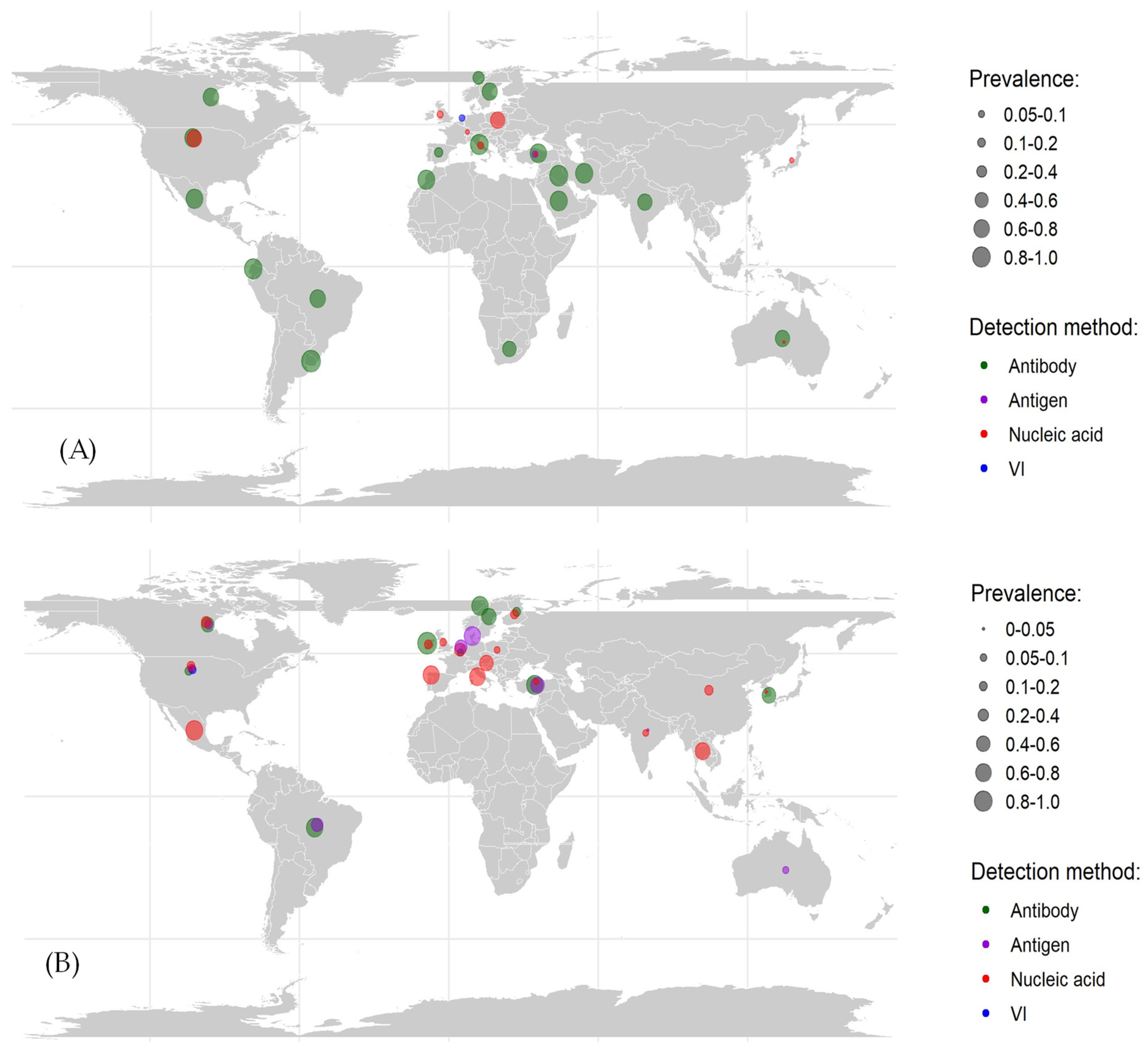
3.2. Prevalence of BRSV
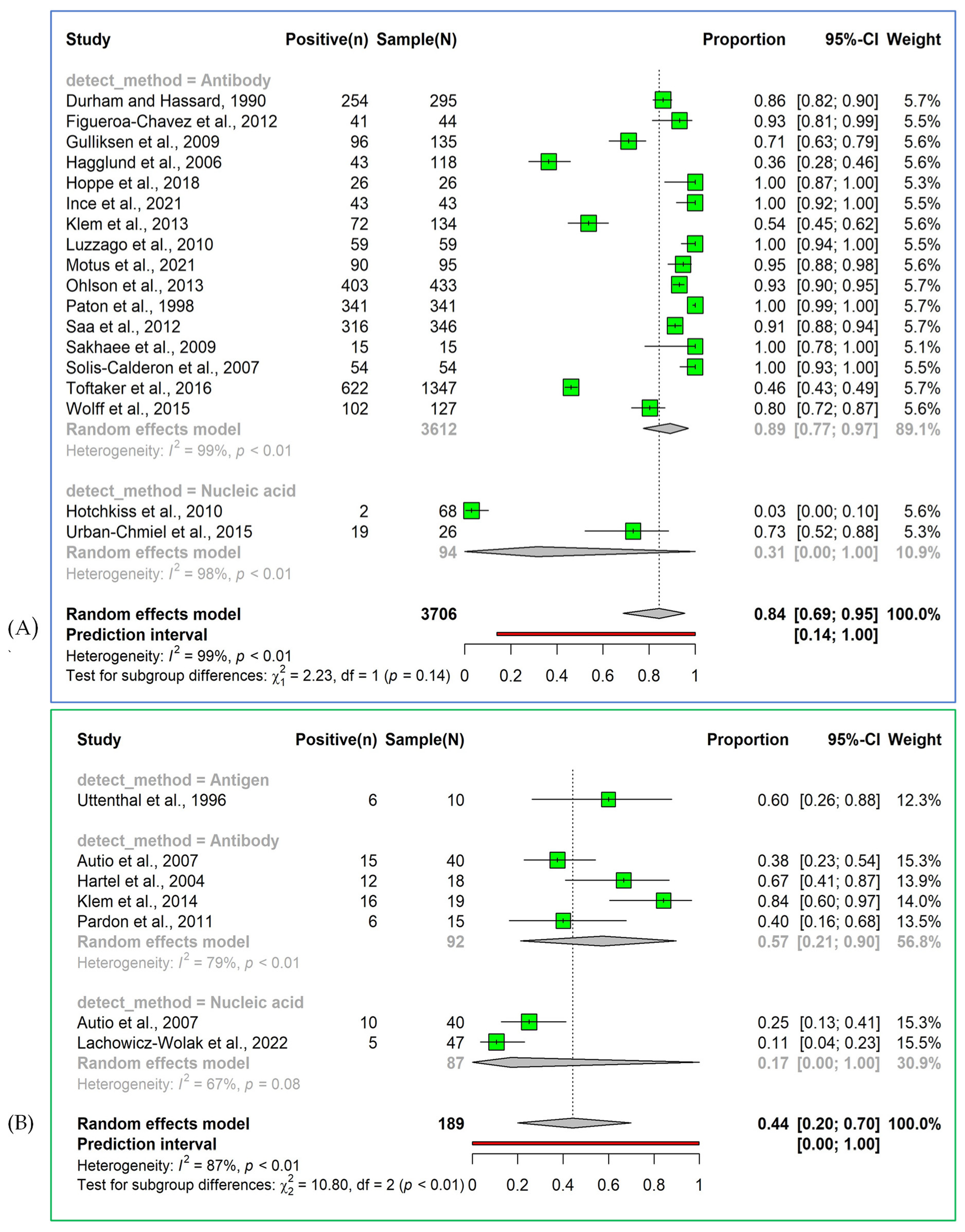
3.2.1. Prevalence of Bovine Respiratory Syncytial Virus Detected by Antibody-Based Detection Methods
3.2.2. Prevalence of Bovine Respiratory Syncytial Virus Detected by Antigen Detection Methods
3.2.3. Prevalence of Bovine Respiratory Syncytial Virus Detected by Nucleic Acid-Based Detection Methods

| Variable Name | Before Outlier Removal | After Outlier Removal |
|---|---|---|
| Number of studies | 10 | 8 * |
| Number of observations | 3626 | 3159 |
| Number of events | 329 | 79 |
| Pooled prevalence | 0.09 (95% CI: 0.01 to 0.23) | 0.03 (95% CI: 0.01 to 0.06) |
| Prediction interval | 0.00 to 0.67 | 0.00 to 0.15 |
| Heterogeneity (I2) | 98.9% (tau2 = 0.07, H = 9.49) | 89.9% (tau2 = 0.01, H = 3.14) |
3.3. BRSV Detection Rate Among Bovine Respiratory Disease Complex (BRDC) Cases
3.3.1. Detection Rate of BRSV Using Antibody Detection Methods in BRD Cases
3.3.2. Detection Rate of the Bovine Respiratory Syncytial Virus Using Antigen Detection Methods in Cattle with Bovine Respiratory Disease Complex Cases
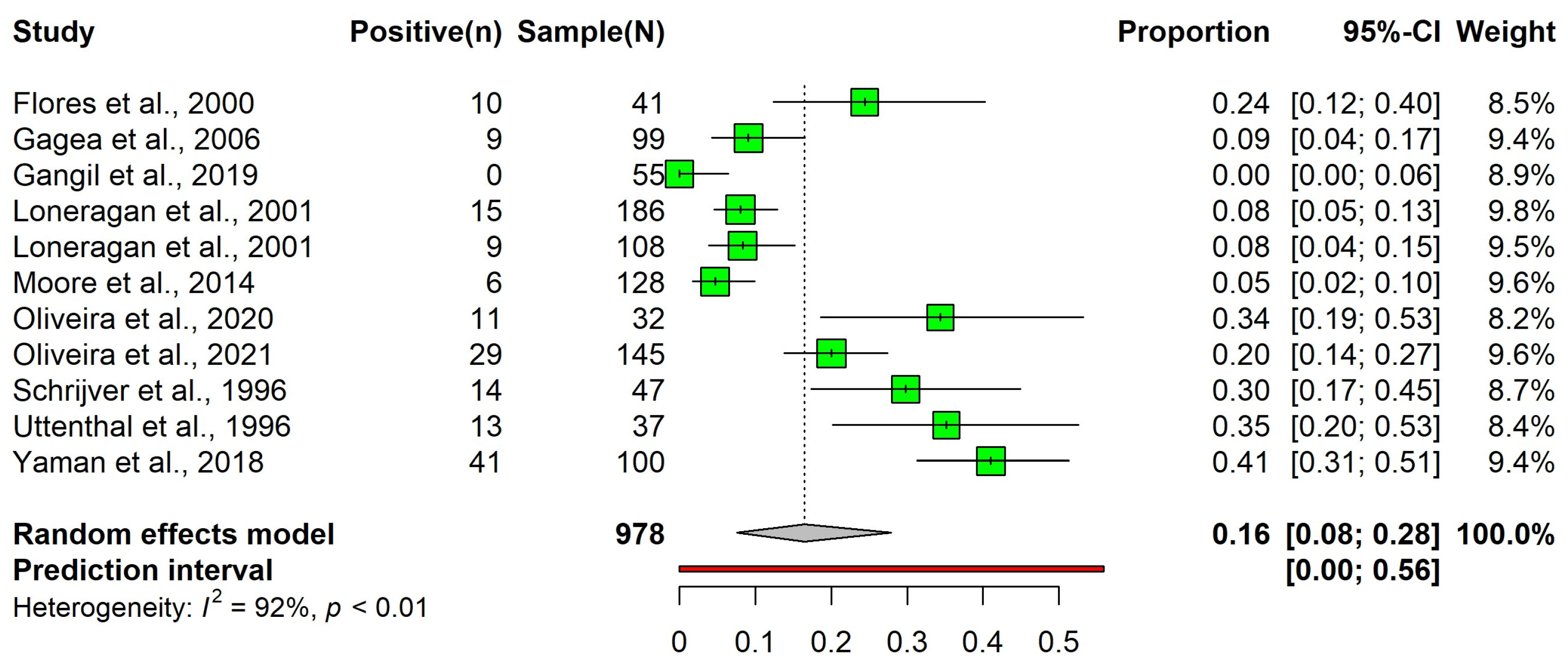
| Variable Name | Before Outlier Removal | After Outlier Removal |
|---|---|---|
| Number of studies | 12 | 11 * |
| Number of observations | 1050 | 978 |
| Pooled detection rate | 0.23 (95% CI: 0.08 to 0.42) | 0.16 (95% CI: 0.08 to 0.28) |
| Prediction interval | 0.00 to 0.88 | 0.00 to 0.56 |
| Heterogeneity (I2) | 96.9% (tau = 0.30) | 91.5% (tau = 0.18) |
3.3.3. Detection Rate of Bovine Respiratory Syncytial Virus Using Nucleic Acid Detection Methods in Cattle with Bovine Respiratory Disease Complex Cases
3.4. Herd-Level Prevalence of Bovine Respiratory Syncytial Virus
3.5. Factors Influencing BRSV Prevalence
3.6. Assessment of Certainty of Evidence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodríguez-Castillo, J.L.; López-Valencia, G.; Monge-Navarro, F.J.; Medina-Basulto, G.E.; Hori-Oshima, S.; Cueto-González, S.A.; Mora-Valle, A.D.L.; Muñoz-Del Real, L.M.; Tinoco-Gracia, L.; Rentería-Evangelista, T.B. Detection and economic impact related to bovine respiratory disease, shrink, and traveling distance in feedlot cattle in Northwest Mexico. Turk. J. Vet. Anim. Sci. 2017, 41, 294–301. [Google Scholar] [CrossRef]
- Lefkowitz, E.J.; Dempsey, D.M.; Hendrickson, R.C.; Orton, R.J.; Siddell, S.G.; Smith, D.B. Virus taxonomy: The database of the International Committee on Taxonomy of Viruses (ICTV). Nucleic Acids Res. 2018, 46, D708–D717. [Google Scholar] [CrossRef] [PubMed]
- Valarcher, J.F.; Schelcher, F.; Bourhy, H. Evolution of bovine respiratory syncytial virus. J. Virol. 2000, 74, 10714–10728. [Google Scholar] [CrossRef]
- Mars, M.H.; Bruschke, C.J.; van Oirschot, J.T. Airborne transmission of BHV1, BRSV, and BVDV among cattle is possible under experimental conditions. Vet. Microbiol. 1999, 66, 197–207. [Google Scholar] [CrossRef]
- Antonis, A.F.; de Jong, M.C.; van der Poel, W.H.; van der Most, R.G.; Stockhofe-Zurwieden, N.; Kimman, T.; Schrijver, R.S. Age-dependent differences in the pathogenesis of bovine respiratory syncytial virus infections related to the development of natural immunocompetence. J. Gen. Virol. 2010, 91, 2497–2506. [Google Scholar] [CrossRef]
- Viuff, B.; Uttenthal, A.; Tegtmeier, C.; Alexandersen, S. Sites of replication of bovine respiratory syncytial virus in naturally infected calves as determined by in situ hybridization. Vet. Pathol. 1996, 33, 383–390. [Google Scholar] [CrossRef]
- Sudaryatma, P.E.; Saito, A.; Mekata, H.; Kubo, M.; Fahkrajang, W.; Mazimpaka, E.; Okabayashi, T. Bovine Respiratory Syncytial Virus Enhances the Adherence of Pasteurella multocida to Bovine Lower Respiratory Tract Epithelial Cells by Upregulating the Platelet-Activating Factor Receptor. Front. Microbiol. 2020, 11, 1676. [Google Scholar] [CrossRef]
- Schreiber, P.; Matheise, J.P.; Dessy, F.; Heimann, M.; Letesson, J.J.; Coppe, P.; Collard, A. High mortality rate associated with bovine respiratory syncytial virus (BRSV) infection in Belgian white blue calves previously vaccinated with an inactivated BRSV vaccine. J. Vet. Med. B Infect. Dis. Vet. Public Health 2000, 47, 535–550. [Google Scholar] [CrossRef]
- Hagglund, S.; Svensson, C.; Emanuelson, U.; Valarcher, J.F.; Alenius, S. Dynamics of virus infections involved in the bovine respiratory disease complex in Swedish dairy herds. Vet. J. 2006, 172, 320–328. [Google Scholar] [CrossRef]
- Brodersen, B.W. Bovine respiratory syncytial virus. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 323–333. [Google Scholar] [CrossRef]
- Chamorro, M.F.; Woolums, A.; Walz, P.H. Vaccination of calves against common respiratory viruses in the face of maternally derived antibodies (IFOMA). Anim. Health Res. Rev. 2016, 17, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Kimman, T.G.; Westenbrink, F.; Schreuder, B.E.; Straver, P.J. Local and systemic antibody response to bovine respiratory syncytial virus infection and reinfection in calves with and without maternal antibodies. J. Clin. Microbiol. 1987, 25, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.A. Bovine parainfluenza-3 virus. Vet. Clin. N. Am. Food Anim. Pract. 2010, 26, 575–593. [Google Scholar] [CrossRef]
- Ferella, A.; Perez Aguirreburualde, M.S.; Margineda, C.; Aznar, N.; Sammarruco, A.; Dus Santos, M.J.; Mozgovoj, M. Bovine respiratory syncytial virus seroprevalence and risk factors in feedlot cattle from Cordoba and Santa Fe, Argentina. Rev. Argent. Microbiol. 2018, 50, 275–279. [Google Scholar] [CrossRef]
- Saa, L.R.; Perea, A.; Jara, D.V.; Arenas, A.J.; Garcia-Bocanegra, I.; Borge, C.; Carbonero, A. Prevalence of and risk factors for bovine respiratory syncytial virus (BRSV) infection in non-vaccinated dairy and dual-purpose cattle herds in Ecuador. Trop. Anim. Health Prod. 2012, 44, 1423–1427. [Google Scholar] [CrossRef]
- Ince, O.B.; Sevik, M.; Ozgur, E.G.; Sait, A. Risk factors and genetic characterization of bovine respiratory syncytial virus in the inner Aegean Region, Turkey. Trop. Anim. Health Prod. 2021, 54, 4. [Google Scholar] [CrossRef]
- Hoppe, I.; Medeiros, A.S.R.; Arns, C.W.; Samara, S.I. Bovine respiratory syncytial virus seroprevalence and risk factors in non-vaccinated dairy cattle herds in Brazil. BMC Vet. Res. 2018, 14, 208. [Google Scholar] [CrossRef]
- Figueroa-Chavez, D.; Segura-Correa, J.C.; Garcia-Marquez, L.J.; Pescador-Rubio, A.; Valdivia-Flores, A.G. Detection of antibodies and risk factors for infection with bovine respiratory syncytial virus and parainfluenza virus 3 in dual-purpose farms in Colima, Mexico. Trop. Anim. Health Prod. 2012, 44, 1417–1421. [Google Scholar] [CrossRef]
- Beaudeau, F.; Bjorkman, C.; Alenius, S.; Frossling, J. Spatial patterns of bovine corona virus and bovine respiratory syncytial virus in the Swedish beef cattle population. Acta Vet. Scand. 2010, 52, 33. [Google Scholar] [CrossRef]
- Elazhary, M.A.; Roy, R.S.; Champlin, R.; Higgins, R.; Marsolais, G. Bovine respiratory syncytial virus in Quebec: Antibody prevalence and disease outbreak. Can. J. Comp. Med. 1980, 44, 299–303. [Google Scholar]
- Pardon, B.; Callens, J.; Maris, J.; Allais, L.; Van Praet, W.; Deprez, P.; Ribbens, S. Pathogen-specific risk factors in acute outbreaks of respiratory disease in calves. J. Dairy Sci. 2020, 103, 2556–2566. [Google Scholar] [CrossRef] [PubMed]
- Kimman, T.G.; Zimmer, G.M.; Westenbrink, F.; Mars, J.; van Leeuwen, E. Epidemiological study of bovine respiratory syncytial virus infections in calves: Influence of maternal antibodies on the outcome of disease. Vet. Rec. 1988, 123, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Gershwin, L.J.; Gunther, R.A.; Anderson, M.L.; Woolums, A.R.; McArthur-Vaughan, K.; Randel, K.E.; Boyle, G.A.; Friebertshauser, K.E.; McInturff, P.S. Bovine respiratory syncytial virus-specific IgE is associated with interleukin-2 and -4, and interferon-gamma expression in pulmonary lymph of experimentally infected calves. Am. J. Vet. Res. 2000, 61, 291–298. [Google Scholar] [CrossRef]
- Larsen, L.E.; Tjornehoj, K.; Viuff, B.; Jensen, N.E.; Uttenthal, A. Diagnosis of enzootic pneumonia in Danish cattle: Reverse transcription-polymerase chain reaction assay for detection of bovine respiratory syncytial virus in naturally and experimentally infected cattle. J. Vet. Diagn. Investig. 1999, 11, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Makoschey, B.; Berge, A.C. Review on bovine respiratory syncytial virus and bovine parainfluenza—Usual suspects in bovine respiratory disease—A narrative review. BMC Vet. Res. 2021, 17, 261. [Google Scholar] [CrossRef] [PubMed]
- Doyle, D.; Credille, B.; Lehenbauer, T.W.; Berghaus, R.; Aly, S.S.; Champagne, J.; Blanchard, P.; Crossley, B.; Berghaus, L.; Cochran, S.; et al. Agreement Among 4 Sampling Methods to Identify Respiratory Pathogens in Dairy Calves with Acute Bovine Respiratory Disease. J. Vet. Intern. Med. 2017, 31, 954–959. [Google Scholar] [CrossRef]
- Meyer, G.; Deplanche, M.; Schelcher, F. Human and bovine respiratory syncytial virus vaccine research and development. Comp. Immunol. Microbiol. Infect. Dis. 2008, 31, 191–225. [Google Scholar] [CrossRef]
- Tasker, L.; Lindsay, R.W.; Clarke, B.T.; Cochrane, D.W.; Hou, S. Infection of mice with respiratory syncytial virus during neonatal life primes for enhanced antibody and T cell responses on secondary challenge. Clin. Exp. Immunol. 2008, 153, 277–288. [Google Scholar] [CrossRef]
- Klem, T.B.; Sjurseth, S.K.; Sviland, S.; Gjerset, B.; Myrmel, M.; Stokstad, M. Bovine respiratory syncytial virus in experimentally exposed and rechallenged calves; viral shedding related to clinical signs and the potential for transmission. BMC Vet. Res. 2019, 15, 156. [Google Scholar] [CrossRef]
- Gershwin, L.J. Bovine respiratory syncytial virus infection: Immunopathogenic mechanisms. Anim. Health Res. Rev. 2007, 8, 207–213. [Google Scholar] [CrossRef]
- Achenbach, J.E.; Topliff, C.L.; Vassilev, V.B.; Donis, R.O.; Eskridge, K.M.; Kelling, C.L. Detection and quantitation of bovine respiratory syncytial virus using real-time quantitative RT-PCR and quantitative competitive RT-PCR assays. J. Virol. Methods 2004, 121, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Timsit, E.; Maingourd, C.; Le Drean, E.; Belloc, C.; Seegers, H.; Douart, A.; Assie, S. Evaluation of a commercial real-time reverse transcription polymerase chain reaction kit for the diagnosis of Bovine respiratory syncytial virus infection. J. Vet. Diagn. Investig. 2010, 22, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Thonur, L.; Maley, M.; Gilray, J.; Crook, T.; Laming, E.; Turnbull, D.; Nath, M.; Willoughby, K. One-step multiplex real time RT-PCR for the detection of bovine respiratory syncytial virus, bovine herpesvirus 1 and bovine parainfluenza virus 3. BMC Vet. Res. 2012, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Sarchet, J.J.; Pollreisz, J.P.; Bechtol, D.T.; Blanding, M.R.; Saltman, R.L.; Taube, P.C. Limitations of bacterial culture, viral PCR, and tulathromycin susceptibility from upper respiratory tract samples in predicting clinical outcome of tulathromycin control or treatment of bovine respiratory disease in high-risk feeder heifers. PLoS ONE 2022, 17, e0247213. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Dhakal, K. NVivo. J. Med. Libr. Assoc. 2022, 110, 270–272. [Google Scholar] [CrossRef]
- Viechtbauer, W. Conducting Meta-Analyses inRwith themetaforPackage. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Barendregt, J.J.; Doi, S.A.; Lee, Y.Y.; Norman, R.E.; Vos, T. Meta-analysis of prevalence. J. Epidemiol. Community Health 2013, 67, 974–978. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Cochran, W.G. The Combination of Estimates from Different Experiments. Biometrics 1954, 10, 101–129. [Google Scholar] [CrossRef]
- Olkin, I.; Dahabreh, I.J.; Trikalinos, T.A. GOSH—A graphical display of study heterogeneity. Res. Synth. Methods 2012, 3, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Ester, M.; Kriegel, H.-P.; Sander, J.; Xu, X. A density-based algorithm for discovering clusters in large spatial databases with noise. In Proceedings of the KDD, Portland, OR, USA, 2–4 August 1996; pp. 226–231. [Google Scholar]
- Peters, J.L.; Sutton, A.J.; Jones, D.R.; Abrams, K.R.; Rushton, L. Comparison of two methods to detect publication bias in meta-analysis. JAMA 2006, 295, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Egger, M.; Moher, D. Addressing reporting biases. In Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2008; pp. 297–333. [Google Scholar]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schunemann, H.J.; Group, G.W. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Solis-Calderon, J.J.; Segura-Correa, J.C.; Aguilar-Romero, F.; Segura-Correa, V.M. Detection of antibodies and risk factors for infection with bovine respiratory syncytial virus and parainfluenza virus-3 in beef cattle of Yucatan, Mexico. Prev. Vet. Med. 2007, 82, 102–110. [Google Scholar] [CrossRef]
- Moore, S.J.; O’Dea, M.A.; Perkins, N.; O’Hara, A.J. Estimation of nasal shedding and seroprevalence of organisms known to be associated with bovine respiratory disease in Australian live export cattle. J. Vet. Diagn. Investig. 2015, 27, 6–17. [Google Scholar] [CrossRef]
- Kale, M.; Dylek, O.; Sybel, H.; Pehlivanoglu, F.; Turutoglu, H. Some Viral and Bacterial Respiratory Tract Infections of Dairy Cattle during the Summer Season. Acta Vet. 2013, 63, 227–236. [Google Scholar] [CrossRef]
- Duman, R.; Yavru, S.; Kale, M.; Avci, O. Seroprevalence of Viral Upper Respiratory Infections in Dairy Cattle. Kafkas Univ. Vet. Fak. Derg. 2009, 15, 539–542. [Google Scholar]
- Autio, T.; Pohjanvirta, T.; Holopainen, R.; Rikula, U.; Pentikainen, J.; Huovilainen, A.; Rusanen, H.; Soveri, T.; Sihvonen, L.; Pelkonen, S. Etiology of respiratory disease in non-vaccinated, non-medicated calves in rearing herds. Vet. Microbiol. 2007, 119, 256–265. [Google Scholar] [CrossRef]
- Hartel, H.; Nikunen, S.; Neuvonen, E.; Tanskanen, R.; Kivela, S.L.; Aho, R.; Soveri, T.; Saloniemi, H. Viral and bacterial pathogens in bovine respiratory disease in Finland. Acta Vet. Scand. 2004, 45, 193–200. [Google Scholar] [CrossRef]
- Obando, C.; Baule, C.; Pedrique, C.; Veracierta, C.; Belak, S.; Merza, M.; Moreno-Lopez, J. Serological and molecular diagnosis of bovine viral diarrhoea virus and evidence of other viral infections in dairy calves with respiratory disease in Venezuela. Acta Vet. Scand. 1999, 40, 253–262. [Google Scholar] [CrossRef]
- Pardon, B.; De Bleecker, K.; Dewulf, J.; Callens, J.; Boyen, F.; Catry, B.; Deprez, P. Prevalence of respiratory pathogens in diseased, non-vaccinated, routinely medicated veal calves. Vet. Rec. 2011, 169, 278. [Google Scholar] [CrossRef] [PubMed]
- Ohlson, A.; Alenius, S.; Traven, M.; Emanuelson, U. A longitudinal study of the dynamics of bovine corona virus and respiratory syncytial virus infections in dairy herds. Vet. J. 2013, 197, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Wolff, C.; Emanuelson, U.; Ohlson, A.; Alenius, S.; Fall, N. Bovine respiratory syncytial virus and bovine coronavirus in Swedish organic and conventional dairy herds. Acta Vet. Scand. 2015, 57, 2. [Google Scholar] [CrossRef] [PubMed]
- Lora, I.; Magrin, L.; Contiero, B.; Ranzato, G.; Cozzi, G. Individual antimicrobial treatments in veal calves: Effect on the net carcasses weight at the slaughterhouse and relationship with the serostatus of the calves upon arrival to the fattening unit. Prev. Vet. Med. 2022, 207, 105715. [Google Scholar] [CrossRef] [PubMed]
- Elvander, M. Severe respiratory disease in dairy cows caused by infection with bovine respiratory syncytial virus. Vet. Rec. 1996, 138, 101–105. [Google Scholar] [CrossRef]
- Graham, D.A.; McShane, J.; Mawhinney, K.A.; McLaren, I.E.; Adair, B.M.; Merza, M. Evaluation of a single dilution ELISA system for detection of seroconversion to bovine viral diarrhea virus, bovine respiratory syncytial virus, parainfluenza-3 virus, and infectious bovine rhinotracheitis virus: Comparison with testing by virus neutralization and hemagglutination inhibition. J. Vet. Diagn. Investig. 1998, 10, 43–48. [Google Scholar] [CrossRef]
- Hazari, S.; Panda, H.K.; Kar, B.C.; Das, B.R. Comparative evaluation of indirect and sandwich ELISA for the detection of antibodies to bovine respiratory syncytial virus (BRSV) in dairy cattle. Comp. Immunol. Microbiol. Infect. Dis. 2002, 25, 59–68. [Google Scholar] [CrossRef]
- Katoch, S.; Dohru, S.; Sharma, M.; Vashist, V.; Chahota, R.; Dhar, P.; Thakur, A.; Verma, S. Seroprevalence of viral and bacterial diseases among the bovines in Himachal Pradesh, India. Vet. World 2017, 10, 1421–1426. [Google Scholar] [CrossRef]
- Klem, T.B.; Gulliksen, S.M.; Lie, K.I.; Loken, T.; Osteras, O.; Stokstad, M. Bovine respiratory syncytial virus: Infection dynamics within and between herds. Vet. Rec. 2013, 173, 476. [Google Scholar] [CrossRef]
- Luzzago, C.; Bronzo, V.; Salvetti, S.; Frigerio, M.; Ferrari, N. Bovine respiratory syncytial virus seroprevalence and risk factors in endemic dairy cattle herds. Vet. Res. Commun. 2010, 34, 19–24. [Google Scholar] [CrossRef]
- Sakhaee, E.; Khalili, M.; Nia, S.K. Serological study of bovine viral respiratory diseases in dairy herds in Kerman province, Iran. Iran. J. Vet. Res. 2009, 10, 49–53. [Google Scholar]
- Toftaker, I.; Toft, N.; Stokstad, M.; Solverod, L.; Harkiss, G.; Watt, N.; O’Brien, A.; Nodtvedt, A. Evaluation of a multiplex immunoassay for bovine respiratory syncytial virus and bovine coronavirus antibodies in bulk tank milk against two indirect ELISAs using latent class analysis. Prev. Vet. Med. 2018, 154, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bidokhti, M.R.; Traven, M.; Fall, N.; Emanuelson, U.; Alenius, S. Reduced likelihood of bovine coronavirus and bovine respiratory syncytial virus infection on organic compared to conventional dairy farms. Vet. J. 2009, 182, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Motus, K.; Rilanto, T.; Viidu, D.A.; Orro, T.; Viltrop, A. Seroprevalence of selected endemic infectious diseases in large-scale Estonian dairy herds and their associations with cow longevity and culling rates. Prev. Vet. Med. 2021, 192, 105389. [Google Scholar] [CrossRef]
- Shirvani, E.; Lotfi, M.; Kamalzadeh, M.; Noaman, V.; Bahriari, M.; Morovati, H.; Hatami, A. Seroepidemiological study of bovine respiratory viruses (BRSV, BoHV-1, PI-3V, BVDV, and BAV-3) in dairy cattle in central region of Iran (Esfahan province). Trop. Anim. Health Prod. 2012, 44, 191–195. [Google Scholar] [CrossRef]
- Paton, D.J.; Christiansen, K.H.; Alenius, S.; Cranwell, M.P.; Pritchard, G.C.; Drew, T.W. Prevalence of antibodies to bovine virus diarrhoea virus and other viruses in bulk tank milk in England and Wales. Vet. Rec. 1998, 142, 385–391. [Google Scholar] [CrossRef]
- Gaeta, N.C.; Ribeiro, B.L.M.; Alemán, M.A.R.; Yoshihara, E.; Marques, E.C.; Hellmeister, A.N.; Pituco, E.M.; Gregory, L. Serological investigation of antibodies against respiratory viruses in calves from Brazilian family farming and their relation to clinical signs of bovine respiratory diseases. Pesqui. Vet. Bras. 2018, 38, 642–648. [Google Scholar] [CrossRef]
- Gulliksen, S.M.; Jor, E.; Lie, K.I.; Loken, T.; Akerstedt, J.; Osteras, O. Respiratory infections in Norwegian dairy calves. J. Dairy Sci. 2009, 92, 5139–5146. [Google Scholar] [CrossRef]
- Klem, T.B.; Rimstad, E.; Stokstad, M. Occurrence and phylogenetic analysis of bovine respiratory syncytial virus in outbreaks of respiratory disease in Norway. BMC Vet. Res. 2014, 10, 15. [Google Scholar] [CrossRef]
- Costa, M.; Garcia, L.; Yunus, A.S.; Rockemann, D.D.; Samal, S.K.; Cristina, J. Bovine respiratory syncytial virus: First serological evidence in Uruguay. Vet. Res. 2000, 31, 241–246. [Google Scholar] [CrossRef]
- Durham, P.J.K.; Hassard, L.E. Prevalence of antibodies to infectious bovine rhinotracheitis, parainfluenza 3, bovine respiratory syncytial, and bovine viral diarrhea viruses in cattle in Saskatchewan and Alberta. Can. Vet. J. 1990, 31, 815–820. [Google Scholar] [PubMed]
- Jimenez-Ruiz, S.; Garcia-Bocanegra, I.; Acevedo, P.; Espunyes, J.; Triguero-Ocana, R.; Cano-Terriza, D.; Torres-Sanchez, M.J.; Vicente, J.; Risalde, M.A. A survey of shared pathogens at the domestic-wild ruminants’ interface in Donana National Park (Spain). Transbound. Emerg. Dis. 2022, 69, 1568–1576. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.A.; Allam, A.M. Seroprevalence of bovine viral diarrhea virus (BVDV), bovine herpes virus type 1 (BHV-1), parainfluenza type 3 virus (PI-3V) and bovine respiratory syncytial virus (BRSV) among non vaccinated cattle. Global Vet. 2013, 10, 348–353. [Google Scholar] [CrossRef]
- Hussain, K.J.; Al-Farwachi, M.I.; Hassan, S.D. Seroprevalence and risk factors of bovine respiratory syncytial virus in cattle in the Nineveh Governorate, Iraq. Vet. World 2019, 12, 1862–1865. [Google Scholar] [CrossRef]
- Durham, P.J.; Hassard, L.E.; Van Donkersgoed, J. Serological studies of infectious bovine rhinotracheitis, parainfluenza 3, bovine viral diarrhea, and bovine respiratory syncytial viruses in calves following entry to a bull test station. Can. Vet. J. 1991, 32, 427–429. [Google Scholar]
- Grubbs, S.T.; Kania, S.A.; Potgieter, L.N. Prevalence of ovine and bovine respiratory syncytial virus infections in cattle determined with a synthetic peptide-based immunoassay. J. Vet. Diagn. Investig. 2001, 13, 128–132. [Google Scholar] [CrossRef]
- Elvander, M.; Edwards, S.; Naslund, K.; Linde, N. Evaluation and application of an indirect ELISA for the detection of antibodies to bovine respiratory syncytial virus in milk, bulk milk, and serum. J. Vet. Diagn. Investig. 1995, 7, 177–182. [Google Scholar] [CrossRef]
- Lynch, J.A.; Derbyshire, J.B. Application of a modified indirect fluorescent antibody test to the detection of antibodies to bovine respiratory syncytial virus in Ontario cattle. Can. J. Vet. Res. 1986, 50, 384–389. [Google Scholar]
- Healy, A.M.; Monaghan, M.L.; Bassett, H.F.; Gunn, H.M.; Markey, B.K.; Collins, J.D. Morbidity and mortality in a large Irish feedlot; microbiological and serological findings in cattle with acute respiratory disease. Br. Vet. J. 1993, 149, 549–560. [Google Scholar] [CrossRef]
- Mahin, L.; Wellemans, G.; Shimi, A. Prevalence of Antibodies to Bovid Herpesvirus-1 (Ibr-Ipv), Bovine Virus Diarrhea, Bovine Respiratory Syncytial Para-Influenza-3, Adeno-a and Adeno-B Viruses in Indigenous and Imported Moroccan Cattle. Ann. Rech. Vet. 1985, 16, 279–283. [Google Scholar]
- Van Vuuren, M. Serological studies of bovine respiratory syncytial virus in feedlot cattle in South Africa. J. S. Afr. Vet. Assoc. 1990, 61, 168–169. [Google Scholar] [PubMed]
- Fulton, R.W.; Purdy, C.W.; Confer, A.W.; Saliki, J.T.; Loan, R.W.; Briggs, R.E.; Burge, L.J. Bovine viral diarrhea viral infections in feeder calves with respiratory disease: Interactions with Pasteurella spp., parainfluenza-3 virus, and bovine respiratory syncytial virus. Can. J. Vet. Res. 2000, 64, 151–159. [Google Scholar] [PubMed]
- Martin, S.W.; Bohac, J.G. The association between serological titers in infectious bovine rhinotracheitis virus, bovine virus diarrhea virus, parainfluenza-3 virus, respiratory syncytial virus and treatment for respiratory disease in Ontario feedlot calves. Can. J. Vet. Res. 1986, 50, 351–358. [Google Scholar] [PubMed]
- Windeyer, M.C.; Leslie, K.E.; Godden, S.M.; Hodgins, D.C.; Lissemore, K.D.; LeBlanc, S.J. Association of bovine respiratory disease or vaccination with serologic response in dairy heifer calves up to three months of age. Am. J. Vet. Res. 2015, 76, 239–245. [Google Scholar] [CrossRef]
- Hoppe, I.; Souza-Pollo, A.; Medeiros, A.S.R.; Samara, S.I.; Carvalho, A.A.B. HoBi-like pestivirus infection in an outbreak of bovine respiratory disease. Res. Vet. Sci. 2019, 126, 184–191. [Google Scholar] [CrossRef]
- Affonso, I.B.; Gatti, S.P.; Alexandrino, B.; Oliveira, M.C.; de Medeiros, A.S.R.; Buzinaro, M.D.; Samara, S.I. Detection of antibodies against bovine respiratory syncytial virus (BRSV) in dairy cattle with different prevalences of bovine herpesvirus type 1 (BoHV-1) in Sao Paulo State, Brazil. Semin. Cienc. Agrar. 2011, 32, 295–299. [Google Scholar] [CrossRef]
- Yesilbag, K.; Gungor, B. Seroprevalence of bovine respiratory viruses in North-Western Turkey. Trop. Anim. Health Prod. 2008, 40, 55–60. [Google Scholar] [CrossRef]
- Tuncer, P.; Yesilbag, K. Serological detection of infection dynamics for respiratory viruses among dairy calves. Vet. Microbiol. 2015, 180, 180–185. [Google Scholar] [CrossRef]
- Yavru, S.; Simsek, A.; Yapkic, O.; Kale, M. Serological evaluation of viral infections in bovine respiratory tract. Acta Vet. 2005, 55, 219–226. [Google Scholar] [CrossRef]
- Rossi, C.R.; Kiesel, G.K. Serological evidence for the association of bovine respiratory syncytial virus with respiratory tract disease in Alabama cattle. Infect. Immun. 1974, 10, 293–298. [Google Scholar] [CrossRef]
- Park, J.-h.; Kim, D. Detection of Respiratory Viral Pathogens and Mycoplasma spp. from Calves with Summer Pneumonia in Korea. J. Vet. Clin. 2019, 36, 185–189. [Google Scholar] [CrossRef]
- Moteane, M.; Babiuk, L.A.; Schiefer, B. Studies on the occurrence and significance of bovine respiratory syncytial virus in Saskatchewan. Can. J. Comp. Med. 1978, 42, 246–248. [Google Scholar] [PubMed]
- Zhang, M.; Hill, J.E.; Godson, D.L.; Ngeleka, M.; Fernando, C.; Huang, Y. The pulmonary virome, bacteriological and histopathological findings in bovine respiratory disease from western Canada. Transbound. Emerg. Dis. 2020, 67, 924–934. [Google Scholar] [CrossRef] [PubMed]
- Toker, E.B.; Yesilbag, K. Molecular characterization and comparison of diagnostic methods for bovine respiratory viruses (BPIV-3, BRSV, BVDV, and BoHV-1) in field samples in northwestern Turkey. Trop. Anim. Health Prod. 2021, 53, 79. [Google Scholar] [CrossRef]
- Fulton, R.W.; Blood, K.S.; Panciera, R.J.; Payton, M.E.; Ridpath, J.F.; Confer, A.W.; Saliki, J.T.; Burge, L.T.; Welsh, R.D.; Johnson, B.J.; et al. Lung pathology and infectious agents in fatal feedlot pneumonias and relationship with mortality, disease onset, and treatments. J. Vet. Diagn. Investig. 2009, 21, 464–477. [Google Scholar] [CrossRef]
- Klima, C.L.; Zaheer, R.; Cook, S.R.; Booker, C.W.; Hendrick, S.; Alexander, T.W.; McAllister, T.A. Pathogens of bovine respiratory disease in North American feedlots conferring multidrug resistance via integrative conjugative elements. J. Clin. Microbiol. 2014, 52, 438–448. [Google Scholar] [CrossRef]
- Chang, Y.; Yue, H.; Tang, C. Prevalence and Molecular Characteristics of Bovine Respiratory Syncytial Virus in Beef Cattle in China. Animals 2022, 12, 3511. [Google Scholar] [CrossRef]
- O’Neill, R.; Mooney, J.; Connaghan, E.; Furphy, C.; Graham, D.A. Patterns of detection of respiratory viruses in nasal swabs from calves in Ireland: A retrospective study. Vet. Rec. 2014, 175, 351. [Google Scholar] [CrossRef]
- Lachowicz-Wolak, A.; Klimowicz-Bodys, M.D.; Ploneczka-Janeczko, K.; Bykowy, M.; Siedlecka, M.; Cinciala, J.; Rypula, K. The Prevalence, Coexistence, and Correlations between Seven Pathogens Detected by a PCR Method from South-Western Poland Dairy Cattle Suffering from Bovine Respiratory Disease. Microorganisms 2022, 10, 1487. [Google Scholar] [CrossRef]
- Saipinta, D.; Panyamongkol, T.; Chuammitri, P.; Suriyasathaporn, W. Reduction in Mortality of Calves with Bovine Respiratory Disease in Detection with Influenza C and D Virus. Animals 2022, 12, 3252. [Google Scholar] [CrossRef]
- Kamdi, B.; Singh, R.; Singh, V.; Singh, S.; Kumar, P.; Singh, K.P.; George, N.; Dhama, K. Immunofluorescence and molecular diagnosis of bovine respiratory syncytial virus and bovine parainfluenza virus in the naturally infected young cattle and buffaloes from India. Microb. Pathog. 2020, 145, 104165. [Google Scholar] [CrossRef] [PubMed]
- Studer, E.; Schonecker, L.; Meylan, M.; Stucki, D.; Dijkman, R.; Holwerda, M.; Glaus, A.; Becker, J. Prevalence of BRD-Related Viral Pathogens in the Upper Respiratory Tract of Swiss Veal Calves. Animals 2021, 11, 1940. [Google Scholar] [CrossRef] [PubMed]
- Lowie, T.; Callens, J.; Maris, J.; Ribbens, S.; Pardon, B. Decision tree analysis for pathogen identification based on circumstantial factors in outbreaks of bovine respiratory disease in calves. Prev. Vet. Med. 2021, 196, 105469. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Shao, Z.; Dai, G.; Li, X.; Xiang, Y.; Jiang, S.; Zhang, Z.; Ren, Y.; Zhu, Z.; Fan, C.; et al. Pathogenic infection characteristics and risk factors for bovine respiratory disease complex based on the detection of lung pathogens in dead cattle in Northeast China. J. Dairy Sci. 2023, 106, 589–606. [Google Scholar] [CrossRef]
- Timurkan, M.O.; Aydin, H.; Sait, A. Identification and Molecular Characterisation of Bovine Parainfluenza Virus-3 and Bovine Respiratory Syncytial Virus—First Report from Turkey. J. Vet. Res. 2019, 63, 167–173. [Google Scholar] [CrossRef]
- Guo, T.; Zhang, J.; Chen, X.; Wei, X.; Wu, C.; Cui, Q.; Hao, Y. Investigation of viral pathogens in cattle with bovine respiratory disease complex in Inner Mongolia, China. Microb. Pathog. 2021, 153, 104594. [Google Scholar] [CrossRef]
- Socha, W.; Larska, M.; Rola, J. Molecular Characterisation of the First Polish Isolates of Bovine Respiratory Syncytial Virus. Bull. Vet. Inst. Pulawy 2009, 53, 569–574. [Google Scholar]
- Urban-Chmiel, R.; Wernicki, A.; Grooms, D.L.; Barbu, N.I.; Rola, J.; Socha, W. Rapid Detection of Bovine Respiratory Syncytial Virus in Poland Using a Human Patient-Side Diagnostic Assay. Transbound. Emerg. Dis. 2015, 62, 407–410. [Google Scholar] [CrossRef]
- Fulton, R.W.; d’Offay, J.M.; Landis, C.; Miles, D.G.; Smith, R.A.; Saliki, J.T.; Ridpath, J.F.; Confer, A.W.; Neill, J.D.; Eberle, R.; et al. Detection and characterization of viruses as field and vaccine strains in feedlot cattle with bovine respiratory disease. Vaccine 2016, 34, 3478–3492. [Google Scholar] [CrossRef]
- Saegerman, C.; Gaudino, M.; Savard, C.; Broes, A.; Ariel, O.; Meyer, G.; Ducatez, M.F. Influenza D virus in respiratory disease in Canadian, province of Quebec, cattle: Relative importance and evidence of new reassortment between different clades. Transbound. Emerg. Dis. 2022, 69, 1227–1245. [Google Scholar] [CrossRef]
- Padalino, B.; Cirone, F.; Zappaterra, M.; Tullio, D.; Ficco, G.; Giustino, A.; Ndiana, L.A.; Pratelli, A. Factors Affecting the Development of Bovine Respiratory Disease: A Cross-Sectional Study in Beef Steers Shipped From France to Italy. Front. Vet. Sci. 2021, 8, 627894. [Google Scholar] [CrossRef] [PubMed]
- Cirone, F.; Padalino, B.; Tullio, D.; Capozza, P.; Lo Surdo, M.; Lanave, G.; Pratelli, A. Prevalence of Pathogens Related to Bovine Respiratory Disease Before and After Transportation in Beef Steers: Preliminary Results. Animals 2019, 9, 1093. [Google Scholar] [CrossRef] [PubMed]
- Pratelli, A.; Cirone, F.; Capozza, P.; Trotta, A.; Corrente, M.; Balestrieri, A.; Buonavoglia, C. Bovine respiratory disease in beef calves supported long transport stress: An epidemiological study and strategies for control and prevention. Res. Vet. Sci. 2021, 135, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Giammarioli, M.; Mangili, P.; Nanni, A.; Pierini, I.; Petrini, S.; Pirani, S.; Gobbi, P.; De Mia, G.M. Highly pathogenic Bovine Respiratory Syncytial virus variant in a dairy herd in Italy. Vet. Med. Sci. 2020, 6, 740–745. [Google Scholar] [CrossRef]
- Fanelli, A.; Cirilli, M.; Lucente, M.S.; Zarea, A.A.K.; Buonavoglia, D.; Tempesta, M.; Greco, G. Fatal Calf Pneumonia Outbreaks in Italian Dairy Herds Involving Mycoplasma bovis and Other Agents of BRD Complex. Front. Vet. Sci. 2021, 8, 742785. [Google Scholar] [CrossRef]
- Goto, Y.; Fukunari, K.; Suzuki, T. Multiplex RT-qPCR Application in Early Detection of Bovine Respiratory Disease in Healthy Calves. Viruses 2023, 15, 669. [Google Scholar] [CrossRef]
- Hotchkiss, E.J.; Dagleish, M.P.; Willoughby, K.; McKendrick, I.J.; Finlayson, J.; Zadoks, R.N.; Newsome, E.; Brulisauer, F.; Gunn, G.J.; Hodgson, J.C. Prevalence of Pasteurella multocida and other respiratory pathogens in the nasal tract of Scottish calves. Vet. Rec. 2010, 167, 555–560. [Google Scholar] [CrossRef]
- Paller, T.; Hostnik, P.; Pogacnik, M.; Toplak, I. The Prevalence of Ten Pathogens Detected by a Real-Time Pcr Method in Nasal Swab Samples Collected from Live Cattle with Respiratory Disease. Slov. Vet. Res. 2017, 54, 101–107. [Google Scholar]
- Flores, E.F.; Weiblen, R.; Medeiros, M.; Botton, S.A.; Irigoyen, L.F.; Driemeier, D.; Schuch, L.F.; Moraes, M. A retrospective search for bovine respiratory syncytial virus (BRSV) antigens in histological specimens by immunofluorescence and immunohistochemistry. Pesqui. Vet. Bras. 2000, 20, 139–143. [Google Scholar] [CrossRef]
- Gagea, M.I.; Bateman, K.G.; van Dreumel, T.; McEwen, B.J.; Carman, S.; Archambault, M.; Shanahan, R.A.; Caswell, J.L. Diseases and pathogens associated with mortality in Ontario beef feedlots. J. Vet. Diagn. Investig. 2006, 18, 18–28. [Google Scholar] [CrossRef]
- Gangil, R.; Kaur, G.; Dwivedi, P.N. Detection of Respiratory Viral Antigens in Nasal Swabs of Bovine by Sandwich ELISA. Ind. J. Ani. Res. 2019, 54, 354–358. [Google Scholar] [CrossRef]
- Loneragan, G.H.; Gould, D.H.; Mason, G.L.; Garry, F.B.; Yost, G.S.; Miles, D.G.; Hoffman, B.W.; Mills, L.J. Involvement of microbial respiratory pathogens in acute interstitial pneumonia in feedlot cattle. Am. J. Vet. Res. 2001, 62, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.J.; O’Dea, M.A.; Perkins, N.; Barnes, A.; O’Hara, A.J. Mortality of live export cattle on long-haul voyages: Pathologic changes and pathogens. J. Vet. Diagn. Investig. 2014, 26, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.H.S.; Dall Agnol, A.M.; Fritzen, J.T.T.; Lorenzetti, E.; Alfieri, A.A.; Alfieri, A.F. Microbial diversity involved in the etiology of a bovine respiratory disease outbreak in a dairy calf rearing unit. Comp. Immunol. Microbiol. Infect. Dis. 2020, 71, 101494. [Google Scholar] [CrossRef]
- Oliveira, T.E.S.; Scuisato, G.S.; Pelaquim, I.F.; Cunha, C.W.; Cunha, L.S.; Flores, E.F.; Pretto-Giordano, L.G.; Lisboa, J.A.N.; Alfieri, A.A.; Saut, J.P.E.; et al. The Participation of a Malignant Catarrhal Fever Virus and Mycoplasma bovis in the Development of Single and Mixed Infections in Beef and Dairy Cattle With Bovine Respiratory Disease. Front. Vet. Sci. 2021, 8, 691448. [Google Scholar] [CrossRef] [PubMed]
- Schrijver, R.S.; Daus, F.; Kramps, J.A.; Langedijk, J.P.; Buijs, R.; Middel, W.G.; Taylor, G.; Furze, J.; Huyben, M.W.; van Oirschot, J.T. Subgrouping of bovine respiratory syncytial virus strains detected in lung tissue. Vet. Microbiol. 1996, 53, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Uttenthal, A.; Jensen, N.P.; Blom, J.Y. Viral aetiology of enzootic pneumonia in Danish dairy herds: Diagnostic tools and epidemiology. Vet. Rec. 1996, 139, 114–117. [Google Scholar] [CrossRef]
- Yaman, T.; Buyukbayram, H.; Ozyildiz, Z.; Terzi, F.; Uyar, A.; Keles, O.F.; Ozsoy, S.Y.; Yener, Z. Detection of Bovine Respiratory Syncytial Virus, Pasteurella Multocida, and Mannheimia Haemolytica by Immunohistochemical Method in Naturally-infected Cattle. J. Vet. Res. 2018, 62, 439–445. [Google Scholar] [CrossRef]
- Tegtmeier, C.; Uttenthal, A.; Friis, N.F.; Jensen, N.E.; Jensen, H.E. Pathological and microbiological studies on pneumonic lungs from Danish calves. Zentralbl Vet. B 1999, 46, 693–700. [Google Scholar] [CrossRef]
- Toftaker, I.; Sanchez, J.; Stokstad, M.; Nodtvedt, A. Bovine respiratory syncytial virus and bovine coronavirus antibodies in bulk tank milk—Risk factors and spatial analysis. Prev. Vet. Med. 2016, 133, 73–83. [Google Scholar] [CrossRef]
- Ohlson, A.; Heuer, C.; Lockhart, C.; Traven, M.; Emanuelson, U.; Alenius, S. Risk factors for seropositivity to bovine coronavirus and bovine respiratory syncytial virus in dairy herds. Vet. Rec. 2010, 167, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Farzinpour, M.; Badiei, K.; Pourjafar, M.; Ghane, M.; Zare, K. Sütçü Siyah Alaca İşletmelerinde Sığır Respiratuvar Sinsityal Virüsü ve Sığır Adenovirüs-3′ün Antikor İzleri, Seroepidemiyolojisi ve Risk Faktörleri. Istanb. Univ. Vet. Fak. Derg. 2016, 42, 5–10. [Google Scholar] [CrossRef]
- Key, D.W.; Derbyshire, J.B. Serological studies of parainfluenza type 3 virus, bovine adenovirus type 3 and bovine respiratory syncytial virus infection in beef calves. Vet. Microbiol. 1984, 9, 587–592. [Google Scholar] [CrossRef]
- Raaperi, K.; Bougeard, S.; Aleksejev, A.; Orro, T.; Viltrop, A. Association of herd BRSV and BHV-1 seroprevalence with respiratory disease and reproductive performance in adult dairy cattle. Acta Vet. Scand. 2012, 54, 4. [Google Scholar] [CrossRef] [PubMed]
- Blodorn, K.; Hagglund, S.; Gavier-Widen, D.; Eleouet, J.F.; Riffault, S.; Pringle, J.; Taylor, G.; Valarcher, J.F. A bovine respiratory syncytial virus model with high clinical expression in calves with specific passive immunity. BMC Vet. Res. 2015, 11, 76. [Google Scholar] [CrossRef] [PubMed]
- Jarrom, D.; Elston, L.; Washington, J.; Prettyjohns, M.; Cann, K.; Myles, S.; Groves, P. Effectiveness of tests to detect the presence of SARS-CoV-2 virus, and antibodies to SARS-CoV-2, to inform COVID-19 diagnosis: A rapid systematic review. BMJ Evid.-Based Med. 2022, 27, 33–45. [Google Scholar] [CrossRef]
- Fulton, R.W.; Confer, A.W. Laboratory test descriptions for bovine respiratory disease diagnosis and their strengths and weaknesses: Gold standards for diagnosis, do they exist? Can. Vet. J. 2012, 53, 754–761. [Google Scholar]
- Werid, G.M.; Hemmatzadeh, F.; Miller, D.; Reichel, M.P.; Messele, Y.E.; Petrovski, K. Comparative Analysis of the Prevalence of Bovine Viral Diarrhea Virus in Cattle Populations Based on Detection Methods: A Systematic Review and Meta-Analysis. Pathogens 2023, 12, 1067. [Google Scholar] [CrossRef]
- Quinting, B.; Robert, B.; Letellier, C.; Boxus, M.; Kerkhofs, P.; Schynts, F.; Collard, A. Development of a 1-step enzyme-linked immunosorbent assay for the rapid diagnosis of bovine respiratory syncytial virus in postmortem specimens. J. Vet. Diagn. Investig. 2007, 19, 238–243. [Google Scholar] [CrossRef]
- West, K.; Bogdan, J.; Hamel, A.; Nayar, G.; Morley, P.S.; Haines, D.M.; Ellis, J.A. A comparison of diagnostic methods for the detection of bovine respiratory syncytial virus in experimental clinical specimens. Can. J. Vet. Res. 1998, 62, 245–250. [Google Scholar]
- Fox, T.; Geppert, J.; Dinnes, J.; Scandrett, K.; Bigio, J.; Sulis, G.; Hettiarachchi, D.; Mathangasinghe, Y.; Weeratunga, P.; Wickramasinghe, D.; et al. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst. Rev. 2022, 11, CD013652. [Google Scholar] [CrossRef] [PubMed]
- Caldow, G.L.; Edwards, S.; Peters, A.R.; Nixon, P.; Ibata, G.; Sayers, R. Associations between viral infections and respiratory disease in artificially reared calves. Vet. Rec. 1993, 133, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Gershwin, L.J. Immunology of bovine respiratory syncytial virus infection of cattle. Comp. Immunol. Microbiol. Infect. Dis. 2012, 35, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.S.; Gershwin, L.J. Systemic and secretory antibody responses to sequential bovine respiratory syncytial virus infections in vaccinated and nonvaccinated calves. Am. J. Vet. Res. 1990, 51, 1596–1602. [Google Scholar] [CrossRef]
- Sudaryatma, P.E.; Nakamura, K.; Mekata, H.; Sekiguchi, S.; Kubo, M.; Kobayashi, I.; Subangkit, M.; Goto, Y.; Okabayashi, T. Bovine respiratory syncytial virus infection enhances Pasteurella multocida adherence on respiratory epithelial cells. Vet. Microbiol. 2018, 220, 33–38. [Google Scholar] [CrossRef]
- Gershwin, L.J.; Gunther, R.A.; Hornof, W.J.; Larson, R.F. Effect of infection with bovine respiratory syncytial virus on pulmonary clearance of an inhaled antigen in calves. Am. J. Vet. Res. 2008, 69, 416–422. [Google Scholar] [CrossRef]
- Frucchi, A.P.S.; Dall Agnol, A.M.; Bronkhorst, D.E.; Beuttemmuller, E.A.; Alfieri, A.A.; Alfieri, A.F. Bovine Coronavirus Co-infection and Molecular Characterization in Dairy Calves With or Without Clinical Respiratory Disease. Front. Vet. Sci. 2022, 9, 895492. [Google Scholar] [CrossRef]
- Timsit, E.; Le Drean, E.; Maingourd, C.; Belloc, C.; Guatteo, R.; Bareille, N.; Seegers, H.; Douart, A.; Sellal, E.; Assie, S. Detection by real-time RT-PCR of a bovine respiratory syncytial virus vaccine in calves vaccinated intranasally. Vet. Rec. 2009, 165, 230–233. [Google Scholar] [CrossRef]
- Sacco, R.E.; McGill, J.L.; Pillatzki, A.E.; Palmer, M.V.; Ackermann, M.R. Respiratory syncytial virus infection in cattle. Vet. Pathol. 2014, 51, 427–436. [Google Scholar] [CrossRef]
- Sarmiento-Silva, R.E.; Nakamura-Lopez, Y.; Vaughan, G. Epidemiology, molecular epidemiology and evolution of bovine respiratory syncytial virus. Viruses 2012, 4, 3452–3467. [Google Scholar] [CrossRef]
- Freeman, S.; Sutton, A. Identifying publication bias in meta-analyses of continuous outcomes. In Leicester: Cochrane Training; University of Leicester: Leicester, UK, 2020. [Google Scholar]
- Shi, X.; Nie, C.; Shi, S.; Wang, T.; Yang, H.; Zhou, Y.; Song, X. Effect comparison between Egger’s test and Begg’s test in publication bias diagnosis in meta-analyses: Evidence from a pilot survey. Int. J. Res. Stud. Biosci. 2017, 5, 14–20. [Google Scholar]
- Werid, G.M.; Miller, D.; Hemmatzadeh, F.; Messele, Y.E.; Petrovski, K. An overview of the detection of bovine respiratory disease complex pathogens using immunohistochemistry: Emerging trends and opportunities. J. Vet. Diagn. Investig. 2024, 36, 12–23. [Google Scholar] [CrossRef]

| Category | Criteria |
|---|---|
| Type of study (Inclusion) | Both cross-sectional and longitudinal epidemiological studies. |
| Target population (Inclusion) | Studies focused on non-vaccinated cattle, including all production systems (e.g., beef, dairy). |
| Outcome measures (Inclusion) | Studies that report the prevalence, incidence, or distribution of bovine respiratory syncytial virus. |
| Diagnostic method (Inclusion) | Studies that used laboratory-confirmed diagnostic methods for bovine respiratory syncytial virus detection. |
| Sample size (Inclusion) | Studies with a sample size of at least 30 cattle and 2 farms or herds. |
| Publication language (Inclusion) | Studies published in English or those available with English translations. |
| Publication type (Inclusion) | Studies published in peer-reviewed journals. |
| Data integrity (Inclusion) | Studies with original or reanalysing previously published data that have data collection date, sample size, or denominator for each reported prevalence or rate. |
| Exclusion criteria | Non-cattle study populations. Vaccinated cattle. Sample sizes smaller than 30. Non-peer-reviewed publications, including conference proceedings and book chapters. Non-epidemiological studies. Studies lacking key details. |
| Variable Name | Pre-Outlier Removal | Post-Outlier Removal |
|---|---|---|
| Number of studies | 36 * | 33 |
| Number of observations | 18,594 | 15,071 |
| Pooled prevalence | 0.63 (95% CI: 0.53 to 0.72) | 0.64 (95% CI: 0.54 to 0.73) |
| Prediction interval | 0.13 to 0.99 | 0.23 to 0.95 |
| Heterogeneity (I2) | 99.3% (tau2 = 0.07, H = 11.81) | 98.6% (tau2 = 0.04, H = 8.59) |
| Variable Name | Before Outlier Removal | After Outlier Removal |
|---|---|---|
| Number of studies | 15 | 14 * |
| Number of observations | 3926 | 3798 |
| Pooled detection rate | 0.38 (95% CI: 0.19 to 0.60) | 0.34 (95% CI: 0.16 to 0.54) |
| Prediction interval | 0.00 to 0.92 | 0.01 to 0.83 |
| Heterogeneity (I2) | 98.5% (tau2 = 0.07, H = 8.23) | 98.0% (tau2 = 0.05, H = 7.14) |
| Variable Name | Before Outlier Removal | After Outlier Removal |
|---|---|---|
| Number of studies | 24 | 22 * |
| Number of observations | 6618 | 6495 |
| Pooled detection rate | 0.18 (95% CI: 0.09 to 0.29) | 0.13 (95% CI: 0.07 to 0.20) |
| Prediction interval | 0.00 to 0.55 | 0.00 to 0.41 |
| Heterogeneity (I2) | 97.4% (tau2 = 0.04, H = 6.20) | 96.3% (tau2 = 0.02, H = 5.18) |
| Thematic Area | Description | Reference |
|---|---|---|
| Age | Older cattle had higher odds of being seropositive | [14,16,17,18,21,46,76] |
| Herd size | Large herds had higher odds of being BRSV positive. | [14,16,46,76,88,89,132,133,134] |
| Respiratory signs | The presence of respiratory signs was associated with a higher prevalence of BRSV. | [14,21,135,136] |
| History of respiratory disease | Cattle previously exposed to BRSV or recent disease occurrence had higher odds of being seropositive. | [16,21,134] |
| Farm characteristics | Distance between farms or farm density of the area affected the prevalence of BRSV. | [15,19,65,132] |
| Co-infection | BRSV was associated with coinfection to BHV1 *, BPI3V ¥, BVDV π, and BAV3 £ | [17,20,21] |
| Geographic location | Geographic location, including farm altitude, affected BRSV prevalence | [15,76,132] |
| Source of an animal | The purchase of animals or introduction of cattle to a herd from another herd increased the odds of being seropositive. | [14,76] |
| Season of the year | Increased prevalence in winter (colder season), compared to summer season. | [21,76] |
| Milk production | Cattle with higher milk production was associated with higher seroprevalence. | [134] |
| Serotype/genotype | BRSV subgroups A and AB were associated with severe respiratory disease | [128] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Werid, G.M.; Wubshet, A.K.; Araya, T.T.; Miller, D.; Hemmatzadeh, F.; Reichel, M.P.; Petrovski, K. Detection of Bovine Respiratory Syncytial Virus in Cattle: A Systematic Review and Meta-Analysis. Ruminants 2024, 4, 491-514. https://doi.org/10.3390/ruminants4040035
Werid GM, Wubshet AK, Araya TT, Miller D, Hemmatzadeh F, Reichel MP, Petrovski K. Detection of Bovine Respiratory Syncytial Virus in Cattle: A Systematic Review and Meta-Analysis. Ruminants. 2024; 4(4):491-514. https://doi.org/10.3390/ruminants4040035
Chicago/Turabian StyleWerid, Gebremeskel Mamu, Ashenafi Kiros Wubshet, Teshale Teklue Araya, Darren Miller, Farhid Hemmatzadeh, Michael P. Reichel, and Kiro Petrovski. 2024. "Detection of Bovine Respiratory Syncytial Virus in Cattle: A Systematic Review and Meta-Analysis" Ruminants 4, no. 4: 491-514. https://doi.org/10.3390/ruminants4040035
APA StyleWerid, G. M., Wubshet, A. K., Araya, T. T., Miller, D., Hemmatzadeh, F., Reichel, M. P., & Petrovski, K. (2024). Detection of Bovine Respiratory Syncytial Virus in Cattle: A Systematic Review and Meta-Analysis. Ruminants, 4(4), 491-514. https://doi.org/10.3390/ruminants4040035