Abstract
From the perspectives of the thermodynamics of irreversible processes and the theory of complex systems, a characterization of longevity and aging and their relationships with the emergence and evolution of cancer was carried out. It was found that: (1) the rate of entropy production could be used as an index of the robustness, plasticity, and aggressiveness of cancer, as well as a measure of biological age; (2) the aging process, as well as the evolution of cancer, goes through what we call a “biological phase transition”; (3) the process of metastasis, which occurs during the epithelial–mesenchymal transition (EMT), appears to be a phase transition that is far from thermodynamic equilibrium and exhibits Shilnikov chaos-like dynamic behavior, which guarantees the robustness of the process and, in turn, its unpredictability; (4) as the ferroptosis process progresses, the complexity of the dynamics that are associated with the emergence and evolution of cancer decreases. The theoretical framework that was developed in this study could contribute to a better understanding of the biophysical and chemical phenomena of longevity and aging and their relationships with cancer.
1. Introduction
Longevity and aging remain two of the most intriguing and captivating topics within human knowledge. Despite the achievements of biomedical sciences, the mechanisms of the aging processes remain unknown and generate great controversy [1]. Aging is manifested through so-called degenerative diseases, such as cardiovascular disorders, cancer, atherosclerosis, diabetes, and senile Alzheimer’s dementia (SDAT), among others [2,3,4]. The incidence rates of all of these degenerative diseases increase exponentially with age.
In 1990, Medvedev [5] compiled around 300 theories on aging, which has continued to grow to this day [6]. There is currently a consensus that the aging process is multifactorial and complex, which is the main difficulty in developing a unified theory.
In general, aging theories can be grouped into three large categories: those that are related to the actions of reactive oxygen species (ROS); those that establish a link between metabolic rate and longevity; and those that try to explain aging from a thermodynamic point of view.
According to Harman’s theory [3,7], the main factor that induces aging processes is the deleterious action of free radicals (ROS) on biopolymers. Free radicals can react with nucleic acids, DNA, proteins, and polysaccharides, which causes structural damage that can lead to their loss of functionality and the appearance of unwanted species. This results in the progressive loss of physiological integrity and, in turn, impaired function and increased vulnerability [8].
According to Sohal’s theory [9], the rate of aging and the metabolic rate of organisms are inversely correlated. The effects of metabolic rate on aging may be mediated by ROS. Antioxidant defenses tend to decline during aging, while ROS-induced damage increases with age.
The third group of theories on aging [10,11,12,13,14], which is no less important than the previous groups, has guided studies on the aging process from a thermodynamic point of view. These systems analysis approaches can offer an integrated picture of phenomena that are as complex and multifaceted as aging.
All of these approaches are closely related and consider various facts, including how aging occurs in people with degenerative or complex diseases. Within the group of so-called degenerative diseases, cancer is the second most common cause of death worldwide, according to the WHO [15], and it is estimated that the number of new cases will increase over the coming years.
As previously stated [16], cancer can be considered as a complex network of cells that have lost their specialization and growth control and that appear during what we call a “biological phase transition”, which leads to spatial and temporal self-organization that is out of thermodynamic equilibrium since it exhibits high robustness and adaptability [17].
The lethal stage of cancer manifests itself during metastasis. The appearance of the metastatic process occurs abruptly by recalling a type of “first-order” phase transition [18]. The chances of survival once metastasis occurs are lower compared to the chances of survival during previous stages due to the greater robustness and higher hierarchical level of metastasis, which competes with the different levels of the hierarchical and functional organization of the organism. Therefore, a tumor is considered cancer according to its ability to metastasize.
There is sufficient evidence [19,20,21] concerning the complexity of cancer but despite the achievements of molecular biology and genomics, the mechanisms of tumor cell growth and the nature of their robustness are still unknown.
Thermodynamic approaches to studying the aging process in biological systems could allow us to see this problem as a whole when considering that the “whole” is more than the sum of its parts. Concerning longevity and aging and their relationships with degenerative diseases, particularly cancer, two basic questions arise: when does the aging process begin, and how does the appearance of degenerative diseases, particularly cancer, manifest as part of the aging process?
Our objective was to explore the general landscape of longevity and aging and their relationships with the appearance and evolution of cancer from the point of view of the thermodynamics of irreversible processes in nonlinear systems, i.e., the thermodynamics of complex processes [22,23]. The work is structured as follows. Section 2 offers a brief introduction to the thermodynamics of complex processes, such as nonlinear systems. Section 3 is dedicated to longevity and aging and their relationships to the emergence and evolution of cancer. The process of ferroptosis and cancer is addressed in Section 4. Finally, some concluding remarks are presented in the final section.
2. Overview of the Thermodynamics of Complex Processes
This section presents an overview of the thermodynamics of irreversible processes in nonlinear dynamic systems and their relationship with complex systems [22,23]. On the one hand, it has been shown that the rate of entropy production constitutes per se a natural Lyapunov function for nonlinear systems [24], which has allowed us to generalize an extremal criterion for natural systems at the macroscopic scale.
On the other hand, an extension has been made to the study of biophysical–chemical systems that have shown that the rate of entropy production represents a physical quantity that can measure the robustness, plasticity, and aggressiveness of cancer [25].
Already in the formulation of the second law within the formalism of classical thermodynamics, it is possible to establish an evolution criterion for the macroscopic processes of natural systems, that is, the entropy production is positive and zero at equilibrium , which represents the general criterion of irreversibility [26]. However, in the classical formulation of the Second Law, the main limitation is that it does not contain time within its formal structure, which imposes a fundamental restriction on the classical formalism of thermodynamics.
To overcome this limitation, the formalism of the so-called thermodynamics of irreversible processes was developed. In the seminal works of Onsager [27,28], de Groot-Mazur [29], and Prigogine [30], the foundations of the thermodynamics of irreversible processes were established. Formally, it is divided into two: the linear region and the non-linear region. Although the formalism of the linear region is solidly established, both in its theoretical foundation and its experimental verification, the nonlinear region is still in its infancy [31].
The formal structure of the thermodynamics of linear irreversible processes is based on the existence of linear relationships between forces and generalized flows [26]. When there is no such phenomenological relationship, then we speak of the non-linear region.
It is important to highlight the fact that the linearity of dynamic systems should not be confused with the existence of linear dependence between flows and generalized forces. For example, in a phenomenon such as heat conduction, typically nonlinear, the phenomenological equation that governs it is the well-known Fourier equation [26], that is, the formalism of linear irreversible processes already deals with phenomena of order natural non-linear nature, consequently, can give rise to complex processes.
The seminal work of Prigogine et al. [32], on the so-called “dissipative structures”, established an appropriate theoretical and practical framework for the thermodynamic approach to biological systems. It constitutes the foundational basis of the so-called Systems Biology [33].
The complexity exhibited by dissipative structures, as a dynamical system, is due to the spatial and/or temporal self-organization [34] that occurs far from thermodynamic equilibrium resulting from a bifurcation [35]. The bifurcations in dynamical systems are the analog of [36] phase transitions in the vicinity of equilibrium and are the consequence of microscopic fluctuations that grow and amplify to the macroscopic level, thus representing the fundamental mechanism of origin of self-organization far from equilibrium [32] and, therefore, of complexity at the macroscopic level [37]. In this way, thermodynamics constitutes the theoretical foundation and, in turn, an essential tool in the study of complexity.
The generalized expression of the Second Law establishes the starting point of thermodynamic formalism [26], thus we have
where is the entropy change of the system per unit time, is the rate of entropy exchange with the surroundings or flow, and is the rate of entropy production due to irreversible processes occurring within the system. Equation (1) can be rewritten as
Thus, the evolution criterion can be generalized as which constitutes one of the postulates on which the formalism of irreversible processes rests and the essence of the Second Law.
To generalize the evolution criterion and formulate an extremal principle, Prigogine demonstrated how the production of entropy per unit of time is a physical magnitude that constitutes a natural Lyapunov function [38], known as the “General Evolution Criterion” [39], as long as there is a linear dependence between the flows and the generalized forces, which is restricted to the linear region of irreversible processes.
At the end of the 19th century, Lyapunov, in parallel with Poincaré, mathematically developed a method known as the Lyapunov function, which allows the evolution and global stability of the dynamics of a system to be known [40].
Let be a fixed point, steady state, of a flow , a function is called Lyapunov function of if for some neighborhood of the following conditions are met:
- for everything in and ;
- The Eulerian derivative, for all in .
In this way it can be affirmed that for all , is globally and asymptotically stable, that is, the system evolves towards a minimum of the function , which constitutes per se an extremal principle.
In previous works [24], we showed, on the one hand, for nonlinear systems, namely chemical reactions, how the rate of entropy production is a Lyapunov function depending on the control parameters, that is, those parameters that determine the quality of the dynamics of a dynamical system. This approach makes it possible to generalize the evolution criterion and thus formulate an extremum criterion, such as
where is the vector of control parameters (analogous to the critical parameters in phase transitions, which determine the quality of the system dynamics).
On the other hand, as an extension to biological systems, we show how the rate of entropy production can be used as an index of robustness, plasticity, and aggressiveness of cancer, and in turn, as a measure of biological age [16,41,42].
Thus, we find that by Ansatz (a term often used in physics-mathematics, which is an estimated solution to an initial equation that describes a physical or mathematical problem), we established a functional dependence of the entropy per unit of time with the fractal dimension of the tumor and its growth rate [25] as:
Formula (4) includes two properties observed in tumors: their growth rate , a measure of aggressiveness, and another, their malignancy related to their morphological characteristics, that is, the fractal dimension ; the ability of the tumor to invade and infiltrate healthy tissue.
According to Prigogine [26] for living organisms the rate of entropy production of a living organism can be measured by its metabolism, through the rate of evolution of energy in the form of heat as
According to Zotin [43], the rate of evolution of energy in the form of heat can be determined in biological systems measured the rate of oxygen consumption , oxidative phosphorylation (OxPhos), through the basal metabolic rate (BMR), and the glycolytic rate , as
For healthy humans, under aerobic conditions, the glycolytic rate term is negligible except in cancer patients where the glycolysis process is predominant [44]. In this case, the rate of entropy production can be determined from Equation (6) as:
The formula from Equation (6) is useful for evaluating the rate of entropy production of living organisms through calorimetric measurements.
3. Longevity and Aging and Their Relationship with the Emergence and Evolution of Cancer
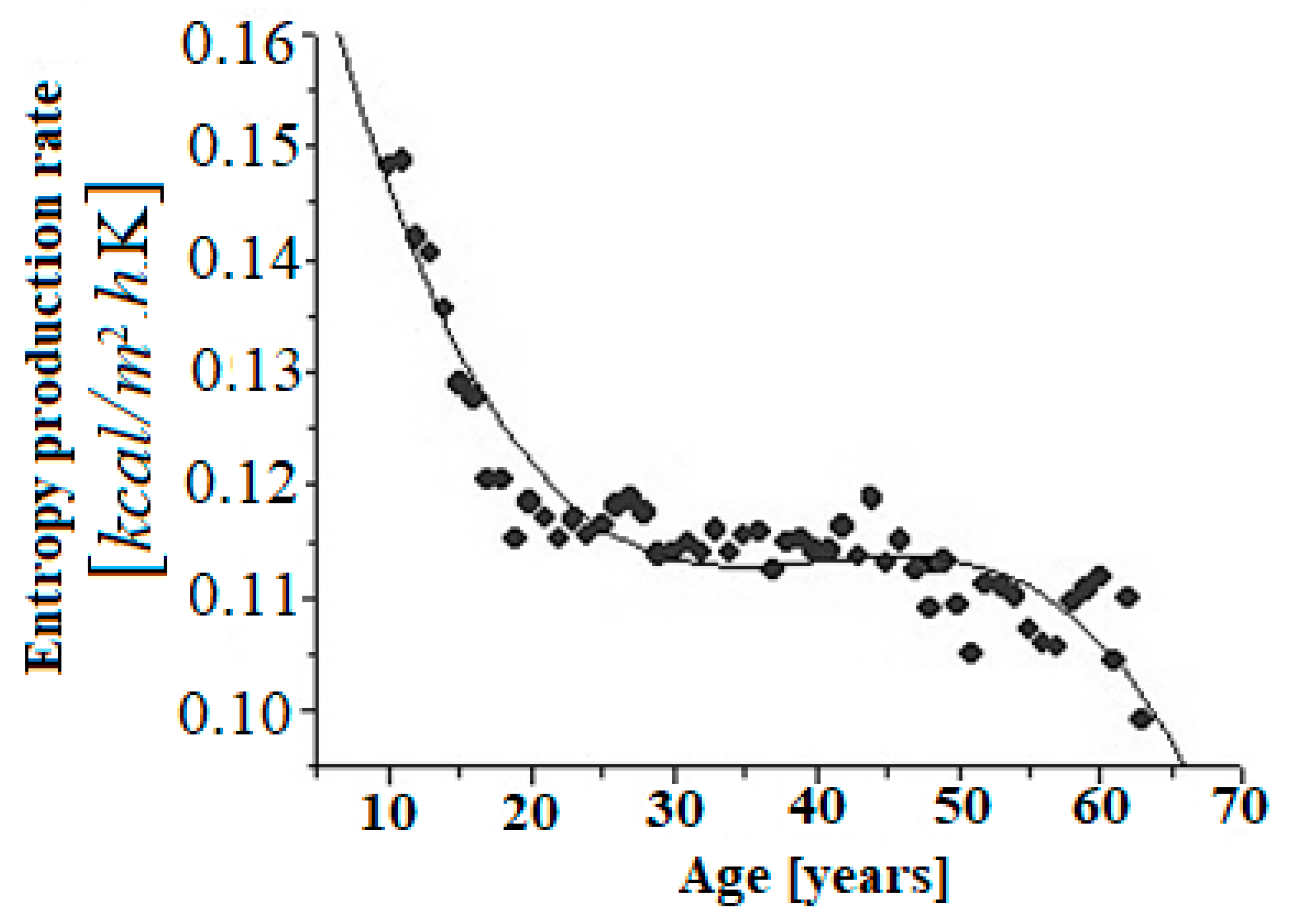
From the data reported by Boothby et al. [45], the rate of entropy production, Equation (6), was evaluated for healthy humans of different ages, as shown in Figure 1.
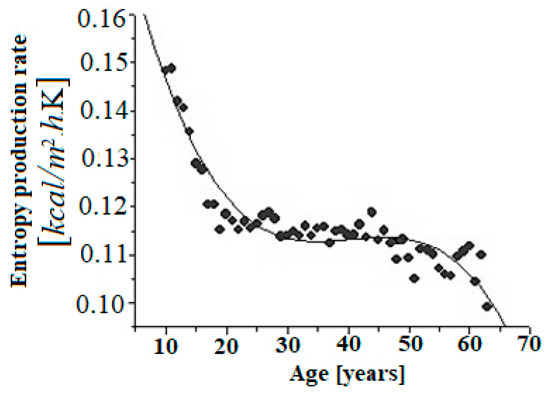
Figure 1.
Dependence of the rate of entropy production per unit surface area of the individual healthy humans under basal conditions. The best fitting curve was determined to be cubic as a function of age with the parameters: , , R-square = 0.88505, SD = 0.00349, N = 54, p < 0.0001.
From the data in Figure 1, a polynomial fit of the following type is found:
where represents the age of the human. The regression, Equation (8), resembles the van der Waals [46] equation of state, which is useful for describing first-order phase transitions. Thus, a parallel can be drawn between the van der Waals equation of state and Equation (8). On the one hand, it is observed (see Figure 1) how the aging process is started around the age of 20, which coincides with what is already that accepted in the literature [47], that is, there appears a phase transition resembling a “first order” phase transition.
Obviously, these calculations are approximations. On one hand, it is observed how the trend from the age of 20 clearly shows that the aging processes begin to be activated. On the other hand, the entropy production per unit of time decreases, which, as we have shown in previous works, implies a decrease in complexity [25], that is, the system is less robust, therefore more sensitive to internal and external perturbations.
The experimental facts indicate this trend, for example, the respiratory capacity of the human being is optimal and begins to decline, interestingly, from the age of twenty [48]. In addition, it has been found that the complexity of the heart rhythm changes with the age of the healthy individual, moving from more complex electrocardiograms to simpler ones [49] and finally to periodic ones. It has also been seen in patients with heart disease, where a certain periodicity appears [50].
This evidence gives us a plausible reason to believe that the aging process goes through what could be called “biological phase transitions” [41].
On the other hand, it is observed that, with increasing age, the rate of entropy production decreases (see Figure 1), that is, the complexity decreases [51], an aspect that has been pointed out by other authors [52].
Thus, the production of entropy per unit of time meets the necessary and sufficient conditions to be a Lyapunov [40,42] function. If we take the age of the subjects as a control parameter (Equation (3)), we find that the Eulerian derivative must satisfy:
Naturally, is related to chronological age, therefore, we have , then it is true that , which can be seen from Equation (8) and as shown in Figure 1. This allows us to state that the rate of entropy production is a Lyapunov function, that is, it shows the directional character of the aging process. Furthermore, it would alternatively be a quantitative indicator of the biological age of humans [47].
We thus come to postulate that the initiation of aging processes occurs naturally for biosystems. This conjecture is in agreement with the theory given by Cutler [53] where each species of mammal is characterized by a particular lifespan. Physiological and psychological changes that occur with aging have been shown to indicate the biological age of the individual [54].
Concerning the second aspect: how do we explain the appearance of degenerative diseases? According to Harman’s theory [3,7] of free radicals, these species, ROS, are generated by chain reactions and are present in the appearance of degenerative diseases such as cancer, atherosclerosis, etc.
In the theory of Sohal [9], the rate of aging and the metabolic rate of organisms are inversely correlated. The effects of metabolic rate on aging may be mediated by ROS. Antioxidant defenses tend to decline during aging, while ROS-induced damage appears to increase with age [55]. Here, precisely the link between the presented thermodynamic framework and the theories of Harman and Sohal [3,7,9] is established.
Particularly in cancer, the significant increase in the rate of glycolysis observed in tumors is well known [44,56]. Fenninger and Mider [57] have observed an elevation in basal metabolic rate in some cancer patients. Altered energy metabolism is proving to be as widespread in cancer cells as many of the other cancer-associated traits that have been accepted as hallmarks of cancer [44], few oncologists or cancer researchers understand the full scope of Warburg’s work [58] despite its great importance. Hence, the regulation of metabolism would be relevant to the senescence process [59], which in turn is key to improving and identifying new cancer therapies in the future.
Consequently, for humans with cancer, it is necessary to use Equation (6) to evaluate the entropy production rate. For this, we use the data reported by Holroyde et al. [60] for patients with metastatic carcinoma. The results showed how the rate of entropy production for cancer patients compared to healthy individuals is higher for the same age range [41]. On the one hand, this may be due to the contribution of the glycolysis process in cancer patients. On the other hand, it can be interpreted as a grade of the robustness of cancer [25,61,62].
As we have mentioned, a common property of cancer is its alteration of glucose metabolism, known as the Warburg effect [63]. It has been suggested that the presence of aerobic glycolysis in highly invasive cancerous tumors is related to their aggressiveness, indicating that the glycolytic phenotype confers a proliferative advantage during the somatic evolution of cancer, in addition to being a crucial component in its malignancy [44].
Despite the limited information available on this aspect, we established as a hypothesis [64] that cancer glycolysis is a self-organized process far from thermodynamic equilibrium, for which sustained oscillations give it high robustness and complexity [23,32,51].
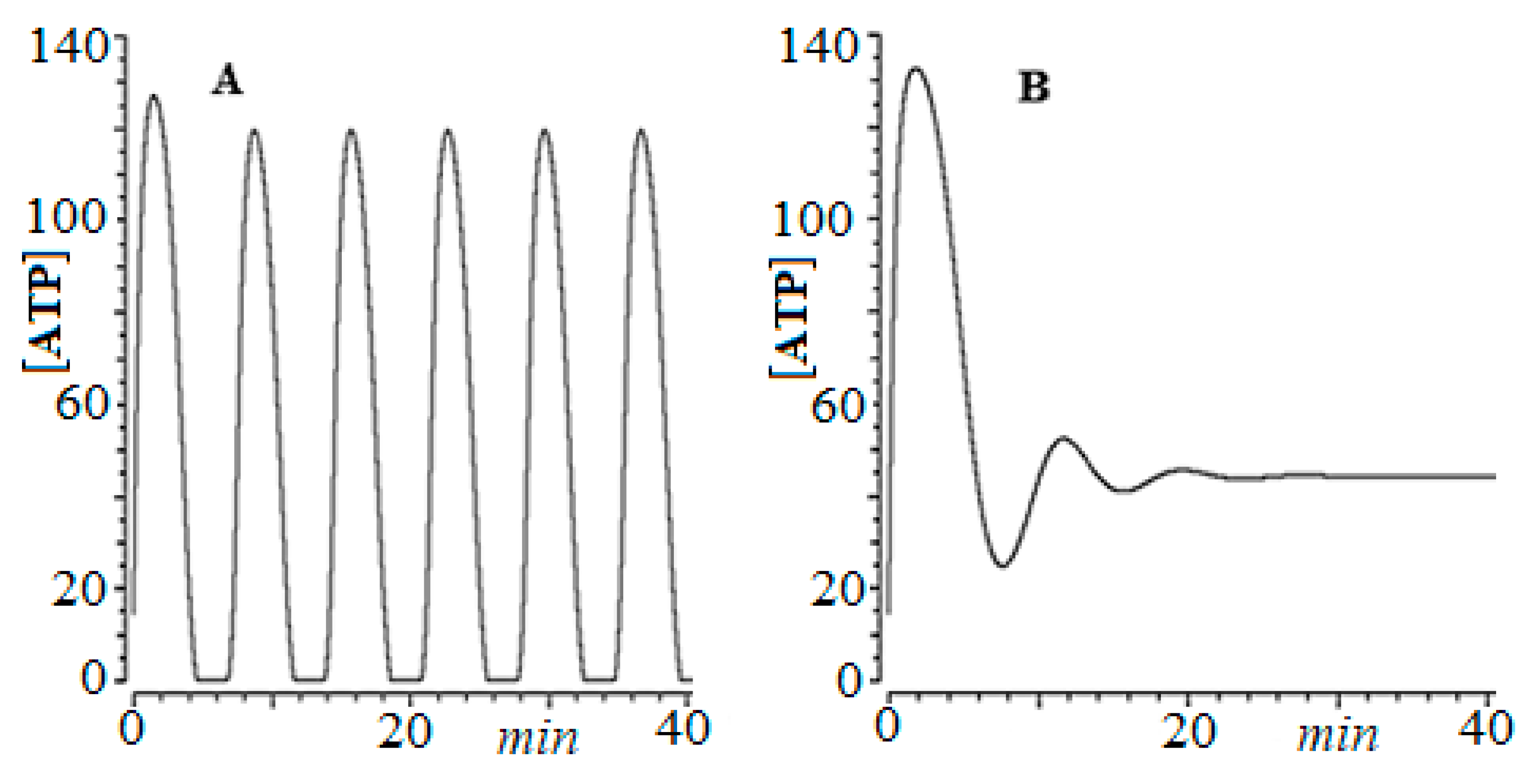
For this, we developed an empirical model of ordinary differential equations, based on HeLa cervical cancer cells [64], which showed that for low glucose concentrations ([Glu]0 = 2.5 mM), as observed in Figure 2A, the system exhibits periodic oscillations, limiting cycle type.
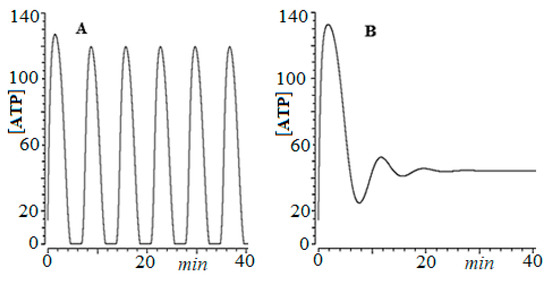
Figure 2.
Time series of ATP concentration vs. time, for HeLa cervical cancer cells. (A) [Glu]0 = 2.5 mM, (B) [Glu]0 = 25.0 mM.
In a later work, Takashi et al. [65] experimentally showed this fact. In other words, the oscillations give the glycolytic process in cancer greater complexity, which ensures its robustness against different therapies.
In general, cancer can be seen as a “failure” of development, involving a network of interacting cells and their microenvironment, losing control over proliferation and cell fate specification [66]. This network of malignant cells, as we mentioned at the beginning, can be considered as a nonlinear dynamic system, self-organized in time and space, far from thermodynamic equilibrium, which presents a high complexity, robustness, and adaptability [17].
Such a process is carried out mainly through the deregulation of critical events that occur during biological transitions. Since phenotypic differentiation and cancer transformation are self-organizing processes, governed by thermodynamics far from equilibrium, fluctuations in control parameters at the bifurcation point are important. Even subtle changes in some critical values can impair the self-organization process, leading to unexpected different states, exhibiting variable robustness and adaptability within the attractive landscape [67].
It is generally accepted that cancer evolves along with three basic steps [68]: avascular, vascular, and metastatic, all emerging after biological phase transitions [16]. The metastatic process consists of sequential, interconnected, and selective steps [43], and many of these are due to an obligatory transition from an epithelial to a mesenchymal phenotype [69].
An epithelial–mesenchymal transition (EMT) is a biological process that allows a polarized epithelial cell, which normally interacts with the basal surface of the membrane, to undergo multiple biochemical changes that allow it to assume a mesenchymal cell phenotype, including an improved migratory capacity, invasiveness, and increased resistance to apoptosis [70].
The current paradigm suggests that EMT drives metastasis by producing mesenchymal cells that escape the primary tumor and migrate to distant sites, thereby reverting to an epithelial state through mesenchymal-epithelial transition (MET). Furthermore, depending on the relationships between cells and their new microenvironment, metastatic foci may eventually spread to other organs and tissues, or enter a state of latency [71]. The EMT process has been observed in multiple epithelial tumors, such as prostate, breast, and colorectal cancer [72] among others.
Although the role of EMT is well documented in the literature [73], there are few reports dealing with the dynamics of EMT [73,74]. Indeed, most dynamical and statistical models of EMT focus on the genetic and biophysical changes associated with EMT [75].
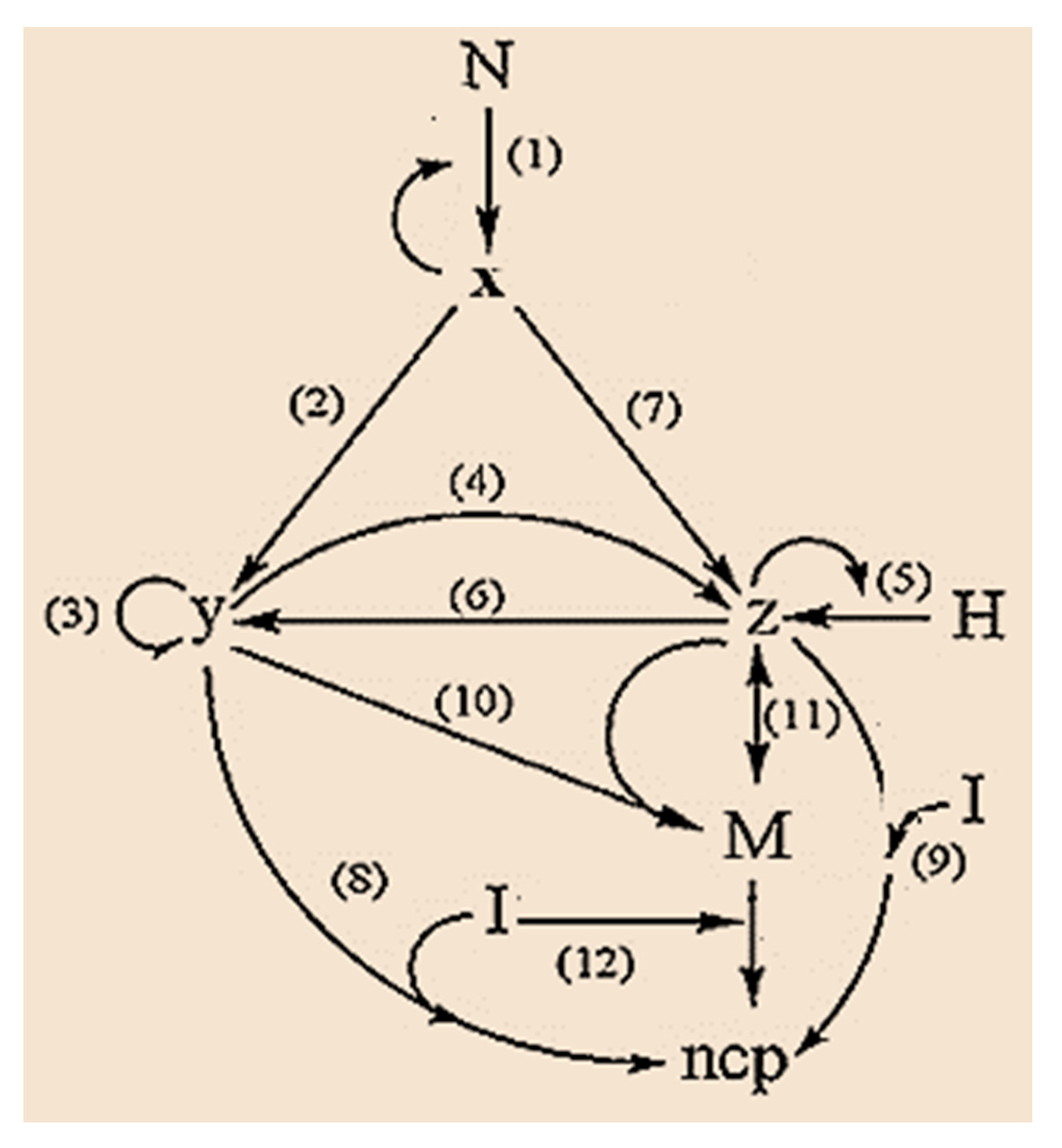
In this sense, we develop an empirical model [76] which rescues the main details of the metastasis process and involves the epithelial–mesenchymal transition process (EMT), which is shown schematically in Figure 3.
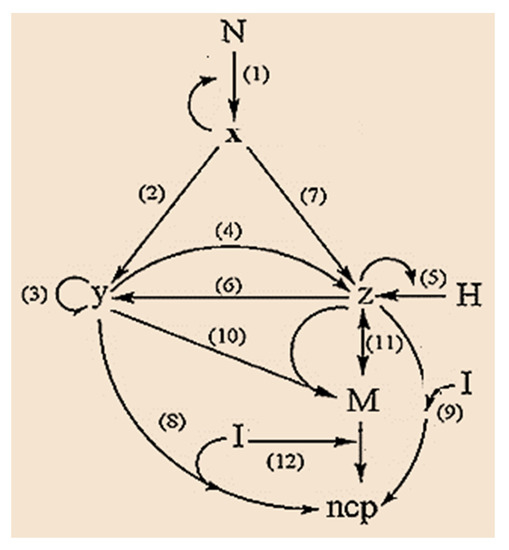
Figure 3.
Graph of the process of epithelial–mesenchymal transition, EMT during metastasis.
In the model, Figure 3, N represents the population of normal cells exposed to the pro-cancer stimulus; H is the population of host cells in the environment surrounding the tumor [77], which exclusively includes epithelial cells; I is the population of immune cells (T lymphocytes (CTL) and natural killer (NK) cells) [78], M is the population of mesenchymal cells. N and H are considered constant (because these cell groups are much more numerous than cancer cells and for practical purposes, their number does not change) and we postulate that the population of immune cells I is the control parameter (since the population of cells immune systems may fluctuate or may be boosted through therapy). The variables: x, y, and z represent the population of epithelial tumor cells in the avascular, vascular and metastatic states, respectively. Finally, ncp represents a noncancerous product due to the action of immune cells.
Steps 1, 3 and 2, 4, and 6 are related to the process of mitosis and apoptosis of proliferating tumor cells, respectively; steps 5 and 7 correspond to the action of the host H [77]; steps 8, 9, and 12 show the action of immune cells I. Finally, steps 10 and 11 are related to EMT. Step 10 represents an intermediate preparatory step of the epithelial cell before its transition to the mesenchymal phenotype [79].
From the model shown in the graph (see Figure 3), using the mathematical methods of chemical kinetics to reduce the network to a system of ordinary differential equations, ODE. The ODE systems, Equation (10) describes the avascular, vascular, and meta-static phases, as well as EMT in tumor dynamics [76] as:
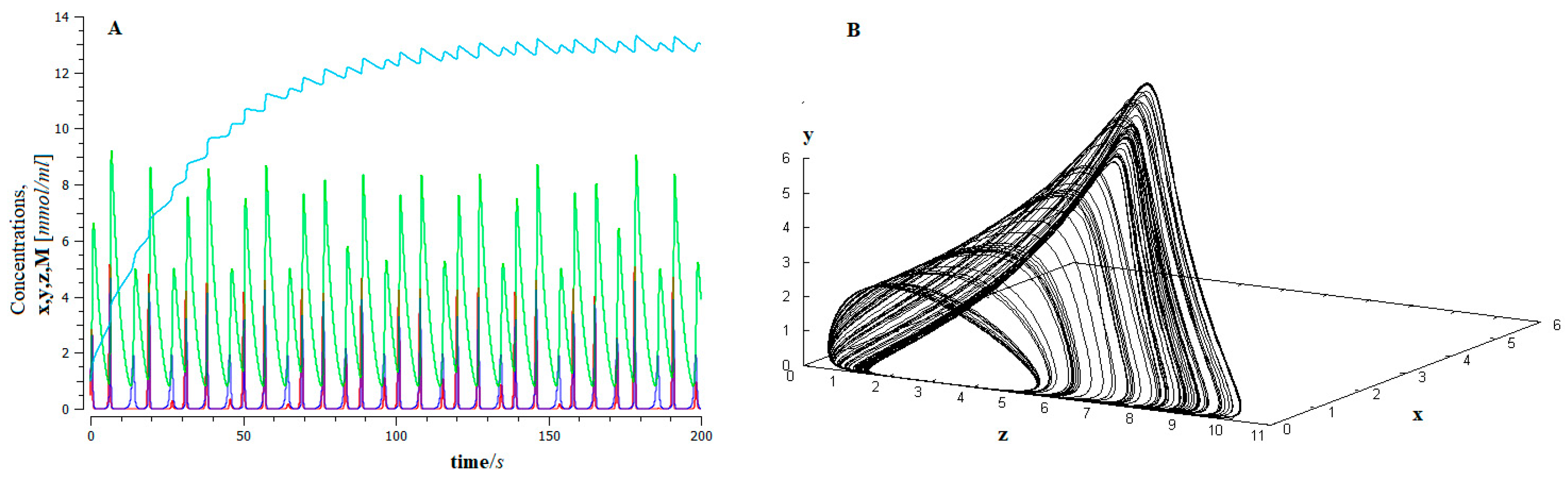
The model shows (see Figure 4) that, for a critical value of the control parameter I, tumor cells exhibit “apparently random behavior” (remnant of Shilnikov-like chaos [80,81] when challenged by immune system activation). That hypothesis has been vindicated by a recent study, demonstrating that a mesenchymal phenotype correlates with immune evasion through reduced immunoproteasome expression, the underlying mechanism of immunoproteasome regulation involving STAT3, STAT1, and miR-200 s [82].
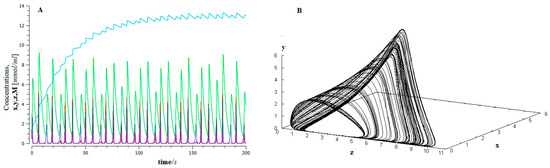
Figure 4.
(A) The time series of the dynamics of the EMT for the proposed model (Figure 3), for control parameter values I = 0.4, x (red) y (dark blue), and z (green), represent the population of epithelial tumor cells in the avascular, vascular and metastatic states, and M is the population of mesenchymal cells (light blue); (B) Chaotic attractor.
This behavior has important biological implications. On the one hand, the high sensitivity of the system to the initial conditions makes long-term predictions about the evolution of the EMT unfeasible, that is, the final forecasts are unlikely (bad forecast).
Furthermore, the system shows a high degree of robustness [83,84]. This implies that cancer cells are resistant to drug treatment, leading to a low response rate, especially when the cancer is in a metastatic state [85].
Additionally, the information created during the evolutionary process, cannot be destroyed [86], which is clinically manifested in the recurrence (relapse) of cancer after a time that has been “eliminated” [87,88]. Mechanisms of drug resistance developed during the multiple transitions affecting cancer development are still maintained even after successful primary chemotherapy intervention and maintain a high rate of clinical recurrence [87,88].
We see how the EMT transition during metastasis appears as a “first order phase transitions” type, for a range of discrete values of the order parameter and a continuous spectrum of transitions from one phenotype to another can be recognized both from the model and from the experimental data. Evaluating EMT as a process characterized by criticality and threshold values can help find treatment strategies aimed at modifying the overall process by targeting singularities. This approach would likely focus on reversing the cancer phenotype rather than simply killing cancer cells, a goal that is hardly achieved with current chemotherapy regimens [89].
4. Ferroptosis and Cancer
Ferroptosis is a process of iron-dependent programmed cell death, characterized by the accumulation of reactive oxygen species (ROS), such as lipid peroxides, radical superoxide, hydroxyl radical, hydrogen peroxide, etc., and it is genetically and biochemically distinct from other forms of regulated cell death, such as apoptosis [90].
Most cancer therapies, such as chemotherapy and radiotherapy, show little effectiveness, especially in the stages of vascular growth and metastasis. It is well known that only 60% of different types of cancer can be cured with conventional therapies, and they are also accompanied by undesirable side effects [91]. On the other hand, it is known that in many types of cancer the process of apoptosis, programmed cell death, is repressed [92].
Three essential characteristics define the ferroptosis process [93], namely: 1. the loss of the ability to repair damage caused by lipid peroxides by glutathione peroxidase GPX4; 2. the availability of iron redox-active; and 3. the oxidation of phospholipids containing polyunsaturated fatty acids (PUFA).
An important issue is whether any type of lipid peroxidation is classified as ferroptosis or whether only certain lethal types of lipid peroxidation should be designated as ferroptosis [94]. Indeed, how lipid peroxidation leads to ferroptosis remains an unsolved mystery [94].
On the one hand, there is evidence that ferroptosis processes are associated with the etiopathogenesis of various degenerative diseases such as cardiovascular disorders, cancer, atherosclerosis, diabetes, and Alzheimer’s dementia (SDAT), among others [2,95], which lead to progressive loss of physiological integrity, leading to functional impairment and increased vulnerability and death [2,3,4].
On the other hand, in recent years, numerous studies have shown the efficacy of cancer elimination by inducing ferroptosis, which is mainly achieved by raising intracellular ROS levels and inactivating the action of GPX4 [96,97,98].
To the best of our knowledge, only a few ferroptosis-related models have been reported [99,100,101]. Kagan et al. [99] developed a continuous model for ferroptosis with a biochemical cascade-based approach. While the model provides an excellent synthesis of the specific processes involved, it excludes the contributions of lipid peroxidation processes involved in ferroptosis.
On the other hand, Agmón et al. [100] performed molecular dynamics simulations of membranes with compositions relevant to ferroptotic sensitivity and showed how the biophysical properties of membranes are altered under competent ferroptotic lipid compositions.
More recently, Konstorum [101] has developed a multistate discrete modeling approach to emphasize the qualitative properties of signaling cascades relevant to ferroptosis. The discrete modeling approach allows the relative importance of different ferroptosis promoters to be explored using a wider range of data than would be available for a detailed kinetic model of the system.
To the authors’ knowledge, there is no model that connects the lipid peroxidation processes involved in ferroptosis with the growth of cancer cells.
Despite there being sufficient evidence and consensus in the literature related to ferroptosis-mediated anticancer effects [102,103], on the one hand, the mechanisms underlying each step of this complex process remain unclear [94,100]. On the other hand, there is no model that connects the lipid peroxidation processes involved in ferroptosis with the growth of cancer cells.
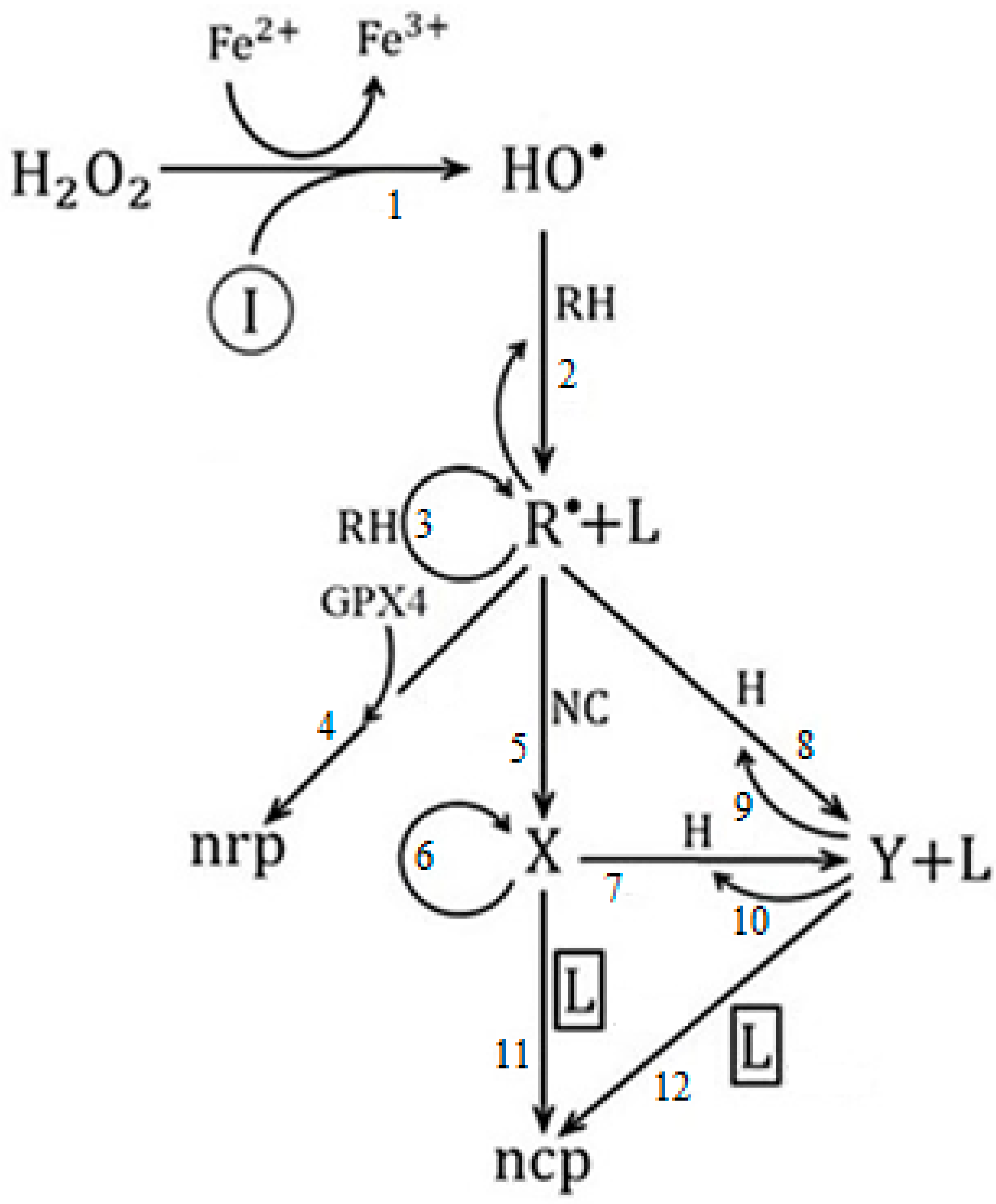
In this sense, we proposed a heuristic model [104] that connects lipid peroxidation, the evolution of cancer cells in the avascular and vascular phases [68], and the ferroptosis process. The model contains three species of populations: -lipid peroxides, -avascular tumor cells, and -vascular tumor cells.
Figure 5 shows the graph structure of the proposed cancer ferroptosis model [104], where a connection is established between lipid peroxidation, the evolution of cancer in the avascular and vascular phases, respectively, and the ferroptosis processes.
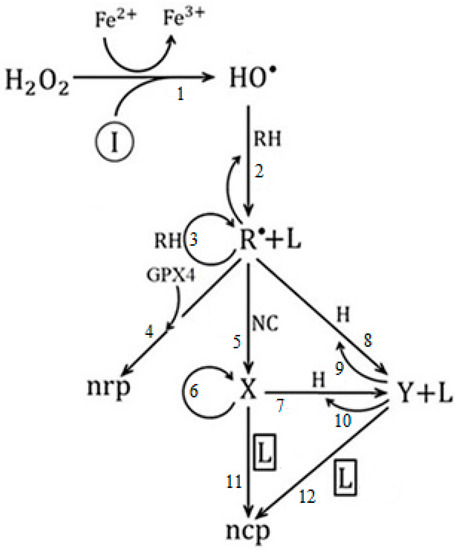
Figure 5.
Graph of the model of ferroptosis of cancer in avascular and vascular growth.
In the model (Figure 5), symbolizes ferroptosis-inducing agents, such as iron-based nanomaterials, e.g., ferumoxytol, amorphous iron nanoparticles, induced ionizing radiation, etc. [105,106]; is hydrogen peroxide; are the hydroxyl radicals; represents polyunsaturated fatty acids (PUFAs); is the population of normal cells exposed to pro-cancer stimuli; it is the population of host cells in the environment surrounding the tumor [77], composed exclusively of epithelial cells; is glutathione peroxidase 4; represents the population of oxidized PUFA fragments [100].
In this sense, it was established as a conjecture that the population of oxidized PUFA fragments are those that favor the ferroptosis process of cancer cells [104]. For this reason, we take as control parameter (CP), that is, whose value changes the quality of the system dynamics.
The variable species represent: -lipid peroxides, -avascular tumor cells, and -vascular tumor cells, respectively. Finally, nrp and ncp represent non-radical products and non-cancerous products, respectively.
Step 1 is associated with the Fenton reaction [107], step 2 is related to the formation of lipid peroxides [108], step 3 is the propagation of lipid peroxidation chain reactions [109], and step 4 is the main cellular mechanism of protection against reactive oxygen species (ROS), which is mediated by the action of glutathione peroxidase 4 [110]. Steps 5, 6 and 7, 10 are related to the process of mitosis and apoptosis of proliferating tumor cells, respectively; steps 8 and 9 correspond to the action of the host cells [77]; finally, steps 11 and 12 are related to ferroptosis and vascular avascular tumor growth, respectively.
In agreement with that model (Figure 5) and the law of mass action governing chemical kinetics, a system of ordinary differential equations ODEs (11) was obtained [104], which describes the ferroptosis avascular and vascular tumor growth:
where,
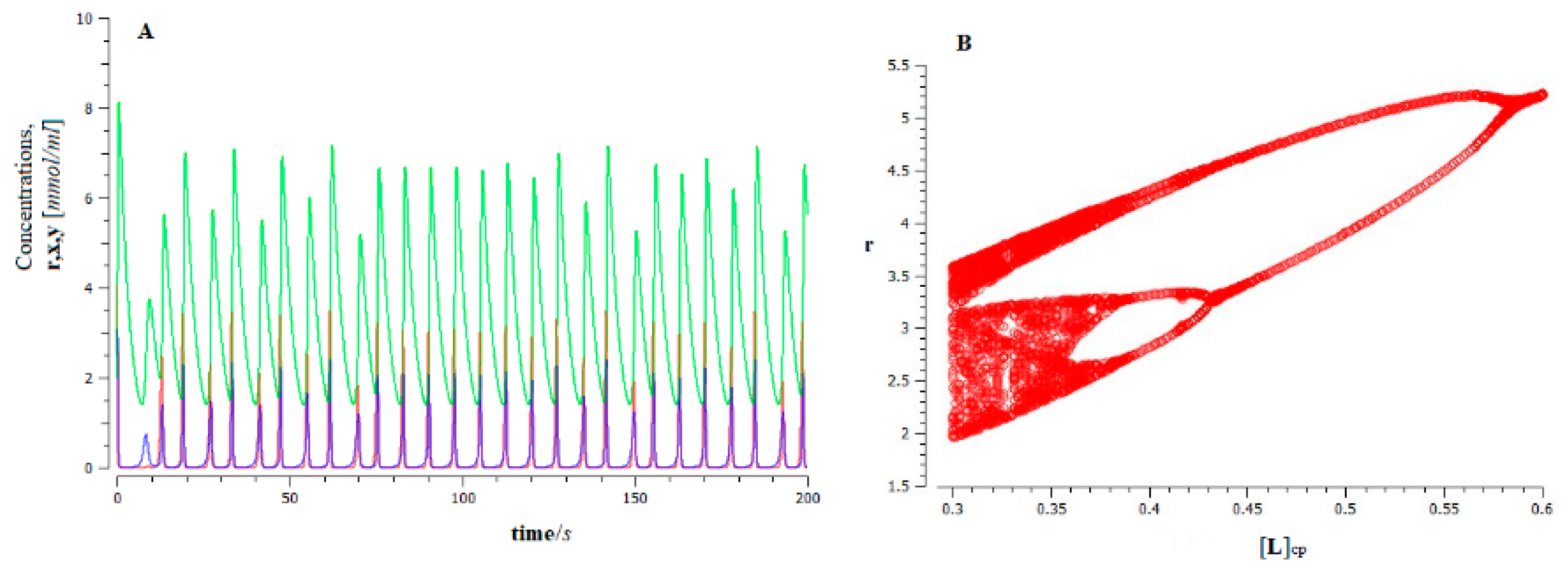
In Figure 6A, the dynamic behavior of the proposed model is shown. It is observed that for low values of the control parameter , tumor cells exhibit an “apparently random behavior” (remnant of Shilnikov-like chaos) [81], with a predominance of the population of vascular tumor cells (green).
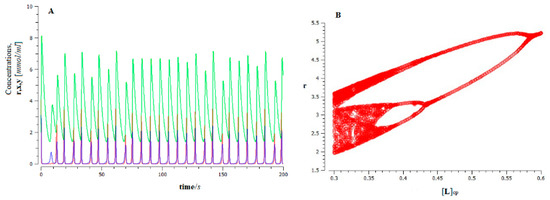
Figure 6.
Dynamics of ferroptosis tumor growth for the proposed model (Figure 5), for the value control parameter; (A) time series r (red), x (dark blue), and y (green), represent the population of lipid peroxides, avascular tumor cells, and vascular tumor cells, respectively; (B) bifurcation diagram obtained from rmax showing the halving scenario (i.e., inverse Feigenbaum) that occurs as inactivation of the population of oxidized PUFA fragments by tumor cells decreases.
This behavior has important biological implications. On the one hand, the high sensitivity of the system to the initial conditions makes long-term predictions unfeasible (bad forecast). In addition, the dynamic system of cancer evolution presents a high degree of complexity [83,84]. This implies that cancer cells are resistant to drug treatment, leading to a low response rate [85].
As can be seen (see Figure 6B), the increase in the population of the oxidized PUFA fragments (control parameter) produces an inverse Feigenbaum scenario (a cascade of saddle-foci Shilnikov’s bifurcations), which leads to the stabilization of the dynamics and a decrease in the complexity of the system.
In fact, at the critical point , a supercritical Andronov–Hopf bifurcation occurs [30], the dynamic analog of a first-order phase transition, leading to a steady state. In this way, we see that, according to our conjecture, there is a fine regulation of the ferroptosis process of cancer cells through the fragments of oxidized PUFAs .
5. Concluding Remarks
In summary, it has been shown how the non-equilibrium thermodynamics formalism and complex systems theory offers an appropriate theoretical framework for the characterization of longevity, aging, and the emergence and evolution of cancer [14,16,17,41,61,62,67,76,111,112,113,114,115]. It was found that:
- The process of metastasis occurs through epithelial–mesenchymal transition (EMT), appears as a phase transition away from thermodynamic equilibrium, and exhibits Shilnikov chaos-like dynamic behavior. This dynamic guarantees the robustness of the process and, in turn, its unpredictability.
- The aging process, as well as the evolution of cancer, goes through what we have called a “biological phase transition”.
- The rate of entropy production can be used as an index of robustness, plasticity, and aggressiveness of cancer. It can also be used as a measure of biological age.
- It was shown that the extent to which the ferroptosis process is strengthened decreases the complexity in the dynamics associated with the emergency and evolution of cancer.
We hope that the theoretical framework developed will contribute to a better understanding of the biophysical-chemical phenomena of longevity and aging and their relationship with cancer. Additionally, the results presented here constitute the theoretical framework herewith for finding optimal pathways for future treatments [116,117,118,119,120].
Author Contributions
Both authors contributed equally to the work. All authors have read and agreed to the published version of the manuscript.
Funding
JMNV’s work was partially funded by the PREI Program of General Directorate of Academic Personnel Affairs (DGAPA) 2019, grant number PREI-DGAPA-2019.
Acknowledgments
Germinal Cocho and A. Alzola in memoriam. One of the authors (J.M.N.-V.) thanks the CEIICH of UNAM Mexico for the financial support. Finally, we thank some anonymous reviewers for their helpful comments and interesting suggestions. All authors have read and agreed to the published version of the manuscript and this acknowledgement.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hayflick, L. Biological Aging Is No Longer an Unsolved Problem. Ann. N. Y. Acad. Sci. 2007, 1100, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N.; Shigenaga, M.K.; Hagen, T.M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Natl. Acad. Sci. USA 1993, 90, 7915–7922. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Free Radical Theory of Aging: An Update: Increasing the Functional Life Span. Ann. N. Y. Acad. Sci. 2006, 1067, 10–21. [Google Scholar] [CrossRef]
- Liochev, S.I. Reactive oxygen species and the free radical theory of aging. Free Radic. Biol. Med. 2013, 60, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, Z.A. An attempt at a rational classification of theories of ageing. Biol. Rev. 1990, 65, 375–398. [Google Scholar] [CrossRef]
- Ghosh, C.; De, A. Basics of aging theories and disease-related aging-an overview. Pharma Tutor 2017, 5, 16–23. [Google Scholar]
- Harman, D. Aging: A Theory Based on Free Radical and Radiation Chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef]
- Cutler, R.G. Aging and oxygen radicals. Physiol. Oxyg. Radic. 1986, 18, 251–285. [Google Scholar]
- Sohal, R.S. Metabolic Rate, Free Radicals and Aging, in Free Radicals. In Molecular Biology, Aging and Disease, Free Radicals in Molecular Biology, Aging, and Disease; Armstrong, D., Ed.; Raven Production: Nashville, TN, USA, 1984; Volume 27. [Google Scholar]
- Miquel, J.; Economos, A.C.; Johnson, J.E., Jr. A Systems Analysis—Thermodynamic View of Cellular and Organismic Aging, Aging and Cell Function; Springer: New York, NY, USA, 1984. [Google Scholar]
- Balmer, R.T. Entropy and Aging in Biological Systems. Chem. Eng. Commun. 1982, 17, 171–181. [Google Scholar] [CrossRef]
- Gladyshev, G.P. The thermodynamic theory of evolution and aging. Adv. Gerontol. 2014, 4, 109–118. [Google Scholar] [CrossRef]
- Aoki, I. Entropy principle for human development, growth and aging. J. Theor. Biol. 1991, 150, 215–223. [Google Scholar] [CrossRef]
- Nieto-Villar, J.M.; Rieumont, J.; Quintana, R.; Miquel, J. Thermodynamic approach to the aging process of biological systems. Rev. CENIC Cienc. Químicas 2003, 34, 149–157. [Google Scholar]
- WHO. Available online: https://www.who.int/health-topics/cancer (accessed on 15 May 2020).
- Montero, S.; Martin, R.; Mansilla, R.; Cocho, G.; Nieto-Villar, J.M. Parameters Estimation in Phase-Space Landscape Reconstruction of Cell Fate: A Systems Biology Approach. In Systems Biology; Humana Press: New York, NY, USA, 2018; pp. 125–170. [Google Scholar]
- Izquierdo-Kulich, E.; Nieto-Villar, J.M. Morphogenesis and complexity of the tumor patterns. In Without Bounds: A Scientific Canvas of Nonlinearity and Complex Dynamics; Springer: Berlin/Heidelberg, 2013; pp. 657–691. [Google Scholar]
- Llanos-Pérez, J.; Betancourt-Mar, J.; Cocho, G.; Mansilla, R.; Nieto-Villar, J.M. Phase transitions in tumor growth: III vascular and metastasis behavior. Phys. A Stat. Mech. Appl. 2016, 462, 560–568. [Google Scholar] [CrossRef]
- Bizzarri, M.; Giuliani, A.; Cucina, A.; D’Anselmi, F.; Soto, A.; Sonnenschein, C. Fractal analysis in a systems biology approach to cancer. Semin. Cancer Biol. 2011, 21, 175–182. [Google Scholar] [CrossRef]
- Gottesman, M.M.; Lavi, O.; Hall, M.D.; Gillet, J.P. Toward a better understanding of the complexity of cancer drug resistance. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 85–102. [Google Scholar] [CrossRef]
- Deisboeck, T.S.; Berens, M.E.; Kansal, A.R.; Torquato, S.; Stemmer-Rachamimov, A.O.; Chiocca, E.A. Pattern of self-organization in tumour systems: Complex growth dynamics in a novel brain tumour spheroid model. Cell Prolif. 2001, 34, 115–134. [Google Scholar] [CrossRef]
- Mansilla, R.; Nieto-Villar, J.M. (Coordinadores). La Termodinámica de los Sistemas Complejos; Centro de Investigaciones Interdisciplinarias en Ciencias y Humanidades: Madrid, Spain, 2017. [Google Scholar]
- Nieto-Villar, J.M.; Betancourt-Mar, J.A.; Izquierdo-Kulich, E.; Tejera, E. Complejidad y Auto-organización en Patrones Naturales; Editorial UH: Havana, Cuba, 2013. [Google Scholar]
- Nieto-Villar, J.M.; Quintana, R.; Rieumont, J. Entropy Production Rate as a Lyapunov Function in Chemical Systems: Proof. Phys. Scr. 2003, 68, 163–165. [Google Scholar] [CrossRef]
- Izquierdo-Kulich, E.; Alonso-Becerra, E.; Nieto-Villar, J.M. Entropy Production Rate for Avascular Tumor Growth. J. Mod. Phys. 2011, 2, 615–620. [Google Scholar] [CrossRef]
- Prigogine, I. Introduction to Thermodynamics of Irreversible Processes, 2nd ed.; Wiley: New York, NY, USA, 1961. [Google Scholar]
- Onsager, L. Reciprocal Relations in Irreversible Processes I. Phys. Rev. 1931, 37, 405–426. [Google Scholar] [CrossRef]
- Onsager, L. Reciprocal Relations in Irre-versible Processes II. Phys. Rev. 1931, 38, 2265–2279. [Google Scholar] [CrossRef]
- De Groot, S.R.; Mazur, P. Non-Equilibrium Thermodynamics; North-Holland Publishing Company: Amsterdam, The Netherlands, 1962. [Google Scholar]
- Prigogine, I. Etude Thermodynamique des Phenomenes irreversibles, Theses d´agregation de l´Enseignement Superieur de l´Universite Libre de Bruxelles, Dunod; Editeurs Paris y Editions Desoer Liege: Paris, France, 1947. [Google Scholar]
- Nieto-Villar, J.M. Una mirada a los sistemas complejos desde la termodinámica. Rev. Mex. Física 2020, 1, 17–24. [Google Scholar] [CrossRef]
- Nicolis, G.; Prigogine, I. Self-Organization in Nonequilibrium Systems; Wiley: New York, NY, USA, 1977. [Google Scholar]
- Bizzarri, M.; Palombo, A.; Cucina, A. Theoretical aspects of systems biology. Prog. Biophys. Mol. Biol. 2013, 112, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Nicolis, G.; Nicolis, C. Foundations of Complex Systems: Emergence, Information and Predicition; World Scientific: Singapore, 2012. [Google Scholar]
- Kuznetsov, Y.A. Elements of Applied Bifurcation Theory; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; Volume 112. [Google Scholar]
- Quintana González, R.L.; Giraldo Gutiérrez, L.; Moreno Piraján, J.C.; Nieto-Villar, J.M. Termodinámica, Ediciones Uniandes; Universidad de Los Andes: Bogotá, Columbia, 2005. [Google Scholar]
- Nicolis, G.; Daems, D. Probabilistic and thermodynamic aspects of dynamical systems. Chaos Interdiscip. J. Nonlinear Sci. 1998, 8, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Prigogine, I. Introduction to Thermodynamics of Irreversible Processes, 3rd ed.; Interscience: New York, NY, USA, 1968. [Google Scholar]
- Glansdorff, P.; Prigogine, I. On a general evolution criterion in macroscopic physics. Physica 1964, 30, 351–374. [Google Scholar] [CrossRef]
- Andronov, A.A.; Khaikin, S.Ė. Theory of Oscillations; Princeton University Press: Princeton, NJ, USA, 1949. [Google Scholar]
- Betancourt-Mar, J.A.; Mansilla, R.; Cocho, G.; Nieto-Villar, J.M. On the relationship between aging & cancer. MOJ Gerontol. Ger. 2018, 3, 163–168. [Google Scholar]
- Betancourt-Mar, J.A.; Cocho, G.; Mansilla, R.; Nieto-Villar, J.M. What Can Be Learned from A Phase Transitions in Tumor Growth? Insights Biomed. 2017, 2, 1. [Google Scholar]
- Zotin, A.I. Thermodynamic Principles and Reaction of Organisms; Nauka: Moscow, Nauka, 1988. (In Russian) [Google Scholar]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Boothby, W.M.; Berkson, J.; Dunn, H.L. Studies of the energy of metabolism of normal individuals: A standard for basal metabolism, with a nomogram for clinical application. Am. J. Physiol. Content 1936, 116, 468–484. [Google Scholar] [CrossRef]
- Van der Waals, J.D. The equation of state for gases and liquids. Nobel Lect. Phys. 1920, 1, 254–265. [Google Scholar]
- De la Fuente, M. The immune system, a marker and modulator of the rate of aging. In Immunology of Aging; Springer: Berlin/Heidelberg, Germany, 2014; pp. 3–23. [Google Scholar]
- Miquel, J. An integrated theory of aging as the result of mitochondrial-DNA mutation in differentiated cells. Arch. Gerontol. Geriatr. 1991, 12, 99–117. [Google Scholar] [CrossRef]
- Lipsitz, L.A.; Goldberger, A.L. Loss of complexity and aging: Potential applications of fractals and chaos theory to senescence. JAMA 1992, 267, 1806–1809. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, P.; Mansilla, R.; Alvarado, P.E.; Avila, F.M.; Gonzalez, A.; Gonzalez, C. A43. evolving technologies in critical care: The Bioelectric Signal of The Electrocardiogram (EKG), Analyzed In Critically Ill Patients, Using Immersion Takens Theorem. Am. J. Respir. Crit. Care Med. 2015, 191, 1. [Google Scholar]
- Betancourt-Mar, J.A.; Rodríguez-Ricard, M.; Mansilla, R.; Cocho, G.; Nieto-Villar, J.M. Entropy production: Evolution criteria, robustness and fractal dimension. Rev. Mex. Física 2016, 62, 164–167. [Google Scholar]
- Kyriazis, M. Practical applications of chaos theory to the modulation of human aging: Nature prefers chaos to regularity. Biogerontology 2003, 4, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Cutler, R.G. Dysdifferentiative hypothesis of aging: A review. In Molecular Biology of Aging: Gene Stability and Gene Expression; Raven Press: New York, NY, USA, 1985; pp. 307–340. [Google Scholar]
- Kirkham, F.; Mills, C.; Nambier, K.; Timeyin, J.; Davies, K.; Kern, F.; Cruickshank, J.; Rajkumar, C. Are you really as old as your arteries? predicting biological age using cardio-ankle vascular index as a marker of vascular stiffness. J. Hypertens. 2017, 35, e19–e20. [Google Scholar] [CrossRef]
- Hecht, F.; Pessoa, C.F.; Gentile, L.B.; Rosenthal, D.; Carvalho, D.P.; Fortunato, R.S. The role of oxidative stress on breast cancer development and therapy. Tumor Biol. 2016, 37, 4281–4291. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Flores, R.; Poff, A.M.; D’Agostino, D.P. Cancer as a metabolic disease: Implications for novel therapeutics. Carcinogenesis 2014, 35, 515–527. [Google Scholar] [CrossRef]
- Fenninger, L.D.; Mider, G. Energy and Nitrogen Metabolism in Cancer. Adv. Cancer Res. 1954, 2, 229–253. [Google Scholar] [CrossRef]
- Tidwell, T.R.; Søreide, K.; Hagland, H.R. Aging, metabolism, and cancer development: From Peto’s paradox to the War-burg effect. Aging Dis. 2017, 8, 662. [Google Scholar] [CrossRef]
- Finkel, T.; Serrano, M.; Blasco, M.A. The common biology of cancer and ageing. Nature 2007, 448, 767–774. [Google Scholar] [CrossRef]
- Holroyde, C.P.; Gabuzda, T.G.; Putnam, R.C.; Paul, P.; Reichard, G.A. Altered glucose metabolism in metastatic carcinoma. Cancer Res. 1975, 35, 3710–3714. [Google Scholar] [PubMed]
- Izquierdo-Kulich, E.; Rebelo, I.; Tejera, E.; Nieto-Villar, J.M. Phase transition in tumor growth: I avascular development. Phys. A Stat. Mech. Appl. 2013 392, 6616–6623. [CrossRef]
- Llanos-Pérez, J.; Betancourt-Mar, A.; De Miguel, M.; Izquierdo-Kulich, E.; Royuela-García, M.; Tejera, E.; Nieto-Villar, J. Phase transitions in tumor growth: II prostate cancer cell lines. Phys. A Stat. Mech. Appl. 2015, 426, 88–92. [Google Scholar] [CrossRef]
- Warburg, O. On Respiratory Impairment in Cancer Cells. Science 1956, 124, 269–270. [Google Scholar] [CrossRef]
- Martin, R.R.; Montero, S.; Silva, E.; Bizzarri, M.; Cocho, G.; Mansilla, R.; Nieto-Villar, J.M. Phase transitions in tumor growth: V what can be expected from cancer glycolytic oscillations? Phys. A Stat. Mech. Appl. 2017, 486, 762–771. [Google Scholar] [CrossRef]
- Amemiya, T.; Shibata, K.; Itoh, Y.; Itoh, K.; Watanabe, M.; Yamaguchi, T. Primordial oscillations in life: Direct observation of glycolytic oscillations in individual HeLa cervical cancer cells. Chaos Interdiscip. J. Nonlinear Sci. 2017, 27, 104602. [Google Scholar] [CrossRef] [PubMed]
- Soto, A.M.; Maffini, M.V.; Sonnenschein, C. Neoplasia as development gone awry: The role of endocrine disruptors. Int. J. Androl. 2007, 31, 288–293. [Google Scholar] [CrossRef]
- Betancourt-Mar, J.A.; Llanos-Pérez, J.A.; Cocho, G.; Mansilla, R.; Martin, R.; Montero, S.; Nieto-Villar, J.M. Phase transi-tions in tumor growth: IV relationship between metabolic rate and fractal dimension of human tumor cells. Phys. A Stat. Mech. Appl. 2017, 473, 344–351. [Google Scholar] [CrossRef]
- Roose, T.; Chapman, S.; Maini, P.K. Mathematical Models of Avascular Tumor Growth. SIAM Rev. 2007, 49, 179–208. [Google Scholar] [CrossRef]
- Cao, H.; Xu, E.; Liu, H.; Wan, L.; Lai, M. Epithelial–mesenchymal transition in colorectal cancer metastasis: A system review. Pathol. Res. Pract. 2015, 211, 557–569. [Google Scholar] [CrossRef]
- Guerra-González, A.; Silva, E.; Montero, S.; Rodríguez, D.J.; Mansilla, R.; Nieto-Villar, J.M. Metástasis: Un hito para el conocimiento, un reto para la ciencia. Rev. Cuba. Med. 2020, 59, 1–20. [Google Scholar]
- MacLean, A.L.; Harrington, H.A.; Stumpf, M.P.H.; Hansen, M.D.H. Epithelial-Mesenchymal Transition in Metastatic Cancer Cell Populations Affects Tumor Dormancy in a Simple Mathematical Model. Biomedicines 2014, 2, 384–402. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef]
- Zhu, W.; Cai, M.Y.; Tong, Z.T.; Dong, S.S.; Mai, S.J.; Liao, Y.J.; Bian, X.W.; Lin, M.C.; Kung, H.F.; Zeng, Y.X.; et al. Overexpression of EIF5A2 promotes colorectal carcinoma cell aggressiveness by upregulating MTA1 through C-myc to induce epithelial-mesenchymal transition. Gut 2012, 61, 562–575. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.; Kohandel, M. Investigating the link between epithelial-mesenchymal transition and the cancer stem cell phenotype: A mathematical approach. J. Theoret. Biol. 2010, 265, 329–335. [Google Scholar] [CrossRef]
- Magi, S.; Iwamoto, K.; Okada-Hatakeyama, M. Current status of mathematical modeling of cancer—From the viewpoint of cancer hallmarks. Curr. Opin. Syst. Biol. 2017, 2, 39–48. [Google Scholar] [CrossRef]
- Guerra, A.; Rodriguez, D.J.; Montero, S.; Betancourt-Mar, J.A.; Martin, R.R.; Silva, E.; Bizzarri, M.; Cocho, G.; Mansilla, R.; Nieto-Villar, J.M. Phase transitions in tumor growth VI: Epithelial–Mesenchymalmal transition. Phys. A Stat. Mechanics Its Appl. 2018, 499, 208–215. [Google Scholar] [CrossRef]
- Brú, A.; Albertos, S.; Subiza, J.L.; García-Asenjo, J.L.; Brú, I. The Universal Dynamics of Tumor Growth. Biophys. J. 2003, 85, 2948–2961. [Google Scholar] [CrossRef]
- Nasir, N.A. Selected Aspects of Cancer Progression: Metastasis. In Apoptosis and Immune Response; Kaiser, H.E., Nasir, A., Eds.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008; Volume 11. [Google Scholar]
- Thiery, J.P.; Sleeman, J.P. Complex networks orchestrate epithelial–mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 2006, 7, 131–142. [Google Scholar] [CrossRef]
- Shilnikov, L. Mathematical Problems of Nonlinear Dynamics: A Tutorial. Int. J. Bifurc. Chaos 1997, 7, 1953–2001. [Google Scholar] [CrossRef]
- Shilnikov, A.L.; Turaev, D.V.; Chua, L.O. Methods of Qualitative Theory in Nonlinear Dynamics; World Scientific: Singapore, 2001. [Google Scholar]
- Tripathi, S.C.; Peters, H.L.; Taguchi, A.; Katayama, H.; Wang, H.; Momin, A.; Jolly, M.K.; Celiktas, M.; Rodriguez-Canales, J.; Liu, H.; et al. Immunoproteasome deficiency is a feature of non-small cell lung cancer with a mesenchymal phenotype and is associated with a poor outcome. Proc. Natl. Acad. Sci. USA 2016, 113, E1555–E1564. [Google Scholar] [CrossRef] [PubMed]
- Betancourt-Mar, J.A.; Nieto-Villar, J.M. Theoretical models for chronotherapy: Periodic perturbations in funnel chaos type. Math. Biosci. Eng. 2007, 4, 177–186. [Google Scholar] [PubMed]
- Kitano, H. Cancer robustness: Tumor tactics. Nature 2003, 426, 125. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lahmy, R.; Riha, C.; Yang, C.; Jakubison, B.L.; van Niekerk, J.; Staub, C.; Wu, Y.; Gates, K.; Dong, D.S.; et al. The Basic Helix-Loop-Helix Transcription Factor E47 Reprograms Human Pancreatic Cancer Cells to a Quies-cent Acinar State with Reduced Tumorigenic Potential. Pancreas 2015, 44, 718–727. [Google Scholar] [CrossRef]
- Volkenstein, M.V. Entropy and Information; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009; Volume 57. [Google Scholar]
- Enderling, H.; Almog, N.; Hlatky, L. Systems Biology of Tumor Dormancy; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Pantel, K.; Alix-Panabiéres, C.; Riethdorf, S. Cancer micrometastasis. Nat. Rev. Clin. Oncol. 2009, 6, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, M.; Cucina, A.; Proietti, S. Tumor reversion: Mesenchymal-epithelial transition as a critical step in managing the tumor microenvironment cross-talk. Curr. Pharm. Des. 2017, 23, 4705–4715. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Schulz, W. Molecular Biology of Human Cancers; Springer Science: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Lane, D.; Levine, A. p53 Research: The past thirty years and the next thirty years. Cold Spring Harb. Perspect Biol. 2010, 2, a000893. [Google Scholar] [CrossRef]
- Dixon, S.J.; Stockwell, B.R. The Hallmarks of Ferroptosis. Annu. Rev. Cancer Biol. 2019, 3, 35–54. [Google Scholar] [CrossRef]
- Feng, H.; Stockwell, B.R. Unsolved mysteries: How does lipid peroxidation cause ferroptosis? PLoS Biol. 2018, 16, e2006203. [Google Scholar] [CrossRef]
- Yan, H.F. Ferroptosis: Mechanisms and links with diseases. Signal Transduct. Target. Ther. 2021, 6, 49. [Google Scholar] [CrossRef]
- Shen, Z.; Song, J.; Yung, B.C. Emerging strategies of cancer therapy based on ferroptosis. Adv. Mater. 2018, 30, 1704007. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Skouta, R.; Kaplan, A.; Yang, W.S.; Hayano, M.; Dixon, S.; Brown, L.M.; Valenzuela, C.A.; Wolpaw, A.; Stockwell, B.R. Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nat. Chem. Biol. 2016, 12, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Zhang, L.; Ma, K.; Riegman, M.; Chen, F.; Ingold, I.; Conrad, M.; Turker, M.Z.; Gao, M.; Jiang, X.; et al. Ultrasmall nanoparticles induce ferroptosis in nutrient-deprived cancer cells and suppress tumor growth. Nat. Nanotechnol. 2016, 11, 977–985. [Google Scholar] [CrossRef]
- Kagan, V.E.; Mao, G.; Qu, F.; Angeli, J.P.F.; Doll, S.; Croix, C.S.; Dar, H.H.; Liu, B.; Tyurin, V.A.; Ritov, V.B.; et al. Oxidized Arachidonic and Adrenic PEs Navigate Cells to Ferroptosis. Nat. Chem. Biol. 2017, 13, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Agmon, E.; Solon, J.; Bassereau, P.; Stockwell, B.R. Modeling the effects of lipid peroxidation during ferroptosis on mem-brane properties. Sci. Rep. 2018, 8, e5155. [Google Scholar] [CrossRef]
- Konstorum, A.; Tesfay, L.; Paul, B.T.; Torti, F.M.; Laubenbacher, R.C.; Torti, S.V. Systems biology of ferroptosis: A modeling approach. J. Theor. Biol. 2020, 493, 110222. [Google Scholar] [CrossRef]
- Dierge, E.; Debock, E.; Guilbaud, C.; Corbet, C.; Mignolet, E.; Mignard, L.; Bastien, E.; Dessy, C.; Larondelle, Y.; Feron, O. Peroxidation of n-3 and n-6 polyunsaturated fatty acids in the acidic tumor environment leads to ferropto-sis-mediated anticancer effects. Cell Metab. 2021, 33, 1–15. [Google Scholar] [CrossRef]
- Hassannia, B.; Vandenabeele, P.; Berghe, T.V. Targeting Ferroptosis to Iron Out Cancer. Cancer Cell 2019, 35, 830–849. [Google Scholar] [CrossRef]
- Nieto-Villar, J.M.; Mansilla, R. Ferroptosis as a Biological Phase Transition I: Avascular and vascular tumor growth, European. J. Biomed. Pharm. Sci. 2021, 8, 63–70. [Google Scholar]
- Wang, S.; Liao, H.; Li, F.; Ling, D. A mini-review and perspective on ferroptosis-inducing strategies in cancer therapy. Chin. Chem. Lett. 2019, 30, 847–852. [Google Scholar] [CrossRef]
- Lei, G.; Zhang, Y.; Koppula, P.; Liu, X.; Zhang, J.; Lin, S.H.; Ajani, J.A.; Xiao, Q.; Liao, Z.; Wang, H.; et al. The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Res. 2020, 30, 146–162. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Li, Y. What Is Responsible for the Initiating Chemistry of Iron-Mediated Lipid Peroxidation: An Update. Chem. Rev. 2007, 107, 748–766. [Google Scholar] [CrossRef] [PubMed]
- Bebber, C.M.; Müller, F.; Prieto Clemente, L.; Weber, J.; Von Karstedt, S. Ferroptosis in Cancer Cell Biology. Cancers 2020, 12, 164. [Google Scholar] [CrossRef] [PubMed]
- Spiteller, G.; Afzal, M. The Action of Peroxyl Radicals, Powerful Deleterious Reagents, Explains Why Neither Cholesterol Nor Saturated Fatty Acids Cause Atherogenesis and Age-Related Diseases. Chem.-A Eur. J. 2014, 20, 14928–14945. [Google Scholar] [CrossRef]
- Dreger, H.; Westphal, K.; Weller, A.; Baumann, G.; Stangl, V.; Meiners, S.; Stangl, K. Nrf2-dependent upregulation of antioxidative enzymes: A novel pathway for proteasome inhibitor-mediated cardioprotection. Cardiovasc. Res. 2003, 83, 354–361. [Google Scholar] [CrossRef]
- Molnar, J.; Thornton, B.; Molnar, A.; Gaal, D.; Luo, L.; Bergmann-Leitner, E. Thermodynamic Aspects of Cancer: Possible Role of Negative Entropy in Tumor Growth, its Relation to Kinetic and Genetic Resistance. Lett. Drug Des. Discov. 2005, 2, 429–438. [Google Scholar] [CrossRef]
- Lucia, U.; Ponzetto, A.; Deisboeck, T.S. A thermodynamic approach to the ‘mitosis/apoptosis’ ratio in cancer. Phys. A Stat. Mech. Its Appl. 2015, 436, 246–255. [Google Scholar] [CrossRef]
- Lucia, U.; Grisolia, G. Second law efficiency for living cells. Front. Biosci. 2017, 9, 270–275. [Google Scholar] [CrossRef]
- Marin, D.; Sabater, B. The cancer Warburg effect may be a testable example of the minimum entropy production rate principle. Phys. Biol. 2017, 14, 024001. [Google Scholar] [CrossRef]
- Montemayor-Aldrete, J.A.; Márquez-Caballé, R.F.; del Castillo-Mussot, M.; Cruz-Peregrino, F. General Thermodynamic Efficiency Loss and Scaling Behavior of Eukaryotic Organisms. Biophys. Rev. Lett. 2020, 15, 143–169. [Google Scholar] [CrossRef]
- Luo, L.-F. Entropy production in a cell and reversal of entropy flow as an anticancer therapy. Front. Phys. China 2009, 4, 122–136. [Google Scholar] [CrossRef]
- Lucia, U. Entropy generation and cell growth with comments for a thermodynamic anticancer approach. Phys. A Stat. Mech. Appl. 2014, 406, 107–118. [Google Scholar] [CrossRef]
- Villar, J.N.; Guerra, A.; Rodriguez, D.; Silva, E.; Mar, J.B.; Cocho, G.; Mansilla, R. Chronotherapy of cancer: Epithelial-mesenchymal transition. MOJ Gerontol. Geriatr. 2019, 4, 124–127. [Google Scholar] [CrossRef]
- Sourailidis, D.; Volos, C.; Moysis, L.; Meletlidou, E.; Stouboulos, I. The Study of Square Periodic Perturbations as an Immunotherapy Process on a Tumor Growth Chaotic Model. Dynamics 2022, 2, 8. [Google Scholar] [CrossRef]
- Jaime, J.C.; Mesa-Álvarez, M.D.; Martin, R.R.; Betancourt-Mar, J.A.; Cocho, G.; Mansilla, R.; Nieto-Villar, J.M. Chronothera-py of cancer: Periodic perturbations in vascular growth and metastasis. Biol. Rhythm Res. 2018, 50, 495–504. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).