Brainwave Dynamics: Neurophysiological Responses to Enclosed Courtyards for Mental Wellbeing in Educational Contexts
Abstract
1. Introduction
- 1.
- How do the spatial configurations of enclosed courtyards in educational settings influence students’ neurophysiological responses, as measured by brainwave activity (e.g., alpha and theta waves)?
- 2.
- What is the relationship between the visual stimuli of enclosed courtyards (e.g., existing natural elements) and psychophysiological outcomes like relaxation or reduced anxiety in students?
2. Shaping Minds: Educational Spaces and Students’ Mental Health
3. The Courtyard Concept: Spatial Configuration and Visual Stimuli
4. Materials and Methods
4.1. Study Area
| Parameter | Description | |||
|---|---|---|---|---|
| Spatial Configuration | Shape and Dimensions | Rectangular courtyard with a narrow, elongated layout. Yellow-marked area in schematic plan indicates the experiment zone where students were seated, as seen in Figure 1. | Approx. 9 m × 17 m (153 m2) |  |
| Enclosure | Surrounded on four sides by multi-story buildings (3–4 stories), creating an enclosed confined space. | Building height: ~12–15 m; height-to-width ratio ≈ 0.8–1.0 | ||
| Visual Stimuli as seen in | Vegetation Coverage | Dense greenery with large canopy trees, shrubs along building edges, and low vegetation near seating. | ~35% of courtyard area |  |
| Hardscape Coverage | Brick paving for central walkway. | ~65% of courtyard area | ||
| Vegetation-to-Hardscape Ratio | Ratio reflecting greenery versus paved/constructed surfaces. | 2:3 (35% vegetation: 65% hardscape) | ||
| Furniture and Shading Structures |
|
| ||
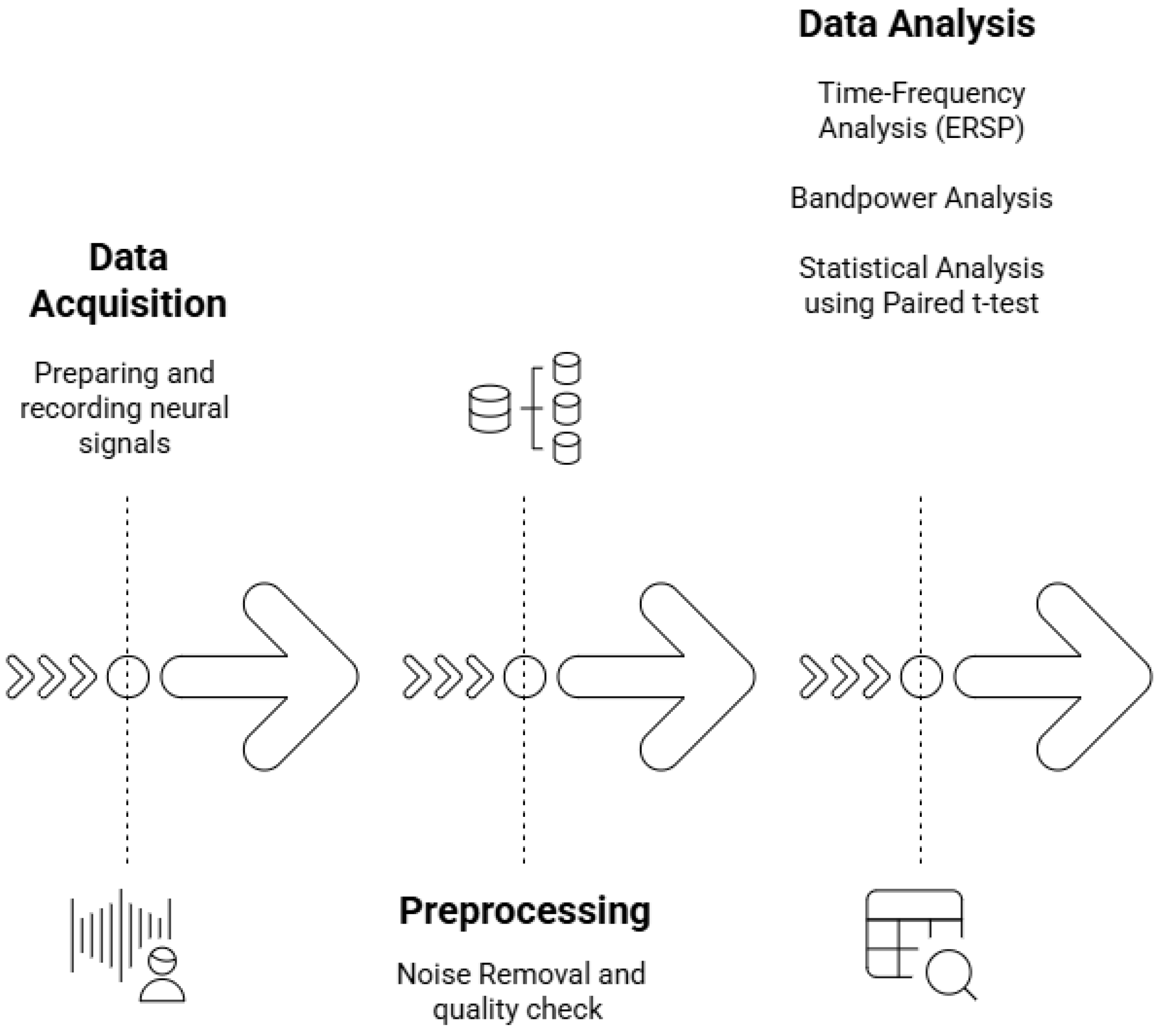
4.2. Study Design
4.3. Participants
4.4. Data Acquisition: Neurophysiological Approach
4.4.1. Apparatus Calibration and Data Preprocessing
4.4.2. Procedures Seen in Figure 3

4.5. Data Processing and Analysis: Psychophysiological Approach (Figure 4)
4.5.1. Data Analyses
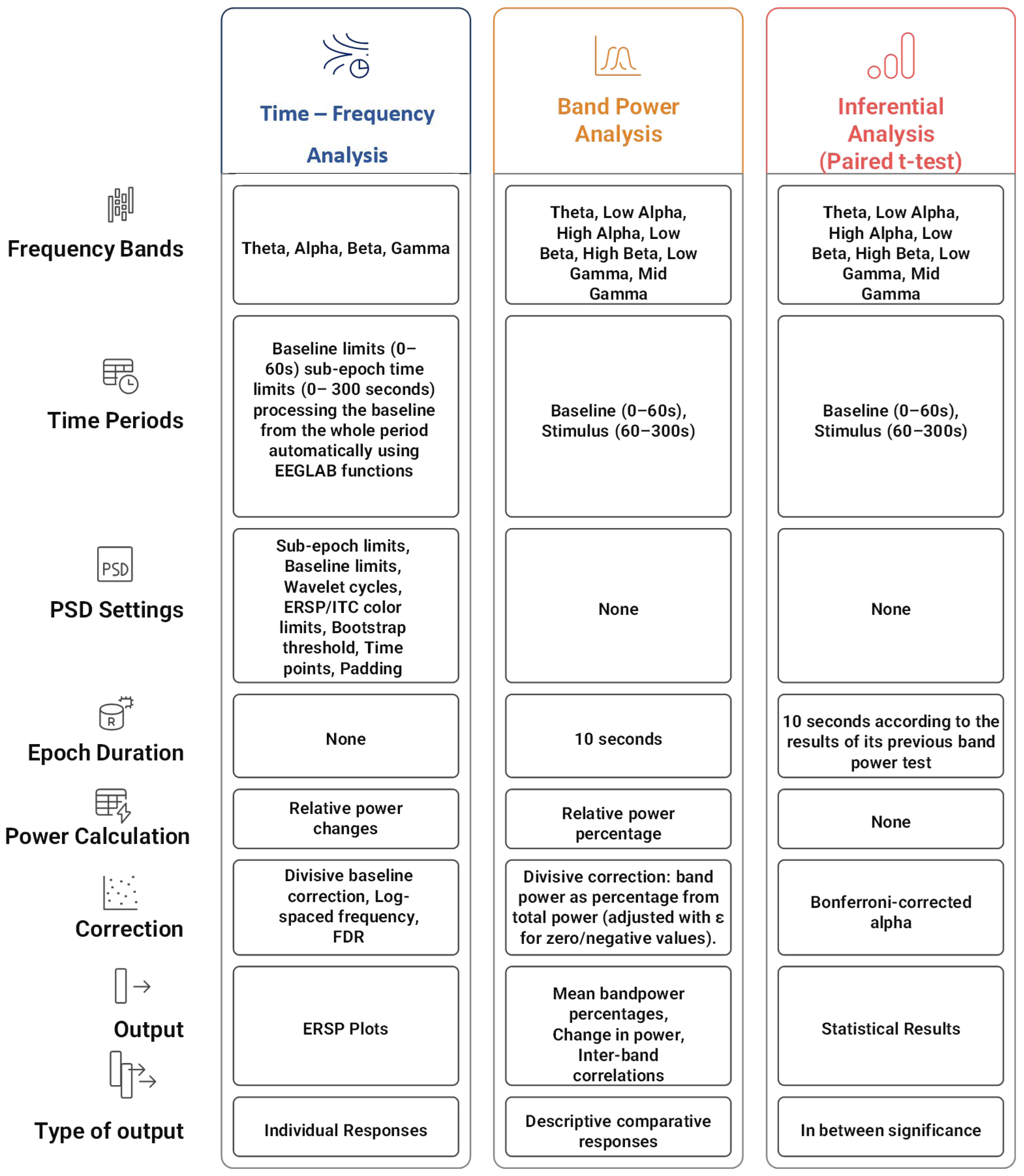
4.5.2. Data Processing
5. Results and Discussion
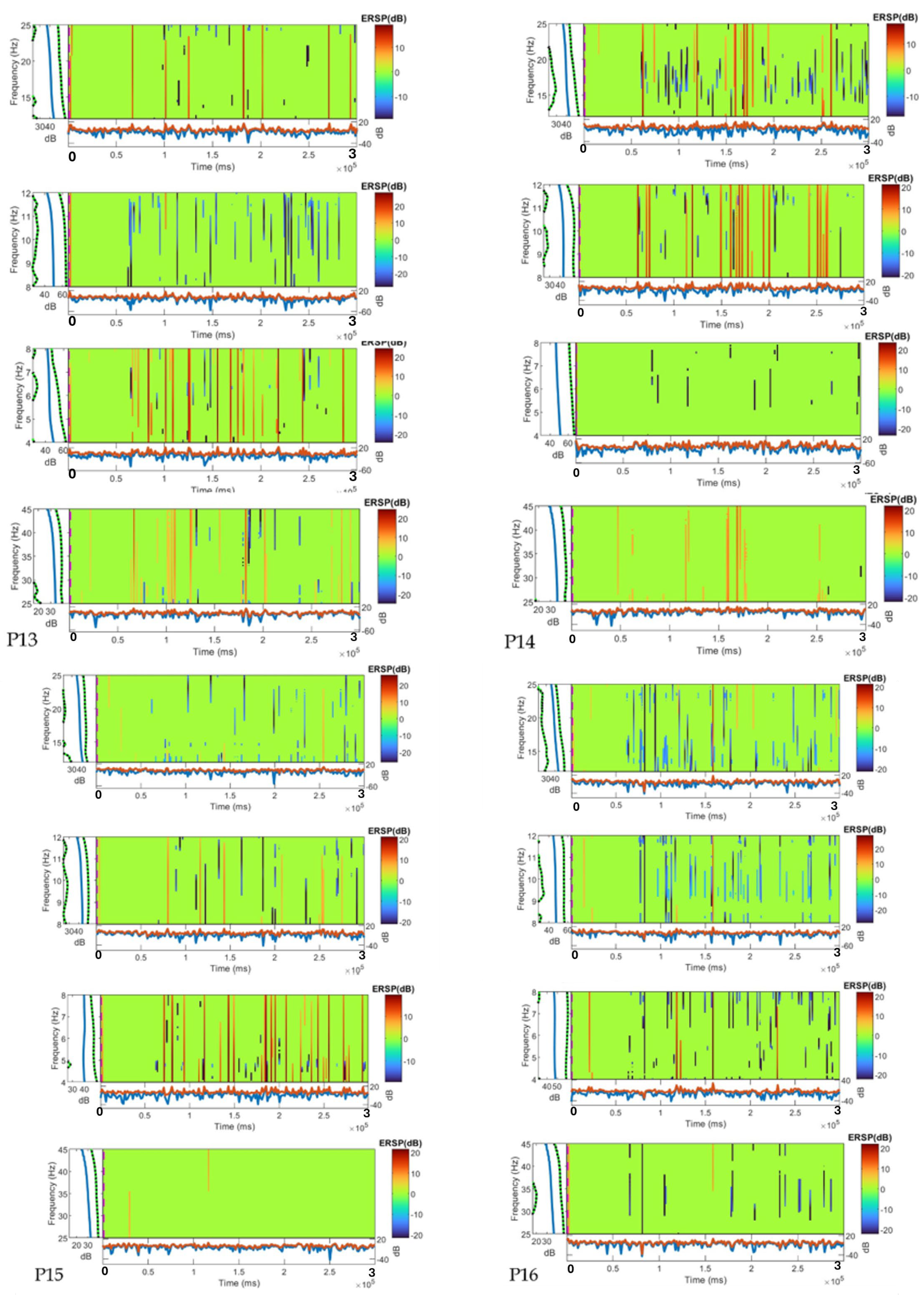
5.1. Time–Frequency Analysis (ERSP)
5.2. Band Power Descriptive Analysis
- Mean Band power Comparison (Figure 8): This bar chart compares the mean power (%) across different brain frequency bands (Theta, LowAlpha, HighAlpha, LowBeta, HighBeta, LowGamma, MidGamma) under two conditions—baseline (blue bars) and stimulus (orange bars). Theta and LowAlpha show the highest power, with stimulus slightly increasing power in Theta and decreasing it in LowAlpha and HighAlpha compared to baseline.
- 2.
- Distribution of Changes (Figure 9): This box plot shows the distribution of percentage changes in power for each frequency band. The median changes are close to zero, with Theta and LowAlpha showing the widest distributions (both positive and negative outliers), while MidGamma has a narrower range and a slight positive shift.
- 3.
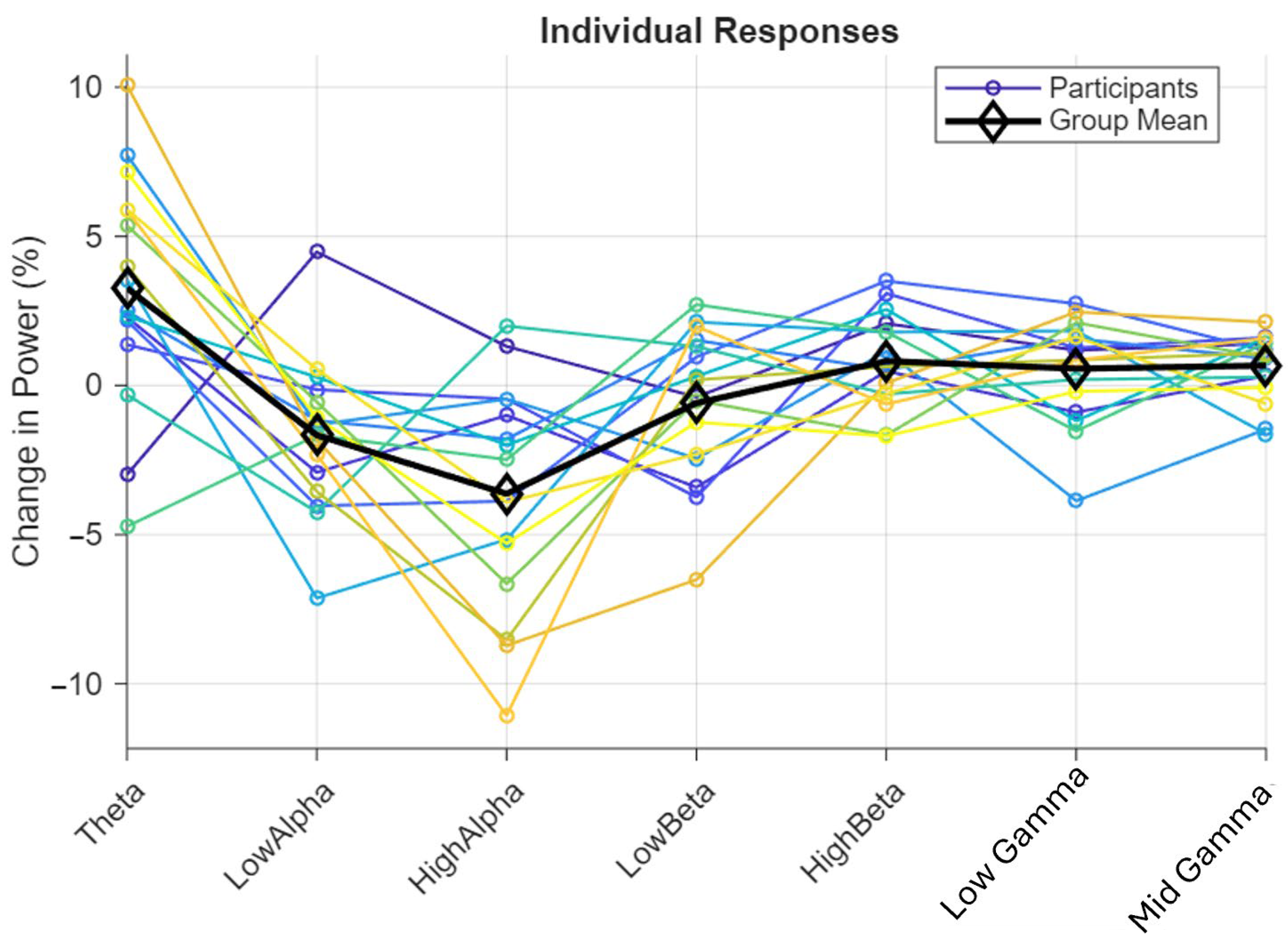
- Individual Responses (Figure 10): This line graph displays the percentage change in power for each frequency band (Theta to MidGamma) across multiple individual responses (different colored lines). The changes vary widely, with some bands (e.g., HighAlpha) showing significant decreases, while others (e.g., LowGamma) show more stability.
- 4.
- Band Change Correlations (Figure 11): The correlation matrix illustrates the pairwise relationships between stimulus-induced power changes across EEG frequency bands in the study cohort. In this visualization, red indicates positive correlations (0 to +1), signifying that power changes in paired bands co-vary in the same direction, while blue denotes negative correlations (−1 to 0), reflecting inverse relationships where power increases in one band associated with decreases in another. The dark-red diagonal confirms the expected perfect positive autocorrelations (r = +1) for each band. For example, dark-blue, where “LowAlpha” meets “Theta,” means that participants who showed increased Low Alpha waves during the stimulus also tended to show decreased Theta waves. The asterisks (*) mark correlations that are statistically significant (less than 5% likely to occur by chance).
5.3. Inferential Statistical Analysis
- ■
- Theta: p = 0.0041, h = 1, t(15) = 3.3868, 95% CI = [0.2651, 6.2650]—significant increase, indicating a reliable shift toward relaxation.
- ■
- LowAlpha: p = 0.0200, h = 0, t(15) = −2.6031, 95% CI = [−3.6581, 0.3257]—not significant, suggesting no reliable change.
- ■
- HighAlpha: p = 0.0015, h = 1, t(15) = −3.8814, 95% CI = [−6.5399, −0.7197]—significant decrease, possibly reflecting a shift from resting to engaged states.
- ■
- LowBeta: p = 0.3662, h = 0, t(15) = −0.9318, 95% CI = [−2.5640, 1.3823]—not significant, indicating stable activity.
- ■
- HighBeta: p = 0.0538, h = 0, t(15) = 2.0927, 95% CI = [−0.3985, 2.0353]—not significant, though near threshold, suggesting possible mild engagement.
- ■
- LowGamma: p = 0.2147, h = 0, t(15) = 1.2957, 95% CI = [−0.7899, 1.9169]—not significant.
- ■
- MidGamma: p = 0.0303, h = 0, t(15) = 2.3924, 95% CI = [−0.2005, 1.5341]—close to significant (p = 0.0303), hinting at possible sensory engagement.
6. Multimodal EEG Analysis: Findings and Synthesis
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reinilä, E.; Kekäläinen, T.; Saajanaho, M.; Kokko, K. The structure of mental well-being and its relationship with generativity in middle adulthood and the beginning of late adulthood. Int. J. Behav. Dev. 2023, 47, 328–338. [Google Scholar] [CrossRef]
- Lin, E. Well-being. In Routledge Encyclopedia of Philosophy; Routledge: London, UK, 2022. [Google Scholar] [CrossRef]
- Rhee, J.H.; Schermer, B.; Cha, S.H. Effects of indoor vegetation density on human well-being for a healthy built environment. Dev. Built Environ. 2023, 14, 100172. [Google Scholar] [CrossRef]
- Baxter, L.; Burnell, K. What is wellbeing and how do we measure and evaluate it? In Archaeology, Heritage, and Wellbeing; Routledge: London, UK, 2022; pp. 7–25. [Google Scholar] [CrossRef]
- Jarden, A.; Roache, A. What Is Wellbeing? Int. J. Environ. Res. Public Health 2023, 20, 5006. [Google Scholar] [CrossRef]
- McCay, L.; Bremer, I.; Endale, T.; Jannati, M.; Yi, J. Urban Design and Mental Health; Springer: Singapore, 2017; pp. 1–24. [Google Scholar] [CrossRef]
- Ulrich, R.S. View Through a Window May Influence Recovery from Surgery. Science 1984, 224, 420–421. [Google Scholar] [CrossRef]
- Kaplan, S. The restorative benefits of nature: Toward an integrative framework. J. Environ. Psychol. 1995, 15, 169–182. [Google Scholar] [CrossRef]
- Closs, L.; Mahat, M.; Imms, W. Learning environments’ influence on students’ learning experience in an Australian Faculty of Business and Economics. Learn. Environ. Res. 2022, 25, 271–285. [Google Scholar] [CrossRef]
- Foellmer, J.; Kistemann, T.; Anthonj, C. Academic Greenspace and Well-Being—Can Campus Landscape be Therapeutic? Evidence from a German University. Wellbeing Space Soc. 2021, 2, 100003. [Google Scholar] [CrossRef]
- Ohly, H.; White, M.P.; Wheeler, B.W.; Bethel, A.; Ukoumunne, O.C.; Nikolaou, V.; Garside, R. Attention Restoration Theory: A systematic review of the attention restoration potential of exposure to natural environments. J. Toxicol. Environ. Health Part B 2016, 19, 305–343. [Google Scholar] [CrossRef]
- Valtchanov, D.; Ellard, C.G. Cognitive and affective responses to natural scenes: Effects of low-level visual properties on preference, cognitive load and eye-movements. J. Environ. Psychol. 2015, 43, 184–195. [Google Scholar] [CrossRef]
- Salameh, M.; Touqan, B.; Suliman, A. Enhancing student satisfaction and academic performance through school courtyard design: A quantitative analysis. Archit. Eng. Des. Manag. 2024, 20, 911–927. [Google Scholar] [CrossRef]
- Norman, J.F.; Carmichael, M.; Hagan, E.; Mishchuk, Y.; Bryant, E.N.; Ramirez, A.B.; Sutton, A. Visual outdoor space perception. Sci. Rep. 2025, 15, 11549. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Luo, M.; Wang, Y.; Wei, Y. Multi-Sensory Interaction and Spatial Perception in Urban Microgreen Spaces: A Focus on Vision, Auditory, and Olfaction. Sustainability 2024, 16, 8809. [Google Scholar] [CrossRef]
- Guo, W.; Wen, H.; Liu, X. Research on the psychologically restorative effects of campus common spaces from the perspective of health. Front. Public Health 2023, 11, 1131180. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, I.; Al Badri, N.; Mushtaha, E.; Omar, O. Evaluating the Impacts of Courtyards on Educational Buildings, Case Study in the University of Sharjah. Sustainability 2021, 14, 141. [Google Scholar] [CrossRef]
- Callaghan, A.; McCombe, G.; Harrold, A.; McMeel, C.; Mills, G.; Moore-Cherry, N.; Cullen, W. The impact of green spaces on mental health in urban settings: A scoping review. J. Ment. Health 2021, 30, 179–193. [Google Scholar] [CrossRef]
- Olszewska, A.A.; Marques, P.F.; Barbosa, F. Enhancing Urban Landscape with Neuroscience Tools: Lessons from the Human Brain. Citygreen 2015, 1, 60. [Google Scholar] [CrossRef]
- Olszewska-Guizzo, A.; Sia, A.; Fogel, A.; Ho, R. Features of urban green spaces associated with positive emotions, mindfulness and relaxation. Sci. Rep. 2022, 12, 20695. [Google Scholar] [CrossRef]
- Jeon, J.Y.; Jo, H.I.; Lee, K. Psycho-physiological restoration with audio-visual interactions through virtual reality simulations of soundscape and landscape experiences in urban, waterfront, and green environments. Sustain. Cities Soc. 2023, 99, 104929. [Google Scholar] [CrossRef]
- Wei, N.; Jiangxu, J.; Mimi, W.; Jin, S.; Gang, L. Research on the Impact of Panoramic Green View Index of Virtual Reality Environments on Individuals’ Pleasure Level Based on EEG Experiment. Landsc. Archit. Front. 2022, 10, 36–51. [Google Scholar] [CrossRef]
- Seiz, A.; Kweon, B.S.; Ellis, C.D.; Oh, H.; Pietro, K. Exploring the Psychophysiological Effects of Viewing Urban Nature through Virtual Reality Using Electroencephalography and Perceived Restorativeness Scale Measures. Sustainability 2023, 15, 13090. [Google Scholar] [CrossRef]
- Aghabozorgi, K.; van der Jagt, A.; Bell, S.; Smith, H. The role of university campus landscape characteristics in students’ mental health. Urban For. Urban Green. 2025, 111, 128863. [Google Scholar] [CrossRef]
- Garg, A.M. Open Spaces in a Institutional Campus Leads to Active Environment. Int. J. Sci. Res. Eng. Manag. 2024, 8, 1–5. [Google Scholar] [CrossRef]
- Stigsdotter, U.K.; Grahn, P. What Makes a Garden a Healing Garden? Available online: https://www.researchgate.net/publication/234072230 (accessed on 2 April 2025).
- Si, B.; binti Mohd Noor, A.I.; Wen, K. Linking University Campus Green Space and Students’ Mental Health and Well-Being: A Systematic Literature Review. Int. J. Acad. Res. Environ. Geogr. 2024, 10, 31–47. [Google Scholar] [CrossRef]
- Olszewska-Guizzo, A.; Fogel, A.; Escoffier, N.; Sia, A.; Nakazawa, K.; Kumagai, A.; Dan, I.; Ho, R. Therapeutic Garden With Contemplative Features Induces Desirable Changes in Mood and Brain Activity in Depressed Adults. Front. Psychiatry 2022, 13, 757056. [Google Scholar] [CrossRef]
- Krefis, A.; Augustin, M.; Schlünzen, K.; Oßenbrügge, J.; Augustin, J. How Does the Urban Environment Affect Health and Well-Being? A Systematic Review. Urban Sci. 2018, 2, 21. [Google Scholar] [CrossRef]
- Choi, H.S.; Bruyns, G.; Zhang, W.; Cheng, T.; Sharma, S. Spatial Cognition and Three-Dimensional Vertical Urban Design Guidelines—Cognitive Measurement and Modelling for Human Centre Design. Urban Sci. 2023, 7, 125. [Google Scholar] [CrossRef]
- Mariani Wigley, I.C.; Nazzari, S.; Pastore, M.; Provenzi, L.; Barello, S. The contribution of environmental sensitivity and connectedness to nature to mental health: Does nature view count? J. Environ. Psychol. 2025, 102, 102541. [Google Scholar] [CrossRef]
- Roe, J.; Aspinall, P. The restorative outcomes of forest school and conventional school in young people with good and poor behaviour. Urban For. Urban Green. 2011, 10, 205–212. [Google Scholar] [CrossRef]
- Gao, T.; Zhang, T.; Zhu, L.; Gao, Y.; Qiu, L. Exploring psychophysiological restoration and individual preference in the different environments based on virtual reality. Int. J. Environ. Res. Public Health 2019, 16, 3102. [Google Scholar] [CrossRef]
- Lau, S.S.Y.; Gou, Z.; Liu, Y. Healthy campus by open space design: Approaches and guidelines. Front. Archit. Res. 2014, 3, 452–467. [Google Scholar] [CrossRef]
- Peker, E.; Ataöv, A. Exploring the ways in which campus open space design influences students’ learning experiences. Landsc. Res. 2020, 45, 310–326. [Google Scholar] [CrossRef]
- Ribeiro, H.; de S. Santana, K.V.; Oliver, S.L. Natural Environments in University Campuses and Students’ Well-being. arXiv 2024. [Google Scholar] [CrossRef]
- Ellard, C. Neuroscience, Wellbeing, and Urban Design: Our Universal Attraction to Vitality. Psychol. Res. Urban Soc. 2020, 3, 6. [Google Scholar] [CrossRef]
- Russo, A. Urban Green Spaces and Healthy Living: A Landscape Architecture Perspective. Urban Sci. 2024, 8, 213. [Google Scholar] [CrossRef]
- Hughes, H.; Franz, J.; Willis, J. School Spaces for Student Wellbeing and Learning Insights from Research and Practice; Springer: Singapore, 2019. [Google Scholar]
- Juba, İ.; Bogenç, Ç. Impact of Courtyard Architecture on Personal Well-Being and Human Health. Türkiye Peyzaj Araştırmaları Derg. 2024, 7, 204–212. [Google Scholar] [CrossRef]
- Nie, X.; Wang, Y.; Zhang, C.; Zhao, Y.; Kirkwood, N. The varied restorative values of campus landscapes to students’ well-being: Evidence from a Chinese University. BMC Public Health 2024, 24, 487. [Google Scholar] [CrossRef]
- Wen, Q.; Zhou, Q.; Ye, H.; Guo, Q.; Shan, J.; Huang, Z. A Perceptual Assessment of the Physical Environment in Teaching Buildings and Its Influence on Students’ Mental Well-Being. Buildings 2024, 14, 1790. [Google Scholar] [CrossRef]
- Karimi, N.; Sajadzadeh, H.; Aram, F. Investigating the Association between Environmental Quality Characteristics and Mental Well-Being in Public Open Spaces. Urban Sci. 2022, 6, 20. [Google Scholar] [CrossRef]
- Cao, Y.; Huang, L. Research on the Healing Effect Evaluation of Campus’ Small-Scale Courtyard Based on the Method of Semantic Differential and the Perceived Restorative Scale. Sustainability 2023, 15, 8369. [Google Scholar] [CrossRef]
- Alnusairat, S.; Al-Shatnawi, Z.; Ayyad, Y.; Alwaked, A.; Abuanzeh, N. Rethinking Outdoor Courtyard Spaces on University Campuses to Enhance Health and Wellbeing: The Anti-Virus Built Environment. Sustainability 2022, 14, 5602. [Google Scholar] [CrossRef]
- Farid, M.; Mohamed, S.; Nasr, S.; Assistant, T. Keywords-Academic Open Spaces (AOS), Social Health, Physical Health, Mental Health. Available online: https://www.iol.co.za/ (accessed on 20 May 2025).
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Chen, Z.; He, Y.; Yu, Y. Attention restoration during environmental exposure via alpha-theta oscillations and synchronization. J. Environ. Psychol. 2020, 68, 101406. [Google Scholar] [CrossRef]
- Chang, S.; Jun, H. Hybrid deep-learning model to recognise emotional responses of users towards architectural design alternatives. J. Asian Archit. Build. Eng. 2019, 18, 381–391. [Google Scholar] [CrossRef]
- Wu, J.; Zhong, Y.; Wang, Y.; Gong, C. Investigating the Relation between Visitor Attention and Visual Quality of Forest Landscape: A Mobile EEG Study. Forests 2022, 13, 1668. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhuo, K.; Wei, W.; Li, F.; Yin, J.; Xu, L. Emotional responses to the visual patterns of urban streets: Evidence from physiological and subjective indicators. Int. J. Environ. Res. Public Health 2021, 18, 9677. [Google Scholar] [CrossRef]
- Li, Z.; Kang, J. Sensitivity analysis of changes in human physiological indicators observed in soundscapes. Landsc. Urban Plan. 2019, 190, 103593. [Google Scholar] [CrossRef]
- Song, R.; Chen, Q.; Zhang, Y.; Jia, Q.; He, H.; Gao, T.; Qiu, L. Psychophysiological restorative potential in cancer patients by virtual reality (VR)-based perception of natural environment. Front. Psychol. 2022, 13, 1003497. [Google Scholar] [CrossRef]
- Ren, H.; Zheng, Z.; Zhang, J.; Wang, Q.; Wang, Y. Electroencephalography (EEG)-Based Comfort Evaluation of Free-Form and Regular-Form Landscapes in Virtual Reality. Appl. Sci. 2024, 14, 933. [Google Scholar] [CrossRef]
- Guo, L.N.; Zhao, R.L.; Ren, A.H.; Niu, L.X.; Zhang, Y.L. Stress recovery of campus street trees as visual stimuli on graduate students in autumn. Int. J. Environ. Res. Public Health 2020, 17, 148. [Google Scholar] [CrossRef]
- Nota, A.; Tecco, S.; Ehsani, S.; Padulo, J.; Baldini, A. Postural stability in subjects with temporomandibular disorders and healthy controls: A comparative assessment. J. Electromyogr. Kinesiol. 2017, 37, 21–24. [Google Scholar] [CrossRef]
- Olszewska-Guizzo, A. Neuroscience for Designing Green Spaces; Routledge: New York, NY, USA, 2023. [Google Scholar] [CrossRef]
- Stevenson, M.P.; Dewhurst, R.; Schilhab, T.; Bentsen, P. Cognitive Restoration in Children Following Exposure to Nature: Evidence From the Attention Network Task and Mobile Eye Tracking. Front. Psychol. 2019, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-Y.; Hammitt, W.E.; Chen, P.-K.; Machnik, L.; Su, W.-C. Psychophysiological responses and restorative values of natural environments in Taiwan. Landsc. Urban Plan 2008, 85, 79–84. [Google Scholar] [CrossRef]
- Ulrich, R.S. Natural Versus Urban Scenes. Environ. Behav. 1981, 13, 523–556. [Google Scholar] [CrossRef]
- Grassini, S.; Revonsuo, A.; Castellotti, S.; Petrizzo, I.; Benedetti, V.; Koivisto, M. Processing of natural scenery is associated with lower attentional and cognitive load compared with urban ones. J. Environ. Psychol. 2019, 62, 1–11. [Google Scholar] [CrossRef]
- Koivisto, M.; Jalava, E.; Kuusisto, L.; Railo, H.; Grassini, S. Top-Down Processing and Nature Connectedness Predict Psychological and Physiological Effects of Nature. Environ. Behav. 2022, 54, 917–945. [Google Scholar] [CrossRef]
- Neale, C.; Aspinall, P.; Roe, J.; Tilley, S.; Mavros, P.; Cinderby, S.; Coyne, R.; Thin, N.; Ward Thompson, C. The impact of walking in different urban environments on brain activity in older people. Cities Health 2020, 4, 94–106. [Google Scholar] [CrossRef]
- Brielmann, A.A.; Buras, N.H.; Salingaros, N.A.; Taylor, R.P. What Happens in Your Brain When You Walk Down the Street? Implications of Architectural Proportions, Biophilia, and Fractal Geometry for Urban Science. Urban Sci. 2022, 6, 3. [Google Scholar] [CrossRef]








| Spatial Form | Description | Psychological Effect |
|---|---|---|
| Plaza | Central plaza for gatherings and events | Fosters social interaction and reduces stress |
| Courtyards | Spaces surrounded by buildings that could include greenery and seating | Encourages relaxation and restoration; enhances collaboration |
| Pedestrian promenades | Pathways connecting campus areas | Promotes mental relaxation and focus |
| Main building quadrangle | Square spaces surrounded by academic buildings | Enhances community; fosters informal learning |
| Amphitheatre | Venue for cultural events | Boosts creativity and mental rejuvenation |
| Lawns and gardens | Green spaces | Provides mental relaxation; enhances creativity |
| Tree-lined pathways | Shaded paths for leisure | Encourages peaceful reflection |
| Aspect of Configuration | Description | Psychological Effect | Brainwave Band Associated |
|---|---|---|---|
| Degree of enclosure | High enclosure (elevated walls) provides shade and cooler environments. | Mental restoration and relaxation | Alpha and Theta |
| Open layouts increase natural illumination. | Improved visual attractiveness; reduced stress | Alpha, Beta, and Gamma | |
| Shape | Rectangular/square courtyards optimize shade and comfort. | Mental restoration; reduced anxiety | Theta and Beta |
| U-shaped courtyards (east–west orientation) improve ventilation. | Enhanced comfort and relaxation | Alpha | |
| Curvilinear shapes promote natural movement and openness. | Increased happiness; reduced tension | Gamma, Beta, and Alpha | |
| Dimensions | Expansive courtyards enhance natural light. | Improved mood; reduced fatigue | Alpha, Beta, and Theta |
| Small courtyards near study areas offer quick access to tranquility. | Better concentration and relaxation | Alpha |
| Visual Stimuli | Description | Psychological Effect | Band Associated |
|---|---|---|---|
| Greenery (vegetation, trees, and shrubs) | High green coverage (GVI 30–90%) | Pleasure; relaxation; stress reduction | Alpha, Beta, and Theta |
| Water elements (fountains and ponds) | Presence of blue spaces (BSs) | Happiness; openness; reduced anxiety | Gamma and Beta |
| Comfortable seating and shade | Encourages social interaction and rest | Sense of belonging; mental restoration | Alpha Theta |
| Natural lighting | Optimized through courtyard shape and orientation | Reduced mental fatigue; improved focus | Alpha and Beta |
| Color variation and naturalness | Brightness, rhythm, and order in landscape design | Relaxation | Alpha |
| Landscape diversity (lawn, forest, and horticulture) | Mixed natural elements | Alleviation of anxiety and depression | Alpha and Beta |
| Landmark features | Visual cues providing feelings of safety | Cognitive wayfinding and navigation | Theta |
| ID | Gender | Experience | Sensitivity | Theta Band | Alpha Band | Beta Band | Gamma Band |
|---|---|---|---|---|---|---|---|
| P1 | Female | Unfamiliar with courtyard; no EEG experience | Moderate | Stable | Random decreases (disengagement) | Sporadic-to-intense increases (attention peaks) | Stable |
| P2 | Female | Unfamiliar with courtyard; no EEG experience | High | Occasional decreases (disengagement) | Rare increases (mild alertness) | Stable | Stable |
| P3 | Female | Unfamiliar with courtyard; no EEG experience | Low | Sporadic increases (enhanced relaxation) | Stable | Sporadic decreases (reduced focus) | Frequent decreases; slight increases |
| P4 | Female | Unfamiliar with courtyard; no EEG experience | Moderate | Stable | Random decreases (disengagement) | Sporadic-to-intense increases (attention peaks) | Occasional increases (cognitive engagement) |
| P5 | Female | Unfamiliar with courtyard; no EEG experience | High | Occasional decreases (disengagement) | Rare increases (mild alertness) | Stable | Stable |
| P6 | Female | Unfamiliar with courtyard; no EEG experience | Low | Occasional decreases (disengagement) | Stable | Sporadic decreases (reduced focus) | Frequent decreases; slight increases |
| P7 | Female | Unfamiliar with courtyard; no EEG experience | Moderate | Stable | Random decreases (disengagement) | Sporadic-to-intense increases (attention peaks) | Occasional increases (cognitive engagement) |
| P8 | Female | Unfamiliar with courtyard; no EEG experience | High | Mix of decreases and pronounced increases (relaxation) | Rare increases (mild alertness) | Stable | Stable |
| P9 | Female | Unfamiliar with courtyard; no EEG experience | Low | Sporadic increases (enhanced relaxation) | Stable | Sporadic decreases (reduced focus) | Frequent decreases; slight increases |
| P10 | Male | Unfamiliar with courtyard; no EEG experience | Moderate | Stable | Random decreases (disengagement) | Sporadic-to-intense increases (attention peaks) | Occasional increases (cognitive engagement) |
| P11 | Male | Unfamiliar with courtyard; no EEG experience | Low | Frequent increases (large relaxation) | Rare increases (mild alertness) | Stable | Stable |
| P12 | Male | Unfamiliar with courtyard; no EEG experience | High | Frequent increases (large relaxation) | Stable | Sporadic decreases (reduced focus) | Frequent decreases; slight increases |
| P13 | Male | Unfamiliar with courtyard; no EEG experience | Moderate | Frequent increases (large relaxation) | Stable | Sporadic-to-intense increases (attention peaks) | Occasional increases (cognitive engagement) |
| P14 | Male | Unfamiliar with courtyard; no EEG experience | High | Occasional decreases (disengagement) | Frequent increases (increased engagement) | Stable | Stable |
| P15 | Male | Unfamiliar with courtyard; no EEG experience | Low | Frequent increases (large relaxation) | Stable | Sporadic decreases (reduced focus) | Predominantly stable |
| P16 | Male | Unfamiliar with courtyard; no EEG experience | Moderate | Frequent increases (large relaxation) | Constant increases (significant engagement) | Strong, frequent increases (heightened cognitive processing) | Uniform, high increases (cognitive engagement) |
| Band Power Analysis with Epoch-Based Power Distribution | Theta | Low Alpha | High Alpha | Low Beta | High Beta | Low Gamma | Mid Gamma |
|---|---|---|---|---|---|---|---|
| Baseline Period (1 min) | |||||||
| Mean (%) | 23.088 | 18.60 | 16.787 | 10.583 | 7.462 | 5.3742 | 3.7035 |
| Stimulus Period (4 min) | |||||||
| Mean (%) | 26.353 | 16.94 | 13.158 | 9.993 | 8.280 | 5.9377 | 4.3703 |
| Change (Stimulus–Baseline) | |||||||
| Mean Δ (%) | +3.265 | −1.666 | −3.630 | −0.59 | +0.81 | 0.5635 | 0.6667 |
| Period | Theta (%) | Low Alpha (%) | High Alpha (%) | Low Beta (%) | High Beta (%) | Low Gamma (%) | Mid Gamma (%) |
|---|---|---|---|---|---|---|---|
| Baseline (1 Min) | 23.088 | 18.607 | 16.787 | 10.583 | 7.4618 | 5.3742 | 3.7035 |
| Stimulus (70–240s) | 26.455 | 16.905 | 13.086 | 9.9942 | 8.243 | 5.9248 | 4.4565 |
| Change (Δ) | +3.3673 | −1.7014 | −3.7016 | −0.58925 | +0.78115 | +0.55065 | +0.75305 |
| Design Aspect | Space Features | EEG Implications | Design Recommendations |
|---|---|---|---|
| Enclosure degree | Enclosed courtyard with visible sky | Enhances Theta activity (linked to psychological restoration) | Avoid overly wide/open courtyards to preserve a sense of containment and refuge. |
| (Height-to-width proportion) | approx. 1:1 (15 m height/~15 m width) |
| |
| Vegetation-to-hardscape ratio | ~35% vegetation/65% hardscape | Sufficient to induce Theta enhancement while supporting mild cognitive engagement |
|
| Spatial configuration and furniture placement | Linear layout with central walkway and benches along vegetation zones | Seating near vegetation and enclosed edges aligns with increased Theta activity |
|
| Acoustic and sensory design | Subtle natural stimuli (leaf rustling and wind) | Mild cognitive activation and sensory engagement: Alpha suppression, low Gamma near to significance, and restoration (Theta increase) |
|
| Familiarity vs. Fascination | Slightly unfamiliar courtyard layout with distinctive planting design | Novelty triggers involuntary attention (Alpha modulation) |
|
| Seasonal and climatic considerations | Comfortable microclimate at 22–26 °C, clear days | Thermal comfort likely enhances restoration |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anwar, R.; Elkhateeb, S.; Afifi, S.; Bayoumi, K. Brainwave Dynamics: Neurophysiological Responses to Enclosed Courtyards for Mental Wellbeing in Educational Contexts. Architecture 2025, 5, 76. https://doi.org/10.3390/architecture5030076
Anwar R, Elkhateeb S, Afifi S, Bayoumi K. Brainwave Dynamics: Neurophysiological Responses to Enclosed Courtyards for Mental Wellbeing in Educational Contexts. Architecture. 2025; 5(3):76. https://doi.org/10.3390/architecture5030076
Chicago/Turabian StyleAnwar, Raneem, Samah Elkhateeb, Samy Afifi, and Karim Bayoumi. 2025. "Brainwave Dynamics: Neurophysiological Responses to Enclosed Courtyards for Mental Wellbeing in Educational Contexts" Architecture 5, no. 3: 76. https://doi.org/10.3390/architecture5030076
APA StyleAnwar, R., Elkhateeb, S., Afifi, S., & Bayoumi, K. (2025). Brainwave Dynamics: Neurophysiological Responses to Enclosed Courtyards for Mental Wellbeing in Educational Contexts. Architecture, 5(3), 76. https://doi.org/10.3390/architecture5030076







