1. Introduction
The
Filoviridae family includes two genera, Ebolavirus and Marburgvirus [
1], both of which have caused numerous outbreaks with high fatality rates over recent decades [
2]. Marburgvirus was first identified in 1967 during outbreaks in Germany and the former Yugoslavia, linked to infected nonhuman primates imported from Uganda. Since then, it has emerged sporadically across East, Central, and South Africa, causing over 14 outbreaks, including major epidemics in the Democratic Republic of the Congo (DRC) (1998–2000) and Angola (2004–2005) [
3]. Recently, new outbreaks have been reported in Guinea (2021), Ghana (2022), Equatorial Guinea and Tanzania (2023), and Rwanda (2024). Ebola viruses were first identified in 1976 during concurrent outbreaks in Zaire (now the DRC) and Sudan (now South Sudan). Since then, Ebola viruses have caused more than 40 outbreaks across sub-Saharan Africa with the 2013–2016 West African epidemic resulting in 28,652 cases and 11,325 deaths [
3].
In addition to their structural and genetic similarities, the diseases caused by these viruses, Ebola virus disease (EVD) and Marburg virus disease (MVD), also exhibit similar clinical manifestations [
4]. Once introduced into human populations, Ebolavirus and Marburgvirus can be transmitted from human to human via direct or indirect contact with the body fluids of an infected individual [
5].
Ebola and Marburg treatment centers (TCs) play a crucial role in responding to these outbreaks by providing a safe environment for patient care and reducing transmission risks through the isolation of infectious patients [
6,
7]. Furthermore, the need for designated treatment centers closer to areas of transmission has been emphasized to enhance the quality of care for patients and mitigate transmission risks [
8].
The Western Africa Ebola outbreak of 2013–2016 and the North Kivu and Ituri outbreak in the DRC in 2018–2019 reported a total of 32.080 accounting for over 90% of the cumulative EVD cases since the virus’s [
9]. The unprecedented scale and duration of these outbreaks catalyzed a surge in scientific research, leading to the development of specific vaccines [
10] and novel experimental treatments [
11]. While clinical care for EVD and MVD has significantly evolved and is well-documented [
12,
13], the design of treatment centers has been poorly addressed.
In 1999, the humanitarian organization Médecins Sans Frontières (MSF) published a summary of their intervention in response to the 1995 Ebola outbreak in Kikwit, DRC [
14]. The MSF team established the isolation ward with three distinct zones, infected, clean and neutral. The repurposing of an existing building into the infected area allowed to hospitalize up to 20 patients. A clean area for healthcare workers (HCWs) and a neutral zone for family members were created with four tents.
In 2007, Always MSF published the lessons learned from their intervention in the Marburg outbreak in Uige, Angola, which occurred in 2005 [
15]. The completed ward featured four distinct structures: two permanent buildings for suspected or probable cases, one permanent building for confirmed cases, and a large temporary ward kept in reserve. According to the authors, the design allowed adequate spacing between suspected and probable cases, thereby reducing the risk of cross-contamination. Separate entry and exit points were established for staff and for patients and relatives. However, the distance between these points, combined with the location of the “clean” nurses’ area at the back of the complex, hindered communication between patients and staff.
In early 2020, Yang Luo and colleagues shared their experience with the construction of the ‘China Ebola TC’ in Liberia during the 2014–2015 Ebola outbreak [
16]. The TC utilized semi-permanent prefabricated structures equipped with air conditioning and video monitoring systems. Patients were separated as suspected, probable, and confirmed cases and occupancy was limited to two patients per room. To minimize infection risks, the TC used two one-way routes for clean and contaminated materials. The center employed a three-zone approach: the green zone (clean area) included offices and stations; the red zone (contaminated area) housed patients; and the yellow zone (semi-contaminated area) served as a buffer with lower contamination risk.
In 2016, Jianping You and Qing Mao shared their experience in establishing an Ebola TC in Monrovia, Liberia, during the late 2014 Ebola outbreak [
17]. This center featured 16 buildings, including clinics, inpatient wards, a disinfection area, a morgue, and training centers, with a total capacity of 100 beds. The TC was constructed with prefabricated boards. It included a three-zone system (contaminated, potentially contaminated, and clean areas), buffering zones, and clean and contaminated pathways to limit cross-contamination. The TC also incorporated features like air conditioning and video monitoring. Initial ventilation issues were noted and later addressed.
Following the 2014–2016 Ebola outbreak in West Africa, MSF undertook a comprehensive review to distill the lessons learned from constructing multiple TCs across Liberia, Sierra Leone, and Guinea [
18,
19]. The objective was to create a compendium of best practices and practical recommendations to guide the design and construction of future centers. The review underscores that effective TC design and construction are dynamic processes that must evolve to meet the specific demands of each outbreak, ensuring both immediate response capabilities and long-term sustainability. Despite the important findings, the review falls short in providing concrete design recommendations.
Besides the MSF’s review and the brief descriptions presented above, scientific literature lacks comprehensive studies on this topic. Considering the important role of these centers and the complexity behind their construction, understanding their evolution is essential for improving the design, efficacy, and safety of future facilities.
This study examines the layouts of Ebola and Marburg treatment centers, designed and built between 2014 and 2023 by various humanitarian organizations, with the aim of describing and documenting the progression in their design and construction methodologies.
2. Materials and Methods
To gather comprehensive data on the design of Ebola and Marburg treatment centers, members of INITIATE
2 project were consulted. INITIATE
2 is a collaborative initiative of the World Health Organization (WHO) and the World Food Programme (WFP), bringing together emergency response actors, research institutions, and academic bodies to develop innovative and standardized solutions and trainings to enhance readiness and response capabilities in outbreak situations [
20]. Participants from INITIATE
2 were invited to share technical drawings, reports, and photographs of treatment centers constructed by their respective organizations over the past decade. To maximize the number of layouts in the analysis, a systematic search was performed in the MEDLINE, Embase, and Google Scholar databases on 14 January 2024, using free-text terms reflecting the eligibility criteria. The search was adapted for each database with ‘MeSH’ filters where appropriate (the search strategy and eligibility criteria are available in the
supplementary information, Tables S1 and S2, respectively). Two authors independently assessed the retrieved references against the eligibility criteria and performed data extraction.
All materials received through the consultation and retrieved through literature review were reproduced using AutoCAD 2021. A unique ID was provided for each TC and standardized coding scheme was developed to ensure consistency across all layouts. Detailed categorization allowed for precise quantification of space allocation and spatial relationships within the centers. In cases of doubt, the provider institution and authors were consulted for clarification. To understand the evolution of design over time, the layouts were divided into three periods: 2014–2017, 2018–2020, and 2021–2023.
A reverse engineering technique [
21] was employed to systematically analyze the technical drawings of treatment centers and extract key design and functionality metrics. Reverse engineering involves deconstructing existing systems, in this case, treatment center layouts, to understand how their design evolved over time and to identify critical elements that contribute to their effectiveness. This approach allowed for the assessment of the spatial dynamics of the centers and how they adapted to the challenges posed by managing infectious diseases. A data extraction tool was developed (available in
supplementary information Table S3) to extract relevant information pertaining to the design and functionality of the treatment centers according to specific spaces as defined in
Table 1. Key metrics included bed capacities, area allocation per functional space and patient, and the ratio of different zones (high-risk, low-risk, outdoor, etc.). Accessibility was evaluated based on standards for healthcare facilities [
22] and focused on features like ramps, wide doorways, and navigability for individuals with disabilities in key areas such as waiting rooms, screening/triage areas, and patient rooms. The treatment centers were categorized as follows based on the construction method used (examples are provided in
Figure S1 in the supplementary information):
Temporary: Centers set-up with tents.
Semi-permanent: Centers built with a combination of concrete slabs for flooring, timber for the structural elements, metal corrugated roofing for protection against the elements, and plastic sheeting for vertical enclosures.
Permanent: Existing buildings repurposed for the outbreak response.
For selected areas, the study assessed the level of air and natural daylight exposure using the window-to-floor ratio as a proxy indicator [
23]. This ratio was calculated by comparing the surface area of openings (such as doors and windows) to the floor surface area. Natural daylight and ventilation levels were considered satisfactory when the window-to-floor ratio was ≥12% or 1/8 [
23]. To ensure robustness, a subset of the layouts and analyses were independently reviewed by external experts in healthcare architecture and infectious prevention and control. Feedback from these experts was incorporated into the final analysis to enhance the reliability and validity of the results.
Table 1.
Definitions for spaces included in the analysis.
Table 1.
Definitions for spaces included in the analysis.
| Physical Spaces Assessed | Functions and Definitions |
|---|
| Ambulance bay | Area reserved for the arrival of the ambulance and the transfer of patients [24]. |
| Waiting area | The place where patients with symptoms wait to be taken care of by health personnel [24]. |
| Screening and triage | Screening is the process in which an individual is evaluated to see whether that person meets a standardized case definition [5].
Triage is the process of sorting patients into categories based on the need for time-sensitive treatment using validated tools. Triage identifies those who require immediate medical intervention, and those who can safely wait. Triage may occur at a health post, primary health center, clinic, or emergency unit. It typically requires close physical contact (within 1 m) with the patient during the assessment [5]. Within Ebola or Marburg treatment centers, often screening and triage take place in the same physical space; therefore, for the sake of this study, these areas were assessed and labeled as screening/triage areas. |
| Morgue | Morgue is the designated area within the treatment center for the temporary storage of deceased patients’ bodies pending final disposition [25]. This space may also feature a designated observation area that allows family members to safely view the deceased. |
| Offices | Dedicated spaces within the treatment center designated for administrative and operational staff to perform non-clinical duties essential to the facility’s functioning. These areas are structured to support tasks such as data management, coordination of services, and communication without direct exposure to the pathogens [26]. |
| Waste area | Waste area is a specifically allocated space within the treatment center dedicated to storage and management of medical and non-medical waste generated by the facility’s operations [27]. |
| Suspect patients’ area | This is a designated section within the treatment center specifically allocated for the isolation and clinical management of individuals who meet the case definition for a suspected case [28]. |
| Confirmed patients’ area | This is a designated section within the treatment center specifically allocated for the isolation and clinical management of individuals who meet the case definition for confirmed case [28]. |
| Intensive care unit | In the context of this study, an “Intensive Care Unit” (ICU) can be defined as a specialized section dedicated to providing advanced medical care and continuous monitoring for patients suffering from severe and life-threatening manifestations of Ebola or Marburg virus diseases. |
| Obstetric and delivery room | In the context of this study, an “Obstetric and Delivery Room” refers to a specially designated area where pregnant women diagnosed with Ebola or Marburg virus diseases receive focused care during labor and delivery. |
| Laboratory service | In the context of this study, “Laboratory Service” refers to a dedicated facility designed to perform a wide range of medical diagnostics, including the detection of Ebola and Marburg viruses using Polymerase Chain Reaction (PCR) testing, as well as various biochemistry analyses and other diagnostic procedures. |
| Children and family spaces | In the context of this study, “Children and Family Spaces” are specialized areas designed to support the unique needs of children and families of patients undergoing treatment for these severe viral infections. |
| Outdoor area | In the context of this study, this is a designated outdoor space for suspect and confirmed patients. |
| Visitor area | In the context of this study, this is a designated space within or outside the treatment center designed to accommodate visitors, allowing for controlled interaction with patients. |
| Low-risk area | In the context of this study, the low-risk area is a designated section within the treatment center allocated for activities and functions that involve no direct exposure to the pathogens. This area, also referred to as the staff area, is where the presence of the virus is not expected. |
| High-risk area | In the context of this study, the high-risk area is a designated section within the treatment center allocated for activities and functions that involve direct exposure to the pathogens. This area, also referred to as the patients’ area, is where the presence of the virus is expected. |
| Donning area | A specifically designated space within the treatment center where healthcare workers and other staff members put on personal protective equipment (PPE) [5]. |
| Doffing area | A specifically designated space within the treatment center where healthcare workers and other staff members take off PPE [5]. |
3. Results
Through consultation, architectural drawings and photographs of 47 treatment centers were obtained: 16 from 2014 to 2017, 19 from 2018 to 2020, and 12 from 2021 to 2023. From the database search, 606 articles were retrieved. After removing duplicates, 512 articles remained for title and abstract screening. Following this, 38 articles were eligible for full-text review. After applying eligibility criteria, one article presenting a TCs built in 2014 was included (
supplementary information Figure S2). The included paper is a conference proceeding describing the role of water, sanitation, and hygiene within a TC built in Sierra Leone [
29]. In total, 48 technical drawings were included for the morphological analysis. More details on the layouts are available in
supplementary information Table S4.
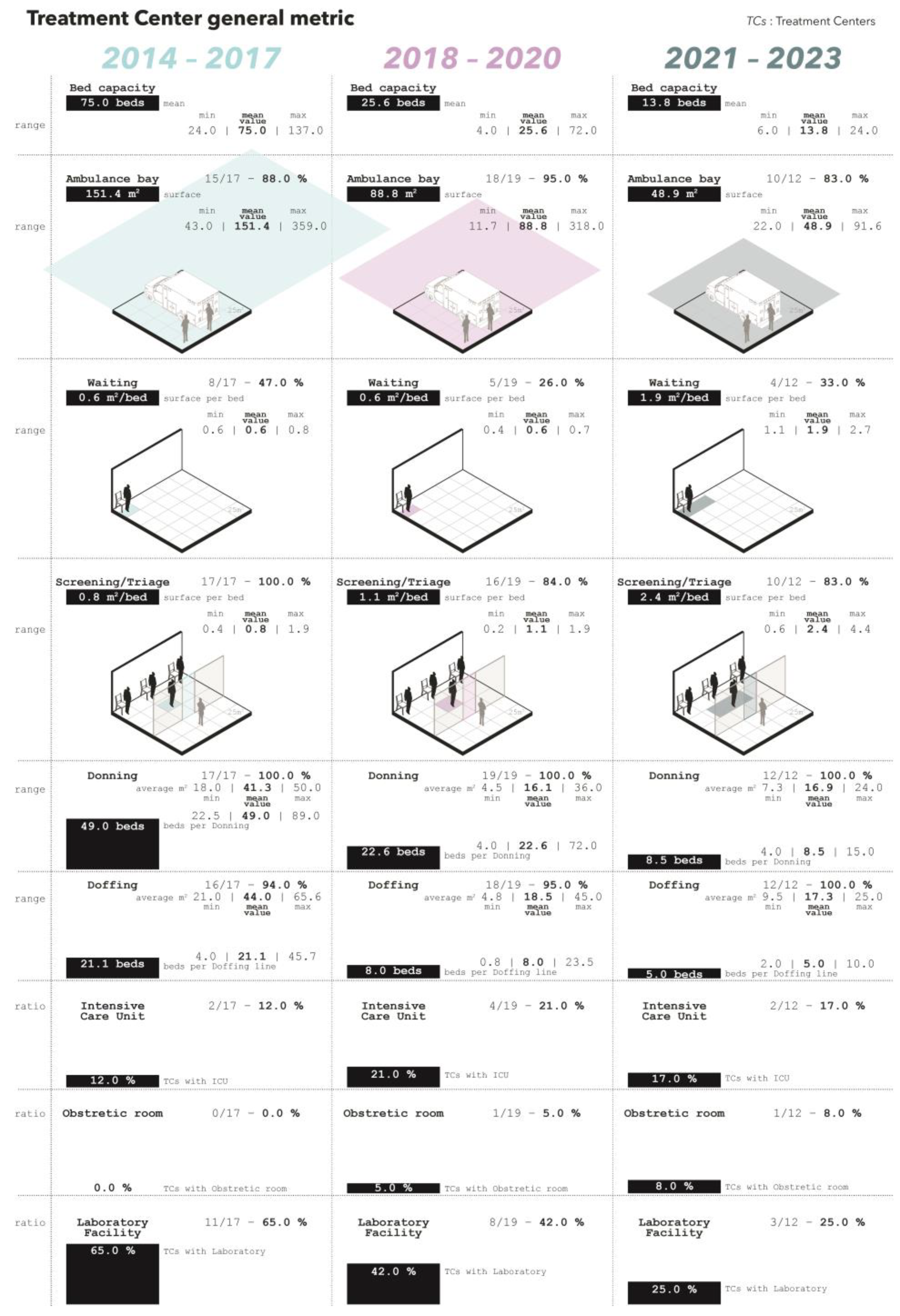
This study analyzes the morphological characteristics of Ebola and Marburg virus treatment centers designed and constructed between 2014 and 2023, focusing on key metrics as presented hereafter. General metrics evolutions are detailed in
Figure 1.
3.1. Bed Capacity
The mean bed capacity of the treatment centers varied across the three periods starting with 75.0 beds in 2014–2017 to 25.6 and 13.8 in 2018–2020 and 2021–2023, respectively.
3.2. Ambulance Bay
An ambulance bay was present in 15 (88%) TCs built between 2014 and 2017. Similarly, between 2018–2020 and 2021–2023, 18 (95%) and 10 (83%) TCs featured a dedicated ambulance bay. The surface dedicated to the ambulance bay varied across the three periods, averaging 151.4 square meters (m2) in 2014–2017, 88.8 m2 in 2018–2020, and 48.9 m2 in 2021–2023. Due to the lack of details in the layouts, it was not possible to estimate the number of vehicles accommodated in the ambulance bays.
3.3. Waiting Area
From 2014 to 2017, eight (47%) facilities incorporated waiting areas. This dropped to five (26%) in 2018–2020 and four (33%) in 2021–2023. The surface area per patient bed increased from 0.6 m2/bed (2014–2020) to 1.9 m2/bed (2021–2023). Approximately 75% of waiting areas were separate from screening/triage sections (2014–2017), reaching 100% in 2018–2020 but dropping to 50% in 2021–2023. Toilet facilities were available in five (29%) centers (2014–2017), two (11%) in 2018–2020, and four (33%) in 2021–2023. Accessibility compliance decreased from 50% (2014 to 2017) to 40% (2018–2020) and 25% (2021–2023).
3.4. Screening/Triage
Although implying two separate activities, the space allocated to screening and triage were consistently merged into a single area throughout the considered periods and available in all TCs built between 2014 and 2017. During 2018–2020 and 2021–2023, 16 (84%) and 10 (83%) TCs included a dedicated screening/triage space, respectively. The average surface per patient bed increased from 0.8 m2/bed (2014–2017) to 1.1 m2/bed (2018–2020) and 2.4 m2/bed (2021–2023). Discharge showers, adjacent to screening/triage, were in three centers (18%) during 2014–2017, two centers (10.5%) during 2018–2020, and two centers (16.7%) in 2021–2023. Accessibility standards were met in 13 (82%) centers (2014–2017), eight (50%) centers (2018–2020), and six (60%) centers (2021–2023).
3.5. Donning
Donning areas (where HCWs put on PPE) were always present across all three study periods, with their locations within the TCs adhering to a pattern of straddling low- and high-risk zones (94% in 2014–2017, 84% in 2018–2020, and 83% in 2021–2023, respectively). In the remaining instances, the donning areas were situated within low-risk zones yet near high-risk areas. The average surface of the donning areas moved from 41.3 m2 in 2014–2017 to 16.1 m2 and 16.9 m2 in 2018–2020 and 2021–2023, respectively. The ratio of the donning area to number of beds changed across the three periods starting at 49.0 beds per donning area in 2014–2017, to 22.6 beds and 8.5 beds in 2018–2020 and 2021–2023, respectively.
3.6. Doffing
Doffing areas (where HCWs take off PPE) were uniformly present throughout the study periods, invariably positioned to bridge the low- and high-risk zones within the TCs. Predominantly, these areas were designed as open structures, shielded only by a roof. Closed rooms, defined by walls on four sides, housed doffing in a minority of facilities, accounting for 19%, 6%, and 33% in the respective periods of 2014–2017, 2018–2020, and 2021–2023. Between 2014 and 2017, the ratio of doffing lines, defined as the allocated space where a single staff member could safely take off the PPE, was one per 21.1 beds. This reduced to one doffing line per 8.0 beds in the 2018–2020 period and further to one per 5.0 beds in 2021–2023. Similarly, the average surface per doffing area decreased from 44 m2 to 18.5 m2 and 17.3 m2 from the oldest to the most recent period. Concurrently, the allocated surface area for each doffing line diminished from 19.5 m2 in 2014–2017 to 9.7 m2 in 2018–2020, and to 8.6 m2 in the 2021–2023 timeframe.
3.7. Intensive Care and Obstetric Rooms
Dedicated rooms for intensive care were only available in two (12%) of the centers built in 2014–2017. This increased to four (21%) and two (17%) in 2018–2020 and 2021–2023, respectively. In 2014–2017 there were no spaces dedicated to obstetric care. In the following two periods, only one center per period had a space allocated to obstetric care corresponding to 5% and 8% of the total facilities built.
3.8. Laboratory Facility
From 2014 to 2017, 11 (65%) treatment centers included a space dedicated to laboratory service. Between 2018 and 2020, eight (42%) facilities presented this feature while only three (25%) presented this feature in the last period.
3.9. Suspected Patients
From 2014 to 2017 all TCs (100%) included beds for suspected patients (
Figure 2). However, only six (35%) of these TCs provided individual rooms, and of these, only 12.5% were equipped with individual toilet and shower facilities. During the period from 2018 to 2020, only 13 (68%) centers provided beds for suspected patients. However, 11 (85%) of these centers provided individual rooms, and of these, 77% were equipped with individual toilet and shower facilities. In the latest timeframe, nine (75%) centers featured beds for suspected patients. All of these beds were located in individual rooms, each equipped with its own toilet and shower facilities.
From 2014 to 2017, the average surface was 9.7 m2 per suspected bed. This expanded to 11.0 m2/ bed in the 2018 to 2020 period and to 12.9 m2/bed in 2020–2023. Similarly, the volume allocated per patient also exhibited a similar trend, with values reported as 29.0 cubic meters per patient (m3/bed) in 2014–2017, decreasing to 28.5 m3/bed in 2018–2020, before increasing to 34.9 m3/bed in 2021–2023. No outdoor areas were available for suspected patients throughout the considered periods.
During the whole period under review, all suspect areas within the TCs relied on natural ventilation. From 2014 to 2017, 16 (94%) facilities provided at least two openings (window and door) per patient. The average window-to-floor ratio was 0.2 in suspect patient’s wards and 0.4 in individual rooms, with 76% of the treatment centers achieving a window-to-floor ratio above 1/8. In the 2018–2020 interval, eight (62%) centers recorded the presence of at least two openings per patient, with individual rooms having an average window-to-floor ratio of 0.3. Nonetheless, 88% of these facilities maintained a window-to-floor ratio above 1/8. From 2021 to 2023, all suspect areas featured at least two openings per patient. The average window-to-floor ratio for individual rooms remained at 0.3, and all the facilities had a window-to-floor ratio above 1/8. When assessing the accessibility of suspected patients’ areas, from 2014 to 2017, 15 (88%) centers met the standards for accessibility. However, only five centers for the 2018–2020 (38%) and 2021–2023 (56%) periods met the accessibility criteria.
During the 2014–2017 period, only one (6%) center offered direct visibility on patients’ rooms from the low-risk area. This increased to four (31%) centers in 2018–2020 and seven (78%) in the last period.
3.10. Confirmed Patients
The analysis indicates that, between 2014 and 2017, 100% of the facilities included beds specifically allocated for confirmed patients (
Figure 3). All these beds were situated in shared wards and 82% included an outdoor area for patients with an average of 17.5 m
2/bed.
Between 2018 and 2020, 18 (95%) TCs provided beds for confirmed patients of which 12 (67%) of these beds were now situated in individual rooms. Among them, 10 (56%) were equipped with individual toilet and shower facilities. The presence of an outdoor area remained steady at 83% and 16.3 m2/bed.
From 2021 to 2023, 11 (92%) of the facilities provided beds for confirmed patients. Notably, 10 (91%) of these centers provided individual rooms, all with individual toilet and shower facilities. The outdoor area was available in only seven (73%) TCs with an average of 34.2 m2/bed.
The average surface per bed increased from 9.2 m2/bed from 2014 to 2017 to 11.0 m2/bed and 13.0 m2/bed in the subsequent periods of 2018–2020 and 2021–2023, respectively. The allocated volume per patient was 27.3 m3/bed during the 2014–2017 period. This decreases to 24.7 m3/bed in the 2018–2020 interval, followed by a rise to 34.3 m3/bed in the 2021–2023 period.
During the whole period under review, all confirmed areas within the TCs were ventilated naturally. From 2014 to 2017, 14 (82%) TCs provided at least two openings per bed. The average window-to-floor ratio was 0.2 with 79% of the TCs achieving a window-to-floor ratio exceeding 1/8. In the 2018–2020 interval, the presence of at least two openings per patient was achieved in 11 (61%) confirmed areas. For shared wards, the average window-to-floor ratio was 0.18 while individual rooms achieved a ratio of 0.29. Of these facilities, 82% maintained a window-to-floor ratio above 1/8. In the period from 2021 to 2023, all confirmed areas featured at least two openings per patient. The average window-to-floor ratio for individual rooms remained steady at 0.29, and 91% of the facilities had a window-to-floor ratio above 1/8.
When assessing the accessibility of confirmed patients’ areas, from 2014 to 2017, 15 (88%) centers met the standards for accessibility. Conversely, only eight (44%) and five (45%) of the centers built from 2018 to 2020 and from 2021 to 2023 met the accessibility criteria, respectively.
In 2014–2017, only 18% of the centers offered direct visibility on patients’ rooms from the low-risk area. This increased to 39% and 73% during 2018–2020 and 2021–2023, respectively. Interestingly, several facilities use transparent screens as nurse stations equipped with biomedical devices (
Figure 4).
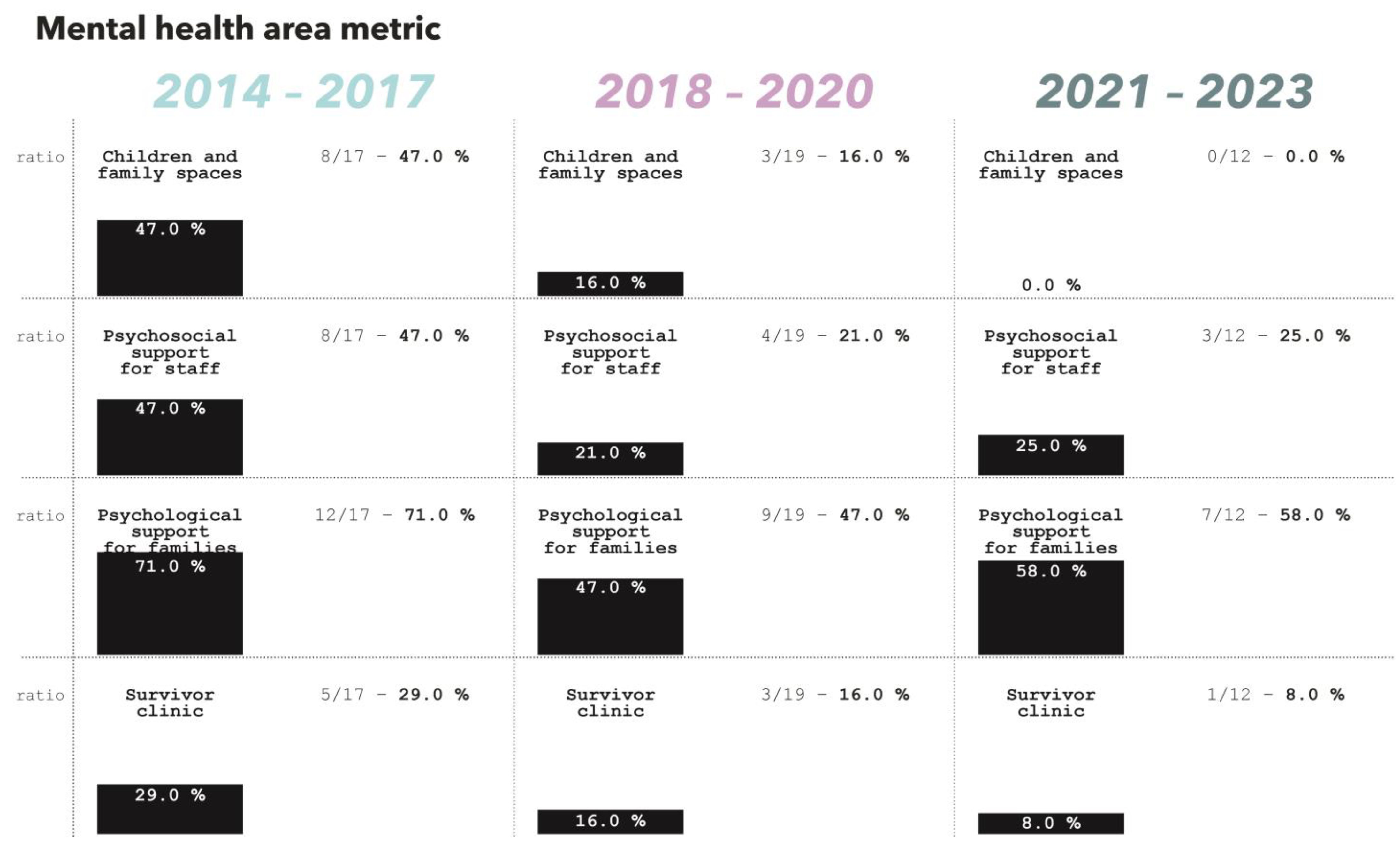
3.11. Mental Health and Psychosocial Support
Spaces and services dedicated to mental health and psychosocial support to families of patients and staff were common across the three studied periods (
Figure 5).
3.11.1. Children and Family Spaces
From 2014 to 2017, eight (47%) TCs included a dedicated space for children and families. Between 2018 and 2020, three (16%) facilities presented this feature while none in the last period.
3.11.2. Visitor Area and Psychosocial Support
Between 2014 and 2017, 15 (88.0%) facilities included a designated visitor area. In 20% of these cases, the visitor area was located within less than 10 m from the patient room/ward. Additionally, 67% of the visitor areas featured a roof.
Between 2018 and 2020, 12 (63%) facilities provided a visitor area. In 75% of these cases, the visitor area was within less than 10 m from the patient room/ward. However, the percentage of visitor areas with a roof decreased to 25%.
During the 2021–2023 period, only two (16.6%) centers provided a visitor area. For both these centers, the visitor area was within less than 10 m from the patient room/ward and in one center the visitor area featured a roof.
The average surface of visitor area per bed increased from 1.3 m2/bed during the years 2014 to 2017, to 7.9 m2/bed and 10 m2/bed in the periods 2018 to 2020 and 2021 to 2023, respectively.
Between 2014 and 2017, eight (47%) centers provided spaces for psychosocial support for staff, and 12 (71%) included spaces dedicated to psychological support for patients’ families. From 2018 to 2020, only four (21%) facilities provided spaces for staff support, and nine (47%) offered areas for family support. In the most recent period, psychosocial support spaces for staff were available in three (25%) centers, while those for family psychological support were present in seven (58%) facilities.
3.11.3. Survivor Clinic
From 2014 to 2017, five (29%) treatment centers included a space for the survivor clinic. Between 2018 and 2020, three (16%) facilities presented this feature while only one (8%) in the last period.
3.12. Supportive Services
A variety of supportive spaces such as offices, waste area and laundry, amongst others, were commonly present throughout the assessed period (more information available in
supplementary information Figure S3).
3.12.1. Office
Between 2014 and 2017, eight (70%) TCs featured a medical office, three (30%) incorporated a logistic office, six (60%) had an administrative office and four (40%) had a psychosocial office. The average surface for office per bed was 1.8 m2/bed with an average surface of 144.5 m2 dedicated to offices.
Between 2018 and 2020, eight (57%) of the facilities included a medical office, with two (14%) featuring a logistic office, five (36%) having an administrative office, and two (14%) providing a psychosocial area. The average surface for office per bed was 2.2 m2/bed with an average surface of 66.8 m2 allocated to offices.
Between 2021 and 2023, nine (82%) TCs presented a medical office. However, logistic and administrative offices were absent, while three (27%) included a psychosocial area. The average surface for office per bed was 2.8 m2/bed with an average surface per center of 30.6 m2.
3.12.2. Waste Area
From 2014 to 2017, 16 (94%) facilities included a waste area on-site. The average surface of these areas was 70.9 m2 corresponding to an average of 0.9 m2/bed. In the subsequent period between 2018 and 2020, 16 (84%) centers featured an on-site waste area, with the average surface of 46 m2 corresponding to 1.5 m2/bed. From 2021 to 2023, 10 (83%) treatment centers incorporated a waste area, with the average surface of 52.2 m2 corresponding to 4.5 m2/bed.
3.12.3. Laundry
From 2014 to 2017, 16 (94%) TCs had a laundry service on-site with an average surface of 1.2 m2/bed. Between 2018 and 2020, 18 (95%) facilities incorporated these services with an average surface of 1.6 m2/bed. During the last considered period, 10 (83%) centers included a laundry space with an average surface of 2.6 m2/bed.
3.12.4. Morgue
Every center built between 2014 and 2017 included a morgue. However, it is important to note that only 10 out of 17 (59%) of these centers featured a designated and secure area for visitors to observe the deceased. In the subsequent period, from 2018 to 2020, 12 out of 19 (63%) TCs were equipped with a morgue, and 11 (92%) of those featuring a morgue included a dedicated visitor area. In the latest phase, 9 out of 12 (75%) TCs had a morgue, and seven (78%) of those featuring a morgue provided a visitor area for safe observation of the deceased.
Concurrently, the average morgue surface per bed increased from 0.7 m2/bed for the 2014–2017 and 2018–2020 periods to 2.8 m2/bed in the subsequent period.
3.13. High-Risk Zone and Low-Risk Zone Ratio
All TCs included in the analysis presented only two zones of risk: low and high risk. Considering only the centers where the number of beds were available, the average space dedicated to the high-risk zone in the 2014 to 2017 period was 50.2 m2/bed. This ratio decreased to 49.6 m2/bed in the 2018 to 2020 period and increased to 94.0 m2/bed in the most recent timeframe. For the low-risk area, the space per bed decreased from 57.1 m2/bed from 2014 to 2017 to 53.4 m2/bed during the 2018 to 2020 period and increased to 78.5 m2/bed for the last period.
Between 2014–2017 and 2018–2020, the ratio of high-risk zones to low-risk zones was constantly observed to be 0.9. The ratio raised to 1.2 in the interval between 2021 and 2023. When considering all the centers regardless of the number of available beds, these ratios change to 1.0, 1.2, and 1.5 for the three considered periods in chronological order.
3.14. Construction Methods
From 2014 to 2017, out of 17 TCs, 14 (82%) operated out of temporary structures while one (6%) was a semi-permanent facility and one (6%) a permanent building. Information was unavailable for one center. Between 2018 and 2020, 11 (58%) of the TCs were in temporary structures, with semi-permanent and permanent structures housing one (5%) and five (26%) of TCs, respectively. Data were missing for two centers. In the most recent period, five (42%) TCs were in permanent buildings whereas temporary structures accounted for only four (33%) facilities. Information was missing for three centers.
4. Discussion
This study has analyzed the evolution of Ebola and Marburg treatment centers from 2014 to 2023, revealing significant changes in spatial dynamics and facility design.
4.1. Bed Capacity
The marked decline in bed capacity over time, from an average of 75 beds between 2014 and 2017 to 13.8 beds between 2021 and 2023, suggests a strategic shift toward smaller, potentially more manageable, and decentralized treatment centers. This could be attributed to lessons learned regarding the optimal size for patient management and strategy to increase early patient’s identification and admission [
30].
4.2. Ambulance Bay
The presence of dedicated ambulance bays in most treatment centers across the three periods underscores their consistent importance. However, the average surface area allocated to ambulance bays decreased significantly, from 151.4 m2 in 2014–2017 to 48.9 m2 in 2021–2023. This reduction likely reflects efforts to optimize space as centers became smaller and more focused.
4.3. Waiting Area and Screening/Triage
The spatial expansion of waiting areas (from 0.6 to 1.9 m2 per patient seat) may be driven by the necessity to ensure physical distancing between patients with an unknown risk status waiting to be screened. The reduction in the number of stand-alone waiting areas (from 100% to 50%), in favor of waiting areas integrated with screening and triage space, suggests an increased focus on ensuring visual contact between waiting patients and medical staff. Furthermore, it seems a good practice to rationalize the use of staff. The inconsistent provision of toilet facilities and the declining adherence to accessibility standards in both waiting areas and screening/triage spaces suggest areas needing improvement.
4.4. Donning and Doffing Areas
The strategic placement of donning and doffing areas straddling low- and high-risk zones has been largely maintained, reflecting the need to ensure a unidirectional flow [
31]. The significant reduction in the surface area allocated to these functions over time might raise concerns about the adequacy of space for safe PPE handling, potentially impacting HCW safety. The extensive use of chlorine for the doffing process and its pungent odor may explain the use of well-ventilated open spaces structure for doffing areas.
4.5. Patient Rooms and Health Services
The evolution of the design of patient rooms, moving from shared wards to individual rooms with dedicated toilets and showers, reflects an improved approach to patient privacy, comfort, and infection control. The increase in surface and volume per patient bed over time further supports this trend, emphasizing the need for adequate space in patient care. The analysis also reveals an increasing attention to natural ventilation, with most TCs achieving adequate window-to-floor ratios. This shift seems to be addressing the concerns about chlorine odors shared in previous articles [
17].
An important aspect of TC design that has evolved considerably is the provision for direct visibility into patients’ rooms from low-risk areas, increasing from 6% (2014–2017) to 31% (2018–2020) and 78% (2021–2023). This feature enhances patient monitoring, improves communication, and potentially reduces psychological stress for both patients and healthcare workers by making the environment more transparent and less intimidating [
32,
33]. It also suggests that modern TC designs are increasingly prioritizing the psychological well-being of patients and operational efficiency for healthcare workers. Interestingly, in the aftermath of the Marburg outbreak in Angola in 2005, MSF was already advocating for design solutions to place the nurses’ station where patients and family members could easily communicate with them to help demystify the isolation area, reduce fear, and prevent rumors [
15].
The sporadic inclusion of intensive care and obstetric rooms, along with the decline in laboratory facilities, highlights gaps in the comprehensive care capacity of these centers. The reduced presence of these facilities in recent periods suggests a potential area for development, ensuring that all necessary services are available within TCs.
Dedicated spaces for children and families decreased from 47% (2014–2017) to 16% (2018–2020), disappearing entirely by 2021–2023. Visitor areas also declined, from 88% in 2014–2017 to 63% in 2018–2020 and just 16.6% in 2021–2023, despite an increase in the average surface per bed. Psychosocial support spaces for staff and families saw a decline as well, with staff support spaces falling from 47% to 25% and family support areas from 71% to 58% over the same periods. A similar negative trend has been observed for accessibility, with patient rooms meeting accessibility standards in only half of the facilities built in the most recent period. These trends highlight ongoing challenges in providing comprehensive care that addresses both physical and emotional needs during outbreaks, underscoring the need for a more integrated and inclusive approach in future treatment center designs [
34]. The reduction in treatment centers with dedicated spaces for survivor clinics, from 29% in 2014–2017 to 8% in 2021–2023, likely reflects the integration of long-term health programs, such as survivor clinics, into the regular health system outside of emergency facilities, as discussed in scientific literature [
35,
36].
4.6. Support Facilities
The varying presence of medical, logistic, and administrative offices, along with changes in their allocated surfaces, reflect ongoing adjustments in the operational needs of TCs. The increasing average surface per bed for these offices in recent periods suggests a recognition of the importance of adequate administrative and logistical support in outbreak management. The presence of designated waste and laundry areas in treatment centers remained high, with the average surface area per bed consistently increasing, highlighting the importance of infection control in TCs.
Morgue facilities were universally included in treatment centers from 2014 to 2017, declining to 63% from 2018 to 2020 and 75% from 2021 to 2023. Secure visitor areas for observing the deceased increased from 59% to 92% and 78%, reflecting the need for dignified, secure, and culturally appropriate facilities. Secure visitor areas have significant socio-cultural and anthropological implications, as they allow for respect of local funeral practices while providing a space for mourning, which is essential for community acceptance during Ebola and Marburg responses [
34].
4.7. Construction Methods
The results demonstrate a clear shift from the reliance on temporary structures in earlier periods to a greater use of permanent facilities over time. Between 2014 and 2017, 82% of treatment centers operated out of temporary structures, while by the period of 2021 to 2023, this dropped to 33%. In contrast, the number of permanent buildings increased from just 6% in 2014 to 2017 to 42% in the most recent period. This trend suggests a gradual integration of more durable, long-term infrastructure as part of the outbreak response, reflecting improvements in preparedness and resilience in health systems. The rise in semi-permanent and permanent facilities likely points to efforts to strengthen healthcare capacity beyond the immediate emergency, aligning with broader health system strengthening strategies.
Several limitations must be acknowledged. The lack of details in some layouts limited the analysis. Data collection was confined to a ten-year period, potentially missing regional variations and smaller-scale outbreaks. The use of the 1/8 window-to-floor ratio, while a standard for assessing natural ventilation and daylight, may not fully account for local preferences in Equatorial African contexts where minimizing direct sunlight is often prioritized. Selection bias may have occurred due to targeted stakeholder consultation. Additionally, reverse engineering focused on physical spaces without assessing clinical outcomes, patient satisfaction, or operational efficiency. Lastly, resource constraints and cultural factors affecting design decisions warrant further investigation.
5. Conclusions
This study provides a comprehensive analysis of the evolution in the design of Ebola and Marburg treatment centers from 2014 to 2023. Significant improvements in spatial dynamics and facility designs reflect a responsive adaptation to emerging challenges in infectious disease management, patient care, and community acceptance. Key changes include a strategic shift toward smaller units, enhanced patient privacy through individual rooms, and the use of transparent screen to ensure direct visibility of patients. The reduction in visitor areas, dedicated spaces for children and families and psychosocial support spaces highlights ongoing challenges in providing holistic care during outbreaks. While the research focuses on outbreaks located in the African region, the design principles and findings can have a broader global significance. The strategies identified for improving infection control, patient privacy, and cultural sensitivity can inform the development of medical centers worldwide, particularly in regions susceptible to infectious disease threats. These principles are crucial for shaping global health preparedness, ensuring that healthcare environments are not only responsive but also adaptable to the needs of diverse populations.
Future research should develop standardized key performance indicators to measure design impacts on health outcomes and patient experiences. Expanding data collection to include direct observations, interviews, and quantitative and qualitative assessments of clinical outcomes, patient satisfaction, and operational efficiency will provide a more holistic understanding of treatment center design implications.