1. Introduction
Cerebrospinal fluid (CSF) shunting remains a cornerstone in the neurosurgical management of paediatric hydrocephalus. The Ventriculoperitoneal (VP) shunt implantation constitutes one of the most commonly performed procedures to alleviate increased intracranial pressure, particularly in cases of obstructive hydrocephalus. Despite substantial advancements in valve technology and an increasingly refined understanding of CSF physiology, overdrainage remains a prevalent and challenging complication, particularly in long-term shunt-dependent patients. CSF overdrainage represents a unique pathophysiological state characterised by sustained intracranial hypovolaemia and hypotension, often associated with the development of slit ventricles, subdural fluid collections, rebound hypertension, and cranial vault deformation. It leads to ventricular collapse and compensatory filling of the intracranial cavity with neural tissue. The result is a nonexpansile, synostotic skull occupied by brain, blood, cerebral vasculature, meninges, and only small amounts of cerebrospinal fluid, allowing no compensatory room for fluctuations in intracranial volume [
1]. In children or young adults, the morphology of the skull can be altered by excessive drainage of CSF following placement of a ventriculoperitoneal (VP) shunt [
2]. In a subset of patients, chronic overdrainage results in characteristic calvarial remodelling and thickening. The first reports of this phenomenon—hyperostosis cranii ex vacuo—were described by Moseley et al. in 1966 in children treated with shunting procedure for hydrocephalus [
3].
Hyperostosis cranii ex vacuo constitutes a physiological, compensatory response to chronically reduced intracranial pressure. It is characterised by diffuse inward calvarial remodelling, with predominant thickening of the inner table of the skull, leading to a net reduction in intracranial volume and contributing to pressure stabilisation. This entity is pathophysiologically distinct from other causes of secondary calvarial hyperostosis, such as those of metabolic, neoplastic, or haematological origin. It should be systematically considered the differential diagnosis of patients presenting with diffuse thickening of the skull, premature suture closure, and who have previously undergone surgical intervention for hydrocephalus [
4]. This phenomenon is particularly concerning in young children, where premature synostosis and diffuse calvarial thickening may disrupt brain growth and increase the risk of long-term neurodevelopmental outcome. Furthermore, this constellation of changes may severely compromise intracranial compliance and predispose to abrupt neurological deterioration in the event of recurrent cerebrospinal fluid accumulation or shunt dysfunction.
The earliest descriptions of diffuse calvarial thickening in paediatric patients treated with ventricular shunting for hydrocephalus were reported by Moseley [
3]. Blaauw and Emery (1969), as well as Anderson et al. (1966) documented cases of cranial vault thickening and premature suture fusion occurring as a structural response to CSF shunting procedure [
5,
6]. Anderson et al. (1970) noted occasional reduction in sellar volume following shunting procedure [
7]. Griscom and Oh identified children who presented with marked skull thickening after ventricular shunting [
8]. These authors concluded that inward growth of the inner table was “an uncommonly seen but physiologically reasonable accompaniment of relief of childhood hydrocephalus” [
2]. None of the initial investigators established a causal relationship between these osseous changes and sustained intracranial hypotension. Recent literature recognises hyperostosis cranii ex vacuo as a distinct pathophysiological entity, emerging as a delayed calvarial remodelling process in the setting of chronic cerebrospinal fluid overdrainage and prolonged intracranial hypopressure.
The pathogenesis of hyperostosis cranii ex vacuo is increasingly understood as a multifactorial, compensatory response to sustained intracranial hypovolaemia, most often secondary to chronic cerebrospinal fluid overdrainage in long-term shunt-dependent patients. Prolonged intracranial hypotension is thought to exert centripetal vector forces on the calvarial vault—predominantly affecting the inner table—leading to a measurable reduction in intracranial volume and sagittal vault length, a phenomenon substantiated by longitudinal neuroimaging studies [
2]. Mechanotransductive signalling via the dura mater, which serves as the endocranial periosteum and is structurally anchored to the cranial base by fibrous reflections, appears to be pivotal in this process. The release of pathological dural tension following ventricular decompression may initiate osteogenic cascades, resulting in appositional compact bone deposition along the inner table [
9]. These alterations are frequently accompanied by a diffuse, smooth, wave-like pattern of pachymeningeal enhancement on MRI, indicative of dural hyperaemia and interstitial oedema secondary to cerebrospinal hypopressure [
10,
11]. Contemporary revisions of the Monro-Kellie doctrine have refuted the notion of a rigid, inelastic calvarium, instead proposing that the skull functions as a dynamic regulatory compartment. Within this framework, hyperostotic remodelling represents an active compensatory mechanism aimed at restoring intracranial pressure homeostasis [
12]. However, such adaptation may prove maladaptive over time, restricting parenchymal re-expansion and predisposing to secondary complications such as rebound intracranial hypertension or premature suture fusion through disrupted cranial mechanobiology [
1].
Despite historical recognition of cranial vault remodelling following cerebrospinal fluid (CSF) diversion, hyperostosis cranii ex vacuo remains an insufficiently characterised and unclassified entity within the spectrum of CSF overdrainage-related pathology. Existing taxonomies of overdrainage syndromes predominantly emphasise ventricular collapse, intracranial hypotension, and cerebrospinal fluid dynamics, while failing to account for long-term osseous adaptations involving the calvarium, skull base, and dura mater. The four currently accepted subtypes: slit ventricle syndrome, subdural fluid collections, acquired Chiari malformation, and rebound intracranial hypertension—each represent different expressions of intracranial hypovolaemia or compliance failure. Moreover, the absence of defined radiological criteria has precluded its systematic identification and nosological inclusion.
The present study aims to address this gap by delineating the radiomorphological features and clinical context of hyperostosis cranii ex vacuo in a cohort of paediatric patients with chronic shunt dependence. Through quantitative assessment of calvarial thickening, sella turcica deformation, pachymeningeal enhancement, and premature cranial suture fusion, we investigate whether this phenotype conforms to the pathophysiological hallmarks of CSF overdrainage. Beyond descriptive characterisation, we further propose preliminary diagnostic criteria and a structured clinical pathway, integrating radiological recognition, intracranial pressure assessment, and tiered neurosurgical management. We hypothesise that hyperostosis cranii ex vacuo constitutes a distinct, underrecognised subtype of CSF overdrainage syndrome, whose formal recognition as the fifth variant may directly inform clinical decision-making, optimise long-term shunt performance, and improve neurodevelopmental outcomes in paediatric hydrocephalus.
2. Materials and Methods
This study was designed as a retrospective, observational analysis conducted at the Department of Pediatric Neurosurgery, University Children’s Hospital, in Kraków, Poland. Clinical, radiological, and procedural data were extracted from institutional electronic medical records spanning the period from years from 2016 to 2025. All imaging was performed and archived according to standardised institutional radiology protocols, utilising computed tomography (CT) and magnetic resonance imaging (MRI) systems calibrated for paediatric neuroimaging.
2.1. Population
The study cohort included nine paediatric patients (aged 7 to 17 years; both sexes) who had been surgically treated for hydrocephalus using cerebrospinal fluid (CSF) shunting systems—either ventriculoperitoneal (VP) or ventriculoatrial (VA) shunts—and who exhibited radiological features consistent with calvarial hyperostosis.
Inclusion criteria were as follows:
- (1)
Age under 18 years;
- (2)
Documented diagnosis of hydrocephalus treated surgically;
- (3)
Radiologically confirmed cranial hyperostosis following shunt implantation;
- (4)
Availability of high-resolution post-shunting CT or MRI scans suitable for morphometric analysis.
Exclusion criteria included the following:
- (1)
Congenital bone metabolism disorders (e.g., osteogenesis imperfecta, hypophosphatasia);
- (2)
Skeletal dysplasias;
- (3)
Endocrinopathies affecting bone turnover (e.g., hyperparathyroidism, Cushing’s disease);
- (4)
Long-term systemic glucocorticoid therapy, due to their potential confounding effects on cranial bone density and morphology.
2.2. Data Collection
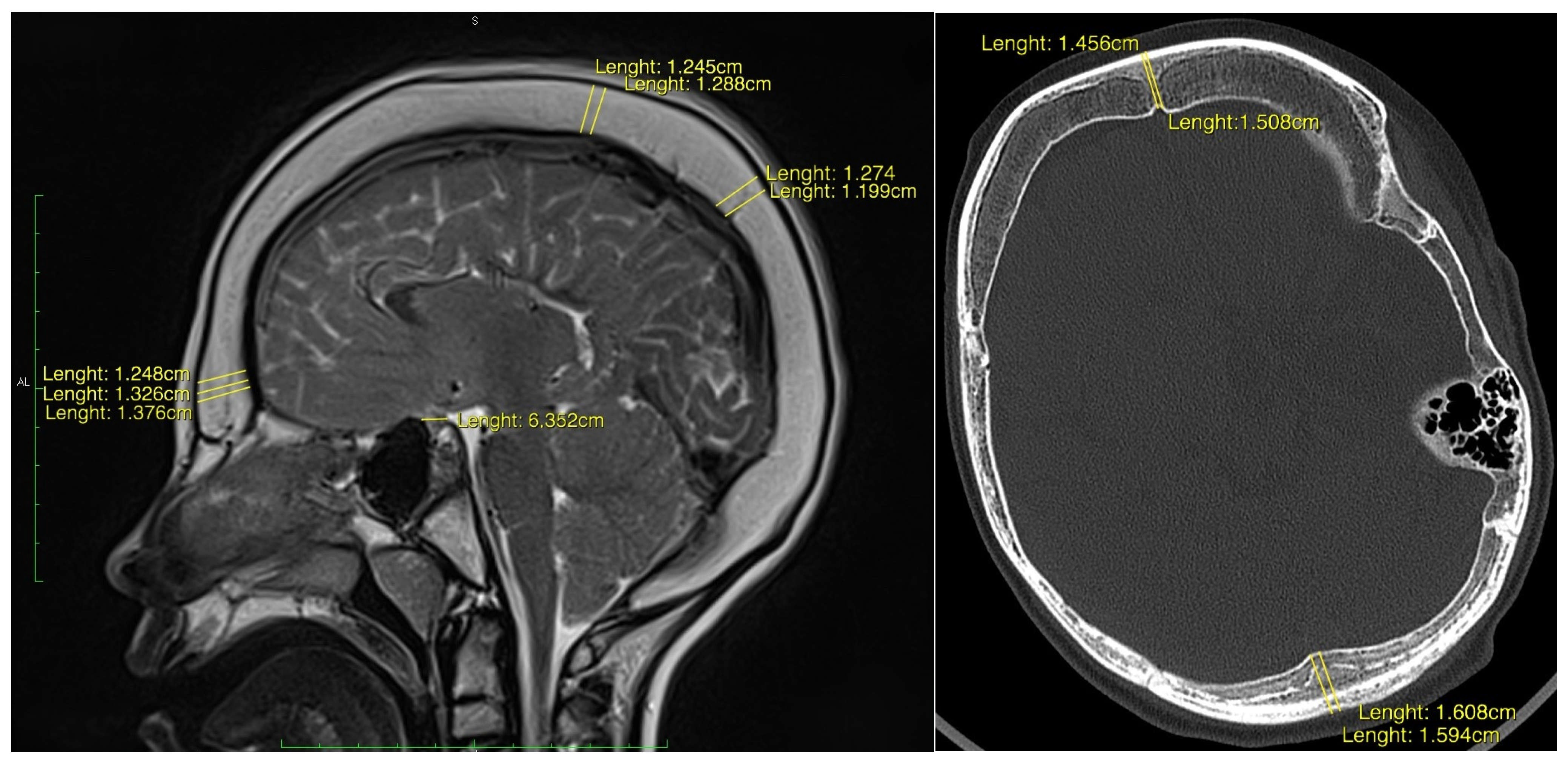
Data collection was performed by qualified clinicians. Acquisitied variables included patient demographics, age at initial shunt implantation, type of shunt system used (VP or VA), history and number of shunt revisions, presence of external ventricular drainage (EVD) or Rickham reservoir placement, latency period between shunt surgery and radiological detection of hyperostosis, incidence of central nervous system (CNS) infections, and age at diagnosis of overdrainage syndrome and cranial hyperostosis. Radiological data were reviewed retrospectively, and morphometric assessments were conducted on the most recent cross-sectional neuroimaging studies (CT or MRI) available. Cranial CT examinations were acquired on a 64-slice multidetector scanner using paediatric protocols with automatic dose modulation, 100–120 kV, and thin-slice reconstruction (0.75–1 mm, increment 0.5 mm) in both bone and soft-tissue kernels. MRI examinations were performed on 1.5 Tesla device with dedicated paediatric head coils, applying standard paediatric brain protocols consistent with paediatric neuroradiology guidelines. Typical acquisition included T1-, T2-, and FLAIR sequences in multiple planes, diffusion-weighted imaging, and susceptibility-sensitive sequences, with slice thickness 3–5 mm and field of view 200–240 mm. Intravenous contrast was administered when clinically indicated: iodinated contrast for CT (1.5–2 mL/kg) and gadolinium-based contrast for MRI (0.1 mmol/kg). Calvarial bone thickness was measured in the axial and coronal planes at three predefined anatomical landmarks: the glabella (frontal bone), the vertex (parietal bone), and the lambda (occipital-parietal suture). The maximum value at each site was recorded, and values were compared against paediatric normative data from the literature [
13].
In cases where contrast-enhanced imaging was available, the presence and degree of dural enhancement were assessed qualitatively.
2.3. Sella Turcica Morphometry
Mid-sagittal CT or MRI images were used to evaluate the morphology of the sella turcica. Reference points were determined on the coronal, sagittal and axial planes to standardise the images. Two lines were determined for the standardisation of measurements, a sagittal line passing through the crista galli and anterior nasal spine on the coronal plane, and a sagittal line passing through the internal occipital protuberance and anterior nasal spine on the axial plane.
Measured parameters included: Sella turcica width (STW): the longest anteroposterior dimension between the tuberculum sellae and dorsum sellae; Sella turcica depth (STD): the perpendicular height from the deepest point of the sella floor to a line connecting the tuberculum and dorsum sellae; Sella turcica length (STL): linear distance from the tuberculum to the dorsum sellae. Sagittal sella surface area was approximated using a geometric model: Rectangular estimation: Area = STW × STD. These values were then compared to age-stratified normative reference data [
14].
2.4. Densitometric Analysis
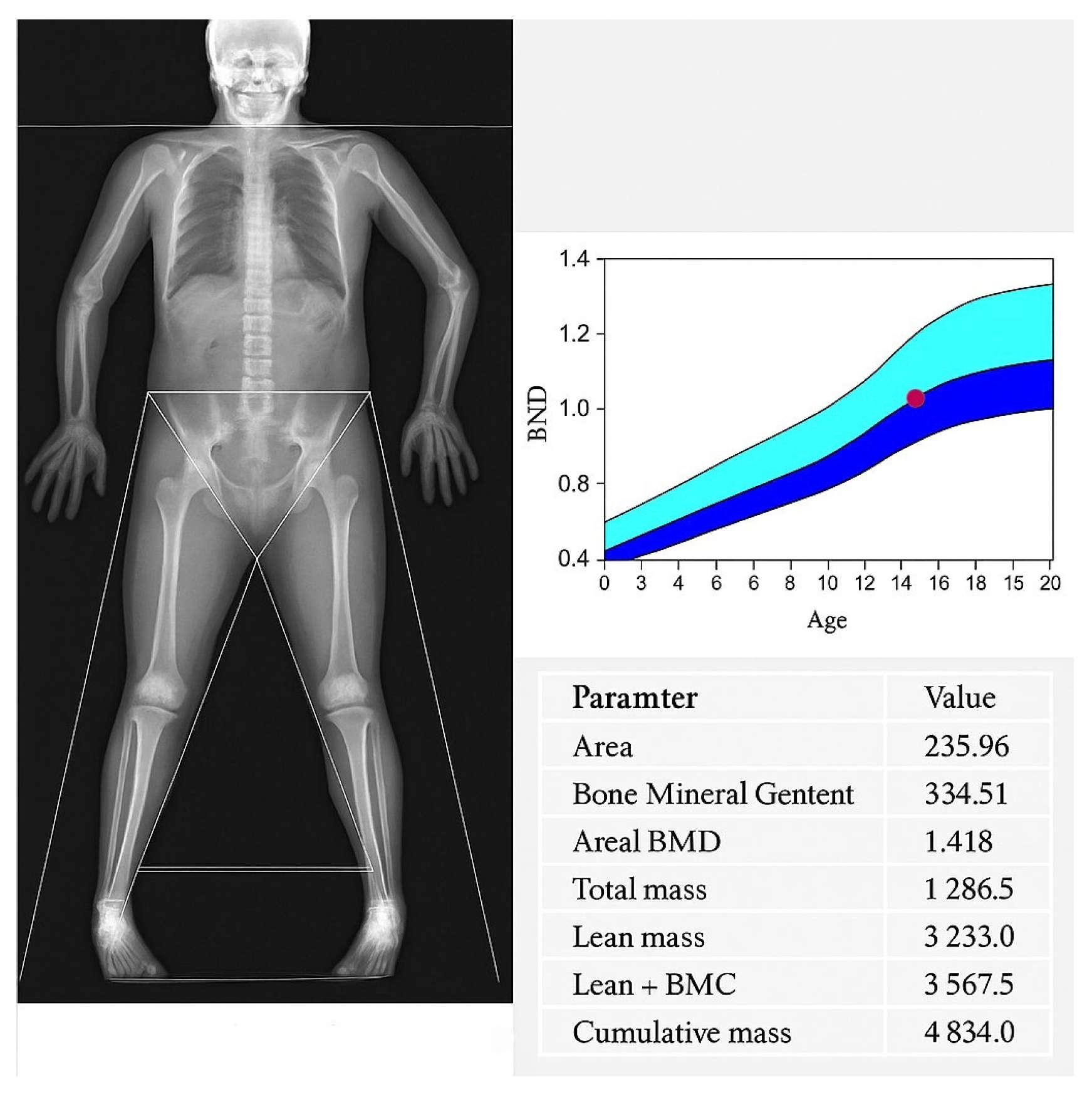
In patients for whom quantitative densitometric evaluation of the calvaria was available, calvarial bone density was assessed within regions affected by hyperostosis. Measurements were interpreted in relation to reference values from paediatric normative cohorts. Cranial bone densitometry was performed using dual energy X ray absorptiometry (DXA) on a Hologic Horizon W system (S/N 307914M) equipped with software version 13.6.1.3 and calibrated according to the TBAR1209—NHANES BCA protocol. All scans were acquired as whole body studies, with subsequent semi-automated segmentation of the “Head” region of interest (ROI) following the manufacturer’s paediatric protocol. Within the head ROI, the following parameters were quantified: Projected area (cm2), Bone mineral content, BMC (g), Areal bone mineral density, aBMD (g/cm2), Total mass (g), Lean mass (g), Combined lean + bone mineral content (g). For each parameter, mean ± standard deviation and observed range were recorded. Each ROI was inspected to ensure exclusion of extraneous soft tissue artefact and correct delineation of the cranial vault margins.
2.5. Study Outcomes
Primary outcome was to assess the association between CSF overdrainage syndrome and the development of cranial hyperostosis in paediatric patients following shunt surgery. Secondary outcomes were as follows: quantitative assessment of calvarial bone thickening (glabella, vertex, lambda) in relation to age- and sex-adjusted normative data. Morphological evaluation of sella turcica dimensions and its potential remodelling in the context of intracranial hypotension. Preliminary densitometric analysis of the affected calvarial bone in selected patients.
2.6. Data Analysis
Descriptive statistics were computed using IBM SPSS Statistics (version 29). Continuous variables were presented as means ± standard deviation (SD), and ranges were reported. Due to the small sample size, no inferential statistical testing was conducted. The analysis was purely quantitative. No formal risk-of-bias tools were applied due to the retrospective nature of the study and absence of control groups.
2.7. Ethics Approval and Data Protection
This study was conducted in accordance with institutional, national, and international ethical standards, including the Declaration of Helsinki and applicable data protection regulations. Ethical approval was not required, as the study involved retrospective analysis of fully anonymised clinical and imaging data obtained during routine medical care, with no impact on diagnostic or therapeutic procedures. All patients and/or their legal guardians were informed during the course of treatment about the potential use of anonymised clinical data for scientific and educational purposes. Patient confidentiality was safeguarded throughout the study. All data were anonymised prior to analysis, and no identifiable personal information was used or stored. Data access was restricted to authorised members of the research team.
4. Discussion
Our series of nine paediatric patients with long-term CSF shunting demonstrated a consistent pattern of diffuse calvarial thickening, premature craniosynostosis, dural enhancement on contrast enhanced imaging, sella turcica shrinkage and preserved physiological bone mineralisation. Eight of nine patients met the diagnostic criteria for CSF overdrainage syndrome. One patient lacked radiological signs of active ventricular narrowing at the time of imaging, although medical history revision suggests the process had occurred previously. All patients exhibited generalised thickening of the cranial bones developed secondary to overdrainage diagnosis at a mean interval of 66 months (range 12–138 months). These findings concur with available research, in which the earliest documented increase in calvarial thickness beyond the normal range was seen on skull radiographs obtained 12 months after ventriculoatrial shunt placement [
6]. Available publications report that hyperostosis develops over a period ranging from 3 years and 2 months to 8 years and 6 months [
6]. Quantitative morphometry revealed an approximate two-fold increase in calvarial thickness across frontal, parietal and occipital regions compared with age matched norms, and 33% of patients exhibited premature fusion of at least one cranial suture. Previous studies have reported mean skull thickness at midfrontal, vertex, and lambda regions averaged 2.5 times normal values for age and sex. The discrepancies between our data and those reports may reflect differences in patient age, measurement techniques or normative reference cohorts. All patients with available post contrast imaging (7/9) demonstrated smooth, wave-like pachymeningeal enhancement, reflecting dural hyperaemia secondary to chronic intracranial hypotension. Radiomorphometric assessment of the sella turcica revealed an average 11% reduction in anteroposterior length and an 18% decrease in estimated surface area in eight out of nine patients. Neurological symptoms were present in nearly 90% of the cohort, including epilepsy, focal deficits, and features of Chiari malformation, suggesting a clinically significant impact of the underlying hypotensive state. Finally, densitometric analysis via paediatric DXA confirmed preservation of normal cranial bone mineral parameters, corroborating that calvarial hypertrophy represents appositional lamellar bone growth within standard BMC and aBMD ranges, attributable to CSF overdrainage rather than intrinsic bone pathology.
These findings of this study align with, and extend, the findings of prior isolated reports on radiographic calvarial thickening in chronically shunted patients. The co-occurrence of clinical signs of hypotensive signs, premature cranial suture closure, reduced parasellar volume, and dural enhancement provides convergent evidence for chronic CSF volume depletion as the primary mechanism. Unlike previous accounts that lacked a unifying pathophysiological framework, our data demonstrates that the observed features fulfil the core diagnostic and mechanistic criteria of CSF overdrainage syndromes. Importantly, by integrating these findings into a structured set of diagnostic criteria and a proposed management algorithm, this study shifts the recognition of hyperostosis cranii ex vacuo from a descriptive radiological observation toward a clinically actionable entity. Such reframing enables early identification, risk stratification, and targeted intervention, thereby enhancing the translational relevance for paediatric neurosurgeons managing long-term shunt-dependent patients. Traditionally classified entities—encompassing slit ventricle syndrome, low pressure headaches, subdural hygromas or haematomas, and craniosynostosis with cranial deformity [
9,
15,
16]. In this context, hyperostosis cranii ex vacuo emerges as an additional, structurally distinct manifestation of the same pressure compliance disturbance. The mean latency of 66 months between shunt implantation and hyperostosis onset further supports a causal relationship.
Cranial vault expansion in chronic CSF overdrainage is driven by persistent traction forces transmitted from the hypotensive intracranial compartment via the dura mater, which functions as the endocranial periosteum and conforms to the brain’s morphology [
3]. The dura is firmly anchored to cranial base by fold-like appendages, known as reflections at the crista galli, the cribriform plate, the lesser wings of the sphenoid, and the petrous temporal crests. Facilitating mechanical coupling between the brain and calvarium [
9]. Release of pathological dural tension stretching and mechanical stress, initiating local osteogenic pathways with bone apposition, leading to increased cranial vault thickness. The skull demonstrated marked expansion of the cancellous space within the bone, but otherwise normal trabeculated bone [
10]. They and Dorst’ suggested that the inner table of the skull is very sensitive to the development of the brain beneath it, and if the stimulation of the neural mass becomes static, the bones of the calvaria thicken but do not enlarge in area [
6].
A secondary mechanism implicates long-term dampening of cerebral pulse pressure by CSF drainage. Both mean intracranial pressure and pulse pressure contribute to skull growth. Chronic CSF diversion thus deprives the vault of these expanding forces, resulting in reduced head circumference, vault thickening and, in some cases, premature suture fusion [
16]. The simultaneous reduction in dural expansion may promote secondary craniosynostosis through loss of tension across developing sutures, mostly affecting sagittal suture [
1]. Albright and Tyler Kabara postulate that the chronic overdrainage dampens normal cerebral pressure waves, decreasing the stimulation of calvarial growth and leading to suture ossification.
Marked reduction in sella turcica, present in the majority of patients, can be interpreted as a consequence of sustained parasellar hypopressure. This observation mirror those in adult spontaneous intracranial hypotension and suggest that detailed sella morphometry may serve as a sensitive radiological adjunct for chronic low CSF pressure, particularly in paediatric patients without overt clinical manifestations.
Smooth, bilateral, wave-like pachymeningeal enhancement was a consistent feature on contrast-enhanced MRI, distinct from the nodular or leptomeningeal patterns of inflammatory or neoplastic disease [
11,
17]. The pachymeninges comprises two fused membranes derived from the embryonic meninx primativa: the periosteum of the inner table of the skull and a meningeal layer. MR imaging is relatively sensitive and specific in the detection the pachymeninges, resulting in characteristic linear dural staining without sulcal or cortical surface enhancement. Radiological pattern, in conjunction with clinical signs of overdrainage is highly suggestive of benign intracranial hypotension [
11]. Under the Monro-Kellie doctrine, sustained intracranial hypotension causes fluid shifts and increased venous capacitance in the subarachnoid space, eventually resulting in dural venous congestion and interstitial oedema [
11].
Despite substantial osseous hypertrophy, paediatric DXA confirmed age-appropriate preservation of bone mineral content and areal bone mineral density This finding excludes primary osteosclerosis or metabolic bone disease and underscores that hyperostosis cranii ex vacuo is an adaptive compensatory phenomenon driven by altered cranial biomechanics rather than systemic pathology. Recognition of this distinction can prevent unnecessary endocrinological investigations and promote multidisciplinary collaboration among neurosurgery, neuroradiology and paediatric neurology.
Hyperostosis cranii ex vacuo is a rarely reported complication of CSF shunting, occurring in only a few patients per 1000 procedures. Its true incidence remains uncertain due to lack of consensus diagnostic criteria and under recognition. The calvarial thickening, premature closure of the sutures, and evidence of shunting procedures should greatly facilitate diagnosis [
4]. To date, no unequivocal association between this condition and CSF overdrainage syndrome has been demonstrated. The absence of a clear definition of overdrainage is reflected in the literature. There is no consensus on diagnostic criteria, and thus, the incidence remains uncertain which reflects on recommendations for prevention, management, and treatment of the condition [
15]. However, even when consensus is reached, an individual patient evaluation remains highly important.
4.1. Clinical Implications of Hyperostosis Cranii Ex Vacuo
In the existing literature, no formal diagnostic criteria or structured management strategies have been established for hyperostosis cranii ex vacuo. The available research includes reports of layered calvarial hyperostosis in the context of spontaneous intracranial hypotension. Given the limited overall understanding of calvarial hyperostosis, any diagnostic guidelines or therapeutic recommendations for hyperostosis cranii ex vacuo must focus on eliminating the underlying aetiology driving calvarial thickening and on preventing overdrainage in paediatric patients treated with shunting systems. Several prior reports provide a conceptual framework linking hyperostosis with overdrainage [
18]. Patients with obstructive hydrocephalus are considered at intermediate risk of developing overdrainage [
19]. Clinical manifestations are highly variable, ranging from mild postural headache to severe, potentially life-threatening states of altered consciousness and responsive acute intracranial hypertension. Most published literature on CSF overdrainage syndromes derives from isolated case reports or small series with limited follow-up; therefore, the efficacy of the described management approaches remains to be validated in prospective studies.
Based on our study and the available evidence, we propose diagnostic criteria for hyperostosis cranii ex vacuo, subdivided into primary criteria, which characterise the condition, and ancillary criteria, which help delineate its radiological and clinical context within the overdrainage spectrum.
Primary criteria:
Calvarial thickening approximately two-fold relative to age- and sex-adjusted normative values, measured at standardised landmarks (glabella, vertex, lambda);
Diffuse thickening of the cranial vault involving the inner table;
Normal densitometric findings, excluding metabolic or systemic bone disease;
Radiological features consistent with CSF overdrainage syndrome.
Ancillary criteria:
Documented low or negative ICP, or evidence of overdrainage on ICP monitoring;
Clinical manifestations of CSF overdrainage;
Premature craniosynostosis;
Pachymeningeal enhancement on contrast-enhanced CT or MRI;
Reduction in the dimensions of the sella turcica;
Brain sagging with herniation of the cerebellar tonsils.
The most common form of CSF overdrainage is the slit ventricle syndrome. Ventricular collapse has been reported in 10–85% of shunted patients [
20]. Although collapsed ventricles are characteristic, they are not pathognomonic and may even occur in asymptomatic patients [
19]. The presence of compartments or persistent ventricular isolation may represent sequelae of this syndrome. Additional radiological findings supporting the diagnosis of overdrainage—and thereby hyperostosis cranii ex vacuo—include: a small posterior fossa, calvarial hyperostosis, dolichocephalic disproportion, sclerotic sutures near the skull base, parenchymal calcifications, and sinus hyperpneumatisation [
19]. In a minority of patients, progressive brain sagging and tonsillar descent through the foramen magnum results in an acquired Chiari I malformation. Clinically, patients with overdrainage may exhibit symptoms resembling shunt malfunction, including postural headaches, nausea, vomiting, or additional features of raised intracranial pressure such as ataxia, seizures, cranial nerve deficits, bradycardia, and systemic hypertension [
21]. A critical clinical distinction lies in the temporal profile and postural dependency of symptoms: in slit ventricle syndrome, headaches often improve in the supine position [
21]. Conversely, the sudden onset of severe neurological symptoms suggests acute shunt malfunction and mandates urgent neurosurgical intervention [
22].
4.2. Management Strategies
Overdrainage syndrome and hyperostosis cranii ex vacuo are both challenging entities for which no consensus treatment strategy currently exists. Multiple therapeutic approaches have been described, ranging from shunt revision and valve pressure augmentation to the use of anti-syphon devices and cranial vault expansion. The overarching aim of treatment is to reduce excessive cerebrospinal fluid (CSF) drainage and to restore cerebral compliance.
Based on available literature, the most frequently employed practice in the management of shunt overdrainage remains reduction in CSF outflow across the valve mechanism [
23]. The most effective means of achieving this is through increasing the valve opening pressure, either by reprogramming an existing programmable valve or by implanting a new programmable system. Valve reprogramming to higher levels usually represents the first therapeutic attempt; however, this is technically complex [
19]. Incremental titration is required, with single-level adjustments performed sequentially. This approach is generally more effective in children, owing to greater cerebral compliance and earlier recognition of the condition. Patients with mild to moderate symptomatology may benefit from shunt upgrading, whereas those with severe or refractory disease often require multiple valve revisions. Valve exchange to prevent chronic overdrainage and to avoid proximal catheter obstruction due to coaptation of the ventricular wall has been reported to be well tolerated, with improved outcomes in terms of ventricular width, symptom relief, and reduced revision rates [
24]. Nevertheless, revision rates remain particularly high in patients shunted before 6 months of age, in whom the risk of secondary craniosynostosis is increased [
22]. A further therapeutic option is the implantation of a device to counteract the syphon effect, ideally in combination with increased valve opening pressure [
23,
25]. In refractory cases, antisiphon devices have provided symptomatic relief in up to 85% of paediatric and adult patients.
Alternative strategies reported in the literature include conservative and pharmacological measures. Pharmacological therapy is generally considered appropriate only in selected patients with hyperostosis and infrequent, non-disabling symptoms, where observation and medical therapy may suffice [
21]. Some authors argue that antimigraine therapy should be viewed merely as a temporising measure while definitive treatment is planned.
Expansion techniques warrant brief consideration. These procedures are relatively uncommon and typically represent a last-resort therapy in the management of overdrainage. Although the evidence base remains scare, cranial vault expansion may be suitable in selected patients, particularly those with secondary craniosynostosis and craniocephalic disproportion. The principal objective of such surgery is to reduce intracranial pressure by increasing intracranial volume [
22]. Expansion is usually reserved for cases unresponsive to shunt reprogramming or hardware substitution [
19]. The greater invasiveness of these operations is counterbalanced by the poor quality of life in refractory patients. Adverse sequelae include the risk of secondary craniosynostosis—reported in 1–10% of cases [
22]—and microcephaly, particularly in children shunted before 6 months of age [
22]. For this reason, vault expansion is generally reserved for patients who have already undergone management with programmable valves and antisiphon devices, or in whom attempts at catheter repositioning have failed [
26].
4.3. Prognosis and Future Implications
Ventricular shunting remains the mainstay of hydrocephalus treatment, providing rapid normalisation of intracranial pressure, limiting neuronal injury, and alleviating symptoms such as vomiting, somnolence, poor feeding, irritability, or seizures. Nevertheless, one of its most frequent complications is overdrainage, caused by an inappropriately low valve opening pressure or the inability of fixed-pressure systems to adapt to the child’s growth. Shunt obstruction or infection require immediate surgical revision to prevent serious neurological sequelae, including acute intracranial hypertension and meningitis [
27]. No universally accepted guidelines currently exist for diagnosing CSF overdrainage. Owing to the wide variability of clinical and radiological manifestations, recognition may be challenging. It is therefore essential for clinicians to familiarise themselves with the full spectrum of imaging features associated with overdrainage in order to prompt timely diagnosis [
18]. The presence of calvarial hyperostosis may be especially valuable in patients with atypical presentations or with equivocal neuroimaging findings [
18]. Our study supports a direct relationship between layered calvarial hyperostosis and overdrainage syndrome. This finding is of clinical significance, as identification of this radiological marker may facilitate the recognition of intracranial hypotension and prompt dedicated diagnostic work-up in the appropriate clinical setting [
18]. Given that osseous remodelling is a chronic rather than acute process, the presence of diffuse hyperostosis cranii in the context of overdrainage indicates a long-standing pathophysiological state and may serve as a surrogate marker of hydrocephalus management quality. Early shunt implantation in neonates and infants carries a risk of complications associated with excessive drainage, leading to calvarial remodelling and premature suture closure. The initial use of programmable valves may mitigate or at least delay the need for surgical re-intervention and subsequent cranioplasty by better adapting to the dynamic intracranial physiology of the growing child. A comprehensive review of the literature demonstrates that shunt overdrainage remains a complex and unresolved dilemma. The primary goal for future research should be the establishment of precise definitions for this syndrome, including the constellation of clinical and radiological findings and the delineation of its distinct subtypes [
27].
Our study highlights hyperostosis cranii ex vacuo as a distinct radiological–clinical entity within the spectrum of CSF overdrainage, with direct implications for the longitudinal care of shunted children. Importantly, calvarial hyperostosis should not be regarded merely as a descriptive imaging feature but rather as a surrogate marker of chronic intracranial hypotension and, by extension, of shunt performance over time. Its recognition has the potential to shift clinical practice from retrospective detection to proactive surveillance and timely intervention.
From a translational standpoint, we propose a preliminary clinical pathway:
Radiological identification—diffuse calvarial thickening (>2× age- and sex-adjusted normative values, inner table involvement, exclusion of systemic bone disease).
Physiological confirmation—demonstration of low/negative ICP or evidence of syphoning on monitoring.
Clinical correlation—assessment of overdrainage symptoms, including postural headaches, neurocognitive changes, and secondary craniosynostosis.
Tiered intervention—stepwise escalation from valve reprogramming to antisiphon devices, with cranial vault expansion reserved for refractory cases with craniocephalic disproportion.
This structured framework underscores the practical relevance of hyperostosis cranii ex vacuo: it enables early recognition, risk stratification, and rational selection of treatment strategies. Beyond the immediate clinical setting, the entity also provides an objective biomarker of shunt-related morbidity and a potential quality indicator for paediatric hydrocephalus management. Future efforts should aim at multicentre, prospective validation of the proposed diagnostic criteria, incorporation into standardised definitions of CSF overdrainage, and evaluation of long-term neurodevelopmental outcomes. Establishing reproducible algorithms and outcome metrics will be essential to formalise hyperostosis cranii ex vacuo as the fifth nosological subtype of CSF overdrainage syndrome and to translate its recognition into measurable improvements in patient care.