Efficacy of Mandibular Advancement Devices in the Treatment of Mild to Moderate Obstructive Sleep Apnea: A Systematic Review
Abstract
1. Introduction
1.1. Diagnosis
1.2. Treatment
1.3. Young Patients
2. Materials and Methods
2.1. Protocol and Registration
2.2. Search Processing
| Article screening strategy |
| Keywords: A: “mandibular advancement device” OR “mandibular advancement splint” OR “oral appliance” OR “dental appliance” OR “MAD” OR “MAS” B: “obstructive sleep apnea” OR “OSA” |
| Boolean Indicators: “A” OR “B” |
| Timespan: within 10 years |
| Electronic databases: Pubmed; Scopus; Web of Science |
2.3. Inclusion Criteria
- Studies that investigated the use of MAD in patients suffering from OSA;
- Randomized clinical trials;
- Studies with open access written in English;
- Full-text articles;
- Studies that were published in the last 10 years.
2.4. Exclusion Criteria
- Animal studies;
- In vitro studies;
- Off-topic studies;
- Reviews, retrospective studies, case series and case reports, letters to the authors or comments;
- Non-English-language studies.
2.5. PICO Question
- Participants: Individuals with mild to moderate OSA, which was confirmed through overnight polysomnography (PSG). Participants: adults (≥18 y) with mild–moderate OSA (AHI 5–30 events/h) diagnosed by polysomnography or type-III HSAT Both male and female.
- Interventions: application of MAD
- Comparisons: different MAD in conservative management (no/standard care, lifestyle, positional) and alternative therapies (CPAP, surgery, weight loss).
- Outcomes: Treatment of OSA to improve quality of life and reduce the risk of cardiovascular complications, apnea-hypopnea index (AHI), oxygen saturation, and sleep quality.
2.6. Data Processing
2.7. Article Identification Procedure
2.8. Study Evaluation
2.9. Quality Assessment
3. Results
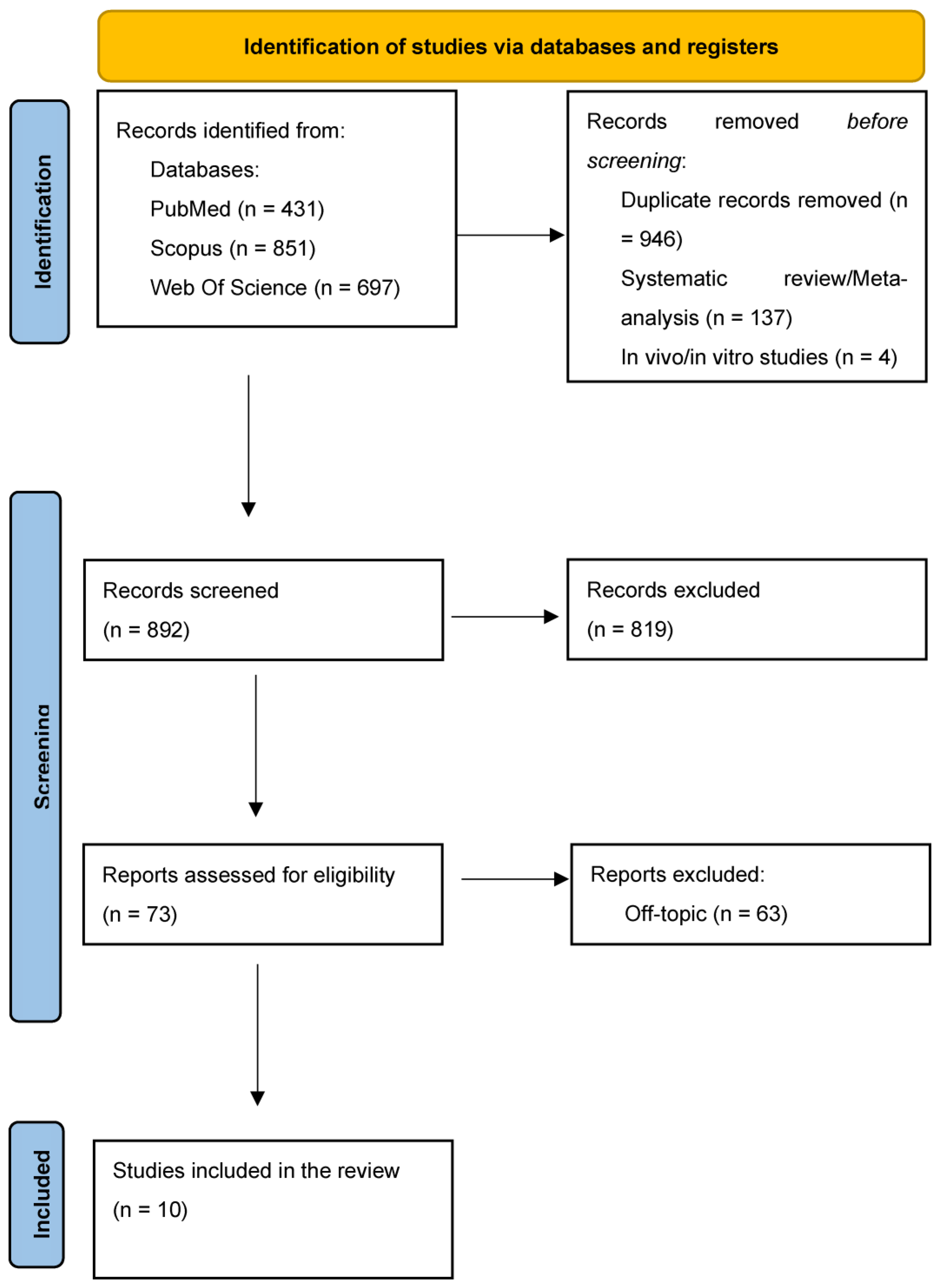
3.1. Study Selection and Characteristics
3.2. Quality Assessment and Risk of Bias
3.3. Clinical Outcomes
4. Discussion
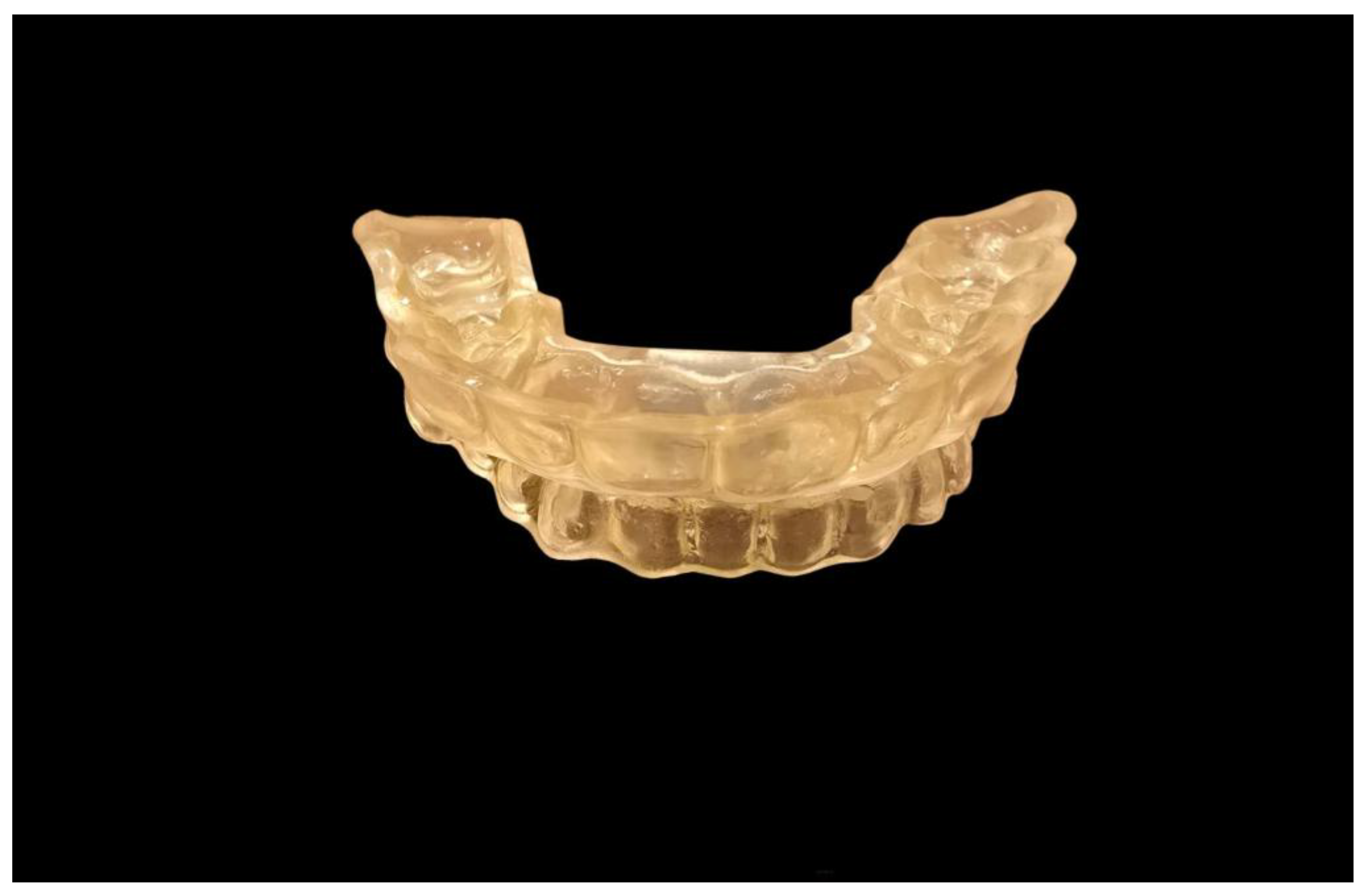
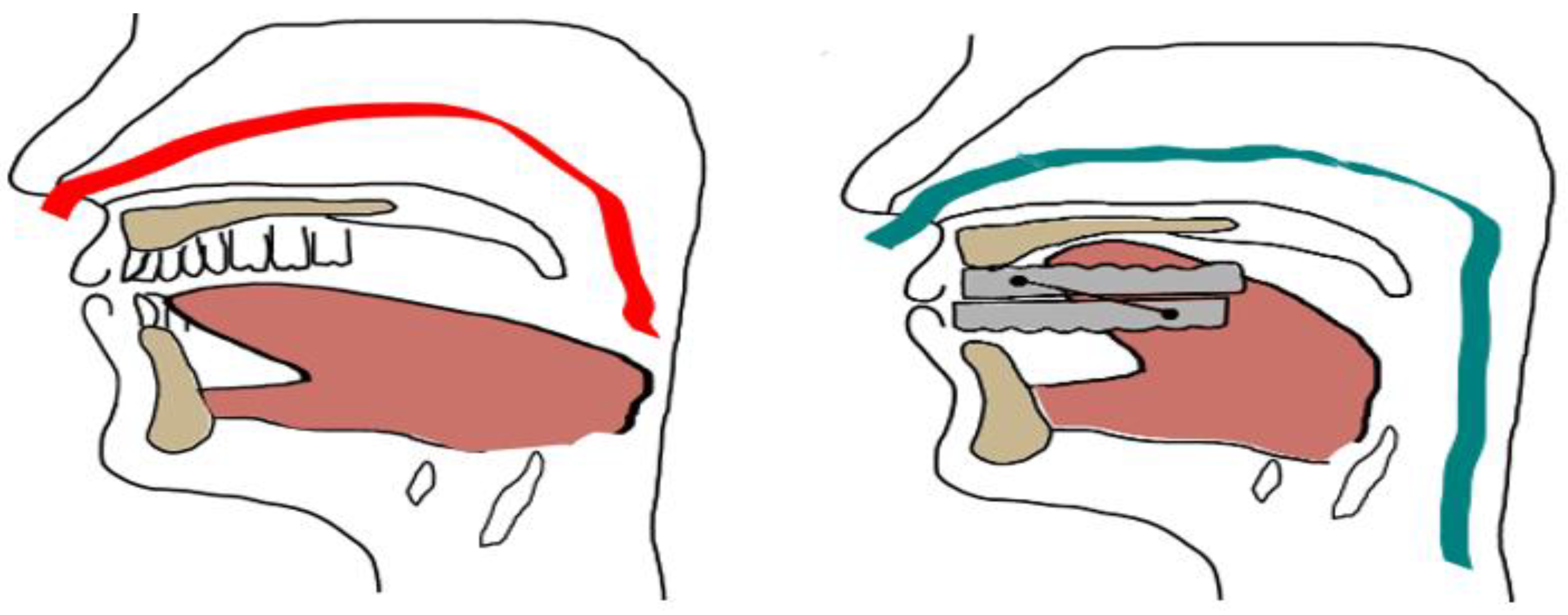
4.1. Types and Mechanisms of Action of MAD
4.2. Clinical Efficacy and Predictive Factors
4.3. Translational Challenges: Tolerability, Adherence, and Multidisciplinary Care
4.4. Limitations of the Study and Future Research
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| OSA | Obstructive sleep apnea |
| MAD | Mandibular advancement devices |
| AHI | Apnea-hypopnea index |
| ESS | Epworth Sleepiness Scale |
| UA | Upper airway |
| PSG | Polysomnography |
| CPAP | Positive pressure airway devices |
References
- Mallampati, S.R.; Gatt, S.P.; Gugino, L.D.; Desai, S.P.; Waraksa, B.; Freiberger, D.; Liu, P.L. A clinical sign to predict difficult tracheal intubation: A prospective study. Can. Anaesth. Soc. J. 1985, 32, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Quinnell, T.G.; Bennett, M.; Jordan, J.; Clutterbuck-James, A.L.; Davies, M.G.; Smith, I.E.; Oscroft, N.; Pittman, M.A.; Cameron, M.; Chadwick, R.; et al. A crossover randomised controlled trial of oral mandibular advancement devices for obstructive sleep apnoea-hypopnoea (TOMADO). Thorax 2014, 69, 938–945. [Google Scholar] [CrossRef]
- Johns, M.W. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef]
- Ghazal, A.; Sorichter, S.; Jonas, I.; Rose, E.C. A randomized prospective long-term study of two oral appliances for sleep apnoea treatment. J. Sleep Res. 2009, 18, 321–328. [Google Scholar] [CrossRef]
- Mehta, A.; Qian, J.; Petocz, P.; Darendeliler, M.A.; Cistulli, P.A. A randomized, controlled study of a mandibular advancement splint for obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2001, 163, 1457–1461. [Google Scholar] [CrossRef]
- Ferguson, K.A.; Ono, T.; Lowe, A.A.; Keenan, S.P.; Fleetham, J.A. A randomized crossover study of an oral appliance vs. nasal-continuous positive airway pressure in the treatment of mild-moderate obstructive sleep apnea. Chest 1996, 109, 1269–1275. [Google Scholar] [CrossRef]
- Ahrens, A.; McGrath, C.; Hägg, U. A systematic review of the efficacy of oral appliance design in the management of obstructive sleep apnoea. Eur. J. Orthod. 2011, 33, 318–324. [Google Scholar] [CrossRef]
- Flemons, W.W.; Douglas, N.J.; Kuna, S.T.; Rodenstein, D.O.; Wheatley, J. Access to Diagnosis and Treatment of Patients with Suspected Sleep Apnea. Am. J. Respir. Crit. Care Med. 2004, 169, 668–672. [Google Scholar] [CrossRef]
- Weaver, T.E.; Grunstein, R.R. Adherence to continuous positive airway pressure therapy: The challenge to effective treatment. Proc. Am. Thorac. Soc. 2008, 5, 173–178. [Google Scholar] [CrossRef]
- De Geest, S.; Sabaté, E. Adherence to Long-Term Therapies: Evidence for Action. Eur. J. Cardiovasc. Nurs. 2003, 2, 323. [Google Scholar] [CrossRef]
- Woehrle, H.; Graml, A.; Weinreich, G. Age- and gender-dependent adherence with continuous positive airway pressure therapy. Sleep Med. 2011, 12, 1034–1036. [Google Scholar] [CrossRef] [PubMed]
- Gabryelska, A.; Turkiewicz, S.; Gajewski, A.; Białasiewicz, P.; Strzelecki, D.; Ditmer, M.; Chałubiński, M.; Sochal, M. Elucidating the Interplay of Hypoxia-Inducible Factor and Circadian Clock Signaling in Obstructive Sleep Apnea Patients. Int. J. Mol. Sci. 2025, 26, 971. [Google Scholar] [CrossRef] [PubMed]
- Basyuni, S.; Barabas, M.; Quinnell, T. An update on mandibular advancement devices for the treatment of obstructive sleep apnoea hypopnoea syndrome. J. Thorac. Dis. 2018, 10, S48–S56. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.K.; Kang, Y.J.; Yoon, W.; Shin, H.-W. Analysing the impact of body position shift on sleep architecture and stage transition: A comprehensive multidimensional study using event-synchronised polysomnography data. J. Sleep Res. 2024, 33, e14115. [Google Scholar] [CrossRef]
- Ingman, T.; Nieminen, T.; Hurmerinta, K. Cephalometric comparison of pharyngeal changes in subjects with upper airway resistance syndrome or obstructive sleep apnoea in upright and supine positions. Eur. J. Orthod. 2004, 26, 321–326. [Google Scholar] [CrossRef]
- Riepponen, A.; Myllykangas, R.; Savolainen, J.; Kilpeläinen, P.; Kellokoski, J.; Pahkala, R. Changes in posterior airway space and hyoid bone position after surgical mandibular advancement. Acta Odontol. Scand. 2017, 75, 73–78. [Google Scholar] [CrossRef]
- Epstein, L.J.; Kristo, D.; Strollo, P.J.; Friedman, N.; Malhotra, A.; Patil, S.P.; Ramar, K.; Rogers, R.; Schwab, R.J.; Weaver, E.M.; et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J. Clin. Sleep Med. 2009, 5, 263–276. [Google Scholar] [CrossRef]
- Ngiam, J.; Balasubramaniam, R.; Darendeliler, M.A.; Cheng, A.T.; Waters, K.; Sullivan, C.E. Clinical guidelines for oral appliance therapy in the treatment of snoring and obstructive sleep apnoea. Aust. Dent. J. 2013, 58, 408–419. [Google Scholar] [CrossRef]
- Ramar, K.; Dort, L.C.; Katz, S.G.; Lettieri, C.J.; Harrod, C.G.; Thomas, S.M.; Chervin, R.D. Clinical Practice Guideline for the Treatment of Obstructive Sleep Apnea and Snoring with Oral Appliance Therapy: An Update for 2015. J. Clin. Sleep Med. 2015, 11, 773–827. [Google Scholar] [CrossRef]
- Sutherland, K.; Deane, S.A.; Chan, A.S.L.; Schwab, R.J.; Ng, A.T.; Darendeliler, M.A.; Cistulli, P.A. Comparative effects of two oral appliances on upper airway structure in obstructive sleep apnea. Sleep 2011, 34, 469–477. [Google Scholar] [CrossRef]
- Vanderveken, O.M.; Devolder, A.; Marklund, M.; Boudewyns, A.N.; Braem, M.J.; Okkerse, W.; Verbraecken, J.A.; Franklin, K.A.; De Backer, W.A.; Van de Heyning, P.H. Comparison of a custom-made and a thermoplastic oral appliance for the treatment of mild sleep apnea. Am. J. Respir. Crit. Care Med. 2008, 178, 197–202. [Google Scholar] [CrossRef]
- Deane, S.A.; Cistulli, P.A.; Ng, A.T.; Zeng, B.; Petocz, P.; Darendeliler, M.A. Comparison of Mandibular Advancement Splint and Tongue Stabilizing Device in Obstructive Sleep Apnea: A Randomized Controlled Trial. Sleep 2009, 32, 648–653. [Google Scholar] [CrossRef]
- Neelapu, B.C.; Kharbanda, O.P.; Sardana, H.K.; Balachandran, R.; Sardana, V.; Kapoor, P.; Gupta, A.; Vasamsetti, S. Craniofacial and upper airway morphology in adult obstructive sleep apnea patients: A systematic review and meta-analysis of cephalometric studies. Sleep Med. Rev. 2017, 31, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Dieltjens, M.; Vanderveken, O.M.; Van de Heyning, P.H.; Braem, M.J. Current opinions and clinical practice in the titration of oral appliances in the treatment of sleep-disordered breathing. Sleep Med. Rev. 2012, 16, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Puech, C.; Badran, M.; Barrow, M.B.; Gozal, D. Cognitive Function, Sleep, and Neuroinflammatory Markers in Mice Exposed to Very Long-Term Intermittent Hypoxia. Int. J. Mol. Sci. 2025, 26, 1815. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, D.J.; Punjabi, N.M. Diagnosis and Management of Obstructive Sleep Apnea: A Review. JAMA 2020, 323, 1389–1400. [Google Scholar] [CrossRef]
- Semelka, M.; Wilson, J.; Floyd, R. Diagnosis and Treatment of Obstructive Sleep Apnea in Adults. Am. Fam. Physician 2016, 94, 355–360. [Google Scholar]
- Gale, D.J.; Sawyer, R.H.; Woodcock, A.; Stone, P.; Thompson, R.; O’Brien, K. Do oral appliances enlarge the airway in patients with obstructive sleep apnoea? A prospective computerized tomographic study. Eur. J. Orthod. 2000, 22, 159–168. [Google Scholar] [CrossRef]
- Flores-Orozco, E.I.; Tiznado-Orozco, G.E.; Díaz-Peña, R.; Orozco, E.I.F.; Galletti, C.; Gazia, F.; Galletti, F. Effect of a Mandibular Advancement Device on the Upper Airway in a Patient with Obstructive Sleep Apnea. J. Craniofac. Surg. 2020, 31, e32–e35. [Google Scholar] [CrossRef]
- Jo, S.Y.; Lee, S.M.; Lee, K.H.; Kim, D.-K. Effect of long-term oral appliance therapy on obstruction pattern in patients with obstructive sleep apnea. Eur. Arch. Oto-Rhino-Laryngol. 2018, 275, 1327–1333. [Google Scholar] [CrossRef]
- Becker, H.F.; Jerrentrup, A.; Ploch, T.; Grote, L.; Penzel, T.; Sullivan, C.E.; Peter, J.H. Effect of Nasal Continuous Positive Airway Pressure Treatment on Blood Pressure in Patients with Obstructive Sleep Apnea. Circulation 2003, 107, 68–73. [Google Scholar] [CrossRef]
- Cartwright, R.D. Effect of Sleep Position on Sleep Apnea Severity. Sleep 1984, 7, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Lamont, J.; Baldwin, D.R.; Hay, K.D.; Veale, A.G. Effect of two types of mandibular advancement splints on snoring and obstructive sleep apnoea. Eur. J. Orthod. 1998, 20, 293–297. [Google Scholar] [CrossRef]
- Ferguson, K.A. The role of oral appliance therapy in the treatment of obstructive sleep apnea. Clin. Chest Med. 2003, 24, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Araghi, M.H.; Chen, Y.-F.; Jagielski, A.; Choudhury, S.; Banerjee, D.; Hussain, S.; Thomas, G.N.; Taheri, S. Effectiveness of Lifestyle Interventions on Obstructive Sleep Apnea (OSA): Systematic Review and Meta-Analysis. Sleep 2013, 36, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Serra-Torres, S.; Bellot-Arcís, C.; Montiel-Company, J.M.; Marco-Algarra, J.; Almerich-Silla, J.M. Effectiveness of mandibular advancement appliances in treating obstructive sleep apnea syndrome: A systematic review. Laryngoscope 2016, 126, 507–514. [Google Scholar] [CrossRef]
- Haskell, J.A.; McCrillis, J.; Haskell, B.S.; Scheetz, J.P.; Scarfe, W.C.; Farman, A.G. Effects of Mandibular Advancement Device (MAD) on Airway Dimensions Assessed with Cone-Beam Computed Tomography. Semin. Orthod. 2009, 15, 132–158. [Google Scholar] [CrossRef]
- Singh, G.D.; Keropian, B.; Pillar, G. Effects of the full breath solution appliance for the treatment of obstructive sleep apnea: A preliminary study. Cranio J. Craniomandib. Pract. 2009, 27, 109–117. [Google Scholar]
- Hoekema, A.; Stegenga, B.; Wijkstra, P.J.; van der Hoeven, J.H.; Meinesz, A.F.; de Bont, L.G.M. Obstructive sleep apnea therapy. J. Dent. Res. 2008, 87, 882–887. [Google Scholar] [CrossRef]
- Vecchierini, M.F.; Léger, D.; Laaban, J.P.; Putterman, G.; Figueredo, M.; Levy, J.; Vacher, C.; Monteyrol, P.J.; Philip, P. Efficacy and compliance of mandibular repositioning device in obstructive sleep apnea syndrome under a patient-driven protocol of care. Sleep Med. 2008, 9, 762–769. [Google Scholar] [CrossRef]
- Giannasi, L.C.; Almeida, F.R.; Nacif, S.R.; De Oliveira, L.V.F. Efficacy of an oral appliance for the treatment of obstructive sleep apnea. Int. J. Prosthodont. 2013, 26, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Ariga, P.; Jain, A.R. Efficacy of Custom-Made Mandibular Advancement Appliance on Patients with Obstructive Sleep Apnea: A Prospective Clinical Trial. 2018. Available online: https://www.semanticscholar.org/paper/Efficacy-of-custom-made-mandibular-advancement-on-A-Agarwal-Ariga/39f2ec0a3bb95b555c9198f497f14c43b4c3464c (accessed on 25 May 2018).
- Durán-Cantolla, J.; Crovetto-Martínez, R.; Alkhraisat, M.-H.; Crovetto, M.; Municio, A.; Kutz, R.; Aizpuru, F.; Miranda, E.; Anitua, E. Efficacy of mandibular advancement device in the treatment of obstructive sleep apnea syndrome: A randomized controlled crossover clinical trial. Med. Oral Patol. Oral Cirugia Bucal 2015, 20, e605–e615. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, L.; Laberge, L.; Beaudry, M.; Laforte, M.; Rompré, P.H.; Lavigne, G.J. Efficacy of two mandibular advancement appliances in the management of snoring and mild-moderate sleep apnea: A cross-over randomized study. Sleep Med. 2009, 10, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Young, T.; Peppard, P.E.; Gottlieb, D.J. Epidemiology of obstructive sleep apnea: A population health perspective. Am. J. Respir. Crit. Care Med. 2002, 165, 1217–1239. [Google Scholar] [CrossRef]
- Candotto, V.; Gabrione, F.; Oberti, L.; Lento, D.; Severino, M. The role of implant-abutment connection in preventing bacterial leakage: A review. J. Biol. Regul. Homeost. Agents 2019, 33, 129–134. [Google Scholar]
- Phillips, C.L.; Grunstein, R.R.; Darendeliler, M.A.; Mihailidou, A.S.; Srinivasan, V.K.; Yee, B.J.; Marks, G.B.; Cistulli, P.A. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: A randomized controlled trial. Am. J. Respir. Crit. Care Med. 2013, 187, 879–887. [Google Scholar] [CrossRef]
- Burudpakdee, C.; Khan, Z.M.; Gala, S.; Nanavaty, M.; Kaura, S. Impact of patient programs on adherence and persistence in inflammatory and immunologic diseases: A meta-analysis. Patient Prefer. Adherence 2015, 9, 435–448. [Google Scholar]
- Prescinotto, R.; Haddad, F.L.M.; Fukuchi, I.; Gregório, L.C.; Cunali, P.A.; Tufik, S.; Bittencourt, L.R.A. Impact of upper airway abnormalities on the success and adherence to mandibular advancement device treatment in patients with Obstructive Sleep Apnea Syndrome. Braz. J. Otorhinolaryngol. 2015, 81, 663–670. [Google Scholar] [CrossRef]
- Severino, M.; Caruso, S.; Rastelli, S.; Gatto, R.; Cutilli, T.; Pittari, L.; Nota, A.; Tecco, S. Hand-Carried Ultrasonography Instrumentation in the Diagnosis of Temporomandibular Joint Dysfunction. Methods Protoc. 2021, 4, 81. [Google Scholar] [CrossRef]
- Bartlett, D.; Wong, K.; Richards, D.; Moy, E.; Espie, C.A.; Cistulli, P.A.; Grunstein, R. Increasing adherence to obstructive sleep apnea treatment with a group social cognitive therapy treatment intervention: A randomized trial. Sleep 2013, 36, 1647–1654. [Google Scholar] [CrossRef]
- Alves, M., Jr.; Baratieri, C.; Mattos, C.T.; Brunetto, D.; Fontes, R.D.C.; Santos, J.R.L.; Ruellas, A.C.D.O. Is the airway volume being correctly analyzed? Am. J. Orthod. Dentofac. Orthop. 2012, 141, 657–661. [Google Scholar] [CrossRef]
- Wang, J.D.J.; Chua, N.Y.M.; Chan, L.-L.; Tan, E.-K. Obstructive Sleep Apnea and Parkinson’s Disease: Bidirectional Clinical and Pathophysiologic Links. Int. J. Mol. Sci. 2025, 26, 3762. [Google Scholar] [CrossRef]
- Marin, J.M.; Carrizo, S.J.; Vicente, E.; Agusti, A.G. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: An observational study. Lancet 2005, 365, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Waldhorn, R.E.; Herrick, T.W.; Nguyen, M.C.; O’Donnell, A.E.; Sodero, J.; Potolicchio, S.J. Long-term compliance with nasal continuous positive airway pressure therapy of obstructive sleep apnea. Chest 1990, 97, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.D.; Patano, A.; Coloccia, G.; Ceci, S.; Inchingolo, A.M.; Marinelli, G.; Malcangi, G.; Montenegro, V.; Laudadio, C.; Palmieri, G.; et al. Genetic Pattern, Orthodontic and Surgical Management of Multiple Supplementary Impacted Teeth in a Rare, Cleidocranial Dysplasia Patient: A Case Report. Med. Kaunas Lith. 2021, 57, 1350. [Google Scholar] [CrossRef]
- Vigié du Cayla, G.; Collet, J.M.; Attali, V.; Kerbrat, J.B.; Benslama, L.; Goudot, P. Long-term effectiveness and side effects of mandibular advancement devices on dental and skeletal parameters. J. Stomatol. Oral Maxillofac. Surg. 2019, 120, 7–10. [Google Scholar] [CrossRef]
- McArdle, N.; Devereux, G.; Heidarnejad, H.; Engleman, H.M.; Mackay, T.W.; Douglas, N.J. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am. J. Respir. Crit. Care Med. 1999, 159, 1108–1114. [Google Scholar] [CrossRef]
- Peppard, P.E.; Young, T.; Palta, M.; Dempsey, J.; Skatrud, J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA 2000, 284, 3015–3021. [Google Scholar] [CrossRef]
- Johnston, C.D.; Gleadhill, I.C.; Cinnamond, M.J.; Gabbey, J.; Burden, D.J. Mandibular advancement appliances and obstructive sleep apnoea: A randomized clinical trial. Eur. J. Orthod. 2002, 24, 251–262. [Google Scholar] [CrossRef]
- Gauthier, L.; Laberge, L.; Beaudry, M.; Laforte, M.; Rompré, P.H.; Lavigne, G.J. Mandibular advancement appliances remain effective in lowering respiratory disturbance index for 2.5-4.5 years. Sleep Med. 2011, 12, 844–849. [Google Scholar] [CrossRef]
- Jayesh, S.R.; Bhat, W.M. Mandibular advancement device for obstructive sleep apnea: An overview. J. Pharm. Bioallied Sci. 2015, 7, S223–S225. [Google Scholar] [CrossRef] [PubMed]
- Petri, N.; Christensen, I.J.; Svanholt, P.; Sonnesen, L.; Wildschiødtz, G.; Berg, S. Mandibular advancement device therapy for obstructive sleep apnea: A prospective study on predictors of treatment success. Sleep Med. 2019, 54, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Garbarino, S.; Guglielmi, O.; Sanna, A.; Mancardi, G.L.; Magnavita, N. Risk of Occupational Accidents in Workers with Obstructive Sleep Apnea: Systematic Review and Meta-analysis. SLEEP 2016, 39, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Dolnicka, A.; Fosse, V.; Raciborska, A.; Śmieszek, A. Building a Therapeutic Bridge Between Dogs and Humans: A Review of Potential Cross-Species Osteosarcoma Biomarkers. Int. J. Mol. Sci. 2025, 26, 5152. [Google Scholar] [CrossRef]
- Ryan, C.F.; Love, L.L.; Peat, D.; Fleetham, J.A.; Lowe, A.A. Mandibular advancement oral appliance therapy for obstructive sleep apnoea: Effect on awake calibre of the velopharynx. Thorax 1999, 54, 972–977. [Google Scholar] [CrossRef]
- Tan, Y.K.; L’Estrange, P.R.; Luo, Y.M.; Smith, C.; Grant, H.R.; Simonds, A.K.; Spiro, S.G.; Battagel, J.M. Mandibular advancement splints and continuous positive airway pressure in patients with obstructive sleep apnoea: A randomized cross-over trial. Eur. J. Orthod. 2002, 24, 239–249. [Google Scholar] [CrossRef]
- Cistulli, P.A.; Gotsopoulos, H.; Marklund, M.; Lowe, A.A. Treatment of snoring and obstructive sleep apnea with mandibular repositioning appliances. Sleep Med. Rev. 2004, 8, 443–457. [Google Scholar] [CrossRef]
- Sharples, L.D.; Clutterbuck-James, A.L.; Glover, M.J.; Bennett, M.S.; Chadwick, R.; Pittman, M.A.; Quinnell, T.G. Meta-analysis of randomised controlled trials of oral mandibular advancement devices and continuous positive airway pressure for obstructive sleep apnoea-hypopnoea. Sleep Med. Rev. 2016, 27, 108–124. [Google Scholar] [CrossRef]
- Anitua, E.; Durán-Cantolla, J.; Almeida, G.Z.; Alkhraisat, M.H. Minimizing the mandibular advancement in an oral appliance for the treatment of obstructive sleep apnea. Sleep Med. 2017, 34, 226–231. [Google Scholar] [CrossRef]
- Karwowska, U.; Kudrycka, A.; Pierzchała, K.; Stawski, R.; Jerczyńska, H.; Białasiewicz, P.; Kuczyński, W. Twelve-Month CPAP Therapy Modulates BDNF Levels in Patients with Severe Obstructive Sleep Apnea: Implications for Metabolic and Treatment Compliance. Int. J. Mol. Sci. 2025, 26, 5855. Available online: https://www.mdpi.com/1422-0067/26/12/5855 (accessed on 30 July 2025). [CrossRef]
- Marklund, M.; Verbraecken, J.; Randerath, W. Non-CPAP therapies in obstructive sleep apnoea: Mandibular advancement device therapy. Eur. Respir. J. 2012, 39, 1241–1247. [Google Scholar] [CrossRef]
- Saletu, A.; Gritsch, F.; Mailath-Pokorny, G.; Gruber, G.; Anderer, P.; Saletu, B. [Objective assessment and therapeutic efficacy of an improved mandibular advancement device for snoring and sleep apnea syndromes with polysomnography]. Wien. Klin. Wochenschr. 2002, 114, 807–815. [Google Scholar]
- Dieltjens, M.; Vanderveken, O.M.; Hamans, E.; Verbraecken, J.A.; Wouters, K.; Willemen, M.; De Backer, W.A.; Van de Heyning, P.H.; Braem, M.J. Treatment of obstructive sleep apnea using a custom-made titratable duobloc oral appliance: A prospective clinical study. Sleep Breath. Schlaf Atm. 2013, 17, 565–572. [Google Scholar] [CrossRef]
- Lin, W.-C.; Winkelman, J.W. Obstructive sleep apnea and severe mental illness: Evolution and consequences. Curr. Psychiatry Rep. 2012, 14, 503–510. [Google Scholar] [CrossRef]
- Yaggi, H.K.; Concato, J.; Kernan, W.N.; Lichtman, J.H.; Brass, L.M.; Mohsenin, V. Obstructive sleep apnea as a risk factor for stroke and death. N. Engl. J. Med. 2005, 353, 2034–2041. [Google Scholar] [CrossRef]
- Faber, J.; Faber, C.; Faber, A.P. Obstructive sleep apnea in adults. Dent. Press J. Orthod. 2019, 24, 99–109. [Google Scholar] [CrossRef]
- Charitos, I.A.; Inchingolo, A.M.; Ferrante, L.; Inchingolo, F.; Inchingolo, A.D.; Castellaneta, F.; Cotoia, A.; Palermo, A.; Scacco, S.; Dipalma, G. The Gut Microbiota’s Role in Neurological, Psychiatric, and Neurodevelopmental Disorders. Nutrients 2024, 16, 4404. [Google Scholar] [CrossRef]
- Young, T.; Palta, M.; Dempsey, J.; Skatrud, J.; Weber, S.; Badr, S. The occurrence of sleep-disordered breathing among middle-aged adults. N. Engl. J. Med. 1993, 328, 1230–1235. [Google Scholar] [CrossRef]
- Peppard, P.E.; Hagen, E.W. The Last 25 Years of Obstructive Sleep Apnea Epidemiology-and the Next 25? Am. J. Respir. Crit. Care Med. 2018, 197, 310–312. [Google Scholar] [CrossRef]
- Guerra, D.; Severino, M.; Caruso, S.; Rastelli, S.; Gatto, R. The Importance of Using Physical Tridimensional Models for the Management and Planning of Extended Osseous Odontogenic Lesions. Dent. J. 2021, 9, 134. [Google Scholar] [CrossRef]
- Sankri-Tarbichi, A.G. Obstructive sleep apnea-hypopnea syndrome: Etiology and diagnosis. Avicenna J. Med. 2012, 2, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Vanek, J.; Prasko, J.; Genzor, S.; Ociskova, M.; Kantor, K.; Holubova, M.; Slepecky, M.; Nesnidal, V.; Kolek, A.; Sova, M. Obstructive sleep apnea, depression and cognitive impairment. Sleep Med. 2020, 72, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, K.; Takaya, H.; Qian, J.; Petocz, P.; Ng, A.T.; Cistulli, P.A. Oral Appliance Treatment Response and Polysomnographic Phenotypes of Obstructive Sleep Apnea. J. Clin. Sleep Med. 2015, 11, 861–868. [Google Scholar] [CrossRef]
- Doff, M.H.J.; Hoekema, A.; Wijkstra, P.J.; van der Hoeven, J.H.; Huddleston Slater, J.J.R.; de Bont, L.G.M.; Stegenga, B. Oral appliance versus continuous positive airway pressure in obstructive sleep apnea syndrome: A 2-year follow-up. Sleep 2013, 36, 1289–1296. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Laforgia, A.; Inchingolo, A.M.; Latini, G.; Pezzolla, C.; Nardelli, P.; Palermo, A.; Inchingolo, F.; Malcangi, G.; Dipalma, G. Rapid palate expansion’s impact on nasal breathing: A systematic review. Int. J. Pediatr. Otorhinolaryngol. 2025, 190, 112248. [Google Scholar] [CrossRef]
- Kharrat, F.; Balasan, N.; Ura, B.; Golino, V.; Campiglia, P.; Peri, G.; Valencic, E.; Qaisiya, M.; de Moura, R.; Di Stazio, M.; et al. Deep Proteomics Analysis Unravels the Molecular Signatures of Tonsillar B Cells in PFAPA and OSAS in the Pediatric Population. Int. J. Mol. Sci. 2025, 26, 6621. Available online: https://www.mdpi.com/1422-0067/26/14/6621 (accessed on 30 July 2025). [CrossRef]
- Horner, R.L. Pathophysiology of Obstructive Sleep Apnea. J. Cardiopulm. Rehabil. Prev. 2008, 28, 289. [Google Scholar] [CrossRef]
- Laforgia, A.; Inchingolo, A.D.; Piras, F.; Colonna, V.; Giorgio, R.V.; Carone, C.; Rapone, B.; Malcangi, G.; Inchingolo, A.M.; Inchingolo, F.; et al. Therapeutic Strategies and Genetic Implications for Periodontal Disease Management: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 7217. [Google Scholar] [CrossRef]
- Kushida, C.A.; Morgenthaler, T.I.; Littner, M.R.; Alessi, C.A.; Bailey, D.; Coleman, J.; Friedman, L.; Hirshkowitz, M.; Kapen, S.; Kramer, M.; et al. Practice parameters for the treatment of snoring and Obstructive Sleep Apnea with oral appliances: An update for 2005. Sleep 2006, 29, 240–243. [Google Scholar] [CrossRef]
- Gagnadoux, F.; Fleury, B.; Vielle, B.; Pételle, B.; Meslier, N.; N’Guyen, X.L.; Trzepizur, W.; Racineux, J.L. Titrated mandibular advancement versus positive airway pressure for sleep apnoea. Eur. Respir. J. 2009, 34, 914–920. [Google Scholar] [CrossRef]
- Park, P.; Jeon, H.W.; Han, D.H.; Won, T.-B.; Kim, D.-Y.; Rhee, C.-S.; Kim, H.J. Therapeutic outcomes of mandibular advancement devices as an initial treatment modality for obstructive sleep apnea. Medicine 2016, 95, e5265. [Google Scholar] [CrossRef] [PubMed]
- Buiret, G.; Bechara, M.; Plouin-Gaudon, I.; Bavozet, F.; Dancea, O.; Pujo, K.; Chidiac, F. Predictive Factors for Efficacious Oral Appliance Therapy in Moderate to Severe Obstructive Sleep Apnea Patients. Laryngoscope 2021, 131, E2089–E2096. [Google Scholar] [CrossRef] [PubMed]
- Marklund, M. Predictors of long-term orthodontic side effects from mandibular advancement devices in patients with snoring and obstructive sleep apnea. Am. J. Orthod. Dentofac. Orthop. 2006, 129, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Hoekema, A.; Doff, M.H.J.; de Bont, L.G.M.; van der Hoeven, J.H.; Wijkstra, P.J.; Pasma, H.R.; Stegenga, B. Predictors of obstructive sleep apnea-hypopnea treatment outcome. J. Dent. Res. 2007, 86, 1181–1186. [Google Scholar] [CrossRef]
- Marshall, N.S.; Wong, K.K.H.; Liu, P.Y.; Cullen, S.R.J.; Knuiman, M.W.; Grunstein, R.R. Sleep apnea as an independent risk factor for all-cause mortality: The Busselton Health Study. Sleep 2008, 31, 1079–1085. [Google Scholar] [CrossRef]
- Hudgel, D.W. Sleep Apnea Severity Classification-Revisited. Sleep 2016, 39, 1165–1166. [Google Scholar] [CrossRef]
- Kaštelan, S.; Kozina, L.; Alaber, M.; Tomić, Z.; Andrešić, M.; Bakija, I.; Bućan, D.; Matejić, T.; Vidović, D. Neuro-Ophthalmological Disorders Associated with Obstructive Sleep Apnoea. Int. J. Mol. Sci. 2025, 26, 6649. [Google Scholar] [CrossRef]
- Machado-Júnior, A.-J.; Signorelli, L.-G.; Zancanella, E.; Crespo, A.-N. Randomized controlled study of a mandibular advancement appliance for the treatment of obstructive sleep apnea in children: A pilot study. Med. Oral Patol. Oral Cirugia Bucal 2016, 21, e403–e407. [Google Scholar] [CrossRef]
- Marques, M.; Genta, P.R.; Azarbarzin, A.; Taranto-Montemurro, L.; Messineo, L.; Hess, L.B.; Demko, G.; White, D.P.; Sands, S.A.; Wellman, A. Structure and severity of pharyngeal obstruction determine oral appliance efficacy in sleep apnoea. J. Physiol. 2019, 597, 5399–5410. [Google Scholar] [CrossRef]
- Carberry, J.C.; Amatoury, J.; Eckert, D.J. Personalized Management Approach for OSA. Chest 2018, 153, 744–755. [Google Scholar] [CrossRef]
- Engleman, H.M.; McDonald, J.P.; Graham, D.; Lello, G.E.; Kingshott, R.N.; Coleman, E.L.; Mackay, T.W.; Douglas, N.J. Randomized crossover trial of two treatments for sleep apnea/hypopnea syndrome: Continuous positive airway pressure and mandibular repositioning splint. Am. J. Respir. Crit. Care Med. 2002, 166, 855–859. [Google Scholar] [CrossRef]
- Shankar Agarwal, S.; Garg, Y.; Kadu, A.; Datana, S.; Kumar, P.; Banari, A. Efficacy of titratable mandibular advancement device versus continuous positive airway pressure therapy in the treatment of obstructive sleep apnea: A clinical crossover trial. Med. J. Armed Forces India 2023, 79, S84–S93. [Google Scholar] [CrossRef]
- Bosschieter, P.F.N.; Uniken, V.J.A.M.; Vonk, P.E.; Ravesloot, M.J.L.; Hoekema, A.; Plooij, J.M.; Lobbezoo, F.; de Vries, N. Equal effect of a noncustom vs. a custom mandibular advancement device in treatment of obstructive sleep apnea. J. Clin. Sleep Med. 2022, 18, 2155–2165. [Google Scholar] [CrossRef] [PubMed]
- Ciavarella, D.; Campobasso, A.; Cazzolla, A.P.; Suriano, C.; Lo Muzio, E.; Guida, L.; Salcuni, F.; Laurenziello, M.; Illuzzi, G.; Burlon, G.; et al. The efficacy of a modified mandibular advancement device for OSA treatment in a group of adult patients. Cranio J. Craniomandib. Pract. 2025, 43, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Fichera, G.; Ronsivalle, V.; Zappalà, G.; Campagna, P.; Quinzi, V.; Lo Giudice, A. Mandibular Advancement Devices (MAD) as a Treatment Alternative for Obstructive Sleep Apnea Syndrome (OSAS). Available online: https://opendentistryjournal.com/VOLUME/15/PAGE/120/FULLTEXT/ (accessed on 16 March 2021).
- On, S.-W.; Kim, D.-K.; Lee, M.H.; Lee, J.H.; Lee, K.C.; Byun, S.-H.; Hong, S.J. Clinical Efficacy of a Position-Responding Mandibular Advancement Device in Patients with Obstructive Sleep Apnea. Clin. Exp. Otorhinolaryngol. 2024, 17, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Pahkala, R.; Seppä, J.; Myllykangas, R.; Tervaniemi, J.; Vartiainen, V.M.; Suominen, A.L.; Muraja-Murro, A. The impact of oral appliance therapy with moderate mandibular advancement on obstructive sleep apnea and upper airway volume. Sleep Breath. Schlaf Atm. 2020, 24, 865–873. [Google Scholar] [CrossRef]
- Marco Pitarch, R.; Selva García, M.; Puertas Cuesta, J.; Marco Algarra, J.; Fernández Julian, E.; Fons Font, A. Effectiveness of a mandibular advancement device in obstructive sleep apnea patients: A prospective clinical trial. Eur. Arch. Oto-Rhino-Laryngol. 2018, 275, 1903–1911. [Google Scholar] [CrossRef]
- Shete, C.S.; Bhad, W.A. Three-dimensional upper airway changes with mandibular advancement device in patients with obstructive sleep apnea. Am. J. Orthod. Dentofac. Orthop. 2017, 151, 941–948. [Google Scholar] [CrossRef]
- Verburg, F.E.; Bollen, K.H.A.; Donker, H.-J.; Kramer, G.J.C. The effectiveness of two types of MADS for OSA therapy. Clin. Oral Investig. 2018, 22, 1995–2003. [Google Scholar] [CrossRef]
- Zhu, N.; Buiret, G. Effects of mandibular advancement devices on the evolution of obstructive sleep apnea. Sleep Breath. Schlaf Atm. 2024, 28, 1127–1135. [Google Scholar] [CrossRef]
- Hoekema, A.; Stegenga, B.; Bakker, M.; Brouwer, W.H.; de Bont, L.G.M.; Wijkstra, P.J.; van der Hoeven, J.H. Simulated driving in obstructive sleep apnoea-hypopnoea; effects of oral appliances and continuous positive airway pressure. Sleep Breath. Schlaf Atm. 2007, 11, 129–138. [Google Scholar] [CrossRef]
- Hoffstein, V. Review of oral appliances for treatment of sleep-disordered breathing. Sleep Breath. Schlaf Atm. 2007, 11, 1–22. [Google Scholar] [CrossRef]
- Somers, V.K.; White, D.P.; Amin, R.; Abraham, W.T.; Costa, F.; Culebras, A.; Daniels, S.; Floras, J.S.; Hunt, C.E.; Olson, L.J.; et al. Sleep apnea and cardiovascular disease: An American Heart Association/american College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation 2008, 118, 1080–1111. [Google Scholar] [PubMed]
- Sullivan, C.E.; Issa, F.G.; Berthon-Jones, M.; Eves, L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet 1981, 1, 862–865. [Google Scholar] [CrossRef] [PubMed]
- Dipalma, G.; Inchingolo, A.D.; Fiore, A.; Balestriere, L.; Nardelli, P.; Casamassima, L.; Di Venere, D.; Palermo, A.; Inchingolo, F.; Inchingolo, A.M. The Differential Impact of Clear Aligners and Fixed Orthodontic Appliances on Periodontal Health: A Systematic Review. Children 2025, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Rastelli, S.; Capogreco, M.; D’Amario, M.; Falisi, G.; Severino, M.; Iacomino, E. Pterygoid implants: A viable alternative for the rehabilitation of the posterior sectors of the atrophic maxilla. Oral Implantol. J. Innov. Adv. Tech. Oral Health 2024, 16, 38–43. [Google Scholar] [CrossRef]
- Rastelli, C.; Falisi, G.; Gatto, R.; Galli, M.; Saccone, E.; Severino, M.; Di Paolo, C. Implant stability in different techniques of surgical sites preparation: An in vitro study. Oral Implantol. 2014, 7, 33–39. [Google Scholar] [CrossRef]
- Cenzato, N.; Farronato, M.; Tartaglia, F.C.; Giannini, L.; Inchingolo, A.M.; Dipalma, G.; Maspero, C.; Inchingolo, F. Soft Tissue Facial Morphology in Growing Patients with Different Occlusal Classes. J. Pers. Med. 2024, 14, 1042. [Google Scholar] [CrossRef]
- Mancini, A.; Chirico, F.; Colella, G.; Piras, F.; Colonna, V.; Marotti, P.; Carone, C.; Inchingolo, A.D.; Inchingolo, A.M.; Inchingolo, F.; et al. Evaluating the success rates and effectiveness of surgical and orthodontic interventions for impacted canines: A systematic review of surgical and orthodontic interventions and a case series. BMC Oral Health 2025, 25, 295. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Inchingolo, A.M.; Campanelli, M.; Carpentiere, V.; de Ruvo, E.; Ferrante, L.; Palermo, A.; Inchingolo, F.; Dipalma, G. Orthodontic treatment in patients with atypical swallowing and malocclusion: A systematic review. J. Clin. Pediatr. Dent. 2024, 48, 14–26. [Google Scholar] [CrossRef]
- Young, T.; Finn, L.; Peppard, P.E.; Szklo-Coxe, M.; Austin, D.; Nieto, F.J.; Stubbs, R.; Hla, K.M. Sleep disordered breathing and mortality: Eighteen-year follow-up of the Wisconsin sleep cohort. Sleep 2008, 31, 1071–1078. [Google Scholar] [CrossRef]
- Lombardo, G.; Pagano, S.; Cianetti, S.; Capobianco, B.; Orso, M.; Negri, P.; Paglia, M.; Friuli, S.; Paglia, L.; Gatto, R.; et al. Sub-ablative laser irradiation to prevent acid demineralisation of dental enamel. A systematic review of literature reporting in vitro studies. Eur. J. Paediatr. Dent. 2019, 20, 295–301. [Google Scholar]
- Chan, A.S.L.; Sutherland, K.; Schwab, R.J.; Zeng, B.; Petocz, P.; Lee, R.W.W.; Darendeliler, M.A.; Cistulli, P.A. The effect of mandibular advancement on upper airway structure in obstructive sleep apnoea. Thorax 2010, 65, 726–732. [Google Scholar] [CrossRef]
- Millman, R.P.; Rosenberg, C.L.; Carlisle, C.C.; Kramer, N.R.; Kahn, D.M.; Bonitati, A.E. The efficacy of oral appliances in the treatment of persistent sleep apnea after uvulopalatopharyngoplasty. Chest 1998, 113, 992–996. [Google Scholar] [CrossRef] [PubMed]
- Laforgia, A.; Inchingolo, A.D.; Riccaldo, L.; Avantario, P.; Buongiorno, S.; Malcangi, G.; Bordea, I.R.; Palermo, A.; Inchingolo, F.; Inchingolo, A.M.; et al. The Use of Platelet-Rich Fibrin (PRF) in the Management of Dry Socket: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 10069. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Inchingolo, A.M.; Latini, G.; Ferrante, L.; Trilli, I.; Del Vecchio, G.; Palmieri, G.; Malcangi, G.; Inchingolo, A.D.; Dipalma, G. Oxidative Stress and Natural Products in Orthodontic Treatment: A Systematic Review. Nutrients 2023, 16, 113. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Inchingolo, A.M.; Avantario, P.; Settanni, V.; Fatone, M.C.; Piras, F.; Di Venere, D.; Inchingolo, A.D.; Palermo, A.; Dipalma, G. The Effects of Periodontal Treatment on Rheumatoid Arthritis and of Anti-Rheumatic Drugs on Periodontitis: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 17228. [Google Scholar] [CrossRef]
- Bartolucci, M.L.; Bortolotti, F.; Raffaelli, E.; D’Antò, V.; Michelotti, A.; Alessandri Bonetti, G. The effectiveness of different mandibular advancement amounts in OSA patients: A systematic review and meta-regression analysis. Sleep Breath. 2016, 20, 911–919. [Google Scholar] [CrossRef]
- Falisi, G.; Foffo, G.; Severino, M.; Di Paolo, C.; Bianchi, S.; Bernardi, S.; Pietropaoli, D.; Rastelli, S.; Gatto, R.; Botticelli, G. SEM-EDX Analysis of Metal Particles Deposition from Surgical Burs after Implant Guided Surgery Procedures. Coatings 2022, 12, 240. [Google Scholar] [CrossRef]
- Fritsch, K.M.; Iseli, A.; Russi, E.W.; Bloch, K.E. Side effects of mandibular advancement devices for sleep apnea treatment. Am. J. Respir. Crit. Care Med. 2001, 164, 813–818. [Google Scholar] [CrossRef]
- Aarab, G.; Lobbezoo, F.; Wicks, D.J.; Hamburger, H.L.; Naeije, M. Short-term effects of a mandibular advancement device on obstructive sleep apnoea: An open-label pilot trial. J. Oral Rehabil. 2005, 32, 564–570. [Google Scholar] [CrossRef]
- Clark, G.T.; Sohn, J.W.; Hong, C.N. Treating obstructive sleep apnea and snoring: Assessment of an anterior mandibular positioning device. J. Am. Dent. Assoc. 2000, 131, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.P.; Ayappa, I.A.; Caples, S.M.; Kimoff, R.J.; Patel, S.R.; Harrod, C.G. Treatment of Adult Obstructive Sleep Apnea with Positive Airway Pressure: An American Academy of Sleep Medicine Systematic Review, Meta-Analysis, and GRADE Assessment. J. Clin. Sleep Med. 2019, 15, 301–334. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.P.; Ayappa, I.A.; Caples, S.M.; Kimoff, R.J.; Patel, S.R.; Harrod, C.G. Treatment of Adult Obstructive Sleep Apnea with Positive Airway Pressure: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2019, 15, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Schwab, R.J.; Kim, C.; Bagchi, S.; Keenan, B.T.; Comyn, F.-L.; Wang, S.; Tapia, I.E.; Huang, S.; Traylor, J.; Torigian, D.A.; et al. Understanding the anatomic basis for obstructive sleep apnea syndrome in adolescents. Am. J. Respir. Crit. Care Med. 2015, 191, 1295–1309. [Google Scholar] [CrossRef]
- Schendel, S.A.; Jacobson, R.; Khalessi, S. Airway growth and development: A computerized 3-dimensional analysis. J. Oral Maxillofac. Surg. 2012, 70, 2174–2183. [Google Scholar] [CrossRef]
- Ng, A.T.; Gotsopoulos, H.; Qian, J.; Cistulli, P.A. Effect of oral appliance therapy on upper airway collapsibility in obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2003, 168, 238–241. [Google Scholar] [CrossRef]
- Botticelli, G.; Severino, M.; Ferrazzano, G.F.; Vittorini Velasquez, P.; Franceschini, C.; Di Paolo, C.; Gatto, R.; Falisi, G. Excision of Lower Lip Mucocele Using Injection of Hydrocolloid Dental Impression Material in a Pediatric Patient: A Case Report. Appl. Sci. 2021, 11, 5819. [Google Scholar] [CrossRef]
- Weaver, T.E.; Sawyer, A. Management of Obstructive Sleep Apnea by Continuous Positive Airway Pressure. Oral Maxillofac. Surg. Clin. N. Am. 2009, 21, 403–412. [Google Scholar] [CrossRef]
- Cistulli, P.A.; Grunstein, R.R. Medical devices for the diagnosis and treatment of obstructive sleep apnea. Expert Rev. Med. Devices 2005, 2, 749–763. [Google Scholar] [CrossRef]
- Garbarino, S.; Pitidis, A.; Giustini, M.; Taggi, F.; Sanna, A. Motor vehicle accidents and obstructive sleep apnea syndrome: A methodology to calculate the related burden of injuries. Chron. Respir. Dis. 2015, 12, 320–328. [Google Scholar] [CrossRef]
- Randerath, W.J.; Verbraecken, J.; Andreas, S.; Bettega, G.; Boudewyns, A.; Hamans, E.; Jalbert, F.; Paoli, J.R.; Sanner, B.; Smith, I.; et al. Non-CPAP therapies in obstructive sleep apnoea. Eur. Respir. J. 2011, 37, 1000–1028. [Google Scholar] [CrossRef]
- Ng, A.; Gotsopoulos, H.; Darendeliler, A.M.; Cistulli, P.A. Oral Appliance Therapy for Obstructive Sleep Apnea. Treat. Respir. Med. 2005, 4, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.H.; Yow, M. Oral Appliances in the Management of Obstructive Sleep Apnea. Sleep Med. Clin. 2019, 14, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Segù, M.; Campagnoli, G.; Di Blasio, M.; Santagostini, A.; Pollis, M.; Levrini, L. Pilot Study of a New Mandibular Advancement Device. Dent. J. 2022, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.D.; Patano, A.; Coloccia, G.; Ceci, S.; Inchingolo, A.M.; Marinelli, G.; Malcangi, G.; Di Pede, C.; Garibaldi, M.; Ciocia, A.M.; et al. Treatment of Class III Malocclusion and Anterior Crossbite with Aligners: A Case Report. Med. Kaunas Lith. 2022, 58, 603. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Patano, A.; Coloccia, G.; Ceci, S.; Inchingolo, A.M.; Marinelli, G.; Malcangi, G.; Montenegro, V.; Laudadio, C.; Pede, C.D.; et al. The Efficacy of a New AMCOP® Elastodontic Protocol for Orthodontic Interceptive Treatment: A Case Series and Literature Overview. Int. J. Environ. Res. Public. Health 2022, 19, 988. [Google Scholar] [CrossRef]
- Verbruggen, A.E.R.; Dieltjens, M.; Wouters, K.; De Volder, I.; Van de Heyning, P.H.; Braem, M.J.; Vanderveken, O.M. Prevalence of residual excessive sleepiness during effective oral appliance therapy for sleep-disordered breathing. Sleep Med. 2014, 15, 269–272. [Google Scholar] [CrossRef]
- Cunha, T.C.A.; Guimarães, T.D.M.; Schultz, T.C.B.; Almeida, F.R.D.; Cunha, T.M.; Simamoto, P.C.; Bittencourt, L.R.A. Predictors of success for mandibular repositioning appliance in obstructive sleep apnea syndrome. Braz. Oral Res. 2017, 31, e37. [Google Scholar] [CrossRef]
- Vanderveken, O.M.; Boudewyns, A.N.; Braem, M.J.; Okkerse, W.; Verbraecken, J.A.; Willemen, M.; Wuyts, F.L.; De Backer, W.A.; Van de Heyning, P.H. Pilot study of a novel mandibular advancement device for the control of snoring. Acta Otolaryngol. 2004, 124, 628–633. [Google Scholar] [CrossRef]
- Iacomino, E.; Rastelli, S.; Capogreco, M.; Severino, M.; Gallottini, S.G.; Grivetto, F. A Pterygoid implants in severe posterior maxillary atrophy: A case report. Oral Implantol. J. Innov. Adv. Tech. Oral Health 2024, 16, 88–94. [Google Scholar]
- Ferguson, K.A.; Cartwright, R.; Rogers, R.; Schmidt-Nowara, W. Oral appliances for snoring and obstructive sleep apnea: A review. Sleep 2006, 29, 244–262. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Lasserson, T.J.; Fleetham, J.; Wright, J. Oral appliances for obstructive sleep apnoea. Cochrane Database Syst. Rev. 2006, 2006, CD004435. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Wang, P.-C.; Hsu, C.-Y.; Cheng, M.; Liou, C.-C.; Chen, N.-H. Nasal resistance in patients with obstructive sleep apnea. ORL J. Oto-Rhino-Laryngol. Its Relat. Spec. 2005, 67, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Dieltjens, M.; Braem, M.J.; Vroegop, A.V.M.T.; Wouters, K.; Verbraecken, J.A.; De Backer, W.A.; Van de Heyning, P.H.; Vanderveken, O.M. Objectively measured vs. self-reported compliance during oral appliance therapy for sleep-disordered breathing. Chest 2013, 144, 1495–1502. [Google Scholar] [CrossRef]
- Inchingolo, F.; Inchingolo, A.M.; Riccaldo, L.; Morolla, R.; Sardano, R.; Di Venere, D.; Palermo, A.; Inchingolo, A.D.; Dipalma, G.; Corsalini, M. Structural and Color Alterations of Teeth following Orthodontic Debonding: A Systematic Review. J. Funct. Biomater. 2024, 15, 123. [Google Scholar] [CrossRef]
- Signorini, L.; Marenzi, G.; Facente, A.; Marrelli, B.; Marano, R.M.; Valletta, A.; Pacifici, L.; Gasparro, R.; Sammartino, G.; Severino, M. Critical Overview on Pure Chitosan-based Scaffolds for Bone Tissue Engineering: Clinical insights in Dentistry. Int. J. Med. Sci. 2023, 20, 1527–1534. [Google Scholar] [CrossRef]
- Björk, A.; Krebs, A.; Solow, B. A Method for Epidemiological Registration of Malocculusion. Acta Odontol. Scand. 1964, 22, 27–41. [Google Scholar] [CrossRef]
- Flegal, K.M.; Carroll, M.D.; Ogden, C.L.; Johnson, C.L. Prevalence and trends in obesity among US adults, 1999-2000. JAMA 2002, 288, 1723–1727. [Google Scholar] [CrossRef]
- Arias, M.A.; García-Río, F.; Alonso-Fernández, A.; Martínez, I.; Villamor, J. Pulmonary hypertension in obstructive sleep apnoea: Effects of continuous positive airway pressure: A randomized, controlled cross-over study. Eur. Heart J. 2006, 27, 1106–1113. [Google Scholar] [CrossRef]
- Lam, B.; Sam, K.; Mok, W.Y.W.; Cheung, M.T.; Fong, D.Y.T.; Lam, J.C.M.; Lam, D.C.L.; Yam, L.Y.C.; Ip, M.S.M. Randomised study of three non-surgical treatments in mild to moderate obstructive sleep apnoea. Thorax 2007, 62, 354–359. [Google Scholar] [CrossRef]
- Maspero, C.; Cappella, A.; Dolci, C.; Cagetti, M.G.; Inchingolo, F.; Sforza, C. Is Orthodontic Treatment with Microperforations Worth It? A Scoping Review. Children 2022, 9, 208. [Google Scholar] [CrossRef]
- Cirulli, N.; Inchingolo, A.D.; Patano, A.; Ceci, S.; Marinelli, G.; Malcangi, G.; Coloccia, G.; Montenegro, V.; Di Pede, C.; Ciocia, A.M.; et al. Innovative Application of Diathermy in Orthodontics: A Case Report. Int. J. Environ. Res. Public. Health 2022, 19, 7448. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Ceci, S.; Patano, A.; Inchingolo, A.M.; Montenegro, V.; Di Pede, C.; Malcangi, G.; Marinelli, G.; Coloccia, G.; Garibaldi, M.; et al. Elastodontic Therapy of Hyperdivergent Class II Patients Using AMCOP® Devices: A Retrospective Study. Appl. Sci. 2022, 12, 3259. [Google Scholar] [CrossRef]
- Inchingolo, F.; Inchingolo, A.D.; Palumbo, I.; Trilli, I.; Guglielmo, M.; Mancini, A.; Palermo, A.; Inchingolo, A.M.; Dipalma, G. The Impact of Cesarean Section Delivery on Intestinal Microbiota: Mechanisms, Consequences, and Perspectives-A Systematic Review. Int. J. Mol. Sci. 2024, 25, 1055. [Google Scholar] [CrossRef]
| Authors (Year) | D1 | D2 | D3 | D4 | D5 | D6 | D7 | Overall |
| Agarwal S.S et al., 2023 [103] |  |  |  |  |  |  |  |  |
| Bosschieter P.F.N. et al., 2022 [104] |  |  |  |  |  |  |  |  |
| Ciavarella D. et al., 2023 [105] |  |  |  |  |  |  |  |  |
| Fichera G. et al., 2020 [106] |  |  |  |  |  |  |  |  |
| On S.W. et al., 2024 [107] |  |  |  |  |  |  |  |  |
| Pahkala R. et al., 2020 [108] |  |  |  |  |  |  |  |  |
| Pitarch R.M. et al., 2018 [109] |  |  |  |  |  |  |  |  |
| Shete C.S. et al., 2017 [110] |  |  |  |  |  |  |  |  |
| Verburg F.E. et al., 2018 [111] |  |  |  |  |  |  |  |  |
| Zhu N. et al., 2024 [112] |  |  |  |  |  |  |  |  |
| Domains: D1: Bias due to confounding. D2: Bias arising from measurement of the exposure. D3: Bias in selection of participants into the study (or into the analysis). D4: Bias due to post-exposure interventions. D5: Bias due to missing data. D6: Bias arising from measurement of the outcome. D7: Bias in Selection of the Reported Result |  Very High Very High High High Some Concerns Some Concerns Low Low No information No information | |||||||
| Authors | Number of Patients | Study Design | AHI (Pre/Post) | ESS (Pre/Post) | Other Parameters |
| Agarwal S.S. et al., 2023 [103] | 30 (18 M, 12 F) | CPAP vs. MAD | Pre: 35 → Post: 25 | Pre: 12 → Post: 8 | Satisfaction higher in MAD vs. CPAP |
| Bosschieter P.F.N. et al., 2022 [104] | 58 | Non-custom MAD vs. Custom MAD | Pre: 16.3 → Post: 10.7 (custom), Pre: 16.3 → Post: 7.8 (non-custom) | Pre-baseline: Not reported → Post: questionnaires indicated improvement post-treatment.” (p = 0.005) | Adherence improvement |
| Ciavarella D. et al., 2023 [105] | 29 (15 M, 14 F) | Customizable MAD | Pre: 28 → Post: 16 | Pre: 15 → Post: 10 | ↑ SpO2, ↑ quality of life |
| Fichera G. et al., 2020 [106] | 18 (15 M, 3 F) | MAD | Pre: 40 → Post: 35 | Pre: 16 → Post: 12 | MAD comparable to CPAP |
| On S.W. et al., 2024 [107] | 14 (<20 years) | Autotitrating MAD | Pre: 32.8 → Post: 12.9 | Pre: 14 → Post: 9 | ↑ SpO2 minima |
| Pahkala R. et al., 2020 [108] | 58 (39 M, 19 F) | MAD | Pre: 40 → Post: 25 | Pre: 16 → Post: 10 | Quality of life, snoring improvement |
| Pitarch R.M. et al., 2018 [109] | 41 | MAD | Pre: 22.5 → Post: 9.1 | Pre: 12 → Post: 7 | Snoring index reduction |
| Shete C.S. et al., 2017 [110] | 37 | MAD | Pre: 30 → Post: 20 | Pre: 15 → Post: 10 | ↑ Airway volume, ↑ SpO2 |
| Verburg F.E. et al., 2018 [111] | 137 | MAD 1 vs. MAD 2 | No significant difference | Not reported | No significant difference in AHI |
| Zhu N. et al., 2024 [112] | 20 | MAD | Pre: >30 → Post: 14.8 | Pre: 12 → Post: 10 | Symptom improvement with titration |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inchingolo, A.D.; Inchingolo, A.M.; Ciocia, C.; Calò, F.; Savastano, S.; Inchingolo, F.; Palermo, A.; Giudice, G.; Di Venere, D.; Marinelli, G.; et al. Efficacy of Mandibular Advancement Devices in the Treatment of Mild to Moderate Obstructive Sleep Apnea: A Systematic Review. Int. J. Transl. Med. 2025, 5, 49. https://doi.org/10.3390/ijtm5040049
Inchingolo AD, Inchingolo AM, Ciocia C, Calò F, Savastano S, Inchingolo F, Palermo A, Giudice G, Di Venere D, Marinelli G, et al. Efficacy of Mandibular Advancement Devices in the Treatment of Mild to Moderate Obstructive Sleep Apnea: A Systematic Review. International Journal of Translational Medicine. 2025; 5(4):49. https://doi.org/10.3390/ijtm5040049
Chicago/Turabian StyleInchingolo, Alessio Danilo, Angelo Michele Inchingolo, Claudia Ciocia, Francesca Calò, Sara Savastano, Francesco Inchingolo, Andrea Palermo, Giuseppe Giudice, Daniela Di Venere, Grazia Marinelli, and et al. 2025. "Efficacy of Mandibular Advancement Devices in the Treatment of Mild to Moderate Obstructive Sleep Apnea: A Systematic Review" International Journal of Translational Medicine 5, no. 4: 49. https://doi.org/10.3390/ijtm5040049
APA StyleInchingolo, A. D., Inchingolo, A. M., Ciocia, C., Calò, F., Savastano, S., Inchingolo, F., Palermo, A., Giudice, G., Di Venere, D., Marinelli, G., & Dipalma, G. (2025). Efficacy of Mandibular Advancement Devices in the Treatment of Mild to Moderate Obstructive Sleep Apnea: A Systematic Review. International Journal of Translational Medicine, 5(4), 49. https://doi.org/10.3390/ijtm5040049











