Twelve-Month CPAP Therapy Modulates BDNF Levels in Patients with Severe Obstructive Sleep Apnea: Implications for Metabolic and Treatment Compliance
Abstract
1. Introduction
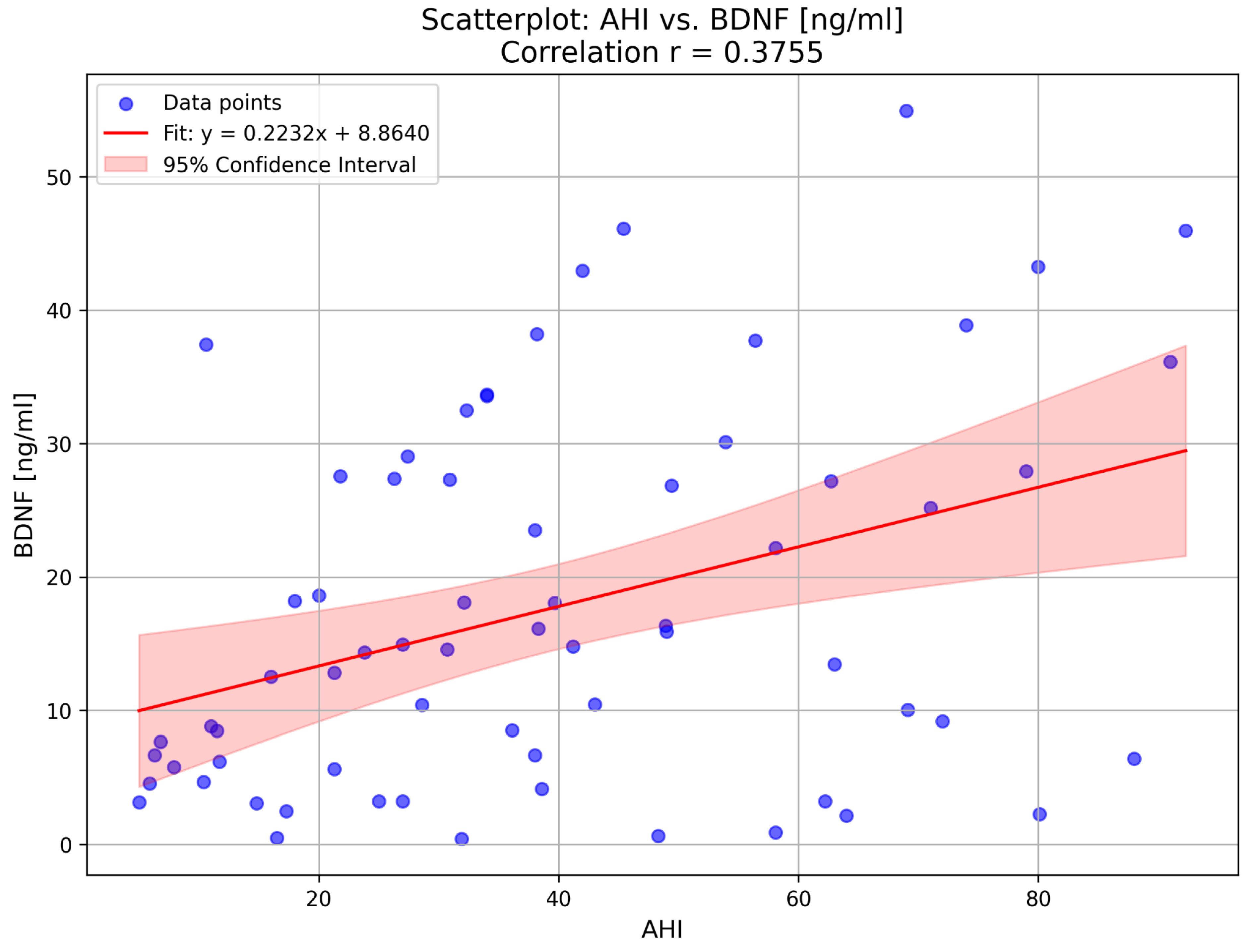
2. Results
3. Discussion
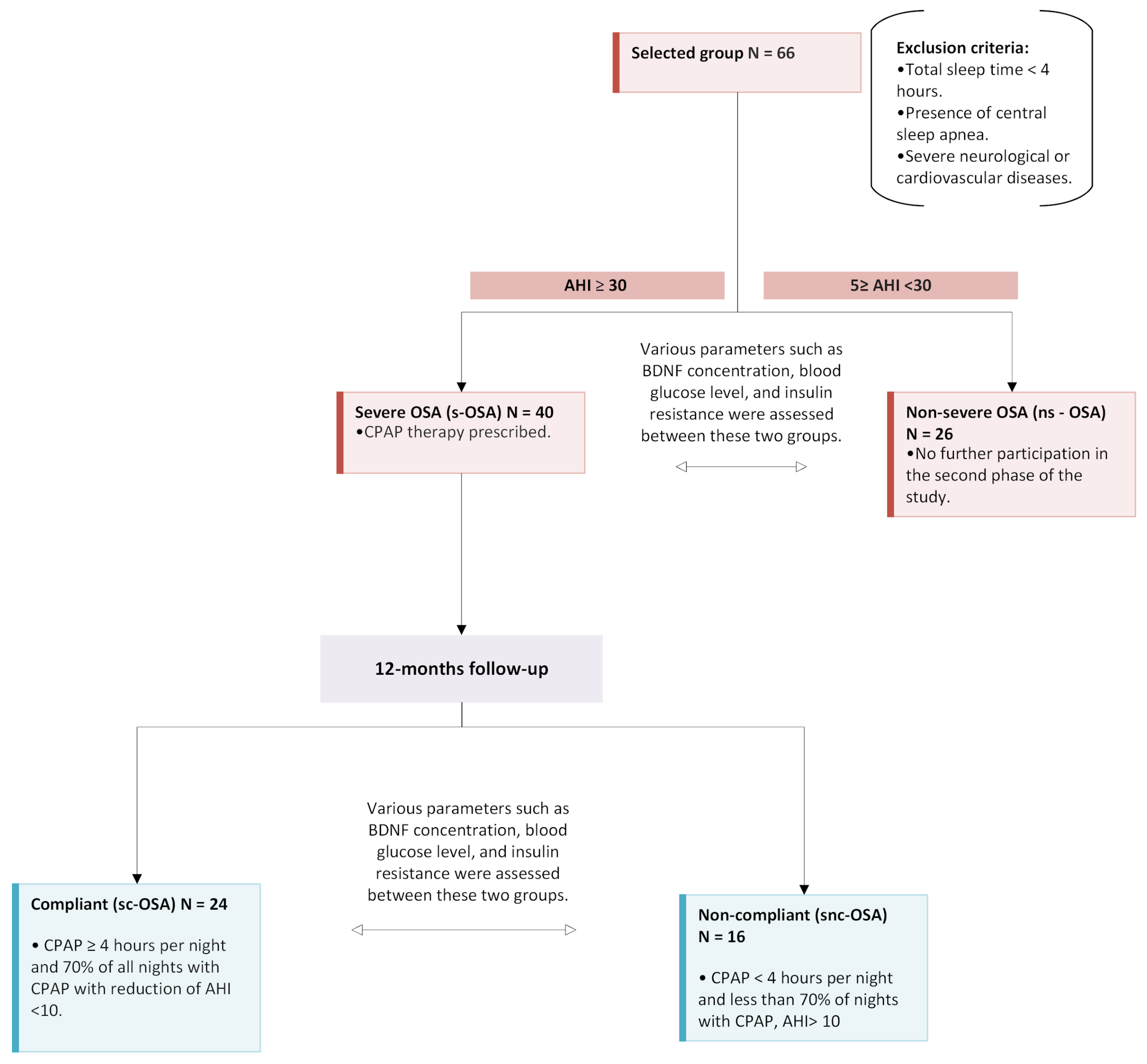
4. Methods and Materials
4.1. Study Population
4.2. Data Collection
4.3. Study Protocol
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AHI | Apnea–Hypopnea Index |
| BDNF | Brain-Derived Neurotrophic Factor |
| BMI | Body Mass Index |
| CPAP | Continuous Positive Airway Pressure |
| DM2 | Diabetes Mellitus type 2 |
| HOMA-IR | Homeostatic Model Assessment—Insulin Resistance |
| NREM | Non-rapid Eye Movement sleep |
| ns-OSA | non-severe Obstructive Sleep Apnea |
| OSA | Obstructive Sleep Apnea |
| PSG | Polysomnography |
| s-OSA | severe Obstructive Sleep Apnea |
| sc-OSA | severe compliant Obstructive Sleep Apnea |
| snc-OSA | severe non-compliant Obstructive Sleep Apnea |
| REM | Rapid Eye Movement sleep |
| TST | Total Sleep Time |
References
- Mei, F.; Nagappan, G.; Ke, Y.; Sacktor, T.C.; Lu, B. BDNF Facilitates L-LTP Maintenance in the Absence of Protein Synthesis Through PKMζ. PLoS ONE 2011, 6, e21568. [Google Scholar] [CrossRef] [PubMed]
- Böyük, B.; Değirmencioğlu, Ş.; Atalay, H.; Güzel, S.; Acar, A.; Çelebi, A.; Ekizoğlu, İ.; Şimşek, Ç. Relationship Between Levels of Brain-Derived Neurotrophic Factor and Metabolic Parameters in Patients With Type 2 Diabetes Mellitus. J. Diabetes Res. 2014, 2014, 978143. [Google Scholar] [CrossRef] [PubMed]
- Ichimura-Shimizu, M.; Kojima, M.; Suzuki, S.; Miyata, M.; Osaki, Y.; Matsui, K.; Mizui, T.; Tsuneyama, K. Brain-derived Neurotrophic Factor Knock-out Mice Develop Non-alcoholic Steatohepatitis. J. Pathol. 2023, 261, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Figurov, A.; Pozzo-Miller, L.; Olafsson, P.; Wang, T.; Lu, B. Regulation of Synaptic Responses to High-Frequency Stimulation and LTP by Neurotrophins in the Hippocampus. Nature 1996, 381, 706–709. [Google Scholar] [CrossRef]
- Golden, E.; Emiliano, A.B.; Maudsley, S.; Windham, B.G.; Carlson, O.D.; Egan, J.M.; Driscoll, I.; Ferrucci, L.; Martin, B.; Mattson, M.P. Circulating Brain-Derived Neurotrophic Factor and Indices of Metabolic and Cardiovascular Health: Data From the Baltimore Longitudinal Study of Aging. PLoS ONE 2010, 5, e10099. [Google Scholar] [CrossRef]
- Fulgenzi, G.; Hong, Z.; Tomassoni-Ardori, F.; Barella, L.F.; Becker, J.; Barrick, C.; Swing, D.A.; Yanpallewar, S.; St. Croix, B.; Wess, J.; et al. Novel Metabolic Role for BDNF in Pancreatic Β-Cell Insulin Secretion. Nat. Commun. 2020, 11, 1950. [Google Scholar] [CrossRef]
- Liao, G.; An, J.J.; Gharami, K.; Waterhouse, E.G.; Vanevski, F.; Jones, K.R.; Xu, B. Dendritically Targeted BDNF MRNA Is Essential for Energy Balance and Response to Leptin. Nat. Med. 2012, 18, 564–571. [Google Scholar] [CrossRef]
- Ċastrén, E. Neurotrophins and Psychiatric Disorders. In Neurotrophic Factors; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Colucci-D’Amato, L.; Speranza, L.; Volpicelli, F. Neurotrophic Factor BDNF, Physiological Functions and Therapeutic Potential in Depression, Neurodegeneration and Brain Cancer. Int. J. Mol. Sci. 2020, 21, 7777. [Google Scholar] [CrossRef]
- Autry, A.E.; Monteggia, L.M. Brain-Derived Neurotrophic Factor and Neuropsychiatric Disorders. Pharmacol. Rev. 2012, 64, 238–258. [Google Scholar] [CrossRef]
- Amidfar, M.; de Oliveira, J.; Kucharska, E.; Budni, J.; Kim, Y. The Role of CREB and BDNF in Neurobiology and Treatment of Alzheimer’s Disease. Life Sci. 2020, 257, 118020. [Google Scholar] [CrossRef]
- Meraz-Ríos, M.A.; Toral-Ríos, D.; Franco-Bocanegra, D.; Villeda-Hernández, J.; Campos-Peña, V. Inflammatory Process in Alzheimer’s Disease. Front. Integr. Neurosci. 2013, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Marosi, K.; Mattson, M.P. BDNF Mediates Adaptive Brain and Body Responses to Energetic Challenges. Trends Endocrinol. Metab. 2014, 25, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Pandit, M.; Behl, T.; Sachdeva, M.; Arora, S. Role of Brain Derived Neurotropic Factor in Obesity. Obes. Med. 2020, 17, 100189. [Google Scholar] [CrossRef]
- Patel, A.; Chong, D.J. Obstructive Sleep Apnea. Clin. Geriatr. Med. 2021, 37, 457–467. [Google Scholar] [CrossRef]
- Seda, G.; Matwiyoff, G.; Parrish, S. Effects of Obstructive Sleep Apnea and CPAP on Cognitive Function. Curr. Neurol. Neurosci. Rep. 2021, 21, 32. [Google Scholar] [CrossRef]
- Vaněk, J.; Praško, J.; Genzor, S.; Ocisková, M.; Kantor, K.; Holubová, M.; Šlepecký, M.; Nesnídal, V.; Kolek, A.; Sova, M. Obstructive Sleep Apnea, Depression and Cognitive Impairment. Sleep Med. 2020, 72, 50–58. [Google Scholar] [CrossRef]
- Kabeloğlu, V.; Şenel, G.B.; Karadeniz, D. Positive Airway Pressure Normalizes Glucose Metabolism in Obstructive Sleep Apnea Independent of Diabetes and Obesity. Ideggyógyászati Szle. 2020, 73, 417–425. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.-J.; Zhao, M.-Q.; Liu, S.-M.; Li, Y.-Z. Changes of Serum Brain-Derived Neurotrophic Factor in Children With Obstructive Sleep Apnoea–Hypopnoea Syndrome Following Adenotonsillectomy. J. Int. Med. Res. 2010, 38, 1942–1951. [Google Scholar] [CrossRef]
- Mok, Y.; Tan, A.K.L.; Hsu, P.P.; Seow, A.; Chan, Y.H.; Wong, H.S.; Poh, Y.; Wong, K. Comparing Treatment Effects of a Convenient Vibratory Positional Device to CPAP in Positional OSA: A Crossover Randomised Controlled Trial. Thorax 2020, 75, 331–337. [Google Scholar] [CrossRef]
- Gabryelska, A.; Sochal, M.; Wasik, B.; Szczepanowski, P.; Białasiewicz, P. Factors Affecting Long-Term Compliance of CPAP Treatment—A Single Centre Experience. J. Clin. Med. 2021, 11, 139. [Google Scholar] [CrossRef]
- McEvoy, R.D.; Antic, N.A.; Heeley, E.; Luo, Y.; Ou, Q.; Zhang, X.; Mediano, O.; Chen, R.; Drager, L.F.; Liu, Z.; et al. CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea. N. Engl. J. Med. 2016, 375, 919–931. [Google Scholar] [CrossRef] [PubMed]
- González-Aquines, A.; Martínez-Roque, D.; Treviño-Herrera, A.B.; Chávez-Luévanos, B.E.; Guerrero-Campos, F.; Góngora-Rivera, F. Obstructive Sleep Apnea Syndrome and Its Relationship with Ischaemic Stroke. Rev. Neurol. 2019, 69, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Lévy, P.; Kohler, M.; McNicholas, W.T.; Barbé, F.; McEvoy, R.D.; Somers, V.K.; Lavie, L.; Pépin, J.L. Obstructive Sleep Apnoea Syndrome. Nat. Rev. Dis. Primers 2015, 1, 15015. [Google Scholar] [CrossRef] [PubMed]
- Filho, G.L.; Genta, P.R.; Pedrosa, R.P.; Drager, L.F.; Martinez, D. Consequências Cardiovasculares Na SAOS. J. Bras. Pneumol. 2010, 36, 38–42. [Google Scholar] [CrossRef]
- Angelico, F.; Del Ben, M.; Augelletti, T.; de Vita, R.; Roma, R.; Violi, F.; Fabiani, M. Obstructive Sleep Apnoea Syndrome and the Metabolic Syndrome in an Internal Medicine Setting. Eur. J. Intern. Med. 2010, 21, 191–195. [Google Scholar] [CrossRef]
- Gabryelska, A.; Turkiewicz, S.; Ditmer, M.; Karuga, F.F.; Strzelecki, D.; Białasiewicz, P.; Sochal, M. BDNF and ProBDNF Serum Protein Levels in Obstructive Sleep Apnea Patients and Their Involvement in Insomnia and Depression Symptoms. J. Clin. Med. 2022, 11, 7135. [Google Scholar] [CrossRef]
- Kielb, S.A.; Ancoli-Israel, S.; Rebok, G.W.; Spira, A.P. Cognition in Obstructive Sleep Apnea-Hypopnea Syndrome (OSAS): Current Clinical Knowledge and the Impact of Treatment. Neuromol. Med. 2012, 14, 180–193. [Google Scholar] [CrossRef]
- Gabryelska, A.; Turkiewicz, S.; Ditmer, M.; Sochal, M. Neurotrophins in the Neuropathophysiology, Course, and Complications of Obstructive Sleep Apnea—A Narrative Review. Int. J. Mol. Sci. 2023, 24, 1808. [Google Scholar] [CrossRef]
- Ditmer, M.; Gabryelska, A.; Turkiewicz, S.; Sochal, M. Investigating the Role of BDNF in Insomnia: Current Insights. Nat. Sci. Sleep 2023, 15, 1045–1060. [Google Scholar] [CrossRef]
- Flores, K.R.; Viccaro, F.; Aquilini, M.; Scarpino, S.; Ronchetti, F.; Mancini, R.; Di Napoli, A.; Scozzi, D.; Rícci, A. Protective Role of Brain Derived Neurotrophic Factor (BDNF) in Obstructive Sleep Apnea Syndrome (OSAS) Patients. PLoS ONE 2020, 15, e0227834. [Google Scholar] [CrossRef]
- Kamińska, M.; O’Sullivan, M.; Mery, V.; Lafontaine, A.; Robinson, A.; Gros, P.; Martin, J.G.; Benedetti, A.; Kimoff, R.J. Inflammatory Markers and BDNF in Obstructive Sleep Apnea (OSA) in Parkinson’s Disease (PD). Sleep Med. 2022, 90, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Staats, R. Regulation of Brain-Derived Neurotrophic Factor (BDNF) During Sleep Apnoea Treatment. Thorax 2005, 60, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Baburao, A.; Souza, G.D. Insulin Resistance in Moderate to Severe Obstructive Sleep Apnea in Nondiabetics and Its Response to Continuous Positive Airway Pressure Treatment. N. Am. J. Med. Sci. 2014, 6, 500. [Google Scholar] [CrossRef] [PubMed]
- Vgontzas, A.N.; Karagkouni, E.; He, F.; Li, Y.; Karataraki, M.; Fernandez-Mendoza, J.; Bixler, E.O. Mild-to-Moderate Obstructive Sleep Apnea and Mortality Risk in a General Population Sample: The Modifying Effect of Age and Cardiovascular/Cerebrovascular Comorbidity. J. Sleep Res. 2024, 33, e13944. [Google Scholar] [CrossRef]
- Abud, R.; Salgueiro, M.; Drake, L.; Barros, T.; Jorquera, J.; Labarca, G. Efficacy of Continuous Positive Airway Pressure (CPAP) Preventing Type 2 Diabetes Mellitus in Patients With Obstructive Sleep Apnea Hypopnea Syndrome (OSAHS) and Insulin Resistance: A Systematic Review and Meta-Analysis. Sleep Med. 2019, 62, 14–21. [Google Scholar] [CrossRef]
- Busarakumtragul, P.; Mekseepralard, C.; Sukhumsirichart, W.; Neruntarat, C. Effects of Obstructive Sleep Apnea on Serum Brain-Derived Neurotrophic Factor Protein, Cortisol, and Lipid Levels. Sleep Breath. 2010, 15, 649–656. [Google Scholar] [CrossRef]
| Overall | s-OSA | ns-OSA | p | |
|---|---|---|---|---|
| N (%) | 66 | 40 (60.6%) | 26 (39.4%) | |
| Male sex, n (%) | 58 (87.9%) | 35 (87.5%) | 23 (88.5%) | 0.61 |
| Age (years) | 57.1 ± 10.2 | 57.4 ± 9.5 | 56.7 ± 11.3 | 0.79 |
| BMI (kg/m2) | 32.4 ± 5.7 | 33.4 ± 5.6 | 30.8 ± 5.5 | 0.1 |
| Neck circumference (cm) | 42.9 ± 4.2 | 43.3 ± 4.6 | 42.2 ± 3.6 | 0.07 |
| TST (hours) | 5.66 ± 0.95 | 5.53 ± 0.99 | 5.86 ± 0.88 | 0.29 |
| REM (hours) | 1.10 ± 0.51 | 0.98 ± 0.44 | 1.29 ± 0.55 | 0.01 |
| REM %TST | 19.4 ± 7.7% | 17.7 ± 7.3% | 21.4 ± 7.8% | 0.00 |
| NREM (hours) | 4.56 ± 0.8 | 4.55 ± 0.92 | 4.57 ± 0.6 | 0.93 |
| NREM %TST | 80.9 ± 7.7% | 82.4 ± 7.3% | 78.6 ± 7.8% | 0.14 |
| Arousal index (per hour) | 44.9 ± 46.0 | 51.6 ± 49.4 | 34.6 ± 38.9 | 0.00 |
| AHI (per hour) | 39.7 ± 23.8 | 54.1 ± 18.5 | 16.6 ± 7.7 | 0.00 |
| Basal SpO2 (%) | 90.3 ± 3.7 | 89.1 ± 4.1 | 92.3 ± 1.9 | 0.00 |
| Time below 90% SpO2 (min) | 126.5 ± 115.1 | 163.9 ± 96.5 | 66.8 ± 119.1 | 0.00 |
| Time below 90% SpO2 (%TST) | 37.4 ± 34.3% | 49.4 ± 2.7% | 19.0 ± 36.3% | 0.00 |
| Glucose (mg/dL) | 110.5 ± 21.2 | 113.9 ± 4.8 | 105.4 ± 13.0 | 0.21 |
| Insulin (mIU/L) | 15.7 ± 11.1 | 17.6 ± 13.5 | 12.9 ± 5.1 | 0.39 |
| HOMA-IR | 4.44 ± 3.63 | 5.12 ± 4.40 | 3.39 ± 1.50 | 0.32 |
| BDNF (ng/mL) | 14.7 (5.9–27.8) | 20.1 (9.0–33.6) | 8.1 (4.6–17.4) | 0.02 |
| Diabetes mellitus t2, n (%) | 9 (13.6%) | 7 (17.5%) | 2 (7.7%) | 0.44 |
| sc-OSAS | snc-OSAS | p | |
|---|---|---|---|
| BMI (kg/m2) | 33.7 ± 5.7 | 30.0 ± 9.3 | 0.30 |
| Glucose (mg/dL) | 111.4 ± 21.6 | 106.4 ± 13.6 | 0.69 |
| Insulin (mIU/L) | 14.6 ± 5.7 | 15.2 ± 7.3 | 0.92 |
| HOMA-IR | 4.0 ± 1.7 | 4.0 ± 1.9 | 0.92 |
| BDNF (ng/mL) | 27.4 (14.8–33.8) | 35.5 (21.7–39.0) | 0.13 |
| Therapeutic pressure in CPAP (mmH2O) | 11.4 | 11.2 | 0.00 |
| AHI (per hour) | 8.6 ± 10.7 | 12.5 ± 18.1 | 0.68 |
| Nights with CPAP (%) | 88.3 ± 9.6% | 44.5 ± 20.0% | 0.00 |
| sc-OSAS (Compliant) n = 24 | p | ||
|---|---|---|---|
| Before CPAP | After 12 Months of CPAP | ||
| BMI (kg/m2) | 34.1 ± 6.2 | 33.7 ± 5.7 | 0.57 |
| Glucose (mg/dL) | 117.3 ± 28.9 | 111.4 ± 21.6 | 0.08 |
| Insulin (mIU/L) | 18.1 ± 11.3 | 14.6 ± 5.7 | 0.09 |
| HOMA-IR | 5.6 ± 4.6 | 4.0 ± 1.7 | 0.04 |
| BDNF (ng/mL) | 24.4 (9.7–34.3) | 27.4 (14.8–33.8) | 0.33 |
| snc-OSAS (Non-Compliant), n = 16 | p | ||
|---|---|---|---|
| Before CPAP | After 12 Months of CPAP | ||
| BMI (kg/m2) | 32.4 ± 4.6 | 30.0 ± 9.3 | 0.36 |
| Glucose (mg/dL) | 108.8 ± 16.4 | 106.4 ± 13.6 | 0.33 |
| Insulin (mIU/L) | 16.9 ± 16.7 | 15.2 ± 7.3 | 0.88 |
| HOMA-IR | 4.4 ± 4.1 | 4.0 ± 1.9 | 0.88 |
| BDNF (ng/mL) | 16.2 (8.5–29.1) | 35.5 (21.7–39.0) | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karwowska, U.; Kudrycka, A.; Pierzchała, K.; Stawski, R.; Jerczyńska, H.; Białasiewicz, P.; Kuczyński, W. Twelve-Month CPAP Therapy Modulates BDNF Levels in Patients with Severe Obstructive Sleep Apnea: Implications for Metabolic and Treatment Compliance. Int. J. Mol. Sci. 2025, 26, 5855. https://doi.org/10.3390/ijms26125855
Karwowska U, Kudrycka A, Pierzchała K, Stawski R, Jerczyńska H, Białasiewicz P, Kuczyński W. Twelve-Month CPAP Therapy Modulates BDNF Levels in Patients with Severe Obstructive Sleep Apnea: Implications for Metabolic and Treatment Compliance. International Journal of Molecular Sciences. 2025; 26(12):5855. https://doi.org/10.3390/ijms26125855
Chicago/Turabian StyleKarwowska, Urszula, Aleksandra Kudrycka, Karol Pierzchała, Robert Stawski, Hanna Jerczyńska, Piotr Białasiewicz, and Wojciech Kuczyński. 2025. "Twelve-Month CPAP Therapy Modulates BDNF Levels in Patients with Severe Obstructive Sleep Apnea: Implications for Metabolic and Treatment Compliance" International Journal of Molecular Sciences 26, no. 12: 5855. https://doi.org/10.3390/ijms26125855
APA StyleKarwowska, U., Kudrycka, A., Pierzchała, K., Stawski, R., Jerczyńska, H., Białasiewicz, P., & Kuczyński, W. (2025). Twelve-Month CPAP Therapy Modulates BDNF Levels in Patients with Severe Obstructive Sleep Apnea: Implications for Metabolic and Treatment Compliance. International Journal of Molecular Sciences, 26(12), 5855. https://doi.org/10.3390/ijms26125855







