Abstract
Background/Objectives: Patients with erythropoietic protoporphyria (EPP) have a decreased activity of the ferrochelatase enzyme which converts protoporphyrin IX (PpIX) into heme, causing PpIX to accumulate in erythrocytes. The ensuing release of PpIX to the skin when exposed to visible light causes a phototoxic reaction with severe pain, erythema, and edema. Erythrocyte PpIX levels in adult EPP patients are rather stable and largely unaffected by pharmaceutical treatments. It is important to be aware of drugs causing an increase in PpIX as this may increase the risk of liver toxicity. Method: The patient had blood samples taken regularly for analyses of PpIX, znPpIX, ALT, ALP, iron, leucocytes, C-reactive protein, and hemoglobin before, during, and after treatment with teriflunomide. Additionally, we tested if teriflunomide increased PpIX in vitro. Results: A female EPP patient was treated for 7 years with teriflunomide for multiple sclerosis attacks. During treatment, her natural PpIX level increased from about 30 µmol/L to about 200 µmol/L, without significant simultaneous changes in hemoglobin, iron levels, alanine transaminase (ALT), or alkaline phosphatase (ALP). The patient experienced no increase in photosensitivity. In vitro addition of teriflunomide did not affect PpIX levels. Discussion: In patients with lead intoxication, the release of PpIX from erythrocytes is very slow. The increase in PpIX during treatment with teriflunomide compared to periods with no medication could be caused by a similar slow PpIX release from the erythrocytes. This theory is supported by the patient’s unchanged light sensitivity and stable levels of hemoglobin, iron, and liver enzymes.
1. Introduction
Erythropoietic protoporphyria (EPP) is a rare, inherited porphyria where protoporphyrin IX (PpIX) accumulates in erythrocytes due to decreased activity of ferrochelatase (FECH) converting PpIX to heme. Exposure to visible light releases the accumulated PpIX into the blood vessel endothelia and skin, triggering an acute phototoxic reaction with severe pain, erythema, and edema [1]. PpIX levels usually increase from birth, through childhood and adolescence, and remain stable in adult EPP patients. Very rarely, EPP is acquired, e.g., in myelodysplastic syndrome, with a moderate increase in PpIX levels. When treated with azacitidine, PpIX levels decrease or may reach normal levels. In pregnant women, PpIX levels fall significantly from early in the pregnancy, with a maximal decrease in PpIX of 66% (median 53%), and with vastly improved phototolerance reported by the patients. Inhibition of the first step in the porphyrin cascade may also lower PpIX. Inhibition of 5-aminolevulinic acid (5-ALA) synthase by Cimetidine may provide up to a 20% decrease in PpIX levels [2], and the glycine transport inhibitor bitopertin may, in a similar fashion, lower PpIX [3]. Patients treated with pharmacological drugs for diseases unrelated to porphyria do not experience increased levels of PpIX, and only iron supplements are known to increase PpIX levels in EPP patients by up to 40% [4]. Some patients with EPP have abnormal liver functions [5]. Overall risk of liver failure for patients with EPP is about 4%, and long-term high levels of PpIX may increase this risk. PpIX is excreted by the liver through the bile. A rapid decrease in PpIX from extracorporeal erythrocyte photodynamic therapy produces elevated alanine aminotransferase (ALT) levels. Treatment of mice with isoniazid causes accumulation of PpIX in the liver and downregulates FECH [6].
Our report presents a case of heavily increased PpIX levels in a patient with EPP during her teriflunomide treatment for multiple sclerosis attacks.
2. Materials and Methods
2.1. Case Report
Our patient gave written consent to publish this case report. Due to her EPP, she had routine blood samples taken regularly at our department laboratory, monitoring blood values and liver enzymes before, during, and after the teriflunomide treatment period. No experimental treatments were performed. Analyses were made of PpIX, zincPpIX, alanine aminotransferase (ALT), alkaline phosphatase (ALP), iron, ferritin, leucocyte count, C-reactive protein, and hemoglobin. Blood PpIX was measured by HPLC. As the erythrocyte volume percentage was constant (36.4) during the whole examination period, erythrocyte PpIX was obtainable.
2.2. Experimental Analysis
To examine if the PpIX blood sample analysis produced incorrect results due to teriflunomide treatment, we added incremental doses of teriflunomide to blood samples from the patient prior to analysis. In vivo, teriflunomide is excreted in the gastrointestinal tract, mainly through the bile, as an unchanged drug. To investigate the effect of teriflunomide itself on PpIX in vitro, we made a basic solution of 10 mg teriflunomide in 1 mL dimethyl sulfoxide. Four identical blood samples (4 mL) from our patient were prepared as follows: one sample contained no teriflunomide solution; to the other 3 samples we, added 40 µL, 80 µL, and 160 µL of the basic solution, respectively (1–5 times the steady concentration in vivo).
3. Results
3.1. Case Report
Our patient is a 57-year-old woman presenting stable PpIX levels (range: 25–30 µmol/L) during the years 2006–2015. From 2015–2023, her PpIX levels increased to 73–201 µmol/L while she was being treated with a daily dose of 14 mg teriflunomide for multiple sclerosis [7,8]. In January 2023, teriflunomide was substituted with rituximab, and within 3–4 months, the patient’s PpIX levels dropped to 28–42 µmol/L. Figure 1 shows the development in PpIX from 2006–2025. The average level of ZincPpIX was 2.75 µmol/L during treatment—29% higher than the post-treatment level of 2.13 µmol/L.
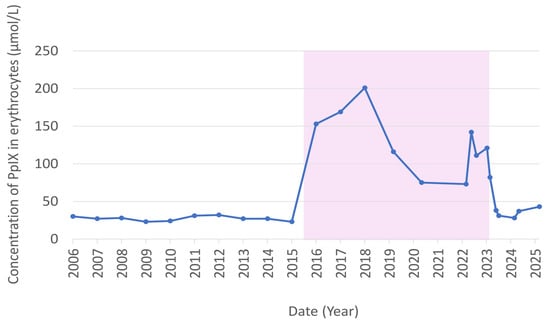
Figure 1.
The development in our EPP patient’s PpIX concentration from 2006–2025. The pink area represents the teriflunomide treatment period.
3.2. Photosensitivity
The patient did not experience any severe increase in photosensitivity during her teriflunomide treatment with heavily increased PpIX levels. She spent approximately 20 h outdoors per week during the whole period. The patient’s self-reported tolerance of 30 min of sun exposure before the onset of EPP-related symptoms did not change over the years. The maximal pain score during the summer season was 7 both during and after teriflunomide treatment (range: 0–10). She used sun protection and had a protection score of 7 during treatment and 8 the year after treatment (range: 0–18). The patient also completed a Quality-of-Life questionnaire developed specifically for patients with EPP (EPPQoL), with a score of 7 during treatment and 8 the year after treatment (range: 0–30) [2] (Suppl.).
3.3. Liver Function
No irregularities were found in the analyses of blood liver enzymes. The plasma ALT was, on average, 30 U/L after treatment and 34 U/L during treatment. Plasma ALP was 64 IU/L after treatment and 59 IU/L during treatment. We found no indications of severe liver damage during the high levels of PpIX.
3.4. Other Blood Analyses
Plasma iron level was 14 µmol/L after treatment and 16 µmol/L during treatment. Plasma ferritin was 33 µg/L after treatment and 48 µg/L during treatment. The plasma ferritin increase of 15 µg/L may have contributed to some increase in PpIX. During a study of iron supplementation in patients with EPP, Heerfordt et al. saw an increase in PpIX from 58 µmol/L to 81 µmol/L, with a similar increase in ferritin levels [4]. Hemoglobin was 7.6 mmol/L after treatment and 7.3 mmol/L during treatment. The blood data suggest no toxicity of the liver or bone marrow during treatment with teriflunomide.
3.5. Experimental Analysis
Adding teriflunomide to blood samples produced a fall in PpIX with increasing volumes added (from 32.2 µmol/L to 28.0 µmol/L). By calculation, half of this small decrease may be caused by the dilution of the blood samples. As no increase in PpIX was observed in the in vitro test, the patient’s heavy increase in PpIX during treatment was not caused by the laboratory’s blood analysis method but might be caused by metabolites.
4. Discussion
With EPP patients, the clinician’s focus is on bringing PpIX to a lower level to manage photosensitivity symptoms [2]; i.e., they are not usually looking for pharmaceutical causes of increased PpIX. Teriflunomide is a pyrimidine synthesis inhibitor with anti-inflammatory properties, and the effect on PpIX has not previously been examined and described. We are not aware of any other pharmaceutical drugs causing a rise in PpIX levels. Increased PpIX may be caused by affected bone marrow, liver toxicity causing decreased excretion, or an affected PpIX blood analysis, and all possibilities were explored in this study. A slow decrease in PpIX after the termination of teriflunomide would be in accordance with the 19 days half-life in vivo [7].
In healthy persons, the level of PpIX is <0.5 µmol/L, and in our EPP cohort, the PpIX levels range from 4 to 250 µmol/L. Our female cohort has an average PpIX level of 38 µmol/L, and the male cohort has an average level of 56 µmol/L [2]. Untreated, our patient’s PpIX levels are within the normal average range for female EPP patients, and her highly elevated PpIX levels during treatment with teriflunomide were quite abnormal.
Figure 1 shows teriflunomide to be both a strong inducer of elevated PpIX levels and of large fluctuations in PpIX levels, despite the patient declaring a stable daily teriflunomide intake of 14 mg. We were unable to discover the cause of the elevated levels of PpIX. Iron levels and hemoglobin were unaltered during and after treatment. Adding teriflunomide to blood samples did not produce elevated PpIX levels either. Iron supplements may cause increased PpIX levels in patients with EPP, and the patient’s plasma ferritin was 15 µg/L higher during treatment with teriflunomide. The increased ferritin level may have increased the PpIX level by about 20 µmol/L, as calculated from data in Heerfordt et al. [4], but offers only a small part of the explanation for the patient’s vastly elevated PpIX levels during teriflunomide treatment.
The patient did not experience any increase in photosensitivity during treatment. Being conscious of sun protection, she primarily went outdoors in the early mornings and late in the day. The patient never travelled to sunny holiday destinations. Additionally, she used self-tanning spray (dihydroxyacetone) as photoprotection. Both during teriflunomide and after treatment, when her eryPpIX level had returned to 30 µmol/L, her EPPQoL was stable, and the score was rather low [2].
It is possible that the effect of teriflunomide on PpIX is caused by increased 5-ALA synthase activity or increased glycine involved in the primary step in the heme synthesis [9]. In this case, the PpIX would increase as EPP patients already have a low FECH activity with diminished conversion of PpIX to heme. Further, teriflunomide may act as am inhibitor of ferrochelatase activity. Just a small inhibition might have a pronounced effect on PpIX levels as ferrochelatase levels are already only at 10–30% in patients with EPP compared to healthy persons [1].
In patients with EPP, the release of PpIX from erythrocytes is relatively fast; in lead-poisoned patients, it is slow [10]. As the patient did not experience an increase in photosensitivity, even with very high PpIX levels, and had no liver toxicity (which might be expected due to high excretion of PpIX), it is likely that teriflunomide stabilizes PpIX to remain inside the erythrocytes, preventing release to plasma and the skin, thereby avoiding liver toxicity and increased photosensitivity.
5. Conclusions
Although we did not find any drug-related liver toxicity of teriflunomide in this case report, the risk of increased PpIX levels and potential harmful effects on the liver should be taken into consideration when administering systemic drugs to patients with erythropoietic protoporphyria.
Author Contributions
Conceptualization, supervision, administration, original draft, methodology, H.C.W.; review writing, A.L.C.; Visualization, validation, review, writing, methodology, I.M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study as no interventions were introduced. Such studies do not require ethics committee approval according to the Danish National Center for Ethics.
Informed Consent Statement
Informed consent to publish this paper was obtained from the patient involved in this study.
Data Availability Statement
Not applicable.
Acknowledgments
We thank our patient for her cooperation and the medical secretary Louise Holbæk Kaihøi for her assistance.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 5-ALA | 5-aminolevulinic acid |
| EPP | Erythropoietic protoporphyria |
| PpIX | Protoporphyrin IX |
| ALT | Alanine transaminase |
| ALP | Alkaline phosphatase |
| FECH | Ferrochelatase |
| EPPQoL | EPP Quality-of-Life (questionnaire developed specifically for patients with EPP) |
References
- Todd, D.J. Erythropoietic protoporphyria. Br. J. Dermatol. 1994, 131, 751–766. [Google Scholar] [CrossRef] [PubMed]
- Wulf, H.C.; Philipsen, P.A.; Lerche, C.M. Cimetidine (H2 histamine antagonist), Fexofenadine (H1 histamine antagonist), and Zinc sulphate in the treatment of erythropoietic protoporphyria. Photodiagnosis Photodyn. Ther. 2025, 53, 104644. [Google Scholar] [CrossRef] [PubMed]
- Pinto, V.M.; Mazzi, F.; De Franceschi, L. Novel therapeutic approaches in thalassemias, sickle cell disease, and other red cell disorders. Blood 2024, 144, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Heerfordt, I.M.; Lerche, C.M.; Philipsen, P.A.; Wulf, H.C. Effects of iron supplements in individuals with erythropoietic protoporphyria. Photodiagnosis Photodyn. Ther. 2024, 47, 104211. [Google Scholar] [CrossRef] [PubMed]
- Minder, A.-E.; Schneider-Yin, X.; Zulewski, H.; Minder, C.E.; Minder, E.I. Afamelanotide is associated with dose-dependent protective effect from liver damage related to erythropoietic protoporphyria. Life 2023, 13, 1066. [Google Scholar] [CrossRef] [PubMed]
- Sachar, M.; Li, F.; Liu, K.; Wang, P.; Lu, J.; Ma, X. Chronic treatment with isoniazid causes protoporphyrin IX accumulation in mouse liver. Chem. Res. Toxicol. 2016, 29, 1293–1297. [Google Scholar] [CrossRef] [PubMed]
- Cada, D.J.; Demaris, K.; Levien, T.L.; Baker, D.E. Teriflunomide. Hosp. Pharm. 2013, 48, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.E. An updated review of teriflunomide’s use in multiple sclerosis. Neurodegener. Dis. Manag. 2021, 11, 387–409. [Google Scholar] [CrossRef] [PubMed]
- Matte, A.; Federti, E.; Winter, M.; Koerner, A.; Harmeier, A.; Mazer, N.; Tomka, T.; Di Paolo, M.L.; De Falco, L.; Andolfo, I.; et al. Bitopertin, a selective oral GLYT1 inhibitor, improves anemia in a mouse model of β-thalassemia. JCI Insight 2019, 4, e130111. [Google Scholar] [CrossRef] [PubMed]
- Piomelli, S.; Lamola, A.A.; Poh-Fitzpatrick, M.F.; Seaman, C.; Harber, L.C. Erythropoietic protoporphyria and lead intoxication: The molecular basis for difference in cutaneous photosensitivity. I: Different rates of disappearance of protoporphyrin from the erythrocytes, both in vivo and in vitro. J. Clin. Investig. 1975, 56, 1519–1527. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).