Fluorescence-Guided Surgery in Head and Neck Squamous Cell Carcinoma (HNSCC)
Abstract
1. Introduction
2. History of Fluorescence-Guided Surgery (FGS)
3. The Technology
3.1. Fluorescent Dye Selection and Administration
3.2. Fluorescence Emission
3.3. Physical Interactions with Cells
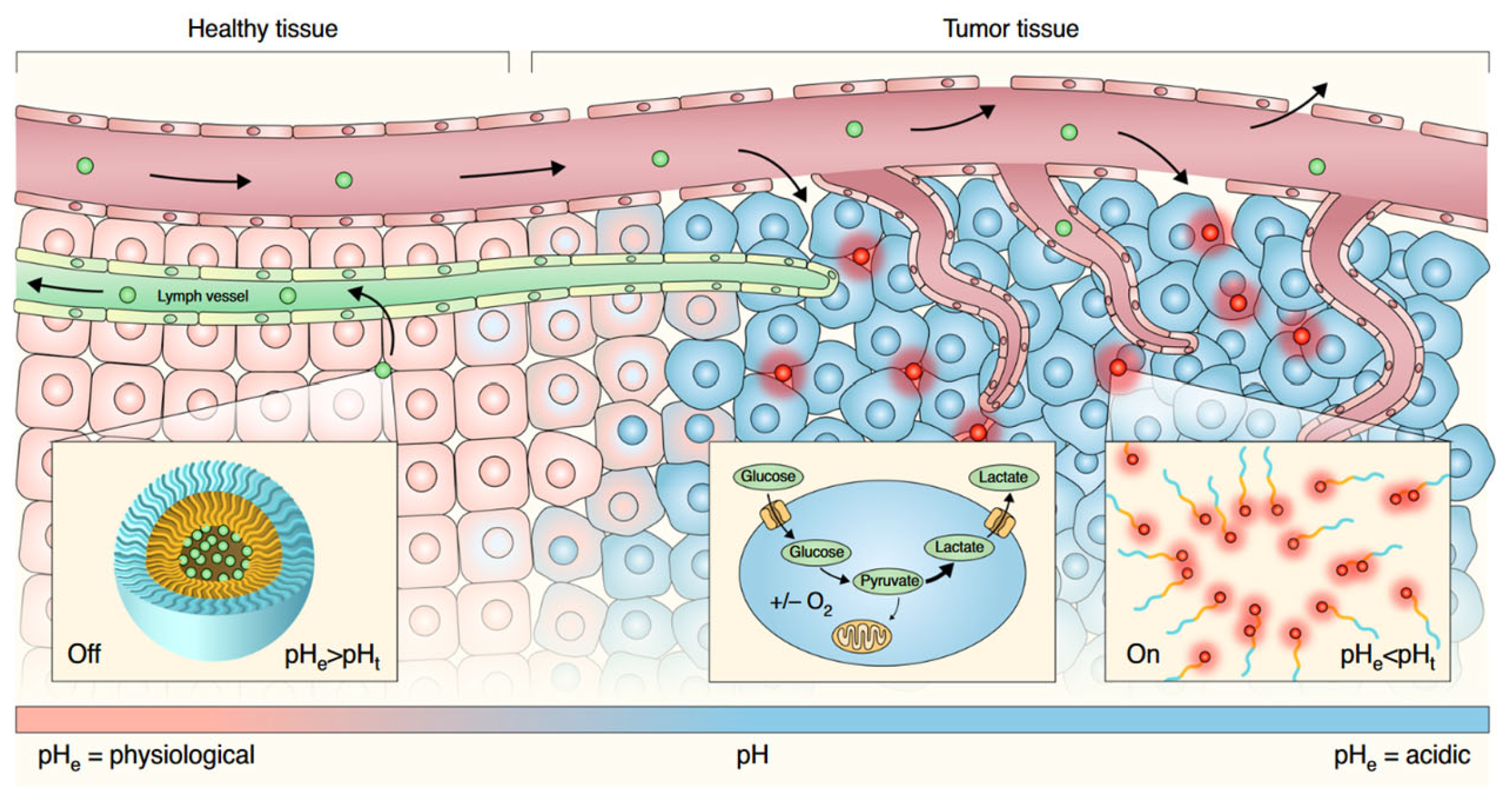
3.4. Tissue Targeting
3.5. Adverse Effects
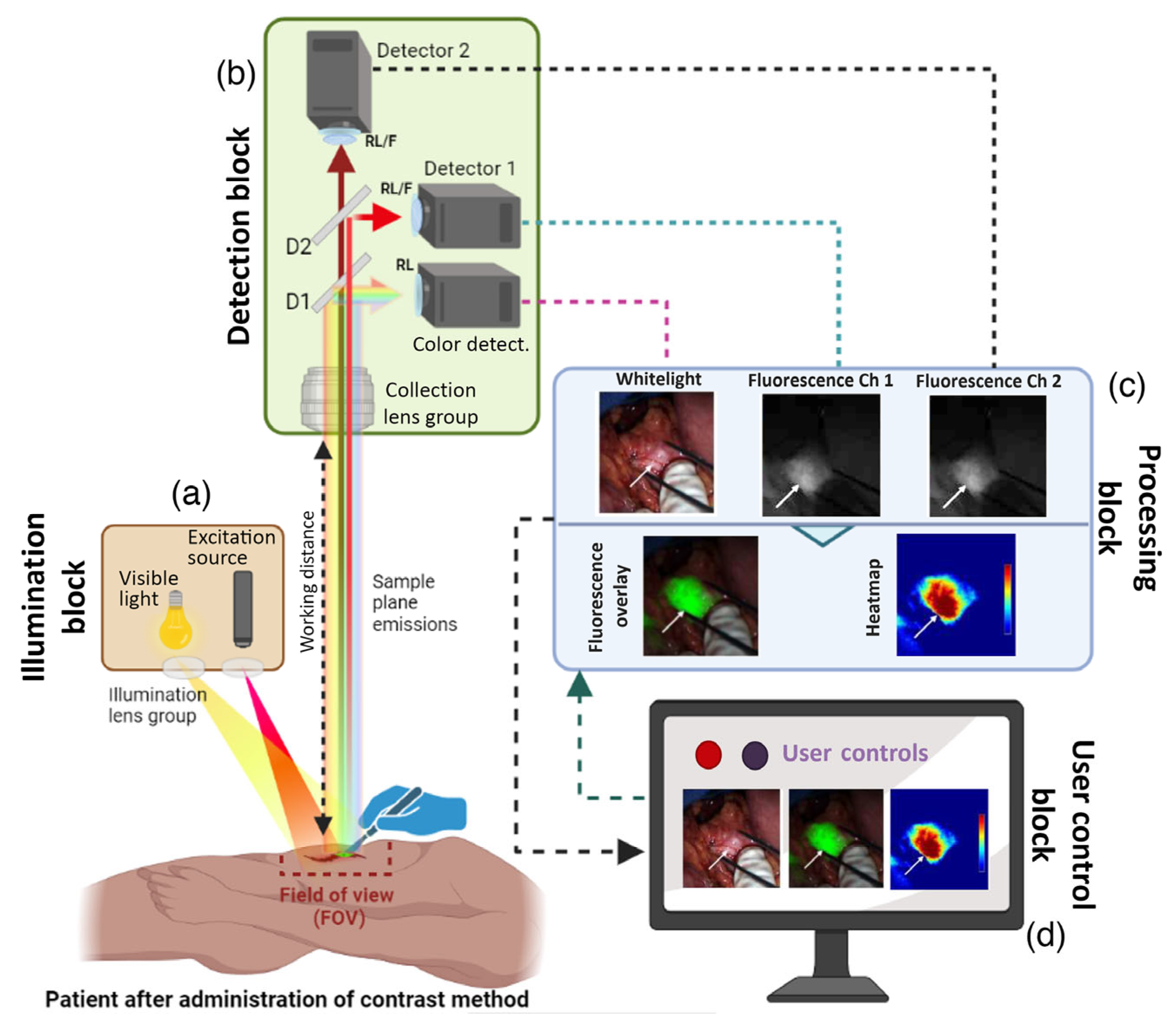
3.6. Imaging and Visualization
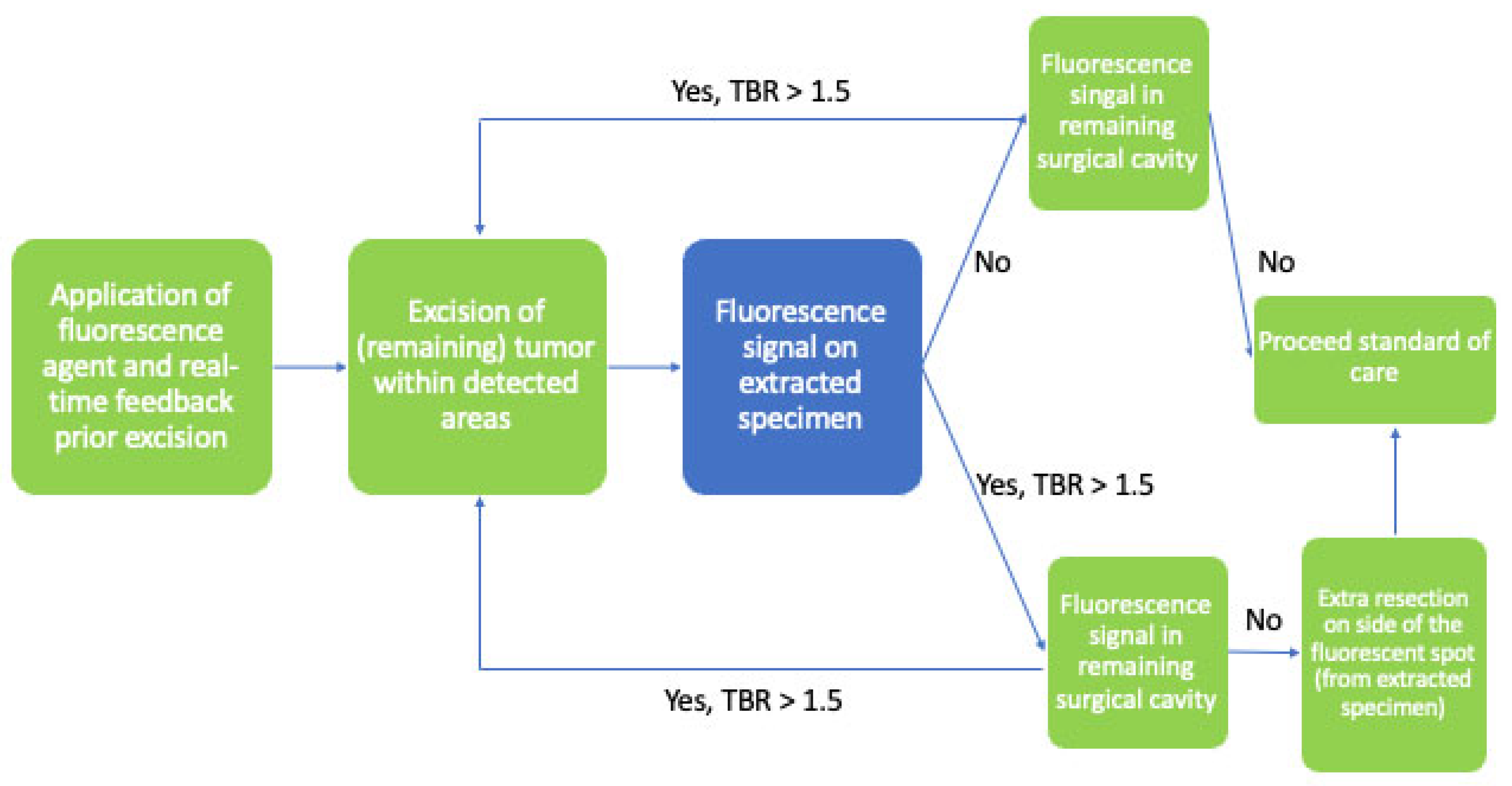
3.7. Surgical Precision
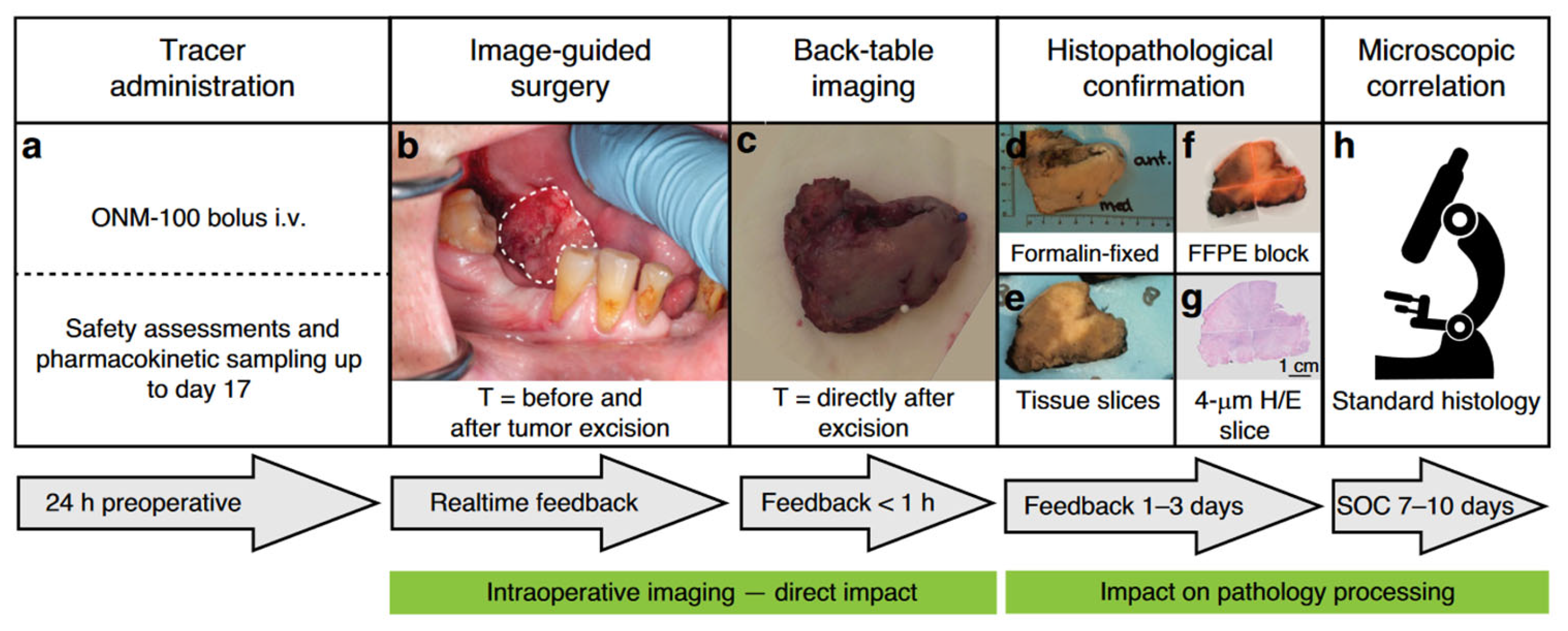
4. Clinical Applications of FGS in HNSCC
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jansen, L.; Moratin, J.; Waldmann, A.; Zaoui, K.; Holleczek, B.; Nennecke, A.; Pritzkuleit, R.; Plinkert, P.K.; Hoffmann, J.; Arndt, V. Mundhöhlen- und Pharynxkarzinome: Inzidenz, Mortalität und Überleben in Deutschland. Bundesgesundheitsblatt—Gesundheitsforschung—Gesundheitsschutz 2021, 64, 941–950. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Forastiere, A.A.; Ismaila, N.; Lewin, J.S.; Nathan, C.A.; Adelstein, D.J.; Eisbruch, A.; Fass, G.; Fisher, S.G.; Laurie, S.A.; Le, Q.-T.; et al. Use of Larynx-Preservation Strategies in the Treatment of Laryngeal Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 1143–1169. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.W.; Teraphongphom, N.T.; Van Den Berg, N.S.; Martin, B.A.; Oberhelman, N.J.; Divi, V.; Kaplan, M.J.; Hong, S.S.; Lu, G.; Ertsey, R.; et al. Determination of Tumor Margins with Surgical Specimen Mapping Using Near-Infrared Fluorescence. Cancer Res. 2018, 78, 5144–5154. [Google Scholar] [CrossRef] [PubMed]
- Dietz, A.; Budach, W. S3-Leitlinie Diagnostik, Therapie, Prävention und Nachsorge des Oro- und Hypopharynxkarzinoms, Langversion 1.0 n.d.; Office des Leitlinienprogrammes Onkologie: Berlin, Germany, 2024. [Google Scholar]
- Batsakis, J.G. Surgical Excision Margins: A Pathologist’s Perspective. Adv. Anat. Pathol. 1999, 6, 140–148. [Google Scholar] [CrossRef]
- Looser, K.G.; Shah, J.P.; Strong, E.W. The significance of “positive” margins in surgically resected epidermoid carcinomas. Head Neck Surg. 1978, 1, 107–111. [Google Scholar] [CrossRef]
- Loree, T.R.; Strong, E.W. Significance of positive margins in oral cavity squamous carcinoma. Am. J. Surg. 1990, 160, 410–414. [Google Scholar] [CrossRef]
- Villemure-Poliquin, N.; Roy, È.-M.; Nguyen, S.; Beauchemin, M.; Audet, N. Tumor Bed Margins Versus Specimen Margins in Oral Cavity Cancer: Too Close to Call? J. Otolaryngol. Head Neck Surg. 2024, 53, 19160216241278653. [Google Scholar] [CrossRef]
- Sivrice, M.E.; Akın, V.; Erkılınç, G.; Yasan, H.; Tüz, M.; Okur, E.; Kumbul, Y.Ç.; Çiriş, İ.M. Frozen Section Evaluation for Surgical Margins in Laryngeal Squamous Cell Carcinoma: Is it a Reliable Method for Partial and Total Laryngectomies? Head Neck Pathol. 2022, 17, 172–177. [Google Scholar] [CrossRef]
- DiNardo, L.J.; Lin, J.; Karageorge, L.S.; Powers, C.N. Accuracy, Utility, and Cost of Frozen Section Margins in Head and Neck Cancer Surgery. Laryngoscope 2000, 110, 1773–1776. [Google Scholar] [CrossRef]
- Olson, S.M.; Hussaini, M.; Lewis, J.S. Frozen section analysis of margins for head and neck tumor resections: Reduction of sampling errors with a third histologic level. Mod. Pathol. 2011, 24, 665–670. [Google Scholar] [CrossRef]
- Maxwell, J.H.; Thompson, L.D.R.; Brandwein-Gensler, M.S.; Weiss, B.G.; Canis, M.; Purgina, B.; Prabhu, A.V.; Lai, C.; Shuai, Y.; Carroll, W.R.; et al. Early Oral Tongue Squamous Cell Carcinoma: Sampling of Margins from Tumor Bed and Worse Local Control. JAMA Otolaryngol. Neck Surg. 2015, 141, 1104. [Google Scholar] [CrossRef]
- Du, E.; Ow, T.J.; Lo, Y.; Gersten, A.; Schiff, B.A.; Tassler, A.B.; Smith, R.V. Refining the utility and role of Frozen section in head and neck squamous cell carcinoma resection. Laryngoscope 2016, 126, 1768–1775. [Google Scholar] [CrossRef] [PubMed]
- Gandour-Edwards, R.F.; Donald, P.J.; Wiese, D.A. Accuracy of intraoperative frozen section diagnosis in head and neck surgery: Experience at a university medical center. Head Neck 1993, 15, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Woolgar, J.A.; Triantafyllou, A. A histopathological appraisal of surgical margins in oral and oropharyngeal cancer resection specimens. Oral Oncol. 2005, 41, 1034–1043. [Google Scholar] [CrossRef]
- Jäckel, M.C.; Ambrosch, P.; Martin, A.; Steiner, W. Impact of re-resection for inadequate margins on the prognosis of upper aerodigestive tract cancer treated by laser microsurgery. Laryngoscope 2007, 117, 350–356. [Google Scholar] [CrossRef]
- Kwok, P.; Gleich, O.; Hübner, G.; Strutz, J. Prognostic importance of “clear versus revised margins” in oral and pharyngeal cancer. Head Neck 2010, 32, 1479–1484. [Google Scholar] [CrossRef]
- Yuen, P.W.; Lam, K.Y.; Chan, A.C.L.; Wei, W.I.; Lam, L.K. Clinicopathological Analysis of Local Spread of Carcinoma of the Tongue 11The study was supported by a research grant from the University of Hong Kong, grant number 337/048/0014 and 335/048/0081. Am. J. Surg. 1998, 175, 242–244. [Google Scholar] [CrossRef]
- Uppaluri, R.; Chernock, R.; Mansour, M.; Jackson, R.; Rich, J.; Pipkorn, P.; Paniello, R.C.; Puram, S.; Zevallos, J.P.; Annino, D.J.; et al. Enhanced pathologic tumor response with two cycles of neoadjuvant pembrolizumab in surgically resectable, locally advanced HPV-negative head and neck squamous cell carcinoma (HNSCC). J. Clin. Oncol. 2021, 39, 6008. [Google Scholar] [CrossRef]
- Shibata, H.; Saito, S.; Uppaluri, R. Immunotherapy for Head and Neck Cancer: A Paradigm Shift from Induction Chemotherapy to Neoadjuvant Immunotherapy. Front. Oncol. 2021, 11, 727433. [Google Scholar] [CrossRef]
- Lybaert, W.; Vermorken, J.B. Editorial: Insights in head and neck cancer: 2021. Front. Oncol. 2023, 13, 1180965. [Google Scholar] [CrossRef]
- Dietz, A.; Vermorken, J.B. Is Reducing the Extent of Local Treatments Justified After Successful Neoadjuvant Therapy? In Critical Issues in Head and Neck Oncology; Vermorken, J.B., Langendijk, J.A., Leemans, C.R., Machiels, J.-P., Nicolai, P., O’Sullivan, B., Eds.; Springer Nature Switzerland: Cham, Switzerland, 2025; pp. 241–259. ISBN 978-3-031-84538-3. [Google Scholar]
- Van Keulen, S.; Nishio, N.; Fakurnejad, S.; Birkeland, A.; Martin, B.A.; Lu, G.; Zhou, Q.; Chirita, S.U.; Forouzanfar, T.; Colevas, A.D.; et al. The Clinical Application of Fluorescence-Guided Surgery in Head and Neck Cancer. J. Nucl. Med. 2019, 60, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Hom, M.E.; Van Den Berg, N.S.; Lwin, T.M.; Lee, Y.-J.; Prilutskiy, A.; Faquin, W.; Yang, E.; Saladi, S.V.; Varvares, M.A.; et al. First Clinical Results of Fluorescence Lifetime-enhanced Tumor Imaging Using Receptor-targeted Fluorescent Probes. Clin. Cancer Res. 2022, 28, 2373–2384. [Google Scholar] [CrossRef] [PubMed]
- Stokes, G.G. XXX. On the change of refrangibility of light. Philos. Trans. R. Soc. Lond. 1852, 142, 463–562. [Google Scholar] [CrossRef]
- Copeman, S.M.; Coke, F.; Gouldesbrough, C. “Activated” (irradiated) fluorescein in the treatment of cancer. Br. Med. J. 1929, 2, 233–242.2. [Google Scholar] [CrossRef]
- Moore, G. Fluorscein as an Agent in the Differentiation of Normal and Malignant Tissues. Science 1947, 106, 130–131. [Google Scholar] [CrossRef]
- Goldhahn, W.E. Tetracycline fluorescence for the delimitation of brain tumors. Arzneimittelforschung 1967, 17, 139–141. [Google Scholar]
- Homasson, J.P.; Bonniot, J.P.; Angebault, M.; Renault, P.; Carnot, F.; Santelli, G. Fluorescence as a guide to bronchial biopsy. Thorax 1985, 40, 38–40. [Google Scholar] [CrossRef]
- Harris, D.M.; Werkhaven, J. Endogenous porphyrin fluorescence in tumors. Lasers Surg. Med. 1987, 7, 467–472. [Google Scholar] [CrossRef]
- Sheridan, R.L.; Schomaker, K.T.; Lucchina, L.C.; Hurley, J.; Yin, L.M.; Tompkins, R.G.; Jerath, M.; Torri, A.; Greaves, K.W.; Bua, D.P.; et al. Burn Depth Estimation by Use of Indocyanine Green Fluorescence: Initial Human Trial. J. Burn Care Rehabil. 1995, 16, 602–604. [Google Scholar] [CrossRef]
- Kitai, T.; Inomoto, T.; Miwa, M.; Shikayama, T. Fluorescence navigation with indocyanine green for detecting sentinel lymph nodes in breast cancer. Breast Cancer Tokyo Jpn. 2005, 12, 211–215. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Tsien, R.Y. Fluorescence-guided surgery with live molecular navigation—A new cutting edge. Nat. Rev. Cancer 2013, 13, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, G.M.; Themelis, G.; Crane, L.M.A.; Harlaar, N.J.; Pleijhuis, R.G.; Kelder, W.; Sarantopoulos, A.; de Jong, J.S.; Arts, H.J.G.; van der Zee, A.G.J.; et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: First in-human results. Nat. Med. 2011, 17, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Gioux, S.; Choi, H.S.; Frangioni, J.V. Image-guided surgery using invisible near-infrared light: Fundamentals of clinical translation. Mol. Imaging 2010, 9, 237–255. [Google Scholar] [CrossRef]
- Barth, C.W.; Gibbs, S. Fluorescence image-guided surgery: A perspective on contrast agent development. In Proceedings of the Molecular-Guided Surgery: Molecules, Devices, and Applications VI; Gibbs, S.L., Pogue, B.W., Gioux, S., Eds.; SPIE: San Francisco, CA, USA, 2020; p. 18. [Google Scholar]
- Dindere, M.E.; Tanca, A.; Rusu, M.; Liehn, E.A.; Bucur, O. Intraoperative Tumor Detection Using Pafolacianine. Int. J. Mol. Sci. 2022, 23, 12842. [Google Scholar] [CrossRef]
- Zhao, H.; Peng, P.; Luo, Z.; Liu, H.; Sun, J.; Wang, X.; Jia, Q.; Yang, Z. Comparison of hexaminolevulinate (HAL)—Guided versus white light transurethral resection for NMIBC: A systematic review and meta-analysis of randomized controlled trials. Photodiagnosis Photodyn. Ther. 2023, 41, 103220. [Google Scholar] [CrossRef]
- Galema, H.A.; Van Ginhoven, T.M.; Franssen, G.J.H.; Hofland, J.; Bouman, C.G.O.T.; Verhoef, C.; Vahrmeijer, A.L.; Hutteman, M.; Hilling, D.E.; Keereweer, S. Fluorescence-guided surgery using methylene blue to improve identification of metastatic small intestinal neuroendocrine tumours. Br. J. Surg. 2023, 110, 541–544. [Google Scholar] [CrossRef]
- OncoNano Medicine. A Study to Evaluate ONM-100, an Intraoperative Fluorescence Imaging Agent for the Detection of Cancer. Available online: https://www.clinicaltrials.gov/study/NCT03735680 (accessed on 1 July 2025).
- Iagaru, A. Panitumumab-IRDye800 and 89Zr-Panitumumab in Identifying Metastatic Lymph Nodes in Patients with Squamous Cell Head and Neck Cancer. Available online: https://clinicaltrials.gov/study/NCT03733210?tab=history&a=4 (accessed on 1 July 2025).
- University Medical Center Groningen. Image Guided Surgery for Margin Assessment of Head and Neck Cancer Using Cetuximab-IRDye800CW cONjugate (ICON). Available online: https://clinicaltrials.gov/study/NCT03134846 (accessed on 1 July 2025).
- University of Texas Southwestern Medical Center. A Phase 2a, Single-dose, Open-label Study to Evaluate Diagnostic Performance and Safety of Pegsitacianine, an Intraoperative Fluorescence Imaging Agent for the Detection of Cancer, in Patients with Unknown Primary Head and Neck Cancer (Illuminate Study). Available online: https://clinicaltrials.gov/study/NCT05576974 (accessed on 1 July 2025).
- FluoGuide A/S An Open-Label, Non-Randomized, Single Center, Single Dose, Exploratory Phase II Trial of FG001 (an Imaging Agent) for Localization of Oral and Oropharyngeal Squamous Cell Carcinoma. Available online: https://www.clinicaltrialsregister.eu/ctr-search/trial/2022-001361-12/DK (accessed on 1 July 2025).
- Demétrio De Souza França, P.; Kossatz, S.; Brand, C.; Karassawa Zanoni, D.; Roberts, S.; Guru, N.; Adilbay, D.; Mauguen, A.; Valero Mayor, C.; Weber, W.A.; et al. A phase I study of a PARP1-targeted topical fluorophore for the detection of oral cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3618–3630. [Google Scholar] [CrossRef]
- Wang, J.; Li, S.; Wang, K.; Zhu, L.; Yang, L.; Zhu, Y.; Zhang, Z.; Hu, L.; Yuan, Y.; Fan, Q.; et al. A c-MET-Targeted Topical Fluorescent Probe cMBP-ICG Improves Oral Squamous Cell Carcinoma Detection in Humans. Ann. Surg. Oncol. 2023, 30, 641–651. [Google Scholar] [CrossRef]
- Jingbo, W.; Yuan, Y.; Tao, X. ASO Author Reflections: Topically Applied Targeted Near-Infrared Fluorescence Imaging Probe Helps Detect Head and Neck Squamous Cell Carcinoma. Ann. Surg. Oncol. 2023, 30, 652. [Google Scholar] [CrossRef]
- Mizushima, T.; Ohnishi, S.; Shimizu, Y.; Hatanaka, Y.; Hatanaka, K.C.; Hosono, H.; Kubota, Y.; Natsuizaka, M.; Kamiya, M.; Ono, S.; et al. Fluorescent imaging of superficial head and neck squamous cell carcinoma using a γ-glutamyltranspeptidase-activated targeting agent: A pilot study. BMC Cancer 2016, 16, 411. [Google Scholar] [CrossRef] [PubMed]
- Chance, B. Near-Infrared Images Using Continuous, Phase-Modulated, and Pulsed Light with Quantitation of Blood and Blood Oxygenation a. Ann. N. Y. Acad. Sci. 1998, 838, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Sevick-Muraca, E.M. A review of performance of near-infrared fluorescence imaging devices used in clinical studies. Br. J. Radiol. 2015, 88, 20140547. [Google Scholar] [CrossRef]
- Vahrmeijer, A.L.; Hutteman, M.; van der Vorst, J.R.; van de Velde, C.J.H.; Frangioni, J.V. Image-guided cancer surgery using near-infrared fluorescence. Nat. Rev. Clin. Oncol. 2013, 10, 507–518. [Google Scholar] [CrossRef]
- Richards-Kortum, R.; Sevick-Muraca, E. Quantitative optical spectroscopy for tissue diagnosis. Annu. Rev. Phys. Chem. 1996, 47, 555–606. [Google Scholar] [CrossRef]
- Dobie, C.; Skropeta, D. Insights into the role of sialylation in cancer progression and metastasis. Br. J. Cancer 2021, 124, 76–90. [Google Scholar] [CrossRef]
- Stowell, S.R.; Ju, T.; Cummings, R.D. Protein Glycosylation in Cancer. Annu. Rev. Pathol. Mech. Dis. 2015, 10, 473–510. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, M.; Portney, N.G.; Cui, D.; Budak, G.; Ozbay, E.; Ozkan, M.; Ozkan, C.S. Zeta potential: A surface electrical characteristic to probe the interaction of nanoparticles with normal and cancer human breast epithelial cells. Biomed. Microdevices 2008, 10, 321–328. [Google Scholar] [CrossRef]
- Le, W.; Chen, B.; Cui, Z.; Liu, Z.; Shi, D. Detection of cancer cells based on glycolytic-regulated surface electrical charges. Biophys. Rep. 2019, 5, 10–18. [Google Scholar] [CrossRef]
- Nishino, M.; Matsuzaki, I.; Musangile, F.Y.; Takahashi, Y.; Iwahashi, Y.; Warigaya, K.; Kinoshita, Y.; Kojima, F.; Murata, S. Measurement and visualization of cell membrane surface charge in fixed cultured cells related with cell morphology. PLoS ONE 2020, 15, e0236373. [Google Scholar] [CrossRef]
- Sun, S.; Wang, Y.; Pang, S.; Wu, X. Combination of the glycated hemoglobin levels and prognostic nutritional index as a prognostic marker in patients with acute coronary syndrome and type 2 diabetes mellitus. Lipids Health Dis. 2024, 23, 12. [Google Scholar] [CrossRef] [PubMed]
- Chehelgerdi, M.; Chehelgerdi, M.; Allela, O.Q.B.; Pecho, R.D.C.; Jayasankar, N.; Rao, D.P.; Thamaraikani, T.; Vasanthan, M.; Viktor, P.; Lakshmaiya, N.; et al. Progressing nanotechnology to improve targeted cancer treatment: Overcoming hurdles in its clinical implementation. Mol. Cancer 2023, 22, 169. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, J.; Marshall, W.F.; Karp, G. Karp’s Cell and Molecular Biology: Concepts and Experiments, 9th ed.; Wiley: Hoboken, NJ, USA, 2020; ISBN 978-1-119-59824-4. [Google Scholar]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: A perfect storm for cancer progression. Nat. Rev. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef]
- Hao, G.; Xu, Z.P.; Li, L. Manipulating extracellular tumour pH: An effective target for cancer therapy. RSC Adv. 2018, 8, 22182–22192. [Google Scholar] [CrossRef]
- Ward, C.; Meehan, J.; Gray, M.E.; Murray, A.F.; Argyle, D.J.; Kunkler, I.H.; Langdon, S.P. The impact of tumour pH on cancer progression: Strategies for clinical intervention. Explor. Target. Anti-Tumor Ther. 2020, 1, 71–100. [Google Scholar] [CrossRef]
- Wu, P.; Zhu, Y.; Liu, S.; Xiong, H. Modular Design of High-Brightness pH-Activatable Near-Infrared BODIPY Probes for Noninvasive Fluorescence Detection of Deep-Seated Early Breast Cancer Bone Metastasis: Remarkable Axial Substituent Effect on Performance. ACS Cent. Sci. 2021, 7, 2039–2048. [Google Scholar] [CrossRef]
- Yokomizo, S.; Henary, M.; Buabeng, E.R.; Fukuda, T.; Monaco, H.; Baek, Y.; Manganiello, S.; Wang, H.; Kubota, J.; Ulumben, A.D.; et al. Topical pH Sensing NIR Fluorophores for Intraoperative Imaging and Surgery of Disseminated Ovarian Cancer. Adv. Sci. 2022, 9, 2201416. [Google Scholar] [CrossRef]
- Kim, S.Y.; Podder, A.; Lee, H.; Cho, Y.-J.; Han, E.H.; Khatun, S.; Sessler, J.L.; Hong, K.S.; Bhuniya, S. Self-assembled amphiphilic fluorescent probe: Detecting pH-fluctuations within cancer cells and tumour tissues. Chem. Sci. 2020, 11, 9875–9883. [Google Scholar] [CrossRef]
- Tian, M.; Wu, R.; Xiang, C.; Niu, G.; Guan, W. Recent Advances in Fluorescent Probes for Cancer Biomarker Detection. Molecules 2024, 29, 1168. [Google Scholar] [CrossRef]
- Zhang, R.R.; Schroeder, A.B.; Grudzinski, J.J.; Rosenthal, E.L.; Warram, J.M.; Pinchuk, A.N.; Eliceiri, K.W.; Kuo, J.S.; Weichert, J.P. Beyond the margins: Real-time detection of cancer using targeted fluorophores. Nat. Rev. Clin. Oncol. 2017, 14, 347–364. [Google Scholar] [CrossRef]
- Baart, V.M.; Van Duijn, C.; Van Egmond, S.L.; Dijckmeester, W.A.; Jansen, J.C.; Vahrmeijer, A.L.; Sier, C.F.M.; Cohen, D. EGFR and αvβ6 as Promising Targets for Molecular Imaging of Cutaneous and Mucosal Squamous Cell Carcinoma of the Head and Neck Region. Cancers 2020, 12, 1474. [Google Scholar] [CrossRef]
- Barberio, M.; Benedicenti, S.; Pizzicannella, M.; Felli, E.; Collins, T.; Jansen-Winkeln, B.; Marescaux, J.; Viola, M.G.; Diana, M. Intraoperative Guidance Using Hyperspectral Imaging: A Review for Surgeons. Diagnostics 2021, 11, 2066. [Google Scholar] [CrossRef]
- Wu, Z.; Doondeea, J.B.; Gholami, A.M.; Janning, M.C.; Lemeer, S.; Kramer, K.; Eccles, S.A.; Gollin, S.M.; Grenman, R.; Walch, A.; et al. Quantitative Chemical Proteomics Reveals New Potential Drug Targets in Head and Neck Cancer. Mol. Cell. Proteomics 2011, 10, M111.011635. [Google Scholar] [CrossRef]
- Abdelazeem, K.N.M.; Nguyen, D.; Corbo, S.; Darragh, L.B.; Matsumoto, M.W.; Van Court, B.; Neupert, B.; Yu, J.; Olimpo, N.A.; Osborne, D.G.; et al. Manipulating the EphB4-ephrinB2 axis to reduce metastasis in HNSCC. Oncogene 2025, 44, 130–146. [Google Scholar] [CrossRef]
- Al-Jamaei, A.A.H.; Subramanyam, R.V.; Helder, M.N.; Forouzanfar, T.; Van Der Meij, E.H.; Al-Jamei, S.; De Visscher, J.G.A.M. A narrative review of the role of Eph receptors in head and neck squamous cell carcinoma. Oral Dis. 2024, 30, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Utispan, K.; Koontongkaew, S. Mucin 1 regulates the hypoxia response in head and neck cancer cells. J. Pharmacol. Sci. 2021, 147, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Odenthal, J.; Rijpkema, M.; Bos, D.; Wagena, E.; Croes, H.; Grenman, R.; Boerman, O.; Takes, R.; Friedl, P. Targeting CD44v6 for fluorescence-guided surgery in head and neck squamous cell carcinoma. Sci. Rep. 2018, 8, 10467. [Google Scholar] [CrossRef]
- Mack, B.; Gires, O. CD44s and CD44v6 Expression in Head and Neck Epithelia. PLoS ONE 2008, 3, e3360. [Google Scholar] [CrossRef] [PubMed]
- Ciulean, I.S.; Fischer, J.; Quaiser, A.; Bach, C.; Abken, H.; Tretbar, U.S.; Fricke, S.; Koehl, U.; Schmiedel, D.; Grunwald, T. CD44v6 specific CAR-NK cells for targeted immunotherapy of head and neck squamous cell carcinoma. Front. Immunol. 2023, 14, 1290488. [Google Scholar] [CrossRef]
- Andersen, A.O.; Christensen, A.; Straede, K.; Lawaetz, M.; Hahn, C.H.; Rubek, N.; Wessel, I.; Lelkaitis, G.; Kiss, K.; Paaske, N.; et al. Optical molecular imaging in oral- and oropharyngeal squamous cell carcinoma using a novel uPAR-targeting near-infrared imaging agent FG001 (ICG-glu-glu-AE105): An explorative phase II clinical trial. Theranostics 2025, 15, 52–67. [Google Scholar] [CrossRef]
- Christensen, A.; Grønhøj, C.; Jensen, J.; Lelkaitis, G.; Kiss, K.; Juhl, K.; Charabi, B.; Mortensen, J.; Kjær, A.; Von Buchwald, C. Expression patterns of uPAR, TF and EGFR and their potential as targets for molecular imaging in oropharyngeal squamous cell carcinoma. Oncol. Rep. 2022, 48, 147. [Google Scholar] [CrossRef]
- Dirheimer, L.; Pons, T.; François, A.; Lamy, L.; Cortese, S.; Marchal, F.; Bezdetnaya, L. Targeting of 3D oral cancer spheroids by αVβ6 integrin using near-infrared peptide-conjugated IRDye 680. Cancer Cell Int. 2024, 24, 228. [Google Scholar] [CrossRef]
- Lauwerends, L.J.; Zweedijk, B.E.; Galema, H.A.; Neijenhuis, L.K.A.; Dekker-Ensink, N.G.; Baatenburg De Jong, R.J.; Verhoef, C.; Bhairosingh, S.S.; Kuppen, P.J.K.; Vahrmeijer, A.L.; et al. Tumour Marker Expression in Head and Neck Malignancies to Identify Potential Targets for Intraoperative Molecular Near-Infrared Imaging. Mol. Diagn. Ther. 2024, 28, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Fan, T.; Wang, J.; Yuan, Y.; Tao, X. Near-infrared imaging of head and neck squamous cell carcinoma using indocyanine green that targets the αvβ6 peptide. J. Biomed. Opt. 2024, 29, 046002. [Google Scholar] [CrossRef] [PubMed]
- Nystrom, M.L.; McCulloch, D.; Weinreb, P.H.; Violette, S.M.; Speight, P.M.; Marshall, J.F.; Hart, I.R.; Thomas, G.J. Cyclooxygenase-2 Inhibition Suppresses αvβ6 Integrin–Dependent Oral Squamous Carcinoma Invasion. Cancer Res. 2006, 66, 10833–10842. [Google Scholar] [CrossRef]
- Sun, J.Y.; Shen, J.; Thibodeaux, J.; Huang, G.; Wang, Y.; Gao, J.; Low, P.S.; Dimitrov, D.S.; Sumer, B.D. In vivo optical imaging of folate receptor-β in head and neck squamous cell carcinoma. Laryngoscope 2014, 124, E312–E319. [Google Scholar] [CrossRef]
- Puré, E.; Blomberg, R. Pro-tumorigenic roles of fibroblast activation protein in cancer: Back to the basics. Oncogene 2018, 37, 4343–4357. [Google Scholar] [CrossRef]
- Wang, C.; Xu, X.; Folaron, M.; Gunn, J.R.; Hodge, S.; Chen, E.Y.; Hoopes, P.J.; Tichauer, K.M.; Samkoe, K.S. Improved Discrimination of Tumors with Low and Heterogeneous EGFR Expression in Fluorescence-Guided Surgery Through Paired-Agent Protocols. Mol. Imaging Biol. 2023, 25, 110–121. [Google Scholar] [CrossRef]
- Li, D.; Li, X.; Li, J.; Wang, Y.; Tan, F.; Li, X. Development of a fibroblast activation protein-targeted PET/NIR dual-modality probe and its application in head and neck cancer. Front. Bioeng. Biotechnol. 2023, 11, 1291824. [Google Scholar] [CrossRef]
- Vaughan, H.J.; Green, J.J.; Tzeng, S.Y. Cancer-Targeting Nanoparticles for Combinatorial Nucleic Acid Delivery. Adv. Mater. 2020, 32, 1901081. [Google Scholar] [CrossRef]
- Nakamura, Y.; Mochida, A.; Choyke, P.L.; Kobayashi, H. Nanodrug Delivery: Is the Enhanced Permeability and Retention Effect Sufficient for Curing Cancer? Bioconjug. Chem. 2016, 27, 2225–2238. [Google Scholar] [CrossRef]
- Rosenthal, E.L.; Moore, L.S.; Tipirneni, K.; de Boer, E.; Stevens, T.M.; Hartman, Y.E.; Carroll, W.R.; Zinn, K.R.; Warram, J.M. Sensitivity and Specificity of Cetuximab-IRDye800CW to Identify Regional Metastatic Disease in Head and Neck Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 4744–4752. [Google Scholar] [CrossRef]
- Rosenthal, E.L.; Warram, J.M.; De Boer, E.; Chung, T.K.; Korb, M.L.; Brandwein-Gensler, M.; Strong, T.V.; Schmalbach, C.E.; Morlandt, A.B.; Agarwal, G.; et al. Safety and Tumor Specificity of Cetuximab-IRDye800 for Surgical Navigation in Head and Neck Cancer. Clin. Cancer Res. 2015, 21, 3658–3666. [Google Scholar] [CrossRef] [PubMed]
- Warram, J.M.; De Boer, E.; Van Dam, G.M.; Moore, L.S.; Bevans, S.L.; Walsh, E.M.; Young, E.S.; Carroll, W.R.; Stevens, T.M.; Rosenthal, E.L. Fluorescence imaging to localize head and neck squamous cell carcinoma for enhanced pathological assessment. J. Pathol. Clin. Res. 2016, 2, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Mukkamala, R.; Lindeman, S.D.; Kragness, K.A.; Shahriar, I.; Srinivasarao, M.; Low, P.S. Design and characterization of fibroblast activation protein targeted pan-cancer imaging agent for fluorescence-guided surgery of solid tumors. J. Mater. Chem. B 2022, 10, 2038–2046. [Google Scholar] [CrossRef]
- Ellington, A.D.; Szostak, J.W. Selection in vitro of single-stranded DNA molecules that fold into specific ligand-binding structures. Nature 1992, 355, 850–852. [Google Scholar] [CrossRef]
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef]
- Camorani, S.; Crescenzi, E.; Fedele, M.; Cerchia, L. Oligonucleotide aptamers against tyrosine kinase receptors: Prospect for anticancer applications. Biochim. Biophys. Acta BBA-Rev. Cancer 2018, 1869, 263–277. [Google Scholar] [CrossRef]
- Affinito, A.; Quintavalle, C.; Esposito, C.L.; Roscigno, G.; Giordano, C.; Nuzzo, S.; Ricci-Vitiani, L.; Scognamiglio, I.; Minic, Z.; Pallini, R.; et al. Targeting Ephrin Receptor Tyrosine Kinase A2 with a Selective Aptamer for Glioblastoma Stem Cells. Mol. Ther. Nucleic Acids 2020, 20, 176–185. [Google Scholar] [CrossRef]
- Santana-Viera, L.; Dassie, J.P.; Rosàs-Lapeña, M.; Garcia-Monclús, S.; Chicón-Bosch, M.; Pérez-Capó, M.; Pozo, L.D.; Sanchez-Serra, S.; Almacellas-Rabaiget, O.; Maqueda-Marcos, S.; et al. Combination of protein and cell internalization SELEX identifies a potential RNA therapeutic and delivery platform to treat EphA2-expressing tumors. Mol. Ther.-Nucleic Acids 2023, 32, 758–772. [Google Scholar] [CrossRef]
- Cheng, S.; Jacobson, O.; Zhu, G.; Chen, Z.; Liang, S.H.; Tian, R.; Yang, Z.; Niu, G.; Zhu, X.; Chen, X. PET imaging of EGFR expression using an 18F-labeled RNA aptamer. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 948–956. [Google Scholar] [CrossRef]
- Thomas, B.J.; Awan, S.Z.; Joshi, T.; Daniels, M.A.; Porciani, D.; Burke, D.H. Anti-EGFR aptamer exhibits direct anti-cancer effects in NSCLC cells harboring EGFR L858R mutations. Npj Precis. Oncol. 2024, 8, 271. [Google Scholar] [CrossRef]
- Wang, D.-L.; Song, Y.-L.; Zhu, Z.; Li, X.-L.; Zou, Y.; Yang, H.-T.; Wang, J.-J.; Yao, P.-S.; Pan, R.-J.; Yang, C.J.; et al. Selection of DNA aptamers against epidermal growth factor receptor with high affinity and specificity. Biochem. Biophys. Res. Commun. 2014, 453, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Tang, C.; Shi, G.; Wang, G.; Du, Y.; Tian, J.; Zhang, H. Novel fluorescent GLUT1 inhibitor for precision detection and fluorescence image-guided surgery in oral squamous cell carcinoma. Int. J. Cancer 2022, 151, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Zamay, G.; Koshmanova, A.; Narodov, A.; Gorbushin, A.; Voronkovskii, I.; Grek, D.; Luzan, N.; Kolovskaya, O.; Shchugoreva, I.; Artyushenko, P.; et al. Visualization of Brain Tumors with Infrared-Labeled Aptamers for Fluorescence-Guided Surgery. J. Am. Chem. Soc. 2024, 146, 24989–25004. [Google Scholar] [CrossRef]
- Kichkailo, A.S.; Narodov, A.A.; Komarova, M.A.; Zamay, T.N.; Zamay, G.S.; Kolovskaya, O.S.; Erakhtin, E.E.; Glazyrin, Y.E.; Veprintsev, D.V.; Moryachkov, R.V.; et al. Development of DNA aptamers for visualization of glial brain tumors and detection of circulating tumor cells. Mol. Ther. Nucleic Acids 2023, 32, 267–288. [Google Scholar] [CrossRef]
- Wang, J.; Fang, X.; Zhang, C.; Ji, H.; Pang, Q.; Li, X.; Luo, Z.; Wu, Q.; Zhang, L. Development of Aptamer-Based Molecular Tools for Rapid Intraoperative Diagnosis and In Vivo Imaging of Serous Ovarian Cancer. ACS Appl. Mater. Interfaces 2021, 13, 16118–16126. [Google Scholar] [CrossRef]
- Li, C.-H.; Kuo, T.-R.; Su, H.-J.; Lai, W.-Y.; Yang, P.-C.; Chen, J.-S.; Wang, D.-Y.; Wu, Y.-C.; Chen, C.-C. Fluorescence-Guided Probes of Aptamer-Targeted Gold Nanoparticles with Computed Tomography Imaging Accesses for in Vivo Tumor Resection. Sci. Rep. 2015, 5, 15675. [Google Scholar] [CrossRef]
- Fasting, C.; Schalley, C.A.; Weber, M.; Seitz, O.; Hecht, S.; Koksch, B.; Dernedde, J.; Graf, C.; Knapp, E.; Haag, R. Multivalency as a Chemical Organization and Action Principle. Angew. Chem. Int. Ed. 2012, 51, 10472–10498. [Google Scholar] [CrossRef]
- Möser, C.; Lorenz, J.; Sajfutdinow, M.; Smith, D. Pinpointed Stimulation of EphA2 Receptors via DNA-Templated Oligovalence. Int. J. Mol. Sci. 2018, 19, 3482. [Google Scholar] [CrossRef]
- Dai, B.; Hu, Y.; Duan, J.; Yang, X.-D. Aptamer-guided DNA tetrahedron as a novel targeted drug delivery system for MUC1-expressing breast cancer cells in vitro. Oncotarget 2016, 7, 38257–38269. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, L.; Wang, L.; Jiang, W. A dual-targeting DNA tetrahedron nanocarrier for breast cancer cell imaging and drug delivery. Talanta 2018, 179, 356–363. [Google Scholar] [CrossRef]
- Van Zundert, I.; Spezzani, E.; Brillas, R.R.; Paffen, L.; Yurchenko, A.; De Greef, T.F.A.; Albertazzi, L.; Bertucci, A.; Patiño, T. Unveiling DNA Origami Interaction Dynamics on Living Cell Surfaces by Single Particle Tracking. bioRxiv 2024, in press. [Google Scholar] [CrossRef]
- Van Schaik, J.E.; Halmos, G.B.; Witjes, M.J.H.; Plaat, B.E.C. An overview of the current clinical status of optical imaging in head and neck cancer with a focus on Narrow Band imaging and fluorescence optical imaging. Oral Oncol. 2021, 121, 105504. [Google Scholar] [CrossRef]
- Steinkamp, P.J.; Voskuil, F.J.; Van Der Vegt, B.; Doff, J.J.; Schepman, K.-P.; De Visscher, S.A.H.J.; Kelder, W.; Jayalakshmi, Y.; Gao, J.; Sumer, B.D.; et al. A Standardized Framework for Fluorescence-Guided Margin Assessment for Head and Neck Cancer Using a Tumor Acidosis Sensitive Optical Imaging Agent. Mol. Imaging Biol. 2021, 23, 809–817. [Google Scholar] [CrossRef]
- Preziosi, A.; Cirelli, C.; Waterhouse, D.; Privitera, L.; De Coppi, P.; Giuliani, S. State of the art medical devices for fluorescence-guided surgery (FGS): Technical review and future developments. Surg. Endosc. 2024, 38, 6227–6236. [Google Scholar] [CrossRef]
- Kanniyappan, U.; Wang, B.; Yang, C.; Ghassemi, P.; Litorja, M.; Suresh, N.; Wang, Q.; Chen, Y.; Pfefer, T.J. Performance test methods for near-infrared fluorescence imaging. Med. Phys. 2020, 47, 3389–3401. [Google Scholar] [CrossRef]
- Kriukova, E.; LaRochelle, E.; Pfefer, T.J.; Kanniyappan, U.; Gioux, S.; Pogue, B.; Ntziachristos, V.; Gorpas, D. Impact of signal-to-noise ratio and contrast definition on the sensitivity assessment and benchmarking of fluorescence molecular imaging systems. J. Biomed. Opt. 2024, 30, S13703. [Google Scholar] [CrossRef]
- Ochoa, M.I.; Ruiz, A.; LaRochelle, E.; Reed, M.; Berber, E.; Poultsides, G.; Pogue, B.W. Assessment of open-field fluorescence guided surgery systems: Implementing a standardized method for characterization and comparison. J. Biomed. Opt. 2023, 28, 096007. [Google Scholar] [CrossRef]
- Van Beurden, F.; Van Willigen, D.M.; Vojnovic, B.; Van Oosterom, M.N.; Brouwer, O.R.; Der Poel, H.G.V.; Kobayashi, H.; Van Leeuwen, F.W.B.; Buckle, T. Multi-Wavelength Fluorescence in Image-Guided Surgery, Clinical Feasibility and Future Perspectives. Mol. Imaging 2020, 19, 153601212096233. [Google Scholar] [CrossRef]
- Yu, M.; Liu, X.; Wang, S.; Qin, Z.; Hu, B.; Li, Z.; Sun, S. Application of NIR Fluorescent Materials in Imaging and Treatment of Tumors of Different Depths. Nanomaterials 2025, 15, 811. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Morsch, M.; Lu, Y.; Shangguan, P.; Han, L.; Wang, Z.; Chen, X.; Song, C.; Liu, S.; et al. Brain-Targeted Aggregation-Induced-Emission Nanoparticles with Near-Infrared Imaging at 1550 nm Boosts Orthotopic Glioblastoma Theranostics. Adv. Mater. 2022, 34, 2106082. [Google Scholar] [CrossRef]
- Darwan, D.; Lim, K.R.G.; Wijaya, H.; Lim, Z.C.; Wang, T.; Ang, W.H.; Tan, Z. Deep Fluorescence Imaging by Laser-Scanning Excitation and Artificial Neural Network Processing. Adv. Opt. Mater. 2020, 8, 2000390. [Google Scholar] [CrossRef]
- Lu, G.; van den Berg, N.S.; Martin, B.A.; Nishio, N.; Hart, Z.P.; van Keulen, S.; Fakurnejad, S.; Chirita, S.U.; Raymundo, R.C.; Yi, G.; et al. Tumour-specific fluorescence-guided surgery for pancreatic cancer using panitumumab-IRDye800CW: A phase 1 single-centre, open-label, single-arm, dose-escalation study. Lancet Gastroenterol. Hepatol. 2020, 5, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Chen, Z.; Zheng, J.; Zhao, Q.; Yuan, Z. Artificial intelligence-aided optical imaging for cancer theranostics. Semin. Cancer Biol. 2023, 94, 62–80. [Google Scholar] [CrossRef]
- Combalia, M.; Garcia, S.; Malvehy, J.; Puig, S.; Mülberger, A.G.; Browning, J.; Garcet, S.; Krueger, J.G.; Lish, S.R.; Lax, R.; et al. Deep learning automated pathology in ex vivo microscopy. Biomed. Opt. Express 2021, 12, 3103. [Google Scholar] [CrossRef]
- Ouyang, W.; Aristov, A.; Lelek, M.; Hao, X.; Zimmer, C. Deep learning massively accelerates super-resolution localization microscopy. Nat. Biotechnol. 2018, 36, 460–468. [Google Scholar] [CrossRef]
- Roberts, H.W.; Donati-Bourne, J.F.; Wilson, V.L.; Wilton, J.C. The Use of Live Fluorescence Staining Techniques in Surgery: A Review. J. Investig. Surg. 2013, 26, 283–293. [Google Scholar] [CrossRef]
- Keereweer, S.; Van Driel, P.B.A.A.; Snoeks, T.J.A.; Kerrebijn, J.D.F.; Baatenburg De Jong, R.J.; Vahrmeijer, A.L.; Sterenborg, H.J.C.M.; Löwik, C.W.G.M. Optical Image-Guided Cancer Surgery: Challenges and Limitations. Clin. Cancer Res. 2013, 19, 3745–3754. [Google Scholar] [CrossRef]
- Pfahl, A.; Radmacher, G.K.; Köhler, H.; Maktabi, M.; Neumuth, T.; Melzer, A.; Gockel, I.; Chalopin, C.; Jansen-Winkeln, B. Combined indocyanine green and quantitative perfusion assessment with hyperspectral imaging during colorectal resections. Biomed. Opt. Express 2022, 13, 3145. [Google Scholar] [CrossRef]
- Chalopin, C.; Maktabi, M.; Köhler, H.; Cervantes-Sanchez, F.; Pfahl, A.; Jansen-Winkeln, B.; Mehdorn, M.; Barberio, M.; Gockel, I.; Melzer, A. Intraoperative Imaging for Procedures of the Gastrointestinal Tract. In Innovative Endoscopic and Surgical Technology in the GI Tract; Horgan, S., Fuchs, K.-H., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 365–379. ISBN 978-3-030-78216-0. [Google Scholar]
- McMahon, J.; O’Brien, C.J.; Pathak, I.; Hamill, R.; McNeil, E.; Hammersley, N.; Gardiner, S.; Junor, E. Influence of condition of surgical margins on local recurrence and disease-specific survival in oral and oropharyngeal cancer. Br. J. Oral Maxillofac. Surg. 2003, 41, 224–231. [Google Scholar] [CrossRef]
- Nagaya, T.; Nakamura, Y.A.; Choyke, P.L.; Kobayashi, H. Fluorescence-Guided Surgery. Front. Oncol. 2017, 7, 314. [Google Scholar] [CrossRef]
- Zhu, Y.; Xiao, T.; He, Y.; Hong, X.; Zhou, T.; Da, M.; Ge, S.; Xie, D.; Wang, Z. Application of near-infrared fluorescence imaging in lingual lymph node screening and drainage pattern observation for tongue cancer. Front. Cell Dev. Biol. 2022, 10, 986575. [Google Scholar] [CrossRef] [PubMed]
- Stone, L.D.; Kasten, B.B.; Rao, S.; Gonzalez, M.L.; Stevens, T.M.; Lin, D.; Carroll, W.; Greene, B.; Moore, L.S.; Fuson, A.; et al. Interim Phase II Results Using Panitumumab-IRDye800CW during Transoral Robotic Surgery in Patients with Oropharyngeal Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2024, 30, 4016–4028. [Google Scholar] [CrossRef] [PubMed]
- Cianchi, F.; Indennitate, G.; Paoli, B.; Ortolani, M.; Lami, G.; Manetti, N.; Tarantino, O.; Messeri, S.; Foppa, C.; Badii, B.; et al. The Clinical Value of Fluorescent Lymphography with Indocyanine Green During Robotic Surgery for Gastric Cancer: A Matched Cohort Study. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2020, 24, 2197–2203. [Google Scholar] [CrossRef]
- Zhong, Q.; Chen, Q.-Y.; Huang, X.-B.; Lin, G.-T.; Liu, Z.-Y.; Chen, J.-Y.; Wang, H.-G.; Weng, K.; Li, P.; Xie, J.-W.; et al. Clinical implications of Indocyanine Green Fluorescence Imaging-Guided laparoscopic lymphadenectomy for patients with gastric cancer: A cohort study from two randomized, controlled trials using individual patient data. Int. J. Surg. Lond. Engl. 2021, 94, 106120. [Google Scholar] [CrossRef]
- Park, J.-H.; Berlth, F.; Wang, C.; Wang, S.; Choi, J.-H.; Park, S.-H.; Suh, Y.-S.; Kong, S.-H.; Park, D.J.; Lee, H.-J.; et al. Mapping of the perigastric lymphatic network using indocyanine green fluorescence imaging and tissue marking dye in clinically advanced gastric cancer. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2022, 48, 411–417. [Google Scholar] [CrossRef]
- Zweedijk, B.E.; Dalmeijer, S.W.R.; Van Manen, L.; Galema, H.A.; Lauwerends, L.J.; Abbasi, H.; Kremer, B.; Verhoef, C.; Robinson, D.J.; Koppes, S.A.; et al. Molecular-Targeted Fluorescence Lymph Node Imaging Could Play a Clinical Role in the Surgical Setting: A Systematic Review. Cancers 2025, 17, 1352. [Google Scholar] [CrossRef]
- Peloso, A.; Franchi, E.; Canepa, M.C.; Barbieri, L.; Briani, L.; Ferrario, J.; Bianco, C.; Quaretti, P.; Brugnatelli, S.; Dionigi, P.; et al. Combined use of intraoperative ultrasound and indocyanine green fluorescence imaging to detect liver metastases from colorectal cancer. HPB 2013, 15, 928–934. [Google Scholar] [CrossRef]
- Van Der Vorst, J.R.; Schaafsma, B.E.; Hutteman, M.; Verbeek, F.P.R.; Liefers, G.; Hartgrink, H.H.; Smit, V.T.H.B.M.; Löwik, C.W.G.M.; Van De Velde, C.J.H.; Frangioni, J.V.; et al. Near-infrared fluorescence-guided resection of colorectal liver metastases. Cancer 2013, 119, 3411–3418. [Google Scholar] [CrossRef]
- Knospe, L.; Gockel, I.; Jansen-Winkeln, B.; Thieme, R.; Niebisch, S.; Moulla, Y.; Stelzner, S.; Lyros, O.; Diana, M.; Marescaux, J.; et al. New Intraoperative Imaging Tools and Image-Guided Surgery in Gastric Cancer Surgery. Diagnostics 2022, 12, 507. [Google Scholar] [CrossRef]
- Jafari, M.D.; Wexner, S.D.; Martz, J.E.; McLemore, E.C.; Margolin, D.A.; Sherwinter, D.A.; Lee, S.W.; Senagore, A.J.; Phelan, M.J.; Stamos, M.J. Perfusion assessment in laparoscopic left-sided/anterior resection (PILLAR II): A multi-institutional study. J. Am. Coll. Surg. 2015, 220, 82–92.e1. [Google Scholar] [CrossRef]
- Liu, D.; Liang, L.; Liu, L.; Zhu, Z. Does intraoperative indocyanine green fluorescence angiography decrease the incidence of anastomotic leakage in colorectal surgery? A systematic review and meta-analysis. Int. J. Color. Dis. 2021, 36, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Arezzo, A.; Bonino, M.A.; Ris, F.; Boni, L.; Cassinotti, E.; Foo, D.C.C.; Shum, N.F.; Brolese, A.; Ciarleglio, F.; Keller, D.S.; et al. Intraoperative use of fluorescence with indocyanine green reduces anastomotic leak rates in rectal cancer surgery: An individual participant data analysis. Surg. Endosc. 2020, 34, 4281–4290. [Google Scholar] [CrossRef] [PubMed]
- Zeh, R.; Sheikh, S.; Xia, L.; Pierce, J.; Newton, A.; Predina, J.; Cho, S.; Nasrallah, M.; Singhal, S.; Dorsey, J.; et al. The second window ICG technique demonstrates a broad plateau period for near infrared fluorescence tumor contrast in glioblastoma. PLoS ONE 2017, 12, e0182034. [Google Scholar] [CrossRef]
- Voskuil, F.J.; Steinkamp, P.J.; Zhao, T.; Van Der Vegt, B.; Koller, M.; Doff, J.J.; Jayalakshmi, Y.; Hartung, J.P.; Gao, J.; Sumer, B.D.; et al. Exploiting metabolic acidosis in solid cancers using a tumor-agnostic pH-activatable nanoprobe for fluorescence-guided surgery. Nat. Commun. 2020, 11, 3257. [Google Scholar] [CrossRef]
- Randall, L.M.; Wenham, R.M.; Low, P.S.; Dowdy, S.C.; Tanyi, J.L. A phase II, multicenter, open-label trial of OTL38 injection for the intra-operative imaging of folate receptor-alpha positive ovarian cancer. Gynecol. Oncol. 2019, 155, 63–68. [Google Scholar] [CrossRef]
- Giuliani, S.; Paraboschi, I.; McNair, A.; Smith, M.; Rankin, K.S.; Elson, D.S.; Paleri, V.; Leff, D.; Stasiuk, G.; Anderson, J. Monoclonal Antibodies for Targeted Fluorescence-Guided Surgery: A Review of Applicability across Multiple Solid Tumors. Cancers 2024, 16, 1045. [Google Scholar] [CrossRef]
- Jiao, J.; Zhang, J.; Yang, F.; Song, W.; Han, D.; Wen, W.; Qin, W. Quicker, deeper and stronger imaging: A review of tumor-targeted, near-infrared fluorescent dyes for fluorescence guided surgery in the preclinical and clinical stages. Eur. J. Pharm. Biopharm. 2020, 152, 123–143. [Google Scholar] [CrossRef]
- Englhard, A.S.; Palaras, A.; Volgger, V.; Stepp, H.; Mack, B.; Libl, D.; Gires, O.; Betz, C.S. Confocal laser endomicroscopy in head and neck malignancies using FITC-labelled EpCAM- and EGF-R-antibodies in cell lines and tumor biopsies. J. Biophotonics 2017, 10, 1365–1376. [Google Scholar] [CrossRef]
- Thariat, J.; Etienne-Grimaldi, M.-C.; Grall, D.; Bensadoun, R.-J.; Cayre, A.; Penault-Llorca, F.; Veracini, L.; Francoual, M.; Formento, J.-L.; Dassonville, O.; et al. Epidermal Growth Factor Receptor Protein Detection in Head and Neck Cancer Patients: A Many-Faceted Picture. Clin. Cancer Res. 2012, 18, 1313–1322. [Google Scholar] [CrossRef]
- Spiegelberg, D.; Kuku, G.; Selvaraju, R.; Nestor, M. Characterization of CD44 variant expression in head and neck squamous cell carcinomas. Tumor Biol. 2014, 35, 2053–2062. [Google Scholar] [CrossRef] [PubMed]
- Orian-Rousseau, V. CD44, a therapeutic target for metastasising tumours. Eur. J. Cancer 2010, 46, 1271–1277. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, J.; Lu, J.; Xiong, H.; Shi, X.; Gong, L. Significance of CD44 expression in head and neck cancer: A systemic review and meta-analysis. BMC Cancer 2014, 14, 15. [Google Scholar] [CrossRef]
- Staibano, S.; Merolla, F.; Testa, D.; Iovine, R.; Mascolo, M.; Guarino, V.; Castellone, M.D.; Di Benedetto, M.; Galli, V.; Motta, S.; et al. OPN/CD44v6 overexpression in laryngeal dysplasia and correlation with clinical outcome. Br. J. Cancer 2007, 97, 1545–1551. [Google Scholar] [CrossRef]
- Brown, R.L.; Reinke, L.M.; Damerow, M.S.; Perez, D.; Chodosh, L.A.; Yang, J.; Cheng, C. CD44 splice isoform switching in human and mouse epithelium is essential for epithelial-mesenchymal transition and breast cancer progression. J. Clin. Investig. 2011, 121, 1064–1074. [Google Scholar] [CrossRef]
- Fakurnejad, S.; Van Keulen, S.; Nishio, N.; Engelen, M.; Van Den Berg, N.S.; Lu, G.; Birkeland, A.; Baik, F.; Colevas, A.D.; Rosenthal, E.L.; et al. Fluorescence molecular imaging for identification of high-grade dysplasia in patients with head and neck cancer. Oral Oncol. 2019, 97, 50–55. [Google Scholar] [CrossRef]
- De Wit, J.G.; Vonk, J.; Voskuil, F.J.; De Visscher, S.A.H.J.; Schepman, K.-P.; Hooghiemstra, W.T.R.; Linssen, M.D.; Elias, S.G.; Halmos, G.B.; Plaat, B.E.C.; et al. EGFR-targeted fluorescence molecular imaging for intraoperative margin assessment in oral cancer patients: A phase II trial. Nat. Commun. 2023, 14, 4952. [Google Scholar] [CrossRef]
- Rakuten Medical, Inc. ASP-1929 Photoimmunotherapy (PIT) Study in Patients with Recurrent Head/Neck Cancer. Available online: https://clinicaltrials.gov/study/NCT05182866 (accessed on 1 July 2025).
- Erasmus University Rotterdam. Fluorescence-guided Surgery Using cRGD-ZW800-1 in Oral Cancer. Available online: https://clinicaltrials.eu/trial/study-on-improving-oral-cancer-surgery-using-crgd-zw800-1-fluorescent-imaging-for-patients-with-oral-cancer/ (accessed on 1 July 2025).
- Vanderbilt-Ingram Cancer Center Evaluating the Use of Dual Imaging Techniques for Detection of Disease in Patients with Head and Neck Cancer. Available online: https://clinicaltrials.gov/study/NCT05945875 (accessed on 1 July 2025).
- Meeks, N.; James, S.; Krishnan, G.; Wodeyar, A.; Tanaka, H.; Kasten, B.B.; Lee, Y.-J.; Hom, M.E.; Rosenthal, E.L.; Warram, J.M. Background Tissue with Native Target Expression Can Determine Presence of Nodal Metastasis in Head and Neck Squamous Cell Carcinoma Patients Infused with Targeted Fluorescent Tracers. Mol. Imaging Biol. 2025, 27, 333–340. [Google Scholar] [CrossRef]
- University Medical Center Groningen. Real-time Margin Assessment in Head and Neck Cancer (LIGHTNING). Available online: https://clinicaltrials.gov/study/NCT05499065 (accessed on 1 July 2025).
- Witjes, M.J.H. Clinical challenges in image-guided surgery in cancer. In Proceedings of the Molecular-Guided Surgery: Molecules, Devices, and Applications XI; Gibbs, S.L., Tichauer, K.M., Eds.; SPIE: San Francisco, CA, USA, 2025; p. 39. [Google Scholar]
- Erasmus Medical Center. Fluorescence-guided Surgery in Laryngeal- and Hypopharyngeal Cancer: A Feasibility Trial (STELLAR). Available online: https://clinicaltrials.gov/study/NCT05752149 (accessed on 1 July 2025).
- Pal, R.; Lwin, T.M.; Krishnamoorthy, M.; Collins, H.R.; Chan, C.D.; Prilutskiy, A.; Nasrallah, M.P.; Dijkhuis, T.H.; Shukla, S.; Kendall, A.L.; et al. Fluorescence lifetime of injected indocyanine green as a universal marker of solid tumours in patients. Nat. Biomed. Eng. 2023, 7, 1649–1666. [Google Scholar] [CrossRef]
- Dirheimer, L.; Cortese, S.; Dolivet, G.; Merlin, J.L.; Marchal, F.; Mastronicola, R.; Bezdetnaya, L. Fluorescence Imaging-Assessed Surgical Margin Detection in Head and Neck Oncology by Passive and Active Targeting. Mol. Diagn. Ther. 2025, 29, 465–481. [Google Scholar] [CrossRef]
- Voskuil, F.J.; De Jongh, S.J.; Hooghiemstra, W.T.R.; Linssen, M.D.; Steinkamp, P.J.; De Visscher, S.A.H.J.; Schepman, K.-P.; Elias, S.G.; Meersma, G.-J.; Jonker, P.K.C.; et al. Fluorescence-guided imaging for resection margin evaluation in head and neck cancer patients using cetuximab-800CW: A quantitative dose-escalation study. Theranostics 2020, 10, 3994–4005. [Google Scholar] [CrossRef]
- Wu, C.; Gleysteen, J.; Teraphongphom, N.T.; Li, Y.; Rosenthal, E. In-vivo optical imaging in head and neck oncology: Basic principles, clinical applications and future directions. Int. J. Oral Sci. 2018, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Alekseev, M.; Rybakov, E.; Shelygin, Y.; Chernyshov, S.; Zarodnyuk, I. A study investigating the perfusion of colorectal anastomoses using fluorescence angiography: Results of the FLAG randomized trial. Color. Dis. Off. J. Assoc. Coloproctol. G. B. Irel. 2020, 22, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Vu, A.; Farah, C. Narrow band imaging: Clinical applications in oral and oropharyngeal cancer. Oral Dis. 2016, 22, 383–390. [Google Scholar] [CrossRef]
- Davies, K.; Connolly, J.M.; Dockery, P.; Wheatley, A.M.; Olivo, M.; Keogh, I. Point of care optical diagnostic technologies for the detection of oral and oropharyngeal squamous cell carcinoma. Surg. J. R. Coll. Surg. Edinb. Irel. 2015, 13, 321–329. [Google Scholar] [CrossRef]
- Green, B.; Tsiroyannis, C.; Brennan, P.A. Optical diagnostic systems for assessing head and neck lesions. Oral Dis. 2016, 22, 180–184. [Google Scholar] [CrossRef]
- Green, B.; Cobb, A.R.M.; Brennan, P.A.; Hopper, C. Optical diagnostic techniques for use in lesions of the head and neck: Review of the latest developments. Br. J. Oral Maxillofac. Surg. 2014, 52, 675–680. [Google Scholar] [CrossRef]
- Arens, C.; Dreyer, T.; Malzahn, K.; Glanz, H. Direct and indirect autofluorescence laryngoscopy in the diagnosis of laryngeal cancer and its precursor lesions. Otolaryngol. Pol. Pol. Otolaryngol. 2004, 58, 197–203. [Google Scholar]
- Vila, P.M.; Park, C.W.; Pierce, M.C.; Goldstein, G.H.; Levy, L.; Gurudutt, V.V.; Polydorides, A.D.; Godbold, J.H.; Teng, M.S.; Genden, E.M.; et al. Discrimination of benign and neoplastic mucosa with a high-resolution microendoscope (HRME) in head and neck cancer. Ann. Surg. Oncol. 2012, 19, 3534–3539. [Google Scholar] [CrossRef]
- Kallaway, C.; Almond, L.M.; Barr, H.; Wood, J.; Hutchings, J.; Kendall, C.; Stone, N. Advances in the clinical application of Raman spectroscopy for cancer diagnostics. Photodiagnosis Photodyn. Ther. 2013, 10, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.F.; Wolthuis, R.; Koljenović, S.; Almeida, R.M.; Puppels, G.J. Fiber-Optic Probes for in Vivo Raman Spectroscopy in the High-Wavenumber Region. Anal. Chem. 2005, 77, 6747–6752. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, Y.; Zheng, B.; Su, L.; Chen, Y.; Ma, S.; Hu, Q.; Zou, X.; Yao, L.; Yang, Y.; et al. Rapid histology of laryngeal squamous cell carcinoma with deep-learning based stimulated Raman scattering microscopy. Theranostics 2019, 9, 2541–2554. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.; Enderle-Ammour, K.; Kurowski, K.; Metzger, M.C.; Poxleitner, P.; Werner, M.; Rothweiler, R.; Beck, J.; Straehle, J.; Schmelzeisen, R.; et al. AI-Based Detection of Oral Squamous Cell Carcinoma with Raman Histology. Cancers 2024, 16, 689. [Google Scholar] [CrossRef]
- Barroso, E.M.; Smits, R.W.H.; Bakker Schut, T.C.; Ten Hove, I.; Hardillo, J.A.; Wolvius, E.B.; Baatenburg De Jong, R.J.; Koljenović, S.; Puppels, G.J. Discrimination between Oral Cancer and Healthy Tissue Based on Water Content Determined by Raman Spectroscopy. Anal. Chem. 2015, 87, 2419–2426. [Google Scholar] [CrossRef]
- Liao, Z.; Lizio, M.G.; Corden, C.; Khout, H.; Rakha, E.; Notingher, I. Feasibility of integrated high-wavenumber Raman imaging and fingerprint Raman spectroscopy for fast margin assessment in breast cancer surgery. J. Raman Spectrosc. 2020, 51, 1986–1995. [Google Scholar] [CrossRef]
- Koljenović, S.; Bakker Schut, T.C.; Wolthuis, R.; De Jong, B.; Santos, L.; Caspers, P.J.; Kros, J.M.; Puppels, G.J. Tissue characterization using high wave number Raman spectroscopy. J. Biomed. Opt. 2005, 10, 031116. [Google Scholar] [CrossRef]
- Grimbergen, M.C.M.; Van Swol, C.F.P.; Van Moorselaar, R.J.A.; Uff, J.; Mahadevan-Jansen, A.; Stone, N. Raman spectroscopy of bladder tissue in the presence of 5-aminolevulinic acid. J. Photochem. Photobiol. B 2009, 95, 170–176. [Google Scholar] [CrossRef]
- Carr, J.A.; Franke, D.; Caram, J.R.; Perkinson, C.F.; Saif, M.; Askoxylakis, V.; Datta, M.; Fukumura, D.; Jain, R.K.; Bawendi, M.G.; et al. Shortwave infrared fluorescence imaging with the clinically approved near-infrared dye indocyanine green. Proc. Natl. Acad. Sci. USA 2018, 115, 4465–4470. [Google Scholar] [CrossRef]
- Lauwerends, L.J.; Abbasi, H.; Bakker Schut, T.C.; Van Driel, P.B.A.A.; Hardillo, J.A.U.; Santos, I.P.; Barroso, E.M.; Koljenović, S.; Vahrmeijer, A.L.; Baatenburg De Jong, R.J.; et al. The complementary value of intraoperative fluorescence imaging and Raman spectroscopy for cancer surgery: Combining the incompatibles. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2364–2376. [Google Scholar] [CrossRef]
- Röhrich, M. Fibroblast Activation Protein Inhibitor PET Imaging in Head and Neck Cancer. PET Clin. 2023, 18, 315–323. [Google Scholar] [CrossRef]
- Linz, C.; Brands, R.C.; Kertels, O.; Dierks, A.; Brumberg, J.; Gerhard-Hartmann, E.; Hartmann, S.; Schirbel, A.; Serfling, S.; Zhi, Y.; et al. Targeting fibroblast activation protein in newly diagnosed squamous cell carcinoma of the oral cavity—Initial experience and comparison to [18F]FDG PET/CT and MRI. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3951–3960. [Google Scholar] [CrossRef]
- Syed, M.; Flechsig, P.; Liermann, J.; Windisch, P.; Staudinger, F.; Akbaba, S.; Koerber, S.A.; Freudlsperger, C.; Plinkert, P.K.; Debus, J.; et al. Fibroblast activation protein inhibitor (FAPI) PET for diagnostics and advanced targeted radiotherapy in head and neck cancers. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2836–2845. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Wu, J.; Han, Y.; Wei, M.; Han, S.; Lin, R.; Sun, Z.; Yang, F.; Jiao, D.; Xie, P.; et al. A novel anti-PSMA human scFv has the potential to be used as a diagnostic tool in prostate cancer. Oncotarget 2016, 7, 59471–59481. [Google Scholar] [CrossRef] [PubMed]
- Mazzocco, C.; Fracasso, G.; Germain-Genevois, C.; Dugot-Senant, N.; Figini, M.; Colombatti, M.; Grenier, N.; Couillaud, F. In vivo imaging of prostate cancer using an anti-PSMA scFv fragment as a probe. Sci. Rep. 2016, 6, 23314. [Google Scholar] [CrossRef] [PubMed]
- Van Driel, P.B.A.A.; Van Der Vorst, J.R.; Verbeek, F.P.R.; Oliveira, S.; Snoeks, T.J.A.; Keereweer, S.; Chan, B.; Boonstra, M.C.; Frangioni, J.V.; Van Bergen En Henegouwen, P.M.P.; et al. Intraoperative fluorescence delineation of head and neck cancer with a fluorescent Anti-epidermal growth factor receptor nanobody. Int. J. Cancer 2014, 134, 2663–2673. [Google Scholar] [CrossRef]
- Mahalingam, S.M.; Dudkin, V.Y.; Goldberg, S.; Klein, D.; Yi, F.; Singhal, S.; O’Neil, K.T.; Low, P.S. Evaluation of a Centyrin-Based Near-Infrared Probe for Fluorescence-Guided Surgery of Epidermal Growth Factor Receptor Positive Tumors. Bioconjug. Chem. 2017, 28, 2865–2873. [Google Scholar] [CrossRef]
- Elliott, J.T.; Marra, K.; Evans, L.T.; Davis, S.C.; Samkoe, K.S.; Feldwisch, J.; Paulsen, K.D.; Roberts, D.W.; Pogue, B.W. Simultaneous In Vivo Fluorescent Markers for Perfusion, Protoporphyrin Metabolism, and EGFR Expression for Optically Guided Identification of Orthotopic Glioma. Clin. Cancer Res. 2017, 23, 2203–2212. [Google Scholar] [CrossRef]
- Gao, Z.; Li, G.; Li, X.; Zhou, J.; Duan, X.; Chen, J.; Joshi, B.P.; Kuick, R.; Khoury, B.; Thomas, D.G.; et al. In vivo near-infrared imaging of ErbB2 expressing breast tumors with dual-axes confocal endomicroscopy using a targeted peptide. Sci. Rep. 2017, 7, 14404. [Google Scholar] [CrossRef]
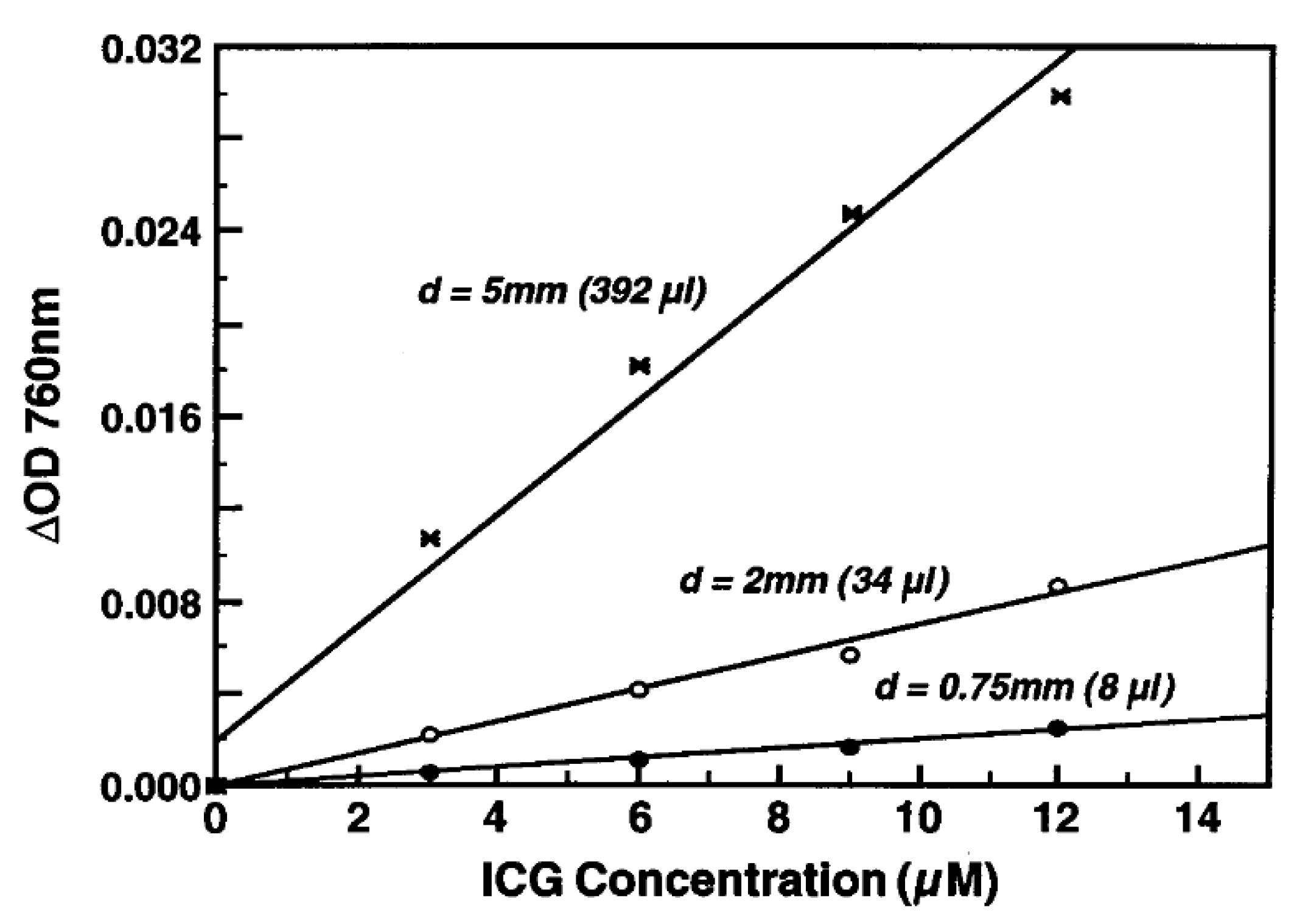
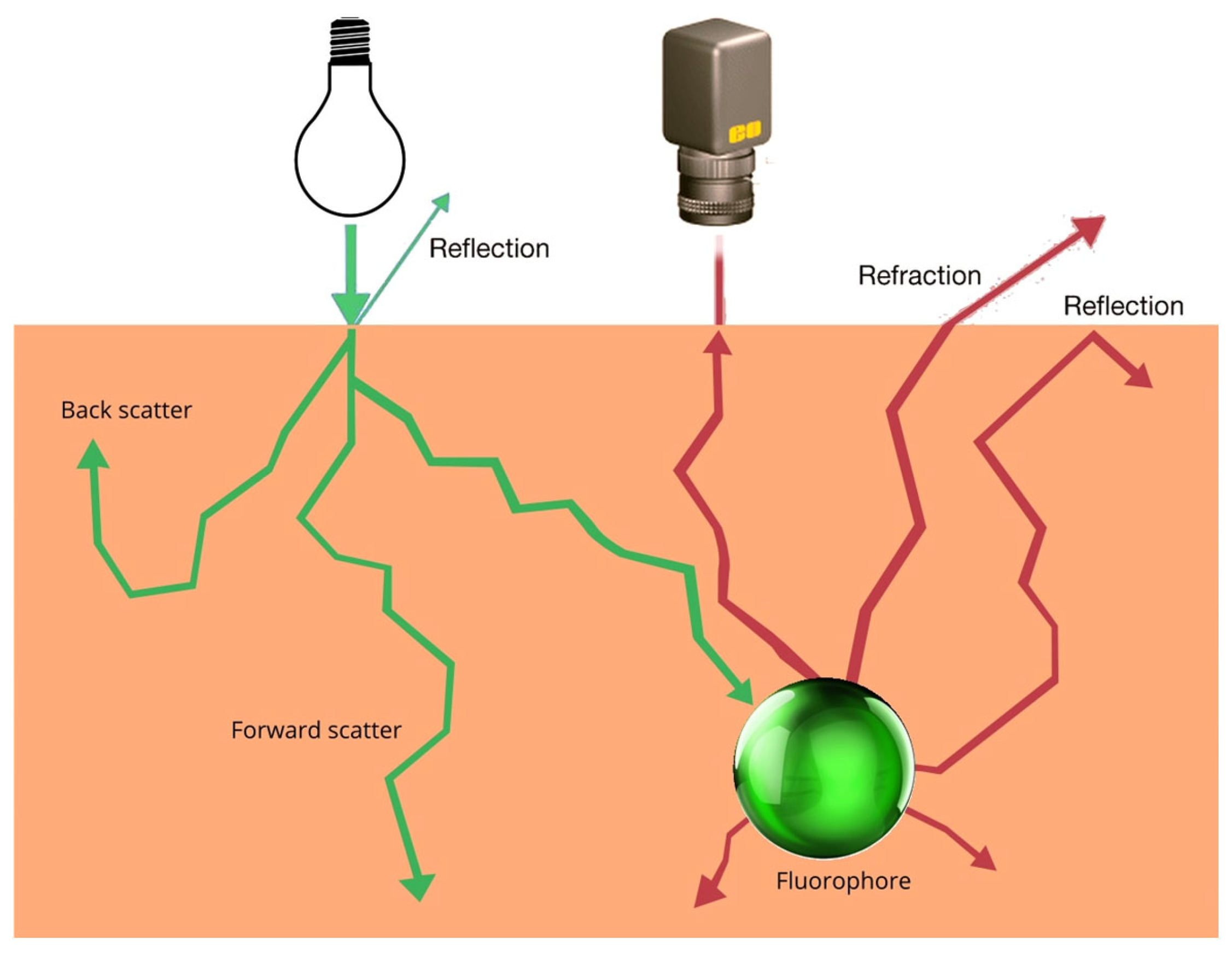
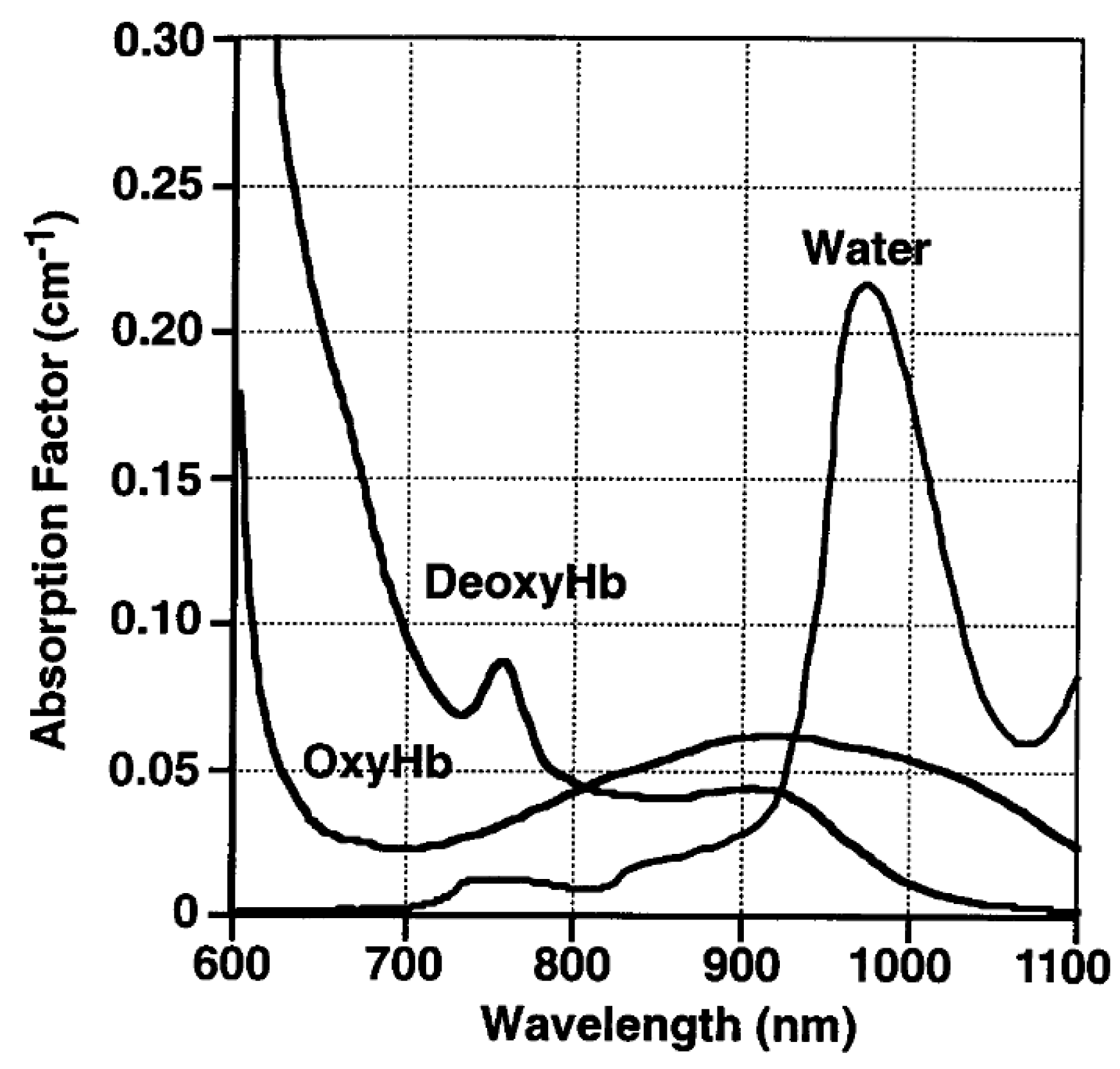
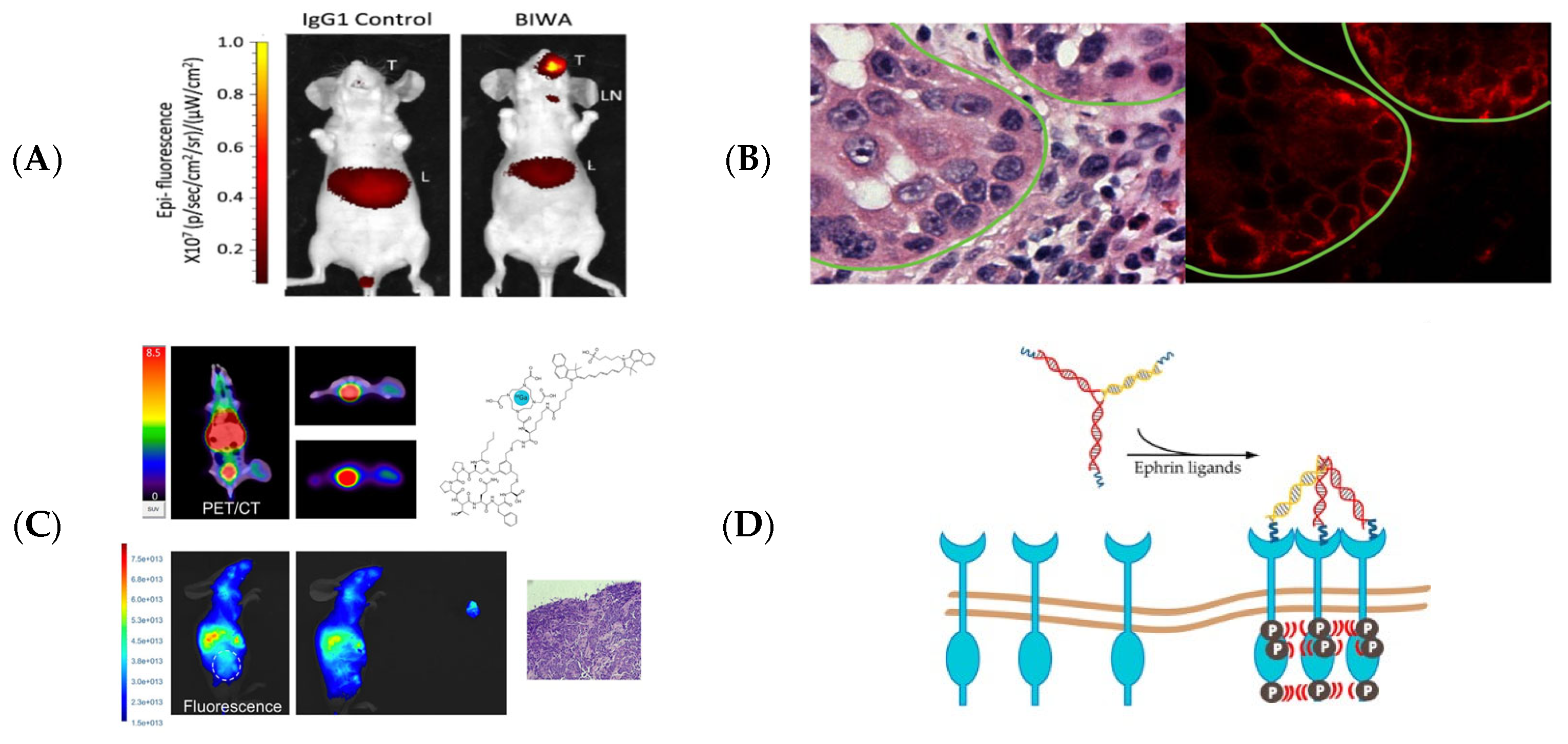

| Agent | Target | Fluorescence | Clinical Trials Registered Number (ClinicalTrials.gov (accessed on 1 July 2025), Clinicaltrialsregister.eu (accessed on 1 July 2025), chictr.org.cn (accessed on 1 July 2025)) | Dose (mg/kg or mg/m2; Cohort Distribution) or Total Mass Administered (mg) | Conjugation (Time Before Operation in h) |
|---|---|---|---|---|---|
| ONM-100 | ICG | NCT03735680 [41] | 1.2 mg/kg (7/13) 0.1 mg/kg (3/13) 0.5 mg/kg (2/13) 0.8 mg/kg (1/13) | 24 ± 8 | |
| 1 mg/kg (3/30) 3 mg/kg (3/30) 2 mg/kg (3/30) 1 mg/kg (6/30) 1 mg/kg (11/30) | 3 ± 2 3 ± 2 6 ± 3 16–80 24 ± 8 | ||||
| Cetuximab-IRDye800 | EGFR | IRDye800CW | NCT03733210 [42] | 2.5 mg/m2. (3/12); 25 mg/m2 (6/12); 62.5 mg/m2 (3/12) (NCT01987375) and 30 mg/m2 (14/14) | 24 ± 8 |
| NCT03134846 [43] | 10 mg/m2 (3/9) 25 mg/m2 (3/9) 50 mg/m2 (3/9) | 24 ± 8 | |||
| Pegsitacianine | ICG | NCT05576974 [44] | 1 mg/kg | 6–300 | |
| FG001 | uPAR | ICG | FG001-CT-003 [45] | 4 mg (4/16) 16 mg (8/16) 36 mg (4/16) | 12 ± 4 |
| PARPi-FL | PAR1 | NCT03085147 [46] | 15 mL 100 nM (3/12) 250 nM (3/12) 500 nM (3/12) 1000 nM (3/12) | 0.1 | |
| cMBP-ICG | c-MET | ICG | ChiCTR2200058058 [47] | 2.5 μM (5/10) 5.0 μM (5/10) | 16 ± 8 |
| Description | Clinical Trial Phase | Clinical Trials Registered Number (ClinicalTrials.gov (accessed on 1 July 2025) or clinicaltrialsregister.eu (accessed on 1 July 2025)) | Patients | State | Results |
|---|---|---|---|---|---|
| ASP-1929 Photoimmunotherapy (PIT) Study in Patients with Recurrent Head/Neck Cancer | Phase II | NCT05182866 [159] | 22 | Recruiting | No results published. |
| Panitumumab-IRDye800 and 89Zr-Panitumumab in Identifying Metastatic Lymph Nodes in Patients with Squamous Cell Head and Neck Cancer | Phase I | NCT03733210 [42] | 14 | Completed 2021 | From 19 lymph nodes that were histopathologic-labeled tumor-positive, 6 were false negatives using panitumumab-IRDye800: 30 mg administered intravenously (IV) and Zirconium Zr-89 panitumumab: 0.8 to 1.2 mCi (29 to 45 Mbq) administered intravenously (IV). Adverse events: Of 14 patients, 57 of the tumor-positive patients and 28.57% of the non-tumor patients were diagnosed with vascular hypertension after administration. |
| Fluorescence-guided Surgery Using cRGD-ZW800-1 in Oral Cancer | Phase II | NCT04191460 [160] | 28 | Recruiting | No results published. |
| Evaluating the Use of Dual Imaging Techniques for Detection of Disease in Patients with Head and Neck Cancer | Phase I | NCT05945875 [161] | 40 | Recruiting | No results published to date, but prior research showed in HNSCC patients infused with a molecularly targeted fluorescent tracer that endogenous expression of the target antigen can be used as a reference standard to detect LN metastasis. Additionally, the performance of the background in determining metastatic LN can be improved by utilizing patient-specific reference standards [162]. |
| Real-time Margin Assessment in Head and Neck Cancer | Phase II | NCT05499065 [163] | 20 | Finished | No results published to date, but University Medical Center Groningen already experimented with cetuximab-800CW tracer for the detection of EGFR, bevazicumab-800CW for VEGF, and others such as ONM-100 as pH-sensitive probes [164]. |
| Fluorescence-guided Surgery in Laryngeal- and Hypopharyngeal Cancer: a Feasibility Trial | Phase II | NCT05752149 [165] | 27 | Not Yet Recruiting | No results published to date. |
| A Study to Evaluate ONM-100, an Intraoperative Fluorescence Imaging Agent for the Detection of Cancer | Phase II | NCT03735680 [41] | 30 | Completed 2021 | 3 patients with administration of 3 mg/kg showed the best tumor-to-background ratio (TBR) of mean 4.022 compared to 1.098 for one patient with 1 mg/kg and 2.280 for 3 patients with 2 mg/kg [41]. 2/3 patients with 3 mg/kg experienced serious adverse events: cellulitis (33%), abscess neck (33%), and superficial vein thrombosis (33%). In all groups, a minimum of 66% had adverse events; in most groups, infusion-related reactions made up most of the other adverse events. |
| A Phase 2a, Single-dose, Open-label Study to Evaluate Diagnostic Performance and Safety of Pegsitacianine, an Intraoperative Fluorescence Imaging Agent for the Detection of Cancer, in Patients with Unknown Primary Head and Neck Cancer (ILLUMINATE STUDY) | Phase II | NCT05576974 [44] | 40 | Recruiting | No results published to date. |
| An open-label, non-randomized, single center, single dose, exploratory phase II trial of FG001 (an imaging agent) for localization of oral and oropharyngeal squamous cell carcinoma | Phase II | FG001-CT-003 [45] | 16 | Completed 2023 | 16 patients undergoing primary surgical resection were systemically administered 36 mg (n = 4), 16 mg (n = 8), or 4 mg (n = 4) of FG001 the evening prior to surgery. Intraoperatively, using a near-infrared imaging system, real-time optical imaging successfully identified all 16 tumors (sensitivity: 100%, mean TBR: 2.99, range: 2.02–3.95), and tumor specificity was confirmed by histology. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blosse, A.; Pirlich, M.; Dietz, A.; Möser, C.; Arnold, K.; Freitag, J.; Neumuth, T.; Smith, D.M.; Kubitschke, H.; Gaenzle, M. Fluorescence-Guided Surgery in Head and Neck Squamous Cell Carcinoma (HNSCC). Int. J. Transl. Med. 2025, 5, 40. https://doi.org/10.3390/ijtm5030040
Blosse A, Pirlich M, Dietz A, Möser C, Arnold K, Freitag J, Neumuth T, Smith DM, Kubitschke H, Gaenzle M. Fluorescence-Guided Surgery in Head and Neck Squamous Cell Carcinoma (HNSCC). International Journal of Translational Medicine. 2025; 5(3):40. https://doi.org/10.3390/ijtm5030040
Chicago/Turabian StyleBlosse, Albrecht, Markus Pirlich, Andreas Dietz, Christin Möser, Katrin Arnold, Jessica Freitag, Thomas Neumuth, David M. Smith, Hans Kubitschke, and Maximilian Gaenzle. 2025. "Fluorescence-Guided Surgery in Head and Neck Squamous Cell Carcinoma (HNSCC)" International Journal of Translational Medicine 5, no. 3: 40. https://doi.org/10.3390/ijtm5030040
APA StyleBlosse, A., Pirlich, M., Dietz, A., Möser, C., Arnold, K., Freitag, J., Neumuth, T., Smith, D. M., Kubitschke, H., & Gaenzle, M. (2025). Fluorescence-Guided Surgery in Head and Neck Squamous Cell Carcinoma (HNSCC). International Journal of Translational Medicine, 5(3), 40. https://doi.org/10.3390/ijtm5030040






