Circulating Extracellular Vesicle-Based Biomarkers: Advances, Clinical Implications and Challenges in Coronary Artery Disease
Abstract
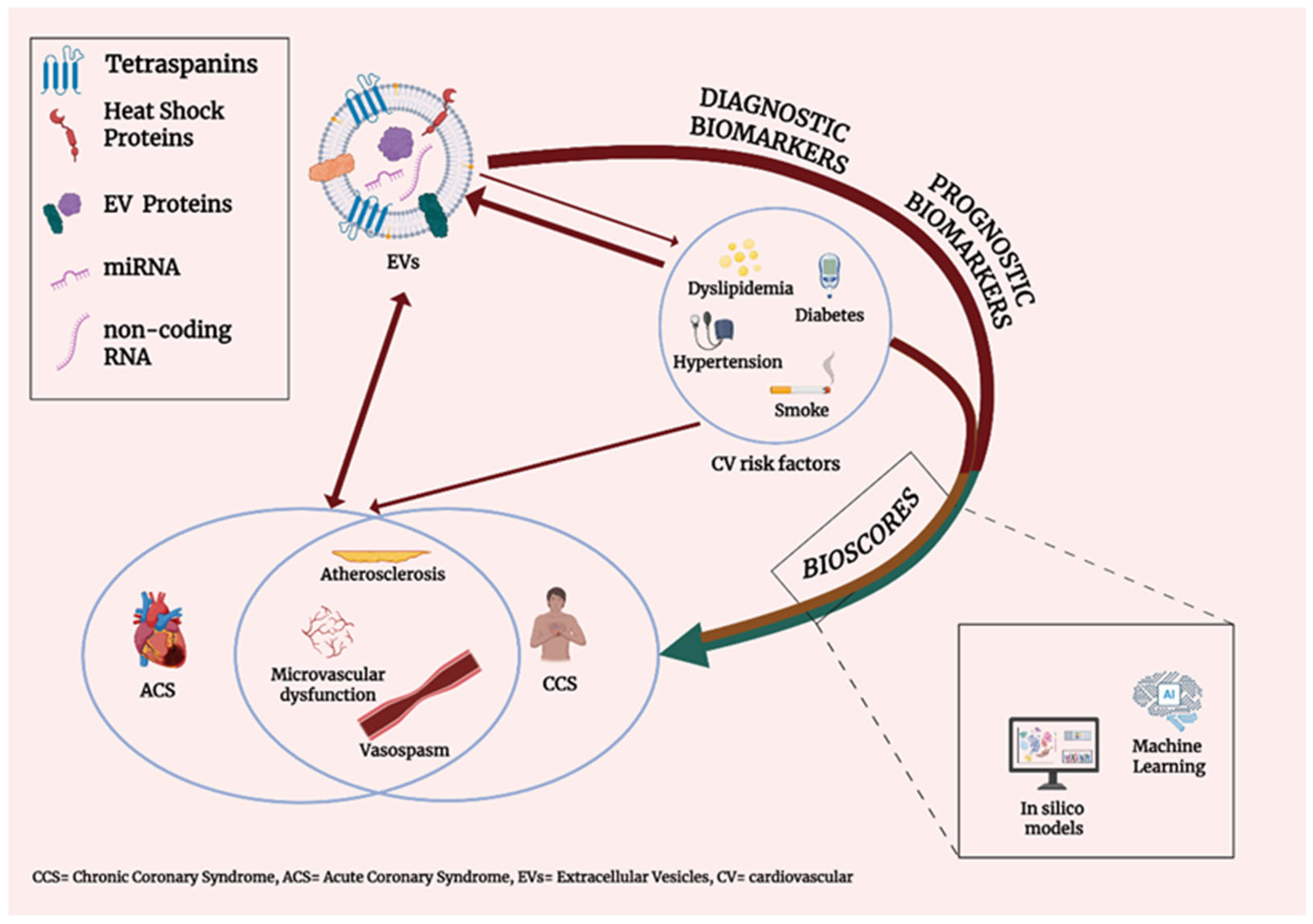
1. Introduction
1.1. Coronary Artery Disease (CAD)
1.2. Extracellular Vesicles
2. Multifaceted Role of EVs as a Tool for Biomarker Discovery in CAD
2.1. Short Historical Overview
2.2. Strategies for Identifying EV Cargo: Emerging Role of Computational Analyses
2.3. ML Models for Biomarker Discovery in CVD
2.4. EVs: An Additional Tool to Define Disease Subtypes
3. miRNAs and circRNAs for Identifying ACS Among CAD Patients
4. Acute Coronary Syndrome (ACS)
| EV Cargo or Epitope | Subjects | Blood Sample; EV Isolation Method | Pathological Conditions; Outcomes | Authors | |
|---|---|---|---|---|---|
| protein | GPIIb, VE-cadherin; ceruloplasmin, transthyretin, fibronectin, PLP1 | STEMI (n = 35), CCS (n = 32) | Venous, before CAG; Precipitation | STEMI; OHCA | [38] |
| epitope | CD62P, CD42a, CD41b, CD31, CD40 | STEMI (n = 30), SAP (n = 38), CTR (n = 30) Validation cohort (n = 80) | Venous; Centrifugation | MI; Diagnosis | [39] |
| miRNA | miR-208a | ACS (n = 500), CTR (n = 200) | Venous; Precipitation | ACS; Diagnosis, Prognosis | [42] |
| protein | CTRC, SRC, CCL17 | STEMI (n = 60), CTR (n = 22); Validation cohort: STEMI (n = 8), UA (n = 8), SAP (n = 8) | Venous; Acoustic trapping | MI; Diagnosis | [45] |
| epitope | N.A. | Stable plaque (n = 15), NSTEMI (n = 15), STEMI (n = 17), CTR (n = 17) | Arterial, during cardiac catheterization; Ultracentrifugation | MI, CAD; Prognosis | [49] |
| protein | ANGPTL6 | MI (n = 20), MI + DM (n = 20), CTR (n = 10) | Arterial (aortic sinus); Ultracentrifugation | MI, DM; Pathophysiology | [61] |
| circRNA | circ_0001535, circ_0000972, circ_0001558 | Sequencing group: MI (n = 15), CTR-NCCP (n = 15); First validation cohort: MI (n = 20), CTR-NCCP (n = 20); Second validation cohort: AMI (n = 85), CTR-NCCP (n = 48) | Venous, fasting; Membrane affinity-based | MI; Diagnosis | [70] |
| lncRNA | MALAT1 and LNC_000226 | MI (n = 90), CTR (n = 88) | Venous; Ultracentrifugation | MI; Diagnosis, Prognosis | [71] |
| miRNA | miR-1915-3p, miR-4507, miR-3656 | MI (n = 6), SCAD (n = 6). Validation cohort: MI (n = 30), SCAD (n = 30) | Venous, before heparin treatment; Ultracentrifugation | MI, SCAD; Differential diagnosis | [73] |
| miRNA | miR-133a-3p | Adult rats | N.A.; Ultracentrifugation | MI; Prognosis | [79] |
| miRNA | miR-186-5p | MI (n = 150), CTR (n = 50) | Venous; Precipitation | MI; Prognosis | [84] |
| miRNA | miR-4516, miR-203 | MI (n = 62), CTR (n = 31) | Venous, before PCI (AMI); Venous, fasting (CTR); Ultracentrifugation | MI; Diagnosis | [89] |
| miRNA | miR-9-5p | STEMI (n = 294) | Venous, ≤24 h after PCI; Ultracentrifugation | STEMI; PCI mortality, N1 polarization in I/R injury | [90] |
| miRNA | miR-9-5p, miR-127-3p | Sequencing group: CTO (n = 29), MI (n = 24) Validation cohort: CTO (n = 35), MI (n = 35), CTR (n = 10) | Arterial (coronary); Precipitation | MI; Differential diagnosis | [91] |
| miRNA | miR-208b-3p, miR-143-3p | ACS-SCD (n = 9), CTR (n = 9); Validation cohort: ACS-SCD (n = 30), CTR (n = 30) | Venous, after admission (ACS-SCD); Venous, fasting (CTR); Ultracentrifugation | ACS; SCD prediction | [94] |
| miRNA | miR-30a | Mice | N.A.; Ultracentrifugation | MI: Pathophysiology | [95] |
| miRNA | miR-152-5p, miR-3681-5p, miR-193a-5p, miR-193b-5p miR-345-5p, miR-125a-5p, miR-365a-3p, miR-4520-2-3p, miR-193b-3p and miR-5579-5p | Sequencing group: STEMI (n = 7), NSTEMI (n = 7), CTR (n = 10); | N.R.; Precipitation | MI (STEMI, NSTEMI); Correlation with echocardiography | [97] |
| lncRNA protein | NEAT1, miR-204, MMP-9 | STEMI (n = 47), UA (n = 24), CTR (n = 27) | Venous, before PCI; Membrane affinity-based | MI; Diagnosis | [98] |
| miRNA | Differentially expressed miRNAs (n = 18) | MI (n = 55), CAD (n = 26), CTRL (n = 37) | N.R.; Precipitation method | MI, SCAD; Differential diagnosis | [99] |
| miRNA | miR-301a-3p, miR-374a-5p, miR-423-5p | STEMI RLVR (n = 5), STEMI AVLR (n = 5) | Venous, after PCI; Precipitation | STEMI; ALVR | [100] |
| miRNA | Differentially expressed miRNAs (n = 77); miR-181a-3p | STEMI (n = 30), CTRL (n = 30); Validation cohort (STEMI n = 20, CTRL n = 24) | Venous; Ultracentrifugation | STEMI; ALVR | [101] |
| N.A. | N.A. | MI (n = 20), CTR (n = 20) | Venous, before CAG; Precipitation | MI; Physiopathology | [102] |
| epitope | CD29, CD41b, CD42a, CD41-CD61 (GP2IIb/IIIa) | STEMI (n = 42) | N.R.; Immuno-magnetic capture | STEMI; risk stratification | [103] |
| miRNA | miR-24-3p | STEMI (n = 8), CTR (n = 8); | Venous, before PCI; SEC | MI; Pathophysiology | [104] |
| miRNA | miR-486-5p | MI (n = 24), CTR (n = 13); Validation cohort: MI (n = 19), CTR (n = 10) | Cardiac (autopsies); Membrane affinity-based | MI; Atherosclerosis severity | [105] |
| epitope | CD9, CD81, CD90, CD144, CCR4, CCR6, CXCR3 | MI (n = 10), CTR (n = 8) | Venous; SEC | MI; Pathophysiology | [106] |
| lncRNA | LIPCAR | STEMI RLVR (n = 5), STEMI AVLR (n = 5) | Venous; Ultracentrifugation | MI; ALVR | [107] |
| protein | SERPIND1, MASP1, FCN2, AMBP, HLA-C | MI (n = 10), SAP (n = 10), CTR (n = 10) | Venous; SEC | MI; Diagnosis | [108] |
| protein | F13A1, TSPAN33, YWHAZ, ITGA2B, GP9, GP5, PPIA | Profiling group: STEMI (n = 5), NSTEMI (n = 5), UA (n = 5), CTR (n = 5); Validation: STEMI (n = 6), NSTEMI (n = 9), CTR (n = 6) | N.R; Precipitation | MI; Diagnosis | [109] |
5. Chronic Coronary Syndrome (CCS)
| EV Cargo or Epitope | Subjects | Blood Sample; EV Isolation Method | Pathological Conditions; Outcomes | Authors | |
|---|---|---|---|---|---|
| protein | Ubiquitinated adenosine A2A receptor | CAD (n = 14), CTR (n = 8) | Venous; Precipitation | CAD+/- hypermocysteinemia | [36] |
| miRNA | miR-140-3p | SCAD (n = 39), CTR (n = 39) | N.R.; Ultracentrifugation | CAD; Physiopathology | [37] |
| protein | Tenascin-C * | CAD (n = 40), CTR (n = 20) | N.R.; N.R.; | CAD; Diagnosis | [41] |
| lncRNA | lncRNA AC100865.1 | SCAD (n = 201), CTR (n = 187) | Arterial (radial); Ultracentrifugation | SCAD; Diagnosis | [43] |
| epitope | CD31+/Annexin V+ | SCAD (n = 200) | Arterial (femoral), before cardiac catheterization; N.R. | SCAD; Prognosis | [44] |
| protein | OST4, PKIG and RPL23 | Database: SCAD (n = 8), MI (n = 10); Validation cohort: CAD (n = 7) | N.R., <48 h admission and prior to CAG; Precipitation | SCAD, MI; Differential diagnosis | [47] |
| circRNA | circ_0001360, circ_0000038 | Sequencing group: CAD (n = 6), CTR (n = 32); Validation cohort: CAD (n = 10), CTR (n = 10) | Venous, fasting; Precipitation | CAD; Diagnosis | [48] |
| epitope | N.A. | CV risk factors (n = 268), Established cardiac disease/Organ damage (n = 138), Acute CV event (n = 8), CTR (n = 132) | Venous; Bead-based immunocapture assay | CV risk profiles; EVaging index | [53] |
| miRNA | let-7b-5p | hyperglycaemic CAD (n = 8), normoglycemic CAD(n = 8); Validation cohort: hyperglycaemic CAD(n = 75), normoglycemic CAD (n = 75) | Venous fasting; Precipitation | CHD with hyperglycaemia | [59] |
| circRNA | circ_0001785 | CAD (n = 31), CTR (n = 24) | N.R.; Ultracentrifugation | CAD; Physiopathology | [66] |
| circRNA | circ_0005540 | Profiling and internal validation: CAD (n = 61), CTR (n = 38); External validation: CAD (n = 47), CTR (n = 51) | N.R.; Membrane affinity-based | CAD; Diagnosis | [67] |
| circRNA | SOCS2-AS1 | Sequencing and training group: CAD (n = 27), CTR (n = 27) Validation cohort: CAD (n = 84), mCAS (n = 48), CTR (n = 41) | Venous, fasting+before CAG; Precipitation | CAD; Diagnosis, Prognosis | [68] |
| circRNA | circ_0075269, circ_0000284 | Sequencing group: CCS (n = 15), NCCP (n = 15); Validation cohorts: CCS (n = 20, 100), NCCP (n = 20, 48); | Venous, after admission; Membrane affinity-based | CCS; Differential diagnosis | [69] |
| miRNA | miR-21-5p, miR-21-3p | CAD (n = 135), CTR (n = 150) | Venous; Precipitation | CAD; Diagnosis | [96] |
| miRNA | miR-942-5p, miR-149-5p, miR-32-5p | SCAD (n = 20), CTR (n = 20) | Venous, fasting; Precipitation | SCAD; Diagnosis | [111] |
| miRNA | let-7c-5p, miR-335-3p, miR-652-3p | SCAD (n = 39), CTR (n = 39) | Venous, fasting; Ultracentrifugation | CAD; Prognosis | [121] |
| circRNA | ENST00000424615.2, ENST00000560769.1 | Sequencing group: CCS (n = 15), CTR (n = 15); Validation cohorts: CCS (n = 20, 100), CTR (n = 20), 48; | N.R.; Membrane affinity-based | CCS; CAD severity | [122] |
| miRNA | miR-382-3p | severe CAD, 3-vessel (n = 129), CTR (n = 114) | Arterial (coronary); Precipitation | CAD; Prognosis | [123] |
| circRNA; miRNA | circSCMH1/miR-874 | SCAD (n = 300), ACS (n = 300), CTR (n = 101) | Venous, fasting; Ultracentrifugation | SCAD, AMI; Carotid plaque stability | [124] |
| miRNA | miR-30e, miR-92a | CAD (n = 42), CTR (n = 42) | Venous; Precipitation | CAD; Diagnosis | [131] |
| protein | SC1, CD14, SG1, PLG, CC, SF2 | CAD (n = 187), CTR (n = 257) | Venous, before MPI; Magnetic bead-based capture | Stress induced ischemia; | [132] |
| epitope; protein | CD42a+, CD62E+; AXL, CD163, IGFBP7, NEMO, resistin, BAFF, perlecan | CAD, prior MI (n = 220) | Venous, fasting; Acoustic trapping | CAD, CFR; Prognosis | [133] |
| miRNA | miR-382-3p, miR-432-5p, miR-200a-3p, miR-3613-3p; miR-125a-5p, miR-185-5p, miR-151a-3p, miR328-3p | Three-vessel CAD (n = 214), no CAD (n = 140) | Arterial (coronary); Precipitation | CAD; Prognosis | [134] |
| miRNA | miR-19a-3p, miR-18a-5p, miR-133a-3p, miR-155-5p, and miR-210-3p | Sequencing group: IHD-DM (n = 6), CTR (n = 6); Validation cohort: IHD-DM (n = 26), CTR (n = 14) | Venous, fasting; Precipitation | IHD in DM; Diagnosis | [135] |
| epitope | CD41+/CD61+, CD142+, CD31+ | CAD (n = 26), CTR (n = 14) | Venous; N.A. | CAD; Diagnosis | [136] |
| miRNA | miR-16-2-3p | DM (n = 3), DM-CMD (n = 3), DM-CAD (n = 3) | Venous; Ultracentrifugation | CAD; Differential diagnosis | [137] |
| miRNA | miR-382-3p, miR-432-5p, miR-200a-3p, mi-3613-3p; miR-125a-5p, miR-185-5p, miR-151a-3p, miR-328-3p | CAD (n = 115) | Venous; Precipitation | CAD; Myocardial perfusion | [138] |
| epitope | CD144+ | INOCA-CMD (n = 34), INOCA-VSA (n = 15), INOCA-mixed endotype (n = 24), NCCP (n = 23) | Venous, before CAG; N.A. | INOCA; Classification | [139] |
| APL membrane | Phosphatidylethanolamine, phosphatidylserine | CAD (n = 19), ACS (n = 24), CTR (n = 24), risk-factor CTR (n = 23) | Venous; SEC | CAD, ACS; thrombosis | [140] |
| APL membrane | Phosphatidylthreonine | CAD (n = 19), ACS (n = 24), CTR (n = 24), risk-factor CTR (n = 23) | Venous; SEC | CAD, ACS; thrombosis | [141] |
6. Ischemia and Non-Obstructive Coronary Artery Disease (INOCA)
7. Post-AMI and Cardiac Remodeling
8. Proteomics
9. Critical Appraisal and Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bernáth-Nagy, D.; Kalinyaprak, M.S.; Giannitsis, E.; Ábrahám, P.; Leuschner, F.; Frey, N.; Krohn, J.B. Circulating extracellular vesicles as biomarkers in the diagnosis, prognosis and therapy of cardiovascular diseases. Front. Cardiovasc. Med. 2024, 11, 1425159. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yuan, Y.; Tang, J. Extracellular Vesicles as Diagnostic Metrics for Cardiovascular Disease: Where We Are and How to Achieve in Clinics. J. Cardiovasc. Transl. Res. 2025. [Google Scholar] [CrossRef] [PubMed]
- Aronson, J.K.; Ferner, R.E. Biomarkers—A General Review. Curr. Protoc. Pharmacol. 2017, 76, 9–23. [Google Scholar] [CrossRef]
- Hill, A.B. Section of Occupational Medicine the Environment and Disease: Association or Causation? Proc. R. Soc. Med. 1965, 58, 295–300. [Google Scholar]
- GBD 2021 Adult BMI Collaborators. Global, regional, and national prevalence of adult overweight and obesity, 1990–2021, with forecasts to 2050: A forecasting study for the Global Burden of Disease Study 2021. Lancet 2025, 405, 813–838. [Google Scholar] [CrossRef]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for the management of chronic coronary syndromes. Eur. Heart J. 2024, 45, 3415–3537. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Marzilli, M.; Merz, C.N.B.; Boden, W.E.; Bonow, R.O.; Capozza, P.G.; Chilian, W.M.; DeMaria, A.N.; Guarini, G.; Huqi, A.; Morrone, D.; et al. Obstructive Coronary Atherosclerosis and Ischemic Heart Disease: An Elusive Link! J. Am. Coll. Cardiol. 2012, 60, 951–956. [Google Scholar] [CrossRef]
- Attiq, A.; Afzal, S.; Ahmad, W.; Kandeel, M. Hegemony of inflammation in atherosclerosis and coronary artery disease. Eur. J. Pharmacol. 2024, 966, 176338. [Google Scholar] [CrossRef]
- Pepine, C.J. ANOCA/INOCA/MINOCA: Open artery ischemia. Am. Heart J. Plus Cardiol. Res. Pract. 2023, 26, 100260. [Google Scholar] [CrossRef]
- Xie, Y.; Jiang, J.; Wang, J. Management of Chronic Coronary Syndrome: 2024 Update. JACC Asia 2025, 5, 327–331. [Google Scholar] [CrossRef]
- Bass, T.A.; Abbott, J.D.; Mahmud, E.; Parikh, S.A.; Aboulhosn, J.; Ashwath, M.L.; Baranowski, B.; Bergersen, L.; Chaudry, H.I.; Coylewright, M.; et al. 2023 ACC/AHA/SCAI Advanced Training Statement on Interventional Cardiology (Coronary, Peripheral Vascular, and Structural Heart Interventions): A Report of the ACC Competency Management Committee. J. Am. Coll. Cardiol. 2023, 81, 14. [Google Scholar] [CrossRef]
- Zdanyte, M.; Wrazidlo, R.W.; Kaltenbach, S.; Groga-Bada, P.; Gawaz, M.; Geisler, T.; Rath, D. Predicting 1-, 3- and 5-year outcomes in patients with coronary artery disease: A comparison of available risk assessment scores. Atherosclerosis 2021, 318, 1–7. [Google Scholar] [CrossRef]
- Katsioupa, M.; Kourampi, I.; Oikonomou, E.; Tsigkou, V.; Theofilis, P.; Charalambous, G.; Marinos, G.; Gialamas, I.; Zisimos, K.; Anastasiou, A.; et al. Novel Biomarkers and Their Role in the Diagnosis and Prognosis of Acute Coronary Syndrome. Life 2023, 13, 1992. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, Y.; Li, Z.; Chen, S.; Fang, F.; Cai, J. Potential roles of microRNAs and long noncoding RNAs as diagnostic, prognostic and therapeutic biomarkers in coronary artery disease. Int. J. Cardiol. 2023, 384, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, A.N.; Pletsch, M.; Chorbajian, A.; Zitser, D.; Rai, V. Biomarkers to monitor the prognosis, disease severity, and treatment efficacy in coronary artery disease. Expert Rev. Cardiovasc. Ther. 2023, 21, 675–692. [Google Scholar] [CrossRef] [PubMed]
- Røsand, Ø.; Høydal, M.A. Cardiac exosomes in ischemic heart disease—A narrative review. Diagnostics 2021, 11, 269. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Vizio, D.D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Van Niel, G.; Carter, D.R.F.; Clayton, A.; Lambert, D.W.; Raposo, G.; Vader, P. Challenges and directions in studying cell–cell communication by extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2022, 23, 369–382. [Google Scholar] [CrossRef]
- Han, C.; Yang, J.; Sun, J.; Qin, G. Extracellular vesicles in cardiovascular disease: Biological functions and therapeutic implications. Pharmacol. Ther. 2022, 233, 108025. [Google Scholar] [CrossRef]
- Stam, J.; Bartel, S.; Bischoff, R.; Wolters, J.C. Isolation of extracellular vesicles with combined enrichment methods. J. Chromatogr. B 2021, 1169, 122604. [Google Scholar] [CrossRef]
- Altıntaş, Ö.; Saylan, Y. Exploring the Versatility of Exosomes: A Review on Isolation, Characterization, Detection Methods, and Diverse Applications. Anal. Chem. 2023, 95, 16029–16048. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Zieren, R.C.; Horie, K.; Kim, C.J.; Mallick, E.; Jing, Y.; Feng, M.; Kuczler, M.D.; Green, J.; Amend, S.R.; et al. Comprehensive evaluation of methods for small extracellular vesicles separation from human plasma, urine and cell culture medium. J. Extracell. Vesicles 2020, 10, e12044. [Google Scholar] [CrossRef] [PubMed]
- Sidhom, K.; Obi, P.O.; Saleem, A. A review of exosomal isolation methods: Is size exclusion chromatography the best option? Int. J. Mol. Sci. 2020, 21, 6466. [Google Scholar] [CrossRef] [PubMed]
- Konoshenko, M.Y.; Lekchnov, E.A.; Bryzgunova, O.E.; Kiseleva, E.; Pyshnaya, I.A.; Laktionov, P.P. Isolation of extracellular vesicles from biological fluids via the aggregation–precipitation approach for downstream mirnas detection. Diagnostics 2021, 11, 384. [Google Scholar] [CrossRef]
- Tiwari, S.; Kumar, V.; Randhawa, S.; Verma, S.K. Preparation and characterization of extracellular vesicles. Am. J. Reprod. Immunol. 2021, 85, e13367. [Google Scholar] [CrossRef]
- Veerman, R.E.; Teeuwen, L.; Czarnewski, P.; Akpinar, G.G.; Sandberg, A.S.; Cao, X.; Pernemalm, M.; Orre, L.M.; Gabrielsson, S.; Eldh, M. Molecular evaluation of five different isolation methods for extracellular vesicles reveals different clinical applicability and subcellular origin. J. Extracell. Vesicles 2021, 10, e12128. [Google Scholar] [CrossRef]
- Comfort, N.; Cai, K.; Bloomquist, T.R.; Strait, M.D.; Ferrante, A.W.; Baccarelli, A.A. Nanoparticle tracking analysis for the quantification and size determination of extracellular vesicles. J. Vis. Exp. 2021, e62447. [Google Scholar] [CrossRef]
- Kurian, T.K.; Banik, S.; Gopal, D.; Chakrabarti, S.; Mazumder, N. Elucidating Methods for Isolation and Quantification of Exosomes: A Review. Mol. Biotechnol. 2021, 63, 249–266. [Google Scholar] [CrossRef]
- Welsh, J.A.; Arkesteijn, G.J.A.; Bremer, M.; Cimorelli, M.; Dignat-George, F.; Giebel, B.; Görgens, A.; Hendrix, A.; Kuiper, M.; Lacroix, R.; et al. A compendium of single extracellular vesicle flow cytometry. J. Extracell. Vesicles 2023, 12, e12299. [Google Scholar] [CrossRef]
- Sluijter, J.P.G.; Davidson, S.M.; Boulanger, C.M.; Buzás, E.I.; Kleijn, D.P.V.D.; Engel, F.B.; Giricz, Z.; Hausenloy, D.J.; Kishore, R.; Lecour, S.; et al. Extracellular vesicles in diagnostics and therapy of the ischaemic heart: Position Paper from the Working Group on Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc. Res. 2018, 114, 19–34. [Google Scholar] [CrossRef]
- Sahoo, S.; Adamiak, M.; Mathiyalagan, P.; Kenneweg, F.; Kafert-Kasting, S.; Thum, T. Therapeutic and Diagnostic Translation of Extracellular Vesicles in Cardiovascular Diseases: Roadmap to the Clinic. Circulation 2021, 143, 1426–1449. [Google Scholar] [CrossRef]
- Saint-Pol, J.; Culot, M. Minimum information for studies of extracellular vesicles (MISEV) as toolbox for rigorous, reproducible and homogeneous studies on extracellular vesicles. Toxicol. Vitr. 2025, 106, 106049. [Google Scholar] [CrossRef]
- Zheng, D.; Huo, M.; Li, B.; Wang, W.; Piao, H.; Wang, Y.; Zhu, Z.; Li, D.; Wang, T.; Liu, K. The Role of Exosomes and Exosomal MicroRNA in Cardiovascular Disease. Front. Cell Dev. Biol. 2021, 8, 616161. [Google Scholar] [CrossRef] [PubMed]
- Femminò, S.; Penna, C.; Margarita, S.; Comità, S.; Brizzi, M.F.; Pagliaro, P. Extracellular vesicles and cardiovascular system: Biomarkers and Cardioprotective Effectors. Vasc. Pharmacol. 2020, 135, 106790. [Google Scholar] [CrossRef] [PubMed]
- Ruf, J.; Vairo, D.; Paganelli, F.; Guieu, R. Extracellular vesicles with ubiquitinated adenosine A2A receptor in plasma of patients with coronary artery disease. J. Cell. Mol. Med. 2019, 23, 6805–6811. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Kang, X.; Su, Y.; Wang, J.; Cui, X.; Bian, Y.; Wu, C. Plasma exosomes from patients with coronary artery disease promote atherosclerosis via impairing vascular endothelial junctions. Sci. Rep. 2024, 14, 29813. [Google Scholar] [CrossRef]
- Zarà, M.; Campodonico, J.; Cosentino, N.; Biondi, M.L.; Amadio, P.; Milanesi, G.; Assanelli, E.; Cerri, S.; Biggiogera, M.; Sandrini, L.; et al. Plasma exosome profile in st-elevation myocardial infarction patients with and without out-of-hospital cardiac arrest. Int. J. Mol. Sci. 2021, 22, 8065. [Google Scholar] [CrossRef]
- Burrello, J.; Bolis, S.; Balbi, C.; Burrello, A.; Provasi, E.; Caporali, E.; Gauthier, L.G.; Peirone, A.; D’Ascenzo, F.; Monticone, S.; et al. An extracellular vesicle epitope profile is associated with acute myocardial infarction. J. Cell. Mol. Med. 2020, 24, 9945–9957. [Google Scholar] [CrossRef]
- Li, C.; Ni, Y.Q.; Xu, H.; Xiang, Q.Y.; Zhao, Y.; Zhan, J.K.; He, J.Y.; Li, S.; Liu, Y.S. Roles and mechanisms of exosomal non-coding RNAs in human health and diseases. Signal Transduct. Target. Ther. 2021, 6, 383. [Google Scholar] [CrossRef]
- Gholipour, A.; Shakerian, F.; Zahedmehr, A.; Oveisee, M.; Maleki, M.; Mowla, S.J.; Malakootian, M. Tenascin-C as a noninvasive biomarker of coronary artery disease. Mol. Biol. Rep. 2022, 49, 9267–9273. [Google Scholar] [CrossRef]
- Bi, S.; Wang, C.; Jin, Y.; Lv, Z.; Xing, X.; Lu, Q. Correlation between serum exosome derived miR-208a and acute coronary syndrome. Int. J. Clin. Exp. Med. 2015, 8, 4275–4280. [Google Scholar]
- Yang, Y.; Cai, Y.; Wu, G.; Chen, X.; Liu, Y.; Wang, X.; Yu, J.; Li, C.; Chen, X.; Jose, P.A.; et al. Plasma long non-coding RNA, CoroMarker, a novel biomarker for diagnosis of coronary artery disease. Clin. Sci. 2015, 129, 675–685. [Google Scholar] [CrossRef]
- Sinning, J.M.; Losch, J.; Walenta, K.; Böhm, M.; Nickenig, G.; Werner, N. Circulating CD31 +/Annexin V + microparticles correlate with cardiovascular outcomes. Eur. Heart J. 2011, 32, 2034–2041. [Google Scholar] [CrossRef] [PubMed]
- Gidlöf, O.; Evander, M.; Rezeli, M.; Marko-Varga, G.; Laurell, T.; Erlinge, D. Proteomic profiling of extracellular vesicles reveals additional diagnostic biomarkers for myocardial infarction compared to plasma alone. Sci. Rep. 2019, 9, 8991. [Google Scholar] [CrossRef] [PubMed]
- Barh, D.; Chaitankar, V.; Yiannakopoulou, E.C.; Salawu, E.O.; Chowbina, S.; Ghosh, P.; Azevedo, V. In Silico Models: From Simple Networks to Complex Diseases. In Animal Biotechnology: Models in Discovery and Translation; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 385–404. ISBN 978-0-12-416002-6. [Google Scholar] [CrossRef]
- Jin, X.; Xu, W.; Wu, Q.; Huang, C.; Song, Y.; Lian, J. Detecting early-warning biomarkers associated with heart-exosome genetic-signature for acute myocardial infarction: A source-tracking study of exosome. J. Cell. Mol. Med. 2024, 28, e18334. [Google Scholar] [CrossRef]
- Zhang, W.; Cui, J.; Li, L.; Zhu, T.; Guo, Z. Identification of Plasma Exosomes hsa_circ_0001360 and hsa_circ_0000038 as Key Biomarkers of Coronary Heart Disease. Cardiol. Res. Pract. 2024, 2024, 5557143. [Google Scholar] [CrossRef]
- Huang, X.; Liu, B.; Guo, S.; Guo, W.; Liao, K.; Hu, G.; Shi, W.; Kuss, M.; Duryee, M.J.; Anderson, D.R.; et al. SERS spectroscopy with machine learning to analyze human plasma derived sEVs for coronary artery disease diagnosis and prognosis. Bioeng. Transl. Med. 2023, 8, e10420. [Google Scholar] [CrossRef]
- Babu, M.; Snyder, M. Multi-omics profiling for health. Mol. Cell. Proteom. 2023, 22, 100561. [Google Scholar] [CrossRef]
- Sethi, Y.; Patel, N.; Kaka, N.; Kaiwan, O.; Kar, J.; Moinuddin, A.; Goel, A.; Chopra, H.; Cavalu, S. Precision Medicine and the future of Cardiovascular Diseases: A Clinically Oriented Comprehensive Review. J. Clin. Med. 2023, 12, 1799. [Google Scholar] [CrossRef]
- Olawade, D.B.; Aderinto, N.; Olatunji, G.; Kokori, E.; David-Olawade, A.C.; Hadi, M. Advancements and applications of Artificial Intelligence in cardiology: Current trends and future prospects. J. Med. Surg. Public Health 2024, 3, 100109. [Google Scholar] [CrossRef]
- Burrello, J.; Goi, J.; Burrello, A.; Vacchi, E.; Rendon-Angel, A.; Lazzarini, E.; Bianco, G.; Limongelli, V.; Vassalli, G.; Cereda, C.W.; et al. Age- and sex-related variations in extracellular vesicle profiling for the assessment of cardiovascular risk: The EVaging index. Npj Aging 2024, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Cavallari, C.; Figliolini, F.; Tapparo, M.; Cedrino, M.; Trevisan, A.; Positello, L.; Rispoli, P.; Solini, A.; Migliaretti, G.; Camussi, G.; et al. miR-130a and Tgfβ Content in Extracellular Vesicles Derived from the Serum of Subjects at High Cardiovascular Risk Predicts their In-Vivo Angiogenic Potential. Sci. Rep. 2020, 10, 706. [Google Scholar] [CrossRef] [PubMed]
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 2018, 17, 83. [Google Scholar] [CrossRef]
- Marx, N.; Federici, M.; Schütt, K.; Müller-Wieland, D.; Ajjan, R.A.; Antunes, M.J.; Christodorescu, R.M.; Crawford, C.; Angelantonio, E.D.; Eliasson, B.; et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur. Heart J. 2023, 44, 4043–4140. [Google Scholar] [CrossRef]
- Bartnik, M.; Rydén, L.; Ferrari, R.; Malmberg, K.; Pyörälä, K.; Simoons, M.; Standl, E.; Soler-Soler, J.; Öhrvik, J.; Manini, M.; et al. The prevalence of abnormal glucose regulation in patients with coronary artery disease across Europe: The Euro Heart Survey on diabetes and the heart. Eur. Heart J. 2004, 25, 1880–1890. [Google Scholar] [CrossRef]
- Zellweger, M.J. Prognostic significance of silent coronary artery disease in type 2 diabetes. Herz 2006, 31, 240–245. [Google Scholar] [CrossRef]
- Han, S.; Fang, J.; Yu, L.; Li, B.; Hu, Y.; Chen, R.; Li, C.; Zhao, C.; Li, J.; Wang, Y.; et al. Serum-derived exosomal hsa-let-7b-5p as a biomarker for predicting the severity of coronary stenosis in patients with coronary heart disease and hyperglycemia. Mol. Med. Rep. 2023, 28, 203. [Google Scholar] [CrossRef]
- Togliatto, G.; Dentelli, P.; Rosso, A.; Lombardo, G.; Gili, M.; Gallo, S.; Gai, C.; Solini, A.; Camussi, G.; Brizzi, M.F. PDGF-BB carried by endothelial Cell-derived extracellular vesicles reduces vascular smooth muscle cell apoptosis in diabetes. Diabetes 2018, 67, 704–716. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, Y.; Zhu, P.; Jia, X.; Wang, C.; Zhang, Q.; Li, H.; Wang, J.; Hou, Y. Differential Proteomic Profiles of Coronary Serum Exosomes in Acute Myocardial Infarction Patients with or Without Diabetes Mellitus: ANGPTL6 Accelerates Regeneration of Endothelial Cells Treated with Rapamycin via MAPK Pathways. Cardiovasc. Drugs Ther. 2024, 38, 13–29. [Google Scholar] [CrossRef]
- D’Ascenzo, F.; Femminò, S.; Ravera, F.; Angelini, F.; Caccioppo, A.; Franchin, L.; Grosso, A.; Comità, S.; Cavallari, C.; Penna, C.; et al. Extracellular vesicles from patients with Acute Coronary Syndrome impact on ischemia-reperfusion injury. Pharmacol. Res. 2021, 170, 105715. [Google Scholar] [CrossRef]
- Femminò, S.; D’ascenzo, F.; Ravera, F.; Comità, S.; Angelini, F.; Caccioppo, A.; Franchin, L.; Grosso, A.; Thairi, C.; Venturelli, E.; et al. Percutaneous coronary intervention (Pci) reprograms circulating extracellular vesicles from acs patients impairing their cardio-protective properties. Int. J. Mol. Sci. 2021, 22, 10270. [Google Scholar] [CrossRef]
- Ward, Z.; Pearson, J.; Schmeier, S.; Cameron, V.; Pilbrow, A. Insights into circular RNAs: Their biogenesis, detection, and emerging role in cardiovascular disease. RNA Biol. 2021, 18, 2055–2072. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Ma, J.; Sun, T.; Zhou, Q.; Wang, W.; Wang, G.; Wu, P.; Wang, H.; Jiang, L.; et al. Exosomal circRNAs: Biogenesis, effect and application in human diseases. Mol. Cancer 2019, 18, 116. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Dang, X.; Liu, D.; Wang, N.; Li, M.; Han, J.; Zhao, J.; Wang, Y.; Huang, M.; Yang, Y.; et al. Exosome-derived circ_0001785 delays atherogenesis through the ceRNA network mechanism of miR-513a-5p/TGFBR3. J. Nanobiotechnol. 2023, 21, 362. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.P.; Pan, Y.H.; Cai, M.Y.; Cen, J.M.; Chen, C.; Zheng, L.; Liu, X.; Xiong, X.D. Plasma-Derived Exosomal Circular RNA hsa_circ_0005540 as a Novel Diagnostic Biomarker for Coronary Artery Disease. Dis. Markers 2020, 2020, 1341918. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Zhang, L.; Lian, X.; Zhu, T.; Zhang, Y.; Gu, N. Circulating Exosomal SOCS2-AS1 Acts as a Novel Biomarker in Predicting the Diagnosis of Coronary Artery Disease. BioMed Res. Int. 2020, 2020, 9182091. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, M.; Han, R.; Yu, Z.; Yuan, W.; Xie, B.; Zhang, Y.; Zhong, J.; Wang, L.; Wang, L.; et al. Circulating Exosomal CircRNAs as Diagnostic Biomarkers for Chronic Coronary Syndrome. Metabolites 2023, 13, 1066. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Yuan, W.; Han, R.; Zhong, J.; Yang, X.; Zheng, M.; Xie, B. Exosomal CircRNAs in Circulation Serve as Diagnostic Biomarkers for Acute Myocardial Infarction. Front. Biosci. Landmark 2024, 29, 149. [Google Scholar] [CrossRef]
- Gu, X.; Hou, J.; Weng, R.; Rao, J.; Liu, S. The Diagnosis and Prognosis Value of Circulating Exosomal lncRNA MALAT1 and LNC_000226 in Patients With Acute Myocardial Infarction: An Observational Study. Immun. Inflamm. Dis. 2024, 12, e70088. [Google Scholar] [CrossRef]
- Moreira-Costa, L.; Barros, A.S.; Lourenço, A.P.; Leite-Moreira, A.F.; Nogueira-Ferreira, R.; Thongboonkerd, V.; Vitorino, R. Exosome-derived mediators as potential biomarkers for cardiovascular diseases: A network approach. Proteomes 2021, 9, 8. [Google Scholar] [CrossRef]
- Su, J.; Li, J.; Yu, Q.; Wang, J.; Li, X.; Yang, J.; Xu, J.; Liu, Y.; Xu, Z.; Ji, L.; et al. Exosomal miRNAs as potential biomarkers for acute myocardial infarction. IUBMB Life 2020, 72, 384–400. [Google Scholar] [CrossRef]
- Li, H.; Li, Z.; Fu, Q.; Fu, S.; Xiang, T. Exploring the landscape of exosomes in heart failure: A bibliometric analysis. Int. J. Surg. 2025, 111, 3356–3372. [Google Scholar] [CrossRef] [PubMed]
- Alles, J.; Fehlmann, T.; Fischer, U.; Backes, C.; Galata, V.; Minet, M.; Hart, M.; Abu-Halima, M.; Grässer, F.A.; Lenhof, H.P.; et al. An estimate of the total number of true human miRNAs. Nucleic Acids Res. 2019, 47, 3353–3364. [Google Scholar] [CrossRef] [PubMed]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. MiRBase: From microRNA sequences to function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Sun, L.; Zhao, P.; Liu, Y.; Zhang, J.; Zhang, Y.; Hong, Y.; Zhu, Y.; Lu, Y.; Zhao, W.; et al. Macrophage migration inhibitory factor facilitates the therapeutic efficacy of mesenchymal stem cells derived exosomes in acute myocardial infarction through upregulating miR-133a-3p. J. Nanobiotechnol. 2021, 19, 61. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Wang, R.; Chen, W.; Cao, Y.D.; Li, L.P.; Chen, X. miR-133a-3p attenuates cardiomyocyte hypertrophy through inhibiting pyroptosis activation by targeting IKKε. Acta Histochem. 2021, 123, 151653. [Google Scholar] [CrossRef]
- Yang, N.; Hou, Y.B.; Cui, T.H.; Yu, J.M.; He, S.F.; Zhu, H.J. Ischemic-Preconditioning Induced Serum Exosomal miR-133a-3p Improved Post-Myocardial Infarction Repair via Targeting LTBP1 and PPP2CA. Int. J. Nanomed. 2024, 19, 9035–9053. [Google Scholar] [CrossRef]
- Liu, N.; Zhen, Z.; Xiong, X.; Xue, Y. Aerobic exercise protects MI heart through miR-133a-3p downregulation of connective tissue growth factor. PLoS ONE 2024, 19, 0296430. [Google Scholar] [CrossRef]
- Kuzmin, V.S.; Ivanova, A.D.; Filatova, T.S.; Pustovit, K.B.; Kobylina, A.A.; Atkinson, A.J.; Petkova, M.; Voronkov, Y.I.; Abramochkin, D.V.; Dobrzynski, H. Micro-RNA 133a-3p induces repolarization abnormalities in atrial myocardium and modulates ventricular electrophysiology affecting ICa,L and Ito currents. Eur. J. Pharmacol. 2021, 908, 174369. [Google Scholar] [CrossRef]
- Zhu, S.; Fang, Z. MicroRNA-127-3p Inhibits Cardiomyocyte Inflammation and Apoptosis after Acute Myocardial Infarction via Targeting CDKN3. Int. Heart J. 2023, 64, 1133–1139. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Y.; Wang, C.; Hu, W.; Zou, S.; Ren, H.; Zuo, Y.; Qu, L. MiR-127-3p enhances macrophagic proliferation via disturbing fatty acid profiles and oxidative phosphorylation in atherosclerosis. J. Mol. Cell. Cardiol. 2024, 193, 36–52. [Google Scholar] [CrossRef]
- Ren, L.; Liu, W.; Chen, S.; Zeng, H. Longitudinal change of serum exosomal miR-186-5p estimates major adverse cardiac events in acute myocardial infarction patients receiving percutaneous coronary intervention. Front. Cardiovasc. Med. 2024, 11, 1341918. [Google Scholar] [CrossRef]
- Ding, J.; Li, H.; Liu, W.; Wang, X.; Feng, Y.; Guan, H.; Chen, Z. miR-186-5p Dysregulation in Serum Exosomes from Patients with AMI Aggravates Atherosclerosis via Targeting LOX-1. Int. J. Nanomed. 2022, 17, 6301–6316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhu, X.; Li, G. E2F1/SNHG7/miR-186-5p/MMP2 axis modulates the proliferation and migration of vascular endothelial cell in atherosclerosis. Life Sci. 2020, 257, 118013. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, T.; Qin, L.; Yin, L. Circ_0068087 Silencing Ameliorates Oxidized Low-Density Lipoprotein-Induced Dysfunction in Vascular Endothelial Cells Depending on miR-186-5p-Mediated Regulation of Roundabout Guidance Receptor 1. Front. Cardiovasc. Med. 2021, 8, 650374. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Chen, S.; Yao, D.; Yan, H. OxLDL-stimulated macrophage exosomes promote proatherogenic vascular smooth muscle cell viability and invasion via delivering miR-186–5p then inactivating SHIP2 mediated PI3K/AKT/mTOR pathway. Mol. Immunol. 2022, 146, 27–37. [Google Scholar] [CrossRef]
- Liu, P.; Wang, S.; Li, K.; Yang, Y.; Man, Y.; Du, F.; Wang, L.; Tian, J.; Su, G. Exosomal microRNA-4516, microRNA-203 and SFRP1 are potential biomarkers of acute myocardial infarction. Mol. Med. Rep. 2023, 27, 124. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Dai, Y.; Han, Y.; Wei, X.; Wei, G.; Chen, W.; Kong, S.; He, Y.; Liu, H.; et al. Neutrophil N1 polarization induced by cardiomyocyte-derived extracellular vesicle miR-9-5p aggravates myocardial ischemia/reperfusion injury. J. Nanobiotechnol. 2024, 22, 632. [Google Scholar] [CrossRef]
- Son, J.H.; Park, J.K.; Bang, J.H.; Kim, D.; Moon, I.; Kong, M.G.; Park, H.W.; Choi, H.O.; Seo, H.S.; Cho, Y.H.; et al. Exosomal miRNAs Differentiate Chronic Total Occlusion from Acute Myocardial Infarction. Int. J. Mol. Sci. 2024, 25, 10223. [Google Scholar] [CrossRef]
- D’Amore, S.; Härdfeldt, J.; Cariello, M.; Graziano, G.; Copetti, M.; Di Tullio, G.; Piglionica, M.; Scialpi, N.; Sabbà, C.; Palasciano, G.; et al. Identification of miR-9-5p as direct regulator of ABCA1 and HDL-driven reverse cholesterol transport in circulating CD14 + cells of patients with metabolic syndrome. Cardiovasc. Res. 2018, 114, 1154–1164. [Google Scholar] [CrossRef]
- Lei, X.; Yang, Y. Oxidized low-density lipoprotein contributes to injury of endothelial cells via the circ_0090231/miR-9-5p/TXNIP axis. Cent. Eur. J. Immunol. 2022, 47, 41–57. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, J.; Wan, H.; Wang, K.; Wu, J.; Cao, Y.; Hu, L.; Yu, Y.; Sun, H.; Yu, Y.; et al. Plasma extracellular vesicles microRNA-208b-3p and microRNA-143-3p as novel biomarkers for sudden cardiac death prediction in acute coronary syndrome. Mol. Omics 2023, 19, 262–273. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Yang, Y.; Pan, Y.; Yuan, Q.; Liu, Y. Murine exosomal miR-30a aggravates cardiac function after acute myocardial infarction via regulating cell fate of cardiomyocytes and cardiac resident macrophages. Int. J. Cardiol. 2024, 414, 132395. [Google Scholar] [CrossRef]
- Sahebi, R.; Gandomi, F.; Shojaei, M.; Farrokhi, E. Exosomal miRNA-21-5p and miRNA-21-3p as key biomarkers of myocardial infarction. Health Sci. Rep. 2024, 7, e2228. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Huang, F.; Liu, Y.; Liu, S.; Tan, G. Exosomal miR-152-5p and miR-3681-5p function as potential biomarkers for ST-segment elevation myocardial infarction. Clinics 2022, 77, 100038. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yan, Y.; Wu, J.; Qi, C.; Liu, J.; Wang, J. Expression level and diagnostic value of exosomal NEAT1/miR-204/MMP-9 in acute ST-segment elevation myocardial infarction. IUBMB Life 2020, 72, 2499–2507. [Google Scholar] [CrossRef]
- Guo, M.; Li, R.; Yang, L.; Zhu, Q.; Han, M.; Chen, Z.; Ruan, F.; Yuan, Y.; Liu, Z.; Huang, B.; et al. Evaluation of exosomal miRNAs as potential diagnostic biomarkers for acute myocardial infarction using next-generation sequencing. Ann. Transl. Med. 2021, 9, 219. [Google Scholar] [CrossRef]
- Eyyupkoca, F.; Ercan, K.; Kiziltunc, E.; Ugurlu, I.B.; Kocak, A.; Eyerci, N. Determination of microRNAs associated with adverse left ventricular remodeling after myocardial infarction. Mol. Cell. Biochem. 2022, 477, 781–791. [Google Scholar] [CrossRef]
- Guan, R.; Zeng, K.; Zhang, B.; Gao, M.; Li, J.; Jiang, H.; Liu, Y.; Qiang, Y.; Liu, Z.; Li, J.; et al. Plasma Exosome miRNAs Profile in Patients With ST-Segment Elevation Myocardial Infarction. Front. Cardiovasc. Med. 2022, 9, 848812. [Google Scholar] [CrossRef]
- Gong, Z.; Wen, M.; Zhang, W.; Yu, L.; Huang, C.; Xu, Y.; Xia, Z.; Xu, M.; Xu, J.; Liang, Q.; et al. Plasma exosomes induce inflammatory immune response in patients with acute myocardial infarction. Arch. Physiol. Biochem. 2023, 129, 1168–1176. [Google Scholar] [CrossRef]
- Zarà, M.; Baggiano, A.; Amadio, P.; Campodonico, J.; Gili, S.; Annoni, A.; Dona, G.D.; Carerj, M.L.; Cilia, F.; Formenti, A.; et al. Circulating Small Extracellular Vesicles Reflect the Severity of Myocardial Damage in STEMI Patients. Biomolecules 2023, 13, 1470. [Google Scholar] [CrossRef] [PubMed]
- Senesi, G.; Lodrini, A.M.; Mohammed, S.; Mosole, S.; Hjortnaes, J.; Veltrop, R.J.A.; Kubat, B.; Ceresa, D.; Bolis, S.; Raimondi, A.; et al. miR-24-3p secreted as extracellular vesicle cargo by cardiomyocytes inhibits fibrosis in human cardiac microtissues. Cardiovasc. Res. 2024, 121, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-Y.; Lee, S.; Park, J.-T.; Lee, S.-J.; Kim, H.-S. Postmortem-Derived Exosomal MicroRNA 486-5p as Potential Biomarkers for Ischemic Heart Disease Diagnosis. Int. J. Mol. Sci. 2024, 25, 9619. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.; Alarcón-Zapata, P.; Guzmán-Gútierrez, E.; Radojkovic, C.; Contreras, H.; Nova-Lampeti, E.; Zúñiga, F.A.; Rodriguez-Alvárez, L.; Escudero, C.; Lagos, P.; et al. Changes in the Release of Endothelial Extracellular Vesicles CD144+, CCR6+, and CXCR3+ in Individuals with Acute Myocardial Infarction. Biomedicines 2024, 12, 2119. [Google Scholar] [CrossRef]
- Turkieh, A.; Beseme, O.; Saura, O.; Charrier, H.; Michel, J.B.; Amouyel, P.; Thum, T.; Bauters, C.; Pinet, F. LIPCAR levels in plasma-derived extracellular vesicles is associated with left ventricle remodeling post-myocardial infarction. J. Transl. Med. 2024, 22, 31. [Google Scholar] [CrossRef]
- Xu, S.; Zhai, Y.; Wang, C.; Zhang, Y.; Liu, X.; Jiang, J.; Mi, Y. Proteomics Analysis of Five Potential Plasma-derived Exosomal Biomarkers for Acute Myocardial Infarction. Curr. Med. Chem. 2025, 32, 4816–4835. [Google Scholar] [CrossRef]
- Zhou, J.; Hou, H.T.; Chen, H.X.; Song, Y.; Zhou, X.L.; Zhang, L.L.; Xue, H.M.; Yang, Q.; He, G.W. Plasma Exosomal Proteomics Identifies Differentially Expressed Proteins as Biomarkers for Acute Myocardial Infarction. Biomolecules 2025, 15, 583. [Google Scholar] [CrossRef]
- Dekker, M.; Waissi, F.; Timmerman, N.; Silvis, M.J.M.; Timmers, L.; Kleijn, D.P.V. de Extracellular vesicles in diagnosing chronic coronary syndromes—The bumpy road to clinical implementation. Int. J. Mol. Sci. 2020, 21, 9128. [Google Scholar] [CrossRef]
- Zhang, P.; Liang, T.; Chen, Y.; Wang, X.; Wu, T.; Xie, Z.; Luo, J.; Yu, Y.; Yu, H. Circulating Exosomal miRNAs as Novel Biomarkers for Stable Coronary Artery Disease. BioMed Res. Int. 2020, 2020, 3593962. [Google Scholar] [CrossRef]
- Yang, J.; Li, X.; Zhang, Y.; Che, P.; Qin, W.; Wu, X.; Liu, Y.; Hu, B. Circ_0090231 knockdown protects vascular smooth muscle cells from ox-LDL-induced proliferation, migration and invasion via miR-942-5p/PPM1B axis during atherosclerosis. Mol. Cell. Biochem. 2024, 479, 2035–2045. [Google Scholar] [CrossRef]
- Wan, H.; You, T.; Luo, W. circ_0003204 Regulates Cell Growth, Oxidative Stress, and Inflammation in ox-LDL-Induced Vascular Endothelial Cells via Regulating miR-942-5p/HDAC9 Axis. Front. Cardiovasc. Med. 2021, 8, 646832. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, S.; Guo, S.; Zhang, X.; Yang, C.; Su, G.; Wan, J. Circ_0004104 participates in the regulation of ox-LDL-induced endothelial cells injury via miR-942-5p/ROCK2 axis. BMC Cardiovasc. Disord. 2022, 22, 517. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Teng, Y.; Tian, M.; Qiu, H.; Zhao, J.; Gao, Q.; Zhang, Y.; Zhuang, J.; Chen, J. Enhancement of LncRNA-HFRL expression induces cardiomyocyte inflammation, proliferation, and fibrosis via the sequestering of miR-149-5p-mediated collagen 22A inhibition. Ann. Transl. Med. 2022, 10, 523. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Xu, J.; Lu, Q.; Feng, X.; Liu, J.; Cui, C.; Song, C. Hsa_circ_0087352 promotes the inflammatory response of macrophages in abdominal aortic aneurysm by adsorbing hsa-miR-149-5p. Int. Immunopharmacol. 2022, 107, 108691. [Google Scholar] [CrossRef]
- Ye, Z.; Yang, S.; Xia, Y.; Hu, R.; Chen, S.; Li, B.; Chen, S.; Luo, X.; Mao, L.; Li, Y.; et al. LncRNA MIAT sponges miR-149-5p to inhibit efferocytosis in advanced atherosclerosis through CD47 upregulation. Cell Death Dis. 2019, 10, 138. [Google Scholar] [CrossRef]
- Wang, M.; Li, C.; Cai, T.; Zhang, A.; Cao, J.; Xin, H. Circ_CHFR Promotes Platelet-Derived Growth Factor-BB-Induced Proliferation, Invasion, and Migration in Vascular Smooth Muscle Cells via the miR-149-5p/NRP2 Axis. Cardiovasc. Pharmacol. 2021, 79, e94–e102. [Google Scholar] [CrossRef]
- Peng, W.; Li, T.; Pi, S.; Huang, L.; Liu, Y. Suppression of circular RNA circDHCR24 alleviates aortic smooth muscle cell proliferation and migration by targeting miR-149-5p/MMP9 axis. Biochem. Biophys. Res. Commun. 2020, 529, 753–759. [Google Scholar] [CrossRef]
- Wang, G.; Li, Y.; Liu, Z.; Ma, X.; Li, M.; Lu, Q.; Li, Y.; Lu, Z.; Niu, L.; Fan, Z.; et al. Circular RNA circ_0124644 exacerbates the ox-LDL-induced endothelial injury in human vascular endothelial cells through regulating PAPP-A by acting as a sponge of miR-149-5p. Mol. Cell. Biochem. 2020, 471, 51–61. [Google Scholar] [CrossRef]
- Han, J.; Cui, X.; Yuan, T.; Yang, Z.; Liu, Y.; Ren, Y.; Wu, C.; Bian, Y. Plasma-derived exosomal let-7c-5p, miR-335–3p, and miR-652–3p as potential diagnostic biomarkers for stable coronary artery disease. Front. Physiol. 2023, 14, 1161612. [Google Scholar] [CrossRef]
- Zheng, M.; Han, R.; Yuan, W.; Chi, H.; Zhang, Y.; Sun, K.; Zhong, J.; Liu, X.; Yang, X. Circulating exosomal lncRNAs in patients with chronic coronary syndromes. Arch. Med. Sci. 2023, 19, 46–56. [Google Scholar] [CrossRef]
- Chang, S.N.; Chen, J.J.; Liu, M.T.; Chung, Y.T.; Lin, S.H.; Liu, C.J.; Li, C.; Lin, J.W. Validation of novel exosomal miRNAs identified by next-generation sequencing in coronary artery disease. J. Formos. Med. Assoc. 2025, in press. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Tian, P.; Xing, L.; Huang, X.; Fu, C.; Xu, X.; Liu, P. Exosomal circSCMH1/miR-874 ratio in serum to predict carotid and coronary plaque stability. Front. Cardiovasc. Med. 2023, 10, 1277427. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, F.; Zhou, L.Y.; Ding, S.L.; Long, B.; Liu, C.Y.; Sun, T.; Fan, Y.Y.; Sun, L.; Li, P.F. MiR-874 regulates myocardial necrosis by targeting caspase-8. Cell Death Dis. 2013, 4, e709. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.J.; Shang, A.Q.; Yang, J.P.; Wang, W.W. microRNA-874 inhibition targeting STAT3 protects the heart from ischemia–reperfusion injury by attenuating cardiomyocyte apoptosis in a mouse model. J. Cell. Physiol. 2019, 234, 6182–6193. [Google Scholar] [CrossRef]
- Ryu, J.; Choe, N.; Kwon, D.H.; Shin, S.; Lim, Y.H.; Yoon, G.; Kim, J.H.; Kim, H.S.; Lee, I.K.; Ahn, Y.; et al. Circular RNA circSmoc1-2 regulates vascular calcification by acting as a miR-874-3p sponge in vascular smooth muscle cells. Mol. Ther. Nucleic Acids 2022, 27, 645–655. [Google Scholar] [CrossRef]
- Li, B.; Xi, W.; Bai, Y.; Liu, X.; Zhang, Y.; Li, L.; Bian, L.; Liu, C.; Tang, Y.; Shen, L.; et al. FTO-dependent m6A modification of Plpp3 in circSCMH1-regulated vascular repair and functional recovery following stroke. Nat. Commun. 2023, 14, 489. [Google Scholar] [CrossRef]
- Yang, L.; Han, B.; Zhang, Z.; Wang, S.; Bai, Y.; Zhang, Y.; Tang, Y.; Du, L.; Xu, L.; Wu, F.; et al. Extracellular vesicle-mediated delivery of circular RNA SCMH1 promotes functional recovery in rodent and nonhuman primate ischemic stroke models. Circulation 2020, 142, 556–574. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, Y.; Cai, Y.; Zhang, Y.; Shen, L.; Xi, W.; Zhou, Z.; Xu, L.; Liu, X.; Han, B.; et al. Circular RNA SCMH1 suppresses KMO expression to inhibit mitophagy and promote functional recovery following stroke. Theranostics 2024, 14, 7292–7308. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.; Zhang, S.; Yan, S.; Wang, Z.; Wang, C.; Zhang, X. MiR-30e and miR-92a are related to atherosclerosis by targeting ABCA1. Mol. Med. Rep. 2019, 19, 3298–3304. [Google Scholar] [CrossRef]
- Dekker, M.; Waissi, F.; van Bennekom, J.; Silvis, M.J.M.; Timmerman, N.; Bank, I.E.M.; Walter, J.E.; Mueller, C.; Schoneveld, A.H.; Schiffelers, R.M.; et al. Plasma extracellular vesicle proteins are associated with stress-induced myocardial ischemia in women presenting with chest pain. Sci. Rep. 2020, 10, 12257. [Google Scholar] [CrossRef]
- Bryl-Górecka, P.; James, K.; Torngren, K.; Haraldsson, I.; Gan, L.M.; Svedlund, S.; Olde, B.; Laurell, T.; Omerovic, E.; Erlinge, D. Microvesicles in plasma reflect coronary flow reserve in patients with cardiovascular disease. Am. J. Physiol.—Heart Circ. Physiol. 2021, 320, H2147–H2160. [Google Scholar] [CrossRef]
- Chang, S.N.; Chen, J.J.; Wu, J.H.; Chung, Y.T.; Chen, J.W.; Chiu, C.H.; Liu, C.J.; Liu, M.T.; Chang, Y.C.; Li, C.; et al. Association between exosomal mirnas and coronary artery disease by next-generation sequencing. Cells 2022, 11, 98. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Qin, Z.; Liu, N.; Zhang, Z.; Lu, Y.; Xu, Y.; Zhang, J.; Tang, J. Diagnostic and Predictive Values of Circulating Extracellular Vesicle-Carried microRNAs in Ischemic Heart Disease Patients With Type 2 Diabetes Mellitus. Front. Cardiovasc. Med. 2022, 9, 813310. [Google Scholar] [CrossRef]
- McGranaghan, P.; Pallinger, É.; Fekete, N.; Maurovich-Horvát, P.; Drobni, Z.; Merkely, B.; Menna, L.; Buzás, E.I.; Hegyesi, H. Modeling the Impact of Extracellular Vesicle Cargoes in the Diagnosis of Coronary Artery Disease. Biomedicines 2024, 12, 2682. [Google Scholar] [CrossRef]
- Liu, Y.; Zhong, C.; Chen, S.; Xue, Y.; Wei, Z.; Dong, L.; Kang, L. Circulating exosomal mir-16-2-3p is associated with coronary microvascular dysfunction in diabetes through regulating the fatty acid degradation of endothelial cells. Cardiovasc. Diabetol. 2024, 23, 60. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Chen, J.; Wu, J.H.; Chung, Y.T.; Chen, J.W.; Liu, M.T.; Chiu, C.H.; Chang, Y.C.; Chang, S.N.; Lin, J.W.; et al. Association of exosomes in patients with compromised myocardial perfusion on functional imaging. J. Formos. Med. Assoc. 2024, 123, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Gąsecka, A.; Szolc, P.; van der Pol, E.; Niewiara, Ł.; Guzik, B.; Kleczyński, P.; Tomaniak, M.; Figura, E.; Zaremba, M.; Grabowski, M.; et al. Endothelial Cell-Derived Extracellular Vesicles Allow to Differentiate Between Various Endotypes of INOCA: A Multicentre, Prospective, Cohort Study. J. Cardiovasc. Transl. Res. 2024, 18, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Protty, M.B.; Tyrrell, V.J.; Allen-Redpath, K.; Soyama, S.; Hajeyah, A.A.; Costa, D.; Choudhury, A.; Mitra, R.; Sharman, A.; Yaqoob, P.; et al. Thrombin Generation Is Associated With Extracellular Vesicle and Leukocyte Lipid Membranes in Atherosclerotic Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 2038–2052. [Google Scholar] [CrossRef]
- Hajeyah, A.A.; Protty, M.B.; Paul, D.; Costa, D.; Omidvar, N.; Morgan, B.; Iwasaki, Y.; McGill, B.; Jenkins, P.V.; Yousef, Z.; et al. Phosphatidylthreonine is a procoagulant lipid detected in human blood and elevated in coronary artery disease. J. Lipid Res. 2024, 65, 100484. [Google Scholar] [CrossRef]
- Mehta, P.K.; Quesada, O.; Al-Badri, A.; Fleg, J.L.; Volgman, A.S.; Pepine, C.J.; Merz, C.N.B.; Shaw, L.J. Ischemia and no obstructive coronary arteries in patients with stable ischemic heart disease. Int. J. Cardiol. 2022, 348, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Monizzi, G.; Di Lenarda, F.; Gallinoro, E.; Bartorelli, A.L. Myocardial Ischemia: Differentiating between Epicardial Coronary Artery Atherosclerosis, Microvascular Dysfunction and Vasospasm in the Catheterization Laboratory. J. Clin. Med. 2024, 13, 4172. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Wei, X.; Lin, Y.; Chen, J.; Yu, D. Pathophysiologic Basis and Diagnostic Approaches for Ischemia With Non-obstructive Coronary Arteries: A Literature Review. Front. Cardiovasc. Med. 2022, 9, 731059. [Google Scholar] [CrossRef] [PubMed]
- Wayne, N.; Singamneni, V.S.; Venkatesh, R.; Cherlin, T.; Verma, S.S.; Guerraty, M.A. Genetic Insights Into Coronary Microvascular Disease. Microcirculation 2025, 32, e12896. [Google Scholar] [CrossRef]
- Mehta, P.K.; Huang, J.; Levit, R.D.; Malas, W.; Waheed, N.; Bairey Merz, C.N. Ischemia and no obstructive coronary arteries (INOCA): A narrative review. Atherosclerosis 2022, 363, 8–21. [Google Scholar] [CrossRef]
- Ford, T.; Zeitz, C.; Spiro, J.; Yong, A.; Layland, J.; Watts, M.; Chan, W.; Girolamo, O.; Marathe, J.A.; Negishi, K.; et al. Functional Coronary Angiography for the Diagnosis of Coronary Vasomotor Disorders. Heart Lung Circ. 2025, in press. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. The extracellular matrix in myocardial injury, repair, and remodeling. J. Clin. Investig. 2017, 127, 1600–1612. [Google Scholar] [CrossRef]
- Leancă, S.A.; Crișu, D.; Petriș, A.O.; Afrăsânie, I.; Genes, A.; Costache, A.D.; Tesloianu, D.N.; Costache, I.I. Left Ventricular Remodeling after Myocardial Infarction: From Physiopathology to Treatment. Life 2022, 12, 1111. [Google Scholar] [CrossRef]
- Limpitikul, W.B.; Silverman, M.G.; Valkov, N.; Park, J.-G.; Yeri, A.; Garcia, F.C.; Li, G.; Gokulnath, P.; Garcia-Contreras, M.; Alsop, E.; et al. Plasma extracellular vesicle cargo microRNAs are associated with heart failure and cardiovascular death following acute coronary syndrome. Extracell. Vesicle 2025, 5, 100070. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Landry, M.P.; Moore, A.; Coreas, R. The protein corona from nanomedicine to environmental science. Nat. Rev. Mater. 2023, 8, 422–438. [Google Scholar] [CrossRef]
- Heidarzadeh, M.; Zarebkohan, A.; Rahbarghazi, R.; Sokullu, E. Protein corona and exosomes: New challenges and prospects. Cell Commun. Signal. 2023, 21, 64. [Google Scholar] [CrossRef]
- Tabatabaeian Nimavard, R.; Sadeghi, S.A.; Mahmoudi, M.; Zhu, G.; Sun, L. Top-Down Proteomic Profiling of Protein Corona by High-Throughput Capillary Isoelectric Focusing-Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2025, 36, 778–786. [Google Scholar] [CrossRef]
- Sadeghi, S.A.; Ashkarran, A.A.; Wang, Q.; Zhu, G.; Mahmoudi, M.; Sun, L. Mass Spectrometry-Based Top-Down Proteomics in Nanomedicine: Proteoform-Specific Measurement of Protein Corona. ACS Nano 2024, 18, 26024–26036. [Google Scholar] [CrossRef]
- Lee, G.Y.; Li, A.A.; Moon, I.; Katritsis, D.; Pantos, Y.; Stingo, F.; Fabbrico, D.; Molinaro, R.; Taraballi, F.; Tao, W.; et al. Protein Corona Sensor Array Nanosystem for Detection of Coronary Artery Disease. Small 2024, 20, 2306168. [Google Scholar] [CrossRef] [PubMed]
- Vélez, P.; Parguiña, A.F.; Ocaranza-Sánchez, R.; Grigorian-Shamagian, L.; Rosa, I.; Alonso-Orgaz, S.; de la Cuesta, F.; Guitián, E.; Moreu, J.; Barderas, M.G.; et al. Identification of a circulating microvesicle protein network involved in ST-elevation myocardial infarction. Thromb. Haemost. 2014, 112, 716–726. [Google Scholar] [CrossRef]
- Bazzan, E.; Tinè, M.; Casara, A.; Biondini, D.; Semenzato, U.; Cocconcelli, E.; Balestro, E.; Damin, M.; Radu, C.M.; Turato, G.; et al. Critical review of the evolution of extracellular vesicles’ knowledge: From 1946 to today 2021. Int. J. Mol. Sci. 2021, 22, 6417. [Google Scholar] [CrossRef]
- Lee, K.; Shao, H.; Weissleder, R.; Lee, H. Acoustic purification of extracellular microvesicles. ACS Nano 2015, 9, 2321–2327. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carcia, V.; De Salve, A.V.; Nonno, C.; Brizzi, M.F. Circulating Extracellular Vesicle-Based Biomarkers: Advances, Clinical Implications and Challenges in Coronary Artery Disease. Int. J. Transl. Med. 2025, 5, 39. https://doi.org/10.3390/ijtm5030039
Carcia V, De Salve AV, Nonno C, Brizzi MF. Circulating Extracellular Vesicle-Based Biomarkers: Advances, Clinical Implications and Challenges in Coronary Artery Disease. International Journal of Translational Medicine. 2025; 5(3):39. https://doi.org/10.3390/ijtm5030039
Chicago/Turabian StyleCarcia, Valeria, Alessandro Vincenzo De Salve, Chiara Nonno, and Maria Felice Brizzi. 2025. "Circulating Extracellular Vesicle-Based Biomarkers: Advances, Clinical Implications and Challenges in Coronary Artery Disease" International Journal of Translational Medicine 5, no. 3: 39. https://doi.org/10.3390/ijtm5030039
APA StyleCarcia, V., De Salve, A. V., Nonno, C., & Brizzi, M. F. (2025). Circulating Extracellular Vesicle-Based Biomarkers: Advances, Clinical Implications and Challenges in Coronary Artery Disease. International Journal of Translational Medicine, 5(3), 39. https://doi.org/10.3390/ijtm5030039







