The Importance of an Adequate Diet in the Treatment and Maintenance of Health in Children with Cystic Fibrosis
Abstract
1. Introduction
2. Etiology
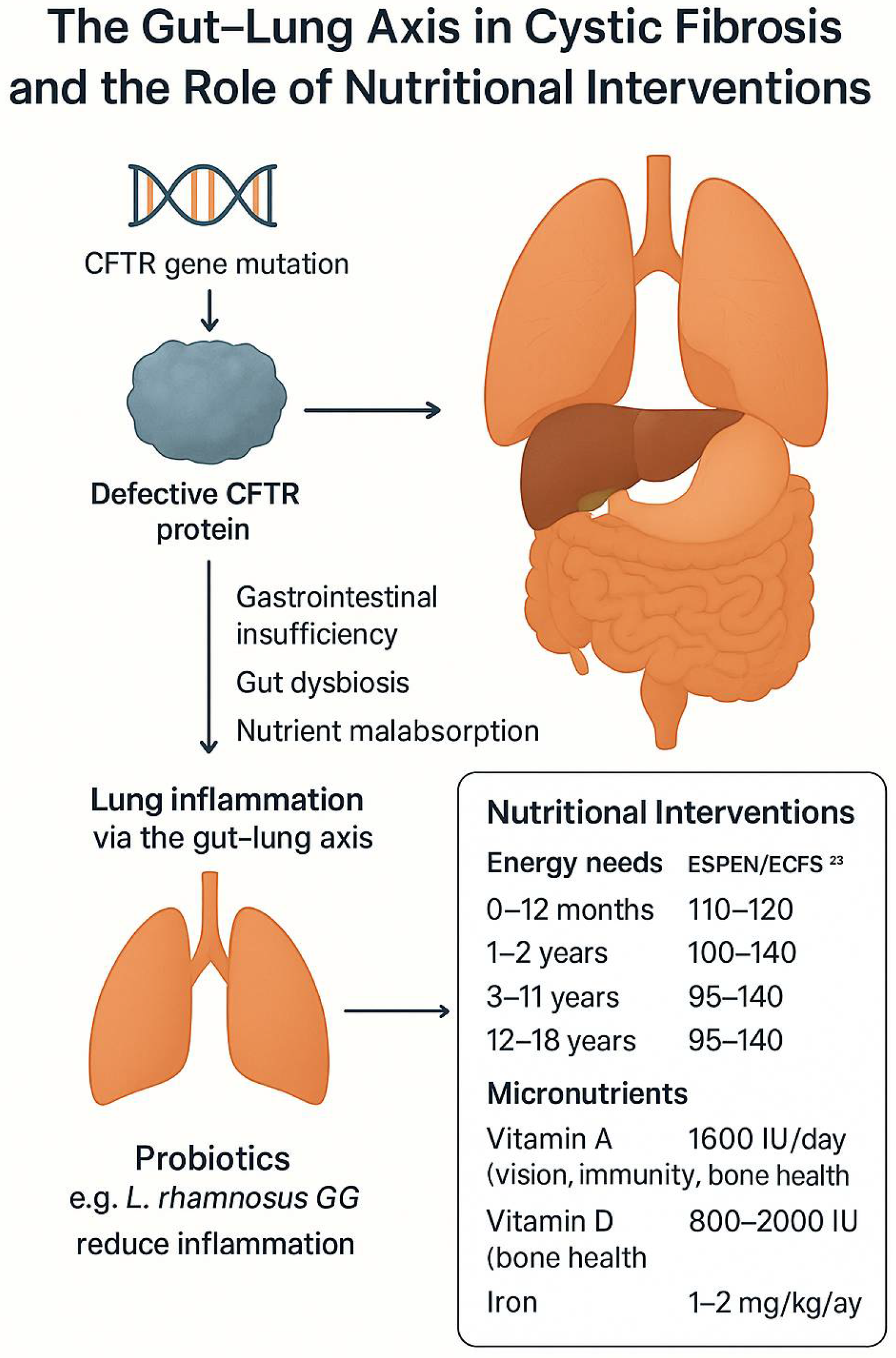
3. Cystic Fibrosis in the Gastrointestinal Tract
4. Probiotics
5. Diagnostic Methods for Gastrointestinal Complications
6. Cystic Fibrosis Pharmacotherapy
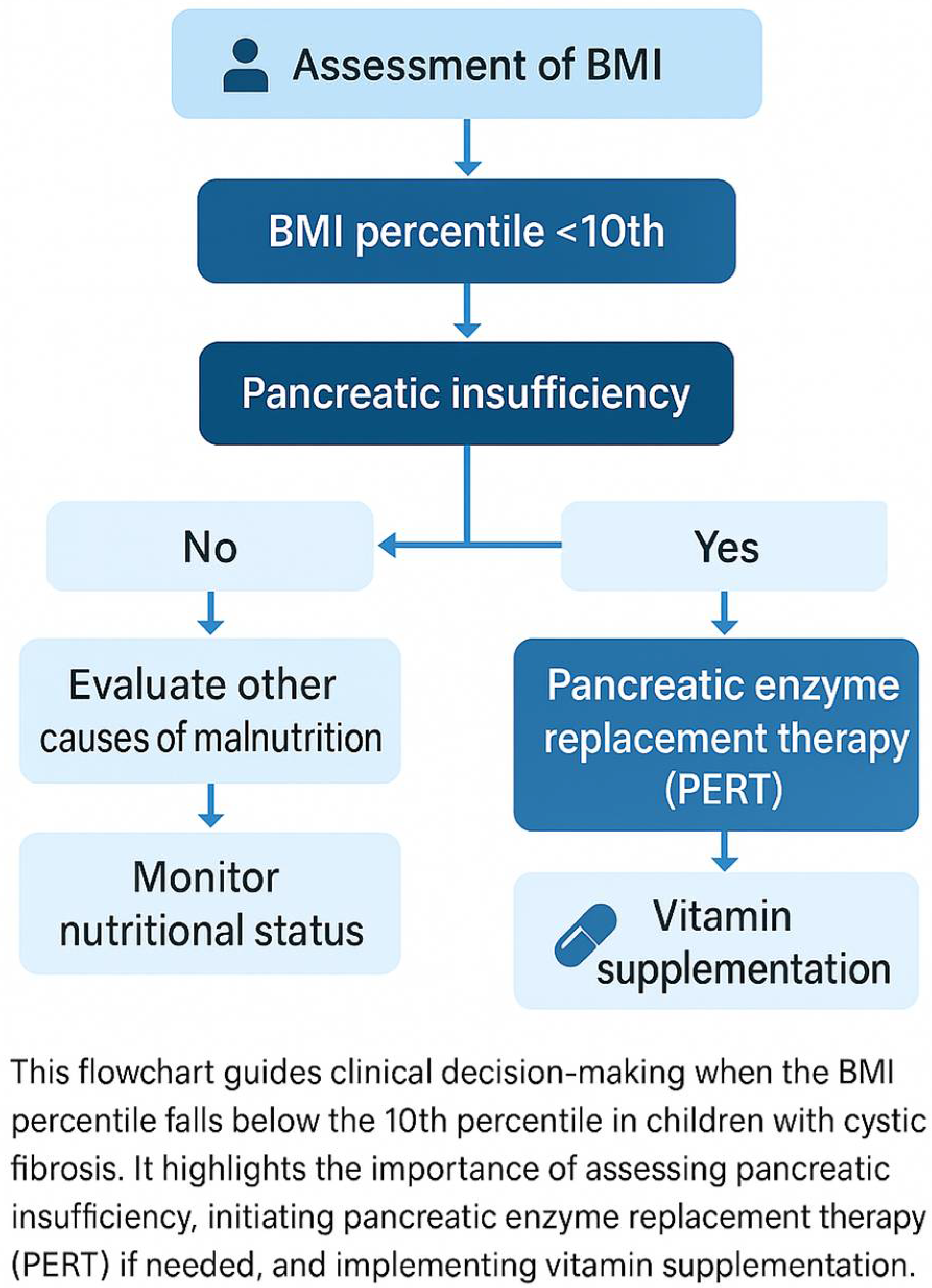
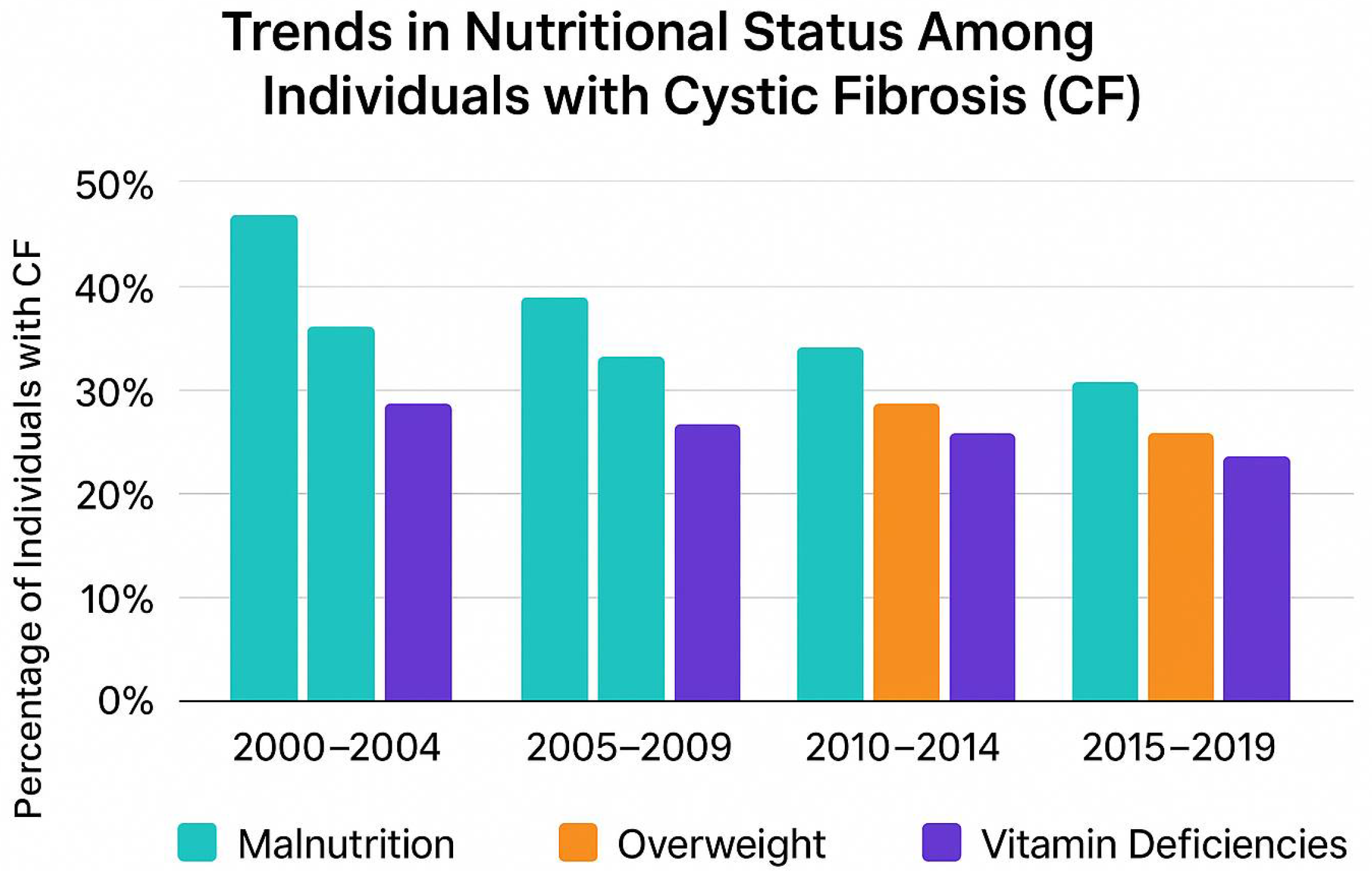
7. The Role of an Appropriate Diet in the Treatment of Cystic Fibrosis in Children
8. Dietary Recommendations
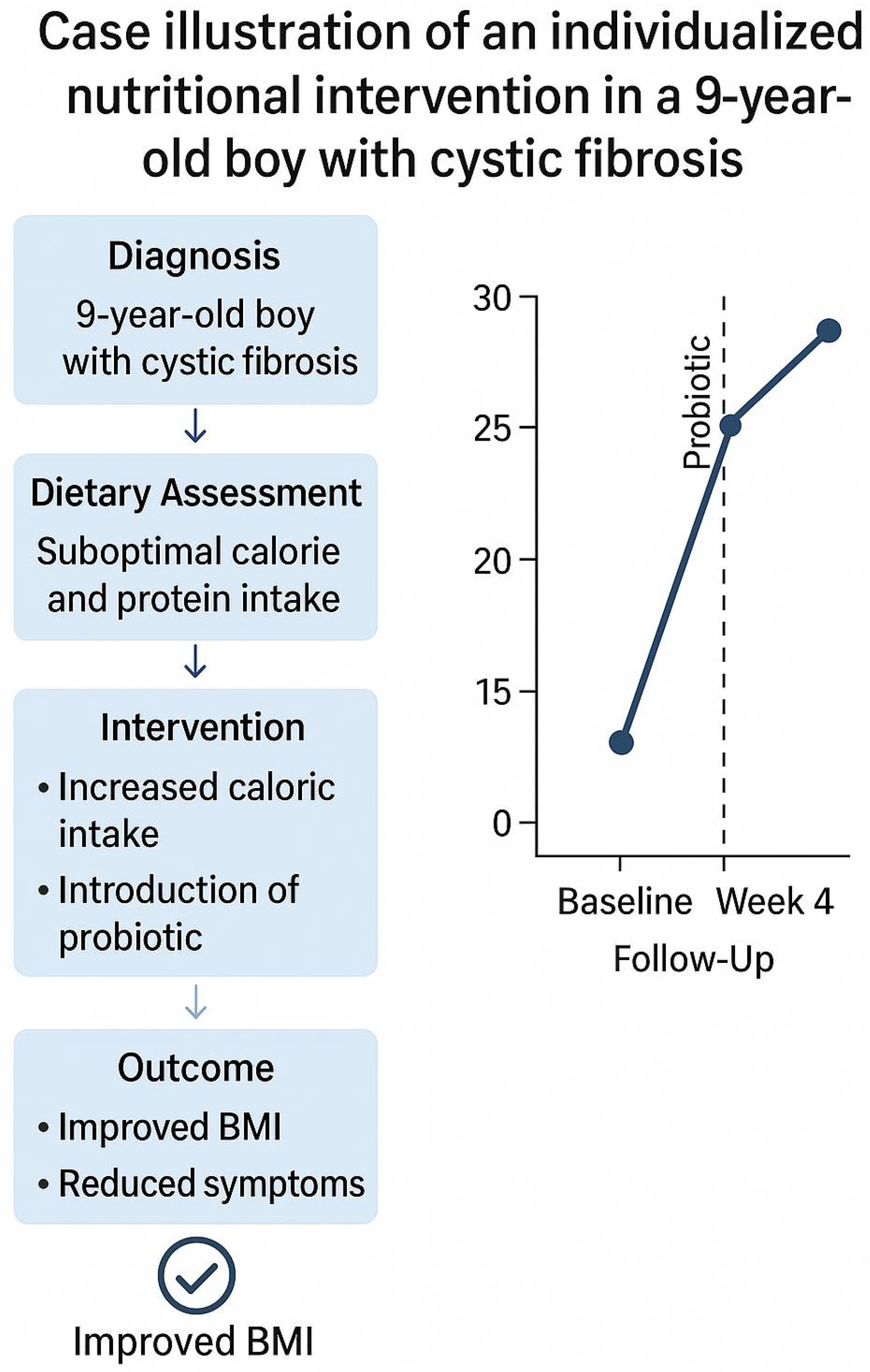
Clinical Case Example: Nutritional Approach in Pediatric Cystic Fibrosis
9. Supplementation of Vitamins and Minerals
10. Enzyme Replacement Therapy
11. Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- At-a-Glance Report. Available online: https://www.ecfs.eu/sites/default/files/At-a-Glance%20report%202021_ECFSPR_09Jun2023.pdf (accessed on 15 May 2025).
- Stoltz, D.A.; Meyerholz, D.K.; Welsh, M.J. Origins of Cystic Fibrosis Lung Disease. N. Engl. J. Med. 2015, 372, 351–362. [Google Scholar] [CrossRef]
- Wilschanski, M.; Munck, A.; Carrion, E.; Cipolli, M.; Collins, S.; Colombo, C.; Declercq, D.; Hatziagorou, E.; Hulst, J.; Kalnins, D.; et al. ESPEN-ESPGHAN-ECFS Guideline on Nutrition Care for Cystic Fibrosis. Clin. Nutr. 2024, 43, 413–445. [Google Scholar] [CrossRef] [PubMed]
- Brewington, J.; Clancy, J.P. Diagnostic Testing in Cystic Fibrosis. Clin. Chest Med. 2016, 37, 31–46. [Google Scholar] [CrossRef]
- Pienkowska, K.; Pust, M.-M.; Gessner, M.; Gaedcke, S.; Thavarasa, A.; Rosenboom, I.; Morán Losada, P.; Minso, R.; Arnold, C.; Hedtfeld, S.; et al. The Cystic Fibrosis Upper and Lower Airway Metagenome. Microbiol. Spectr. 2023, 11, e0363322. [Google Scholar] [CrossRef] [PubMed]
- Kharrazi, M.; Yang, J.; Bishop, T.; Lessing, S.; Young, S.; Graham, S.; Pearl, M.; Chow, H.; Ho, T.; Currier, R.; et al. Newborn Screening for Cystic Fibrosis in California. Pediatrics 2015, 136, 1062–1072. [Google Scholar] [CrossRef]
- Simmonds, N.J. Is It Cystic Fibrosis? The Challenges of Diagnosing Cystic Fibrosis. Paediatr. Respir. Rev. 2019, 31, 6–8. [Google Scholar] [CrossRef]
- Mishra, A.; Greaves, R.; Massie, J. The Relevance of Sweat Testing for the Diagnosis of Cystic Fibrosis in the Genomic Era. Clin. Biochem. Rev. 2005, 26, 135–153. [Google Scholar] [PubMed]
- Introduction: From the Discovery of the CFTR Gene in 1989 Through to 2014|Request PDF. Available online: https://www.researchgate.net/publication/364848628_Introduction_From_the_discovery_of_the_CFTR_gene_in_1989_through_to_2014 (accessed on 10 September 2024).
- Li, L.; Somerset, S. Digestive System Dysfunction in Cystic Fibrosis: Challenges for Nutrition Therapy. Dig Liver Dis. 2014, 46, 865–874. [Google Scholar] [CrossRef]
- Sathe, M.; Houwen, R. Is Meconium Ileus Associated with Worse Outcomes in Cystic Fibrosis? J. Cyst. Fibros. 2019, 18, 746. [Google Scholar] [CrossRef]
- Marson, F.A.L. Disease-Modifying Genetic Factors in Cystic Fibrosis. Curr. Opin. Pulm. Med. 2018, 24, 296–308. [Google Scholar] [CrossRef]
- Dodge, J.A.; Turck, D. Cystic Fibrosis: Nutritional Consequences and Management. Best. Pract. Res. Clin. Gastroenterol. 2006, 20, 531–546. [Google Scholar] [CrossRef] [PubMed]
- Castellani, C.; Assael, B.M. Cystic Fibrosis: A Clinical View. Cell Mol. Life Sci. 2017, 74, 129–140. [Google Scholar] [CrossRef]
- Farrell, P.M.; White, T.B.; Ren, C.L.; Hempstead, S.E.; Accurso, F.; Derichs, N.; Howenstine, M.; McColley, S.A.; Rock, M.; Rosenfeld, M.; et al. Diagnosis of Cystic Fibrosis: Consensus Guidelines from the Cystic Fibrosis Foundation. J. Pediatr. 2017, 181S, S4–S15.e1. [Google Scholar] [CrossRef] [PubMed]
- Matamouros, S.; Hayden, H.S.; Hager, K.R.; Brittnacher, M.J.; Lachance, K.; Weiss, E.J.; Pope, C.E.; Imhaus, A.-F.; McNally, C.P.; Borenstein, E.; et al. Adaptation of Commensal Proliferating Escherichia Coli to the Intestinal Tract of Young Children with Cystic Fibrosis. Proc. Natl. Acad. Sci. USA 2018, 115, 1605–1610. [Google Scholar] [CrossRef]
- Eng, A.; Hayden, H.S.; Pope, C.E.; Brittnacher, M.J.; Vo, A.T.; Weiss, E.J.; Hager, K.R.; Leung, D.H.; Heltshe, S.L.; Raftery, D.; et al. Infants with Cystic Fibrosis Have Altered Fecal Functional Capacities with Potential Clinical and Metabolic Consequences. BMC Microbiol. 2021, 21, 247. [Google Scholar] [CrossRef]
- Nielsen, S.; Needham, B.; Leach, S.T.; Day, A.S.; Jaffe, A.; Thomas, T.; Ooi, C.Y. Disrupted Progression of the Intestinal Microbiota with Age in Children with Cystic Fibrosis. Sci. Rep. 2016, 6, 24857. [Google Scholar] [CrossRef] [PubMed]
- Schippa, S.; Iebba, V.; Santangelo, F.; Gagliardi, A.; De Biase, R.V.; Stamato, A.; Bertasi, S.; Lucarelli, M.; Conte, M.P.; Quattrucci, S.; et al. Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Allelic Variants Relate to Shifts in Faecal Microbiota of Cystic Fibrosis Patients. PLoS ONE 2013, 8, e61176. [Google Scholar] [CrossRef]
- Thavamani, A.; Salem, I.; Sferra, T.J.; Sankararaman, S. Impact of Altered Gut Microbiota and Its Metabolites in Cystic Fibrosis. Metabolites 2021, 11, 123. [Google Scholar] [CrossRef]
- Rahmani, P.; Rohani, P.; Kariman, A.; Motamed, F.; Modaresi, M.R.; Eftekhari, K.; Ayati, M.; Sohouli, M.H. The Impact of Probiotics on Pulmonary, Gastrointestinal, and Growth Outcomes in Pediatric Cystic Fibrosis: A Randomized Controlled Trial. BMC Pediatr. 2025, 25, 430. [Google Scholar] [CrossRef]
- Resting Energy Expenditure in Cystic Fibrosis Patients Decreases after Lung Transplantation, Which Improves Applicability of Prediction Equations for Energy Requirement. Available online: https://www.researchgate.net/publication/340590345_Resting_energy_expenditure_in_cystic_fibrosis_patients_decreases_after_lung_transplantation_which_improves_applicability_of_prediction_equations_for_energy_requirement (accessed on 10 September 2024).
- Anderson, J.L.; Miles, C.; Tierney, A.C. Effect of Probiotics on Respiratory, Gastrointestinal and Nutritional Outcomes in Patients with Cystic Fibrosis: A Systematic Review. J. Cyst. Fibros. 2017, 16, 186–197. [Google Scholar] [CrossRef]
- Asensio-Grau, A.; Calvo-Lerma, J.; Ferriz-Jordán, M.; García-Hernández, J.; Heredia, A.; Andrés, A. Effect of Lactobacillaceae Probiotics on Colonic Microbiota and Metabolite Production in Cystic Fibrosis: A Comparative In Vitro Study. Nutrients 2023, 15, 3846. [Google Scholar] [CrossRef]
- Madan, J.C.; Koestler, D.C.; Stanton, B.A.; Davidson, L.; Moulton, L.A.; Housman, M.L.; Moore, J.H.; Guill, M.F.; Morrison, H.G.; Sogin, M.L.; et al. Serial Analysis of the Gut and Respiratory Microbiome in Cystic Fibrosis in Infancy: Interaction between Intestinal and Respiratory Tracts and Impact of Nutritional Exposures. mBio 2012, 3, e00251-12. [Google Scholar] [CrossRef]
- Hoen, A.G.; Li, J.; Moulton, L.A.; O’Toole, G.A.; Housman, M.L.; Koestler, D.C.; Guill, M.F.; Moore, J.H.; Hibberd, P.L.; Morrison, H.G.; et al. Associations between Gut Microbial Colonization in Early Life and Respiratory Outcomes in Cystic Fibrosis. J. Pediatr. 2015, 167, 138–147.e1–3. [Google Scholar] [CrossRef]
- Antosca, K.M.; Chernikova, D.A.; Price, C.E.; Ruoff, K.L.; Li, K.; Guill, M.F.; Sontag, N.R.; Morrison, H.G.; Hao, S.; Drumm, M.L.; et al. Altered Stool Microbiota of Infants with Cystic Fibrosis Shows a Reduction in Genera Associated with Immune Programming from Birth. J. Bacteriol. 2019, 201, e00274-19. [Google Scholar] [CrossRef]
- Duytschaever, G.; Huys, G.; Bekaert, M.; Boulanger, L.; De Boeck, K.; Vandamme, P. Dysbiosis of Bifidobacteria and Clostridium Cluster XIVa in the Cystic Fibrosis Fecal Microbiota. J. Cyst. Fibros. 2013, 12, 206–215. [Google Scholar] [CrossRef]
- Debray, D.; Kelly, D.; Houwen, R.; Strandvik, B.; Colombo, C. Best Practice Guidance for the Diagnosis and Management of Cystic Fibrosis-Associated Liver Disease. J. Cyst. Fibros. 2011, 10 (Suppl. S2), S29–S36. [Google Scholar] [CrossRef]
- Freswick, P.N.; Reid, E.K.; Mascarenhas, M.R. Pancreatic Enzyme Replacement Therapy in Cystic Fibrosis. Nutrients 2022, 14, 1341. [Google Scholar] [CrossRef] [PubMed]
- Walkowiak, J.; Lisowska, A.; Przyslawski, J.; Grzymislawski, M.; Krawczynski, M.; Herzig, K.H. Faecal Elastase-1 Test Is Superior to Faecal Lipase Test in the Assessment of Exocrine Pancreatic Function in Cystic Fibrosis. Acta Paediatr. 2004, 93, 1042–1045. [Google Scholar] [CrossRef]
- Declercq, D.; Van Biervliet, S.; Robberecht, E. Nutrition and Pancreatic Enzyme Intake in Patients With Cystic Fibrosis With Distal Intestinal Obstruction Syndrome. Nutr. Clin. Pract. 2015, 30, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Perano, S.J.; Couper, J.J.; Horowitz, M.; Martin, A.J.; Kritas, S.; Sullivan, T.; Rayner, C.K. Pancreatic Enzyme Supplementation Improves the Incretin Hormone Response and Attenuates Postprandial Glycemia in Adolescents With Cystic Fibrosis: A Randomized Crossover Trial. J. Clin. Endocrinol. Metab. 2014, 99, 2486–2493. [Google Scholar] [CrossRef] [PubMed]
- Sosinski, L.M.; Martin H., C.; Neugebauer, K.A.; Ghuneim, L.J.; Guzior, D.V.; Castillo-Bahena, A.; Mielke, J.; Thomas, R.; McClelland, M.; Conrad, D.; et al. A Restructuring of Microbiome Niche Space Is Associated with Elexacaftor-Tezacaftor-Ivacaftor Therapy in the Cystic Fibrosis Lung. J. Cyst. Fibros. 2022, 21, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Harvey, C.; Weldon, S.; Elborn, S.; Downey, D.G.; Taggart, C. The Effect of CFTR Modulators on Airway Infection in Cystic Fibrosis. Int. J. Mol. Sci. 2022, 23, 3513. [Google Scholar] [CrossRef]
- Elborn, J.S.; Blasi, F.; Burgel, P.-R.; Peckham, D. Role of Inhaled Antibiotics in the Era of Highly Effective CFTR Modulators. Eur. Respir. Rev. 2023, 32, 220154. [Google Scholar] [CrossRef] [PubMed]
- Rogers, G.B.; Taylor, S.L.; Hoffman, L.R.; Burr, L.D. The Impact of CFTR Modulator Therapies on CF Airway Microbiology. J. Cyst. Fibros. 2020, 19, 359–364. [Google Scholar] [CrossRef]
- Durfey, S.L.; Pipavath, S.; Li, A.; Vo, A.T.; Ratjen, A.; Carter, S.; Morgan, S.J.; Radey, M.C.; Grogan, B.; Salipante, S.J.; et al. Combining Ivacaftor and Intensive Antibiotics Achieves Limited Clearance of Cystic Fibrosis Infections. mBio 2021, 12, e0314821. [Google Scholar] [CrossRef]
- Lindsay, S.; Larry, B.; Imre, N.; Dana, A. Modulator-Refractory Cystic Fibrosis: Defining the Scope and Challenges of an Emerging at-Risk Population. Ther. Adv. Respir. Dis. 2024, 18, 17534666241297877. [Google Scholar] [CrossRef]
- Ridley, K.; Condren, M. Elexacaftor-Tezacaftor-Ivacaftor: The First Triple-Combination Cystic Fibrosis Transmembrane Conductance Regulator Modulating Therapy. J. Pediatr. Pharmacol. Ther. 2020, 25, 192–197. [Google Scholar] [CrossRef]
- Thornton, C.S.; Acosta, N.; Surette, M.G.; Parkins, M.D. Exploring the Cystic Fibrosis Lung Microbiome: Making the Most of a Sticky Situation. J. Pediatr. Infect. Dis. Soc. 2022, 11, S13–S22. [Google Scholar] [CrossRef]
- Allaire, N.E.; Griesenbach, U.; Kerem, B.; Lueck, J.D.; Stanleigh, N.; Oren, Y.S. Gene, RNA, and ASO-Based Therapeutic Approaches in Cystic Fibrosis. J. Cyst. Fibros. 2023, 22 (Suppl. S1), S39–S44. [Google Scholar] [CrossRef]
- Zampoli, M.; Morrow, B.M.; Paul, G. Real-World Disparities and Ethical Considerations with Access to CFTR Modulator Drugs: Mind the Gap! Front. Pharmacol. 2023, 14, 1163391. [Google Scholar] [CrossRef] [PubMed]
- McGarry, M.E.; Gibb, E.R.; Laguna, T.A.; O’Sullivan, B.P.; Sawicki, G.S.; Zobell, J.T. How Many Billions Is Enough? Prioritizing Profits over Patients with Cystic Fibrosis. Pediatr. Pulmonol. 2023, 58, 1595–1597. [Google Scholar] [CrossRef]
- Werblińska, A.; Zielińska, D.; Szlanga, L.; Skrzypczak, P.; Bryl, M.; Piwkowski, C.; Gabryel, P. The Impact of Nutritional Support on Outcomes of Lung Cancer Surgery-Narrative Review. J. Clin. Med. 2025, 14, 3197. [Google Scholar] [CrossRef]
- da Silva Filho, L.V.R.F.; Zampoli, M.; Cohen-Cymberknoh, M.; Kabra, S.K. Cystic Fibrosis in Low and Middle-Income Countries (LMIC): A View from Four Different Regions of the World. Paediatr. Respir. Rev. 2021, 38, 37–44. [Google Scholar] [CrossRef]
- Guo, J.; Garratt, A.; Hill, A. Worldwide Rates of Diagnosis and Effective Treatment for Cystic Fibrosis. J. Cyst. Fibros. 2022, 21, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Solomon, M.; Bozic, M.; Mascarenhas, M.R. Nutritional Issues in Cystic Fibrosis. Clin. Chest Med. 2016, 37, 97–107. [Google Scholar] [CrossRef]
- Corey, M.; McLaughlin, F.J.; Williams, M.; Levison, H. A Comparison of Survival, Growth, and Pulmonary Function in Patients with Cystic Fibrosis in Boston and Toronto. J. Clin. Epidemiol. 1988, 41, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Yankaskas, J.R.; Marshall, B.C.; Sufian, B.; Simon, R.H.; Rodman, D. Cystic Fibrosis Adult Care: Consensus Conference Report. Chest 2004, 125, 1S–39S. [Google Scholar] [CrossRef]
- Turck, D.; Braegger, C.P.; Colombo, C.; Declercq, D.; Morton, A.; Pancheva, R.; Robberecht, E.; Stern, M.; Strandvik, B.; Wolfe, S.; et al. ESPEN-ESPGHAN-ECFS Guidelines on Nutrition Care for Infants, Children, and Adults with Cystic Fibrosis. Clin. Nutr. 2016, 35, 557–577. [Google Scholar] [CrossRef] [PubMed]
- Diet, Nutrition and the Prevention of Chronic Diseases; WHO Technical Report Series; World Health Organization: Geneva, Switzerland, 2003; Volume 916, i–viii, 1–149, backcover.
- Stallings, V.A.; Stark, L.J.; Robinson, K.A.; Feranchak, A.P.; Quinton, H.; Clinical Practice Guidelines on Growth and Nutrition Subcommittee; Ad Hoc Working Group. Evidence-Based Practice Recommendations for Nutrition-Related Management of Children and Adults with Cystic Fibrosis and Pancreatic Insufficiency: Results of a Systematic Review. J. Am. Diet. Assoc. 2008, 108, 832–839. [Google Scholar] [CrossRef]
- Principato, L.; Pice, G.; Pezzi, A. Understanding Food Choices in Sustainable Healthy Diets—A Systematic Literature Review on Behavioral Drivers and Barriers. Environ. Sci. Policy 2025, 163, 103975. [Google Scholar] [CrossRef]
- Anastasiou, K.; Baker, P.; Hadjikakou, M.; Hendrie, G.A.; Lawrence, M. A Conceptual Framework for Understanding the Environmental Impacts of Ultra-Processed Foods and Implications for Sustainable Food Systems. J. Clean. Prod. 2022, 368, 133155. [Google Scholar] [CrossRef]
- Fao, I. The State of Food Security and Nutrition in the World 2023; FAO: Rome, Italy; IFAD: Rome, Italy; UNICEF: Mumbai, India; WFP: Rome, Italy; WHO: Geneva, Switzerland, 2023; ISBN 978-92-5-137226-5. [Google Scholar]
- Sutherland, R.; Katz, T.; Liu, V.; Quintano, J.; Brunner, R.; Tong, C.W.; Collins, C.E.; Ooi, C.Y. Dietary Intake of Energy-Dense, Nutrient-Poor and Nutrient-Dense Food Sources in Children with Cystic Fibrosis. J. Cyst. Fibros. 2018, 17, 804–810. [Google Scholar] [CrossRef]
- Matel, J.L. Nutritional Management of Cystic Fibrosis. JPEN J. Parenter. Enter. Nutr. 2012, 36, 60S–67S. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, B.A.; Goldsweig, B.K.; Sidhaye, A.; Blackman, S.M.; Schindler, T.; Moran, A. Cystic Fibrosis Related Diabetes: Nutrition and Growth Considerations. J. Cyst. Fibros. 2019, 18 (Suppl. S2), S32–S37. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, M.; Mandecka, A.; Regulska-Ilow, B. Nutritional status disorders in cystic fibrosis–dietary recommendations and supplementation. A review. Med. Og. Nauk. Zdr. 2017, 23, 115–121. [Google Scholar] [CrossRef]
- Engelen, M.P.K.J.; Com, G.; Deutz, N.E.P. Protein Is an Important but Undervalued Macronutrient in the Nutritional Care of Patients with Cystic Fibrosis. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 515–520. [Google Scholar] [CrossRef]
- Szentpetery, S.; Fernandez, G.S.; Schechter, M.S.; Jain, R.; Flume, P.A.; Fink, A.K. Obesity in Cystic Fibrosis: Prevalence, Trends and Associated Factors Data from the US Cystic Fibrosis Foundation Patient Registry. J. Cyst. Fibros. 2022, 21, 777–783. [Google Scholar] [CrossRef]
- Kuneš, J.; Hojná, S.; Mráziková, L.; Montezano, A.; Touyz, R.M.; Maletínská, L. Obesity, Cardiovascular and Neurodegenerative Diseases: Potential Common Mechanisms. Physiol. Res. 2023, 72, S73–S90. [Google Scholar] [CrossRef] [PubMed]
- El Attar, M.M.; Azab, N.M.; El Dine Hamed, D.H.; Tawfik, A.S.A. Growth Assessment in Egyptian Children with Cystic Fibrosis: A Single Center Study. Egypt. Pediatr. Assoc. Gaz. 2017, 65, 21–24. [Google Scholar] [CrossRef]
- ECFS Patient Registry. Available online: https://www.ecfs.eu/projects/ecfs-patient-registry/project (accessed on 10 September 2024).
- Gaskin, K.J. Nutritional Care in Children with Cystic Fibrosis: Are Our Patients Becoming Better? Eur. J. Clin. Nutr. 2013, 67, 558–564. [Google Scholar] [CrossRef]
- Bodirsky, B.L.; Dietrich, J.P.; Martinelli, E.; Stenstad, A.; Pradhan, P.; Gabrysch, S.; Mishra, A.; Weindl, I.; Le Mouël, C.; Rolinski, S.; et al. The Ongoing Nutrition Transition Thwarts Long-Term Targets for Food Security, Public Health and Environmental Protection. Sci. Rep. 2020, 10, 19778. [Google Scholar] [CrossRef]
- Ng, M.; Gakidou, E.; Lo, J.; Abate, Y.H.; Abbafati, C.; Abbas, N.; Abbasian, M.; ElHafeez, S.A.; Abdel-Rahman, W.M.; Abd-Elsalam, S.; et al. Global, Regional, and National Prevalence of Adult Overweight and Obesity, 1990–2021, with Forecasts to 2050: A Forecasting Study for the Global Burden of Disease Study 2021. Lancet 2025, 405, 813–838. [Google Scholar] [CrossRef]
- Micronutrients and Their Effects on Horticultural Crop Quality, Productivity and Sustainability. Sci. Hortic. 2024, 323, 112512. [CrossRef]
- Frantzen, T.; Barsky, S.; LaVecchia, G.; Marowitz, M.; Wang, J. Evolving Nutritional Needs in Cystic Fibrosis. Life 2023, 13, 1431. [Google Scholar] [CrossRef] [PubMed]
- Sinaasappel, M.; Stern, M.; Littlewood, J.; Wolfe, S.; Steinkamp, G.; Heijerman, H.G.M.; Robberecht, E.; Döring, G. Nutrition in Patients with Cystic Fibrosis: A European Consensus. J. Cyst. Fibros. 2002, 1, 51–75. [Google Scholar] [CrossRef]
- Dorlöchter, L.; Aksnes, L.; Fluge, G. Faecal Elastase-1 and Fat-Soluble Vitamin Profiles in Patients with Cystic Fibrosis in Western Norway. Eur. J. Nutr. 2002, 41, 148–152. [Google Scholar] [CrossRef]
- Rana, M.; Wong-See, D.; Katz, T.; Gaskin, K.; Whitehead, B.; Jaffe, A.; Coakley, J.; Lochhead, A. Fat-Soluble Vitamin Deficiency in Children and Adolescents with Cystic Fibrosis. J. Clin. Pathol. 2014, 67, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Greer, R.M.; Buntain, H.M.; Lewindon, P.J.; Wainwright, C.E.; Potter, J.M.; Wong, J.C.; Francis, P.W.; Batch, J.A.; Bell, S.C. Vitamin A Levels in Patients with CF Are Influenced by the Inflammatory Response. J. Cyst. Fibros. 2004, 3, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Hakim, F.; Kerem, E.; Rivlin, J.; Bentur, L.; Stankiewicz, H.; Bdolach-Abram, T.; Wilschanski, M. Vitamins A and E and Pulmonary Exacerbations in Patients with Cystic Fibrosis. J. Pediatr. Gastroenterol. Nutr. 2007, 45, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Loukou, I.; Moustaki, M.; Sardeli, O.; Plyta, M.; Katsagoni, C.N.; Douros, K. Association of Vitamin A Status with Lung Function in Children and Adolescents with Cystic Fibrosis. Pediatr. Investig. 2021, 5, 125–129. [Google Scholar] [CrossRef]
- Daley, T.; Hughan, K.; Rayas, M.; Kelly, A.; Tangpricha, V. Vitamin D Deficiency and Its Treatment in Cystic Fibrosis. J. Cyst. Fibros. 2019, 18 (Suppl. S2), S66–S73. [Google Scholar] [CrossRef]
- El Miedany, Y.; Toth, M.; Mohamed El Gaafary, M.; Mahran, S.A.; Hassan, W.; Hassan Abu-Zaid, M.; Elwakil, W.; Basyoni Selim, W.; Ahmed Sultan, E.; Saber Ibraheem, G.; et al. Vitamin D Management Update: Evidence-Based Guidelines for Vitamin D Optimization by the Egyptian Academy for Bone and Muscle Health. Egypt. Rheumatol. Rehabil. 2025, 52, 34. [Google Scholar] [CrossRef]
- Sermet-Gaudelus, I.; Bianchi, M.L.; Garabédian, M.; Aris, R.M.; Morton, A.; Hardin, D.S.; Elkin, S.L.; Compston, J.E.; Conway, S.P.; Castanet, M.; et al. European Cystic Fibrosis Bone Mineralisation Guidelines. J. Cyst. Fibros. 2011, 10 (Suppl. S2), S16–S23. [Google Scholar] [CrossRef]
- Maqbool, A.; Stallings, V.A. Update on Fat-Soluble Vitamins in Cystic Fibrosis. Curr. Opin. Pulm. Med. 2008, 14, 574. [Google Scholar] [CrossRef] [PubMed]
- Sommerburg, O.; Hämmerling, S.; Schneider, S.P.; Okun, J.; Langhans, C.-D.; Leutz-Schmidt, P.; Wielpütz, M.O.; Siems, W.; Gräber, S.Y.; Mall, M.A.; et al. CFTR Modulator Therapy with Lumacaftor/Ivacaftor Alters Plasma Concentrations of Lipid-Soluble Vitamins A and E in Patients with Cystic Fibrosis. Antioxidants 2021, 10, 483. [Google Scholar] [CrossRef]
- Bergeron, C.; Potter, K.J.; Boudreau, V.; Ouliass, B.; Bonhoure, A.; Lacombe, J.; Mailhot, M.; Lavoie, A.; Ferron, M.; Ferland, G.; et al. Low Vitamin K Status in Adults with Cystic Fibrosis Is Associated with Reduced Body Mass Index, Insulin Secretion, and Increased Pseudomonal Colonization. Appl. Physiol. Nutr. Metab. 2023, 48, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, K.A.; Schall, J.I.; Stallings, V.A. Suboptimal Vitamin K Status despite Supplementation in Children and Young Adults with Cystic Fibrosis. Am. J. Clin. Nutr. 2010, 92, 660–667. [Google Scholar] [CrossRef]
- Declercq, D.; Van Braeckel, E.; Marchand, S.; Van Daele, S.; Van Biervliet, S. Sodium Status and Replacement in Children and Adults Living with Cystic Fibrosis: A Narrative Review. J. Acad. Nutr. Diet. 2020, 120, 1517–1529. [Google Scholar] [CrossRef]
- Cystic Fibrosis Foundation; Borowitz, D.; Robinson, K.A.; Rosenfeld, M.; Davis, S.D.; Sabadosa, K.A.; Spear, S.L.; Michel, S.H.; Parad, R.B.; White, T.B.; et al. Cystic Fibrosis Foundation Evidence-Based Guidelines for Management of Infants with Cystic Fibrosis. J. Pediatr. 2009, 155, S73–S93. [Google Scholar] [CrossRef]
- Al-Ghimlas, F.; Faughnan, M.E.; Tullis, E. Metabolic Alkalosis in Adults with Stable Cystic Fibrosis. Open Respir. Med. J. 2012, 6, 59–62. [Google Scholar] [CrossRef]
- Uijterschout, L.; Nuijsink, M.; Hendriks, D.; Vos, R.; Brus, F. Iron Deficiency Occurs Frequently in Children with Cystic Fibrosis. Pediatr. Pulmonol. 2014, 49, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Ehrhardt, P.; Miller, M.G.; Littlewood, J.M. Iron Deficiency in Cystic Fibrosis. Arch. Dis. Child. 1987, 62, 185–187. [Google Scholar] [CrossRef]
- Shteinberg, M.; Haq, I.J.; Polineni, D.; Davies, J.C. Cystic Fibrosis. Lancet 2021, 397, 2195–2211. [Google Scholar] [CrossRef] [PubMed]
- Kałużna-Czyż, M.; Grzybowska-Chlebowczyk, U.; Woś, H.; Więcek, S. Serum Hepcidin Level as a Marker of Iron Status in Children with Cystic Fibrosis. Mediat. Inflamm. 2018, 2018, 3040346. [Google Scholar] [CrossRef]
- Gore, A.P.; Kwon, S.H.; Stenbit, A.E. A Roadmap to the Brittle Bones of Cystic Fibrosis. J. Osteoporos. 2010, 2011, 926045. [Google Scholar] [CrossRef]
- Sands, D.; Mielus, M.; Umławska, W.; Lipowicz, A.; Oralewska, B.; Walkowiak, J. Evaluation of Factors Related to Bone Disease in Polish Children and Adolescents with Cystic Fibrosis. Adv. Med. Sci. 2015, 60, 315–320. [Google Scholar] [CrossRef]
- Lynch, S.; Pfeiffer, C.M.; Georgieff, M.K.; Brittenham, G.; Fairweather-Tait, S.; Hurrell, R.F.; McArdle, H.J.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND)-Iron Review. J. Nutr. 2018, 148, 1001S–1067S. [Google Scholar] [CrossRef]
- Lin, P.-H.; Sermersheim, M.; Li, H.; Lee, P.H.U.; Steinberg, S.M.; Ma, J. Zinc in Wound Healing Modulation. Nutrients 2017, 10, 16. [Google Scholar] [CrossRef]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health 2018, 21, 27–32. [Google Scholar] [CrossRef]
- Ibs, K.-H.; Rink, L. Zinc-Altered Immune Function. J. Nutr. 2003, 133, 1452S–1456S. [Google Scholar] [CrossRef] [PubMed]
- Ataee, P.; Najafi, M.; Gharagozlou, M.; Aflatounian, M.; Mahmoudi, M.; Khodadad, A.; Farahmand, F.; Motamed, F.; Fallahi, G.H.; Kalantari, N.; et al. Effect of Supplementary Zinc on Body Mass Index, Pulmonary Function and Hospitalization in Children with Cystic Fibrosis. Turk. J. Pediatr. 2014, 56, 127–132. [Google Scholar] [PubMed]
- Maqbool, A.; Schall, J.I.; Zemel, B.S.; Garcia-Espana, J.F.; Stallings, V.A. Plasma Zinc and Growth Status in Preadolescent Children with Cystic Fibrosis. J. Pediatr. Gastroenterol. Nutr. 2006, 43, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Van Biervliet, S.; Vande Velde, S.; Van Biervliet, J.P.; Robberecht, E. The Effect of Zinc Supplements in Cystic Fibrosis Patients. Ann. Nutr. Metab. 2008, 52, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Abdulhamid, I.; Beck, F.W.J.; Millard, S.; Chen, X.; Prasad, A. Effect of Zinc Supplementation on Respiratory Tract Infections in Children with Cystic Fibrosis. Pediatr. Pulmonol. 2008, 43, 281–287. [Google Scholar] [CrossRef]
- Berry, A.J. Pancreatic Enzyme Replacement Therapy during Pancreatic Insufficiency. Nutr. Clin. Pract. 2014, 29, 312–321. [Google Scholar] [CrossRef]
- Schindler, T.; Michel, S.; Wilson, A.W.M. Nutrition Management of Cystic Fibrosis in the 21st Century. Nutr. Clin. Pract. 2015, 30, 488–500. [Google Scholar] [CrossRef]
- Ratchford, T.L.; Teckman, J.H.; Patel, D.R. Gastrointestinal Pathophysiology and Nutrition in Cystic Fibrosis. Expert. Rev. Gastroenterol. Hepatol. 2018, 12, 853–862. [Google Scholar] [CrossRef]
| Age Group | Energy Needs (% RDA) | Fat (%) | Protein (%) | Carbohydrates (%) | Vitamin A (μg/d) | Vitamin D (IU/d) | Vitamin E (IU/d) | Vitamin K (μg/d) |
|---|---|---|---|---|---|---|---|---|
| Infants (0–12 m) | 110–120% | 35–40% | 10–15% | 45–55% | 400–500 | 400–800 | 50 | 0.3–1.0 |
| Toddlers (1–3 y) | 120–140% | 35–40% | 10–20% | 40–50% | 300–400 | 600–1000 | 100 | 1–5 |
| Children (4–8 y) | 130–150% | 35–40% | 15–20% | 40–45% | 400–500 | 800–1200 | 200 | 5–10 |
| Adolescents (9–18) | 140–200% | 35–40% | 20% | 40–45% | 600–700 | 1000–2000 | 400 | 10 |
| Nutrient | Clinical Manifestations | Recommended Monitoring | Supplementation/Intervention |
|---|---|---|---|
| Vitamin A | Night blindness, xerophthalmia, epithelial dysfunction | Serum retinol levels | 1 mg/kg/day β-carotene (max 10 mg/day); monitor levels with modulators |
| Vitamin D | Osteopenia, rickets, muscle cramps, poor bone mineralization | Serum 25(OH)D | 400 IU/day (infants) to 1000–2000 IU/day (older children/adolescents) |
| Vitamin E | Neuropathy, ataxia, hemolytic anemia | α-tocopherol to cholesterol ratio | 50 IU/day (infants), 100–400 IU/day (older children) |
| Vitamin K | Coagulation disorders, bleeding, impaired bone health | Prothrombin time (indirect), INR | 0.3–1.0 mg/day (infants); 1–10 mg/day (older children); ↑ during antibiotics |
| Sodium | Growth disturbances, hyponatremia, muscle weakness | Serum/urine sodium, clinical assessment | Routine salt supplementation individualized by age and sweat loss |
| Iron | Anemia, growth delay, reduced immunity | Ferritin (consider inflammatory markers) | 1–2 mg/kg/day elemental iron; monitor ferritin and CRP |
| Zinc | Growth retardation, poor immunity, poor appetite | Serum zinc levels | Supplement if deficient; doses up to 5 mg/kg/day in severe cases |
| Calcium | Osteoporosis, bone fractures, poor mineralization | Dietary intake + bone density (DEXA) | Dietary enrichment + ensure adequate vitamin D + K status |
| Age Group | Recommended Lipase Units per Meal | Maximum Daily Dose | Notes |
|---|---|---|---|
| Infants (0–12 m) | 2000–4000 U per 120 mL milk | ≤10,000 U/kg/day | Based on volume of milk or formula |
| Toddlers (1–4 y) | 2000–4000 U/g fat | ≤10,000 U/kg/day | Dosing adjusted to fat content |
| Children (4–8 y) | 500 U/kg/meal | ≤2500 U/kg/meal | Titrate based on stool consistency and weight gain |
| Adolescents (9–18) | 500–1000 U/kg/meal | ≤10,000 U/kg/day | Higher doses may be split across meals/snacks |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazur, M.; Malik, A.; Pytka, M.; Popiołek-Kalisz, J. The Importance of an Adequate Diet in the Treatment and Maintenance of Health in Children with Cystic Fibrosis. Int. J. Transl. Med. 2025, 5, 38. https://doi.org/10.3390/ijtm5030038
Mazur M, Malik A, Pytka M, Popiołek-Kalisz J. The Importance of an Adequate Diet in the Treatment and Maintenance of Health in Children with Cystic Fibrosis. International Journal of Translational Medicine. 2025; 5(3):38. https://doi.org/10.3390/ijtm5030038
Chicago/Turabian StyleMazur, Michał, Agnieszka Malik, Monika Pytka, and Joanna Popiołek-Kalisz. 2025. "The Importance of an Adequate Diet in the Treatment and Maintenance of Health in Children with Cystic Fibrosis" International Journal of Translational Medicine 5, no. 3: 38. https://doi.org/10.3390/ijtm5030038
APA StyleMazur, M., Malik, A., Pytka, M., & Popiołek-Kalisz, J. (2025). The Importance of an Adequate Diet in the Treatment and Maintenance of Health in Children with Cystic Fibrosis. International Journal of Translational Medicine, 5(3), 38. https://doi.org/10.3390/ijtm5030038







