Abstract
Apolipoprotein A-I (apoA-I)-coated nanoemulsion particles target scavenger receptors. Adsorbed apoA-I (from the bloodstream) mediates/facilitates this targeted molecular contact, which is followed by receptor-mediated endocytosis and subsequent transcytosis of these same nanoemulsion (nanocarrier) particles across the blood–brain barrier (BBB). When the right drugs are added in advance to these high-density lipoprotein (HDL)-like nanocarriers, multifunctional combination treatment is achieved. This medication penetrates the BBB and targets particular cell-surface scavenger receptors, mainly class B type I (SR-BI). As a result, these (drug-carrying) nanoemulsions may find application in the biomedical therapy of complex medical disorders, such as dementia, as well as some aspects of aging. According to recent research, sustained inflammatory stimulation in the gut, such as via serum amyloid A (SAA), may cause the release of proinflammatory cytokines. Thus, using this “HDL-like” nanoemulsion vehicle to target drugs early (or even proactively) toward a major SAA receptor (like SR-BI), which is implicated in SAA-mediated cell-signaling processes that lead to aging and/or cognitive decline (and eventually Alzheimer’s disease or dementia), may be a useful preventive and therapeutic strategy.
1. Introduction
An ability to deliver therapeutically active agents to diseased tissues and cells, while avoiding damage to healthy tissues and cells, has presented a difficult and long-standing problem for physicians treating patients (e.g., [1,2,3]). In an effort to overcome this problem, much biomedical research (over the last few decades) has been directed to targeting drug-delivery toward the pathogenic cascades involved in age-related chronic disease [4,5,6].
Using targeting of anticancer drugs as a useful example regarding ligand–receptor interaction, in the course of ordinary chemotherapy the antineoplastic drugs reach the tumor tissue with poor specificity and dose-limiting toxicity. To overcome this problem, the typical overexpression of receptors in human cancers lends itself to efficient (active) uptake of particulate substances via receptor-mediated endocytosis, whereby extracellular particles gain entry into the intracellular environment. In general, the antineoplastic drug bound to the targeted nanoparticle carrier begins passage into the tumor cell by virtue of specific ligand–receptor interactions. Once localized at the tumor cell surface, the targeted drug–nanoparticle carrier complexes may exert their cytosolic action either at the plasma membrane or following internalization. Accordingly, dissociation of the drug from the nanoparticle can occur at the (adjacent) extracellular space, at the cell surface, or in lysosomes by lysosomal enzymes—resulting in the release of free drug into the cytosol (cf. [1]).
Most mammalian cell-surface receptors, which mediate endocytosis, exhibit two common ligand-binding characteristics: high-affinity and narrow specificity. The ligand-binding properties of some later-characterized (mammalian) receptors, that is, the “multiligand lipoprotein receptors” which include scavenger receptors, do not conform to a narrow binding specificity. These receptors bind, with high affinity, both lipoprotein and non-lipoprotein ligands and participate in a wide variety of biological processes (e.g., [1] and see below).
Lipid nanoparticles (i.e., non-lamellar lipid nanostructures) can be utilized as drug, protein/peptide, or nucleic acid targeted-delivery nanosystems, which are able to carry a wide variety of hydrophilic, hydrophobic, and amphiphilic small molecules and biomacromolecules [7]. The persistence of lipid nanoparticles (e.g., drug nanocarriers), in the bloodstream, is strongly affected by physical interactions with specific blood-circulating components. These components are called opsonins (which include complement proteins, immunoglobulins, and numerous other biogenic molecules). Surface binding of opsonins (i.e., opsonization) promotes the removal of foreign particles from the circulation within seconds to minutes via the mononuclear phagocytic system (MPS), also known as the reticuloendothelial system (RES), and by phagocytic macrophages located in the liver. This natural body-defense process, unfortunately, also promotes the removal of drug nanocarriers from the bloodstream [8]. Specific surface features of nanocarriers play a key role in the opsonization process. For example, it has been reported that charged particles undergo higher opsonization as compared to neutrally charged and (most notably) nonionic particles. The consequence of avoiding opsonization is the prolongation of the particle (i.e., nanocarrier) persistence in the bloodstream from a few seconds or minutes to, instead, up to a many-fold longer duration. Such enhanced prolongation of nanocarrier persistence in the circulation is consistent with observations of nonionic nanoemulsion drug delivery characteristics. Accordingly, this targeted (nonionic) nanoemulsion therapeutic could be used for repurposing of many drugs and treatments for both oncology and neurodegeneration [1]. (At the same time, it should also be mentioned that there is a need to monitor for potential side effects of lipid nanoemulsion particle-mediated transport in relation to the targeting of other cell types—for example, outside the brain, in the liver, and/or on macrophages.)
An intricate interplay exists between the vascular system and degenerative diseases, which has profound implications for disease progression and management (e.g., pharmacological targeting [see below]). Dysfunction in the vascular system—characterized by impaired blood flow, endothelial dysfunction, and vascular inflammation—emerges as a common denominator of degenerative diseases across multiple organ systems [9,10,11]. Accordingly, it has been widely reported that endothelial modulation and repair is feasible by using pharmacological targeting (e.g., [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27]). In addition, within the nervous system, the influence of vascular factors on neurodegenerative diseases is emphasized by the observed critical roles of cerebral blood flow regulation as well as blood–brain barrier (BBB) status [10,27,28]. Importantly, nanoemulsion formulations [see below] are well-suited for targeting their drug cargo toward major receptor sites on the BBB—which influence its functional capacity (cf. [29,30,31]).
2. BBB Scavenger Receptors, “apo A-I” Affinity for BBB, Colloidal-Nanocarrier Targeting of SR-BI, and Colloidal Lipid Self-Assembly
Adsorption of apolipoprotein (apo)A-I from the circulation onto the surface of specific colloidal lipid particles (following intravenous injection of such colloidal nanocarriers) has already been regularly demonstrated in the research literature. These apoA-I-coated colloidal nanocarriers therefore target several BBB scavenger receptors [12,32,33]. During the subsequent receptor-mediated endocytosis and then, in turn, transcytosis of the nanocarrier particles across the blood–brain barrier, the adsorbed apoA-I mediates/facilitates this specific molecular interaction/sequence. [34,35]. The addition of the right medicine or drugs to these biomimetic nanocarriers beforehand results in a multifunctional combination therapy. By targeting particular cell-surface scavenger receptors, mainly class B type I (SR-BI), this therapeutic nanoemulsion crosses the BBB [1,34]. These various authors claim that improving plasma apoA-I delivery may alter or increase transcytosis across the BBB, which could then help increase the transport of therapeutic drugs in conjunction with “HDL-like synthetic particles” across the BBB to treat neurodegenerative diseases like Alzheimer’s disease [4,34]. It has been reported in the biomedical literature that the lipid shells and contents of naturally occurring HDL versus artificial biomimetic (nanoemulsion) nanocarrier particles share structural similarities. This allows the nanocarrier particles to partially mimic or simulate the known heterogeneity (i.e., subpopulations or subspecies) of native HDL particles [1]. As a result, “colloidal drug nanocarriers” have the potential to be employed in the biomedical management of complex medical disorders (such as tumor growth), and it is further suggested that these scavenger receptor-seeking nanocarriers may be crucial locally in controlling atherosclerotic lesion development in the arterial wall in humans (cf. [35]). The ultimate objective of “targeted chemotherapy” of atherosclerotic lesions in the cerebrovasculature, at different stages of development (using selected drugs) in humans, appears particularly well suited for an appropriate colloidal drug-nanocarrier vehicle (cf. below); namely, SR-BI/CLA-1 [the human SR-BI ortholog] has emerged as the most likely candidate receptor for primary involvement in ligand–receptor binding of selected nanoemulsion (drug-carrying) vehicles [1].
With regard to prospective human clinical trials involving the CLA-1 [human SR-BI ortholog]/SR-BI receptor for targeted chemotherapy of atherosclerosis, three potential routes for drug delivery have already been proposed in the biomedical literature, i.e., via macrophages or platelets or the liver—all of which entail receptor-mediated processes where SR-BI/CLA-1 plays a key role. This situation is well suited to a small group of (protein-free) “actively targeted” lipid (nanoemulsion) nanocarriers for which SR-BI/CLA-1 emerged as the most likely candidate receptor for primary or major involvement in ligand–receptor binding of such nanoemulsions at selected target cells. For example, investigators have found that i.v. administration of “recombinant chylomicrons” (comprising commercially available lipids to form these manufactured lipid-nanoemulsion particles, and which were designed and expected to acquire apolipoproteins upon incubation with serum) led to selective targeting to liver parenchymal cells. Specifically, incorporation of drug into “recombinant chylomicrons” resulted in a 40-fold increased liver uptake, and sharply reduced extrahepatic deposition, as compared to free drug [1].
Furthermore, as concerns lipid nanoparticles and/or colloidal-drug nanocarriers in the bloodstream, many of their overlapping biophysical mechanisms also occur in another example of a related physiological process; specifically, these lipid-based biophysical mechanisms are readily encountered when examining the intestinal processing of fats in humans. Fat entering the intestine undergoes emulsification through the action of various cholesterol derivatives—the bile salts (cf. [1]). After subsequent enzymatic degradation (of the emulsified fat), the monoglycerides, cholesterol derivatives (bile salts), cholesterol, or other lipid components then spontaneously form “mixed micelles” in the intestinal lumen. Besides supplying the bile salts for the above fat emulsification process, the liver bile also displays similar self-assembly of related ‘mixed’ micelles (containing cholesterol derivatives [bile salts], cholesterol, and also phospholipid) as well as ‘simple’ micelles (containing only cholesterol derivatives [bile salts] and cholesterol), in addition to other colloidal structures. Therefore, simple micelles coexist with the mixed micelles, as well as with cholesterol-rich (lipid-bilayer) vesicles, in bile under most physiological conditions. The small unilamellar vesicles have been shown to be important carriers of cholesterol in bile, and to serve as a major source of cholesterol for nucleation; in addition, cholesterol nucleation in bile involves several lipid structural transitions probably beginning with vesicle aggregation and/or fusion, and subsequent formation of “liquid crystals” (cf. Section 7).
The above description of the molecular interplay among cholesterol-rich simple micelles, mixed micelles, unilameller vesicles, “liquid crystals”, and solid cholesterol crystals in both model and human biles are analogous to, and further support, the earlier proposed [1] molecular lipid interplay within certain biocompatible, colloidal-lipid (nanoemulsion) nanocarrier formulations. Hence, the self-assembling mixed-lipid (nanoemulsion) nanoparticle population, measured in the stable (Filmix®) nanocarrier agent (cf. Section 5 and Section 7 [see below]), likely largely represents a colloidal dispersion of lipid “liquid crystals”—and can include more than one type of liquid-crystalline structure (cf. [36]). For example, liquid-crystalline cubic phases are formed spontaneously when certain amphiphilic lipids (commonly monoglycerides) are placed in an aqueous environment. These self-assembled cubic phases formed by monoglycerides are resistant to changes in temperature, and such dispersions of cubic phases at the nanometer scale have been previously explored by other scientists for use as drug-delivery systems. This particular feature of monoglycerides is significant in this context, as they represent the largest single-lipid component (by weight) of the powdered solid lipid mixture utilized to create the (Filmix® nanoemulsion) nanocarrier agent (cf. Section 5 and Section 7). While monoglycerides generally exhibit low solubility in water, they possess free hydroxyl groups that can form hydrogen bonds with water, surfactants, cosolvents, and so on. As polar lipids, monoglycerides typically: (1) provide improved solvent properties for drugs; (2) function as “cosurfactants” that enhance the mutual solubility of excipients [inactive ingredients]; (3) facilitate water absorption; and (4) encourage the self-dispersibility of lipid formulations. Also, it is worth noting that cholesterol and its esters change the packing structure of lipids, and in high local concentrations, they are known to induce the formation of a liquid-crystal phase (under some conditions, a liquid-crystal phase may also coexist with a solid phase). Furthermore, Kuntsche et al. [36] have synthesized lipid nanoparticles in the liquid-crystalline (or mesomorphic) phase using cholesterol esters with saturated acyl chains. The researchers were inspired by the understanding that numerous cholesterol esters are natural lipid compounds capable of forming liquid-crystalline phases (thermotropic mesophases), which led them to explore their potential as a delivery system for lipophilic drugs via liquid-crystalline nanoparticles [36]. In line with the aforementioned findings and considerations, the significant presence of cholesterol esters and cholesterol in the (Filmix® nanoemulsion) lipid nanocarrier formulation likely contributes to the established stability of its (liquid-crystalline) nanoparticles within this nanocarrier agent [1].
3. “HDL-like” Nanocarrier, Serum Amyloid A (SAA) Inflammatory Effects, and SAA Versus HDL-Mimetic Targeting of SR-BI
Widespread inflammation and oxidative stress, which involve various pathophysiological pathways, are triggered by factors that increase the risk of dementia. Recent research indicates that particularly proinflammatory cytokines may be released as a result of a sustained inflammatory stimulus in the gut, such as via serum amyloid A (SAA) [37,38]. Consequently, targeting a key SAA receptor (such as CLA-1, the human SR-BI ortholog) that is involved in SAA-mediated cell signaling processes associated with cognitive decline (including Alzheimer’s disease or late-onset dementia) could be a viable strategy for prevention and treatment. By increasing the concentration level of HDL or, more preferably, the (synthetic, exogenously administered) “HDL-like” [nanocarrier] nanoemulsion particle concentration (see below) in the blood, relative to circulating free SAA, the clinician may be able to prevent SAA-mediated endothelial dysfunction (and other SAA-mediated proinflammatory processes) [39]. This proposed strategy requires further experimental validation.
Hence (in Abstract; Section 1, paragraphs 2,4,5; Section 2, paragraphs 1,2; Section 3, paragraph 1), it has been repeatedly summarized that various pathophysiological pathways are activated by factors that increase the risk of dementia. At the same time, however, the proposed "HDL-like") nanocarrier strategy carries a risk from potential side effects of lipid nanoemulsion particle-mediated transport in relation to the targeting of other cell types (e.g., outside the brain). Such potential side effects need to be indicated when considering the role of apoA-I in Alzheimer’s disease. In addition, Xie et al. recently [35] attempted to elucidate many of the physiological functions and metabolic pathways of apoA-I, integrating its associations with Alzheimer’s disease-related pathologies, risk factors, and potential therapeutic targets. These authors believe that by elucidating the mechanisms by which apoA-1 traverses between peripheral systems and the CNS, along with its modulation of Alzheimer’s disease pathology, could provide novel insights into Alzheimer’s disease progression [35].
The above-described “HDL-like” (nanoemulsion) nanocarriers are not to be confused with “native” lipoproteins. Attempted commercial utilization of native lipoproteins, as a drug-delivery vehicle, is fraught with unnecessary difficulties—such as the complications associated with large-scale isolation, biosafety issues, and hence the excessive cost involved in the development of lipoprotein–drug combinations/formulations. Instead, employing only nonionic lipids in the “HDL-like” nanocarrier, for creating the drug-delivery vehicle, avoids such unnecessary difficulties [1].
4. Biological Aging, Chronic Illness, Dementia, Diabetes/Obesity and Drug Targeting
As with many chronic illnesses, the most significant contributing factor to the development of Alzheimer’s disease and related dementias (ADRD) is aging [4]. Even with normal aging, cognitive decline and some neurodegenerative alterations are evident, indicating that comparable pathophysiological pathways may be engaged in neurodegenerative illness as well as in older adults who do not experience dementia over their lifetimes [40,41]. The underlying pathophysiology of ADRD has been linked to biological aging processes, according to recent evaluations of clinical and translational studies. Therefore, in a mostly unexplored approach, ADRD and age-related cognitive decline may both be ameliorated by concentrating on the fundamental processes that underpin biological aging [40]. The field of biology pertaining to aging has made significant strides in identifying the pathophysiological mechanisms underlying biological aging and multisystem organ deterioration [40,42]. For instance, the BBB may be compromised by oxidative stress and systemic inflammation induced by cerebral vascular risk factors. Serum amyloid A (SAA) is one example of a proinflammatory cytokine that may be generated in response to prolonged inflammatory stimulation in the gut, according to more recent research [43]. Meanwhile, because of the increased permeability of the BBB caused by aging and/or dysfunction, such proinflammatory cytokines might enter the brain tissue and cause glia reactivity [44]. Similarly, it has been observed that neuroinflammation after traumatic brain injury is a persistent reaction to an acute insult and is often associated with proinflammatory cytokine production and activated microglia [45,46]. In addition, young adults who have moderate to severe head trauma are more than twice as likely to acquire Alzheimer’s disease or a similar dementia in the future. Since SAA-mediated cell signaling processes are linked to normal aging brain alterations [40] and/or actual dementia [11], adopting early (or even proactive) drug targeting toward a major SAA receptor (like CLA-1 [human SR-BI ortholog]) may serve to inhibit such SAA-mediated processes and thereby provide an effective preventive and therapeutic strategy [4].
Besides aging, the long-established link between the incidence of Alzheimer’s disease and diabetes (as well as obesity) [47] has also brought attention to GLUT-1, the primary facilitative glucose transporter protein in the brain, and its role in and likely contribution to neurodegenerative illness [48]. It was known more than 20 years ago that the normal human brain endothelium displays a high density of GLUT-1, but the cerebral microvessels of patients with Alzheimer’s disease had a much lower GLUT-1 density than age-matched controls. In subsequent thorough experiments, researchers found that decreased GLUT-1 expression (at the BBB) exacerbates the cerebrovascular degeneration, neuropathology, and cognitive function linked to Alzheimer’s disease. This suggests that (cerebral endothelial) GLUT-1 may be another therapeutic target for the vasculo-neuronal dysfunction and degeneration associated with Alzheimer’s disease. (Currently, GLUT-1 remains an experimental therapeutic target).
In addition, brain glucose dysregulation has been shown by other researchers to be a crucial step in the pathophysiology of Alzheimer’s disease, often closely reflecting the severity of the disease’s pathology and symptom expression. However, it is also possible for problems in brain glucose homeostasis to start years before clinical symptoms appear [48]. Accordingly, “pure” Alzheimer’s disease is far less prevalent than mixed dementias, where vascular alterations are present along with both protein tau tangles (in neurons) and extracellular amyloid-beta plaques, as seen by MRI scans in the clinic or by neuropathological evaluation at autopsy. Apart from the effects of senile plaques on the vasculature, such tau pathological changes (in neurons) can affect the biology of brain endothelial cells, which in turn can cause changes in the brain’s microvasculature (such as abnormal spiraling morphologies, decreased blood vessel diameters, and increased overall blood vessel density in the cerebral cortex). Hence, it is noteworthy to emphasize here that because vascular pathology is a frequent co-factor, such pathology may represent a promising early intervention target using the proposed (“HDL-like”) nanocarrier strategy. Also, the distinctive “senile plaques” that form in Alzheimer’s disease are extracellular deposits that are mostly made up of insoluble aggregates of amyloid-β protein (Aβ) fibrils that have been invaded by astrocytes and reactive microglia. Microglia have been identified as scavengers responsible for clearing Aβ fibril deposits of Alzheimer’s disease and, therefore, microglial scavenger receptors have already been identified as novel targets for therapeutic interventions in Alzheimer’s disease (cf. [1]).
With regard to blood glucose, the brain is an extremely energy-demanding organ whose operation is primarily dependent upon a reliable and effective energy source. Under physiological settings, the human brain utilizes up to 20% of the oxygen the body uses while we are awake, and 25% of the glucose that is in the blood when we are awake. Fatty acid and ketone body oxidation can only slightly replace glucose as the brain’s energy source. Because the brain is unable to absorb free fatty acids from the blood, it must rely on astrocytes to convert carbohydrates into fatty acids. As a result, all tissues—aside from the brain—may convert from utilizing glucose to instead using fatty acids, thereby conserving glucose for the brain. Regrettably, a fatty diet’s elevated blood fatty-acid levels hinder the liver’s ability to metabolize glucose, a phenomenon known as “glucose sparing.” This effect upregulates glucose to a hazardous level and plays a major role in the development of “type 2” diabetes. It is now increasingly clear that diabetes-related CNS disorders may be significantly impacted by blood–brain barrier dysfunction [49].
5. Manufacturing Considerations and Methodology
Among the many factors to be considered when designing an actual process for manufacturing the desired targeted nanoemulsion described above, it has been found that particular attention needs to be paid to the energetic method used to form the nanoemulsion itself. The importance of the amount and type of energy input into a manufacturing process relates to its potential physicochemical effect on nanoemulsion stability (i.e., it can introduce instability within the colloidal system [see below]).
Briefly, in the context of colloid science (and specifically with reference to nanoemulsions in general), “stability” is defined as a state in which the free energy of a colloidal dispersion is higher than that of the comparable bulk material. (In addition, a state of subdivision known as “colloidal” occurs when the particles, droplets, or bubbles scattered in a different phase have at least one dimension ranging from 1 to 1000 nm.) When a significant energy barrier impedes the removal of the colloidal state, the colloidal system becomes metastable and has the potential to stay in that condition for an extended period of time. Thus, the entire issue of colloidal system preparation and stability is intimately related to the elements that result in free-energy barriers high enough to stop the colloidal state from disintegrating. (Therefore, the word “metastable” refers to the equilibrium condition of the colloidal system, which corresponds to a local minimum of free energy.) The dispersion will stay in a metastable condition indefinitely if the free-energy barrier is large enough in comparison to the random thermal motion (Brownian motion) of the nanoemulsion particles. Accordingly, it is claimed that this dispersion, or nanoemulsion, is “colloidally stable” [1,50]. In summary, (lipid) nanoemulsions represent systems that are thermodynamically unstable, but they can still exhibit lengthy kinetic stability under particular conditions. Certain lipid nanoemulsions are especially well suited as colloidal drug-delivery vehicles for pharmaceutical applications because of their extended stability, small droplet size (as well as small liquid-crystal size), and availability of low-energy production methods [1,4]. In the present multidisciplinary paper, the term “LCM/ND” is used to accurately trace the chronological development (and functional conversion) of the “lipid-coated microbubble/nanoparticle-derived” (LCM/ND) colloidal system: (1) from its early biomedical application as an imaging agent (which focuses primarily on the less numerous micron-scale colloidal species) to (2) the later adaptation of the same mixed-lipid (e.g., Filmix®) colloidal system (which focuses increasingly upon the much more numerous nanoscale colloidal species) for nanomedical application as an LCM/ND drug-delivery vehicle. Furthermore, newer models of a few chosen particle-size-analysis instruments (see below) have revealed that over 99% of the same mixed-lipid colloidal species are documented to be smaller than 300 nm in diameter, whereas approximately 90% of the total LCM/ND colloidal species are actually smaller than 200 nm in diameter (detectable via optical-particle-counter data) [1].
Nanoemulsions are commonly formed, in industry, by some chosen “high-energy” manufacturing method [e.g., ultrasonic dispersion]. However, such “high-energy methods” often introduce a host of additional processing variables which are difficult to control by most entrepreneurial groups.
Contrariwise, the (previously patented [51,52]) LOW-energy manufacturing technology, of Cav-Con Inc., avoids most of the above-mentioned (processing and/or formulation) complications by only using simple mechanical shaking, a (scalable) filtration step, and selected (biobased, nonionic, stable) lipid components [e.g., monoglycerides, cholesterol derivatives] in the nanoemulsion film [8].
There has previously been much research on the physical characterization of the actual size distribution of the LCM/ND lipid nanoemulsion particles, i.e., the nanoemulsion made using the aforementioned “low-energy” production approach [1,50,53]. Five distinct optical particle counter models, all manufactured by Particle Measuring Systems (Boulder, CO), were used in these investigations to measure the scattered light. It is possible to conclude that the LCM/ND lipid nanoemulsion produced did not exhibit particle size variation under the various concentration settings because all of the data (from the five separate particle counters) were almost identical. There was no discernible shift in the size distribution over a minimum of one month [50,53]. This type of nanoemulsion has about 10 billion particles (less than 0.1 µm in diameter) per milliliter, as determined by optical particle counts. At least 90% of the entire population of nanoemulsion particles has a diameter of less than 0.2 µm (cf. Figure 1).
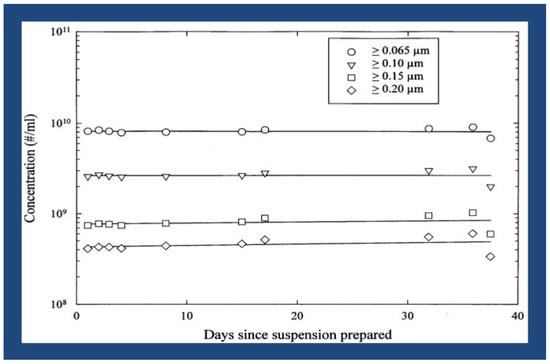
Figure 1.
LCM/ND nanoemulsion stability over time. (Reprinted from Ref. [4]).
The LCM/ND (mixed-lipid) nanoemulsion drug-delivery vehicle [i.e., the aqueous Filmix® suspension] is stable in vitro for more than 1 year when stored refrigerated. More specifically, the product specifications (i.e., particle size analyses) remain constant for many months when the LCM/ND agent is stored at room temperature, and for over 1 year when the LCM/ND agent is stored refrigerated (but not frozen). In addition, more detailed (collaborative) multidisciplinary analyses of the LCM/ND nanoemulsion colloidal system were carried out, particularly in submicron size range. This experiment series was performed (at CT Associates, Bloomington, MN) by preparing different dilutions of the LCM/ND nanoemulsion agent, then injecting the various dilutions into water at different rates using the dilution flow/system set up for their S100 instrument. All of the measurements were found to be essentially identical, indicating that the LCM/ND nanoemulsion particles did not change size when subjected to the different concentration conditions [1].
As previously mentioned, the lipid composition of LCM/ND nanoemulsion particles is comparable to that of chylomicron remnant particles [1] and HDL [see above]. Furthermore, it has been noted that the endocytic pathway or pathways used in the intracellular uptake of these three kinds of nanoparticles exhibit exceptional selectivity and speed of nanoparticle uptake. The size distribution of the LCM/ND nanoemulsion should then most likely fall within the same diameter range as that of chylomicron remnants. In particular, the remnant particles are of special interest because their size distribution is known to exhibit both larger sizes and a wider range (i.e., more polydispersity) than that of HDL or modified HDL particles. As a result, this polydispersity offers additional insight into the size restrictions or limitations imposed by the endocytic pathways involved in the active uptake of chylomicron remnant particles and most likely also of LCM/ND nanomulsion particles. Other researchers have already established, from in vivo and in vitro studies (using both reconstituted chylomicron remnant particles and chylomicron remnant-like emulsions), that the diameter range of the natural chylomicron remnants is roughly 100–200 nm (i.e., ~0.1–0.2 µm). [This reported diameter range for chylomicron remnants also is in agreement with the finding that the routine clearance of these particles in the human body, by the liver (i.e., liver sinusoids → endothelial fenestrae → space of Disse → hepatocytes), requires passage through endothelial fenestrae having diameters often ranging up to 200 nm (0.2 μm)] [1]. Hence, multidisciplinary analyses of the entire LCM/ND nanoemulsion’s particle size distribution were undertaken, with special attention given to the submicron size range, in order to determine what proportion of the LCM/ND nanoemulsion’s particle population actually falls within this diameter range of 0.1–0.2 μm (e.g., Figure 1).
Because there is no proof that the LCM/ND lipid nanoemulsion particles combine or coalesce into any “superparticle or microbubble-like” structure larger than 5 μm, the risk of embolism is minimal [1]. It was shown that the acute intravenous LD50 for dogs and rabbits was higher than 4.8 mL/kg. Moreover, at a dosage of 4.8 (mL/kg), no overt toxicity or fatalities were observed [4]. As specifically relates to these acute intravenous toxicity investigations (of the isotonic LCM/ND nanoemulsion agent administered i.v. into dogs and rabbits), these studies were carried out at an independent GLP contractor with regard to in vivo testing. The abbreviation “GLP” denotes that the research carried out by the aforementioned contractor complies with 21 CFR Part 58′s Good Laboratory Practices Regulations, which is intended to be submitted to the U.S. Food and Drug Administration in support of an Investigational New Drug Application (INDA). Thus, it is expected from this statement that there is no GLP regulation deviations that could compromise the study’s integrity or quality [4]. Further animal (range-finding subchronic intravenous) toxicology studies [1], using the same (isotonic) lipid nanoemulsion agent, yielded toxicological outcomes—at intravenous doses of 0.14 mL/kg given three times a week for six weeks (in rats) and i.v. doses of 0.48 mL/kg given three times a week for three months (in rabbits)—as follows: the histology of the adrenals, bladder, brain, heart, kidney, liver, lungs, marrow, pituitary, spleen, thyroid, and ureters did not change adversely, nor did the blood chemistry, liver functions, hematology, and coagulation profile [1,53].
6. Future Nanocarrier-Assisted Drug Delivery for Possible Targeted Treatment of Neurodegenerative Disease via GluN3A-Containing Receptors
Despite the availability of “limited treatment” for Alzheimer’s disease (from 2–3 decades ago), using acetylcholinesterase (AChE) inhibitors or memantine as an N-methyl-D-aspartate (NMDA) receptor antagonist, the prevalence of Alzheimer’s disease as a cause of death has continued to rise (Terpstra K et al. 2025 [preprint doi:10.2643/chemrxiv-2025-f9pnk]). These authors also report that development of small-molecule inhibitors of NMDA receptors (or of AChE) has continued in efforts to improve treatment of Alzheimer’s disease. While NMDA receptors have been studied extensively by neuroscientists for decades, the atypical subunit GluN3A (of the NMDA receptor family) was partially elucidated—including its potential role in cognition [see below]—only relatively recently in the biomedical literature.
The anatomical expression profile of GluN3A-containing receptors [see below], which includes the ventral hippocampus region, suggests a potential involvement in brain processes relevant to psychiatry—including cognition. The lack of a targeted pharmacological (i.e., drug-delivery) vehicle of sufficient quality, to be used for in vivo experiments, has impeded progress in our understanding at the systems level. While behavior of knock-out mice does support the hypothesis of GluN3A’s involvement in cognition, much more needs to be learned about GluN3A’s impact on physiology and behaviors (such as cognition) that are related to symptoms of mental illness (cf. von Heimendahl 2025 [https://www.opnme.com/opn2experts/cns-03-glun3a-psychiatric-symptoms (accessed on: 1 July 2025)]).
Some specific examples of recent publications, supporting the belief that GluN3A-containing receptors are involved in psychiatric symptoms of neurodegenerative disease, include as follows: Yu et al. [54] report that deficiency of NMDA receptor’s atypical subunit GluN3A is a trigger for chronic neuronal hyperactivity and disruption of Ca2+ homeostasis, leading to sporadic (or late-onset) Alzheimer’s disease phenotypes. In a separate study, Bossi et al. [55] conclude that GluN3A-containing excitatory glycine receptors represent a novel and widespread signaling modality in the adult brain (with attributes that strikingly depart from those of conventional NMDA receptors (NMDAR)). Most recently, Hurley et al. [56] state that their experiments, using male and female mice, demonstrated that GluN3A subunits are enriched in the adult ventral hippocampus. These authors also found that GluN3A knock-out mice enhanced both NMDAR-dependent calcium influx and NMDAR-dependent long-term potentiation in the ventral hippocampus. These investigators further state that, together, their data reveal a novel role for GluN3A (which can contribute to forming functional excitatory glycine receptors) in the control of ventral hippocampal circuits in the mature brain [56]. Finally, a related study by Zhong et al. [57] provides much added details to support this investigator group’s beliefs of the following: (1) GluN3A is involved in psychiatric symptoms of neurodegenerative disease; (2) is critical for Ca2+ homeostasis; (3) its deficiency is pathogenic for Alzheimer’s disease. These authors examined physiological and functional changes in GluN3A knock-out (KO) mice during aging. The GluN3A KO mouse brain displayed age-dependent moderate but persistent neuronal hyperactivity, elevated intracellular Ca2+, neuroinflammation, impaired synaptic integrity/plasticity, and neuronal loss. Accordingly, GluN3A KO mice developed olfactory dysfunction followed by psychological/cognitive deficits (—but before any detectable Aβ/tau pathology). In addition, memantine (administered at preclinical stage) prevented/attenuated Alzheimer’s disease syndromes. These authors also found that the brains of patients with Alzheimer’s disease show reduced GluN3A expression. To conclude, these investigators proposed that chronic “degenerative excitotoxicity” leads to sporadic (i.e., late-onset) Alzheimer’s disease, while GluN3A represents a primary pathogenic factor, an early biomarker, and an amyloid-independent therapeutic target. They also state that unlike many transgenic Alzheimer’s-disease mouse models, the GluN3A KO mouse develops virtually all major pathophysiology and age-dependent psychological, cognitive symptoms seen in (human) Alzheimer’s-disease patients [57].
In summary, while the above examples of peer-reviewed publications (from the recent biomedical literature) provide a clear indication that GluN3A is likely involved in the development of neurodegenerative disease, the continuing lack of a “selective pharmacological modulator” (or ‘targeted pharmacological drug-delivery vehicle’) of sufficient quality would, unfortunately, probably impede any substantial increases in understanding at the systems level. However, a newly developed (i.e., optimized) ‘targeted pharmacological vehicle’ may help circumvent this obstacle [see below].
Specifically, lipid nanoparticles (also known as non-lamellar lipid nanostructures) can be used as targeted-delivery nanosystems for drugs, proteins/peptides, or nucleic acids. These nanosystems can transport a wide range of small molecules and biomacromolecules that are hydrophilic, hydrophobic, and amphiphilic [7]. For historical context, it was previously noted that lipophilic medications have been administered intravascularly for many years using lipid-based dispersion systems, including liposomes, micellar systems, and simple emulsions [1]. The colloidal stability of a small number of these lipid-based liquid–crystalline dispersion systems in excess water, however, is a crucial characteristic that makes it possible for the predispersion of liquid-crystalline systems in blood plasma as submicron particles appropriate for intravenous drug delivery. Glycerides, cholesterol, and cholesterol esters are among the materials that are known to display this type of phase behavior; importantly, these substances are all found in significant amounts in the LCM/ND nanoemulsion formulations that have been previously discussed [1,4,8]. Since the LCM/ND nanoemulsion particles are recognized by receptors on a variety of Alzheimer’s-related cell types (e.g., [1,4]), the previously mentioned “selective pharmacological modulator” obstacle may now possibly be avoided when using these LCM/ND nanoemulsion formulations for the targeted delivery of GluN3A (or specific modulators and/or drugs). (Currently, the author is not aware of any specific pharmacological agents or ligands that modulate GluN3A; therefore, this speculative therapeutic approach is still hypothetical.)
7. Concluding Remarks
7.1. Various Patented (Past, Present, and/or Pending) Formulations of Lipid Colloids
It is useful at this point to consider several selected, patented, colloid types/classes—which help to delineate, protect, and/or guide commercialization—relating to certain chemotherapeutic lipid nanoemulsions for clinical application. Nanoemulsions can include more than one category of stable colloidal species in this context, so that a concise review of the formal definitions can be helpful here. The term “emulsion” may also be used to refer to “colloidal dispersions of ‘liquid crystals’ in a liquid”. Additionally, the term “liquid crystal” itself is defined as a phase that has mobility like that of a liquid, as well as a high degree of order like that in a crystal—also known as a mesophase or a mesomorphic phase (cf. [1]). The present examination will compare relevant, published patent specifications (and/or claims) as concerns several key lipid nanoemulsion components.
For historical perspective, it was reviewed earlier that past lipid-based dispersion systems (such as simple emulsions, micellar systems, and liposomes) have been utilized for intravascular administration of lipophilic drugs for several decades [1]. However, an important attribute of a limited number of these lipid-based liquid–crystalline dispersion systems is that they are colloidally stable in excess water, thereby allowing for the predispersion of liquid–crystalline systems in blood plasma in the form of submicron particles suitable for intravenous drug delivery. Materials known to exhibit such phase behavior include glycerides, cholesterol, and cholesterol esters—all of which are present in substantial concentrations in the LCM/ND nanoemulsion formulations described in Section 3, Section 4, and Section 6 above as well as Section 7.
For purposes of comparison, the category of colloids designated as “solid lipid nanoparticles” (or SLN), all with a solid lipid matrix, has already been studied for more than three decades, and is the topic of numerous published reports concerning improved drug-delivery vehicles. In addition to matrix lipid and drug, the many different examples of such solid nanoparticles all contain surfactants (e.g., often phospholipids) as stabilizers. Measurements of SLN sizes, reported in the literature, routinely yield average diameters in the nanometer range (for the most part between 0.1 and 0.2 μm). As explained in various reviews, SLN are made from solid lipids, that is, lipids that are solid at room temperature and also at body temperature. Hence, the lipids involved can be triglycerides, complex glyceride mixtures, or even waxes. Since incorporated drugs are located between fatty acid chains (i.e., between the lipid layers and also in crystal imperfections), a highly ordered crystal lattice cannot accommodate large amounts of drug. Accordingly, the use of more complex lipids (mono-, di-, triglycerides, and/or different carbon-chain lengths) is more sensible for higher drug loading. Specifically, the spatially different lipids lead to larger distances between the fatty acid chains of the glycerides and/or in the vicinity of general imperfections in the crystal lattice and thus offer more room for the accommodation of guest molecules [1]. In view of all the above considerations regarding SLN, a major advantage of specifically a lipid nanoemulsion drug-delivery vehicle becomes more apparent. Namely, a self-assembling mixed-lipid nanoemulsion, such as the LCM/ND nanoemulsion formulation, largely represents a “colloidal dispersion of ‘liquid’ crystals” (i.e., represents a lipid-based liquid-crystalline dispersion system). Further indirect support for the utility of employing lipid nanoparticles, existing in the liquid-crystalline phase, is derived from additional drug-delivery work by other investigators [36]; specifically, this research work relates to their studies of particularly cholesterol esters with saturated acyl chains as well as incorporation of model drugs into the dispersions. These authors concluded that due to the highly viscous, liquid-crystalline state of their matrix lipid (using cholesterol myristate as a model cholesterol ester), these cholesterol ester nanoparticles show promise as delivery systems for lipophilic drugs [36]. Such liquid-crystalline lipid nanoparticles offer a desirable compromise between highly fluid (and larger) emulsion droplets and the rigid crystalline matrix of SLN that usually severely limits their drug-loading capacity [1]. In addition, the above combined considerations indicate that the constituent cholesterol esters of LCM/ND lipid nanoemulsion(s) probably reside, along with its triglycerides, for the most part within the nonpolar core of the liquid-crystalline lipid nanoparticles.
Regarding development of practical biomedical applications, the closely related (protein-free) lipid nanocarriers have often functioned in the past as a useful tool that facilitates human studies of chylomicron metabolism. More specifically, these related nanoemulsion vehicles display “active targeting” properties closely resembling those of “recombinant chylomicrons”, which is an approach that has been validated in human subject studies. This useful research approach has revealed chylomicron metabolism defects in malignant hypertension, systemic lupus erythematosus, and heart transplant recipients and has also been used to investigate effects of lipid-lowering drugs on chylomicron metabolism. Accordingly, in human subjects having coronary artery disease or other conditions that are associated with coronary artery disease development, it was found that a diminished elimination/removal of “chylomicron-like” nanoemulsions was measurable. This pattern of results (and related findings) implies that deficiencies in chylomicron intravascular catabolism are involved in atherogenesis [1].
7.2. Functional Characteristics of LCM/ND Nanoemulsions
Notice that unlike other (past or present) patented lipid nanoemulsion vehicles (cf. [1]), the LCM/ND nanoemulsion formulation(s) already described (in Section 3, Section 4, and Section 6) display various added advantages. This type of nanoemulsion was originally developed to selectively and actively target tumors, as well as certain (noncancerous) proliferative processes, via receptor-mediated endocytosis.
This endocytic fate of LCM/ND nanoemulsion particles, in tumor cells, was further elucidated earlier by determination of their subcellular localization using chemical markers for various organelles. When C6 tumor cells were examined by confocal fluorescence microscopy using dual-channel recording, it was found that 70 ± 20% of the internalized LCM/ND particles were associated with acidic compartments (which comprise endosomes and/or lysosomes). Moreover, the interactions of the LCM/ND particles with separate perivascular tumor (i.e., 9L gliosarcoma) cells appear to propagate from the circumferential area, immediately surrounding blood vessels, to the outer periphery of the tumor. This particle-movement pattern reflects the growth properties of the 9L tumor which is known to spread profusely in the brain parenchyma by perivascular invasion. This data on the distribution of LCM/ND particles in the brain of rats bearing 9L tumors show that these particles interact preferentially with tumors, even when they are remote from the site of implantation. This finding also indicates that tissue injury created from implanting tumor cells is not a requisite for LCM/ND attraction (i.e., LCM/ND nanoemulsion targeting). Furthermore, other data reviewed in these same past publications indicate that LCM/ND particles are rapidly removed from the circulation by tumors. Specifically, the maximum accumulation of LCM/ND particles in the tumor area occurs within the first 30 min after i.v. injection. At least four different types of experimental tumors in rats (C6 glioma, 9L gliosarcoma, Novikoff hepatoma, and Walker-256 carcinosarcoma), as well as several spontaneous tumors in dogs, do interact with LCM/ND particles in a preferential manner—suggesting that LCM/ND particle affinity may be active for many types of tumor cells. Most C6 and 9L tumor cells examined appear to have LCM/ND particles in their cytoplasm 2 min after LCM/ND nanoemulsion injection. This uptake was observed by confocal laser scanning microscopy both in brain tumors in vivo and tumor cells in culture. The time course of internalization and the temperature dependency of the process are both consistent with endocytosis, as is the documented inhibition of LCM/ND particle uptake by energy blockers, since endocytosis is a temperature-dependent active-uptake process (cf. [1]).
In addition, the LCM/ND formulation [51,52] utilizes only saturated glycerides in the “particularly preferred form” of this type of nanoemulsion—which results in an added benefit, namely, saturated acyl chains are incapable of undergoing peroxidation and hence the acceptable storage life of this nanoemulsion formulation is increased. As concerns competing patents issued in this Field, notice that there are some specific acyl chain lengths shared with the different glycerides (both unsaturated and saturated) utilized in these various competing lipid nanoemulsions, that is, they overlap in the range of 10–12 carbons long. However, LCM/ND nanoemulsions do not contain any phospholipid (of human or other origin). Consequently, while an earlier comparison of several selected patents versus LCM/ND nanoemulsion formulation(s) revealed an occasional similarity regarding either a lipid structural property or a nanoemulsion functional property [1], there are still important differences in the molecular composition of these separate types of lipid nanoemulsion. Contrariwise, within the specific category of “LCM/ND” nanoemulsion formulations, any differences in (key lipid) composition are minor and have been found to be inconsequential with regard to maintenance of adequate nanoemulsion stability and overall functional capacity—based on the pertinent published (and/or public domain) reports. Hence, unlike the other patented lipid nanoemulsion vehicles, the LCM/ND nanoemulsion formulations: (1) have the advantage of being able to selectively and actively target tumors, as well as certain (noncancerous) proliferative processes, through receptor-mediated endocytosis; (2) utilize only saturated glycerides in the “particularly preferred form” of this type of nanoemulsion; and (3) both do not require nor contain any phospholipid in this nanoemulsion formulation. The early (issued) patents by D’Arrigo [51,52], covering the category of LCM/ND technology, were originally employed to protect an i.v. imaging agent and, thereafter, were utilized by D’Arrigo and coworkers in the development of a targeted drug-delivery nanocarrier [1].
Finally, the molecular biology and biomedical topics treated in this book chapter can mostly be summarized as follows. The BBB is mostly made up of endothelial cells, which are severely compromised in a number of neurological conditions, including numerous neurodegenerative diseases. As Alzheimer’s disease progresses, there is evidence of an early BBB breakdown and/or (endothelial) dysfunction that occurs prior to dementia, neurodegeneration, and/or brain atrophy. Researchers have found that focusing on the BBB can affect how the neurological disorder develops. Thus, it becomes possible to prevent and treat common dementias with vascular-targeted treatments. The relative risk for Alzheimer’s disease is raised by midlife vascular-risk factors, including obesity, diabetes, dyslipidemia, cardiovascular disease and hypertension. These various co-morbidities are all accompanied by low and/or malfunctioning HDL, which itself is a risk factor for Alzheimer’s disease. Specifically, HDL regulates vascular health by increasing endothelial cell survival and integrity as well as by modifying vasorelaxation, inflammation, and oxidative stress—all in addition to its well-documented participation in lipid transport). In conclusion, it is possible that the previously described, multifunctional LCM/ND nanoemulsion could be used as a targeted, apoA-I-based (SR-BI mediated) therapeutic agent, for common (late-onset) dementias; namely, SR-BI has already been identified as a major receptor for HDL (with their major apolipoprotein, i.e., apoA-I) as well as for the multifunctional LCM/ND nanoemulsion. In this particular targeted-delivery approach, the self-assembled HDL-related “lipid nanoemulsion particle” structure itself (after i.v. injection) likely binds to apoA-I in blood plasma; subsequently, such apoA-I-targeted LCM/ND nanoemulsion particles are recognized by SR-BI receptors on various Alzheimer’s-related cell types. (With regard to the current stage of development of the LCM/ND platform, clinical trials have not yet been initiated, and the LCM/ND technology currently remains in the preclinical phase).
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflicts of interest. J.S.D. is employed at Cav-Con Inc.
Abbreviation List
| AChE | acetylcholinesterase |
| Aβ fibril | amyloid-β protein fibril |
| BBB | blood–brain barrier |
| GLUT-1 | glucose transporter protein |
| HDL | high-density lipoprotein |
| LCM/ND nanoemulsion | “Lipid-coated microbubble/nanoparticle-derived” nanoemulsion |
| MPS | mononuclear phagocytic system |
| rHDL | reconstituted high-density lipoprotein |
| RES | reticuloendothelial system |
| SAA | serum amyloid A |
| SLN | solid lipid nanoparticle |
| SR-BI | scavenger receptor class B type I |
References
- D’Arrigo, J.S. Stable Nanoemulsions: Self-Assembly in Nature and Nanomedicine; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Lacko, A.G.; Nair, M.; Paranjape, S.; Mooberry, L.; McConathy, W.J. Trojan horse meets magic bullet to spawn a novel, highly effective drug delivery model. Chemotherapy 2006, 52, 171–173. [Google Scholar] [CrossRef]
- Lacko, A.G.; Nair, M.; Prokai, L.; McConathy, W.J. Prospects and challenges of the development of lipoprotein-based formulations for anti-cancer drugs. Expert Opin. Drug Deliv. 2007, 4, 665–675. [Google Scholar] [CrossRef]
- D’Arrigo, J.S. Overlapping receptor-based pathogenic cascades in degenerative disease: Implications ranging from tumor targeting to aging and dementia therapeutics. Int. J. Transl. Med. 2024, 4, 152–162. [Google Scholar] [CrossRef]
- Wang, W.; Hassan, M.M.; Mao, G. Colloidal perspective on targeted drug delivery to the central nervous system. Langmuir 2023, 39, 3235–3245. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Brugger, A.; Khare, A.; Chaubal, M.; Papadopoulos, P.; Rabinow, B.; Kipp, J.; Ning, J. Suspensions for intravenous (I.V.) injection: A review of development, preclinical and clinical aspects. Adv. Drug Deliv. Rev. 2008, 60, 939–954. [Google Scholar] [CrossRef]
- Chountoulesi, M.; Pispas, S.; Tseti, I.K.; Demetzos, C. Lyotropic liquid crystalline nanostructures as drug delivery systems and vaccine platforms. Pharmaceuticals 2022, 15, 429. [Google Scholar] [CrossRef]
- D’Arrigo, J.S. Targeting Drug Delivery Toward Degenerative Disease. U.S. provisional Patent application. # 63/560,061; Application field on:, 1 March 2024. [Google Scholar]
- De Marchi, F.; Munitic, I.; Vidatic, L.; Papić, E.; Rački, V.; Nimac, J.; Jurak, I.; Novotni, G.; Rogelj, B.; Vuletic, V.; et al. Overlapping neuroimmune mechanisms and therapeutic targets in neurodegenerative disorders. Biomedicines 2023, 11, 2793. [Google Scholar] [CrossRef]
- Sheikh, M.S.; Yano, S.; Tabassum, S.; Nagai, A. The role of the vascular system in degenerative diseases: Mechanisms and implications. Int. J. Mol. Sci. 2024, 25, 2169. [Google Scholar] [CrossRef]
- Weaver, D.F. Thirty risk factors for Alzheimer’s disease unified by a common neuroimmune-neuroinflammation mechanism. Brain Sci. 2024, 14, 41. [Google Scholar] [CrossRef]
- Almer, G.; Mangge, H.; Zimmer, A.; Prassl, R. Lipoprotein-related and apolipoprotein-mediated delivery systems for drug targeting and and imaging. Curr. Med. Chem. 2015, 22, 3631–3651. [Google Scholar] [CrossRef]
- Bredesen, D.E. Reversal of cognitive decline: A novel therapeutic program. Aging 2014, 6, 707–717. [Google Scholar] [CrossRef]
- Carradori, D.; Gaudin, A.; Brambilla, D.; Andrieux, K. Application of nanomedicine to the CNS diseases. Int. Rev. Neurobiol. 2016, 130, 73–113. [Google Scholar]
- D’Arrigo, J.S. Nanotherapy for Alzheimer’s disease and vascular dementia: Targeting senile endothelium. Adv. Colloid Interface Sci. 2018, 251, 44–54. [Google Scholar] [CrossRef] [PubMed]
- De Boer, A.G.; van der Sandt Gaillard, P.J. The role of drug transporters at the Blood–Brain Barrier. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 629–656. [Google Scholar] [CrossRef] [PubMed]
- Di Marco, L.Y.; Venneri, A.; Farkas, E.; Evans, P.C.; Marzo, A.; Frangi, A.F. Vascular dyfunction in the pathogenesis of Alzheimer’s disease—A review of endothelium-mediated mechanisms and ensuing vicious circles. Neurobiol. Dis. 2015, 82, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Goldwaser, E.L.; Acharya, N.K.; Sarkar, A.; Godsey, G.; Nagele, R.G.; Shea, T. Breakdown of the cerebrovasculature and blood-brain barrier: A mechanistic link between diabetes mellitus and Alzheimer’s disease. J. Alzheimer’s Dis. 2016, 54, 445–456. [Google Scholar] [CrossRef]
- Hostenbach, S.; D’haeseleer, M.; Kooijman, R.; De Keyser, J. The pathophysiological role of astrocytic endothelin-1. Prog. Neurobiol. 2016, 144, 88–102. [Google Scholar] [CrossRef]
- Koizumi, K.; Wang, G.; Park, L. Endothelial dysfunction and amyloid-β-induced neurovascular alterations. Cell. Mol. Neurobiol. 2016, 36, 155–165. [Google Scholar] [CrossRef]
- Koster, K.P.; Thomas, R.; Morris, A.W.; Tai, L.M. Epidermal growth factor prevents oligomeric amyloid-β-induced angiogenesis deficits in vitro. J. Cereb. Blood Flow Metab. 2016, 36, 1865–1871. [Google Scholar] [CrossRef]
- Preston, J.E.; Abbott, J.; Begley, D.J. Transcytosis of macromolecules at the blood-brain barrier. Adv. Pharmacol. 2014, 71, 147–163. [Google Scholar]
- Qosa, H.; Mohamed, A.; Al Rihani, S.B.; Batarseh, Y.S.; Duong, Q.-V.; Keller, J.N.; Kaddoumi, A.; Carro, E. High-throughput screening for identification of blood-brain barrier integrity enhancers: A drug repurposing opportunity to rectify vascular amyloid toxicity. J. Alzheimer’s Dis. 2016, 53, 1499–1516. [Google Scholar] [CrossRef]
- Salmina, A.B.; Inzhutova, A.I.; Malinovskaya, N.A.; Petrova, M.M. Endothelial dysfunction and repair in Alzheimer-type neurodegeneration: Neuronal and glial control. Alzheimer’s Dis. 2010, 22, 17–36. [Google Scholar] [CrossRef] [PubMed]
- Srimanee, A.; Regberg, J.; Hallbrink, M.; Vajragupta, O.; Langel, Ü. Role of scavenger receptors in peptide-based delivery of plasmid DNA across a blood-brain barrier model. Int. J. Pharm. 2016, 500, 128–135. [Google Scholar] [CrossRef]
- Tong, X.K.; Hamel, E. Simvastatin restored vascular reactivity, endothelial function and reduced string vessel pathology in a mouse model of cerebrovascular disease. Cereb. Blood Flow Metabol. 2015, 35, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Zenaro, E.; Piacentino, G.; Constantin, G. The blood-brain barrier in Alzheimer’s disease. Neurobiol. Dis. 2016, 107, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Masserini, M. Nanoparticles for brain drug delivery. ISRN Biochem. 2013, 2013, 238428. [Google Scholar] [CrossRef]
- Chami, B.; Barrie, N.; Cai, X.; Wang, X.; Paul, M.; Morton-Chandra, R.; Sharland, A.; Dennis, J.M.; Freedman, S.B.; Witting, P.K. Serum amyloid A receptor blockade and incorporation into high-density lipoprotein modulates its pro-inflammatory and pro-thrombotic activities on vascular endothelial cells. Int. J. Mol. Sci. 2015, 16, 11101–11124. [Google Scholar] [CrossRef]
- Matsuo, K.; Nishihara, H. Rebuilding insight into the pathophysiology of Alzheimer’s disease through new blood-brain barrier models. Neural Regener. Res. 2024, 19, 1954–1960. [Google Scholar] [CrossRef]
- Tian, X.; Zhao, Y.; Zhu, Y.; Cui, M. Association between elevated blood-brain barrier permeability and the risk of progressive cognitive decline: A longitudinal study. Arch. Gerontol. Geriatr. 2024, 124, 105441. [Google Scholar] [CrossRef]
- Mahringer, A.; Reichel, V.; Ott, M.; MacLean, C.; Reimold, I.; Hollnack-Pusch, E.; Fricker, G. Overcoming the blood-brain barrier—The challenge of brain drug targeting. J. Nanoneurosci. 2012, 2, 5–19. [Google Scholar] [CrossRef]
- Petri, B.; Bootz, A.; Khalansky, A.; Hekmatara, T.; Müller, R.; Uhl, R.; Kreuter, J.; Gelperina, S. Chemotherapy of brain tumour using doxorubicin bound to surfactant-coated poly(butyl cyanoacrylate) nanoparticles: Revisiting the role of surfactants. J. Control. Release 2007, 117, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Fung, K.Y.; Wang, C.; Nyegaard, S.; Heit, B.; Fairn, G.D.; Lee, W.L. SR-BI mediated transcytosis of HDL in brain microvascular endothelial cells is independent of caveoliv, clathrin, and PDZK1. Front. Physiol. 2017, 8, 841. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Jiang, G.; Huang, L.; Sun, S.; Wan, Y.; Li, F.; Wu, B.; Zhang, Y.; Li, X.; Xiong, B.; et al. The Role of APOA-I in Alzheimer’s Disease: Bridging Peripheral Tissues and the Central Nervous System. Pharmaceuticals 2025, 18, 790. [Google Scholar] [CrossRef] [PubMed]
- Kuntsche, J.; Westesen, K.; Drechsler, M.; Koch, M.H.J.; Bunjes, H. Supercooled smectic nanoparticles: A potential novel carrier system for poorly water soluble drugs. Pharm. Res. 2004, 21, 1834–1843. [Google Scholar] [CrossRef]
- Calabrese, V.; Giordano, J.; Signorile, A.; Ontario, M.L.; Castorina, S.; De Pasquale, C.; Eckert, G.; Calabrese, E.J. Major pathogenic mechanisms in vascular dementia: Roles of cellular stress response and hormesis in neuroprotection. J. Neurosci. Res. 2016, 94, 1588–1603. [Google Scholar] [CrossRef]
- D’Arrigo, J.S. Biomimetic nanocarrier targeting drug(s) to upstream-receptor mechanisms in dementia: Focusing on linking pathogenic cascades. Biomimetics 2020, 5, 11. [Google Scholar] [CrossRef]
- D’Arrigo, J.S. Arterial elasticity: Linking of cardiovascular risks, pulse pressure, dementia, aging, and drug targeting. OBM Neurobiol. 2022, 6, 117. [Google Scholar] [CrossRef]
- Gonzales, M.M.; Garbarino, V.R.; Pollet, E.; Palavicini, J.P.; Kellogg, D.L.; Kraig, E.; Orr, M.E. Biological aging processes underlying cognitive decline and neurodegenerative disease. J. Clin. Investig. 2022, 132, e158453. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.J. Normal aging induces changes in the brain and neurodegeneration progress: Review of the structural, biochemical, metabolic, cellular, and molecular changes. Front. Aging Neurosci. 2022, 14, 931536. [Google Scholar] [CrossRef]
- Lindsay, H.G.; Hendrix, C.J.; Murcia, J.D.G.; Haynie, C.; Weber, K.S. The role of atypical chemokine receptors in neuroinflammation and neurodegenerative disorders. Int. J. Mol. Sci. 2023, 24, 16493. [Google Scholar] [CrossRef]
- Mou, Y.; Du, Y.; Zhou, L.; Yue, J.; Hu, X.; Liu, Y.; Chen, S.; Lin, X.; Zhang, G.; Xiao, H.; et al. Gut microbiota interact with the brain through systemic chronic inflammation: Implications on neuroinflammation, neurodegeneration, and aging. Front. Immunol. 2022, 13, 796288. [Google Scholar] [CrossRef]
- Takata, F.; Nakagawa, S.; Matsumoto, J.; Dohgu, S. Blood-brain barrier dysfunction amplifies the development of neuroinflammation: Understanding of cellular events in brain microvascular endothelial cells for prevention and treatment of BBB dysfunction. Front. Cell. Neurosci. 2021, 15, 661838. [Google Scholar] [CrossRef]
- Hartigh, L.J.D.; May, K.S.; Zhang, X.S.; Chait, A.; Blaser, M.J. Serum amyloid A and metabolic disease: Evidence for a critical role in chronic inflammatory conditions. Front. Cardiovasc. Med. 2023, 10, 1197432. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of neuroinflammation in neurodegeneration development. Nat. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef] [PubMed]
- Lemche, E.; Killick, R.; Mitchell, J.; Caton, P.W.; Choudhary, P.; Howard, J.K. Molecular mechanisms linking type 2 diabetes mellitus and late-onset Alzheimer’s disease: A systematic review and qualitative meta-analysis. Neurobiol. Dis. 2024, 196, 106485. [Google Scholar] [CrossRef] [PubMed]
- D’Arrigo, J.S. Targeting early dementia: Using lipid cubic phase nanocarriers to cross the blood-brain barrier. Biomimetics 2018, 3, 4. [Google Scholar] [CrossRef]
- Blaszczyk, J.W. Pathogenesis of dementia. Int. J. Mol. Sci. 2023, 24, 543. [Google Scholar] [CrossRef]
- D’Arrigo, J.S. Biobased nanoemulsion methodology aimed at nanotargeted drug delivery for dementia. Nano Prog. 2021, 3, 11–18. [Google Scholar] [CrossRef]
- D’Arrigo, J.S. Surfactant Mixtures, Stable Gas-in-Liquid Emulsions, and Methods for the Production of such Emulsions from Said Mixtures. U.S. Patent 4,684,479, 4 August 1987. [Google Scholar]
- D’Arrigo, J.S. Method for the Production of Medical-Grade Lipid-Coated Microbubbles, Paramagnetic Labeling of such Microbubbles and Therapeutic Uses of Microbubbles. U.S. Patent 5,215.680, 1 June 1993. [Google Scholar]
- D’Arrigo, J.S. Biobased nanoemulsions for targeted drug delivery to treat dementia and aging. Aging Pathobiol. Ther. 2023, 5, 107–111. [Google Scholar] [CrossRef]
- Yu, S.P.; Jiang, M.; Berglund, K.; Ling, W. Strain hypothesis and additional evidence of the GluN3A deficiency-mediated pathogenesis of Alzheimer’s disease. Alzheimers Dement. 2023, 19, 4267–4269. [Google Scholar] [CrossRef]
- Bossi, S.; Dhanasak, D.; Ellis-Davies, G.C.R.; Frontera, J.; de Brito Van Velze, M.; Lourenço, J.; Murillo, A.; Luján, R.; Casado, M.; Perez-Otaño, I.; et al. GluN3A excitatory glycine receptors control adult cortical and amygdalar circuits. Neuron 2022, 110, 2438–2454. [Google Scholar] [CrossRef]
- Hurley, E.P.; Mukherjee, B.; Fang, L.Z.; Barnes, J.R.; Barron, J.C.; Nafar, F.; Hirasawa, M.; Parsons, M.P. GluN3A and excitatory glycine receptors in the adult hippocampus. J. Neurosci. 2024, 44, e0401242024. [Google Scholar] [CrossRef]
- Zhong, W.; Wu, A.; Berglund, K.; Gu, X.; Jiang, M.Q.; Talati, J.; Zhao, J.; Wei, L.; Yu, S.P. Pathogenesis of sporadic Alzheimer’s disease by deficiency of NMDA receptor subunit GluN3A. Alzheimer’s Dement. 2022, 18, 222–239. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).