Abstract
Cancer continues to be a leading cause of global mortality, necessitating innovative therapeutic strategies to address its complexity and heterogeneity. Protein engineering has emerged as a transformative approach in developing cancer biotherapeutics, enabling the creation of highly specific, potent, and adaptable treatments. This paper provides a comprehensive review of the state-of-the-art in protein engineering, highlighting key techniques such as directed evolution, rational design, and hybrid approaches that underpin the development of monoclonal antibodies, bispecific antibodies, and novel fusion proteins. Case studies of FDA-approved therapies, including engineered monoclonal antibodies like trastuzumab and bispecific T-cell engagers such as blinatumomab, are discussed to illustrate the impact of these advancements. Furthermore, emerging trends, including AI-driven protein design and synthetic biology applications, are explored alongside their potential to revolutionize future cancer treatments. Challenges such as immunogenicity, stability, and scalability are critically evaluated, offering insights into potential solutions and future research directions. By synthesizing advancements in protein science and oncology, this paper aims to guide researchers and clinicians in harnessing the full potential of engineered proteins for cancer therapy.
1. Introduction
Cancer continues to be one of the most significant global health challenges, responsible for nearly 10 million deaths in 2020 and representing a leading cause of morbidity and mortality worldwide [1]. The disease’s complexity, driven by genetic mutations, tumor heterogeneity, and the dynamic interplay between cancer cells and their microenvironment, has made it notoriously difficult to treat effectively [2]. Traditional therapeutic approaches, such as chemotherapy and radiation therapy, while effective in some cases, often suffer from a lack of specificity, leading to systemic toxicity, severe side effects, and limited efficacy against advanced or metastatic cancers [3]. These limitations have spurred the search for innovative and targeted therapeutic strategies that can address the unique molecular and cellular characteristics of individual tumors [4].
In recent decades, biotherapeutics have emerged as a transformative force in oncology, offering highly specific and personalized treatment options [5]. Unlike conventional therapies, biotherapeutics are designed to precisely target cancer cells while sparing healthy tissues, thereby minimizing adverse effects and improving patient outcomes [6]. Among these, protein-based therapies—such as monoclonal antibodies [7], bispecific antibodies [8], and fusion proteins [9]—have gained prominence due to their ability to modulate immune responses, block oncogenic signaling pathways, and deliver cytotoxic payloads directly to tumor cells. The success of therapies like trastuzumab (Herceptin) for HER2-positive breast cancer and rituximab (Rituxan) for B-cell malignancies has underscored the potential of protein-based approaches in revolutionizing cancer treatment [10,11].
At the heart of this biotherapeutic revolution lies protein engineering, a discipline that combines principles of molecular biology, structural biology, and computational science to design and optimize proteins with enhanced therapeutic properties [12]. Protein engineering encompasses a diverse array of techniques, including directed evolution, rational design, and de novo protein design, each offering unique advantages for tailoring proteins to specific clinical needs [13]. Directed evolution, for instance, mimics natural selection in the laboratory to generate proteins with improved binding affinity or stability, while rational design leverages structural and functional insights to create proteins with precise molecular interactions [14]. More recently, the integration of artificial intelligence (AI) and machine learning has further accelerated the pace of innovation, enabling the rapid prediction and optimization of protein structures with unprecedented accuracy [15,16].
The application of protein engineering in oncology has led to the development of a new generation of cancer biotherapeutics with enhanced efficacy, specificity, and versatility [17]. For example, bispecific T-cell engagers (BiTEs), such as blinatumomab, have demonstrated remarkable success in redirecting the immune system to target and eliminate cancer cells [18]. Similarly, antibody-drug conjugates (ADCs) combine the specificity of monoclonal antibodies with the potency of cytotoxic drugs, offering a powerful tool for targeted cancer therapy [19]. Beyond these established modalities, emerging approaches such as synthetic biology and protein-based vaccines hold promise for further expanding the therapeutic arsenal against cancer [20,21]. Despite these advancements, significant challenges remain in the development and clinical translation of engineered protein therapeutics. Issues such as immunogenicity, protein stability, manufacturing scalability, and tumor resistance continue to hinder the widespread adoption of these therapies [22]. Addressing these challenges will require interdisciplinary collaboration, innovative engineering strategies, and a deeper understanding of the biological mechanisms underlying cancer progression and treatment resistance.
This review aims to provide a comprehensive overview of the latest advances in protein engineering for cancer biotherapeutics, highlighting key methodologies, successful case studies, and emerging trends. We will explore the transformative impact of engineered proteins on cancer therapy, critically evaluate the challenges facing the field, and discuss future directions for research and development. By synthesizing insights from protein science, oncology, and biotechnology, this review seeks to inform and inspire researchers, clinicians, and industry stakeholders in their efforts to harness the full potential of protein engineering for next-generation cancer treatments.
2. Foundations of Protein Engineering
Protein engineering has emerged as a cornerstone of modern biotechnology, enabling the design and optimization of proteins with tailored functions for therapeutic, diagnostic, and industrial applications [12]. In the context of cancer biotherapeutics, protein engineering provides the tools to create highly specific, potent, and adaptable molecules capable of targeting complex disease mechanisms [23]. This section provides an overview of the key techniques, tools, and technologies that underpin protein engineering (Figure 1), highlighting their applications and contributions to the development of next-generation cancer therapies (Table 1).
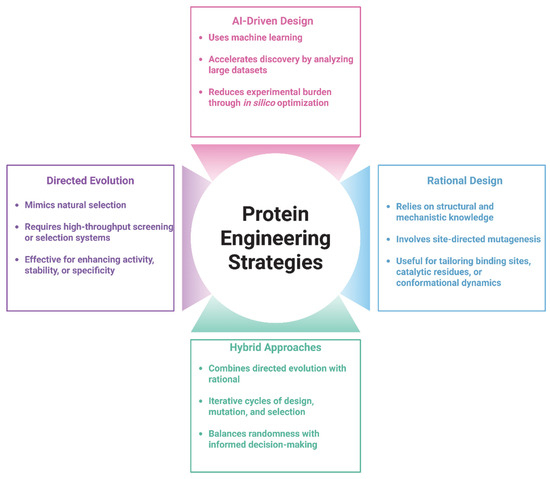
Figure 1.
Overview of key strategies in protein engineering. Protein engineering approaches are broadly categorized into Directed Evolution, Rational Design, AI-Driven Design, and Hybrid Approaches. Directed Evolution relies on iterative rounds of mutagenesis and selection, while Rational Design utilizes structural knowledge for targeted modifications. AI-Driven Design leverages machine learning to predict functional variants, and Hybrid Approaches integrate multiple strategies to enhance efficiency and precision in protein optimization. (Illustration created with BioRender.com; https://app.biorender.com, accessed on 25 June 2025.)

Table 1.
Comparison of key protein engineering strategies [24,25,26,27].
2.1. Directed Evolution: Mimicking Natural Selection in the Lab
Directed evolution is a robust and widely adopted strategy for engineering proteins with improved or entirely new functions by simulating the principles of Darwinian evolution under controlled laboratory conditions [26]. This technique systematically mimics natural selection by introducing genetic diversity into a target gene—typically through methods such as random mutagenesis, error-prone PCR, or DNA recombination techniques like DNA shuffling [28]. The resulting library of gene variants encodes a vast array of protein sequences, each potentially possessing unique structural and functional characteristics [29].
Following the generation of this diverse genetic pool, the variants are expressed and subjected to high-throughput screening or selection protocols designed to identify those exhibiting enhanced performance based on specific criteria, such as increased enzymatic activity, improved substrate specificity, greater thermostability, or higher binding affinity [30,31]. Importantly, the selection criteria are tailored to the desired application, making the process highly versatile across multiple fields, including biotechnology, synthetic biology, and therapeutic development [32]. Directed evolution has profoundly impacted protein engineering, particularly in the development of biologics and therapeutic proteins and one notable application involves optimizing monoclonal antibodies to improve their affinity for tumor-associated antigens, thereby increasing their efficacy in targeted cancer therapies [33,34]. Additionally, enzymes evolved through this process have been utilized in industrial biocatalysis, often demonstrating improved activity under non-natural conditions, such as extreme pH or temperature [35].
What distinguishes directed evolution from rational design is its ability to explore vast areas of protein sequence space without requiring detailed structural or mechanistic knowledge of the target protein [26]. The iterative cycle of mutation and selection enables the accumulation of beneficial mutations across generations, often revealing unanticipated adaptive solutions that would be difficult to predict computationally [36]. This evolutionary approach not only expands the functional repertoire of proteins but also provides insights into the relationship between sequence, structure, and function.
2.2. Rational Design: Precision Engineering Based on Structural Insights
Rational design represents a targeted approach to protein engineering that leverages detailed structural and mechanistic information to make deliberate, hypothesis-driven modifications aimed at achieving specific functional outcomes [37]. Unlike directed evolution, which relies on random mutagenesis and empirical selection, rational design begins with a deep understanding of the protein’s three-dimensional conformation [38], often obtained through high-resolution structural biology techniques such as X-ray crystallography [39], nuclear magnetic resonance (NMR) spectroscopy [40], and cryo-electron microscopy (cryo-EM) [41]. These methodologies provide atomic-level insights into the spatial arrangement of amino acid residues and the nature of a protein’s interactions with ligands, substrates, cofactors, or other biomolecules [42]. Using this structural information as a foundation, researchers employ a range of computational tools to model protein dynamics, stability, and binding interactions. Molecular dynamics (MD) simulations allow for the exploration of conformational flexibility and stability over time, while molecular docking algorithms predict how candidate molecules—such as drugs or substrates—interact with the protein’s active or binding sites [43]. Computational mutagenesis can then be used to virtually introduce specific amino acid substitutions and assess their potential impact on the protein’s function, stability, or specificity before conducting experimental validations [44].
This methodical approach has proven especially valuable in designing therapeutic proteins and enzymes where precision is paramount. For example, rational design has been utilized to reduce the immunogenicity of biotherapeutics by identifying and modifying epitopes recognized by the human immune system without compromising functional efficacy [45]. It has also enabled the engineering of proteins with enhanced thermal stability for industrial applications and improved binding specificity for therapeutic targets, such as in the development of antibody-drug conjugates or receptor antagonists [46,47]. Rational design is particularly effective when there is substantial structural and functional knowledge about the target protein, including its folding, domain organization, and active site architecture [48]. Although it is inherently limited by the accuracy of available structural data and predictive models, recent advances in machine learning [49], cryo-EM resolution [50], and integrative modeling [51] have significantly expanded its applicability. Moreover, rational design is often used in conjunction with directed evolution in a hybrid approach, wherein initial rational modifications are further optimized through evolutionary cycles, combining the precision of design with the adaptive power of selection [52].
2.3. Hybrid Approaches: Combining the Best of Both Worlds
Hybrid approaches in protein engineering strategically combine the complementary strengths of directed evolution and rational design to enhance the efficiency and effectiveness of protein optimization [53]. While directed evolution excels at navigating vast sequence spaces without requiring prior knowledge, rational design offers precision based on structural and mechanistic insights, each method also has inherent limitations [54]. By integrating these strategies, researchers can capitalize on the exploratory power of evolution while maintaining the targeted accuracy of design. One of the most common implementations of this synergy involves using computational and structural insights to inform the design of focused mutational libraries for directed evolution [55]. Instead of relying on completely random mutagenesis, rational design principles are used to identify functionally relevant residues or structurally important regions of a protein [25]. Targeted mutagenesis is then applied to these sites, significantly reducing library size and improving the likelihood of obtaining beneficial variants. This focused approach streamlines the screening process and enhances the efficiency of experimental workflows [56].
Conversely, directed evolution can also inform rational design. By analyzing the mutations that accumulate in functional variants during evolutionary selection, researchers can identify critical residues or structural motifs that contribute significantly to protein performance and these empirically derived insights can guide subsequent rational modifications, such as introducing stabilizing mutations or engineering novel binding interfaces [14]. In this way, directed evolution not only produces improved proteins but also generates valuable data that enhances our understanding of protein structure–function relationships [24]. Hybrid strategies have proven particularly powerful in the engineering of complex biomolecules that require simultaneous optimization of multiple parameters, such as binding affinity, specificity, solubility, and stability [57]. Notably, the design of bispecific antibodies, which must coordinate the interaction of two distinct antigen-binding sites within a single molecule, often relies on hybrid approaches to balance functional efficacy with structural integrity [58]. Similarly, fusion protein engineered by linking domains from different proteins benefits from this integrative strategy to ensure that each domain retains its function while the overall architecture remains stable and biologically active [59].
Recent advances in machine learning [60], structural prediction tools (such as AlphaFold) [61], and high-throughput screening technologies have further enhanced the potential of hybrid methods [62]. These innovations facilitate more accurate identification of mutation hotspots, better prediction of mutational effects, and more refined library construction. As such, hybrid approaches represent a highly adaptable and powerful paradigm for modern protein engineering, capable of addressing the growing complexity of biotherapeutic and industrial enzyme development.
2.4. Tools and Technologies in Protein Engineering
2.4.1. CRISPR-Based Systems for Directed Modifications
The emergence of CRISPR-Cas systems has transformed the landscape of genetic engineering, offering unprecedented precision, efficiency, and versatility in manipulating protein-coding genes [63]. Initially derived from bacterial adaptive immune systems, the CRISPR-Cas9 platform has become a cornerstone of modern molecular biology, enabling targeted gene editing across a wide range of organisms [64]. In the field of protein engineering, CRISPR-based technologies allow for the direct and programmable alteration of genomic DNA, facilitating the rational modification of protein sequences within their native genomic context [65].
One of the primary advantages of CRISPR-Cas9 is its ability to introduce site-specific double-strand breaks at user-defined loci, guided by a single guide RNA (sgRNA) that recognizes complementary DNA sequences [66]. This targeted cleavage can be harnessed to induce precise insertions, deletions, or substitutions via homology-directed repair (HDR) or non-homologous end joining (NHEJ), enabling the systematic modification of protein-encoding genes [67]. Researchers can thus introduce mutations to alter amino acid residues, fuse novel functional domains, or delete regulatory elements to fine-tune protein expression and function.
In cancer biotherapeutics, CRISPR has been particularly instrumental in the engineering of immune cells for adoptive cell therapies. A prominent example is the generation of chimeric antigen receptor (CAR) T cells, where CRISPR is employed to insert synthetic receptor genes into T cells, enabling them to recognize and destroy tumor cells with high specificity [68,69]. Moreover, CRISPR can be used to disrupt immune checkpoint genes (e.g., PD-1) or endogenous T-cell receptors to enhance the persistence, potency, and safety of CAR T cells in clinical applications [70]. Beyond conventional genome editing, next-generation CRISPR technologies—such as base editing and prime editing—have significantly expanded the precision toolkit available to protein engineers. Base editors enable the conversion of specific nucleotide bases without inducing double-strand breaks, thereby allowing single amino acid substitutions to be made with minimal genomic disruption [71]. Prime editing further extends this capability by facilitating the insertion, deletion, or replacement of sequences at targeted sites, using a reverse transcriptase fused to a Cas9 nickase. These methods are particularly valuable for introducing subtle but functionally important changes in protein-coding regions, such as tuning enzyme active sites or modulating post-translational modification motifs [72].
Collectively, CRISPR-based systems provide a powerful and flexible platform for engineering proteins in vivo, bridging the gap between gene editing and protein function. As delivery technologies and editing efficiencies continue to improve, CRISPR is poised to play an increasingly central role in the design of next-generation therapeutics, synthetic biology constructs, and functional genomics studies.
2.4.2. Advances in AI and Machine Learning for Protein Design
The integration of AI and machine learning into protein engineering is ushering in a new era of computationally driven design, dramatically enhancing speed, accuracy, and scope of therapeutic protein development [73]. These technologies leverage large-scale biological data and complex statistical models to uncover patterns that are often imperceptible to traditional analytical methods, enabling the prediction and generation of protein structures, functions, and interactions with remarkable efficiency [74].
One of the most transformative applications of AI in protein engineering has been in the field of structure prediction. Deep learning models, particularly those based on neural network architectures, have achieved unprecedented accuracy in predicting the three-dimensional conformations of proteins from their primary amino acid sequences [75]. Notably, AlphaFold2, developed by DeepMind, and RoseTTAFold (Figure 2), developed by the Baker Lab, have demonstrated that AI can reliably infer atomic-level protein structures, rivaling experimental techniques such as X-ray crystallography and cryo-electron microscopy in many cases [76,77]. These tools have significantly lowered the barrier to structural characterization, enabling researchers to model proteins for which experimental data are unavailable and to design proteins with novel folds and functions. Building upon this success, DeepMind released AlphaFold 3 in 2024, which extends predictions to include protein interactions with DNA, RNA, ligands, and antibodies. This version surpasses traditional physics-based tools in accuracy for biomolecular interactions [78]. Tools such as AlphaFold2 and RoseTTAFold have demonstrated notable accuracy in certain benchmark settings; however, challenges remain in predicting dynamics, multimeric complexes, and post-translational modifications. These AI approaches complement experimental methods and may aid in the design of therapeutics when used in conjunction with empirical validation [79].
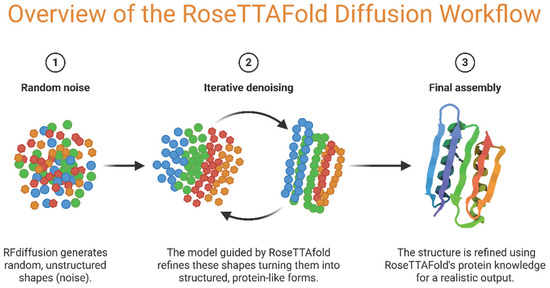
Figure 2.
Schematic overview of the RoseTTAFold Diffusion workflow for protein structure generation. (1) The process begins with random noise, where RFDiffusion generates unstructured shapes. (2) Through iterative denoising, the RoseTTAFold model progressively transforms these noisy inputs into structured, protein-like intermediates. (3) The final structure is assembled and refined using RoseTTAFold’s protein structure knowledge, producing realistic protein models. (Illustration created with BioRender.com; https://app.biorender.com, accessed on 25 June 2025.)
AlphaFold2 achieved a median GDT_TS score of 92.4 across CASP14 targets, demonstrating near-experimental accuracy for many single-chain structures [80]. RoseTTAFold, an alternative model, reached comparable accuracy but with improved speed for certain use cases. However, these tools remain limited in modeling conformational flexibility, protein–protein interactions, and post-translational modifications, highlighting the need for experimental validation [81].
In the context of cancer biotherapeutics, AI and machine learning are being employed to design protein therapeutics with enhanced tumor-targeting abilities [82], improved stability [15], and reduced off-target effects [83]. For example, AI models can analyze vast immunological datasets to predict epitopes that elicit minimal immune responses, aiding in the development of low-immunogenicity proteins [84]. Similarly, machine learning algorithms can be trained on pharmacokinetic and pharmacodynamic data to optimize drug-like properties such as half-life [85], solubility [86], and biodistribution [87].
Machine learning is also instrumental in processing and interpreting data from high-throughput screening and directed evolution experiments where via analyzing millions of sequence-function pairs, machine learning models can identify key sequence features associated with enhanced activity or binding affinity, thereby guiding the rational design of new protein variants [88]. Furthermore, generative models such as variational autoencoders and generative adversarial networks are being explored for de novo protein design, enabling the creation of entirely novel proteins with tailored functions [89].
AI-powered platforms are also transforming high-throughput virtual screening processes. Machine learning models can analyze large libraries of protein variants to identify those with optimal properties, such as binding affinity, stability, and solubility. A study demonstrated the use of machine learning to optimize the stability and expression of therapeutic antibodies, leading to the identification of high-performing variants. This approach significantly reduces the time and cost associated with experimental screening [90].
Importantly, the continued success of AI and machine learning in protein engineering depends on the availability of high-quality datasets and the integration of interdisciplinary knowledge spanning bioinformatics, structural biology, and systems biology. As computational models become more sophisticated and experimental validation pipelines more streamlined, AI is expected to play a central role in the next generation of protein therapeutics, particularly in the personalization of cancer treatment strategies and the rapid response to emerging biological threats.
2.4.3. High-Throughput Screening and Automation
High-throughput screening technologies form a critical component of modern protein engineering workflows, enabling the rapid evaluation of large libraries of protein variants generated through directed evolution [91], rational design, or hybrid approaches [92]. The primary goal of high-throughput screening is to efficiently identify candidates with desirable biochemical or biophysical properties, such as enhanced catalytic activity [93], increased binding affinity [94], or improved stability [95], from among thousands to millions of engineered variants.
Recent advancements in laboratory automation, microfluidics, and assay miniaturization have significantly increased the throughput, accuracy, and scalability of screening platforms [96,97,98]. Robotic liquid handling systems, coupled with multi-well plate formats and automated readout technologies (e.g., fluorescence, luminescence, or absorbance-based assays), allow for the parallel testing of protein variants under standardized and reproducible conditions [99]. These systems can be integrated with downstream analytical tools such as mass spectrometry or high-performance liquid chromatography (HPLC) to provide detailed characterization of variant functions and structure [100]. Microfluidic technologies have further enhanced screening efficiency by enabling the compartmentalization and analysis of single protein variants in picoliter-scale droplets and these droplet-based systems can perform reactions at ultra-high throughput, often exceeding 106 variants per day, while minimizing reagent consumption and assay costs [101]. This capability is particularly valuable in directed evolution experiments, where the sequence space is vast, and exploration efficiency is paramount. Automation not only accelerates the screening process but also ensures consistency, reduces human error, and facilitates the integration of machine learning algorithms for data-driven decision-making [102]. By coupling high-throughput screening platforms with computational tools, researchers can identify sequence-function correlations and iteratively refine libraries to converge on optimal protein designs more rapidly [103]. This convergence of automation and AI-driven analytics is transforming protein engineering into a data-rich, feedback-optimized process that supports rational and empirical innovation alike.
In therapeutic development, high-throughput screening platforms are indispensable for screening engineered proteins such as enzymes, antibodies, or cytokines for key pharmaceutical properties, including solubility, aggregation resistance, immunogenicity, and activity under physiological conditions. As technologies continue to evolve, the integration of high-throughput screening with advanced gene synthesis, protein expression systems, and real-time analytics will further enhance the speed and precision with which the next generation biotherapeutics are developed.
2.4.4. Synthetic Biology and De Novo Protein Design
Synthetic biology is revolutionizing the field of protein engineering by enabling the creation of novel biomolecules that transcend the constraints of natural evolution [104]. Central to this innovation is de novo protein design, a computational approach that allows for the generation of entirely new protein sequences engineered to fold into predefined three-dimensional structures with specific functions [105]. Unlike traditional protein engineering, which modifies existing natural proteins, de novo design constructs proteins from first principles, guided by biophysical models of folding and function [106].
Advanced computational algorithms, such as Rosetta and newer deep learning-based frameworks, play a pivotal role in predicting how amino acid sequences will fold and interact and these tools enable the rational construction of stable protein scaffolds, which can be functionalized to perform desired biological tasks [27]. De novo designed proteins have demonstrated a wide range of functionalities, including bespoke binding interfaces, novel catalytic centers, and structural elements optimized for specific environmental conditions [107]. In the context of cancer therapeutics, de novo protein design offers unique opportunities to develop next-generation biologics with improved precision and minimized side effects. For instance, artificial binding domains can be engineered to target tumor-specific antigens with high affinity and selectivity, while avoiding cross-reactivity with healthy tissues [108]. This level of specificity is especially valuable in the design of therapeutic agents such as synthetic cytokines, targeted protein degraders, or immune cell engagers. Furthermore, de novo scaffolds can be engineered to possess superior stability, reduce immunogenicity, and customizable pharmacokinetics, making them highly attractive for in vivo applications [109,110].
One prominent application of synthetic biology is the engineering of immune cells, particularly CAR T cells, to improve their specificity and efficacy against tumors. Traditional CAR T cells have shown success in hematological malignancies but face challenges in solid tumors due to antigen heterogeneity and the immunosuppressive tumor microenvironment. To address these issues, researchers have developed synthetic Notch (synNotch) receptors that allow T cells to recognize specific antigens and, upon activation, induce the expression of customized therapeutic programs [111]. For instance, a study demonstrated that synNotch CAR T cells could be programmed to produce IL-2 locally within the tumor microenvironment, enhancing T cell infiltration and tumor clearance without systemic toxicity [112]. Synthetic biology enables the construction of protein-based circuits that can sense and respond to specific cues within the tumor microenvironment. These circuits can be designed to activate therapeutic responses, such as the release of cytotoxic agents, only in the presence of tumor-specific signals, thereby minimizing off-target effects. [112,113].
Synthetic biology also supports the modular assembly of complex protein constructs by integrating de novo designed elements with natural domains and this modularity facilitates the engineering of multifunctional proteins, such as synthetic receptors or signal transduction components, tailored for specific therapeutic goals [114,115]. Additionally, synthetic circuits incorporating engineered proteins can be used to program cellular behavior, offering a new dimension in cancer immunotherapy and regenerative medicine [116]. As computational power and algorithmic accuracy continue to improve, de novo protein design is expected to expand its role in biotherapeutic innovation. Its potential to create truly novel functionalities—untethered from the limitations of natural evolutions, as a transformative tool in the quest for highly effective and precisely targeted cancer treatments.
3. Key Applications of Engineered Proteins in Cancer Therapeutics
Engineered proteins have fundamentally transformed cancer treatment by enabling the design of therapeutic agents that are highly specific, potent, and adaptable. These proteins, shaped through advances in molecular biology, synthetic biology, and protein design, play a central role in a wide array of targeted and immune-based therapies. This section provides an overview of the principal applications of engineered proteins in cancer therapeutics, with a focus on monoclonal antibodies, bispecific and multispecific antibodies, engineered cytokines, nanobodies, and protein-based delivery systems (Table 2). Through selected examples from peer-reviewed studies and FDA-approved therapeutics, the clinical significance and innovative potential of these modalities are highlighted.

Table 2.
Summary of engineered protein modalities in cancer therapeutics.
3.1. Monoclonal Antibodies
Monoclonal antibodies (mAbs) represent one of the most successful and extensively applied forms of protein engineering in oncology (Table 3). Their ability to recognize tumor-associated antigens with high affinity has enabled the selective targeting of cancer cells while minimizing damage to normal tissues [126]. Advances in antibody engineering have led to the development of humanized and fully human mAbs, significantly reducing immunogenic responses and improving pharmacological profiles [127]. Furthermore, engineering of the Fc region has optimized antibody interactions with immune effector cells, enhancing mechanisms such as antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC), while also extending serum half-life through improved neonatal Fc receptor binding [128].
One of the most impactful uses of engineered mAbs in oncology is immune checkpoint inhibition and antibodies targeting inhibitory molecules such as PD-1, PD-L1, and CTLA-4 have revolutionized immunotherapy by reactivating exhausted T cells and restoring anti-tumor immune responses [129]. Notable examples include pembrolizumab (Keytruda) and nivolumab (Opdivo), both of which target PD-1 and have shown significant clinical benefits across a range of malignancies, including advanced melanoma and non-small cell lung cancer [130,131]. The phase Ib KEYNOTE-001 trial (NCT01295827) demonstrated that pembrolizumab provides durable antitumor responses and a favorable safety profile in advanced melanoma. With a median follow-up of 55 months, 5-year overall survival reached 34% in all patients and 41% in treatment-naive patients. Most responses were long-lasting, with 73% still ongoing at data cut-off [132].
In addition to checkpoint inhibitors, several FDA-approved mAbs have become standard-of-care treatments for specific cancer types. Trastuzumab (Herceptin), a humanized IgG1 monoclonal antibody targeting the HER2 receptor, has become integral to the management of HER2-positive breast and gastric cancers [117]. It exerts its effects by blocking HER2-driven signaling and promoting immune-mediated cell killing [10]. Another widely used example is rituximab (Rituxan), a chimeric anti-CD20 antibody employed in the treatment of B-cell malignancies such as non-Hodgkin lymphoma and chronic lymphocytic leukemia [133]. Rituximab mediates tumor cell elimination through a combination of ADCC, CDC, and direct induction of apoptosis [134]. These therapeutics underscore the central role of monoclonal antibodies in the era of targeted cancer treatment and highlight the clinical power of protein engineering.
Trastuzumab (approved in 1998) and rituximab (approved in 1997) are among the first FDA-approved monoclonal antibodies. The 2024 “Antibodies to Watch” report highlights those 16 new monoclonal antibody therapeutics received their first global approval in 2023 (as of 17 November), split evenly between cancer and non-cancer indications, with the U.S. leading approvals (9), followed by China and the E.U. (5 each), Japan (4), and Canada (4). The report also identifies 26 candidates under regulatory review and projects 23 more investigational antibodies will enter review by end-2024 and an analysis of clinical-phase success rates, based on datasets through 2019, reveals overall approval probabilities ranging from 14% to 32% [135].
3.2. Bispecific and Multispecific Antibodies
Bispecific and multispecific antibodies represent a sophisticated class of engineered therapeutic proteins designed to engage multiple targets simultaneously, offering novel mechanisms of action that extend beyond the capabilities of conventional monoclonal antibodies [136]. Bispecific antibodies are engineered to bind two distinct antigens or epitopes, allowing them to bridge cells or signaling pathways in a controlled and therapeutically advantageous manner. This dual-targeting capability has been harnessed to modulate complex immunological interactions and enhance specificity in tumor targeting [137].
A prominent application of bispecific antibodies is found in the development of bispecific T-cell engagers (BiTEs), which function by physically linking T cells to tumor cells, thereby initiating targeted immune-mediated cytotoxicity [138]. One of the most clinically advanced BiTEs is blinatumomab (Blincyto), a CD19/CD3 bispecific antibody approved for the treatment of relapsed or refractory B-cell acute lymphoblastic leukemia (ALL) [139]. Blinatumomab operates simultaneously binding to CD19 on malignant B cells and CD3 on T cells (Figure 3A), effectively redirecting cytotoxic T cells to lyse CD19-positive cancer cells. This mechanism bypasses traditional antigen presentation pathways, enabling rapid and robust immune responses against leukemic cells [118]. In the randomized, phase III TOWER trial, adult patients with relapsed or refractory Philadelphia-negative B-cell precursor ALL receiving blinatumomab experienced a median overall survival of 7.7 months versus 4.0 months with standard chemotherapy (hazard ratio for death, 0.71; 95% CI, 0.55–0.93; p = 0.01), along with higher complete remission rates (34% vs. 16%) and improved event-free survival (6-month estimates, 31% vs. 12%) [140]. A recent study introduced a bispecific CD40 agonistic antibody, BiA9*2_HF, which not only stimulates CD40 signaling to activate antigen-presenting cells but also allows for the rapid formation of antibody-peptide conjugates. This dual functionality exemplifies the potential of modular design in creating versatile therapeutic agents [119]. Mosunetuzumab is a prime example of a protein-engineered therapeutic representing the growing class of bispecific antibodies designed for targeted immunotherapy (Figure 3B), as it is engineered to simultaneously bind CD20 on malignant B cells and CD3 on T cells, thereby redirecting T cell cytotoxicity toward tumor cells and offering a novel mechanism of action distinct from conventional monoclonal antibodies; this dual specificity is achieved through sophisticated antibody engineering techniques that ensure correct chain pairing, structural stability, and functional activity, and with its FDA approval for relapsed or refractory follicular lymphoma, mosunetuzumab exemplifies how advances in protein engineering can be harnessed to create next-generation immunotherapies with improved specificity and clinical efficacy [141].
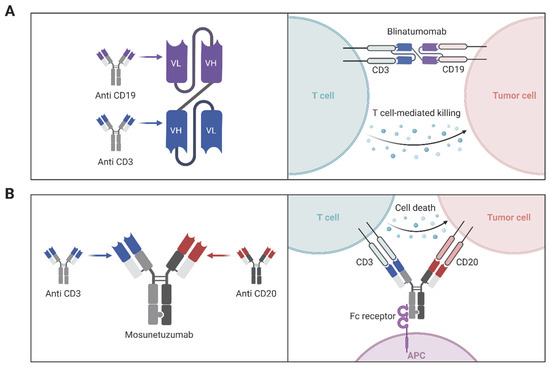
Figure 3.
Protein-engineered bispecific antibodies for T cell-mediated tumor killing. (A) Schematic representation of the bispecific T-cell engager (BiTE) blinatumomab, designed using protein engineering to fuse the variable regions of two monoclonal antibodies: one targeting CD3 on T cells and the other targeting CD19 on tumor cells. This single-chain bispecific format brings T cells into proximity with tumor cells, enabling cytotoxic killing. (B) Structure and mechanism of the full-length bispecific antibody mosunetuzumab, which simultaneously binds CD3 on T cells and CD20 on tumor cells and retains an Fc region to engage Fc receptors on antigen-presenting cells (APCs), enhancing immune activation and cell death. (Illustration created with BioRender.com; https://app.biorender.com, accessed on 25 June 2025.)
Building on the success of bispecific antibodies, research is now advancing toward the development of trispecific antibodies, which can engage three separate targets simultaneously [142]. These next generation constructions aim to enhance therapeutic efficacy, overcome resistance mechanisms, and achieve more nuanced immune modulation [143]. For example, a trispecific antibody engineered to target CD38, CD3, and CD28 has shown promising results in preclinical models of multiple myeloma. This molecule is designed to activate T cells through CD3 and CD28 co-stimulation while directing them toward CD38-expressing tumor cells, thereby enhancing both the activation and cytolytic function of the immune effector cells [144]. Zhao et al. report the design and preclinical evaluation of a novel CD19/CD22/CD3 trispecific antibody that markedly enhances antitumor efficacy and overcomes immune escape in B-ALL. By site-specifically fusing an anti-CD19 scFv (FMC63) and an anti-CD22 nanobody (Nb25) onto defined sites of a CD3-binding Fab (SP34), the optimized construct promotes optimal immune-synapse formation. Compared with corresponding bispecific antibodies or blinatumomab alone, this construct induced superior T-cell cytotoxicity and cytokine release against CD19+ and/or CD22+ target cells, achieved long-term eradication of patient-derived B-ALL xenografts, and significantly prolonged survival in PDX models. This work highlights a broadly applicable strategy for structurally optimizing multispecific T-cell engagers to address antigen heterogeneity and resistance [145].
Such trispecific platforms exemplify the versatility of protein engineering in creating highly tailored immunotherapeutics capable of addressing complex and heterogeneous tumor environments. Overall, bispecific and multispecific antibodies reflect the growing sophistication of antibody engineering technologies. By integrating multi-target binding with controlled immune activation, these agents provide powerful tools for precision oncology and are poised to play an increasingly prominent role in the next generation of cancer therapies.
Compared to monoclonal antibodies, bispecific formats offer the advantage of dual targeting, which may overcome resistance mechanisms associated with single epitope recognition. However, their complex design often leads to reduced stability and more stringent manufacturing requirements. Additionally, the therapeutic window for bispecifics is often narrower, requiring precise dose optimization to mitigate off-target effects [146]. Despite increased interest, only a limited number of bispecific antibodies have reached clinical approval, with high attrition in early-phase trials due to stability, toxicity, or manufacturing complexity [147].

Table 3.
Clinically approved antibody-based cancer therapies with protein engineering modifications.
Table 3.
Clinically approved antibody-based cancer therapies with protein engineering modifications.
| Antibody Name | Engineering Strategy | Target(s) | Mechanism/Engineering Purpose | Cancer Indication(s) | FDA Approval Year | Ref. |
|---|---|---|---|---|---|---|
| Trastuzumab (Herceptin) | Humanized IgG1 | HER2 | Humanization to reduce immunogenicity | HER2+ breast, gastric cancer | 1998 | [148] |
| Atezolizumab (Tecentriq) | Fc-engineered IgG1 | PD-L1 | Fc mutation reduces ADCC to preserve immune cells | NSCLC, urothelial carcinoma, | 2016 | [149] |
| Durvalumab (Imfinzi) | Fc-engineered IgG1 | PD-L1 | Reduced Fc effector function | NSCLC, SCLC, bladder cancer | 2017 | [150] |
| Blinatumomab (Blincyto) | Bispecific T cell engager | CD3/CD19 | BiTE format links T cells to B cells | B-cell ALL | 2014 | [151] |
| Mosunetuzumab | Bispecific antibody | CD20/CD3 | Redirects T cells to B cells | Follicular lymphoma | 2022 | [152] |
| Glofitamab | Bispecific antibody | CD20/CD3 | 2:1 binding format for enhanced avidity | DLBCL | 2023 | [153] |
| Brentuximab vedotin (Adcetris) | ADC | CD30 | MMAE cytotoxin via cleavable linker | Hodgkin lymphoma | 2011 | [154] |
| Trastuzumab emtansine (Kadcyla) | ADC | HER2 | DM1 payload conjugated to trastuzumab | HER2+ breast cancer | 2013 | [155] |
| Trastuzumab deruxtecan (Enhertu) | ADC | HER2 | Topoisomerase I inhibitor payload | HER2+ breast, gastric, lung cancers | 2019 | [156] |
| Sacituzumab govitecan (Trodelvy) | ADC | Trop-2 | SN-38 (irinotecan active form) conjugated | TNBC, urothelial carcinoma | 2020 | [157] |
| Margetuximab | Fc-engineered anti-HER2 | HER2 | Fc domain optimized for better FcγRIIIa binding (enhanced ADCC) | HER2+ breast cancer | 2020 | [158] |
| Elranatamab | Bispecific antibody | CD3/BCMA | Engages T cells with BCMA-expressing myeloma cells | Multiple myeloma | 2023 | [159] |
3.3. Engineered Cytokines and Fusion Proteins
Cytokines are key regulators of immune activity and play an essential role in orchestrating anti-tumor responses, however, their clinical application has historically been hampered by significant challenges, including severe systemic toxicity, short in vivo half-lives, and limited specificity [160]. Through protein engineering, researchers have developed cytokine variants and multifunctional fusion proteins that address these limitations, thereby unlocking new therapeutic potential for these potent immunomodulators [161].
One of the major advancements in this field involves the engineering of interleukin variants such as IL-2 and IL-15 to improve their therapeutic index. These cytokines are critical for the activation and proliferation of T cells and natural killer (NK) cells but can cause substantial toxicity due to their interaction with high-affinity receptor subunits expressed on non-target cells [162]. To mitigate this, engineered variants with selectively reduced affinity for the α-chain of the IL-2 receptor (IL-2Rα) have been created, thereby biasing cytokine activity toward effector immune cells and away from regulatory or endothelial cells [163]. A notable example is NKTR-214 (bempegaldesleukin), an IL-2 variant that extends serum half-life and preferentially activates CD8+ T cells and NK cells [120]. Clinical trials in melanoma and renal cell carcinoma have demonstrated encouraging results, particularly when used in combination with immune checkpoint inhibitors [120]. Walker et al. describe a preclinical study in which the CD122-biased IL-2 agonist bempegaldesleukin (NKTR-214) was combined with a single high-dose of radiotherapy (RT) in murine models of bilateral MCA-205 fibrosarcoma and CT26 colorectal cancer. While each modality alone had limited efficacy, their combination produced dramatic synergy: cure rates rose from minimal levels with monotherapy to 45–85% with RT + NKTR-214, dependent on CD8+ T cells. Mechanistically, combination therapy amplified tumor-specific CD8+ responses, enhanced T-cell trafficking to both irradiated and distant (abscopal) lesions and induced favorable changes in effector versus regulatory T-cell ratios. These data support RT + NKTR-214 as a potent inducer of systemic antitumor immunity and rationale for clinical evaluation in patients with advanced solid tumors [164]. One notable example is the combination of pembrolizumab, an anti-PD-1 monoclonal antibody, with lenvatinib, a multi-kinase inhibitor. This regimen has demonstrated significant clinical benefits in patients with advanced renal cell carcinoma. In the phase 3 CLEAR study, patients receiving the pembrolizumab–lenvatinib combination exhibited improved progression-free survival and overall survival compared to those treated with sunitinib, a standard therapy for renal cell carcinoma [165].
Beyond monomeric cytokines, protein engineering has enabled the creation of multifunctional fusion proteins that combine immune-stimulatory cytokines with tumor-targeting domains [166]. These constructs aim to increase the local concentration of cytokines at tumor sites while reducing systemic exposure and associated toxicity [161]. Immunocytokines, such as L19/IL-2, exemplify this approach by fusing IL-2 to an antibody fragment that specifically binds to the extra-domain B (ED-B) of fibronectin, a marker of tumor vasculature [121]. In preclinical models of solid tumors, L19-IL2 has demonstrated enhanced tumor localization, improved immune cell recruitment, and significant anti-tumor efficacy [121]. These fusion proteins represent a strategic advancement in cytokine therapy, offering enhanced selectivity and potency through modular design.
Expanding on the concept of targeted fusion proteins, our recent research has explored the use of vascular endothelial growth factor (VEGF) as a delivery vehicle for cytotoxic agents and our study detailed the development of a novel fusion protein combining mouse VEGF with the heminecrolysin (HNc) toxin [9]. This VEGF–HNc fusion protein was designed to target VEGF receptors, which are commonly overexpressed in tumor vasculature, thereby delivering the toxin directly to the tumor site which In vitro and in vivo experiments demonstrated that the fusion protein exhibited significant cytotoxicity against VEGFR-expressing cells and effectively reduced tumor growth in a mouse model [9]. These findings suggest that VEGF-based fusion proteins could serve as a promising strategy for selectively targeting tumor vasculature and enhancing the specificity of cancer therapeutics [9].
One notable example is the development of immunocytokines, which are fusion proteins combining cytokines with tumor-targeting antibody fragments. NHS-IL12 is an immunocytokine that fuses IL-12 with the NHS76 antibody, targeting DNA-histone complexes in necrotic tumor regions. Preclinical studies have demonstrated that NHS-IL12 elicits robust Th1 immune responses, enhancing the activity of NK cells and CD8+ T lymphocytes, leading to significant tumor suppression in various solid tumor models [122].
In addition to multi-functional fusion proteins, modular protein platforms have emerged as powerful tools for the rapid assembly of customized therapeutic proteins. These platforms utilize standardized protein domains that can be recombined in various configurations to target different cancer antigens or modulate distinct therapeutic pathways [167]. For instance, the SpyMask system enables the combinatorial assembly of bispecific binders, facilitating the creation of bispecific antibodies that can simultaneously engage two different targets. This modular approach allows for the efficient generation of diverse bispecific antibodies with potential applications in cancer immunotherapy [168]. While engineered cytokines enhance immune activation and tumor infiltration, they often induce systemic immune-related adverse events (irAEs), including cytokine release syndrome and vascular leak. Several efforts, such as biased cytokine receptor targeting and tumor-restricted delivery using fusion proteins, have been developed to mitigate these toxicities while preserving efficacy [169]. Engineered cytokine therapies such as bempegaldesleukin (NKTR-214) entered Phase III trials but were ultimately discontinued due to lack of significant efficacy [170]. This highlights a key challenge in balancing immune stimulation and systemic toxicity, with few engineered cytokine drugs receiving approval despite promising preclinical data.
Together, engineered cytokines and fusion proteins reflect a growing trend toward precision immunomodulation in cancer therapy. By refining the pharmacokinetics, receptor specificity, and tumor-targeting capabilities of these agents, protein engineering continues to advance the safety and effectiveness of cytokine-based interventions in oncology.
3.4. Nanobodies and Single-Domain Antibodies
Nanobodies, also known as single-domain antibodies, are the variable domains of heavy-chain-only antibodies naturally found in camelids [171]. Due to their small size (~15 kDa), high stability, and strong antigen-binding affinity, nanobodies have emerged as versatile tools in cancer diagnostics and therapeutics [172]. Their unique properties, including superior tissue penetration and rapid systemic clearance, make them particularly advantageous for applications requiring precise targeting and minimal off-target effects [173].
One prominent application of nanobodies is in molecular imaging. For instance, a Phase I study evaluated the use of a 68Ga- anti-HER2 nanobody for PET/CT imaging in patients with HER2-positive breast carcinoma. The study demonstrated that 68Ga-HER2-nanobody PET/CT is a safe procedure with favorable biodistribution and high tumor-to-background contrast, enabling effective visualization of HER2-positive lesions [174]. Building upon these findings, a subsequent Phase II trial confirmed the repeatability and tumor uptake of 68Ga HER2 single-domain antibody PET/CT in patients with breast carcinoma, reinforcing the potential of nanobody-based imaging agents in clinical settings [123].
Beyond diagnostics, nanobodies have been engineered as carriers for cytotoxic agents in targeted cancer therapy. A notable example is the development of nanobody-based immunotoxins targeting the epidermal growth factor receptor (EGFR), which is overexpressed in various cancers, including colorectal cancer. In a recent study, researchers produced and characterized several immunotoxin designs using a nanobody against EGFR (VHH 7D12) as the targeting domain. These nanobody-drug conjugates exhibited potent anti-tumor activity in preclinical models, highlighting their therapeutic potential [124]. The modular nature of nanobodies also allows for the creation of multivalent biparatopic constructs, enabling simultaneous targeting of antigens or epitopes. This approach can enhance therapeutic efficacy and reduce the likelihood of resistance development. For example, Fan et al. developed a tetravalent biparatopic EGFR-targeting nanobody-drug conjugate (S7 ADC) designed to overcome resistance mutations that limit the efficacy of existing anti-EGFR therapies like cetuximab. This construction combines two nanobodies (7D12 and 9G8) targeting distinct EGFR epitopes and is conjugated to the cytotoxic agent monomethyl auristatin E (MMAE). S7 ADC demonstrated potent antitumor activity in vitro and in vivo, including against EGFR mutants such as S492R and G465R. Additionally, the engineered Fc domain with an E430G mutation enhanced complement-dependent cytotoxicity, further boosting therapeutic efficacy. These findings highlight the potential of multivalent nanobody-based ADCs to address therapeutic resistance in EGFR-driven cancers [175].
In summary, nanobodies and single-domain antibodies represent a significant advancement in the field of cancer therapeutics. Their unique structural and functional attributes facilitate precise tumor targeting, effective imaging, and the delivery of therapeutic payloads, thereby offering new avenues for the diagnosis and treatment of various malignancies.
3.5. Protein-Based Drug Delivery Systems
The convergence of protein engineering and nanotechnology has led to the development of sophisticated drug delivery systems designed to enhance the efficacy and specificity of cancer therapeutics while minimizing systemic toxicity. Engineered proteins serve as versatile platforms for the targeted delivery of chemotherapeutic agents and nucleic acid-based drugs, offering promising avenues for improving cancer treatment outcomes.
One notable example is the ADC trastuzumab emtansine (T-DM1), which combines the HER2-targeting capabilities of trastuzumab with the cytotoxic agent emtansine (DM1). T-DM1 has demonstrated significant clinical benefits in patients with HER2-positive metastatic breast cancer who have previously received trastuzumab and taxane chemotherapy. In the phase 3 EMILIA trial, T-DM1 significantly improved overall survival compared to the combination of capecitabine and lapatinib, with a median overall survival of 29.9 months versus 25.9 months, respectively. These findings underscore the potential of ADCs to deliver potent chemotherapeutic agents directly to tumor cells, thereby enhancing therapeutic efficacy while reducing off-target effects [176].
Beyond ADCs, engineered protein nanostructures have emerged as innovative carriers for the delivery of nucleic acid-based therapeutics [177]. Ferritin, a naturally occurring iron storage protein, can be reassembled into nanocages capable of encapsulating various therapeutic payloads [125]. Studies have demonstrated the utility of ferritin-based nanoparticles in delivering small interfering RNA (siRNA) to tumor cells. For instance, a study reported the development of gold nanocluster-assisted delivery of nerve growth factor (NGF) siRNA for the treatment of pancreatic cancer. The gold nanocluster-siRNA complex enhanced the stability and tumor accumulation of siRNA, leading to efficient NGF gene silencing and significant tumor regression in preclinical models [178].
These advancements highlight the versatility of protein-based delivery systems in cancer therapy. By leveraging the specificity of engineered proteins and the structural advantages of nanocarriers, these systems can facilitate the targeted delivery of a wide range of therapeutics, including chemotherapeutic agents and nucleic acids. As research progresses, the integration of protein engineering with nanotechnology holds great promise for the development of next-generation cancer treatments that are both effective and precise.
One persistent limitation across engineered therapeutic platforms is tumor heterogeneity, which allows for the emergence of resistant clones lacking the targeted antigen. Additionally, immune checkpoint upregulation and immunosuppressive microenvironments pose barriers to sustained responses. These mechanisms necessitate combination strategies or multimodal constructions [179].
4. Challenges and Limitations and Future
While protein engineering has revolutionized cancer biotherapeutics, several challenges and limitations must be addressed to fully realize its potential. These include issues related to protein stability, immunogenicity, manufacturing scalability, and regulatory hurdles. This section critically evaluates these challenges and discusses potential strategies to overcome them. This section critically evaluates these challenges and discusses potential strategies to overcome them. A summary of the key barriers and corresponding engineering strategies is provided in Table 4.
4.1. Protein Stability and Folding in Therapeutic Contexts
Protein engineering has significantly advanced cancer biotherapeutics; however, challenges related to protein stability and folding persist, particularly concerning aggregation, misfolding, and degradation [180]. Engineered proteins, especially those with complex architectures like bispecific antibodies, are susceptible to aggregation and misfolding during production and storage [181] and such instability can lead to reduced activity, increased immunogenicity, and potential toxicity. For instance, studies have shown that bispecific antibodies may aggregate due to their intricate structures, posing challenges for their stability and shelf life [182].
To address these challenges, various strategies have been employed to enhance protein stability. Computational modeling and directed evolution techniques are used to design proteins with improved folding and reduced aggregation tendencies. Introducing stabilizing mutations, optimizing expression systems, and refining purification processes can significantly improve protein stability [183]. For example, computational approaches have been utilized to identify mutations that enhance the stability of therapeutic antibodies, leading to improved shelf life and efficacy [184,185]. Additionally, formulation strategies play a crucial role in maintaining protein stability and adjusting pH levels, ionic strength, and buffer compositions can influence protein folding and aggregation [186]. Studies have demonstrated that certain formulations can suppress aggregation during long-term storage, thereby preserving the therapeutic integrity of engineered proteins [187,188]. While protein engineering has revolutionized cancer therapy, addressing the challenges of protein stability and folding remains essential. Through a combination of computational design, strategic mutations, and optimized formulations, the stability and efficacy of therapeutic proteins can be significantly enhanced.
4.2. Immunogenicity and Strategies for Immune Evasion
Immunogenicity remains a significant hurdle in the development and clinical application of protein-based therapeutics and the immune system may recognize these engineered proteins as foreign, leading to the production of anti-drug antibodies (ADAs) that can neutralize therapeutic effects, alter pharmacokinetics, and potentially cause adverse reactions [189]. Several factors contribute to the immunogenicity of therapeutic proteins. These include the presence of non-human sequences, such as those derived from murine antibodies, post-translational modifications, and protein aggregation [190]. Even fully humanized proteins can elicit immune responses due to novel structural features or high concentrations [191]. For instance, a clinical trial involving a novel cytokine fusion protein reported the development of ADAs in patients, leading to reduced therapeutic efficacy [192].
To mitigate immunogenicity, various protein engineering strategies have been employed. Deimmunization involves modifying immunogenic epitopes to reduce T-cell recognition, while humanization replaces non-human sequences with human counterparts to minimize immune responses and computational tools have been instrumental in identifying and altering immunogenic regions [193]. For example, a study demonstrated the successful deimmunization of a therapeutic antibody through computational epitope mapping and site-directed mutagenesis, resulting in reduced immunogenicity in preclinical models [194]. Furthermore, optimizing protein formulations and delivery routes can further minimize immune recognition and adjusting factors such as pH, ionic strength, and buffer composition can influence protein stability and reduce aggregation, thereby decreasing immunogenic potential [195]. Moreover, alternative delivery methods, such as subcutaneous administration, may lower the risk of immune responses compared to intravenous routes [196]. Several protein therapeutics fail in late-stage development due to immunogenicity, suboptimal pharmacokinetics, or low response rates [189]. For example, oportuzumab monatox (a recombinant fusion protein for bladder cancer) administered weekly for six weeks and then monthly showed a complete response (CR) rate of 40% at 3 months among patients with carcinoma in situ. This rate declined to 17% in 12 months. Treatment-emergent adverse events (TEAEs) occurred in 88% of patients, with serious adverse events in 9% and a 2% treatment discontinuation rate and not approved despite completing Phase III trials [197].
Focusing on immunogenicity is crucial for the successful development of protein therapeutics. Through a combination of protein engineering techniques, computational modeling, and formulation optimization, it is possible to design therapeutic proteins with reduced immunogenic potential, enhancing their safety and efficacy in clinical applications.
4.3. Manufacturing and Scalability Issues
Manufacturing engineered proteins at scale presents significant challenges, including high costs, complex purification processes, and variability in product quality and these issues are particularly pronounced in the production of advanced therapeutics such as bispecific antibodies and chimeric antigen receptor CAR T cells [198,199]. The choice of expression system is critical in determining protein yield, folding, and post-translational modifications where mammalian cells are often preferred for producing complex proteins due to their ability to perform human-like post-translational modifications [200]. However, they are costly and present scalability challenges and bacterial and yeast systems offer higher yields and lower costs but may lack the necessary modifications for certain therapeutic proteins [22].
Purification of engineered proteins to meet regulatory standards is a complex and expensive process and impurities, such as host cell proteins or aggregates, must be removed to ensure safety and efficacy [201]. Advanced chromatography techniques, including mixed-mode anion exchange and optimized Protein A chromatography, have been employed to improve the purification of bispecific antibodies, reducing impurities and increasing yield [202]. The production of CAR T cells exemplifies the complexities of scaling up engineered protein manufacturing and traditional CAR T cell manufacturing involves multiple manual steps, including T cell isolation, genetic modification using viral vectors, expansion, and quality control, all under stringent Good Manufacturing Practice (GMP) conditions. This process is labor-intensive, time-consuming, and costly, often taking several weeks per patient [203]. To address these challenges, the industry is shifting towards automated and closed-system manufacturing platforms, and these systems aim to reduce manual interventions, minimize contamination risks, and improve reproducibility [204]. For instance, the integration of automated bioreactors and modular purification systems has shown promise in enhancing the scalability and efficiency of CAR T cell production [205]. While significant progress has been made in the manufacturing of engineered proteins, challenges related to expression systems, purification processes, and scalability persist. Continued advancements in automation, process optimization, and innovative purification strategies are essential to overcome these hurdles and meet the growing demand for protein-based therapeutics.
The development and approval of protein-based therapeutics entail navigating intricate regulatory frameworks and managing substantial costs, factors that can impede accessibility and affordability. Regulatory agencies, such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), mandate comprehensive preclinical and clinical data to substantiate the safety, efficacy, and quality of engineered proteins. The inherent complexity of these biologics often necessitates additional studies, including immunogenicity assessments and long-term stability evaluations. For instance, the approval process for blinatumomab (Blincyto), a bispecific T-cell engager targeting CD19 and CD3, involved extensive clinical trials to demonstrate its safety and efficacy in patients with relapsed or refractory ALL [206]. The high costs associated with developing and manufacturing protein-based therapeutics further challenge their accessibility, particularly in low-resource settings. Strategies to mitigate these costs include optimizing production processes, leveraging biosimilars, and implementing value-based pricing models [207,208]. Biosimilars, which are highly similar to approved biologic products, offer a promising avenue for cost reduction. For example, the introduction of biosimilars has been projected to reduce direct spending on biologic drugs by approximately $54 billion from 2017 to 2026 in the United States [209]. Additionally, adopting value-based pricing models, where the cost of a therapeutic is aligned with its clinical benefit, can enhance affordability and patient access [210].
The complexity of manufacturing engineered proteins depends heavily on the expression platform. Bacterial systems offer low-cost, high-yield production but lack post-translational modifications, limiting their utility for complex biologics [211]. Yeast and mammalian systems provide more accurate folding and glycosylation but are more expensive and time intensive [212]. For CAR T cells and bispecific antibodies, automated and closed-system platforms, such as point-of-care manufacturing and bioreactor-based expansion, are increasingly used to reduce variability and scale production [203]. Despite these advances, the manufacturing cost for many protein therapeutics remains high. For example, CAR T-cell therapy can cost USD 373,000–475,000 per patient, not including hospitalization and supportive care [213]. The emergence of biosimilars and platform manufacturing is expected to lower costs; the U.S. FDA projects biosimilar savings could decrease direct spending on biologic drugs by USD 54 billion over a 10-year period (2017–2026) [214]. Addressing manufacturing scalability is essential not only for broader clinical adoption but also for equitable global access.

Table 4.
Key challenges in protein engineering for cancer therapy and proposed solutions [17,22,180,189,215].
Table 4.
Key challenges in protein engineering for cancer therapy and proposed solutions [17,22,180,189,215].
| Challenge | Underlying Issue | Example Case | Proposed Engineering Strategies |
|---|---|---|---|
| Protein Stability | Aggregation, misfolding, or degradation during production or storage | Bispecific antibodies prone to aggregation | Stabilizing mutations; computational folding models; optimized expression systems and formulations |
| Immunogenicity | Host immune system recognizes engineered protein as foreign | Anti-drug antibodies (ADAs) generated against cytokine fusion proteins | Deimmunization; humanization; epitope masking; computational T-cell epitope mapping |
| Manufacturing Scale-Up | Low yield, complex purification, batch variability | CAR T-cell therapies; bispecific antibody production | Automation; closed-system bioreactors; mammalian expression platforms; advanced chromatography |
| Regulatory Complexity | Need for extensive safety, efficacy, and stability data | Approval of bispecifics like blinatumomab required extended trials | Early engagement with regulators; adaptive trial design; real-world evidence generation |
| Cost and Accessibility | High development and production costs limit patient access | Limited access to ADCs and CAR T-cell therapies in low-resource settings | Biosimilar development; value-based pricing; process optimization for cost reduction |
4.4. Future Perspectives and Research Directions
The future of protein engineering in cancer biotherapeutics is poised for major breakthroughs (Figure 4), driven by innovations such as quantum computing [216,217], ultra-stable and non-immunogenic proteins [218], strategies targeting the tumor microenvironment (TME) [219], and collaborative frameworks spanning academia, industry, and regulatory bodies. Quantum computing holds the potential to overcome classical computational limitations by enabling large-scale simulations of protein folding and interactions, which can accelerate the design of optimized therapeutic proteins with improved stability and reduced immunogenicity [216]. Complementing this, advances in computational modeling, directed evolution, and synthetic biology are enabling the creation of ultra-stable therapeutic proteins that resist degradation and evade immune detection, with several studies demonstrating enhanced drug properties and reduced anti-drug antibody formation which discussed here. Addressing the challenges of the TME, engineered proteins like bispecific antibodies that target dual immunosuppressive pathways (e.g., PD-L1 and TGF-β) and responsive protein-based nanoparticles are improving therapeutic delivery and immune activation at tumor sites. These strategies offer enhanced efficacy over traditional monotherapies and reduce systemic toxicity. Driving these innovations forward are collaborative efforts such as the Structural Genomics Consortium, which shares structural and functional protein data openly to accelerate discovery, and regulatory initiatives like the FDA’s Oncology Center of Excellence, which supports adaptive clinical trial designs and dose optimization for safer and faster approvals. Additionally, global partnerships such as the International Cancer Proteogenome Consortium are leveraging international expertise and resources to develop affordable and personalized cancer therapies. Altogether, the integration of emerging technologies, advanced design strategies, and cross-sector collaboration is shaping a transformative future for protein-based cancer treatments.
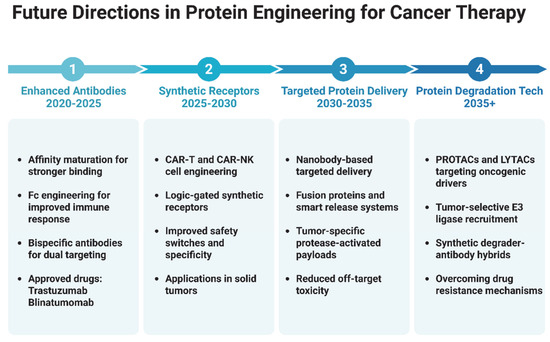
Figure 4.
Illustrative timeline outlining key future directions in protein engineering for cancer therapy. Advancements include enhanced antibodies, synthetic receptors such as CAR T and CAR-NK technologies, targeted protein delivery using nanobodies and fusion proteins, and the development of protein degradation technologies such as PROTACs, LYTACs, and synthetic degrader-antibody hybrids. These innovations aim to improve specificity, reduce toxicity, and overcome resistance in cancer treatment. (Illustration created with BioRender.com; https://app.biorender.com, accessed on 25 June 2025.)
5. Conclusions
Protein engineering has emerged as a transformative force in cancer therapeutics, enabling the development of highly specific, potent, and adaptable biotherapeutics that address the complexity and heterogeneity of cancer. This review has highlighted the remarkable advancements in the field, from the design of monoclonal antibodies and bispecific T-cell engagers to the creation of engineered cytokines, nanobodies, and protein-based drug delivery systems. These innovations have not only improved patient outcomes but have also expanded the therapeutic arsenal available to clinicians and researchers. The integration of cutting-edge technologies, such as computational modeling, artificial intelligence, and synthetic biology, has accelerated the pace of discovery and optimization in protein engineering. These tools have enabled the design of proteins with enhanced stability, reduced immunogenicity, and novel functionalities, paving the way for next-generation cancer therapies. Furthermore, the exploration of emerging trends, such as quantum computing, ultra-stable protein designs, and strategies to overcome the tumor microenvironment, holds immense promise for addressing current limitations and unlocking new therapeutic possibilities.
The transformative potential of protein-engineered biotherapeutics in oncology cannot be overstated. By harnessing the power of engineered proteins, researchers and clinicians are moving closer to realizing the vision of precision oncology—tailoring treatments to the unique molecular and cellular characteristics of individual tumors. The success of FDA-approved therapies, such as trastuzumab, blinatumomab, and immune checkpoint inhibitors, underscores the clinical impact of these advancements and serves as a testament to the power of protein engineering. However, significant challenges remain, including issues related to protein stability, immunogenicity, manufacturing scalability, and regulatory hurdles. Addressing these challenges will require interdisciplinary collaboration, innovative engineering strategies, and continued investment in research and development. Collaborative efforts between academia, industry, and regulatory bodies will be essential to accelerate the translation of scientific discoveries into clinical applications and ensure that these life-saving therapies reach patients worldwide.
Looking ahead, the roadmap for future innovations in protein engineering for cancer therapeutics is both exciting and ambitious. Advances in quantum computing, AI-driven protein design, and synthetic biology are poised to revolutionize the field, enabling the creation of even more sophisticated and effective therapies. At the same time, a focus on addressing global health disparities and improving access to biotherapeutics will be critical to maximizing their impact. In conclusion, protein engineering has fundamentally reshaped the landscape of cancer therapeutics, offering new hope to patients and transforming the way we approach cancer treatment. As the field continues to evolve, the integration of emerging technologies, collaborative efforts, and a commitment to innovation will drive the next wave of breakthroughs, bringing us closer to a future where cancer is no longer a life-threatening disease but a manageable condition.
Author Contributions
Literature review and writing, Z.N. and A.S.; writing, editing, and supervision, A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Jacquemin, V.; Antoine, M.; Dom, G.; Detours, V.; Maenhaut, C.; Dumont, J.E. Dynamic Cancer Cell Heterogeneity: Diagnostic and Therapeutic Implications. Cancers 2022, 14, 280. [Google Scholar] [CrossRef]
- Liu, B.L.; Zhou, H.Y.; Tan, L.C.; Siu, K.T.H.; Guan, X.Y. Exploring treatment options in cancer: Tumor treatment strategies. Signal Transduct. Target. Ther. 2024, 9, 175. [Google Scholar] [CrossRef] [PubMed]
- El-Tanani, M.; Rabbani, S.A.; Babiker, R.; Rangraze, I.; Kapre, S.; Palakurthi, S.S.; Alnuqaydan, A.M.; Aljabali, A.A.; Rizzo, M.; El-Tanani, Y.; et al. Unraveling the tumor microenvironment: Insights into cancer metastasis and therapeutic strategies. Cancer Lett. 2024, 591, 216894. [Google Scholar] [CrossRef] [PubMed]
- Ghemrawi, R.; Abuamer, L.; Kremesh, S.; Hussien, G.; Ahmed, R.; Mousa, W.; Khoder, G.; Khair, M. Revolutionizing Cancer Treatment: Recent Advances in Immunotherapy. Biomedicines 2024, 12, 2158. [Google Scholar] [CrossRef]
- Papiez, M.A.; Krzysciak, W. Biological Therapies in the Treatment of Cancer-Update and New Directions. Int. J. Mol. Sci. 2021, 22, 11694. [Google Scholar] [CrossRef]
- Zahavi, D.; Weiner, L. Monoclonal Antibodies in Cancer Therapy. Antibodies 2020, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Eskafi, A.H.; Oghalaei, A.; Mahboudi, F.; Ghaderi, H.; Behdani, M.; Shoari, A.; Kazemi-Lomedasht, F. Investigation of the therapeutic potential of recombinant bispecific bivalent anti-PD-L1/VEGF nanobody in inhibition of angiogenesis. Immunopharm. Immunot. 2023, 45, 197–202. [Google Scholar] [CrossRef]
- Naderiyan, Z.; Sotoudeh, N.; Shoari, A.; Ghaderi, H.; Habibi-Anbouhi, M.; Moazzami, R.; Cohan, R.A.; Behdani, M. In Vitro and In Vivo Studies of a Heminecrolysin Toxin-VEGF Fusion Protein as a Novel Therapeutic for Solid Tumor Targeting. Mol. Biotechnol. 2023, 65, 766–773. [Google Scholar] [CrossRef]
- Mercogliano, M.F.; Bruni, S.; Mauro, F.L.; Schillaci, R. Emerging Targeted Therapies for HER2-Positive Breast Cancer. Cancers 2023, 15, 1987. [Google Scholar] [CrossRef]
- Salles, G.; Barrett, M.; Foa, R.; Maurer, J.; O’Brien, S.; Valente, N.; Wenger, M.; Maloney, D.G. Rituximab in B-Cell Hematologic Malignancies: A Review of 20 Years of Clinical Experience. Adv. Ther. 2017, 34, 2232–2273. [Google Scholar] [CrossRef]
- Tobin, P.H.; Richards, D.H.; Callender, R.A.; Wilson, C.J. Protein engineering: A new frontier for biological therapeutics. Curr. Drug Metab. 2014, 15, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Ndochinwa, G.O.; Wang, Q.Y.; Okoro, N.O.; Amadi, O.C.; Nwagu, T.N.; Nnamchi, C.I.; Moneke, A.N.; Odiba, A.S. New advances in protein engineering for industrial applications: Key takeaways. Open Life Sci. 2024, 19, 20220856. [Google Scholar] [CrossRef] [PubMed]
- Yuen, C.M.; Liu, D.R. Dissecting protein structure and function using directed evolution. Nat. Methods 2007, 4, 995–997. [Google Scholar] [CrossRef]
- Son, A.; Park, J.; Kim, W.; Yoon, Y.; Lee, S.; Park, Y.; Kim, H. Revolutionizing Molecular Design for Innovative Therapeutic Applications through Artificial Intelligence. Molecules 2024, 29, 4626. [Google Scholar] [CrossRef]
- Ocana, A.; Pandiella, A.; Privat, C.; Bravo, I.; Luengo-Oroz, M.; Amir, E.; Gyorffy, B. Integrating artificial intelligence in drug discovery and early drug development: A transformative approach. Biomark. Res. 2025, 13, 45. [Google Scholar] [CrossRef]
- Kintzing, J.R.; Filsinger Interrante, M.V.; Cochran, J.R. Emerging Strategies for Developing Next-Generation Protein Therapeutics for Cancer Treatment. Trends Pharmacol. Sci. 2016, 37, 993–1008. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, M.; Ren, F.; Meng, X.; Yu, J. The landscape of bispecific T cell engager in cancer treatment. Biomark. Res. 2021, 9, 38. [Google Scholar] [CrossRef]
- Akram, F.; Ali, A.M.; Akhtar, M.T.; Fatima, T.; Shabbir, I.; ul Haq, I. The journey of antibody-drug conjugates for revolutionizing cancer therapy: A review. Bioorgan Med. Chem. 2025, 117, 118010. [Google Scholar] [CrossRef] [PubMed]
- Roybal, K.T.; Lim, W.A. Synthetic Immunology: Hacking Immune Cells to Expand Their Therapeutic Capabilities. Annu. Rev. Immunol. 2017, 35, 229–253. [Google Scholar] [CrossRef]
- Liao, H.C.; Liu, S.J. Advances in nucleic acid-based cancer vaccines. J. Biomed. Sci. 2025, 32, 10. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Barwal, A.; Sharma, N.; Mir, D.S.; Kumar, P.; Kumar, V. Therapeutic proteins: Developments, progress, challenges, and future perspectives. 3 Biotech 2024, 14, 112. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Morales, J.; Vanella, R.; Appelt, E.A.; Whillock, S.; Paulk, A.M.; Shusta, E.V.; Hackel, B.J.; Liu, C.C.; Nash, M.A. Protein Engineering and High-Throughput Screening by Yeast Surface Display: Survey of Current Methods. Small Sci. 2023, 3, 2300095. [Google Scholar] [CrossRef]
- McLure, R.J.; Radford, S.E.; Brockwell, D.J. High-throughput directed evolution: A golden era for protein science. Trends Chem. 2022, 4, 378–391. [Google Scholar] [CrossRef]
- Korendovych, I.V. Rational and Semirational Protein Design. Methods Mol. Biol. 2018, 1685, 15–23. [Google Scholar] [CrossRef]
- Selles Vidal, L.; Isalan, M.; Heap, J.T.; Ledesma-Amaro, R. A primer to directed evolution: Current methodologies and future directions. RSC Chem. Biol. 2023, 4, 271–291. [Google Scholar] [CrossRef]
- Yao, J.W.; Wang, X.G. Artificial intelligence in de novo protein design. Med. Nov. Technol. Devices 2025, 26, 100366. [Google Scholar] [CrossRef]
- Cobb, R.E.; Chao, R.; Zhao, H. Directed Evolution: Past, Present and Future. AIChE J. 2013, 59, 1432–1440. [Google Scholar] [CrossRef]
- Romero, P.A.; Arnold, F.H. Exploring protein fitness landscapes by directed evolution. Nat. Rev. Mol. Cell Biol. 2009, 10, 866–876. [Google Scholar] [CrossRef]
- Xiao, H.; Bao, Z.; Zhao, H. High Throughput Screening and Selection Methods for Directed Enzyme Evolution. Ind. Eng. Chem. Res. 2015, 54, 4011–4020. [Google Scholar] [CrossRef]
- Nannemann, D.P.; Birmingham, W.R.; Scism, R.A.; Bachmann, B.O. Assessing directed evolution methods for the generation of biosynthetic enzymes with potential in drug biosynthesis. Future Med. Chem. 2011, 3, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Cobb, R.E.; Sun, N.; Zhao, H. Directed evolution as a powerful synthetic biology tool. Methods 2013, 60, 81–90. [Google Scholar] [CrossRef]
- Boder, E.T.; Midelfort, K.S.; Wittrup, K.D. Directed evolution of antibody fragments with monovalent femtomolar antigen-binding affinity. Proc. Natl. Acad. Sci. USA 2000, 97, 10701–10705. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Chandley, P.; Rohatgi, S. Recent Advances in the Development of Monoclonal Antibodies and Next-Generation Antibodies. Immunohorizons 2023, 7, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Hibbert, E.G.; Dalby, P.A. Directed evolution strategies for improved enzymatic performance. Microb. Cell Factories 2005, 4, 29. [Google Scholar] [CrossRef]
- Neylon, C. Chemical and biochemical strategies for the randomization of protein encoding DNA sequences: Library construction methods for directed evolution. Nucleic Acids Res. 2004, 32, 1448–1459. [Google Scholar] [CrossRef]
- Marshall, S.A.; Lazar, G.A.; Chirino, A.J.; Desjarlais, J.R. Rational design and engineering of therapeutic proteins. Drug Discov. Today 2003, 8, 212–221. [Google Scholar] [CrossRef]
- Tiwari, M.K.; Singh, R.; Singh, R.K.; Kim, I.W.; Lee, J.K. Computational approaches for rational design of proteins with novel functionalities. Comput. Struct. Biotechnol. J. 2012, 2, e201209002. [Google Scholar] [CrossRef]
- Maveyraud, L.; Mourey, L. Protein X-ray Crystallography and Drug Discovery. Molecules 2020, 25, 1030. [Google Scholar] [CrossRef]
- Ntountaniotis, D. Reactions in NMR Tubes as Key Weapon in Rational Drug Design. Methods Mol. Biol. 2018, 1824, 417–430. [Google Scholar] [CrossRef]
- Cebi, E.; Lee, J.; Subramani, V.K.; Bak, N.; Oh, C.; Kim, K.K. Cryo-electron microscopy-based drug design. Front. Mol. Biosci. 2024, 11, 1342179. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Z.; Scheuring, S. A structural biology compatible file format for atomic force microscopy. Nat. Commun. 2025, 16, 1671. [Google Scholar] [CrossRef] [PubMed]
- Salo-Ahen, O.M.H.; Alanko, I.; Bhadane, R.; Bonvin, A.M.J.J.; Honorato, R.V.; Hossain, S.; Juffer, A.H.; Kabedev, A.; Lahtela-Kakkonen, M.; Larsen, A.S.; et al. Molecular Dynamics Simulations in Drug Discovery and Pharmaceutical Development. Processes 2021, 9, 71. [Google Scholar] [CrossRef]
- Ferreira, P.; Fernandes, P.A.; Ramos, M.J. Modern computational methods for rational enzyme engineering. Chem Catal. 2022, 2, 2481–2498. [Google Scholar] [CrossRef]
- Harris, C.T.; Cohen, S. Reducing Immunogenicity by Design: Approaches to Minimize Immunogenicity of Monoclonal Antibodies. BioDrugs 2024, 38, 205–226. [Google Scholar] [CrossRef] [PubMed]
- Pongsupasa, V.; Anuwan, P.; Maenpuen, S.; Wongnate, T. Rational-Design Engineering to Improve Enzyme Thermostability. Methods Mol. Biol. 2022, 2397, 159–178. [Google Scholar] [CrossRef]
- Shi, H.; Fu, M.; Zhang, T.; Zhang, X.; Yao, L.; Xue, C.; Tang, C. Rational Design of Formate Dehydrogenase for Enhanced Thermal Stability and Catalytic Activity in Bioelectrocatalysis. J. Agric. Food Chem. 2024, 72, 23333–23344. [Google Scholar] [CrossRef]
- Huggins, D.J.; Sherman, W.; Tidor, B. Rational approaches to improving selectivity in drug design. J. Med. Chem. 2012, 55, 1424–1444. [Google Scholar] [CrossRef]
- Li, Y.C.; Zhang, R.F.; Yan, X.Y.; Fan, K.L. Machine learning facilitating the rational design of nanozymes. J. Mater. Chem. B 2023, 11, 6466–6477. [Google Scholar] [CrossRef]
- de Oliveira, T.M.; van Beek, L.; Shilliday, F.; Debreczeni, J.E.; Phillips, C. Cryo-EM: The Resolution Revolution and Drug Discovery. SLAS Discov. 2021, 26, 17–31. [Google Scholar] [CrossRef]
- Garibsingh, R.A.; Ndaru, E.; Garaeva, A.A.; Shi, Y.; Zielewicz, L.; Zakrepine, P.; Bonomi, M.; Slotboom, D.J.; Paulino, C.; Grewer, C.; et al. Rational design of ASCT2 inhibitors using an integrated experimental-computational approach. Proc. Natl. Acad. Sci. USA 2021, 118, e2104093118. [Google Scholar] [CrossRef] [PubMed]
- Nepali, K.; Sharma, S.; Sharma, M.; Bedi, P.M.; Dhar, K.L. Rational approaches, design strategies, structure activity relationship and mechanistic insights for anticancer hybrids. Eur. J. Med. Chem. 2014, 77, 422–487. [Google Scholar] [CrossRef]
- Phintha, A.; Chaiyen, P. Rational and mechanistic approaches for improving biocatalyst performance. Chem. Catal. 2022, 2, 2614–2643. [Google Scholar] [CrossRef]
- Molina-Espeja, P.; Vina-Gonzalez, J.; Gomez-Fernandez, B.J.; Martin-Diaz, J.; Garcia-Ruiz, E.; Alcalde, M. Beyond the outer limits of nature by directed evolution. Biotechnol. Adv. 2016, 34, 754–767. [Google Scholar] [CrossRef]
- Spirov, A.; Holloway, D. Using evolutionary computations to understand the design and evolution of gene and cell regulatory networks. Methods 2013, 62, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Huttanus, H.M.; Triola, E.H.; Velasquez-Guzman, J.C.; Shin, S.M.; Granja-Travez, R.S.; Singh, A.; Dale, T.; Jha, R.K. Targeted mutagenesis and high-throughput screening of diversified gene and promoter libraries for isolating gain-of-function mutations. Front. Bioeng. Biotechnol. 2023, 11, 1202388. [Google Scholar] [CrossRef]
- Shi, J.H.; Yuan, B.; Yang, H.Q.; Sun, Z.T. Recent advances on protein engineering for improved stability. Biodesign Res. 2025, 7, 100005. [Google Scholar] [CrossRef]
- Iwasaki, Y.W.; Tharakaraman, K.; Subramanian, V.; Khongmanee, A.; Hatas, A.; Fleischer, E.; Rurak, T.T.; Ngok-Ngam, P.; Tit-Oon, P.; Ruchirawat, M.; et al. Generation of bispecific antibodies by structure-guided redesign of IgG constant regions. Front. Immunol. 2022, 13, 1063002. [Google Scholar] [CrossRef]
- Chen, X.; Zaro, J.L.; Shen, W.C. Fusion protein linkers: Property, design and functionality. Adv. Drug Deliv. Rev. 2013, 65, 1357–1369. [Google Scholar] [CrossRef]
- Alanazi, W.; Meng, D.; Pollastri, G. Advancements in one-dimensional protein structure prediction using machine learning and deep learning. Comput. Struct. Biotechnol. J. 2025, 27, 1416–1430. [Google Scholar] [CrossRef]
- Malhotra, Y.; John, J.; Yadav, D.; Sharma, D.; Rawal, K.; Mishra, V.; Chaturvedi, N. Advancements in protein structure prediction: A comparative overview of AlphaFold and its derivatives. Comput. Biol. Med. 2025, 188, 109842. [Google Scholar] [CrossRef]
- Ye, L.; Yang, C.; Yu, H. From molecular engineering to process engineering: Development of high-throughput screening methods in enzyme directed evolution. Appl. Microbiol. Biotechnol. 2018, 102, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Bhokisham, N.; Laudermilch, E.; Traeger, L.L.; Bonilla, T.D.; Ruiz-Estevez, M.; Becker, J.R. CRISPR-Cas System: The Current and Emerging Translational Landscape. Cells 2023, 12, 1103. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A. CRISPR-Cas9: A fascinating journey from bacterial immune system to human gene editing. Prog. Mol. Biol. Transl. Sci. 2021, 178, 63–83. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.; Shirguppe, S.; Perez-Pinera, P. Protein Engineering Technologies for Development of Next-Generation Genome Editors. Curr. Opin. Biomed. Eng. 2023, 28, 100514. [Google Scholar] [CrossRef]
- Asmamaw, M.; Zawdie, B. Mechanism and Applications of CRISPR/Cas-9-Mediated Genome Editing. Biol. Targets Ther. 2021, 15, 353–361. [Google Scholar] [CrossRef]
- Liao, H.; Wu, J.; VanDusen, N.J.; Li, Y.; Zheng, Y. CRISPR-Cas9-mediated homology-directed repair for precise gene editing. Mol. Ther. Nucleic Acids 2024, 35, 102344. [Google Scholar] [CrossRef]
- Lei, T.; Wang, Y.; Zhang, Y.; Yang, Y.; Cao, J.; Huang, J.; Chen, J.; Chen, H.; Zhang, J.; Wang, L.; et al. Leveraging CRISPR gene editing technology to optimize the efficacy, safety and accessibility of CAR T-cell therapy. Leukemia 2024, 38, 2517–2543. [Google Scholar] [CrossRef]
- Dimitri, A.; Herbst, F.; Fraietta, J.A. Engineering the next-generation of CAR T-cells with CRISPR-Cas9 gene editing. Mol. Cancer 2022, 21, 78. [Google Scholar] [CrossRef]
- Rupp, L.J.; Schumann, K.; Roybal, K.T.; Gate, R.E.; Ye, C.J.; Lim, W.A.; Marson, A. CRISPR/Cas9-mediated PD-1 disruption enhances anti-tumor efficacy of human chimeric antigen receptor T cells. Sci. Rep. 2017, 7, 737. [Google Scholar] [CrossRef]
- Pickar-Oliver, A.; Gersbach, C.A. The next generation of CRISPR-Cas technologies and applications. Nat. Rev. Mol. Cell Biol. 2019, 20, 490–507. [Google Scholar] [CrossRef]
- Fu, Y.; He, X.; Gao, X.D.; Li, F.; Ge, S.; Yang, Z.; Fan, X. Prime editing: Current advances and therapeutic opportunities in human diseases. Sci. Bull. 2023, 68, 3278–3291. [Google Scholar] [CrossRef] [PubMed]
- Son, A.; Park, J.; Kim, W.; Lee, W.; Yoon, Y.; Ji, J.; Kim, H. Integrating Computational Design and Experimental Approaches for Next-Generation Biologics. Biomolecules 2024, 14, 1073. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, Q.; Nasif, K.F.A.; Xie, Y.; Deng, B.; Niu, S.; Pouriyeh, S.; Dai, Z.; Chen, J.; Xie, C.Y. AI-Driven Deep Learning Techniques in Protein Structure Prediction. Int. J. Mol. Sci. 2024, 25, 8426. [Google Scholar] [CrossRef] [PubMed]
- Pearce, R.; Zhang, Y. Deep learning techniques have significantly impacted protein structure prediction and protein design. Curr. Opin. Struct. Biol. 2021, 68, 194–207. [Google Scholar] [CrossRef]
- Yang, Z.; Zeng, X.; Zhao, Y.; Chen, R. AlphaFold2 and its applications in the fields of biology and medicine. Signal Transduct. Target. Ther. 2023, 8, 115. [Google Scholar] [CrossRef]
- Baek, M.; DiMaio, F.; Anishchenko, I.; Dauparas, J.; Ovchinnikov, S.; Lee, G.R.; Wang, J.; Cong, Q.; Kinch, L.N.; Schaeffer, R.D.; et al. Accurate prediction of protein structures and interactions using a three-track neural network. Science 2021, 373, 871–876. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- Agarwal, V.; McShan, A.C. The power and pitfalls of AlphaFold2 for structure prediction beyond rigid globular proteins. Nat. Chem. Biol. 2024, 20, 950–959. [Google Scholar] [CrossRef]
- Bouatta, N.; Sorger, P.; AlQuraishi, M. Protein structure prediction by AlphaFold2: Are attention and symmetries all you need? Biol. Crystallogr. 2021, 77, 982–991. [Google Scholar] [CrossRef]
- Krishna, R.; Wang, J.; Ahern, W.; Sturmfels, P.; Venkatesh, P.; Kalvet, I.; Lee, G.R.; Morey-Burrows, F.S.; Anishchenko, I.; Humphreys, I.R.; et al. Generalized biomolecular modeling and design with RoseTTAFold All-Atom. Science 2024, 384, eadl2528. [Google Scholar] [CrossRef] [PubMed]
- You, Y.J.; Lai, X.; Pan, Y.; Zheng, H.R.; Vera, J.; Liu, S.; Deng, S.; Zhang, L. Artificial intelligence in cancer target identification and drug discovery. Signal Transduct. Target. Ther. 2022, 7, 156. [Google Scholar] [CrossRef]
- Rehman, A.U.; Li, M.; Wu, B.; Ali, Y.; Rasheed, S.; Shaheen, S.; Liu, X.; Luo, R.; Zhang, J. Role of artificial intelligence in revolutionizing drug discovery. Fundam. Res. 2024, 5, 1273–1287. [Google Scholar] [CrossRef]
- Grewal, S.; Hegde, N.; Yanow, S.K. Integrating machine learning to advance epitope mapping. Front. Immunol. 2024, 15, 1463931. [Google Scholar] [CrossRef]
- Fan, J.; Shi, S.; Xiang, H.; Fu, L.; Duan, Y.; Cao, D.; Lu, H. Predicting Elimination of Small-Molecule Drug Half-Life in Pharmacokinetics Using Ensemble and Consensus Machine Learning Methods. J. Chem. Inf. Model. 2024, 64, 3080–3092. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Mahdi, M.S.; Radi, U.K.; Jihad, A.; Abdulhussein, A.H.; Ahmad, I.; Mansuri, N.; Abdalrahman, M.A.; Alkhayyat, A.; Faisal, A. Machine learning based modeling for estimation of drug solubility in supercritical fluid by adjusting important parameters. Chemom. Intell. Lab. Syst. 2024, 254, 105241. [Google Scholar] [CrossRef]
- Eugster, R.; Orsi, M.; Buttitta, G.; Serafini, N.; Tiboni, M.; Casettari, L.; Reymond, J.L.; Aleandri, S.; Luciani, P. Leveraging machine learning to streamline the development of liposomal drug delivery systems. J. Control. Release 2024, 376, 1025–1038. [Google Scholar] [CrossRef] [PubMed]
- Vanella, R.; Kovacevic, G.; Doffini, V.; Fernandez de Santaella, J.; Nash, M.A. High-throughput screening, next generation sequencing and machine learning: Advanced methods in enzyme engineering. Chem. Commun. 2022, 58, 2455–2467. [Google Scholar] [CrossRef]
- Khakzad, H.; Igashov, I.; Schneuing, A.; Goverde, C.; Bronstein, M.; Correia, B. A new age in protein design empowered by deep learning. Cell Syst. 2023, 14, 925–939. [Google Scholar] [CrossRef]
- Minot, M.; Reddy, S.T. Meta learning addresses noisy and under-labeled data in machine learning-guided antibody engineering. Cell Syst. 2024, 15, 4–18.e14. [Google Scholar] [CrossRef]
- Shoari, A.; Khalili-Tanha, G.; Coban, M.A.; Radisky, E.S. Structure and computation-guided yeast surface display for the evolution of TIMP-based matrix metalloproteinase inhibitors. Front. Mol. Biosci. 2023, 10, 1321956. [Google Scholar] [CrossRef] [PubMed]
- Lutz, S. Beyond directed evolution-semi-rational protein engineering and design. Curr. Opin. Biotechnol. 2010, 21, 734–743. [Google Scholar] [CrossRef]
- Vasina, M.; Velecky, J.; Planas-Iglesias, J.; Marques, S.M.; Skarupova, J.; Damborsky, J.; Bednar, D.; Mazurenko, S.; Prokop, Z. Tools for computational design and high-throughput screening of therapeutic enzymes. Adv. Drug Deliv. Rev. 2022, 183, 114143. [Google Scholar] [CrossRef]
- Zhu, Z.; Cuozzo, J. Review article: High-throughput affinity-based technologies for small-molecule drug discovery. SLAS Discov. 2009, 14, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Bommarius, A.S.; Broering, J.M.; Chaparro-Riggers, J.F.; Polizzi, K.M. High-throughput screening for enhanced protein stability. Curr. Opin. Biotechnol. 2006, 17, 606–610. [Google Scholar] [CrossRef]
- Cheong, R.; Paliwal, S.; Levchenko, A. High-content screening in microfluidic devices. Expert Opin. Drug Discov. 2010, 5, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Murthy, T.; Lim, J. Life Sciences discovery and technology highlights. SLAS Technol. 2025, 30, 100235. [Google Scholar] [CrossRef]
- Battersby, B.J.; Trau, M. Novel miniaturized systems in high-throughput screening. Trends Biotechnol. 2002, 20, 167–173. [Google Scholar] [CrossRef]
- Dörr, M.; Fibinger, M.P.C.; Last, D.; Schmidt, S.; Santos-Aberturas, J.; Böttcher, D.; Hummel, A.; Vickers, C.; Voss, M.; Bornscheuer, U.T. Fully automatized high-throughput enzyme library screening using a robotic platform. Biotechnol. Bioeng. 2016, 113, 1421–1432. [Google Scholar] [CrossRef]
- Leet, J.E.; Belcastro, J.V.; Dowling, C.J.; Nemeth, G.A.; Weller, H.N. HPLC Biogram Analysis: A Powerful Tool Used for Hit Confirmation in Early Drug Discovery. J. Biomol. Screen. 2015, 20, 681–687. [Google Scholar] [CrossRef]
- Weng, L.; Spoonamore, J.E. Droplet Microfluidics-Enabled High-Throughput Screening for Protein Engineering. Micromachines 2019, 10, 734. [Google Scholar] [CrossRef] [PubMed]
- Kouba, P.; Kohout, P.; Haddadi, F.; Bushuiev, A.; Samusevich, R.; Sedlar, J.; Damborsky, J.; Pluskal, T.; Sivic, J.; Mazurenko, S. Machine Learning-Guided Protein Engineering. ACS Catal. 2023, 13, 13863–13895. [Google Scholar] [CrossRef]
- Zhang, Y.; Minagawa, Y.; Kizoe, H.; Miyazaki, K.; Iino, R.; Ueno, H.; Tabata, K.V.; Shimane, Y.; Noji, H. Accurate high-throughput screening based on digital protein synthesis in a massively parallel femtoliter droplet array. Sci. Adv. 2019, 5, eaav8185. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Luo, Y.; Zhao, H. Synthetic biology: Putting synthesis into biology. Wiley Interdiscip. Rev. Syst. Biol. Med. 2011, 3, 7–20. [Google Scholar] [CrossRef]
- Zhou, W.; Smidlehner, T.; Jerala, R. Synthetic biology principles for the design of protein with novel structures and functions. FEBS Lett. 2020, 594, 2199–2212. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Kortemme, T. Recent advances in de novo protein design: Principles, methods, and applications. J. Biol. Chem. 2021, 296, 100558. [Google Scholar] [CrossRef]
- Korendovych, I.V.; DeGrado, W.F. De novo protein design, a retrospective. Q. Rev. Biophys. 2020, 53, e3. [Google Scholar] [CrossRef]
- Miyamoto, K.; Tadokoro, T.; Matsumoto, A. Unique E2-binding specificity of artificial RING fingers in cancer cells. Sci. Rep. 2024, 14, 2545. [Google Scholar] [CrossRef]
- Lu, H.; Cheng, Z.; Hu, Y.; Tang, L.V. What Can De Novo Protein Design Bring to the Treatment of Hematological Disorders? Biology 2023, 12, 166. [Google Scholar] [CrossRef]
- Quijano-Rubio, A.; Ulge, U.Y.; Walkey, C.D.; Silva, D.A. The advent of de novo proteins for cancer immunotherapy. Curr. Opin. Chem. Biol. 2020, 56, 119–128. [Google Scholar] [CrossRef]
- Shirzadian, M.; Moori, S.; Rabbani, R.; Rahbarizadeh, F. SynNotch CAR-T cell, when synthetic biology and immunology meet again. Front. Immunol. 2025, 16, 1545270. [Google Scholar] [CrossRef] [PubMed]
- Allen, G.M.; Frankel, N.W.; Reddy, N.R.; Bhargava, H.K.; Yoshida, M.A.; Stark, S.R.; Purl, M.; Lee, J.; Yee, J.L.; Yu, W.; et al. Synthetic cytokine circuits that drive T cells into immune-excluded tumors. Science 2022, 378, eaba1624. [Google Scholar] [CrossRef] [PubMed]
- Crunkhorn, S. Synthetic cytokine circuit targets solid tumours. Nat. Rev. Drug Discov. 2023, 22, 99. [Google Scholar] [CrossRef]
- Plaper, T.; Rihtar, E.; Zeleznik Ramuta, T.; Forstneric, V.; Jazbec, V.; Ivanovski, F.; Bencina, M.; Jerala, R. The art of designed coiled-coils for the regulation of mammalian cells. Cell Chem. Biol. 2024, 31, 1460–1472. [Google Scholar] [CrossRef]
- Burgos-Morales, O.; Gueye, M.; Lacombe, L.; Nowak, C.; Schmachtenberg, R.; Horner, M.; Jerez-Longres, C.; Mohsenin, H.; Wagner, H.J.; Weber, W. Synthetic biology as driver for the biologization of materials sciences. Mater. Today Bio 2021, 11, 100115. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Yin, H.; Zhang, D.; Zhang, M.; Chao, X.; Scimeca, L.; Wu, M.R. Synthetic biology approaches for improving the specificity and efficacy of cancer immunotherapy. Cell. Mol. Immunol. 2024, 21, 436–447. [Google Scholar] [CrossRef]
- Valabrega, G.; Montemurro, F.; Aglietta, M. Trastuzumab: Mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann. Oncol. 2007, 18, 977–984. [Google Scholar] [CrossRef]
- Hoffman, L.M.; Gore, L. Blinatumomab, a Bi-Specific Anti-CD19/CD3 BiTE((R)) Antibody for the Treatment of Acute Lymphoblastic Leukemia: Perspectives and Current Pediatric Applications. Front. Oncol. 2014, 4, 63. [Google Scholar] [CrossRef]
- Mebrahtu, A.; Lauren, I.; Veerman, R.; Akpinar, G.G.; Lord, M.; Kostakis, A.; Astorga-Wells, J.; Dahllund, L.; Olsson, A.; Andersson, O.; et al. A bispecific CD40 agonistic antibody allowing for antibody-peptide conjugate formation to enable cancer-specific peptide delivery, resulting in improved T proliferation and anti-tumor immunity in mice. Nat. Commun. 2024, 15, 9542. [Google Scholar] [CrossRef]
- Diab, A.; Tannir, N.M.; Bentebibel, S.E.; Hwu, P.; Papadimitrakopoulou, V.; Haymaker, C.; Kluger, H.M.; Gettinger, S.N.; Sznol, M.; Tykodi, S.S.; et al. Bempegaldesleukin (NKTR-214) plus Nivolumab in Patients with Advanced Solid Tumors: Phase I Dose-Escalation Study of Safety, Efficacy, and Immune Activation (PIVOT-02). Cancer Discov. 2020, 10, 1158–1173. [Google Scholar] [CrossRef]
- Ongaro, T.; Gouyou, B.; Stringhini, M.; Corbellari, R.; Neri, D.; Villa, A. A novel format for recombinant antibody-interleukin-2 fusion proteins exhibits superior tumor-targeting properties in vivo. Oncotarget 2020, 11, 3698–3711. [Google Scholar] [CrossRef]
- Minnar, C.M.; Lui, G.; Gulley, J.L.; Schlom, J.; Gameiro, S.R. Preclinical and clinical studies of a tumor targeting IL-12 immunocytokine. Front. Oncol. 2023, 13, 1321318. [Google Scholar] [CrossRef]
- Gondry, O.; Caveliers, V.; Xavier, C.; Raes, L.; Vanhoeij, M.; Verfaillie, G.; Fontaine, C.; Glorieus, K.; De Greve, J.; Joris, S.; et al. Phase II Trial Assessing the Repeatability and Tumor Uptake of [68Ga]Ga-HER2 Single-Domain Antibody PET/CT in Patients with Breast Carcinoma. J. Nucl. Med. 2024, 65, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Narbona, J.; Hernandez-Baraza, L.; Gordo, R.G.; Sanz, L.; Lacadena, J. Nanobody-Based EGFR-Targeting Immunotoxins for Colorectal Cancer Treatment. Biomolecules 2023, 13, 1042. [Google Scholar] [CrossRef]
- Palombarini, F.; Di Fabio, E.; Boffi, A.; Macone, A.; Bonamore, A. Ferritin Nanocages for Protein Delivery to Tumor Cells. Molecules 2020, 25, 825. [Google Scholar] [CrossRef]
- Delgado, M.; Garcia-Sanz, J.A. Therapeutic Monoclonal Antibodies against Cancer: Present and Future. Cells 2023, 12, 2837. [Google Scholar] [CrossRef] [PubMed]
- Harding, F.A.; Stickler, M.M.; Razo, J.; DuBridge, R.B. The immunogenicity of humanized and fully human antibodies: Residual immunogenicity resides in the CDR regions. MAbs 2010, 2, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Oldham, R.J.; Teal, E.; Beers, S.A.; Cragg, M.S. Fc-Engineering for Modulated Effector Functions-Improving Antibodies for Cancer Treatment. Antibodies 2020, 9, 64. [Google Scholar] [CrossRef]
- Almawash, S. Revolutionary Cancer Therapy for Personalization and Improved Efficacy: Strategies to Overcome Resistance to Immune Checkpoint Inhibitor Therapy. Cancers 2025, 17, 880. [Google Scholar] [CrossRef]
- Kwok, G.; Yau, T.C.; Chiu, J.W.; Tse, E.; Kwong, Y.L. Pembrolizumab (Keytruda). Hum. Vaccin. Immunother. 2016, 12, 2777–2789. [Google Scholar] [CrossRef]
- Lim, J.S.; Soo, R.A. Nivolumab in the treatment of metastatic squamous non-small cell lung cancer: A review of the evidence. Ther. Adv. Respir. Dis. 2016, 10, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.S.; Hwu, W.J.; Kefford, R.; Wolchok, J.D.; Hersey, P.; Joseph, R.; Weber, J.S.; et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann. Oncol. 2019, 30, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Payandeh, Z.; Bahrami, A.A.; Hoseinpoor, R.; Mortazavi, Y.; Rajabibazl, M.; Rahimpour, A.; Taromchi, A.H.; Khalil, S. The applications of anti-CD20 antibodies to treat various B cells disorders. Biomed. Pharmacother. 2019, 109, 2415–2426. [Google Scholar] [CrossRef]
- Hauptrock, B.; Hess, G. Rituximab in the treatment of non-Hodgkin’s lymphoma. Biol. Targets Ther. 2008, 2, 619–633. [Google Scholar] [CrossRef][Green Version]
- Crescioli, S.; Kaplon, H.; Chenoweth, A.; Wang, L.; Visweswaraiah, J.; Reichert, J.M. Antibodies to watch in 2024. MAbs 2024, 16, 2297450. [Google Scholar] [CrossRef]
- Herrera, M.; Pretelli, G.; Desai, J.; Garralda, E.; Siu, L.L.; Steiner, T.M.; Au, L. Bispecific antibodies: Advancing precision oncology. Trends Cancer 2024, 10, 893–919. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.M.; Lee, J.H.; Ko, S.; Hong, S.S.; Jin, H.E. Mechanism of Action and Pharmacokinetics of Approved Bispecific Antibodies. Biomol. Ther. 2024, 32, 708–722. [Google Scholar] [CrossRef]
- Huehls, A.M.; Coupet, T.A.; Sentman, C.L. Bispecific T-cell engagers for cancer immunotherapy. Immunol. Cell Biol. 2015, 93, 290–296. [Google Scholar] [CrossRef]
- Wu, J.; Fu, J.; Zhang, M.; Liu, D. Blinatumomab: A bispecific T cell engager (BiTE) antibody against CD19/CD3 for refractory acute lymphoid leukemia. J. Hematol. Oncol. 2015, 8, 104. [Google Scholar] [CrossRef]
- Kantarjian, H.; Stein, A.; Gokbuget, N.; Fielding, A.K.; Schuh, A.C.; Ribera, J.M.; Wei, A.; Dombret, H.; Foa, R.; Bassan, R.; et al. Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2017, 376, 836–847. [Google Scholar] [CrossRef]
- Marischen, L.; Fritsch, J.; Ilic, J.; Wahl, L.; Bertsch, T.; Knop, S.; Bold, A. Two Are Better than One: The Bi-Specific Antibody Mosunetuzumab Reveals an Improved Immune Response of Vgamma9Vdelta2 T Cells Targeting CD20 in Malignant B Cells in Comparison to the Mono-Specific Antibody Obinutuzumab. Int. J. Mol. Sci. 2025, 26, 1262. [Google Scholar] [CrossRef]
- Tapia-Galisteo, A.; Compte, M.; Alvarez-Vallina, L.; Sanz, L. When three is not a crowd: Trispecific antibodies for enhanced cancer immunotherapy. Theranostics 2023, 13, 1028–1041. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Seung, E.; Xu, L.; Rao, E.; Lord, D.M.; Wei, R.R.; Cortez-Retamozo, V.; Ospina, B.; Posternak, V.; Ulinski, G.; et al. Trispecific antibodies enhance the therapeutic efficacy of tumor-directed T cells through T cell receptor co-stimulation. Nat. Cancer 2020, 1, 86–98. [Google Scholar] [CrossRef]
- Grab, A.L.; Kim, P.S.; John, L.; Bisht, K.; Wang, H.; Baumann, A.; Van de Velde, H.; Sarkar, I.; Shome, D.; Reichert, P.; et al. Pre-Clinical Assessment of SAR442257, a CD38/CD3xCD28 Trispecific T Cell Engager in Treatment of Relapsed/Refractory Multiple Myeloma. Cells 2024, 13, 879. [Google Scholar] [CrossRef]
- Zhao, L.; Li, S.; Wei, X.; Qi, X.; Liu, D.; Liu, L.; Wen, F.; Zhang, J.S.; Wang, F.; Liu, Z.L.; et al. A novel CD19/CD22/CD3 trispecific antibody enhances therapeutic efficacy and overcomes immune escape against B-ALL. Blood 2022, 140, 1790–1802. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, X.; Wang, X.; Yuan, K.; Wang, G.; Hu, L.; Zhang, G.; Pei, W.; Wang, L.; Sun, C.; et al. Bispecific antibodies in cancer therapy: Target selection and regulatory requirements. Acta Pharm. Sin. B 2023, 13, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Firestone, R.; Lesokhin, A.M.; Usmani, S.Z. An Embarrassment of Riches: Three FDA-Approved Bispecific Antibodies for Relapsed Refractory Multiple Myeloma. Blood Cancer Discov. 2023, 4, 433–436. [Google Scholar] [CrossRef]
- Greenblatt, K.; Khaddour, K. Trastuzumab. In StatPearls; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Markham, A. Atezolizumab: First Global Approval. Drugs 2016, 76, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Syed, Y.Y. Erratum to: Durvalumab: First Global Approval. Drugs 2017, 77, 1817. [Google Scholar] [CrossRef]
- Przepiorka, D.; Ko, C.W.; Deisseroth, A.; Yancey, C.L.; Candau-Chacon, R.; Chiu, H.J.; Gehrke, B.J.; Gomez-Broughton, C.; Kane, R.C.; Kirshner, S.; et al. FDA Approval: Blinatumomab. Clin. Cancer Res. 2015, 21, 4035–4039. [Google Scholar] [CrossRef]
- Kang, C. Mosunetuzumab: First Approval. Drugs 2022, 82, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Shirley, M. Glofitamab: First Approval. Drugs 2023, 83, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Richardson, N.C.; Kasamon, Y.L.; Chen, H.; de Claro, R.A.; Ye, J.; Blumenthal, G.M.; Farrell, A.T.; Pazdur, R. FDA Approval Summary: Brentuximab Vedotin in First-Line Treatment of Peripheral T-Cell Lymphoma. Oncologist 2019, 24, e180–e187. [Google Scholar] [CrossRef] [PubMed]
- Ballantyne, A.; Dhillon, S. Trastuzumab emtansine: First global approval. Drugs 2013, 73, 755–765. [Google Scholar] [CrossRef]
- Keam, S.J. Trastuzumab Deruxtecan: First Approval. Drugs 2020, 80, 501–508. [Google Scholar] [CrossRef]
- Syed, Y.Y. Sacituzumab Govitecan: First Approval. Drugs 2020, 80, 1019–1025. [Google Scholar] [CrossRef]
- Markham, A. Margetuximab: First Approval. Drugs 2021, 81, 599–604. [Google Scholar] [CrossRef]
- Dhillon, S. Elranatamab: First Approval. Drugs 2023, 83, 1621–1627. [Google Scholar] [CrossRef]
- Boersma, B.; Poinot, H.; Pommier, A. Stimulating the Antitumor Immune Response Using Immunocytokines: A Preclinical and Clinical Overview. Pharmaceutics 2024, 16, 974. [Google Scholar] [CrossRef]
- VanDyke, D.; Iglesias, M.; Tomala, J.; Young, A.; Smith, J.; Perry, J.A.; Gebara, E.; Cross, A.R.; Cheung, L.S.; Dykema, A.G.; et al. Engineered human cytokine/antibody fusion proteins expand regulatory T cells and confer autoimmune disease protection. Cell Rep. 2022, 41, 111478. [Google Scholar] [CrossRef]
- Shi, W.; Liu, N.; Lu, H. Advancements and challenges in immunocytokines: A new arsenal against cancer. Acta Pharm. Sin. B 2024, 14, 4649–4664. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Sun, J.; Yuan, Y.; Ji, D.; Sun, Y.; Liu, Y.; Li, S.; Zhu, X.; Wu, X.; Hu, J.; et al. Proximity-enabled covalent binding of IL-2 to IL-2Ralpha selectively activates regulatory T cells and suppresses autoimmunity. Signal Transduct. Target. Ther. 2023, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.M.; Rolig, A.S.; Charych, D.H.; Hoch, U.; Kasiewicz, M.J.; Rose, D.C.; McNamara, M.J.; Hilgart-Martiszus, I.F.; Redmond, W.L. NKTR-214 immunotherapy synergizes with radiotherapy to stimulate systemic CD8(+) T cell responses capable of curing multi-focal cancer. J. Immunother. Cancer 2020, 8, e000464. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Eto, M.; Motzer, R.; De Giorgi, U.; Buchler, T.; Basappa, N.S.; Mendez-Vidal, M.J.; Tjulandin, S.; Hoon Park, S.; Melichar, B.; et al. Lenvatinib plus pembrolizumab versus sunitinib as first-line treatment of patients with advanced renal cell carcinoma (CLEAR): Extended follow-up from the phase 3, randomised, open-label study. Lancet Oncol. 2023, 24, 228–238. [Google Scholar] [CrossRef]
- Yi, M.; Li, T.Y.; Niu, M.K.; Zhang, H.X.; Wu, Y.Z.; Wu, K.M.; Dai, Z.J. Targeting cytokine and chemokine signaling pathways for cancer therapy. Signal Transduct. Target. Ther. 2024, 9, 176. [Google Scholar] [CrossRef]
- Ma, W.W.; Saccardo, A.; Roccatano, D.; Aboagye-Mensah, D.; Alkaseem, M.; Jewkes, M.; Di Nezza, F.; Baron, M.; Soloviev, M.; Ferrari, E. Modular assembly of proteins on nanoparticles. Nat. Commun. 2018, 9, 1489. [Google Scholar] [CrossRef]
- Driscoll, C.L.; Keeble, A.H.; Howarth, M.R. SpyMask enables combinatorial assembly of bispecific binders. Nat. Commun. 2024, 15, 2403. [Google Scholar] [CrossRef]
- Baldo, B.A. Side effects of cytokines approved for therapy. Drug Saf. 2014, 37, 921–943. [Google Scholar] [CrossRef]
- Sznol, M.; Rizvi, N. Teaching an old dog new tricks: Re-engineering IL-2 for immuno-oncology applications. J. Immunother. Cancer 2023, 11, e006346. [Google Scholar] [CrossRef]
- Shoari, A.; Tahmasebi, M.; Khodabakhsh, F.; Cohan, R.A.; Oghalaie, A.; Behdani, M. Angiogenic biomolecules specific nanobodies application in cancer imaging and therapy; review and updates. Int. Immunopharmacol. 2022, 105, 108585. [Google Scholar] [CrossRef]
- Zhu, H.; Ding, Y. Nanobodies: From Discovery to AI-Driven Design. Biology 2025, 14, 547. [Google Scholar] [CrossRef] [PubMed]
- Jovcevska, I.; Muyldermans, S. The Therapeutic Potential of Nanobodies. BioDrugs 2020, 34, 11–26. [Google Scholar] [CrossRef]
- Keyaerts, M.; Xavier, C.; Heemskerk, J.; Devoogdt, N.; Everaert, H.; Ackaert, C.; Vanhoeij, M.; Duhoux, F.P.; Gevaert, T.; Simon, P.; et al. Phase I Study of 68Ga-HER2-Nanobody for PET/CT Assessment of HER2 Expression in Breast Carcinoma. J. Nucl. Med. 2016, 57, 27–33. [Google Scholar] [CrossRef]
- Fan, J.; Zhuang, X.; Yang, X.; Xu, Y.; Zhou, Z.; Pan, L.; Chen, S. A multivalent biparatopic EGFR-targeting nanobody drug conjugate displays potent anticancer activity in solid tumor models. Signal Transduct. Target. Ther. 2021, 6, 320. [Google Scholar] [CrossRef]
- Dieras, V.; Miles, D.; Verma, S.; Pegram, M.; Welslau, M.; Baselga, J.; Krop, I.E.; Blackwell, K.; Hoersch, S.; Xu, J.; et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): A descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2017, 18, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, J.; Gui, X.; Zheng, Y.; Schaar, E.; Liu, G.; Shi, J. Bioengineered nanotechnology for nucleic acid delivery. J. Control. Release 2023, 364, 124–141. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.F.; Tang, L.X.; Xie, Y.Z.Y.; Xianyu, Y.L.; Zhang, L.M.; Wang, P.; Hamada, Y.; Jiang, K.; Zheng, W.F.; Jiang, X.Y. Gold nanoclusters-assisted delivery of siRNA for effective treatment of pancreatic cancer. Nat. Commun. 2017, 8, 15130. [Google Scholar] [CrossRef]
- Proietto, M.; Crippa, M.; Damiani, C.; Pasquale, V.; Sacco, E.; Vanoni, M.; Gilardi, M. Tumor heterogeneity: Preclinical models, emerging technologies, and future applications. Front. Oncol. 2023, 13, 1164535. [Google Scholar] [CrossRef]
- Rahban, M.; Ahmad, F.; Piatyszek, M.A.; Haertle, T.; Saso, L.; Saboury, A.A. Stabilization challenges and aggregation in protein-based therapeutics in the pharmaceutical industry. RSC Adv. 2023, 13, 35947–35963. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Y.; Park, J.; Liu, X.; Hu, Y.; Wang, T.; McFarland, K.; Betenbaugh, M.J. Design and Production of Bispecific Antibodies. Antibodies 2019, 8, 43. [Google Scholar] [CrossRef]
- Ingavat, N.; Dzulkiflie, N.; Liew, J.M.; Wang, X.; Leong, E.; Loh, H.P.; Ng, S.K.; Yang, Y.; Zhang, W. Investigation on environmental factors contributing to bispecific antibody stability and the reversal of self-associated aggregates. Bioresour. Bioprocess. 2024, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Sumida, K.H.; Nunez-Franco, R.; Kalvet, I.; Pellock, S.J.; Wicky, B.I.M.; Milles, L.F.; Dauparas, J.; Wang, J.; Kipnis, Y.; Jameson, N.; et al. Improving Protein Expression, Stability, and Function with ProteinMPNN. J. Am. Chem. Soc. 2024, 146, 2054–2061. [Google Scholar] [CrossRef] [PubMed]
- Seeliger, D.; Schulz, P.; Litzenburger, T.; Spitz, J.; Hoerer, S.; Blech, M.; Enenkel, B.; Studts, J.M.; Garidel, P.; Karow, A.R. Boosting antibody developability through rational sequence optimization. MAbs 2015, 7, 505–515. [Google Scholar] [CrossRef]
- Warszawski, S.; Borenstein Katz, A.; Lipsh, R.; Khmelnitsky, L.; Ben Nissan, G.; Javitt, G.; Dym, O.; Unger, T.; Knop, O.; Albeck, S.; et al. Optimizing antibody affinity and stability by the automated design of the variable light-heavy chain interfaces. PLoS Comput. Biol. 2019, 15, e1007207. [Google Scholar] [CrossRef]
- Nugrahadi, P.P.; Hinrichs, W.L.J.; Frijlink, H.W.; Schoneich, C.; Avanti, C. Designing Formulation Strategies for Enhanced Stability of Therapeutic Peptides in Aqueous Solutions: A Review. Pharmaceutics 2023, 15, 935. [Google Scholar] [CrossRef] [PubMed]
- Svilenov, H.L.; Winter, G. Formulations That Suppress Aggregation During Long-Term Storage of a Bispecific Antibody are Characterized by High Refoldability and Colloidal Stability. J. Pharm. Sci. 2020, 109, 2048–2058. [Google Scholar] [CrossRef]
- Svilenov, H.; Winter, G. The ReFOLD assay for protein formulation studies and prediction of protein aggregation during long-term storage. Eur. J. Pharm. Biopharm. 2019, 137, 131–139. [Google Scholar] [CrossRef]
- Baker, M.P.; Reynolds, H.M.; Lumicisi, B.; Bryson, C.J. Immunogenicity of protein therapeutics: The key causes, consequences and challenges. Self/Nonself 2010, 1, 314–322. [Google Scholar] [CrossRef]
- Kuriakose, A.; Chirmule, N.; Nair, P. Immunogenicity of Biotherapeutics: Causes and Association with Posttranslational Modifications. J. Immunol. Res. 2016, 2016, 1298473. [Google Scholar] [CrossRef]
- Howard, E.L.; Goens, M.M.; Susta, L.; Patel, A.; Wootton, S.K. Anti-Drug Antibody Response to Therapeutic Antibodies and Potential Mitigation Strategies. Biomedicines 2025, 13, 299. [Google Scholar] [CrossRef]
- Rudman, S.M.; Jameson, M.B.; McKeage, M.J.; Savage, P.; Jodrell, D.I.; Harries, M.; Acton, G.; Erlandsson, F.; Spicer, J.F. A phase 1 study of AS1409, a novel antibody-cytokine fusion protein, in patients with malignant melanoma or renal cell carcinoma. Clin. Cancer Res. 2011, 17, 1998–2005. [Google Scholar] [CrossRef] [PubMed]
- Zinsli, L.V.; Stierlin, N.; Loessner, M.J.; Schmelcher, M. Deimmunization of protein therapeutics—Recent advances in experimental and computational epitope prediction and deletion. Comput. Struct. Biotechnol. J. 2021, 19, 315–329. [Google Scholar] [CrossRef]
- Parker, A.S.; Choi, Y.; Griswold, K.E.; Bailey-Kellogg, C. Structure-guided deimmunization of therapeutic proteins. J. Comput. Biol. 2013, 20, 152–165. [Google Scholar] [CrossRef]
- Lou, H.; Feng, M.; Hageman, M.J. Advanced Formulations/Drug Delivery Systems for Subcutaneous Delivery of Protein-Based Biotherapeutics. J. Pharm. Sci. 2022, 111, 2968–2982. [Google Scholar] [CrossRef]
- Bittner, B.; Richter, W.; Schmidt, J. Subcutaneous Administration of Biotherapeutics: An Overview of Current Challenges and Opportunities. BioDrugs 2018, 32, 425–440. [Google Scholar] [CrossRef] [PubMed]
- Beinfeld, M.; Atlas, S.J.; Touchette, D.; McKenna, A.; Rind, D.; Pearson, S.D. The effectiveness and value of nadofaragene firadenovec, oportuzumab monatox, and pembrolizumab for BCG-unresponsive non-muscle-invasive bladder cancer. J. Manag. Care Spec. Pharm. 2021, 27, 797–804. [Google Scholar] [CrossRef]
- Fesnak, A.D. The Challenge of Variability in Chimeric Antigen Receptor T cell Manufacturing. Regen. Eng. Transl. Med. 2020, 6, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Sifniotis, V.; Cruz, E.; Eroglu, B.; Kayser, V. Current Advancements in Addressing Key Challenges of Therapeutic Antibody Design, Manufacture, and Formulation. Antibodies 2019, 8, 36. [Google Scholar] [CrossRef]
- Gray, D. Overview of protein expression by mammalian cells. Curr. Protoc. Protein Sci. 1997, 10, 5.9.1–5.9.18. [Google Scholar] [CrossRef]
- Ito, T.; Lutz, H.; Tan, L.; Wang, B.; Tan, J.; Patel, M.; Chen, L.; Tsunakawa, Y.; Park, B.; Banerjee, S. Host cell proteins in monoclonal antibody processing: Control, detection, and removal. Biotechnol. Prog. 2024, 40, e3448. [Google Scholar] [CrossRef]
- Liang, X.; He, Q.; Qin, G.; Li, G.; Li, Q.; Tan, H.; Wang, Z.; Fan, M.; Xu, D. Effectively removing the homodimer in bispecific antibodies by weak partitioning mode of anion exchange chromatography. J. Chromatogr. B 2023, 1225, 123767. [Google Scholar] [CrossRef] [PubMed]
- Abou-El-Enein, M.; Elsallab, M.; Feldman, S.A.; Fesnak, A.D.; Heslop, H.E.; Marks, P.; Till, B.G.; Bauer, G.; Savoldo, B. Scalable Manufacturing of CAR T cells for Cancer Immunotherapy. Blood Cancer Discov. 2021, 2, 408–422. [Google Scholar] [CrossRef]
- Melocchi, A.; Schmittlein, B.; Sadhu, S.; Nayak, S.; Lares, A.; Uboldi, M.; Zema, L.; di Robilant, B.N.; Feldman, S.A.; Esensten, J.H. Automated manufacturing of cell therapies. J. Control. Release 2025, 381, 113561. [Google Scholar] [CrossRef]
- Lee, J.S.Z.; Prabhu, A.V.; Wu, Y.Y.; Bin Abdul Rahim, A.A.; Chen, S.; Naing, M.W.; Liu, D. Transition from manual to automated processes for autologous T cell therapy manufacturing using bioreactor with expandable culture area. Sci. Rep. 2025, 15, 15819. [Google Scholar] [CrossRef] [PubMed]
- Mirfakhraie, R.; Dehaghi, B.K.; Ghorbi, M.D.; Ghaffari-Nazari, H.; Mohammadian, M.; Salimi, M.; Ardakani, M.T.; Parkhideh, S. All about blinatumomab: The bispecific T cell engager immunotherapy for B cell acute lymphoblastic leukemia. Hematol. Transfus. Cell Ther. 2024, 46, 192–200. [Google Scholar] [CrossRef]
- Bas, T.G.; Duarte, V. Biosimilars in the Era of Artificial Intelligence-International Regulations and the Use in Oncological Treatments. Pharmaceuticals 2024, 17, 925. [Google Scholar] [CrossRef]
- Love, J.C.; Love, K.R.; Barone, P.W. Enabling global access to high-quality biopharmaceuticals. Curr. Opin. Chem. Eng. 2013, 2, 383–390. [Google Scholar] [CrossRef]
- Yang, J.; Carioto, J.; Pyenson, B.; Smith, R.; Jacobson, N.; Pittinger, S.; Shelbaya, A. Greater uptake, an alternative reimbursement methodology needed to realize cost-saving potential of oncology biosimilars in the United States. J. Manag. Care Spec. Pharm. 2021, 27, 1642–1651. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Garcia, Z.; Chen, D.; Liu, H.; Trelstad, P. Cost and supply considerations for antibody therapeutics. MAbs 2025, 17, 2451789. [Google Scholar] [CrossRef]
- Niazi, S.K.; Magoola, M. Advances in Escherichia coli-based therapeutic protein expression: Mammalian conversion, continuous manufacturing, and cell-free production. Biologics 2023, 3, 380–401. [Google Scholar] [CrossRef]
- Schutz, A.; Bernhard, F.; Berrow, N.; Buyel, J.F.; Ferreira-da-Silva, F.; Haustraete, J.; van den Heuvel, J.; Hoffmann, J.E.; de Marco, A.; Peleg, Y.; et al. A concise guide to choosing suitable gene expression systems for recombinant protein production. STAR Protoc. 2023, 4, 102572. [Google Scholar] [CrossRef] [PubMed]
- Geethakumari, P.R.; Ramasamy, D.P.; Dholaria, B.; Berdeja, J.; Kansagra, A. Balancing Quality, Cost, and Access During Delivery of Newer Cellular and Immunotherapy Treatments. Curr. Hematol. Malig. Rep. 2021, 16, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, A.W.; Hlavka, J.P.; Case, S.R. Biosimilar Cost Savings in the United States: Initial Experience and Future Potential. Rand Health Q. 2018, 7, 3. [Google Scholar] [PubMed]
- Sugiki, T.; Fujiwara, T.; Kojima, C. Latest approaches for efficient protein production in drug discovery. Expert Opin. Drug Dis. 2014, 9, 1189–1204. [Google Scholar] [CrossRef]
- Khatami, M.H.; Mendes, U.C.; Wiebe, N.; Kim, P.M. Gate-based quantum computing for protein design. PLoS Comput. Biol. 2023, 19, e1011033. [Google Scholar] [CrossRef]
- Doga, H.; Raubenolt, B.; Cumbo, F.; Joshi, J.; DiFilippo, F.P.; Qin, J.; Blankenberg, D.; Shehab, O. A Perspective on Protein Structure Prediction Using Quantum Computers. J. Chem. Theory Comput. 2024, 20, 3359–3378. [Google Scholar] [CrossRef]
- Ebrahimi, S.B.; Samanta, D. Engineering protein-based therapeutics through structural and chemical design. Nat. Commun. 2023, 14, 2411. [Google Scholar] [CrossRef]
- Peng, K.; Fu, Y.X.; Liang, Y. Engineering cytokines for tumor-targeting and selective T cell activation. Trends Mol. Med. 2025, 31, 373–387. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).