Decoding the CD36-Centric Axis in Gastric Cancer: Insights into Lipid Metabolism, Obesity, and Hypercholesterolemia
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Acquisition
2.1.1. Gene Expression and Clinical Metadata Acquisition
2.1.2. Gene Sets for Obesity and Hypercholesterolemia
2.2. Bioinformatic Analysis Pipeline
2.2.1. Differential Gene Expression Analysis and Volcano Plot with FDR Correction
2.2.2. Identification of Common Genetic Signatures
2.2.3. Pathway Analysis and Functional Annotation
2.2.4. Protein–Protein Interaction Network Analysis
2.3. CD36 Expression Analysis and Clinicopathological Correlations in Gastric Cancer
2.3.1. Survival Analysis
2.3.2. Clinical Parameter Analysis
2.3.3. Statistical Analysis
2.3.4. Data Visualization
3. Results
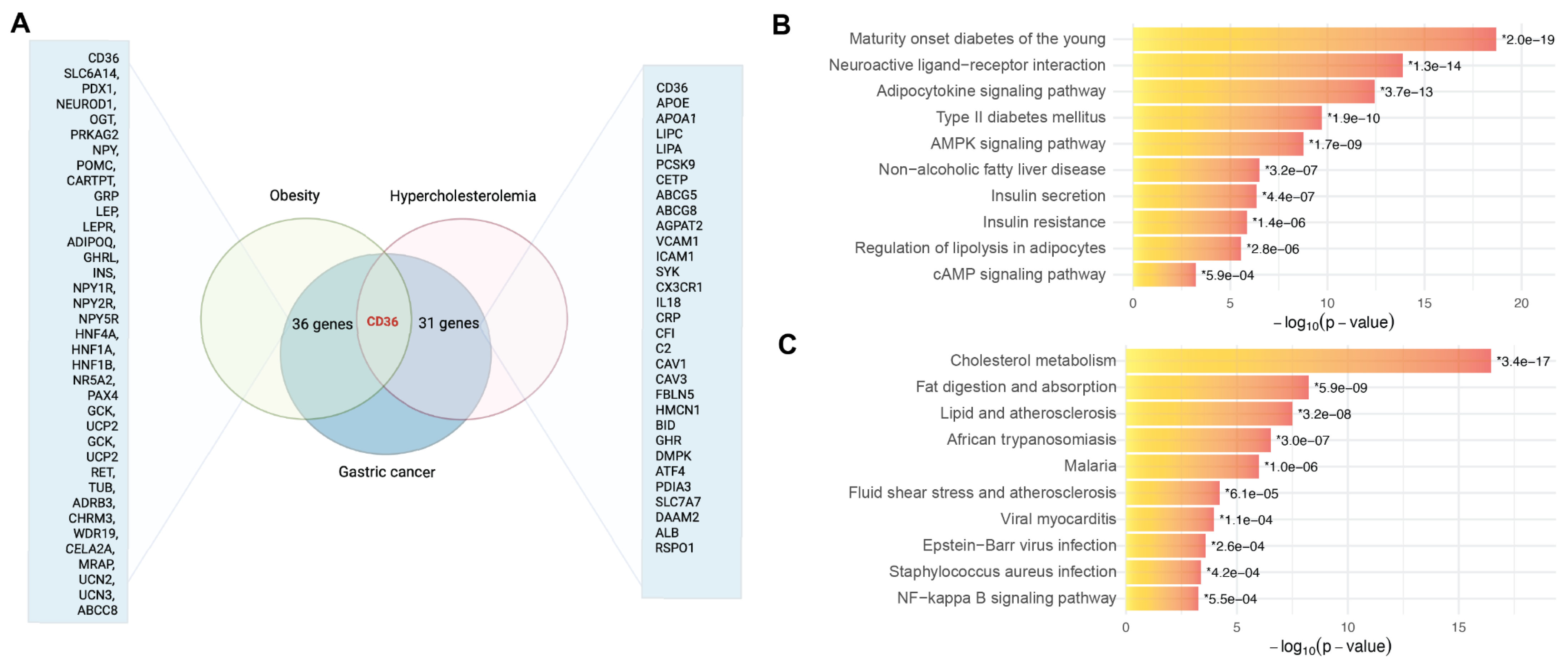
3.1. CD36 Identified as a Molecular Intersection Linking Gastric Cancer with Obesity and Hypercholesterolemia
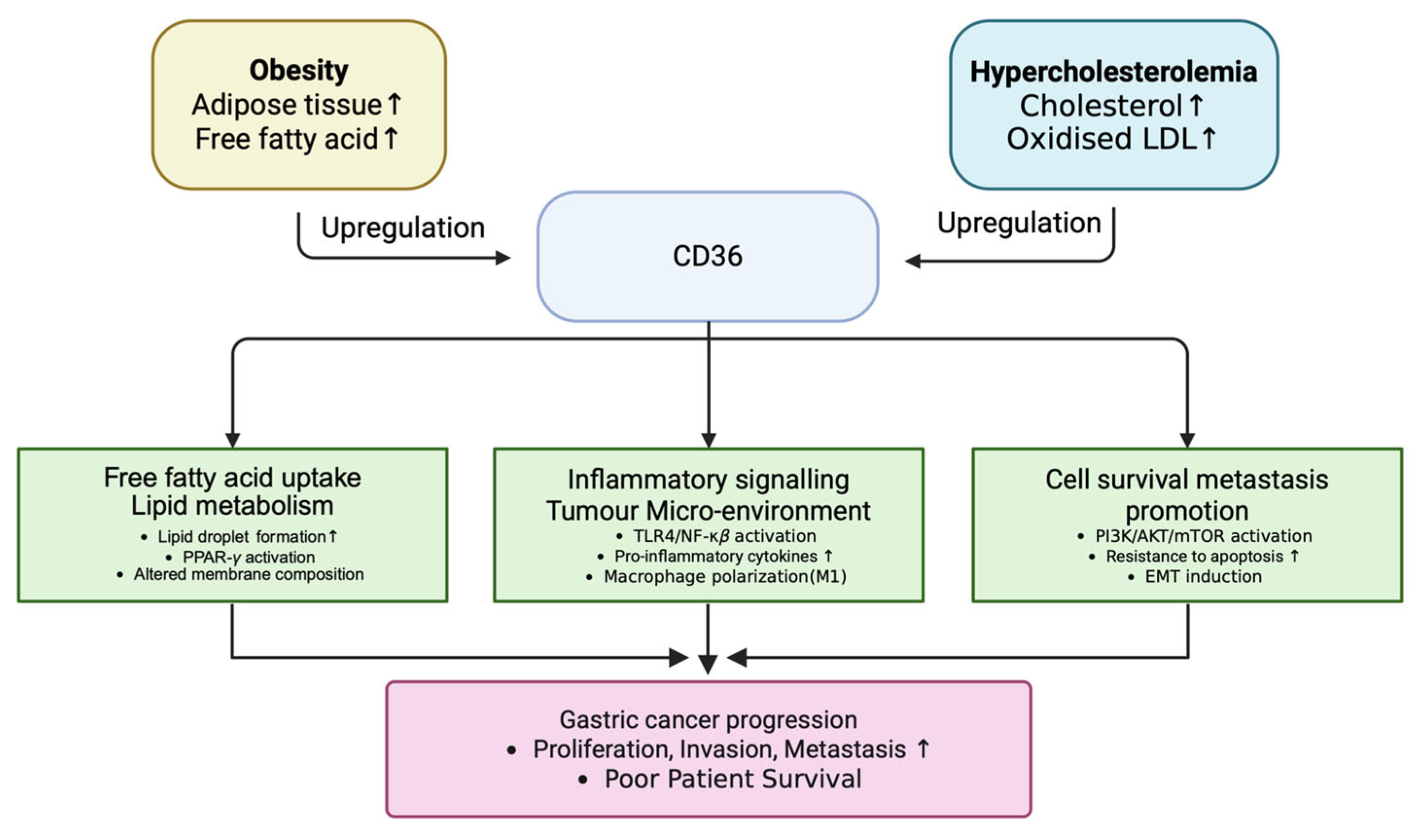
3.2. CD36 as a Central Mediator of Metabolic Reprogramming, Inflammation, and Tumor Progression in Gastric Cancer
3.3. CD36 Expression Correlates with Clinical Stage, Race, and Prognosis in Gastric Cancer
3.4. Transcriptomic Profiling Highlights Mortality-Associated Differential Expression Patterns
4. Discussion
4.1. Mechanistic Insights from Genetic Intersections
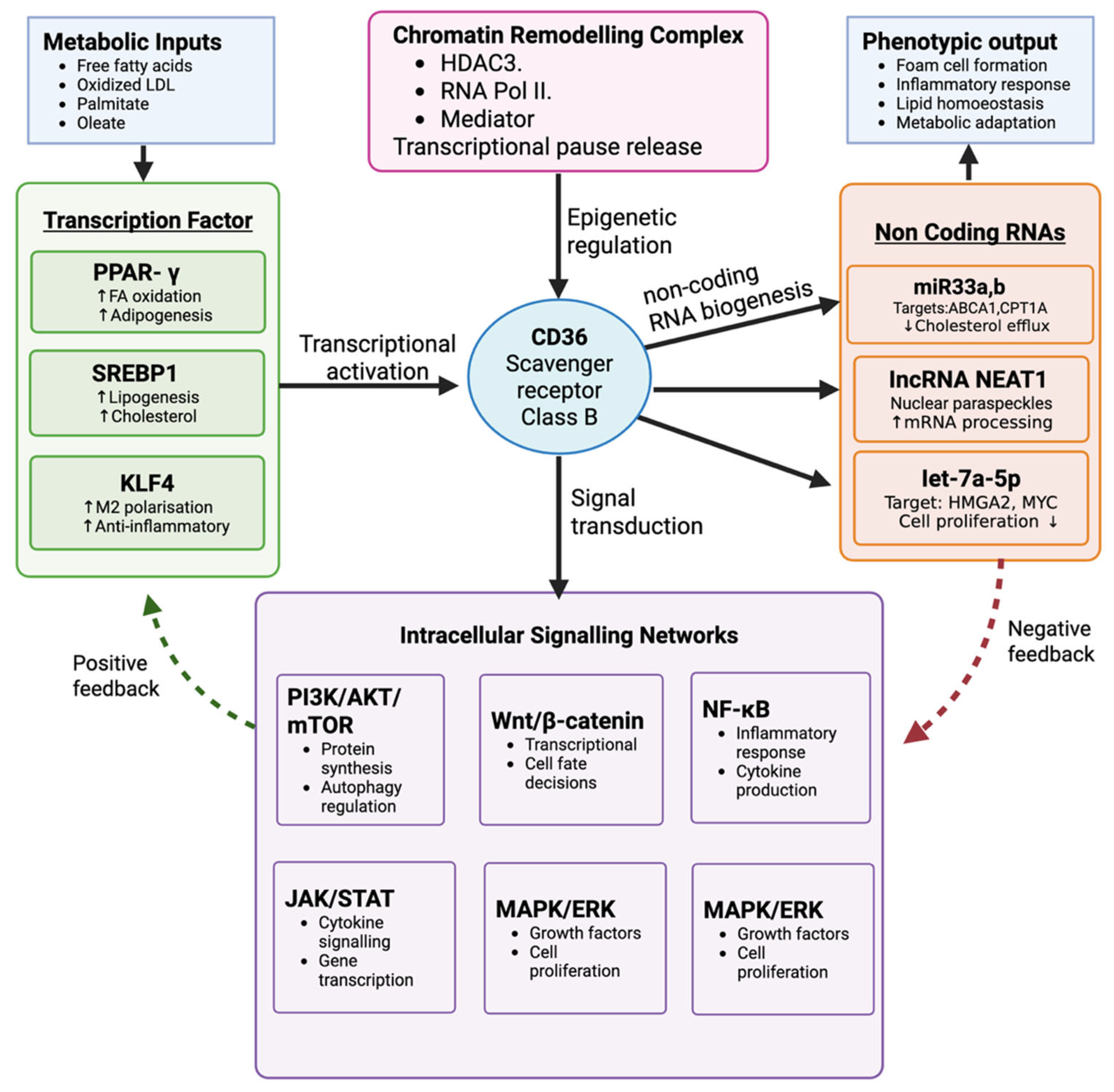
4.2. CD36-Centered Mechanistic Pathway
4.3. Additional Molecular Connections Beyond CD36
4.3.1. Hypercholesterolemia–Gastric Cancer Pathway
4.3.2. Obesity–Gastric Cancer Pathway
4.4. Clinical Implications
4.4.1. Therapeutic Targeting of CD36 and Its Axis
4.4.2. Immunometabolic Targeting and Biomarker Applications
4.4.3. PPAR-γ Modulation and Metabolic Reprogramming
4.5. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morgan, E.; Arnold, M.; Camargo, M.C.; Gini, A.; Kunzmann, A.T.; Matsuda, T.; Meheus, F.; Verhoeven, R.H.A.; Vignat, J.; Laversanne, M.; et al. The current and future incidence and mortality of gastric cancer in 185 countries, 2020-40: A population-based modelling study. EClinicalMedicine 2022, 47, 101404. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Liu, J.L.; Lu, X.; Yang, Q. Epigenetic regulation of energy metabolism in obesity. J. Mol. Cell Biol. 2021, 13, 480–499. [Google Scholar] [CrossRef] [PubMed]
- Conti, C.B.; Agnesi, S.; Scaravaglio, M.; Masseria, P.; Dinelli, M.E.; Oldani, M.; Uggeri, F. Early Gastric Cancer: Update on Prevention, Diagnosis and Treatment. Int. J. Environ. Res. Public Health 2023, 20, 2149. [Google Scholar] [CrossRef] [PubMed]
- Sisic, L.; Crnovrsanin, N.; Nienhueser, H.; Jung, J.O.; Schiefer, S.; Haag, G.M.; Bruckner, T.; Schneider, M.; Muller-Stich, B.P.; Buchler, M.W.; et al. Perioperative chemotherapy with 5-FU, leucovorin, oxaliplatin, and docetaxel (FLOT) for esophagogastric adenocarcinoma: Ten years real-life experience from a surgical perspective. Langenbecks Arch. Surg. 2023, 408, 81. [Google Scholar] [CrossRef]
- Gajria, D.; Chandarlapaty, S. HER2-amplified breast cancer: Mechanisms of trastuzumab resistance and novel targeted therapies. Expert. Rev. Anticancer. Ther. 2011, 11, 263–275. [Google Scholar] [CrossRef]
- Riedl, J.M.; Moik, F.; Esterl, T.; Kostmann, S.M.; Gerger, A.; Jost, P.J. Molecular diagnostics tailoring personalized cancer therapy-an oncologist’s view. Virchows Arch. 2024, 484, 169–179. [Google Scholar] [CrossRef]
- Wadhwa, R.; Taketa, T.; Sudo, K.; Blum-Murphy, M.; Ajani, J.A. Ramucirumab: A novel antiangiogenic agent. Future Oncol. 2013, 9, 789–795. [Google Scholar] [CrossRef]
- Di Carlo, S.; Franceschilli, M.; Rossi, P.; Cavallaro, G.; Cardi, M.; Vinci, D.; Sibio, S. Perforated gastric cancer: A critical appraisal. Discov. Oncol. 2021, 12, 15. [Google Scholar] [CrossRef]
- Bozkurt, E.; Omeroglu, S.; Capkinoglu, E.; Guven, O.; Mihmanli, M. Management of Gastric Tumor Perforation with Conservative Treatment during Neoadjuvant Chemotherapy. JCPSP-J. Coll. Physicians Surg. Pak. 2022, 32, 117–118. [Google Scholar]
- Anthony, M.; Goyal, U. Definitive chemoradiation with dose escalation for locally advanced gastric cancer: Case studies. Cureus 2020, 12, e11040. [Google Scholar] [CrossRef]
- Song, Z.; Wu, Y.; Yang, J.; Yang, D.; Fang, X. Progress in the treatment of advanced gastric cancer. Tumor Biol. 2017, 39, 1010428317714626. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, T.; Piao, Y. Role of nutritional care and general guidance for patients with advanced or metastatic gastric cancer. Future Oncol. 2021, 17, 3101–3109. [Google Scholar] [CrossRef] [PubMed]
- Izuishi, K.; Mori, H. Recent Strategies for Treating Stage IV Gastric Cancer: Roles of Palliative Gastrectomy, Chemotherapy, and Radiotherapy. J. Gastrointest. Liver Dis. 2016, 25, 87–94. [Google Scholar] [CrossRef]
- Katano, A.; Yamashita, H. Long-Term Survival After Repeated Salvage Chemoradiation Therapy for Metastatic Lymph Node Recurrence in Advanced Gastric Cancer: A Case Report. Cureus 2023, 15, e36649. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.Q.; Nguyen, H.N.M.; Ngo, D.-H.; Phan, P.-H.; Vo, T.S. The Suppressive Activity of Water Mimosa Extract on Human Gastric Cancer Cells. Appl. Sci. 2022, 12, 6817. [Google Scholar] [CrossRef]
- Yang, T.-W.; Wang, C.-C.; Hung, W.-C.; Liu, Y.-H.; Sung, W.-W.; Tsai, M.-C. Improvement in the mortality-to-incidence ratios for gastric cancer in developed countries with high health expenditures. Front. Public Health 2021, 9, 713895. [Google Scholar] [CrossRef]
- Mamun, T.I.; Younus, S.; Rahman, M.H. Gastric cancer-Epidemiology, modifiable and non-modifiable risk factors, challenges and opportunities: An updated review. Cancer Treat. Res. Commun. 2024, 41, 100845. [Google Scholar] [CrossRef]
- Yang, L.; Ying, X.; Liu, S.; Lyu, G.; Xu, Z.; Zhang, X.; Li, H.; Li, Q.; Wang, N.; Ji, J. Gastric cancer: Epidemiology, risk factors and prevention strategies. Chin. J. Cancer Res. 2020, 32, 695–704. [Google Scholar] [CrossRef]
- Collaborators, G.B.D.R.F. Global burden and strength of evidence for 88 risk factors in 204 countries and 811 subnational locations, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2162–2203. [Google Scholar]
- Pati, S.; Irfan, W.; Jameel, A.; Ahmed, S.; Shahid, R.K. Obesity and Cancer: A Current Overview of Epidemiology, Pathogenesis, Outcomes, and Management. Cancers 2023, 15, 485. [Google Scholar] [CrossRef]
- Fu, Y.; Zou, T.; Shen, X.; Nelson, P.J.; Li, J.; Wu, C.; Yang, J.; Zheng, Y.; Bruns, C.; Zhao, Y.; et al. Lipid metabolism in cancer progression and therapeutic strategies. MedComm 2021, 2, 27–59. [Google Scholar] [CrossRef] [PubMed]
- Hajian, M. Kaggle. Gene Expression in Gastric Cancer. 2022. Available online: https://www.kaggle.com/datasets/mahdiehhajian/gene-expression-in-gastric-cancer (accessed on 27 April 2025).
- Jiang, M.; Wu, N.; Xu, B.; Chu, Y.; Li, X.; Su, S.; Chen, D.; Li, W.; Shi, Y.; Gao, X.; et al. Fatty acid-induced CD36 expression via O-GlcNAcylation drives gastric cancer metastasis. Theranostics 2019, 9, 5359–5373. [Google Scholar] [CrossRef]
- Luo, X.; Zheng, E.; Wei, L.; Zeng, H.; Qin, H.; Zhang, X.; Liao, M.; Chen, L.; Zhao, L.; Ruan, X.Z.; et al. The fatty acid receptor CD36 promotes HCC progression through activating Src/PI3K/AKT axis-dependent aerobic glycolysis. Cell Death Dis. 2021, 12, 328. [Google Scholar] [CrossRef]
- Zhou, X.; Su, M.; Lu, J.; Li, D.; Niu, X.; Wang, Y. CD36: The Bridge between Lipids and Tumors. Molecules 2024, 29, 531. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Sun, W.; Sun, F.; Yin, G.; Liang, P.; Chen, S.; Liu, X.; Jiang, T.; Zhang, F. Biological Mechanisms and Related Natural Inhibitors of CD36 in Nonalcoholic Fatty Liver. Drug Des. Devel Ther. 2022, 16, 3829–3845. [Google Scholar] [CrossRef]
- Li, S.; Yuan, H.; Li, L.; Li, Q.; Lin, P.; Li, K. Oxidative Stress and Reprogramming of Lipid Metabolism in Cancers. Antioxidants 2025, 14, 201. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, J.; Cui, W.; Silverstein, R.L. CD36, a signaling receptor and fatty acid transporter that regulates immune cell metabolism and fate. J. Exp. Med. 2022, 219, e20211314. [Google Scholar] [CrossRef]
- Guerrero-Rodriguez, S.L.; Mata-Cruz, C.; Perez-Tapia, S.M.; Velasco-Velazquez, M.A. Role of CD36 in cancer progression, stemness, and targeting. Front. Cell Dev. Biol. 2022, 10, 1079076. [Google Scholar] [CrossRef]
- Silverstein, R.L.; Febbraio, M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci. Signal. 2009, 2, re3. [Google Scholar] [CrossRef]
- Liao, X.; Yan, S.; Li, J.; Jiang, C.; Huang, S.; Liu, S.; Zou, X.; Zhang, G.; Zou, J.; Liu, Q. CD36 and its role in regulating the tumor microenvironment. Curr. Oncol. 2022, 29, 8133–8145. [Google Scholar] [CrossRef]
- Ferracini, M.; Rios, F.J.; Pecenin, M.; Jancar, S. Clearance of apoptotic cells by macrophages induces regulatory phenotype and involves stimulation of CD36 and platelet-activating factor receptor. Mediat. Inflamm. 2013, 2013, 950273. [Google Scholar] [CrossRef] [PubMed]
- Nath, A.; Li, I.; Roberts, L.R.; Chan, C. Elevated free fatty acid uptake via CD36 promotes epithelial-mesenchymal transition in hepatocellular carcinoma. Sci. Rep. 2015, 5, 14752. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Cai, X.; Long, L.; Xie, L.; Ma, H.; Zhou, Y.; Liu, S.; Zeng, C. CD36 promotes the epithelial–mesenchymal transition and metastasis in cervical cancer by interacting with TGF-β. J. Transl. Med. 2019, 17, 352. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, Z.; Xu, E.; Shen, X.; Wang, X.; Li, Z.; Yu, H.; Chen, K.; Hu, Q.; Xia, X. Apolipoprotein C-II induces EMT to promote gastric cancer peritoneal metastasis via PI3K/AKT/mTOR pathway. Clin. Transl. Med. 2021, 11, e522. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, J.; Vaikari, V.P.; Beckford, J.S.; Wu, S.; Akhtari, M.; Alachkar, H. Apolipoprotein C2-CD36 promotes leukemia growth and presents a targetable axis in acute myeloid leukemia. Blood Cancer Discov. 2020, 1, 198–213. [Google Scholar] [CrossRef]
- Brishti, M.A.; Raghavan, S.; Lamar, K.; Singh, U.P.; Collier, D.M.; Leo, M.D. Diabetic endothelial cell glycogen synthase kinase 3β activation induces VCAM1 ectodomain shedding. Int. J. Mol. Sci. 2023, 24, 14105. [Google Scholar] [CrossRef]
- Li, S.; He, R.-C.; Wu, S.-G.; Song, Y.; Zhang, K.-L.; Tang, M.-L.; Bei, Y.-R.; Zhang, T.; Lu, J.-B.; Ma, X. LncRNA PSMB8-AS1 instigates vascular inflammation to aggravate atherosclerosis. Circ. Res. 2024, 134, 60–80. [Google Scholar] [CrossRef]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef]
- Simon, L.; Campos, A.; Leyton, L.; Quest, A.F.G. Caveolin-1 function at the plasma membrane and in intracellular compartments in cancer. Cancer Metastasis Rev. 2020, 39, 435–453. [Google Scholar] [CrossRef]
- Gore, A.V.; Swift, M.R.; Cha, Y.R.; Lo, B.; McKinney, M.C.; Li, W.; Castranova, D.; Davis, A.; Mukouyama, Y.S.; Weinstein, B.M. Rspo1/Wnt signaling promotes angiogenesis via Vegfc/Vegfr3. Development 2011, 138, 4875–4886. [Google Scholar] [CrossRef]
- Lee, H.K.; Chaboub, L.S.; Zhu, W.; Zollinger, D.; Rasband, M.N.; Fancy, S.P.; Deneen, B. Daam2-PIP5K is a regulatory pathway for Wnt signaling and therapeutic target for remyelination in the CNS. Neuron 2015, 85, 1227–1243. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Liang, J. Activating transcription factor 4: A regulator of stress response in human cancers. Front. Cell Dev. Biol. 2024, 12, 1370012. [Google Scholar] [CrossRef]
- Garcia-Miranda, A.; Garcia-Hernandez, A.; Castaneda-Saucedo, E.; Navarro-Tito, N.; Maycotte, P. Adipokines as Regulators of Autophagy in Obesity-Linked Cancer. Cells 2022, 11, 3230. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, J.H.; Lee, Y.J. The Role of Adipokines in Tumor Progression and Its Association with Obesity. Biomedicines 2024, 12, 97. [Google Scholar] [CrossRef]
- Kern, L.; Mittenbuhler, M.J.; Vesting, A.J.; Ostermann, A.L.; Wunderlich, C.M.; Wunderlich, F.T. Obesity-Induced TNFalpha and IL-6 Signaling: The Missing Link between Obesity and Inflammation-Driven Liver and Colorectal Cancers. Cancers 2018, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Naik, A.; Monjazeb, A.M.; Decock, J. The Obesity Paradox in Cancer, Tumor Immunology, and Immunotherapy: Potential Therapeutic Implications in Triple Negative Breast Cancer. Front. Immunol. 2019, 10, 1940. [Google Scholar] [CrossRef] [PubMed]
- Schiliro, C.; Firestein, B.L. Mechanisms of Metabolic Reprogramming in Cancer Cells Supporting Enhanced Growth and Proliferation. Cells 2021, 10, 1056. [Google Scholar] [CrossRef]
- Marino, A.; Hausenloy, D.J.; Andreadou, I.; Horman, S.; Bertrand, L.; Beauloye, C. AMP-activated protein kinase: A remarkable contributor to preserve a healthy heart against ROS injury. Free Radic. Biol. Med. 2021, 166, 238–254. [Google Scholar] [CrossRef]
- Makhijani, P.; Basso, P.J.; Chan, Y.T.; Chen, N.; Baechle, J.; Khan, S.; Furman, D.; Tsai, S.; Winer, D.A. Regulation of the immune system by the insulin receptor in health and disease. Front. Endocrinol. 2023, 14, 1128622. [Google Scholar] [CrossRef]
- Wang, A.J.; Wang, S.; Wang, B.J.; Xiao, M.; Guo, Y.; Tang, Y.; Zhang, J.; Gu, J. Epigenetic Regulation Associated With Sirtuin 1 in Complications of Diabetes Mellitus. Front. Endocrinol. 2020, 11, 598012. [Google Scholar] [CrossRef]
- Kuda, O.; Pietka, T.A.; Demianova, Z.; Kudova, E.; Cvacka, J.; Kopecky, J.; Abumrad, N.A. Sulfo-N-succinimidyl oleate (SSO) inhibits fatty acid uptake and signaling for intracellular calcium via binding CD36 lysine 164: SSO also inhibits oxidized low density lipoprotein uptake by macrophages. J. Biol. Chem. 2013, 288, 15547–15555. [Google Scholar] [CrossRef]
- Mansor, L.S.; Sousa Fialho, M.D.L.; Yea, G.; Coumans, W.A.; West, J.A.; Kerr, M.; Carr, C.A.; Luiken, J.; Glatz, J.F.C.; Evans, R.D.; et al. Inhibition of sarcolemmal FAT/CD36 by sulfo-N-succinimidyl oleate rapidly corrects metabolism and restores function in the diabetic heart following hypoxia/reoxygenation. Cardiovasc. Res. 2017, 113, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Wen, L.; Tian, Y.; Ma, L.; Wen, Z.; Kun, Y.; Xu, M.; Liu, X. Sulfosuccinimidyl oleate ameliorates the high-fat diet-induced obesity syndrome by reducing intestinal and hepatic absorption. Front. Pharmacol. 2023, 14, 1193006. [Google Scholar] [CrossRef] [PubMed]
- Bodart, V.; Febbraio, M.; Demers, A.; McNicoll, N.; Pohankova, P.; Perreault, A.; Sejlitz, T.; Escher, E.; Silverstein, R.L.; Lamontagne, D.; et al. CD36 mediates the cardiovascular action of growth hormone-releasing peptides in the heart. Circ. Res. 2002, 90, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Tokudome, T.; Kishimoto, I. The cardiovascular action of hexarelin. J. Geriatr. Cardiol. 2014, 11, 253–258. [Google Scholar]
- Febbraio, M.; Silverstein, R.L. CD36: Implications in cardiovascular disease. Int. J. Biochem. Cell Biol. 2007, 39, 2012–2030. [Google Scholar] [CrossRef]
- Park, Y.M.; Febbraio, M.; Silverstein, R.L. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. J. Clin. Investig. 2009, 119, 136–145. [Google Scholar] [CrossRef]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Bertrand, L.; Pollak, M.; Viollet, B. Metformin: From mechanisms of action to therapies. Cell Metab. 2014, 20, 953–966. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- Szwed, A.; Kim, E.; Jacinto, E. Regulation and metabolic functions of mTORC1 and mTORC2. Physiol. Rev. 2021, 101, 1371–1426. [Google Scholar] [CrossRef] [PubMed]
- Grygiel-Gorniak, B. Peroxisome proliferator-activated receptors and their ligands: Nutritional and clinical implications--a review. Nutr. J. 2014, 13, 17. [Google Scholar] [CrossRef]
- Monsalve, F.A.; Pyarasani, R.D.; Delgado-Lopez, F.; Moore-Carrasco, R. Peroxisome proliferator-activated receptor targets for the treatment of metabolic diseases. Mediat. Inflamm. 2013, 2013, 549627. [Google Scholar] [CrossRef] [PubMed]
- Parsons, S.J.; Parsons, J.T. Src family kinases, key regulators of signal transduction. Oncogene 2004, 23, 7906–7909. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.L.; Iida, M.; Dunn, E.F. The role of Src in solid tumors. Oncologist 2009, 14, 667–678. [Google Scholar] [CrossRef]
- Handberg, A.; Norberg, M.; Stenlund, H.; Hallmans, G.; Attermann, J.; Eriksson, J.W. Soluble CD36 (sCD36) clusters with markers of insulin resistance, and high sCD36 is associated with increased type 2 diabetes risk. J. Clin. Endocrinol. Metab. 2010, 95, 1939–1946. [Google Scholar] [CrossRef]
- Handberg, A.; Hojlund, K.; Gastaldelli, A.; Flyvbjerg, A.; Dekker, J.M.; Petrie, J.; Piatti, P.; Beck-Nielsen, H.; Investigators, R. Plasma sCD36 is associated with markers of atherosclerosis, insulin resistance and fatty liver in a nondiabetic healthy population. J. Intern. Med. 2012, 271, 294–304. [Google Scholar] [CrossRef]
- Puchalowicz, K.; Rac, M.E. The Multifunctionality of CD36 in Diabetes Mellitus and Its Complications-Update in Pathogenesis, Treatment and Monitoring. Cells 2020, 9, 1877. [Google Scholar] [CrossRef]
- Febbraio, M.; Abumrad, N.A.; Hajjar, D.P.; Sharma, K.; Cheng, W.; Pearce, S.F.; Silverstein, R.L. A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J. Biol. Chem. 1999, 274, 19055–19062. [Google Scholar] [CrossRef]
- Koch, M.; Hussein, F.; Woeste, A.; Grundker, C.; Frontzek, K.; Emons, G.; Hawighorst, T. CD36-mediated activation of endothelial cell apoptosis by an N-terminal recombinant fragment of thrombospondin-2 inhibits breast cancer growth and metastasis in vivo. Breast Cancer Res. Treat. 2011, 128, 337–346. [Google Scholar] [CrossRef]
- Podrez, E.A.; Byzova, T.V.; Febbraio, M.; Salomon, R.G.; Ma, Y.; Valiyaveettil, M.; Poliakov, E.; Sun, M.; Finton, P.J.; Curtis, B.R.; et al. Platelet CD36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype. Nat. Med. 2007, 13, 1086–1095. [Google Scholar] [CrossRef]
- Vorchheimer, D.A.; Becker, R. Platelets in atherothrombosis. Mayo Clin. Proc. 2006, 81, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, S.O.; Lennon, D.J.; Febbraio, M.; Podrez, E.A.; Hazen, S.L.; Silverstein, R.L. A CD36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell Metab. 2006, 4, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.R.; Stuart, L.M.; Wilkinson, K.; van Gils, J.M.; Deng, J.; Halle, A.; Rayner, K.J.; Boyer, L.; Zhong, R.; Frazier, W.A.; et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat. Immunol. 2010, 11, 155–161. [Google Scholar] [CrossRef]
- Xu, S.; Jay, A.; Brunaldi, K.; Huang, N.; Hamilton, J.A. CD36 enhances fatty acid uptake by increasing the rate of intracellular esterification but not transport across the plasma membrane. Biochemistry 2013, 52, 7254–7261. [Google Scholar] [CrossRef]
- Ma, X.; Xiao, L.; Liu, L.; Ye, L.; Su, P.; Bi, E.; Wang, Q.; Yang, M.; Qian, J.; Yi, Q. CD36-mediated ferroptosis dampens intratumoral CD8(+) T cell effector function and impairs their antitumor ability. Cell Metab. 2021, 33, 1001–1012.E5. [Google Scholar] [CrossRef] [PubMed]
- Hayasaka, T.; Fuda, H.; Hui, S.P.; Chiba, H. Imaging Mass Spectrometry Reveals a Decrease of Cardiolipin in the Kidney of NASH Model Mice. Anal. Sci. 2016, 32, 473–476. [Google Scholar] [CrossRef]
- Su, P.; Wang, Q.; Bi, E.; Ma, X.; Liu, L.; Yang, M.; Qian, J.; Yi, Q. Enhanced Lipid Accumulation and Metabolism Are Required for the Differentiation and Activation of Tumor-Associated Macrophages. Cancer Res. 2020, 80, 1438–1450. [Google Scholar] [CrossRef]
- Zhang, Y.; Kurupati, R.; Liu, L.; Zhou, X.Y.; Zhang, G.; Hudaihed, A.; Filisio, F.; Giles-Davis, W.; Xu, X.; Karakousis, G.C.; et al. Enhancing CD8(+) T Cell Fatty Acid Catabolism within a Metabolically Challenging Tumor Microenvironment Increases the Efficacy of Melanoma Immunotherapy. Cancer Cell 2017, 32, 377–391 e379. [Google Scholar] [CrossRef]
- Wang, H.; Franco, F.; Tsui, Y.C.; Xie, X.; Trefny, M.P.; Zappasodi, R.; Mohmood, S.R.; Fernandez-Garcia, J.; Tsai, C.H.; Schulze, I.; et al. CD36-mediated metabolic adaptation supports regulatory T cell survival and function in tumors. Nat. Immunol. 2020, 21, 298–308. [Google Scholar] [CrossRef]
- Pascual, G.; Avgustinova, A.; Mejetta, S.; Martin, M.; Castellanos, A.; Attolini, C.S.; Berenguer, A.; Prats, N.; Toll, A.; Hueto, J.A.; et al. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature 2017, 541, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Sag, D.; Carling, D.; Stout, R.D.; Suttles, J. Adenosine 5′-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J. Immunol. 2008, 181, 8633–8641. [Google Scholar] [CrossRef]
- Bonen, A.; Parolin, M.L.; Steinberg, G.R.; Calles-Escandon, J.; Tandon, N.N.; Glatz, J.F.; Luiken, J.J.; Heigenhauser, G.J.; Dyck, D.J. Triacylglycerol accumulation in human obesity and type 2 diabetes is associated with increased rates of skeletal muscle fatty acid transport and increased sarcolemmal FAT/CD36. FASEB J. 2004, 18, 1144–1146. [Google Scholar] [CrossRef]
- Glatz, J.F.; Luiken, J.J.; Bonen, A. Membrane fatty acid transporters as regulators of lipid metabolism: Implications for metabolic disease. Physiol. Rev. 2010, 90, 367–417. [Google Scholar] [CrossRef] [PubMed]
- Aguer, C.; Mercier, J.; Man, C.Y.; Metz, L.; Bordenave, S.; Lambert, K.; Jean, E.; Lantier, L.; Bounoua, L.; Brun, J.F.; et al. Intramyocellular lipid accumulation is associated with permanent relocation ex vivo and in vitro of fatty acid translocase (FAT)/CD36 in obese patients. Diabetologia 2010, 53, 1151–1163. [Google Scholar] [CrossRef]
- Holloway, G.P.; Bezaire, V.; Heigenhauser, G.J.; Tandon, N.N.; Glatz, J.F.; Luiken, J.J.; Bonen, A.; Spriet, L.L. Mitochondrial long chain fatty acid oxidation, fatty acid translocase/CD36 content and carnitine palmitoyltransferase I activity in human skeletal muscle during aerobic exercise. J. Physiol. 2006, 571, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Nickerson, J.G.; Alkhateeb, H.; Benton, C.R.; Lally, J.; Nickerson, J.; Han, X.X.; Wilson, M.H.; Jain, S.S.; Snook, L.A.; Glatz, J.F.C.; et al. Greater transport efficiencies of the membrane fatty acid transporters FAT/CD36 and FATP4 compared with FABPpm and FATP1 and differential effects on fatty acid esterification and oxidation in rat skeletal muscle. J. Biol. Chem. 2009, 284, 16522–16530. [Google Scholar] [CrossRef]
- Greco, D.; Kotronen, A.; Westerbacka, J.; Puig, O.; Arkkila, P.; Kiviluoto, T.; Laitinen, S.; Kolak, M.; Fisher, R.M.; Hamsten, A.; et al. Gene expression in human NAFLD. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G1281–G1287. [Google Scholar] [CrossRef]
- Zhou, J.; Febbraio, M.; Wada, T.; Zhai, Y.; Kuruba, R.; He, J.; Lee, J.H.; Khadem, S.; Ren, S.; Li, S.; et al. Hepatic fatty acid transporter Cd36 is a common target of LXR, PXR, and PPARgamma in promoting steatosis. Gastroenterology 2008, 134, 556–567. [Google Scholar] [CrossRef]
- Miquilena-Colina, M.E.; Lima-Cabello, E.; Sanchez-Campos, S.; Garcia-Mediavilla, M.V.; Fernandez-Bermejo, M.; Lozano-Rodriguez, T.; Vargas-Castrillon, J.; Buque, X.; Ochoa, B.; Aspichueta, P.; et al. Hepatic fatty acid translocase CD36 upregulation is associated with insulin resistance, hyperinsulinaemia and increased steatosis in non-alcoholic steatohepatitis and chronic hepatitis C. Gut 2011, 60, 1394–1402. [Google Scholar] [CrossRef]
- Feng, W.W.; Zuppe, H.T.; Kurokawa, M. The Role of CD36 in Cancer Progression and Its Value as a Therapeutic Target. Cells 2023, 12, 1605. [Google Scholar] [CrossRef] [PubMed]
- Giuppi, M.; La Salvia, A.; Evangelista, J.; Ghidini, M. The Role and Expression of Angiogenesis-Related miRNAs in Gastric Cancer. Biology 2021, 10, 146. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Liao, W.X.; Huang, S.Z.; Yu, Y.F.; Wen, J.Y.; Chen, J.; Lin, D.G.; Wu, X.Y.; Jiang, N.; Li, X. Prognostic and immunological role of CD36: A pan-cancer analysis. J. Cancer 2021, 12, 4762–4773. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Chen, Z.; Zuo, Q.; Kang, Y. Regulation of CD8+ T cells by lipid metabolism in cancer progression. Cell Mol. Immunol. 2024, 21, 1215–1230. [Google Scholar] [CrossRef]
- Suri, C.; Pande, B.; Suhasini Sahithi, L.; Swarnkar, S.; Khelkar, T.; Verma, H.K. Metabolic crossroads: Unravelling immune cell dynamics in gastrointestinal cancer drug resistance. Cancer Drug Resist. 2025, 8, 7. [Google Scholar] [CrossRef]
- Christofides, A.; Konstantinidou, E.; Jani, C.; Boussiotis, V.A. The role of peroxisome proliferator-activated receptors (PPAR) in immune responses. Metabolism 2021, 114, 154338. [Google Scholar] [CrossRef]
- Blanquicett, C.; Roman, J.; Hart, C.M. Thiazolidinediones as anti-cancer agents. Cancer Ther. 2008, 6, 25–34. [Google Scholar]
- Chi, T.; Wang, M.; Wang, X.; Yang, K.; Xie, F.; Liao, Z.; Wei, P. PPAR-gamma Modulators as Current and Potential Cancer Treatments. Front. Oncol. 2021, 11, 737776. [Google Scholar] [CrossRef]



| Category | Intervention/ Application | Mechanism of Action | Target Pathway | Clinical Status | Evidence Level | Potential Applications | Limitations/Challenges |
|---|---|---|---|---|---|---|---|
| CD36 Direct Antagonists | Sulfosuccinimidyl oleate (SSO) [52,53,54] | Irreversible CD36 inhibition | Fatty acid uptake blockade | Preclinical | In vitro/animal studies | Metabolic disorders, cancer metabolism | Limited bioavailability, specificity concerns |
| Hexarelin [55,56] | CD36 receptor antagonism | Scavenger receptor function | Phase I/II trials | Clinical evidence | Cardiovascular disease, atherosclerosis | Potential off-target effects | |
| Anti-CD36 monoclonal antibodies [57,58] | Direct receptor blockade | Multiple CD36 functions | Preclinical | Experimental | Cancer immunotherapy, metabolic syndrome | Antibody delivery challenges | |
| Downstream Pathway Inhibitors | AMPK activators (Metformin) [59,60] | Metabolic reprogramming | AMPK-mTOR axis | FDA-approved | Extensive clinical data | Type 2 diabetes, cancer prevention | Variable response rates |
| mTOR inhibitors (Rapamycin analogs) [61,62] | Protein synthesis inhibition | mTOR signaling cascade | FDA-approved | Phase III trials | Cancer therapy, metabolic disorders | Immunosuppressive effects | |
| PPAR modulators [63,64] | Transcriptional regulation | Nuclear receptor signaling | FDA-approved | Clinical evidence | Metabolic syndrome, NASH | Potential cardiovascular risks | |
| Src kinase inhibitors [65,66] | Signal transduction blockade | CD36-Src pathway | Phase II/III trials | Clinical development | Cancer, inflammatory diseases | Broad kinase inhibition | |
| Non-invasive Biomarkers | Plasma-soluble CD36 (sCD36) [67,68,69] | Circulating receptor fragment | Membrane shedding | Clinical validation ongoing | Observational studies | Cardiovascular risk assessment | Standardization needed |
| Serum CD36+ extracellular vesicles [70,71] | Vesicle-associated CD36 | Cellular communication | Research phase | Proof-of-concept | Cancer progression monitoring | Technical complexity | |
| Platelet CD36 expression [72,73] | Flow cytometry analysis | Thrombotic function | Clinical research | Case–control studies | Atherothrombosis risk | Platelet activation variability | |
| Monocyte CD36 levels [74,75] | Cell surface expression | Immune cell phenotyping | Research application | Observational data | Inflammatory disease monitoring | Inter-individual variation | |
| Immunotherapy Predictors | CD36 tumor expression [76,77,78] | Immunohistochemistry/RNA-seq | T cell dysfunction pathway | Biomarker development | Retrospective analyses | Anti-PD-1/PD-L1 response prediction | Tumor heterogeneity |
| CD36+ tumor-associated macrophages [79,80,81] | Flow cytometry/imaging | M2 polarization status | Research phase | Preclinical evidence | Combination immunotherapy | Sampling accessibility | |
| Circulating CD36+ immune cells [82,83] | Peripheral blood analysis | Systemic immune suppression | Early development | Pilot studies | Treatment stratification | Need for validation cohorts | |
| Metabolic Drug Predictors | Adipose CD36 expression [84,85,86] | Tissue biopsy analysis | Fatty acid metabolism | Research application | Metabolic studies | Insulin sensitizer response | Invasive sampling required |
| Muscle CD36 localization [87,88] | Immunofluorescence | Substrate utilization | Research phase | Exercise physiology | Metabolic flexibility assessment | Biopsy limitations | |
| Hepatic CD36 levels [89,90,91] | Imaging/biopsy | Lipid accumulation | Clinical research | NAFLD studies | Steatosis treatment response | Sampling challenges |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutta, P.; Saha, D.; Giri, A.; Bhatnagar, A.R.; Chakraborty, A. Decoding the CD36-Centric Axis in Gastric Cancer: Insights into Lipid Metabolism, Obesity, and Hypercholesterolemia. Int. J. Transl. Med. 2025, 5, 26. https://doi.org/10.3390/ijtm5030026
Dutta P, Saha D, Giri A, Bhatnagar AR, Chakraborty A. Decoding the CD36-Centric Axis in Gastric Cancer: Insights into Lipid Metabolism, Obesity, and Hypercholesterolemia. International Journal of Translational Medicine. 2025; 5(3):26. https://doi.org/10.3390/ijtm5030026
Chicago/Turabian StyleDutta, Preyangsee, Dwaipayan Saha, Atanu Giri, Aseem Rai Bhatnagar, and Abhijit Chakraborty. 2025. "Decoding the CD36-Centric Axis in Gastric Cancer: Insights into Lipid Metabolism, Obesity, and Hypercholesterolemia" International Journal of Translational Medicine 5, no. 3: 26. https://doi.org/10.3390/ijtm5030026
APA StyleDutta, P., Saha, D., Giri, A., Bhatnagar, A. R., & Chakraborty, A. (2025). Decoding the CD36-Centric Axis in Gastric Cancer: Insights into Lipid Metabolism, Obesity, and Hypercholesterolemia. International Journal of Translational Medicine, 5(3), 26. https://doi.org/10.3390/ijtm5030026







