Novel Roles and Therapeutic Approaches Linking Platelets and Megakaryocytes to Non-Hemostatic and Thrombotic Disease
Abstract
1. Introduction
2. Conventional and Non-Conventional Roles of Platelets in Disease
2.1. Platelets in Hemostasis and Thrombosis
2.2. Role of Platelets as Immune Cells in Non-Hemostatic Disease
2.3. Role of Platelets in Non-Hemostatic, Nonimmune Disorders
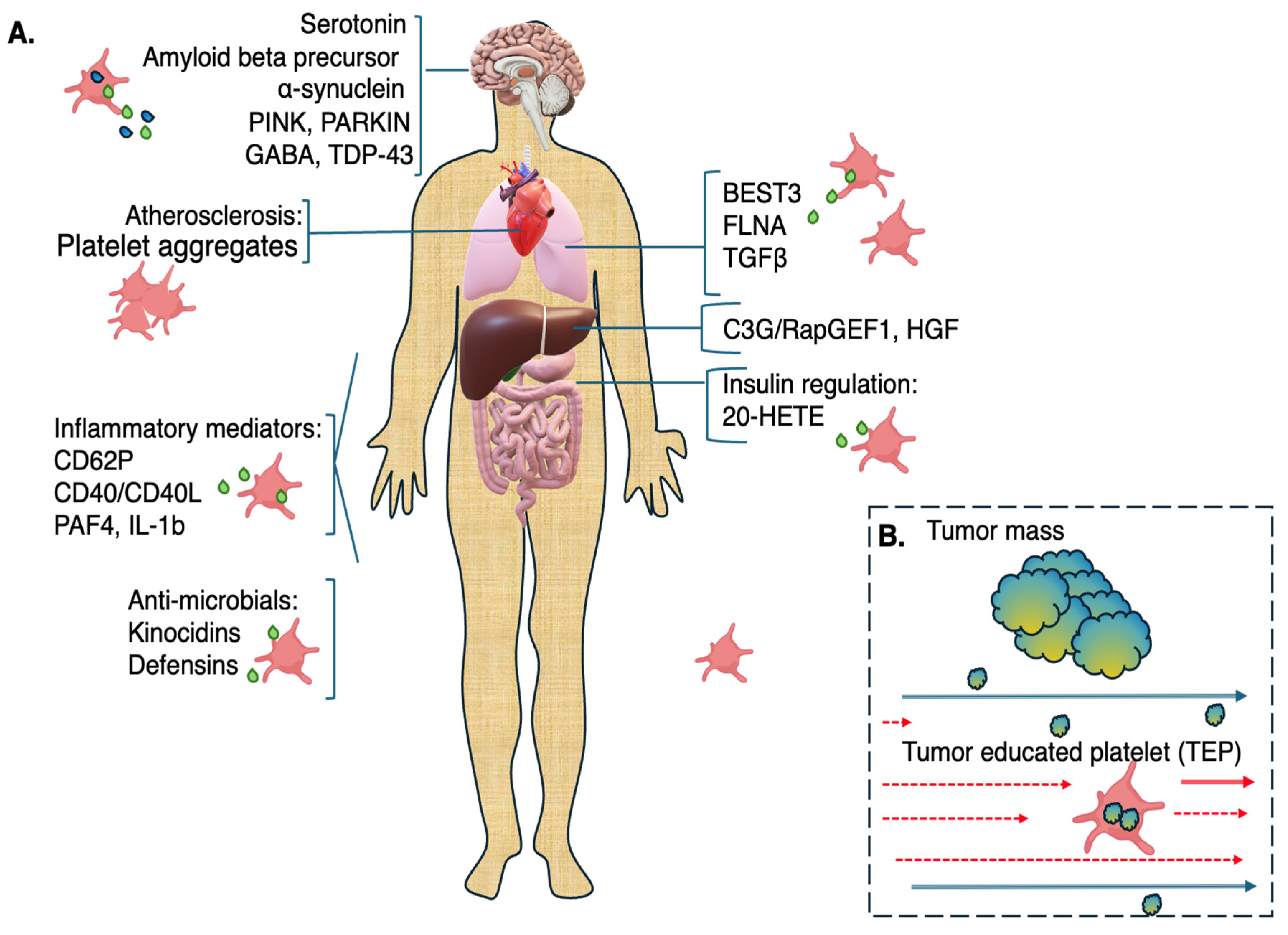
2.4. Role of Platelets in the Nervous System
2.5. Role of Platelets in Diabetes
2.6. Role of Platelets in Fibrotic Disease and Cancer
3. Platelet Derivatives for Potential Therapeutic Application
3.1. Potential of Stem Cell-Differentiated MKs and Platelets for Translational Medicine
3.2. Role of Platelets as Diagnostic Sensors
3.3. Challenges to Implementation of Platelet-Based Diagnostics
3.4. Platelet-Rich Plasma for Regenerative Medicine
3.5. Platelet and MK Extracellular Microvesicles (EVs)
3.6. Platelet Membranes in Nanorobotic Therapeutics
4. Future Perspectives
Funding
Data Availability Statement
Conflicts of Interest
References
- Conrad, C.; Magnen, M.; Tsui, J.; Wismer, H.; Naser, M.; Venkataramani, U.; Samad, B.; Cleary, S.J.; Qiu, L.; Tian, J.J.; et al. Decoding functional hematopoietic progenitor cells in the adult human lung. Blood 2025, 145, 1975–1986. [Google Scholar] [CrossRef] [PubMed]
- Lefrancais, E.; Ortiz-Munoz, G.; Caudrillier, A.; Mallavia, B.; Liu, F.; Sayah, D.M.; Thornton, E.E.; Headley, M.B.; David, T.; Coughlin, S.R.; et al. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature 2017, 544, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Zucker-Franklin, D.; Philipp, C.S. Platelet production in the pulmonary capillary bed: New ultrastructural evidence for an old concept. Am. J. Pathol. 2000, 157, 69–74. [Google Scholar] [CrossRef]
- Eckly, A.; Heijnen, H.; Pertuy, F.; Geerts, W.; Proamer, F.; Rinckel, J.Y.; Leon, C.; Lanza, F.; Gachet, C. Biogenesis of the demarcation membrane system (DMS) in megakaryocytes. Blood 2014, 123, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Krammer, T.L.; Zeibig, S.; Schrottmaier, W.C.; Pirabe, A.; Goebel, S.; Diendorfer, A.B.; Holthoff, H.P.; Assinger, A.; Hackl, M. Comprehensive Characterization of Platelet-Enriched MicroRNAs as Biomarkers of Platelet Activation. Cells 2022, 11, 1254. [Google Scholar] [CrossRef]
- Supernat, A.; Popeda, M.; Pastuszak, K.; Best, M.G.; Gresner, P.; Veld, S.I.; Siek, B.; Bednarz-Knoll, N.; Rondina, M.T.; Stokowy, T.; et al. Transcriptomic landscape of blood platelets in healthy donors. Sci. Rep. 2021, 11, 15679. [Google Scholar] [CrossRef]
- Lan, W.; Li, J.; Ye, Z.; Liu, Y.; Luo, S.; Lu, X.; Cao, Z.; Chen, Y.; Chen, H.; Li, Z. A subset of megakaryocytes regulates development of hematopoietic stem cell precursors. EMBO J. 2024, 43, 1722–1739. [Google Scholar] [CrossRef]
- Liu, C.; Huang, B.; Wang, H.; Zhou, J. The heterogeneity of megakaryocytes and platelets and implications for ex vivo platelet generation. Stem Cells Transl. Med. 2021, 10, 1614–1620. [Google Scholar] [CrossRef]
- Carrelha, J.; Mazzi, S.; Winroth, A.; Hagemann-Jensen, M.; Ziegenhain, C.; Hogstrand, K.; Seki, M.; Brennan, M.S.; Lehander, M.; Wu, B.; et al. Alternative platelet differentiation pathways initiated by nonhierarchically related hematopoietic stem cells. Nat. Immunol. 2024, 25, 1007–1019. [Google Scholar] [CrossRef]
- Rodriguez-Fraticelli, A.E.; Wolock, S.L.; Weinreb, C.S.; Panero, R.; Patel, S.H.; Jankovic, M.; Sun, J.; Calogero, R.A.; Klein, A.M.; Camargo, F.D. Clonal analysis of lineage fate in native haematopoiesis. Nature 2018, 553, 212–216. [Google Scholar] [CrossRef]
- Swieringa, F.; Heemskerk, J.W.M.; Assinger, A. Platelet activation and signaling in thrombus formation. Blood 2025. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Stewart Iv, J.H. Platelet’s plea to Immunologists: Please do not forget me. Int. Immunopharmacol. 2024, 143, 113599. [Google Scholar] [CrossRef]
- Speth, C.; Rambach, G.; Wurzner, R.; Lass-Florl, C.; Kozarcanin, H.; Hamad, O.A.; Nilsson, B.; Ekdahl, K.N. Complement and platelets: Mutual interference in the immune network. Mol. Immunol. 2015, 67, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Antoniak, S.; Mackman, N. Platelets and viruses. Platelets 2021, 32, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Portier, I.; Campbell, R.A. Role of Platelets in Detection and Regulation of Infection. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 70–78. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Sulaiman, G.M.; Mohammed, H.A.; Dawood, R.A.; Albuhadily, A.K.; Al-Gareeb, A.I.; Abomughaid, M.M.; Klionsky, D.J. Alterations in the Processing of Platelet APP (Amyloid Beta Precursor Protein) in Alzheimer Disease: The Possible Nexus. Neuropsychopharmacol. Rep. 2025, 45, e12525. [Google Scholar] [CrossRef]
- Nicolai, L.; Pekayvaz, K.; Massberg, S. Platelets: Orchestrators of immunity in host defense and beyond. Immunity 2024, 57, 957–972. [Google Scholar] [CrossRef]
- Rodriguez Moore, G.; Melo-Escobar, I.; Stegner, D.; Bracko, O. One immune cell to bind them all: Platelet contribution to neurodegenerative disease. Mol. Neurodegener. 2024, 19, 65. [Google Scholar] [CrossRef]
- Salauddin, M.; Bhattacharyya, D.; Samanta, I.; Saha, S.; Xue, M.; Hossain, M.G.; Zheng, C. Role of TLRs as signaling cascades to combat infectious diseases: A review. Cell. Mol. Life Sci. 2025, 82, 122. [Google Scholar] [CrossRef]
- Mack, A.; Vanden Hoek, T.; Du, X. Thromboinflammation and the Role of Platelets. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 1175–1180. [Google Scholar] [CrossRef]
- Scherlinger, M.; Richez, C.; Tsokos, G.C.; Boilard, E.; Blanco, P. The role of platelets in immune-mediated inflammatory diseases. Nat. Rev. Immunol. 2023, 23, 495–510. [Google Scholar] [CrossRef]
- Yacoub, D.; Benslimane, N.; Al-Zoobi, L.; Hassan, G.; Nadiri, A.; Mourad, W. CD154 is released from T-cells by a disintegrin and metalloproteinase domain-containing protein 10 (ADAM10) and ADAM17 in a CD40 protein-dependent manner. J. Biol. Chem. 2013, 288, 36083–36093. [Google Scholar] [CrossRef] [PubMed]
- Elzey, B.D.; Tian, J.; Jensen, R.J.; Swanson, A.K.; Lees, J.R.; Lentz, S.R.; Stein, C.S.; Nieswandt, B.; Wang, Y.; Davidson, B.L.; et al. Platelet-mediated modulation of adaptive immunity. A communication link between innate and adaptive immune compartments. Immunity 2003, 19, 9–19. [Google Scholar] [CrossRef]
- Bendas, G.; Gobec, M.; Schlesinger, M. Modulating Immune Responses: The Double-Edged Sword of Platelet CD40L. In Seminars in Thrombosis and Hemostasis; Thieme Medical Publishers: New York, NY, USA, 2024. [Google Scholar] [CrossRef]
- Thienel, U.; Loike, J.; Yellin, M.J. CD154 (CD40L) induces human endothelial cell chemokine production and migration of leukocyte subsets. Cell. Immunol. 1999, 198, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Cognasse, F.; Duchez, A.C.; Audoux, E.; Ebermeyer, T.; Arthaud, C.A.; Prier, A.; Eyraud, M.A.; Mismetti, P.; Garraud, O.; Bertoletti, L.; et al. Platelets as Key Factors in Inflammation: Focus on CD40L/CD40. Front. Immunol. 2022, 13, 825892. [Google Scholar] [CrossRef]
- Chapman, L.M.; Aggrey, A.A.; Field, D.J.; Srivastava, K.; Ture, S.; Yui, K.; Topham, D.J.; Baldwin, W.M., 3rd; Morrell, C.N. Platelets present antigen in the context of MHC class I. J. Immunol. 2012, 189, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Pariser, D.N.; Hilt, Z.T.; Ture, S.K.; Blick-Nitko, S.K.; Looney, M.R.; Cleary, S.J.; Roman-Pagan, E.; Saunders, J., 2nd; Georas, S.N.; Veazey, J.; et al. Lung megakaryocytes are immune modulatory cells. J. Clin. Investig. 2021, 131, e137377. [Google Scholar] [CrossRef]
- Ortiz-Munoz, G.; Yu, M.A.; Lefrancais, E.; Mallavia, B.; Valet, C.; Tian, J.J.; Ranucci, S.; Wang, K.M.; Liu, Z.; Kwaan, N.; et al. Cystic fibrosis transmembrane conductance regulator dysfunction in platelets drives lung hyperinflammation. J. Clin. Investig. 2020, 130, 2041–2053. [Google Scholar] [CrossRef]
- Bain, W.; Olonisakin, T.; Yu, M.; Qu, Y.; Hulver, M.; Xiong, Z.; Li, H.; Pilewski, J.; Mallampalli, R.K.; Nouraie, M.; et al. Platelets inhibit apoptotic lung epithelial cell death and protect mice against infection-induced lung injury. Blood Adv. 2019, 3, 432–445. [Google Scholar] [CrossRef]
- Kraemer, B.F.; Campbell, R.A.; Schwertz, H.; Cody, M.J.; Franks, Z.; Tolley, N.D.; Kahr, W.H.; Lindemann, S.; Seizer, P.; Yost, C.C.; et al. Novel anti-bacterial activities of beta-defensin 1 in human platelets: Suppression of pathogen growth and signaling of neutrophil extracellular trap formation. PLoS Pathog. 2011, 7, e1002355. [Google Scholar] [CrossRef]
- Bayer, A.; Lammel, J.; Tohidnezhad, M.; Lippross, S.; Behrendt, P.; Kluter, T.; Pufe, T.; Cremer, J.; Jahr, H.; Rademacher, F.; et al. The Antimicrobial Peptide Human Beta-Defensin-3 Is Induced by Platelet-Released Growth Factors in Primary Keratinocytes. Mediat. Inflamm. 2017, 2017, 6157491. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, S.; Ghosh, S.K.; Ferrari, B.; Wyrick, J.M.; Podrez, E.A.; Weinberg, A.; Sieg, S.F. Human beta-Defensin-3 is Associated with Platelet-Derived Extracellular Vesicles and is a Potential Contributor to Endothelial Dysfunction. Front. Mol. Biosci. 2022, 9, 824954. [Google Scholar] [CrossRef]
- Greinacher, A.; Warkentin, T.E. Platelet factor 4 triggers thrombo-inflammation by bridging innate and adaptive immunity. Int. J. Lab. Hematol. 2023, 45 (Suppl. 2), 11–22. [Google Scholar] [CrossRef] [PubMed]
- Bakogiannis, C.; Sachse, M.; Stamatelopoulos, K.; Stellos, K. Platelet-derived chemokines in inflammation and atherosclerosis. Cytokine 2019, 122, 154157. [Google Scholar] [CrossRef] [PubMed]
- Machlus, K.R.; Johnson, K.E.; Kulenthirarajan, R.; Forward, J.A.; Tippy, M.D.; Soussou, T.S.; El-Husayni, S.H.; Wu, S.K.; Wang, S.; Watnick, R.S.; et al. CCL5 derived from platelets increases megakaryocyte proplatelet formation. Blood 2016, 127, 921–926. [Google Scholar] [CrossRef]
- Chatterjee, M.; Gawaz, M. Platelet-derived CXCL12 (SDF-1alpha): Basic mechanisms and clinical implications. J. Thromb. Haemost. 2013, 11, 1954–1967. [Google Scholar] [CrossRef]
- Noval Rivas, M.; Kocaturk, B.; Franklin, B.S.; Arditi, M. Platelets in Kawasaki disease: Mediators of vascular inflammation. Nat. Rev. Rheumatol. 2024, 20, 459–472. [Google Scholar] [CrossRef]
- Ponomarev, E.D. Fresh Evidence for Platelets as Neuronal and Innate Immune Cells: Their Role in the Activation, Differentiation, and Deactivation of Th1, Th17, and Tregs during Tissue Inflammation. Front. Immunol. 2018, 9, 406. [Google Scholar] [CrossRef]
- Lindemann, S.; Tolley, N.D.; Dixon, D.A.; McIntyre, T.M.; Prescott, S.M.; Zimmerman, G.A.; Weyrich, A.S. Activated platelets mediate inflammatory signaling by regulated interleukin 1beta synthesis. J. Cell Biol. 2001, 154, 485–490. [Google Scholar] [CrossRef]
- Duerschmied, D.; Suidan, G.L.; Demers, M.; Herr, N.; Carbo, C.; Brill, A.; Cifuni, S.M.; Mauler, M.; Cicko, S.; Bader, M.; et al. Platelet serotonin promotes the recruitment of neutrophils to sites of acute inflammation in mice. Blood 2013, 121, 1008–1015. [Google Scholar] [CrossRef]
- Kanova, M.; Kohout, P. Serotonin-Its Synthesis and Roles in the Healthy and the Critically Ill. Int. J. Mol. Sci. 2021, 22, 4837. [Google Scholar] [CrossRef] [PubMed]
- Reed, G.L.; Fitzgerald, M.L.; Polgar, J. Molecular mechanisms of platelet exocytosis: Insights into the “secrete” life of thrombocytes. Blood 2000, 96, 3334–3342. [Google Scholar] [PubMed]
- Kravcenko, U.; Ruwolt, M.; Kroll, J.; Yushkevich, A.; Zenkner, M.; Ruta, J.; Lotfy, R.; Wanker, E.E.; Rosenmund, C.; Liu, F.; et al. Molecular architecture of synaptic vesicles. Proc. Natl. Acad. Sci. USA 2024, 121, e2407375121. [Google Scholar] [CrossRef]
- Yamamoto, H.; Gurney, M.E. Human platelets contain brain-derived neurotrophic factor. J. Neurosci. 1990, 10, 3469–3478. [Google Scholar] [CrossRef] [PubMed]
- Burnouf, T.; Walker, T.L. The multifaceted role of platelets in mediating brain function. Blood 2022, 140, 815–827. [Google Scholar] [CrossRef]
- Rawish, E.; Langer, H.F. Platelets and the Role of P2X Receptors in Nociception, Pain, Neuronal Toxicity and Thromboinflammation. Int. J. Mol. Sci. 2022, 23, 6585. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-beta Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef]
- Evin, G.; Li, Q.X. Platelets and Alzheimer’s disease: Potential of APP as a biomarker. World J. Psychiatry 2012, 2, 102–113. [Google Scholar] [CrossRef]
- Van Nostrand, W.E.; Schmaier, A.H.; Farrow, J.S.; Cines, D.B.; Cunningham, D.D. Protease nexin-2/amyloid beta-protein precursor in blood is a platelet-specific protein. Biochem. Biophys. Res. Commun. 1991, 175, 15–21. [Google Scholar] [CrossRef]
- Bush, A.I.; Martins, R.N.; Rumble, B.; Moir, R.; Fuller, S.; Milward, E.; Currie, J.; Ames, D.; Weidemann, A.; Fischer, P.; et al. The amyloid precursor protein of Alzheimer’s disease is released by human platelets. J. Biol. Chem. 1990, 265, 15977–15983. [Google Scholar] [CrossRef]
- Kniewallner, K.M.; de Sousa, D.M.B.; Unger, M.S.; Mrowetz, H.; Aigner, L. Platelets in Amyloidogenic Mice Are Activated and Invade the Brain. Front. Neurosci. 2020, 14, 129. [Google Scholar] [CrossRef] [PubMed]
- Donner, L.; Toska, L.M.; Kruger, I.; Groniger, S.; Barroso, R.; Burleigh, A.; Mezzano, D.; Pfeiler, S.; Kelm, M.; Gerdes, N.; et al. The collagen receptor glycoprotein VI promotes platelet-mediated aggregation of beta-amyloid. Sci. Signal 2020, 13, eaba9872. [Google Scholar] [CrossRef]
- Alqahtani, S.M.; Al-Kuraishy, H.M.; Al Gareeb, A.I.; Albuhadily, A.K.; Alexiou, A.; Papadakis, M.; Hemeda, L.R.; Faheem, S.A.; El-Saber Batiha, G. Unlocking Alzheimer’s Disease: The Role of BDNF Signaling in Neuropathology and Treatment. Neuromol. Med. 2025, 27, 36. [Google Scholar] [CrossRef]
- Want, A.; Nan, X.; Kokkali, E.; Barde, Y.A.; Morgan, J.E. Brain-derived neurotrophic factor released from blood platelets prevents dendritic atrophy of lesioned adult central nervous system neurons. Brain Commun. 2023, 5, fcad046. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.N.; Tan, M.S.; Yu, J.T.; Xie, A.M.; Tan, L. The Role of Reelin Signaling in Alzheimer’s Disease. Mol. Neurobiol. 2016, 53, 5692–5700. [Google Scholar] [CrossRef]
- Belaidi, A.A.; Bush, A.I.; Ayton, S. Apolipoprotein E in Alzheimer’s disease: Molecular insights and therapeutic opportunities. Mol. Neurodegener. 2025, 20, 47. [Google Scholar] [CrossRef] [PubMed]
- Lopera, F.; Marino, C.; Chandrahas, A.S.; O’Hare, M.; Villalba-Moreno, N.D.; Aguillon, D.; Baena, A.; Sanchez, J.S.; Vila-Castelar, C.; Ramirez Gomez, L.; et al. Resilience to autosomal dominant Alzheimer’s disease in a Reelin-COLBOS heterozygous man. Nat. Med. 2023, 29, 1243–1252. [Google Scholar] [CrossRef]
- Katsuyama, Y.; Hattori, M. REELIN ameliorates Alzheimer’s disease, but how? Neurosci. Res. 2024, 208, 8–14. [Google Scholar] [CrossRef]
- Yi, L.X.; Zeng, L.; Wang, Q.; Tan, E.K.; Zhou, Z.D. Reelin links Apolipoprotein E4, Tau, and Amyloid-beta in Alzheimer’s disease. Ageing Res. Rev. 2024, 98, 102339. [Google Scholar] [CrossRef]
- Gowert, N.S.; Kruger, I.; Klier, M.; Donner, L.; Kipkeew, F.; Gliem, M.; Bradshaw, N.J.; Lutz, D.; Kober, S.; Langer, H.; et al. Loss of Reelin protects mice against arterial thrombosis by impairing integrin activation and thrombus formation under high shear conditions. Cell Signal. 2017, 40, 210–221. [Google Scholar] [CrossRef]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef] [PubMed]
- Beura, S.K.; Panigrahi, A.R.; Yadav, P.; Singh, S.K. Role of platelet in Parkinson’s disease: Insights into pathophysiology & theranostic solutions. Ageing Res. Rev. 2022, 80, 101681. [Google Scholar] [CrossRef]
- Muneeb, M.; Abdallah, D.M.; El-Abhar, H.S.; Wadie, W.; Ahmed, K.A.; Abul Fadl, Y.S. Antiplatelet therapy as a novel approach in Parkinson’s disease: Repositioning Ticagrelor to alleviate rotenone-induced parkinsonism via modulation of ER stress, apoptosis, and autophagy. Neuropharmacology 2025, 269, 110346. [Google Scholar] [CrossRef]
- Navarro, S.; Talucci, I.; Gob, V.; Hartmann, S.; Beck, S.; Orth, V.; Stoll, G.; Maric, H.M.; Stegner, D.; Nieswandt, B. The humanized platelet glycoprotein VI Fab inhibitor EMA601 protects from arterial thrombosis and ischaemic stroke in mice. Eur. Heart J. 2024, 45, 4582–4597. [Google Scholar] [CrossRef] [PubMed]
- Begni, B.; Tremolizzo, L.; D’Orlando, C.; Bono, M.S.; Garofolo, R.; Longoni, M.; Ferrarese, C. Substrate-induced modulation of glutamate uptake in human platelets. Br. J. Pharmacol. 2005, 145, 792–799. [Google Scholar] [CrossRef]
- Gautam, D.; Naik, U.P.; Naik, M.U.; Yadav, S.K.; Chaurasia, R.N.; Dash, D. Glutamate Receptor Dysregulation and Platelet Glutamate Dynamics in Alzheimer’s and Parkinson’s Diseases: Insights into Current Medications. Biomolecules 2023, 13, 1609. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Jiang, Q.; Yang, B.; Hu, X.; Shen, G.; Shen, W.; Xu, J. Platelet mitochondria, a potent immune mediator in neurological diseases. Front. Physiol. 2023, 14, 1210509. [Google Scholar] [CrossRef]
- Leiter, O.; Walker, T.L. Platelets in Neurodegenerative Conditions-Friend or Foe? Front. Immunol. 2020, 11, 747. [Google Scholar] [CrossRef]
- Luthi-Carter, R.; Cappelli, S.; Le Roux-Bourdieu, M.; Tentillier, N.; Quinn, J.P.; Petrozziello, T.; Gopalakrishnan, L.; Sethi, P.; Choudhary, H.; Bartolini, G.; et al. Location and function of TDP-43 in platelets, alterations in neurodegenerative diseases and arising considerations for current plasma biobank protocols. Sci. Rep. 2024, 14, 21837. [Google Scholar] [CrossRef]
- Lee, S.H.; Pham, D.; Kosa, E.; Agbas, A. Human Platelet Derived Mitochondrial OPA-1 Isoforms and Interaction with TDP-43 in Neurodegenerative Diseases. Mo. Med. 2024, 121, 87–92. [Google Scholar]
- Leiter, O.; Walker, T.L. Platelets: The missing link between the blood and brain? Prog. Neurobiol. 2019, 183, 101695. [Google Scholar] [CrossRef]
- Karwen, T.; Kolczynska-Matysiak, K.; Gross, C.; Loffler, M.C.; Friedrich, M.; Loza-Valdes, A.; Schmitz, W.; Wit, M.; Dziaczkowski, F.; Belykh, A.; et al. Platelet-derived lipids promote insulin secretion of pancreatic beta cells. EMBO Mol. Med. 2023, 15, e16858. [Google Scholar] [CrossRef] [PubMed]
- Kolczynska-Matysiak, K.; Karwen, T.; Loeffler, M.; Hawro, I.; Kassouf, T.; Stegner, D.; Sumara, G. Dense but not alpha granules of platelets are required for insulin secretion from pancreatic beta cells. Biochem. Biophys. Res. Commun. 2024, 734, 150753. [Google Scholar] [CrossRef] [PubMed]
- Russo, I.; Penna, C.; Musso, T.; Popara, J.; Alloatti, G.; Cavalot, F.; Pagliaro, P. Platelets, diabetes and myocardial ischemia/reperfusion injury. Cardiovasc. Diabetol. 2017, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Schneider, D.J. Factors contributing to increased platelet reactivity in people with diabetes. Diabetes Care 2009, 32, 525–527. [Google Scholar] [CrossRef]
- Agarwal, S.; Saha, S.; Ghosh, R.; Sarmadhikari, D.; Asthana, S.; Maiti, T.K.; Khadgawat, R.; Guchhait, P. Elevated glycosylation of CD36 in platelets is a risk factor for oxLDL-mediated platelet activation in type 2 diabetes. FEBS J. 2024, 291, 376–391. [Google Scholar] [CrossRef]
- Zhong, H.; Waresi, M.; Jia, X.; Ge, J. Enhanced STIM1 expression drives platelet hyperactivity in diabetes. Biochem. Biophys. Res. Commun. 2025, 753, 151510. [Google Scholar] [CrossRef]
- Amalia, M.; Puteri, M.U.; Saputri, F.C.; Sauriasari, R.; Widyantoro, B. Platelet Glycoprotein-Ib (GPIb) May Serve as a Bridge between Type 2 Diabetes Mellitus (T2DM) and Atherosclerosis, Making It a Potential Target for Antiplatelet Agents in T2DM Patients. Life 2023, 13, 1473. [Google Scholar] [CrossRef]
- Casari, M.; Siegl, D.; Deppermann, C.; Schuppan, D. Macrophages and platelets in liver fibrosis and hepatocellular carcinoma. Front. Immunol. 2023, 14, 1277808. [Google Scholar] [CrossRef]
- Kanikarla Marie, P.; Fowlkes, N.W.; Afshar-Kharghan, V.; Martch, S.L.; Sorokin, A.; Shen, J.P.; Morris, V.K.; Dasari, A.; You, N.; Sood, A.K.; et al. The Provocative Roles of Platelets in Liver Disease and Cancer. Front. Oncol. 2021, 11, 643815. [Google Scholar] [CrossRef]
- Kodama, T.; Takehara, T.; Hikita, H.; Shimizu, S.; Li, W.; Miyagi, T.; Hosui, A.; Tatsumi, T.; Ishida, H.; Tadokoro, S.; et al. Thrombocytopenia exacerbates cholestasis-induced liver fibrosis in mice. Gastroenterology 2010, 138, 2487–2498.e7. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Teramoto, H.; Ichihara, A. Purification and characterization of a growth factor from rat platelets for mature parenchymal hepatocytes in primary cultures. Proc. Natl. Acad. Sci. USA 1986, 83, 6489–6493. [Google Scholar] [CrossRef]
- Pietrapiana, D.; Sala, M.; Prat, M.; Sinigaglia, F. Met identification on human platelets: Role of hepatocyte growth factor in the modulation of platelet activation. FEBS Lett. 2005, 579, 4550–4554. [Google Scholar] [CrossRef] [PubMed]
- Baquero, C.; Iniesta-Gonzalez, M.; Palao, N.; Fernandez-Infante, C.; Cueto-Remacha, M.; Mancebo, J.; de la Camara-Fuentes, S.; Rodrigo-Faus, M.; Valdecantos, M.P.; Valverde, A.M.; et al. Platelet C3G protects from liver fibrosis, while enhancing tumor growth through regulation of the immune response. J. Pathol. 2025, 265, 502–517. [Google Scholar] [CrossRef]
- Darbousset, R.; Senkpeil, L.; Kuehn, J.; Balu, S.; Miglani, D.; Dillon, E.; Fromson, C.; Elahee, M.; Jarrot, P.A.; Montesi, S.B.; et al. A GPVI-platelet-neutrophil-NET axis drives systemic sclerosis. bioRxiv 2025. [Google Scholar] [CrossRef]
- Chong, D.L.W.; Mikolasch, T.A.; Sahota, J.; Rebeyrol, C.; Garthwaite, H.S.; Booth, H.L.; Heightman, M.; Denneny, E.K.; Jose, R.J.; Khawaja, A.A.; et al. Investigating the role of platelets and platelet-derived transforming growth factor-beta in idiopathic pulmonary fibrosis. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2023, 325, L487–L499. [Google Scholar] [CrossRef]
- Carrington, R.; Jordan, S.; Wong, Y.J.; Pitchford, S.C.; Page, C.P. A novel murine model of pulmonary fibrosis: The role of platelets in chronic changes induced by bleomycin. J. Pharmacol. Toxicol. Methods 2021, 109, 107057. [Google Scholar] [CrossRef]
- Lee, C.T.; Adegunsoye, A. Anticoagulation and Pulmonary Fibrosis: Friends, Foes, or Functional Allies? Chest 2021, 159, 1321–1323. [Google Scholar] [CrossRef]
- Kolonics-Farkas, A.M.; Sterclova, M.; Mogulkoc, N.; Kus, J.; Hajkova, M.; Muller, V.; Jovanovic, D.; Tekavec-Trkanjec, J.; Littnerova, S.; Hejduk, K.; et al. Anticoagulant Use and Bleeding Risk in Central European Patients with Idiopathic Pulmonary Fibrosis (IPF) Treated with Antifibrotic Therapy: Real-World Data from EMPIRE. Drug Saf. 2020, 43, 971–980. [Google Scholar] [CrossRef]
- Borok, Z.; Horie, M.; Flodby, P.; Wang, H.; Liu, Y.; Ganesh, S.; Firth, A.L.; Minoo, P.; Li, C.; Beers, M.F.; et al. Grp78 Loss in Epithelial Progenitors Reveals an Age-linked Role for Endoplasmic Reticulum Stress in Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2020, 201, 198–211. [Google Scholar] [CrossRef]
- Habiel, D.M.; Espindola, M.S.; Coelho, A.L.; Hogaboam, C.M. Modeling Idiopathic Pulmonary Fibrosis in Humanized Severe Combined Immunodeficient Mice. Am. J. Pathol. 2018, 188, 891–903. [Google Scholar] [CrossRef]
- Xu, X.R.; Yousef, G.M.; Ni, H. Cancer and platelet crosstalk: Opportunities and challenges for aspirin and other antiplatelet agents. Blood 2018, 131, 1777–1789. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, Z.; Tian, Y.; Li, Z.; Liu, Z.; Zhu, S. The critical role of platelet in cancer progression and metastasis. Eur. J. Med. Res. 2023, 28, 385. [Google Scholar] [CrossRef]
- Demers, M.; Wagner, D.D. Targeting platelet function to improve drug delivery. Oncoimmunology 2012, 1, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.; Zhang, L.; Zhong, D. Chemotherapy-induced thrombocytopenia: Literature review. Discov. Oncol. 2023, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Zhao, K.; Li, X. Platelet stimulation-regulated expression of ILK and ITGB3 contributes to intrahepatic cholangiocarcinoma progression through FAK/PI3K/AKT pathway activation. Cell. Mol. Life Sci. 2024, 82, 19. [Google Scholar] [CrossRef]
- Cho, M.S.; Bottsford-Miller, J.; Vasquez, H.G.; Stone, R.; Zand, B.; Kroll, M.H.; Sood, A.K.; Afshar-Kharghan, V. Platelets increase the proliferation of ovarian cancer cells. Blood 2012, 120, 4869–4872. [Google Scholar] [CrossRef]
- Labelle, M.; Begum, S.; Hynes, R.O. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 2011, 20, 576–590. [Google Scholar] [CrossRef]
- Velez, J.; Enciso, L.J.; Suarez, M.; Fiegl, M.; Grismaldo, A.; Lopez, C.; Barreto, A.; Cardozo, C.; Palacios, P.; Morales, L.; et al. Platelets promote mitochondrial uncoupling and resistance to apoptosis in leukemia cells: A novel paradigm for the bone marrow microenvironment. Cancer Microenviron. 2014, 7, 79–90. [Google Scholar] [CrossRef]
- Demers, M.; Ho-Tin-Noe, B.; Schatzberg, D.; Yang, J.J.; Wagner, D.D. Increased efficacy of breast cancer chemotherapy in thrombocytopenic mice. Cancer Res. 2011, 71, 1540–1549. [Google Scholar] [CrossRef]
- Bottsford-Miller, J.; Choi, H.J.; Dalton, H.J.; Stone, R.L.; Cho, M.S.; Haemmerle, M.; Nick, A.M.; Pradeep, S.; Zand, B.; Previs, R.A.; et al. Differential platelet levels affect response to taxane-based therapy in ovarian cancer. Clin. Cancer Res. 2015, 21, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Song, A.B.; Al-Samkari, H. Chemotherapy-induced thrombocytopenia: Modern diagnosis and treatment. Br. J. Haematol. 2025, 206, 1062–1066. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.P. On the horizon: Upcoming new agents for the management of ITP. Hematol. Am. Soc. Hematol. Educ. Program 2024, 2024, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Arnold, D.M.; Patriquin, C.J.; Nazy, I. Thrombotic microangiopathies: A general approach to diagnosis and management. CMAJ 2017, 189, E153–E159. [Google Scholar] [CrossRef]
- Zhong, Z.; Chen, C.; Wang, N.; Qiu, Y.; Li, X.; Liu, S.; Wu, H.; Tang, X.; Fu, Y.; Chen, Q.; et al. Generation of Functioning Platelets from Mature Megakaryocytes Derived from CD34(+) Umbilical Cord Blood Cells. Stem Cells Dev. 2024, 33, 677–691. [Google Scholar] [CrossRef]
- Kasirer-Friede, A.; Shattil, S.J. Genetic Instruction of Megakaryocytes and Platelets Derived from Human Induced Pluripotent Stem Cells for Studies of Integrin Regulation. Methods Mol. Biol. 2021, 2217, 237–249. [Google Scholar] [CrossRef]
- Chen, C.; Wang, N.; Zhang, X.; Fu, Y.; Zhong, Z.; Wu, H.; Wei, Y.; Duan, Y. Highly efficient generation of mature megakaryocytes and functional platelets from human embryonic stem cells. Stem Cell Res. Ther. 2024, 15, 454. [Google Scholar] [CrossRef]
- Takayama, N.; Nishimura, S.; Nakamura, S.; Shimizu, T.; Ohnishi, R.; Endo, H.; Yamaguchi, T.; Otsu, M.; Nishimura, K.; Nakanishi, M.; et al. Transient activation of c-MYC expression is critical for efficient platelet generation from human induced pluripotent stem cells. J. Exp. Med. 2010, 207, 2817–2830. [Google Scholar] [CrossRef]
- Takayama, N.; Eto, K. In vitro generation of megakaryocytes and platelets from human embryonic stem cells and induced pluripotent stem cells. Methods Mol. Biol. 2012, 788, 205–217. [Google Scholar] [CrossRef]
- Kayama, A.; Eto, K. Mass production of iPSC-derived platelets toward the clinical application. Regen. Ther. 2024, 25, 213–219. [Google Scholar] [CrossRef]
- Chen, S.J.; Hashimoto, K.; Fujio, K.; Hayashi, K.; Paul, S.K.; Yuzuriha, A.; Qiu, W.Y.; Nakamura, E.; Kanashiro, M.A.; Kabata, M.; et al. A let-7 microRNA-RALB axis links the immune properties of iPSC-derived megakaryocytes with platelet producibility. Nat. Commun. 2024, 15, 2588. [Google Scholar] [CrossRef]
- Chen, L.; Liu, J.; Chen, K.; Su, Y.; Chen, Y.; Lei, Y.; Si, J.; Zhang, J.; Zhang, Z.; Zou, W.; et al. SET domain containing 2 promotes megakaryocyte polyploidization and platelet generation through methylation of alpha-tubulin. J. Thromb. Haemost. 2024, 22, 1727–1741. [Google Scholar] [CrossRef]
- Strassel, C.; Brouard, N.; Mallo, L.; Receveur, N.; Mangin, P.; Eckly, A.; Bieche, I.; Tarte, K.; Gachet, C.; Lanza, F. Aryl hydrocarbon receptor-dependent enrichment of a megakaryocytic precursor with a high potential to produce proplatelets. Blood 2016, 127, 2231–2240. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, C.; Blasczyk, R. Generation of HLA Universal Megakaryocytes and Platelets by Genetic Engineering. Front. Immunol. 2021, 12, 768458. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, N.; Kanda, J.; Nakamura, S.; Kitano, T.; Hishizawa, M.; Kondo, T.; Shimizu, S.; Shigemasa, A.; Hirai, H.; Arai, Y.; et al. iPLAT1: The first-in-human clinical trial of iPSC-derived platelets as a phase 1 autologous transfusion study. Blood 2022, 140, 2398–2402. [Google Scholar] [CrossRef]
- Ingrungruanglert, P.; Phodang, S.; Amarinthnukrowh, P.; Meehart, P.; Pratedrat, P.; Suratannon, N.; Shotelersuk, V.; Suphapeetiporn, K.; Israsena, N. Gene correction of Wiskott-Aldrich-syndrome iPS cells rescues proplatelet defects and improves platelet size. Thromb. Haemost. 2024. [Google Scholar] [CrossRef]
- Lee, B.C.; Zhou, Y.; Bresciani, E.; Ozkaya, N.; Dulau-Florea, A.; Carrington, B.; Shin, T.H.; Baena, V.; Syed, Z.A.; Hong, S.G.; et al. A RUNX1-FPDMM rhesus macaque model reproduces the human phenotype and predicts challenges to curative gene therapies. Blood 2023, 141, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Stone, A.P.; Nascimento, T.F.; Barrachina, M.N. The bone marrow niche from the inside out: How megakaryocytes are shaped by and shape hematopoiesis. Blood 2022, 139, 483–491. [Google Scholar] [CrossRef]
- Landry, P.; Plante, I.; Ouellet, D.L.; Perron, M.P.; Rousseau, G.; Provost, P. Existence of a microRNA pathway in anucleate platelets. Nat. Struct. Mol. Biol. 2009, 16, 961–966. [Google Scholar] [CrossRef]
- Huang, W.; Zhao, S.; Xu, W.; Zhang, Z.; Ding, X.; He, J.; Liang, W. Presence of intra-tumoral CD61+ megakaryocytes predicts poor prognosis in non-small cell lung cancer. Transl. Lung Cancer Res. 2019, 8, 323–331. [Google Scholar] [CrossRef]
- Ding, S.; Dong, X.; Song, X. Tumor educated platelet: The novel BioSource for cancer detection. Cancer Cell Int. 2023, 23, 91. [Google Scholar] [CrossRef]
- Campolo, F.; Sesti, F.; Feola, T.; Puliani, G.; Faggiano, A.; Tarsitano, M.G.; Tenuta, M.; Hasenmajer, V.; Ferretti, E.; Verrico, M.; et al. Platelet-derived circRNAs signature in patients with gastroenteropancreatic neuroendocrine tumors. J. Transl. Med. 2023, 21, 548. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, J.; Tian, Y.; Fu, Y.; Tian, S.; Li, Q.; Yang, J.; Zhang, L. Zwitterionic microgel preservation platform for circulating tumor cells in whole blood specimen. Nat. Commun. 2023, 14, 4958. [Google Scholar] [CrossRef]
- Denis, M.M.; Tolley, N.D.; Bunting, M.; Schwertz, H.; Jiang, H.; Lindemann, S.; Yost, C.C.; Rubner, F.J.; Albertine, K.H.; Swoboda, K.J.; et al. Escaping the nuclear confines: Signal-dependent pre-mRNA splicing in anucleate platelets. Cell 2005, 122, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Sanchez, M.; Diaz, T.; Pascual, C.; Antequera, D.; Herrero-San Martin, A.; Llamas-Velasco, S.; Villarejo-Galende, A.; Bartolome, F.; Carro, E. Platelet Proteomic Analysis Revealed Differential Pattern of Cytoskeletal- and Immune-Related Proteins at Early Stages of Alzheimer’s Disease. Mol. Neurobiol. 2018, 55, 8815–8825. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Du, M.Z.; Liao, Y.; Zu, R.; Rao, L.; Xiang, R.; Zhang, X.; Liu, S.; Zhang, P.; Leng, P.; et al. Deciphering the Significance of Platelet-Derived Chloride Ion Channel Gene (BEST3) Through Platelet-Related Subtypes Mining for Non-Small Cell Lung Cancer. J. Cell. Mol. Med. 2024, 28, e70233. [Google Scholar] [CrossRef]
- Zu, R.; Ren, H.; Yin, X.; Zhang, X.; Rao, L.; Xu, P.; Wang, D.; Li, Y.; Luo, H. The FLNA Gene in Tumour-Educated Platelets Can Be Utilised to Identify High-Risk Populations for NSCLCs. J. Cell. Mol. Med. 2025, 29, e70544. [Google Scholar] [CrossRef]
- Benariba, M.A.; Hannachi, K.; Wang, S.; Zhang, Y.; Wang, X.; Wang, L.; Zhou, N. Liposome-encapsulated lambda exonuclease-based amplification system for enhanced detection of miRNA in platelet-derived microvesicles of non-small cell lung cancer. J. Mater. Chem. B 2025, 13, 2666–2673. [Google Scholar] [CrossRef]
- Sheng, M.; Dong, Z.; Xie, Y. Identification of tumor-educated platelet biomarkers of non-small-cell lung cancer. Onco. Targets Ther. 2018, 11, 8143–8151. [Google Scholar] [CrossRef]
- Jopek, M.A.; Sieczczynski, M.; Pastuszak, K.; Lapinska-Szumczyk, S.; Jassem, J.; Zaczek, A.J.; Rondina, M.T.; Supernat, A. Impact of clinical factors on accuracy of ovarian cancer detection via platelet RNA profiling. Blood Adv. 2025, 9, 979–989. [Google Scholar] [CrossRef]
- Wei, S.; Zhou, J.; Dong, B. A novel risk model consisting of nine platelet-related gene signatures for predicting prognosis, immune features and drug sensitivity in glioma. Hereditas 2024, 161, 52. [Google Scholar] [CrossRef]
- Sol, N.; In ‘t Veld, S.; Vancura, A.; Tjerkstra, M.; Leurs, C.; Rustenburg, F.; Schellen, P.; Verschueren, H.; Post, E.; Zwaan, K.; et al. Tumor-Educated Platelet RNA for the Detection and (Pseudo)progression Monitoring of Glioblastoma. Cell Rep. Med. 2020, 1, 100101. [Google Scholar] [CrossRef]
- Rafanan, J.; Ghani, N.; Kazemeini, S.; Nadeem-Tariq, A.; Shih, R.; Vida, T.A. Modernizing Neuro-Oncology: The Impact of Imaging, Liquid Biopsies, and AI on Diagnosis and Treatment. Int. J. Mol. Sci. 2025, 26, 917. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Hu, J.; Zhao, Z.; Lu, H.; Han, Y.; Li, B.; Ouyang, Z. Development of an accurate breast cancer detection classifier based on platelet RNA. Sci. Rep. 2024, 14, 30733. [Google Scholar] [CrossRef]
- D’Ambrosi, S.; Nilsson, R.J.; Wurdinger, T. Platelets and tumor-associated RNA transfer. Blood 2021, 137, 3181–3191. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Kawai, K.; Tsuno, N.H.; Sunami, E.; Kitayama, J. Impact of preoperative thrombocytosis on the survival of patients with primary colorectal cancer. World J. Surg. 2012, 36, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Lai, Y.; Myers, R.E.; Li, B.; Hyslop, T.; London, J.; Chatterjee, D.; Palazzo, J.P.; Burkart, A.L.; Zhang, K.; et al. Preoperative platelet count associates with survival and distant metastasis in surgically resected colorectal cancer patients. J. Gastrointest. Cancer. 2013, 44, 293–304. [Google Scholar] [CrossRef]
- Karp, J.M.; Modrek, A.S.; Ezhilarasan, R.; Zhang, Z.Y.; Ding, Y.; Graciani, M.; Sahimi, A.; Silvestro, M.; Chen, T.; Li, S.; et al. Deconvolution of the tumor-educated platelet transcriptome reveals activated platelet and inflammatory cell transcript signatures. JCI Insight 2024, 9, e178719. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, W.; Wang, Z.; Wang, Y.; Li, J.; Wang, Y.; Xu, F.; Chen, Y. Mass-tagged self-assembled nanoprobe reveals the transport of PD-L1 from cancer cells to tumor-educated platelets. Anal. Chim. Acta. 2024, 1331, 343312. [Google Scholar] [CrossRef]
- Ma, L.; Guo, H.; Zhao, Y.; Liu, Z.; Wang, C.; Bu, J.; Sun, T.; Wei, J. Liquid biopsy in cancer current: Status, challenges and future prospects. Signal Transduct. Target Ther. 2024, 9, 336. [Google Scholar] [CrossRef]
- Stejskal, P.; Goodarzi, H.; Srovnal, J.; Hajduch, M.; van ‘t Veer, L.J.; Magbanua, M.J.M. Circulating tumor nucleic acids: Biology, release mechanisms, and clinical relevance. Mol. Cancer 2023, 22, 15. [Google Scholar] [CrossRef] [PubMed]
- Teruel-Montoya, R.; Kong, X.; Abraham, S.; Ma, L.; Kunapuli, S.P.; Holinstat, M.; Shaw, C.A.; McKenzie, S.E.; Edelstein, L.C.; Bray, P.F. MicroRNA expression differences in human hematopoietic cell lineages enable regulated transgene expression. PLoS ONE 2014, 9, e102259. [Google Scholar] [CrossRef]
- Wolfsberger, W.; Dietz, C.; Foster, C.; Oleksyk, T.; Washington, A.V.; Lynch, D. The First Comprehensive Description of the Platelet Single Cell Transcriptome. bioRxiv 2024. [Google Scholar] [CrossRef]
- Thibord, F.; Johnson, A.D. Sources of variability in the human platelet transcriptome. Thromb. Res. 2023, 231, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Heemskerk, J.W.M.; Swieringa, F. Combining human platelet proteomes and transcriptomes: Possibilities and challenges. Platelets 2023, 34, 2224454. [Google Scholar] [CrossRef] [PubMed]
- Moss, J.; Ben-Ami, R.; Shai, E.; Gal-Rosenberg, O.; Kalish, Y.; Klochendler, A.; Cann, G.; Glaser, B.; Arad, A.; Shemer, R.; et al. Megakaryocyte- and erythroblast-specific cell-free DNA patterns in plasma and platelets reflect thrombopoiesis and erythropoiesis levels. Nat. Commun. 2023, 14, 7542. [Google Scholar] [CrossRef]
- Best, M.G.; Sol, N.; In ‘t Veld, S.; Vancura, A.; Muller, M.; Niemeijer, A.N.; Fejes, A.V.; Tjon Kon Fat, L.A.; Huis In ‘t Veld, A.E.; Leurs, C.; et al. Swarm Intelligence-Enhanced Detection of Non-Small-Cell Lung Cancer Using Tumor-Educated Platelets. Cancer Cell 2017, 32, 238–252.e239. [Google Scholar] [CrossRef]
- Bensa, A.; Previtali, D.; Sangiorgio, A.; Boffa, A.; Salerno, M.; Filardo, G. PRP Injections for the Treatment of Knee Osteoarthritis: The Improvement Is Clinically Significant and Influenced by Platelet Concentration: A Meta-analysis of Randomized Controlled Trials. Am. J. Sports Med. 2025, 53, 745–754. [Google Scholar] [CrossRef]
- Smith, J.; Rai, V. Platelet-Rich Plasma in Diabetic Foot Ulcer Healing: Contemplating the Facts. Int. J. Mol. Sci. 2024, 25, 12864. [Google Scholar] [CrossRef]
- Corsini, A.; Perticarini, L.; Palermi, S.; Bettinsoli, P.; Marchini, A. Re-Evaluating Platelet-Rich Plasma Dosing Strategies in Sports Medicine: The Role of the “10 Billion Platelet Dose” in Optimizing Therapeutic Outcomes-A Narrative Review. J. Clin. Med. 2025, 14, 2714. [Google Scholar] [CrossRef]
- Sharun, K.; Banu, S.A.; El-Husseiny, H.M.; Abualigah, L.; Pawde, A.M.; Dhama, K.; Amarpal. Exploring the applications of platelet-rich plasma in tissue engineering and regenerative medicine: Evidence from goat and sheep experimental research. Connect. Tissue Res. 2024, 65, 364–382. [Google Scholar] [CrossRef] [PubMed]
- Shome, S.; Kodieswaran, M.; Dadheech, R.; Chevella, M.; Sensharma, S.; Awasthi, S.; Bandyopadhyay, A.; Mandal, B.B. Recent advances in platelet-rich plasma and its derivatives: Therapeutic agents for tissue engineering and regenerative medicine. Prog. Biomed. Eng. 2024, 6, 012004. [Google Scholar] [CrossRef]
- Kawase, T.; Mourao, C.F.; Fujioka-Kobayashi, M.; Mastrogiacomo, M. Editorial: Recent advances in platelet-concentrate therapy in regenerative medicine. Front. Bioeng. Biotechnol. 2024, 12, 1518095. [Google Scholar] [CrossRef] [PubMed]
- Khandan-Nasab, N.; Torkamanzadeh, B.; Abbasi, B.; Mohajeri, T.; Oskuee, R.K.; Sahebkar, A. Application of Platelet-Rich Plasma-Based Scaffolds in Soft and Hard Tissue Regeneration. Tissue Eng. Part B Rev. 2025. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, P.; Xue, X.; Zhang, Z.; Wang, L.; Jiang, Y.; Zhang, C.; Zhou, H.; Lv, S.; Shen, W.; et al. The role of platelet-rich plasma in biomedicine: A comprehensive overview. iScience 2025, 28, 111705. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.; Lejmi, E.; Fontana, P.; Morel, P.; Gonelle-Gispert, C.; Buhler, L. A focus on the role of platelets in liver regeneration: Do platelet-endothelial cell interactions initiate the regenerative process? J. Hepatol. 2015, 63, 1263–1271. [Google Scholar] [CrossRef]
- Meglei, A.Y.; Nedorubova, I.A.; Basina, V.P.; Chernomyrdina, V.O.; Nedorubov, A.A.; Kuznetsova, V.S.; Vasilyev, A.V.; Kutsev, S.I.; Goldshtein, D.V.; Bukharova, T.B. Collagen-Platelet-Rich Plasma Mixed Hydrogels as a pBMP2 Delivery System for Bone Defect Regeneration. Biomedicines 2024, 12, 2461. [Google Scholar] [CrossRef]
- Lourenco, E.S.; Rocha, N.R.S.; de Lima Barbosa, R.; Mello-Machado, R.C.; de Souza Lima, V.H.; Leite, P.E.C.; Pereira, M.R.; Casado, P.L.; Kawase, T.; Mourao, C.F.; et al. Investigating the Biological Efficacy of Albumin-Enriched Platelet-Rich Fibrin (Alb-PRF): A Study on Cytokine Dynamics and Osteoblast Behavior. Int. J. Mol. Sci. 2024, 25, 11531. [Google Scholar] [CrossRef]
- Qiu, D.; Wang, L.; Wang, L.; Dong, Y. Human platelet lysate: A potential therapeutic for intracerebral hemorrhage. Front. Neurosci. 2024, 18, 1517601. [Google Scholar] [CrossRef]
- Nebie, O.; Carvalho, K.; Barro, L.; Delila, L.; Faivre, E.; Renn, T.Y.; Chou, M.L.; Wu, Y.W.; Nyam-Erdene, A.; Chou, S.Y.; et al. Human platelet lysate biotherapy for traumatic brain injury: Preclinical assessment. Brain 2021, 144, 3142–3158. [Google Scholar] [CrossRef]
- Fan, Z.; Gan, Y.; Hu, Y. The potential utilization of platelet-derived extracellular vesicles in clinical treatment. Platelets 2024, 35, 2397592. [Google Scholar] [CrossRef] [PubMed]
- Lou, C.; Cai, X. The emerging roles of platelet-derived extracellular vesicles in disease. Ann. Med. 2025, 57, 2499029. [Google Scholar] [CrossRef]
- Vasina, E.M.; Cauwenberghs, S.; Staudt, M.; Feijge, M.A.; Weber, C.; Koenen, R.R.; Heemskerk, J.W. Aging- and activation-induced platelet microparticles suppress apoptosis in monocytic cells and differentially signal to proinflammatory mediator release. Am. J. Blood Res. 2013, 3, 107–123. [Google Scholar]
- Miyazawa, B.; Trivedi, A.; Togarrati, P.P.; Potter, D.; Baimukanova, G.; Vivona, L.; Lin, M.; Lopez, E.; Callcut, R.; Srivastava, A.K.; et al. Regulation of endothelial cell permeability by platelet-derived extracellular vesicles. J. Trauma Acute Care Surg. 2019, 86, 931–942. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Meng, X.; Cao, F.; Wang, J.; Yang, M. Exosomes derived from platelet-rich plasma promote diabetic wound healing via the JAK2/STAT3 pathway. iScience 2023, 26, 108236. [Google Scholar] [CrossRef]
- Esmaeilzadeh, A.; Yeganeh, P.M.; Nazari, M.; Esmaeilzadeh, K. Platelet-derived extracellular vesicles: A new-generation nanostructured tool for chronic wound healing. Nanomedicine 2024, 19, 915–941. [Google Scholar] [CrossRef] [PubMed]
- Spakova, T.; Janockova, J.; Rosocha, J. Characterization and Therapeutic Use of Extracellular Vesicles Derived from Platelets. Int. J. Mol. Sci. 2021, 22, 9701. [Google Scholar] [CrossRef]
- Das, S.; Thompson, W.; Papoutsakis, E.T. Engineered and hybrid human megakaryocytic extracellular vesicles for targeted non-viral cargo delivery to hematopoietic (blood) stem and progenitor cells. Front. Bioeng. Biotechnol. 2024, 12, 1435228. [Google Scholar] [CrossRef]
- Yu, H.; Ben-Akiva, E.; Meyer, R.A.; Green, J.J. Biomimetic Anisotropic-Functionalized Platelet-Membrane-Coated Polymeric Particles for Targeted Drug Delivery to Human Breast Cancer Cells. ACS Appl. Mater. Interfaces. 2025, 17, 351–362. [Google Scholar] [CrossRef]
- Xie, L.; Gan, F.; Hu, Y.; Zheng, Y.; Lan, J.; Liu, Y.; Zhou, X.; Zheng, J.; Zhou, X.; Lou, J. From Blood to Therapy: The Revolutionary Application of Platelets in Cancer-Targeted Drug Delivery. J. Funct. Biomater. 2025, 16, 15. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, T.; Lu, W.; Lin, Y.; Zhou, M.; Cai, X. Hybrid Cell Membrane-Engineered Nanocarrier for Triple-Action Strategy to Address Pseudomonas aeruginosa Infection. Adv. Sci. 2025, 12, e2411261. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Di, X.; Li, F.; Rong, Z.; Lian, W.; Li, Z.; Chen, T.; Wang, W.; Zhong, Q.; Sun, G.; et al. Platelet Membrane-Coated HGF-PLGA Nanoparticles Promote Therapeutic Angiogenesis and Tissue Perfusion Recovery in Ischemic Hindlimbs. ACS Appl. Bio. Mater. 2025, 8, 399–409. [Google Scholar] [CrossRef]
- Pan, Y.; Hu, D.; Chen, H.; Wang, M.; Dong, X.; Wan, G.; Tang, H.; Wang, H.; Chen, H. PLGA nanocarriers biomimetic of platelet membranes and their interactions with the placental barrier. Int. J. Pharm. 2025, 671, 125225. [Google Scholar] [CrossRef]
- Li, Y.; Wu, C.; Yang, R.; Tang, J.; Li, Z.; Yi, X.; Fan, Z. Application and Development of Cell Membrane Functionalized Biomimetic Nanoparticles in the Treatment of Acute Ischemic Stroke. Int. J. Mol. Sci. 2024, 25, 8539. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Gul, A.; Zhao, H.; Qian, R.; Fang, L.; Huang, C.; Xi, L.; Wang, L.; Cheang, U.K. Hybrid Membrane Biomimetic Photothermal Nanorobots for Enhanced Chemodynamic-Chemotherapy-Immunotherapy. ACS Appl. Mater. Interfaces 2025, 17, 5784–5798. [Google Scholar] [CrossRef]
- Safdar, A.; Wang, P.; Muhaymin, A.; Nie, G.; Li, S. From bench to bedside: Platelet biomimetic nanoparticles as a promising carriers for personalized drug delivery. J. Control. Release 2024, 373, 128–144. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, Z.; Zhang, F.; Sun, L.; Luan, H.; Fang, Z.; Dedrick, J.L.; Zhang, Y.; Tang, C.; Zhu, A.; et al. Inhalable biohybrid microrobots: A non-invasive approach for lung treatment. Nat. Commun. 2025, 16, 666. [Google Scholar] [CrossRef]
- Vidallon, M.L.P.; Moon, M.J.; Liu, H.; Song, Y.; Crawford, S.; Teo, B.M.; McFadyen, J.D.; Bishop, A.I.; Tabor, R.F.; Peter, K.; et al. Engineering Hyperechogenic Colloids with Clot-Targeting Capabilities from Platelet-Derived Membranes. ACS Appl. Mater. Interfaces 2024, 16, 55142–55154. [Google Scholar] [CrossRef]
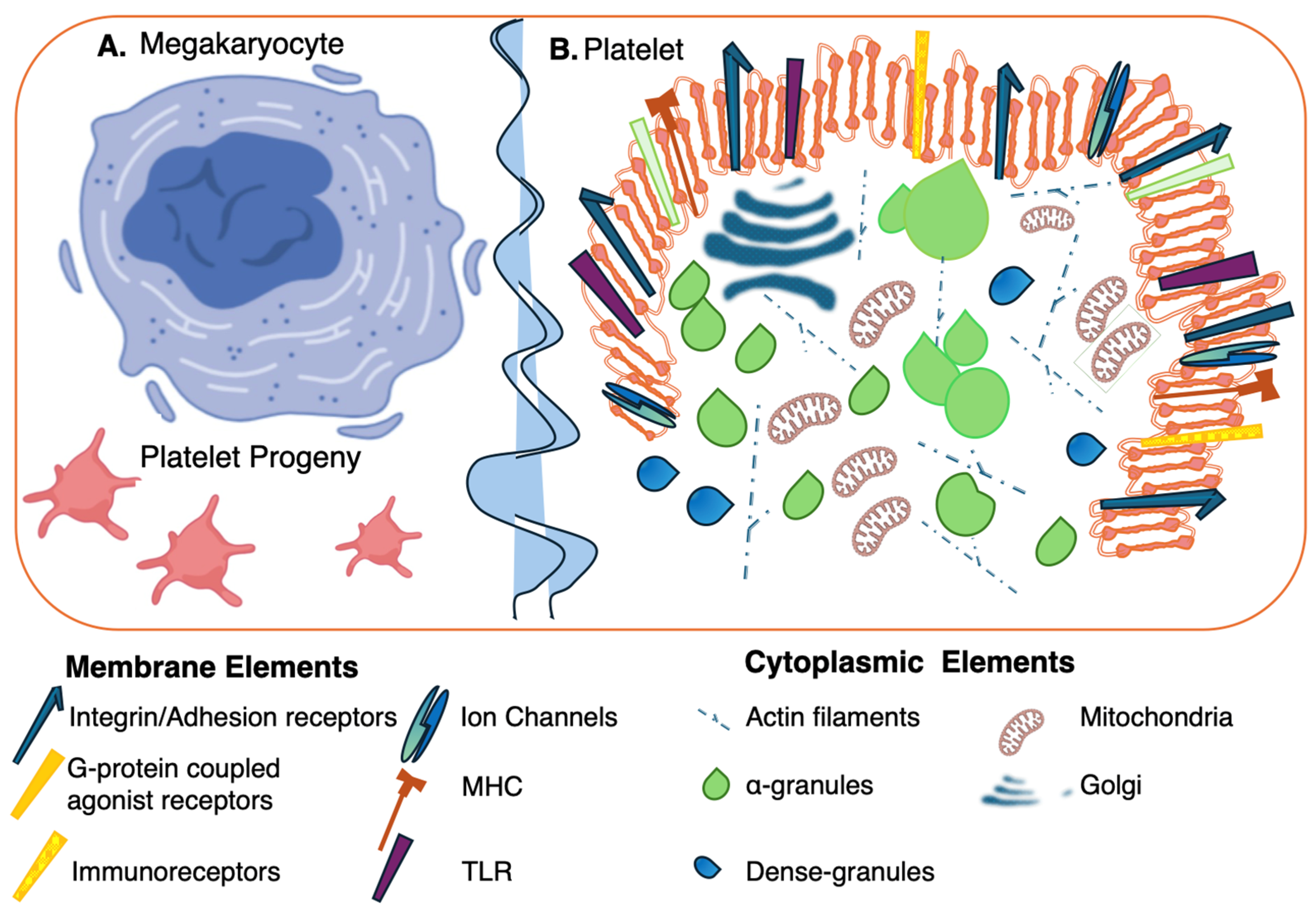
| Molecule | Function | Platelet Connection | Disease Relevance | References |
|---|---|---|---|---|
| P-selectin (CD62P) | Adhesion molecule that binds leukocyte PSGL-1 and possibly Mac-1 | Expressed on activated platelets; stored in α-granules; mediates platelet–leukocyte co-aggregates | Seen in SLE, rheumatoid arthritis, and antiphospholipid syndrome; marker of platelet activation in infections | [21] |
| CD40/CD40L | Immune co-stimulatory dyad; regulates inflammatory and humoral immune responses | Expressed and released by activated platelets; source of sCD40L; promotes endothelial activation and leukocyte recruitment | Involved in bacterial/viral clearance; contributes to vascular and neuronal inflammation in chronic inflammatory diseases | [15,22,23,24,25,26] |
| MHC Class I | Antigen presentation to CD8+ T cells | Platelets and megakaryocytes (MKs) present antigens via MHC I; link to adaptive immune activation | Triggers antimicrobial responses and interferon production | [27] |
| MHC Class II, CD11c | Antigen presentation to CD4+ T cells | More highly expressed in lung-resident MKs, suggesting APC-like phenotype | Suggests enhanced immune surveillance role for lung MKs | [28] |
| CFTR | Ion channel involved in epithelial fluid transport | Hyperactivation shown by CFTR-deficient platelets; impact on inflammatory lung response | Aggravates inflammation in CF; potential marker and modulator target in CFTR therapy | [29] |
| TRPC6 | Cation channel implicated in calcium signaling and platelet activation | Platelet activation and lung injury reduced by inhibition in CF models | Target for reducing CF-related lung damage and platelet hyperactivation | [29] |
| Thrombopoietin receptor (mpl) | Regulator of platelet production | Impaired platelet regulation and increased susceptibility to lung injury in mpl-/- mice | Impaired defense in Pseudomonas infection; relevance in infection control and platelet homeostasis | [30] |
| β-defensins | Antimicrobial peptides that disrupt microbial membranes | β-defensin-1 and β-defensin-3 expressed and released by platelets, contributing to antimicrobial activity | Role in innate immunity and defense against pathogens | [31,32,33] |
| CXCL4 (PF4) | Chemokine that attracts monocytes, modulates T cell function, and can have antimicrobial properties | Abundantly stored in α-granules; released upon activation | Implicated in inflammatory diseases, thrombosis, and infection-related immune responses | [34,35] |
| CCL5 (RANTES) | Recruits leukocytes such as T cells and monocytes to sites of inflammation | Released by activated platelets; synergizes with CXCL4 | Elevated in autoimmune diseases, cardiovascular disease, and infections | [36] |
| CXCL12 (SDF-1α) | Attracts hematopoietic and immune cells; supports vascular repair | Released from platelet granules; promotes leukocyte recruitment | Key in inflammation, cancer metastasis, and vascular diseases | [37] |
| IL-1β | Proinflammatory cytokine that initiates and amplifies inflammatory responses | Released by activated platelets and MKs | Found in platelet-driven inflammation in cardiovascular and neuroinflammatory diseases, and Kawasaki disease | [38,39,40] |
| Serotonin (5-HT) | Monoamine neurotransmitter with vasoconstrictive and proinflammatory properties | Stored in dense granules; released during platelet activation | Contributes to vascular tone, platelet aggregation, and inflammation in pulmonary and cardiovascular diseases | [41,42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasirer-Friede, A. Novel Roles and Therapeutic Approaches Linking Platelets and Megakaryocytes to Non-Hemostatic and Thrombotic Disease. Int. J. Transl. Med. 2025, 5, 25. https://doi.org/10.3390/ijtm5030025
Kasirer-Friede A. Novel Roles and Therapeutic Approaches Linking Platelets and Megakaryocytes to Non-Hemostatic and Thrombotic Disease. International Journal of Translational Medicine. 2025; 5(3):25. https://doi.org/10.3390/ijtm5030025
Chicago/Turabian StyleKasirer-Friede, Ana. 2025. "Novel Roles and Therapeutic Approaches Linking Platelets and Megakaryocytes to Non-Hemostatic and Thrombotic Disease" International Journal of Translational Medicine 5, no. 3: 25. https://doi.org/10.3390/ijtm5030025
APA StyleKasirer-Friede, A. (2025). Novel Roles and Therapeutic Approaches Linking Platelets and Megakaryocytes to Non-Hemostatic and Thrombotic Disease. International Journal of Translational Medicine, 5(3), 25. https://doi.org/10.3390/ijtm5030025






