Abstract
Diagnosis and management of proliferative diabetic retinopathy are reliant upon retinal imaging. A systematic literature review of non-invasive imaging to guide diagnosis and treatment of proliferative diabetic retinopathy was performed. There is a trend of moving away from invasive (e.g., fundus fluorescein angiography) to non-invasive (e.g., wide-field optical coherence tomography (OCT), OCT angiography and colour fundus photography) imaging modalities to allow for more objective assessments that can be readily repeated in a time-efficient manner without compromising patient safety. Such quantitative assessments generating large amounts of data could benefit from artificial intelligence approaches to aid clinical decision making. These non-invasive imaging modalities continue to improve both in terms of the quality of image acquisition and progress in image interpretation. It is important that newer non-invasive imaging modalities are appropriately validated in large-scale prospective observational studies or randomised clinical trials.
1. Introduction
Diabetic retinopathy (DR) is a leading cause of vision impairment in people aged 20–74 years [1,2]. DR is a disease of global significance affecting populations in low, middle, and high-income countries. With the projected proportion of people living with diabetes expected to rise to 700 million by 2045, the global burden of diabetic-related eye disease will increase correspondingly, placing significant demand on healthcare systems [3].
The term DR describes microvascular and neural abnormalities seen in diabetes-affected eyes which may progress to proliferative diabetic retinopathy (PDR), characterised by the presence of neovascularization on the optic disc (NVD) or elsewhere (NVE) [4,5,6]. The new blood vessels that develop in PDR can be described by the histopathologic definition where there is a breach of the internal limiting membrane (ILM) and growth of the new vessels into the posterior hyaloid [7]. This is distinct from one of the features of severe non-proliferative DR (NPDR), where intraretinal microvascular abnormalities (IRMA) do not extend into the vitreous [8]. Important causes of vision loss in eyes with PDR include vitreous haemorrhage and tractional retinal detachment [9,10].
The disruption of the blood–retinal barrier (BRB) in DR states leads to an accumulation of blood components in the extravascular retinal parenchyma [11]. The grading of DR severity is based on the identification of features seen in the disease state which are hypothesised to result from breakdown in the BRB and underlying capillary nonperfusion or tissue hypoxia that drives an ischemic pathologic state which affects the structure and function of the retina [8,11,12]. Pathological features seen in DR include microaneurysms, retinal dot and blot haemorrhages, cotton wool spots, hard exudates, venous beading, IRMAs, attenuation or drop-out of retinal capillaries, and neovascularization at the vitreoretinal interface associated with tractional retinal detachment, pre-retinal haemorrhage, and vitreous haemorrhage (VH) (Table 1) [13,14,15].

Table 1.
Summary of findings in diabetic retinopathy by non-invasive imaging.
Classification systems have been developed which define features seen in DR to provide a standard for clinically staging the disease [13,16]. The Airlie House classification of DR stages based on colour fundus photography was published in 1968 and has since evolved into the modified Airlie House classification [13]. This system has been widely adopted into both clinical practice and are used in large multi-centre clinical research trials [15,17,18,19,20].
These earlier studies which helped to define grading systems and guide standard therapies at the time preceded modern therapeutic options available today [13,14,20,21]. Similarly, the imaging technologies at the time of the seminal studies in DR have now been proceeded by newer advancements in non-invasive imaging modalities, such as Fourier domain optical coherence tomography (FD-OCT) and ultrawide field (UWF) colour fundus imaging, each with advanced angiography capabilities. These technologies offer greater imaging efficiencies, processing abilities, high resolution scanning, and allow for follow-up with accurate sequential image alignment.
This review will systematically present new developments in current and evolving non-invasive imaging modalities used to diagnose PDR. Advances in non-invasive imaging technologies are also described in the context of disease response to modern therapies for PDR including to anti-vascular endothelial growth factor (anti VEGF), pan-retinal photocoagulation (PRP), and pars plana vitrectomy (PPV).
2. Methods: Systematic Literature Search
2.1. Selection of Studies
A systematic literature search was conducted on 1 June 2021 using Ovid Medline, Embase, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials and PubMed databases to identify potentially eligible studies. The literature search included papers published between 1947–1 June 2021. Further references were identified by manually searching included articles by citation tracking and consulting experts in the field. The following multipurpose search terms were used which looked in the Title, Original Title, Abstract, Subject Heading, Floating Sub-heading, Keywords, Name of Substance, Supplementary Concept Words, Synonyms and Unique Identifier fields: ‘proliferative diabetic retinopathy’ OR ‘new vessels elsewhere’ OR ‘new vessels at the disc’ OR ‘PDR’ OR ‘NVE’ OR ‘NVD’; AND ‘diagnosis’ OR ‘management’ OR ‘therapies’ OR ‘therapy’ OR ‘treatment*’; AND ‘color fundus imaging’ OR ‘colour fundus imaging’ OR ‘optical coherence tomography’ OR ‘OCT’ OR ‘optical coherence tomography angiography’ OR ‘OCTA’.
The preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines were followed in reporting. The modified PRISMA flow chart of the literature search and selection of studies for systematic review is reported in Figure 1.
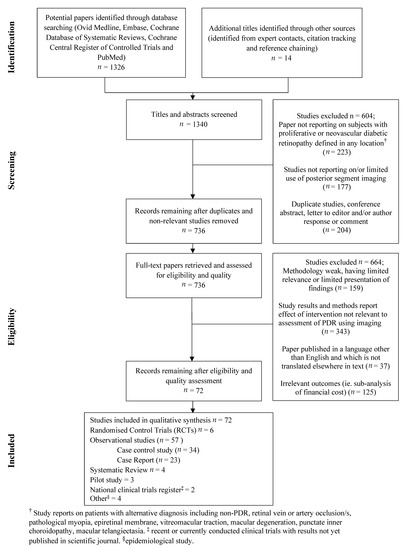
Figure 1.
Modified PRISMA flow diagram of systematic literature search.
2.2. Eligibility Criteria and Study Content
Duplicated or non-relevant studies were excluded. No date or language restrictions were applied. One thousand three hundred and forty titles and abstracts were retrieved and assessed against pre-determined inclusion and exclusion criteria. Paper abstracts were reviewed by a single reviewer for exclusion criteria. Full publications were retrieved for all citations accepted at the initial screening. Full publications were then re-screened and reviewed using pre-piloted eligibility and exclusion criteria (Figure 1). Differences were discussed and agreed with the input of an adjudicator where necessary.
Pilot studies, national clinical trials methods (where full-text publications of results were not yet available) were eligible for selection. Conference abstracts without full publication of results were excluded. Studies were excluded if they did not report on subjects with proliferative or neovascular diabetic retinopathy defined in any location, or if the study did not report on posterior segment imaging tools or intervention relevant to the assessment of PDR. Anterior segment complications, such as iris rubeosis, were not included, and only posterior segment imaging modalities were included in this review.
3. Invasive Fundus Imaging Modalities to Guide Diagnosis of PDR
3.1. Fundus Fluorescein Angiography (FFA)
FFA is an invasive procedure whereby fluorescein dye is injected in a vein such as the antecubital fossa and then retinal perfusion and leakage associated with disease states affecting the microvasculature can be assessed with a series of fundus photographs [22]. FFA is the gold standard imaging technique for the assessment of PDR, characterised by fluorescein leakage from neovascular tissue, increasing in intensity and area over time. FFA can also identify areas of peripheral retinal and macular non-perfusion (Figure 2).
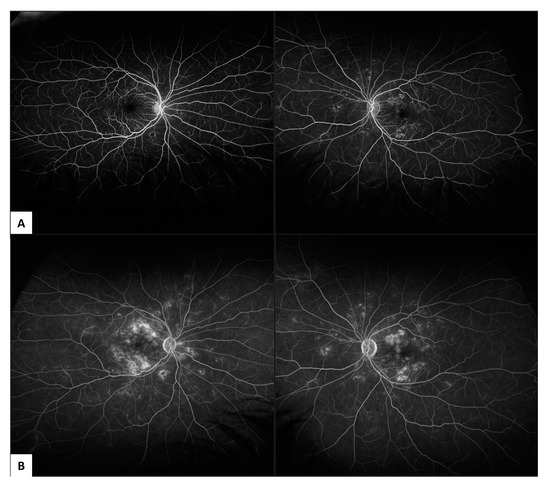
Figure 2.
Ultrawide field (UWF) fluorescein fundus angiography (FFA) in non-proliferative diabetic retinopathy (NPDR) captured on 200° Optos (Optos California, Optos PLC, Dunfermline, United Kingdom): (A) showing early phase leakage at 1-minute following fluorescein dye injection; (B) showing late phase leakage at 6 minutes following fluorescein dye injection.
Limitations of FFA imaging are that patients require intravenous injection of an exogenous dye. This may be contraindicated in some diseases with end-stage microvascular disease including diabetic nephropathy. Some patients may develop life threatening severe allergy. The FFA procedure takes longer to perform and may be less well-tolerated by patients when compared to other non-invasive imaging procedures, limiting the number of times the test can be repeated.
Furthermore, FFA has a relatively low spatial resolution compared with newer OCT-A machines [23]. Importantly, FFA is unable to separately distinguish superficial from deep capillary networks due to its limits of two-dimensions, and retinal details become progressively obscured over the time-course of FFA imaging due to extravasation of the dye [24]. Whilst FFA may better identify microaneurysms, some of which may be missed by OCT-A in some studies, it is unable to identify the histological layer of positive findings, nor reliably follow-up identified areas of retina in serially aligned scans [23].
3.2. Indocyanine Green Angiography (ICG-A)
ICG-A is an invasive test similar to FFA with a different dye. Indocyanine green (ICG) dye has been histologically localised to both the choroidal intravascular space as well as extravasating into the choroidal stroma and accumulating within the RPE [25]. ICG therefore passes through local blood and pigmentary obscurations, adding to its utility in imaging the deep retinal and choroidal layers.
ICG-A can identify hypo-fluorescence in zones which represent a filling delay or defect at the level of the choriocapillaris which have been observed in DR [26]. Retinal avascular zones are more likely to be identified by FFA compared to ICG-A. Studies in DR have shown no leakage of ICG on angiographic studies in eyes with known diffuse diabetic macular oedema [27].
Limitations of ICG-A are similar to FFA; the principal of these being that repeated imaging is limited by the potential morbidity from an invasive dye test. Additionally, images are only able to be captured in two-dimensions. ICG-A is not routinely recommended for the diagnosis or management of PDR.
3.3. Ophthalmic B-Scan Ultrasonography
Ophthalmic B-scan ultrasonography has a role in imaging in the context of DR where VH obscures the direct view of the retina [28]. B-scan ultrasound can demonstrate whether a tractional retinal detachment accompanies other retinal pathology such as VH, a thickened posterior hyaloid, posterior vitreous detachment, or sub-retinal haemorrhage. Although examination with a B-scan may be considered less invasive by comparison to FFA and ICG angiography, it does require contact and manipulation of the ultrasound probe position with the patients closed eye making it more invasive than other non-touch, non-invasive imaging modalities.
4. Non-Invasive Fundus Imaging Modalities to Guide Diagnosis and Treatment of PDR
4.1. Colour Fundus Imaging
Seminal studies from the 1980s that established a gold-standard evidence base to inform management of DR include the Diabetic Retinopathy Study (DRS) [20], the Diabetic Retinopathy Vitrectomy Study (DRVS) [14,29], and the Early Treatment Diabetic Retinopathy Study (ETDRS) [21]. These early studies used 30-degree fundus cameras to image the posterior pole of the retina. The ETDRS defined seven-standard, central 30-degree photographic fields for viewing which was used for grading the severity of DR.
Pathologic features of DR which are captured by colour fundus imaging include haemorrhages, microaneurysms, IRMA, venous beading, cotton wool spots, hard exudates, drusen, retinal thickening, papillary swelling, new vessels on the disc (Figure 3) and NVE, pre-retinal and subretinal haemorrhage, vitreous haemorrhage, and tractional retinal detachment (Table 1). The extended modified Airlie House DR classification grading scale characterises the location and extent of these specific retinal lesions, which have been shown to be highly predictive of disease severity and the risk of DR progression over time. It is the accepted gold standard for grading which is used widely in research settings including large multi-centre clinical trials. Aspects of the classification system also form a basis for estimated risk of progression of DR to guide follow-up and treatment decisions in clinical practice.
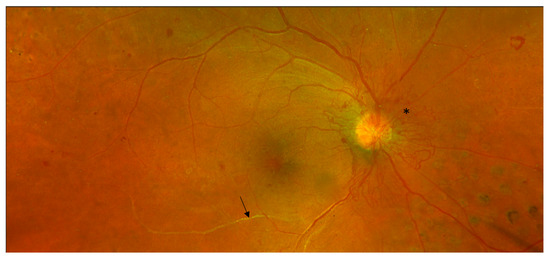
Figure 3.
Non-invasive Optos (Optos California, Optos PLC, Dunfermline, UK) colour fundus photograph of proliferative diabetic retinopathy. Sheathed retinal arteriole inferior to macula (arrow). Neovascularisation of the disc (*). Scattered intraretinal haemorrhages. Previous grid retinal laser nasal to disc (right eye).
The modified Airlie House classification uses non-simultaneous stereoscopic pairs of seven-standard photographic fields plus additional photographs to better define the grading scale for hard exudates, soft exudates, arteriovenous nicking, retinal elevation, and vitreous haemorrhage. The modified Airlie House classification also separates arterial abnormalities previously pooled as venous characteristics. The seven-standard ETDRS photographic fields are; field one centred on the optic disc, field two centred on the macula, field three temporal to the macula, fields four to seven are tangential to horizontal lines passing through the upper and lower poles of the disc and to a vertical line passing through the disc centre. Besides the seven-standard fields, an optional eighth field was added in the ETDRS for follow-up to document new vessels, fibrous proliferations, pre-retinal haemorrhage, or vitreous haemorrhage outside of the standard defined seven fields [13].
Since the advent of commercially available UWF fundus imaging systems, numerous studies have compared images captured from UWF to the stereoscopic seven-standard ETDRS fields. The level of agreeability between the imaging fields for grading DR severity has been demonstrated to be high, but UWF images have also been demonstrated to provide additional prognostic value with regard to the risks of DR onset, progression and outcomes compared to areas only visualised by seven-standard field ETDRS photography.
Newer imaging modalities, such as models of the Optos fundus camera (Optos plc, Dunfermline, Scotland, UK), enable mydriatic non-simultaneous stereoscopic 200-degree UWF images of the retina [22]. High-resolution UWF scanning laser ophthalmoscopes enable approximately 80–85% of the retinal surface to be captured in a single image. The benefits of this newer technology pertain to added efficiencies with reducing total imaging time, the number of images required, greater ease of image evaluation, reduced rates of poor-resolution images and a reduction in the areas of retina missed by imaging as result of operator error. Such benefits and commercial availability of these UWF imaging systems have meant that they are now widespread in clinical practice (Figure 4) and are used by newer clinical trials.
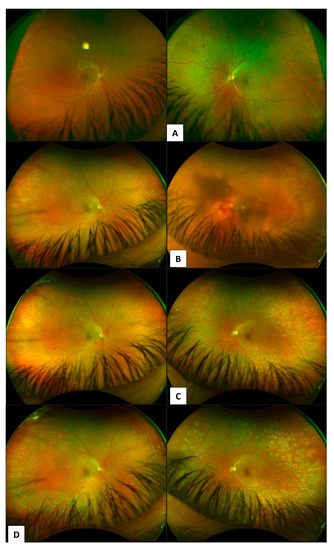
Figure 4.
Case series of patient showing progression of proliferative diabetic retinopathy. Diagnosis (A) to 5 years follow-up patient re-presents with vitreous haemorrhage (B). (C) 1-month post vitrectomy and pan-retinal endolaser photocoagulation for management of VH due to PDR. (D) right eye showing grid laser 360 PRP and left eye showing PRP delivered with endolaser during vitrectomy.
Limitations of technology at the time when the original ETDRS criteria was developed meant that the far retinal periphery was not able to be systematically captured by imaging systems. Over the past thirty years since the ETDRS, numerous retinal imaging studies and clinical observations have demonstrated the significance of PPL as pathologic features of DR which are not evaluated by seven-standard field ETDRS photography. A lesion is considered to be predominantly peripheral if more than half of the area of the lesion being graded is in the peripheral retinal field compared to the modified seven-standard ETDRS photographic fields. A 4-year follow-up study by Silva et al. [22] found that eyes which had no PDR at baseline but did have PPLs on UWF imaging had a 3.2 increased risk of DR progression compared to eyes without PPLs (p = 0.005). Additionally, eyes with PPL were found to have a 4.7-fold increased risk for progression to PDR over 4 years even after correcting for diabetes duration and HbA1c levels [22]. This finding is supported by other studies similarly using UWF imaging [7,15,30].
Intraoperative visualization of areas of traction and source of PDR can assist vitreo-retinal surgery (Figure 5 and Figure 6).
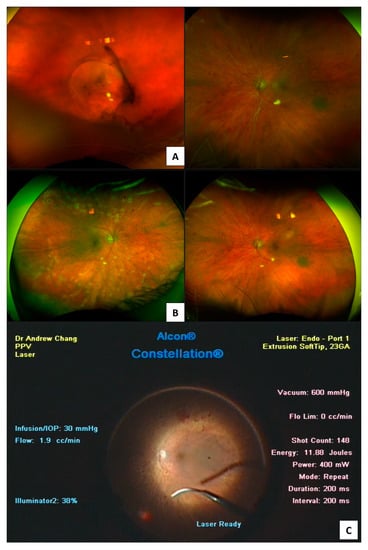
Figure 5.
Proliferative diabetic retinopathy presents in (A) with vitreous haemorrhage in the right eye. Post vitrectomy Optos (Optos California, Optos PLC, Dunfermline, UK) colour fundus photograph 200° (B) showing targeted endolaser to areas of neovascularisation during vitrectomy (C).
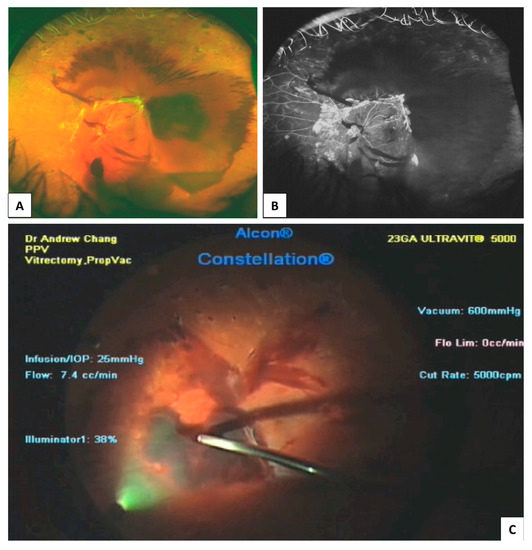
Figure 6.
Traction retinal detachment and vitreous haemorrhage in proliferative diabetic retinopathy. Colour fundus photograph (A); Fluorescein angiography (B); Intraoperative video stills from recording during vitrectomy surgery showing pre-retinal, posterior hyaloid haemorrhage (C).
4.2. Optical Coherence Tomography (OCT)
OCT is a non-invasive imaging technique with three-dimensional volume information which provides micron level high-resolution cross-sectional retinal images. Segmentation of OCT allows for clearer differentiation of retinal layers, however earlier OCT used a shorter wavelength of 800 nm, which had limited penetration beyond the RPE to the choriocapillaris. Newer models have longer wavelength capabilities to 1050 nm which allow better visualization of the choroidal structures [3]. OCT is now the current clinical standard for observing the structural changes seen in diabetic macular oedema (DMO) [3,31].
Commercially available spectral-domain (SD-OCT) machines; Heidelberg Spectralis (Heidelberg Engineering, Heidelberg, Germany), Zeiss Cirrus (Carl Zeiss Meditec, Dublin, CA, USA), swept-source OCT (SS-OCT, Topcon, Tokyo, Japan), and Optovue RTVue (Optovue Inc., Fremont, CA, USA) machines, and one time-domain OCT Zeiss Stratus (Carl Zeiss Meditec) are used by clinical studies described in the body of this review [32].
With OCT technology it is possible to non-invasively evaluate cross-sectional images of the posterior vitreous and retinal layers to assess the ILM and posterior hyaloid for breaches in keeping with diagnostic criterion for NVE in the PDR stage of disease [24]. A summary of non-invasive imaging features to help diagnose PDR is included in Table 2. Assessment of the vitreoretinal interface plays an important role in management decisions for DR, both for prognostication and timeliness for interventional therapies. In assessment of eyes with contraction of fibrovascular tissue and tractional retinal detachment for consideration of vitrectomy, imaging of the extent of the traction in particular macular involvement is essential [33]. Macular threatening traction or direct involvement is an indication for vitrectomy and membrane segmentation and delamination to relieve the traction to restore the macular anatomy [34]. Inner retinal traction and disruption of neural layers is well imaged with OCT [35,36].

Table 2.
Studies reporting diagnostic features of proliferative diabetic retinopathy by non-invasive imaging modalities, published within the past 5 years (2016–1 June 2021).
Techniques used for imaging an extended field with earlier OCT technology have been described by Mishra et al. [37] in 2017, where the authors used a wide-field 12 mm × 12 mm radial swept source OCT together with a convex +90 diopter double aspheric noncontact slit-lamp lens placed between the eye and the OCT machine to theoretically expand the imaging field by increasing the imaging light incidence angle. This technique however has been shown to have a higher incidence of rim artefacts and reflection on OCT images, and, as each pixel is magnified without the enhanced hardware capabilities of the OCT system, there is some loss of retinal architectural detail and resolution [37]. Whilst this technique provides a panoramic OCT view of a large segment of fundus to define the vitreoretinal relationship at one time, there are compromises in resolution and image quality which may be further degraded by poor media or lens clarity, poor patient fixation and smaller pupil size. OCT and OCTA technologies have been further enhanced since the years of recruitment in these studies (2016–2017) [8,37] with faster scan rates, wider fields of view, denser scan volumes, pseudocolour imaging acquisition modules and enhanced depth range of OCT imaging now available. Summaries of published non-invasive imaging changes after panretinal laser photocoagulation (Table 3) and anti-VEGF therapy (Table 4) are included.

Table 3.
Studies reporting response to pan-retinal photocoagulation treatment in proliferative diabetic retinopathy by non-invasive imaging modalities, published within the past 5 years (2016–1 June 2021).

Table 4.
Studies reporting response to intravitreal anti-VEGF therapy in proliferative diabetic retinopathy by non-invasive imaging modalities, published within the past 5 years (2016–1 June 2021).
4.3. OCTA
The OCT-A uses multiple, sequential OCT B-scans in cross-section to produce reconstructed images of the network of blood vessels enabling three different vascular layers to be visualised: the superficial capillary plexus, the deep capillary plexus, and the choriocapillaris. OCT-A allows quantitative, serial assessments of areas of capillary non-perfusion, including high-resolution images which are aligned to allow ease of monitoring disease progression at specific identified retinal areas. This feature makes OCT-A better suited to follow-up for DR with less risk to the patient when compared to invasive imaging modalities [23]. There is currently a lack of clinical studies which examine the role of OCT-A in DR peripheral lesions. Most recent (2020) studies in PDR patients using OCT-A principally examine the posterior pole [12,38].
A classification system distinguishing NVE by topographic distribution, origin, and morphologic features has been described by Pan et al. [8,64]. This system relies on a combination of FA and OCTA to describe NVE as either originating from the venous side (Type 1) with tethering to the posterior hyaloid surface, from capillary networks (Type 2), or from intraretinal microvascular abnormalities (type 3) where the posterior hyaloid was still attached to the retina, as opposed to vitreoschisis or partial PVD in Type 1 NVE. The clinical implications of this classification system are that the three types of NVE may have varying topographical distribution features, and the authors have described distinguishable risk for complications including vitreous haemorrhage and traction retinal detachment, and differing responsiveness to treatment observed in a recently published study [64]. Limitations of the study [8] describing this classification of NVE according to OCTA features include the cross-sectional study design with a small cohort (35 eyes), and the reliance on only a small field of OCTA view (6 mm × 6 mm); therefore, NVE in the periphery were not included.
Recently, OCT-A has been suggested to more accurately measure areas of capillary dropout across the total, superficial, and deep capillary plexus in regions of ischaemia compared to images obtained by FFA [24].
Wide-field swept-source OCT-A has been used to provide information on microvascular perfusion in DR and may have a role in detecting peripheral capillary dropout, however it is not selective to excluding larger vessels so may not distinguish between different stages of DR progression [12].
OCT-A may have a role in better understanding the integrity of microvascular perfusion and how this relates to the maintenance of oxygen delivery to the retina, which has not yet been adequately explored in patients with PDR. Vitrectomy has been shown to improve rates of hypoxia and reduce VEGF production from ischaemic areas of retina [65]. Reduced VEGF levels reduces the stimulus for neovascularization processes as in diabetic neovascularization. Vitrectomy results in changes in oxygen flux across the retina and removes VEGF away from the retina at a faster rate than eyes without vitrectomy [65,66]. Therefore, vitrectomy could have additional benefits in diabetic eyes with retinal ischaemia [67]. There is presently a lack of OCT-A evidence in PDR patients post-vitrectomy (Table 5).

Table 5.
Studies reporting response to pars plana vitrectomy surgery in proliferative diabetic retinopathy by non-invasive imaging modalities, published within the past 5 years (2016–1 June 2021).
5. Future Directions
5.1. AI Approaches
Screening for DR is currently based on human grading, which is labour intensive. Extrapolating the current UK annual DR screening protocol globally would require 2.2 billion retinal images to be graded in 2030 [69]. Emerging automated retinal image analysis systems are artificial intelligence machine learning algorithms that may provide a cost-effective alternative to human grading for sight-threatening complications of DR, such as PDR.
The use of computerised, automated platforms to detect clinically relevant biomarkers such as pan-retinal microaneurysm count in PDR have been proposed to enable assessment of disease response to therapy and for grading of different stages of disease progression [30]. Further angiographic metrics which may be analysed include retinal non-perfusion areas and retinal leakage [70].
Abdelsalam, 2020 [71] described AI methods which can accurately classify fundus OCTA in diabetic patients to distinguish DR with or without NPDR with a sensitivity and specificity of >96% in a small sample. The methodology uses an artificial neural network (ANN) as an automatic classifier to distinguish between normal subjects without diabetes (n = 40), diabetics without DR (n = 30), and mild to moderate NPDR subjects (n = 30) [71]. The ANN are a set of algorithms modelled closely to the human brain which are designed to perform non-linear statistical modelling using a biologically inspired paradigm where a computer learns from observational data feeds. OCTA images of the mean of the intercapillary areas as are shown to the ANN which develops predictive models from dichotomous outcomes. This methodology relies on features of the FAZ based on the previously established generalisation that DR patients have an enlarged FAZ region, reduced vascular density and circularity index [71,72].
With the increase in the number of acquired OCT scans and the technical complexity associated with analysis of layer segmentation in DR, new automated techniques for detection of local tissue alterations are becoming more relevant.
AI algorithms which integrate both imaging and clinical parameters for decision support may have an advantage over imaging algorithms alone. This would require collecting a structured minimum dataset of patient-centred outcomes in electronic medical records. Additionally, this would require exchange of data across different imaging and electronic medical record platforms that may be facilitated by initiatives such as FHIR (Fast Healthcare Interoperability Resources).
5.2. Development of Other Non-Invasive Imaging Modalities
As well as wide-field OCT, OCTA and colour fundus photography other non-invasive imaging modalities have been suggested for the diagnosis and monitoring of PDR. For example, there has been interest in hyperspectral imaging, which is similar to conventional retinal photography, but instead of using a single white light flash, a series of images is captured using different wavelengths of light in a fraction of a second. These images are then stacked to yield an image cube with spectral (wavelength) and spatial dimensions that can be interrogated to identify features that are not discernible with conventional retinal photography. Exploratory parameters include retinal non-perfusion and retinal nerve fibre layer thinning [73]. In 2020, Vaz-Pereira et al. [39] reported high-contrast near infrared reflectance imaging with a field of view of 30° was able to observe changes in the neovascular complexes at the disc and elsewhere in 20 eyes with PDR.
5.3. Training, Education, and Equipment Maintenance
The transition from invasive to non-invasive imaging modalities to guide diagnosis and treatment of proliferative diabetic retinopathy requires both the image acquisition and image interpretation skills of healthcare professionals to be updated. This can be facilitated by updates in training curricula for both doctors and allied healthcare professionals. With modern web-based education tools, there is the opportunity for this teaching to be delivered remotely and for high-income countries to develop partnerships with low-middle income countries. There are also opportunities to share expertise with clinicians and patients who are located remotely via telemedicine approaches.
It is also important to consider the high costs of acquiring and maintaining new imaging devices as well as networking them. This might limit the uptake of such devices particularly in low-middle income countries.
5.4. Validation in Prospective Observational Studies and Randomised Clinical Trials
The non-invasive imaging modalities are at different stages of clinical validation. It is important that they have been proven to be effective in large-scale prospective observational studies or randomised clinical trials. Large-scale observational studies offer the opportunity to explore the utility of novel non-invasive imaging modalities. A number of currently running clinical trials have included exploratory analyses with wide-field OCT, OCTA and colour fundus photography and we await the results with interest [74,75,76]. In a recent multi-centre, head-to-head, real-world validation study of seven automated AI DR screening systems, the algorithms showed significant performance differences; for example, one system missed 25% of cases of PDR. These results argue for rigorous testing of all such algorithms on local real-world data before clinical implementation [77].
6. Conclusions
Diagnosis and management of proliferative diabetic retinopathy are reliant upon retinal imaging. There is a trend of moving away from invasive (e.g., FFA) to non-invasive (e.g., wide-field OCT, OCTA, and colour fundus photography) imaging modalities to allow for more objective assessments that can be readily repeated in a time-efficient manner without compromising patient safety. Such quantitative assessments generating large amounts of data could benefit from AI approaches to aid clinical decision making. These non-invasive imaging modalities continue to improve both in terms of the quality of image acquisition and progress in image interpretation. It is important that newer, non-invasive imaging modalities are appropriately validated in large-scale prospective observational studies or randomised clinical trials.
Author Contributions
All authors, E.B., H.M. and A.C. actively participated in conceptualisation, methodology, and curation of data for the manuscript. Original draft preparation by E.B. was reviewed and edited by H.M. and A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
H.M. and A.C. are consultants for Allergan, Bayer, Novartis, and Roche. E.B. reports no conflicts of interest. None of the authors have any proprietary interest in any material or method presented. All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria, educational grants, participation in speakers’ bureaux, membership, employment, consultancies, stock ownership, other equity interest, expert testimony, or patent-licensing arrangements), or non-financial interests (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
References
- Keel, S.; Xie, J.; Foreman, J.; van Wijngaarden, P.; Taylor, H.R.; Dirani, M. The Prevalence of Diabetic Retinopathy in Australian Adults with Self-Reported Diabetes: The National Eye Health Survey. Ophthalmology 2017, 124, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Mathur, R.; Bhaskaran, K.; Edwards, E.; Lee, H.; Chaturvedi, N.; Smeeth, L.; Douglas, I. Population trends in the 10-year incidence and prevalence of diabetic retinopathy in the UK: A cohort study in the Clinical Practice Research Datalink 2004–2014. BMJ Open 2017, 7, e014444. [Google Scholar] [CrossRef]
- Amoaku, W.M.; Ghanchi, F.; Bailey, C.; Banerjee, S.; Banerjee, S.; Downey, L.; Gale, R.; Hamilton, R.; Khunti, K.; Posner, E.; et al. Diabetic retinopathy and diabetic macular oedema pathways and management: UK Consensus Working Group. Eye 2020, 34, 1–51. [Google Scholar] [CrossRef]
- Ramchandran, R.; Bawany, M.H.; Ding, L.; Sharma, G.; Wykoff, C.C.; Kuriyan, A.E. Automated vessel density detection in fluorescein angiography images correlates with vision in proliferative diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2020, 61, 5312. [Google Scholar]
- Chhablani, J.; Sharma, A.; Goud, A.; Peguda, H.K.; Rao, H.L.; Begum, V.U.; Barteselli, G. Neurodegeneration in type 2 diabetes: Evidence from spectral-domain optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6333–6338. [Google Scholar] [CrossRef] [Green Version]
- Duh, E.J.; Sun, J.; Stitt, A.W. Diabetic retinopathy: Current understanding, mechanisms, and treatment strategies. JCI Insight 2017, 2, e93751. [Google Scholar] [CrossRef]
- Fan, W.; Nittala, M.G.; Velaga, S.B.; Hirano, T.; Wykoff, C.C.; Ip, M.; Lampen, S.I.; van Hemert, J.; Fleming, A.; Verhoek, M.; et al. Distribution of Nonperfusion and Neovascularization on Ultrawide-Field Fluorescein Angiography in Proliferative Diabetic Retinopathy (RECOVERY Study): Report 1. Am. J. Ophthalmol. 2019, 206, 154–160. [Google Scholar] [CrossRef]
- Pan, J.; Chen, D.; Yang, X.; Zou, R.; Zhao, K.; Cheng, D.; Huang, S.; Zhou, T.; Yang, Y.; Chen, F. Characteristics of Neovascularization in Early Stages of Proliferative Diabetic Retinopathy by Optical Coherence Tomography Angiography. Am. J. Ophthalmol. 2018, 192, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Rush, R.B.; Del Valle Penella, A.; Reinauer, R.M.; Rush, S.W.; Bastar, P.G. Internal Limiting Membrane Peeling during Vitrectomy for Diabetic Vitreous Hemorrhage: A Randomized Clinical Trial. RETINA 2020, 41, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Marcus, D.M.; Singh, H.; Farooq, A.; Starnes, D.; Walia, H. Endolaserless vitrectomy with intravitreal aflibercept injection (IAI) for proliferative diabetic retinopathy (PDR)-related vitreous hemorrhage (LASER LESS TRIAL). Investig. Ophthalmol. Vis. Sci. 2017, 58, 5036. [Google Scholar]
- Curtis, T.M.; Gardiner, T.A.; Stitt, A.W. Microvascular lesions of diabetic retinopathy: Clues towards understanding pathogenesis? Eye 2009, 23, 1496–1508. [Google Scholar] [CrossRef] [Green Version]
- Tan, B.; Chua, J.; Lin, E.; Cheng, J.; Gan, A.; Yao, X.; Wong, D.W.; Sabanayagam, C.; Wong, D.; Chan, C.M.; et al. Quantitative Microvascular Analysis with Wide-Field Optical Coherence Tomography Angiography in Eyes with Diabetic Retinopathy. JAMA Netw. Open 2020, 3, e1919469. [Google Scholar] [CrossRef]
- Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs—An extension of the modified Airlie House classification: ETDRS report number 10. Ophthalmology 1991, 98, 786–806. [Google Scholar] [CrossRef]
- The Diabetic Retinopathy Vitrectomy Study Research Group. Early vitrectomy for severe vitreous hemorrhage in diabetic retinopathy: Two-year results of a randomized trial—Diabetic Retinopathy Vitrectomy Study report 2. Arch. Ophthalmol. 1985, 103, 1644–1652. [Google Scholar] [CrossRef]
- Silva, P.S.; Cruz, A.J.D.; Ledesma, M.G.; van Hemert, J.; Radwan, A.; Cavallerano, J.; Aiello, L.M.; Sun, J.K. Diabetic Retinopathy Severity and Peripheral Lesions Are Associated with Nonperfusion on Ultrawide Field Angiography. Ophthalmology 2015, 122, 2465–2472. [Google Scholar] [CrossRef]
- Wilkinson, C.; Ferris, F.; Klein, R.; Lee, P.; Agardh, C.D.; Davis, M.; Dills, D.; Kampik, A.; Pararajasegaram, R.; Verdaguer, J.T. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003, 110, 1677–1682. [Google Scholar] [CrossRef]
- Gross, J.G.; Glassman, A.R.; Liu, D.; Sun, J.K.; Antoszyk, A.N.; Baker, C.W.; Bressler, N.M.; Elman, M.J.; Ferris, F.L.; Gardner, T.W.; et al. Five-Year Outcomes of Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy. JAMA Ophthalmol. 2018, 136, 1138–1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preti, R.C.; Vasquez Ramirez, L.M.; Ribeiro Monteiro, M.L.; Pelayes, D.E.; Takahashi, W.Y. Structural and functional assessment of macula in patients with high-risk proliferative diabetic retinopathy submitted to panretinal photocoagulation and associated intravitreal bevacizumab injections: A comparative, randomised, controlled trial. Ophthalmologica 2013, 230, 1–8. [Google Scholar] [CrossRef]
- Nikkhah, H.; Ghazi, H.; Razzaghi, M.R.; Karimi, S.; Ramezani, A.; Soheilian, M. Extended targeted retinal photocoagulation versus conventional pan-retinal photocoagulation for proliferative diabetic retinopathy in a randomized clinical trial. Int. Ophthalmol. 2017, 38, 313–321. [Google Scholar] [CrossRef]
- The Diabetic Retinopathy Study Research Group. Photocoagulation Treatment of Proliferative Diabetic Retinopathy: Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS Report Number 8. Ophthalmology 1981, 88, 583–600. [Google Scholar] [CrossRef]
- Flynn, H.W.; Chew, E.Y.; Simons, B.D.; Barton, F.B.; Remaley, N.A.; Ferris, F.L. Pars Plana Vitrectomy in the Early Treatment Diabetic Retinopathy Study. Ophthalmology 1992, 99, 1351–1357. [Google Scholar] [CrossRef]
- Silva, P.S.; Cavallerano, J.; Haddad, N.M.N.; Kwak, H.; Dyer, K.H.; Omar, A.F.; Shikari, H.; Aiello, L.M.; Sun, J.K. Peripheral Lesions Identified on Ultrawide Field Imaging Predict Increased Risk of Diabetic Retinopathy Progression over 4 Years. Ophthalmology 2015, 122, 949–956. [Google Scholar] [CrossRef]
- Elnahry, A.G.; Ramsey, D.J. Automated Image Alignment for Comparing Microvascular Changes Detected by Fluorescein Angiography and Optical Coherence Tomography Angiography in Diabetic Retinopathy. Semin. Ophthalmol. 2021, 36, 757–764. [Google Scholar] [CrossRef]
- Ishibazawa, A.; Nagaoka, T.; Yokota, H.; Takahashi, A.; Omae, T.; Song, Y.S.; Takahashi, T.; Yoshida, A. Characteristics of retinal neovascularization in proliferative diabetic retinopathy imaged by optical coherence tomography angiography. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6247–6255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, A.A.; Morse, L.; Handa, J.T.; Morales, R.B.; Tucker, R.; Hjelmeland, L.; A Yannuzzi, L. Histologic localization of indocyanine green dye in aging primate and human ocular tissues with clinical angiographic correlation. Ophthalmology 1998, 105, 1060–1068. [Google Scholar] [CrossRef]
- Shiragami, C.; Shiraga, F.; Matsuo, T.; Tsuchida, Y.; Ohtsuki, H. Risk factors for diabetic choroidopathy in patients with diabetic retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2002, 240, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.A.; Calucci, D.; Orefice, J.L. Indocyanine Green Angiography for The Detection Of Macular “Treatable Lesions” In Patients with Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2011, 52, 583. [Google Scholar]
- Mohamed, I.E.; Mohamed, M.A.; Yousef, M.; Mahmoud, M.Z.; Alonazi, B. Use of ophthalmic B-scan ultrasonography in determining the causes of low vision in patients with diabetic retinopathy. Eur. J. Radiol. Open 2018, 5, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Diabetic Retinopathy Vitrectomy Study Research Group. Early Vitrectomy for Severe Vitreous Hemorrhage in Diabetic Retinopathy. Four-year results of a randomized trial. Diabetic Retinopathy Study report 5. Arch. Ophthalmol. 1990, 108, 958–964. [Google Scholar] [CrossRef]
- Babiuch, A.; Wykoff, C.C.; Hach, J.; Srivastava, S.; E Talcott, K.; Yu, H.J.; Nittala, M.; Sadda, S.; Ip, M.S.; Le, T.; et al. Longitudinal panretinal microaneurysm dynamics on ultra-widefield fluorescein angiography in eyes treated with intravitreal aflibercept for proliferative diabetic retinopathy in the recovery study. Br. J. Ophthalmol. 2020, 105, 1111–1115. [Google Scholar] [CrossRef] [PubMed]
- Shimura, M.; Kitano, S.; Muramatsu, D.; Fukushima, H.; Takamura, Y.; Matsumoto, M.; Kokado, M.; Kogo, J.; Sasaki, M.; Morizane, Y.; et al. Real-world management of treatment-naïve diabetic macular oedema: 2-year visual outcome focusing on the starting year of intervention from STREAT-DMO study. Br. J. Ophthalmol. 2020, 104, 1755–1761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gross, J.G.; Glassman, A.R.; Jampol, L.M.; Inusah, S.; Aiello, L.P.; Antoszyk, A.N.; Baker, C.W.; Berger, B.B.; Bressler, N.M.; Browning, D.; et al. Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: A randomized clinical trial. JAMA 2015, 314, 2137–2146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, C.C.; Yang, C.H.; Yeh, P.T.; Yang, C.M. Macular tractional retinoschisis in proliferative diabetic retinopathy: Clinical characteristics and surgical outcome. Ophthalmologica 2013, 231, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Inigo, Y.J.; Acaba, L.A.; Berrocal, M.H. Surgical management of retinal diseases: Proliferative diabetic retinopathy and traction retinal detachment. Dev. Ophthalmol. 2014, 54, 196–203. [Google Scholar]
- Ophir, A.; Martinez, M.R.; Mosqueda, P.; Trevino, A. Vitreous traction and epiretinal membranes in diabetic macular oedema using spectral-domain optical coherence tomography. Eye 2010, 24, 1545–1553. [Google Scholar] [CrossRef]
- Chhablani, J.K.; Kim, J.S.; Cheng, L.; Kozak, I.; Freeman, W. External limiting membrane as a predictor of visual improvement in diabetic macular edema after pars plana vitrectomy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2012, 250, 1415–1420. [Google Scholar] [CrossRef]
- Mishra, D.K.; Shanmugam, M.P.; Ramanjulu, R.; Sagar, P. Comparison of standard and “innovative wide-field” optical coherence tomography images in assessment of vitreoretinal interface in proliferative diabetic retinopathy: A pilot study. Indian J. Ophthalmol. 2020, 69, 99–102. [Google Scholar] [CrossRef]
- Um, T.; Seo, E.J.; Kim, Y.J.; Yoon, Y.H. Optical coherence tomography angiography findings of type 1 diabetic patients with diabetic retinopathy, in comparison with type 2 patients. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 281–288. [Google Scholar] [CrossRef]
- Vaz-Pereira, S.; Monteiro-Grillo, M.; Engelbert, M. Near-infrared reflectance imaging of neovascularization in proliferative diabetic retinopathy. Int. J. Retin. Vitr. 2020, 6, 59. [Google Scholar] [CrossRef]
- Schwartz, R.; Khalid, H.; Sivaprasad, S.; Nicholson, L.; Anikina, E.; Sullivan, P.; Patel, P.J.; Balaskas, K.; Keane, P.A. Objective Evaluation of Proliferative Diabetic Retinopathy Using OCT. Ophthalmol. Retin. 2020, 4, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Levine, E.S.; Arya, M.; Chaudhari, J.; Greig, E.C.; Alibhai, A.Y.; Baumal, C.R.; Witkin, A.J.; Duker, J.S.; Waheed, N.K. Repeatability and reproducibility of vessel density measurements on optical coherence tomography angiography in diabetic retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 1687–1695. [Google Scholar] [CrossRef] [PubMed]
- Kase, S.; Endo, H.; Takahashi, M.; Saito, M.; Yokoi, M.; Ito, Y.; Katsuta, S.; Sonoda, S.; Sakamoto, T.; Ishida, S.; et al. Alteration of choroidal vascular structure in diabetic retinopathy. Br. J. Ophthalmol. 2020, 104, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Hoshiyama, K.; Hirabayashi, K.; Wakabayashi, M.; Toriyama, Y.; Tokimitsu, M.; Murata, T. Vitreoretinal Interface Slab in OCT Angiography for Detecting Diabetic Retinal Neovascularization. Ophthalmol. Retin. 2020, 4, 588–594. [Google Scholar] [CrossRef]
- Ashraf, M.; Sampani, K.; Clermont, A.; Abu-Qamar, O.; Rhee, J.; Silva, P.S.; Aiello, L.P.; Sun, J.K. Vascular Density of Deep, Intermediate and Superficial Vascular Plexuses Are Differentially Affected by Diabetic Retinopathy Severity. Investig. Opthalmology Vis. Sci. 2020, 61, 53. [Google Scholar] [CrossRef]
- Wang, H.; Tao, Y. Choroidal structural changes correlate with severity of diabetic retinopathy in diabetes mellitus. BMC Ophthalmol. 2019, 19, 186. [Google Scholar] [CrossRef] [Green Version]
- Motulsky, E.H.; Liu, G.; Shi, Y.; Zheng, F.; Flynn Jr, H.W.; Gregori, G.; Rosenfeld, P.J. Widefield swept-source optical coherence tomography angiography of proliferative diabetic retinopathy. Ophthalmic Surg. Lasers Imaging Retin. 2019, 50, 474–484. [Google Scholar] [CrossRef]
- La Mantia, A.; Kurt, R.A.; Mejor, S.; Egan, C.; Tufail, A.; Keane, P.A.; Sim, D.A. Comparing fundus fluorescein angiography and swept-source optical coherence tomography angiography in the evaluation of diabetic macular perfusion. Retina 2019, 39, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, C.C.; Hsu, H.M.; Yang, C.M.; Yang, C.H. Correlation of retinal vascular perfusion density with dark adaptation in diabetic retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 1401–1410. [Google Scholar] [CrossRef]
- Hirano, T.; Kitahara, J.; Toriyama, Y.; Kasamatsu, H.; Murata, T.; Sadda, S. Quantifying vascular density and morphology using different swept-source optical coherence tomography angiographic scan patterns in diabetic retinopathy. Br. J. Ophthalmol. 2019, 103, 216–221. [Google Scholar] [CrossRef]
- Cui, Y.; Zhu, Y.; Wang, J.C.; Lu, Y.; Zeng, R.; Katz, R.; Wu, D.M.; Vavvas, D.G.; Husain, D.; Miller, J.W.; et al. Imaging artifacts and segmentation errors with wide-field swept-source optical coherence tomography angiography in diabetic retinopathy. Transl. Vis. Sci. Technol. 2019, 8, 18. [Google Scholar] [CrossRef] [Green Version]
- Plasencia, M.A.; Abreu-Gonzalez, R.; Culebras, M.A.G. Structure–Function Correlation Using OCT Angiography And Microperimetry In Diabetic Retinopathy. Clin. Ophthalmol. 2019, 13, 2181–2188. [Google Scholar] [CrossRef] [PubMed]
- Savastano, M.C.; Federici, M.; Falsini, B.; Caporossi, A.; Minnella, A.M. Detecting papillary neovascularization in proliferative diabetic retinopathy using optical coherence tomography angiography. Acta Ophthalmol. 2018, 96, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, E.S.; Yu, S.Y. Longitudinal changes in retinal microvasculature after panretinal photocoagulation in diabetic retinopathy using swept-source OCT angiography. Sci. Rep. 2021, 11, 216. [Google Scholar] [CrossRef] [PubMed]
- Vergmann, A.S.; Sorensen, K.T.; Torp, T.L.; Kawasaki, R.; Wong, T.; Peto, T.; Grauslund, J. Optical coherence tomography angiography measured area of retinal neovascularization is predictive of treatment response and progression of disease in patients with proliferative diabetic retinopathy. Int. J. Retin. Vitr. 2020, 6, 49. [Google Scholar] [CrossRef]
- Russell, J.F.; Al-Khersan, H.; Shi, Y.; Scott, N.L.; Hinkle, J.W.; Fan, K.C.; Lyu, C.; Feuer, W.J.; Gregori, G.; Rosenfeld, P.J. Retinal Nonperfusion in Proliferative Diabetic Retinopathy Before and After Panretinal Photocoagulation Assessed by Widefield OCT Angiography. Am. J. Ophthalmol. 2020, 213, 177–185. [Google Scholar] [CrossRef]
- Lupidi, M.; Gujar, R.; Cerquaglia, A.; Chhablani, J.; Fruttini, D.; Muzi, A.; Corbucci, R.; Fiore, T.; Coscas, F.; Coscas, G.; et al. OCT-Angiography as a reliable prognostic tool in laser-treated proliferative diabetic retinopathy: The RENOCTA Study. Eur. J. Ophthalmol. 2020. Online ahead of print. [Google Scholar] [CrossRef]
- Zacharias, L.C.; Azevedo BM, S.; de Araujo, R.B.; Ciongoli, M.R.; Hatanaka, M.; Preti, R.C.; Monteiro, M.L.R. Effect of panretinal photocoagulation on the peripapillary retinal nerve fiber layer in diabetic retinopathy patients. Clinics 2019, 74, e1163. [Google Scholar] [CrossRef] [Green Version]
- Mirshahi, A.; Ghassemi, F.; Fadakar, K.; Mirshahi, R.; Bazvand, F.; Riazi-Esfahani, H. Effects of panretinal photocoagulation on retinal vasculature and foveal avascular zone in diabetic retinopathy using optical coherence tomography angiography: A pilot study. J. Curr. Ophthalmol. 2019, 31, 287–291. [Google Scholar] [CrossRef]
- Lorusso, M.; Milano, V.; Nikolopoulou, E.; Ferrari, L.M.; Cicinelli, M.V.; Querques, G.; Ferrari, T.M. Panretinal Photocoagulation Does Not Change Macular Perfusion in Eyes With Proliferative Diabetic Retinopathy. Ophthalmic Surg. Lasers Imaging Retin. 2019, 50, 174–178. [Google Scholar] [CrossRef]
- Choi, W.; Kang, H.G.; Choi, E.Y.; Kim, S.S.; Koh, H.J.; Kim, M. Effect of intravitreal bevacizumab injection before panretinal photocoagulation on the prevention of macular edema aggravation in proliferative diabetic retinopathy. J. Clin. Med. 2020, 9, 3772. [Google Scholar] [CrossRef] [PubMed]
- Chatziralli, I.; Dimitriou, E.; Theodossiadis, G.; Kazantzis, D.; Theodossiadis, P. Intravitreal ranibizumab alone or in combination with panretinal photocoagulation for the treatment of proliferative diabetic retinopathy with coexistent macular edema: Long-term outcomes of a prospective study. Acta Diabetol. 2020, 57, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Bressler, N.M.; Beaulieu, W.T.; Bressler, S.B.; Glassman, A.R.; Melia, B.M.; Jampol, L.M.; Jhaveri, C.D.; Salehi-Had, H.; Velez, G.; Sun, J.K.; et al. Anti–vascular endothelial growth factor therapy and risk of traction retinal detachment in eyes with proliferative diabetic retinopathy: Pooled Analysis of Five DRCR Retina Network Randomized Clinical Trials. Retina 2020, 40, 1021–1028. [Google Scholar] [CrossRef]
- Arevalo, J.F.; Lasave, A.F.; Kozak, I.; Al Rashaed, S.; Al Kahtani, E.; Maia, M.; Farah, M.E.; Cutolo, C.; Brito, M.; Osorio, C.; et al. Preoperative Bevacizumab for Tractional Retinal Detachment in Proliferative Diabetic Retinopathy: A Prospective Randomized Clinical Trial. Am. J. Ophthalmol. 2019, 207, 279–287. [Google Scholar] [CrossRef]
- Pan, J.; Chen, F.; Chen, D.; Yang, X.; Wang, J.; Chen, Z.; He, X.; Zhou, T.; Zheng, J.; Chen, H. Novel Three Types of Neovascularization Elsewhere Determine the Differential Clinical Features of Proliferative Diabetic Retinopathy. Retina 2020, 41, 1265–1274. [Google Scholar] [CrossRef]
- Stefansson, E. Physiology of retinal oxygenation. Acta Ophthalmol. 2015, 93. [Google Scholar] [CrossRef]
- Stefánsson, E. Ocular Oxygenation and the Treatment of Diabetic Retinopathy. Surv. Ophthalmol. 2006, 51, 364–380. [Google Scholar] [CrossRef] [PubMed]
- Sharma, T.; Fong, A.; Lai, T.Y.; Lee, V.; Das, S.; Lam, D. Surgical treatment for diabetic vitreoretinal diseases: A review. Clin. Exp. Ophthalmol. 2016, 44, 340–354. [Google Scholar] [CrossRef]
- Elhamid, A.H.A.; Mohamed, A.A.E.A.; Khattab, A.M. Intravitreal Aflibercept injection with Panretinal photocoagulation versus early Vitrectomy for diabetic vitreous hemorrhage: Randomized clinical trial. BMC Ophthalmol. 2020, 20, 130–139. [Google Scholar] [CrossRef]
- Heydon, P.; Egan, C.; Bolter, L.; Chambers, R.; Anderson, J.; Aldington, S.; Stratton, I.M.; Scanlon, P.H.; Webster, L.; Mann, S.; et al. Prospective evaluation of an artificial intelligence-enabled algorithm for automated diabetic retinopathy screening of 30 000 patients. Br. J. Ophthalmol. 2021, 105, 723–728. [Google Scholar] [CrossRef]
- Babiuch, A.S.; Wykoff, C.C.; Srivastava, S.K.; Talcott, K.; Zhou, B.; Hach, J.; Hu, M.; Reese, J.L.; Ehlers, J.P. Retinal leakage index dynamics on ultra-widefield fluorescein angiography in eyes treated with intravitreal aflibercept for proliferative diabetic retinopathy in the recovery study. Retina 2020, 40, 2175–2183. [Google Scholar] [CrossRef] [PubMed]
- Abdelsalam, M.M. Effective blood vessels reconstruction methodology for early detection and classification of diabetic retinopathy using OCTA images by artificial neural network. Informatics Med. Unlocked 2020, 20, 100390. [Google Scholar] [CrossRef]
- Akil, H.; Karst, S.; Heisler, M.; Etminan, M.; Navajas, E.; Maberley, D. Application of optical coherence tomography angiography in diabetic retinopathy: A comprehensive review. Can. J. Ophthalmol. 2019, 54, 519–528. [Google Scholar] [CrossRef]
- Reshef, E.R.; Miller, J.B.; Vavvas, D.G. Hyperspectral Imaging of the Retina: A Review. Int. Ophthalmol. Clin. 2020, 60, 85–96. [Google Scholar] [CrossRef]
- Gillies, M.C. Laser Therapy Combined with Intravitreal Aflibercept vs Intravitreal Aflibercept Monotherapy (LADAMO). Available online: https://clinicaltrials.gov/ct2/show/NCT024325472019 (accessed on 1 June 2021).
- Lujan, B.J.; Calhoun, C.T.; Glassman, A.R.; Googe, J.M.; Jampol, L.M.; Melia, M.; Schlossman, D.K.; Sun, J.K. Optical Coherence Tomography Angiography Quality Across Three Multicenter Clinical Studies of Diabetic Retinopathy. Transl. Vis. Sci. Technol. 2021, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Maturi, R.K.; Glassman, A.R.; Josic, K.; Antoszyk, A.N.; Blodi, B.A.; Jampol, L.M.; Marcus, D.M.; Martin, D.F.; Melia, M.; Salehi-Had, H.; et al. Effect of Intravitreous Anti–Vascular Endothelial Growth Factor vs Sham Treatment for Prevention of Vision-Threatening Complications of Diabetic Retinopathy. JAMA Ophthalmol. 2021, 139, 701–712. [Google Scholar] [CrossRef]
- Lee, A.Y.; Yanagihara, R.T.; Lee, C.S.; Blazes, M.; Jung, H.C.; Chee, Y.E.; Gencarella, M.D.; Gee, H.; Maa, A.Y.; Cockerham, G.C.; et al. Multicenter, Head-to-Head, Real-World Validation Study of Seven Automated Artificial Intelligence Diabetic Retinopathy Screening Systems. Diabetes Care 2021, 44, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).