Abstract
Angiogenesis is essential during development or when tissue restoration and oxygenation is required. Limited or excessive formation of blood vessels is a hallmark of several pathologies, and many angiogenesis-related pathways are being studied to highlight potential targets for effective angiogenesis-stimulating or inhibiting therapeutic approaches. A few studies point to the adrenergic system as a significant regulator of angiogenesis, directly or indirectly. Functional adrenergic receptors are expressed on endothelial cells and affect their response to the adrenergic system. The latter can also upregulate the release of growth factors by mural cells of the vessel wall, blood cells or cancer cells, thus subsequently affecting endothelial cell functions and angiogenesis. In the present study we summarize up-to-date literature on the known effects of the adrenergic receptors on physiological and pathological angiogenesis.
1. Introduction
1.1. Angiogenesis
Angiogenesis is the formation of new blood vessels from pre-existing ones. It is a complex process with many stages and mechanisms, which occurs throughout life in healthy conditions, such as embryo development and wound healing, but also in pathological ones, such as cancer and diabetes. During angiogenesis, vascular and predominantly endothelial cells play the most profound role. The onset of angiogenesis requires selective activation of endothelial cells from the pre-existing network of capillaries by pro-angiogenic growth factors. Among the latter, vascular endothelial growth factor A (VEGFA) is the most important, guiding various steps during new vessel sprouting. The newly formed vessels are stabilized by mural cell (pericytes and vascular smooth muscle cells) recruitment by platelet derived growth factor (PDGF) [1,2,3].
Blood cells may also affect angiogenesis by secreting various pro-angiogenic molecules. For example, in inflammation, monocytes that express VEGFA increase the permeability of the endothelial monolayer and thus initiate angiogenesis. Monocytes also express PDGF, which promotes the proliferation of both the endothelial and vascular mural cells [4].
A significant initiator of angiogenesis is hypoxia, which occurs in cancer and other pathologies. Hypoxia leads to upregulation of VEGFA secretion by cancer or other types of cells, which then activates endothelial cells to form new vessels [2,5].
1.2. Adrenergic Receptors
Adrenergic receptors (ARs) belong to the superfamily of G protein-coupled receptors, also referred to as receptors with seven transmembrane domains. They are divided into two distinct subtypes, α and β ARs. Alpha ARs are divided into α1 and α2 ARs, subdivided into α1A, α1B, α1D and α2A, α2B, α2C respectively, while beta ARs are classified as β1, β2 and β3. Norepinephrine and epinephrine are the natural, endogenous ligands of ARs that mediate the effects of the sympathetic nervous system. Agonist binding to ARs induces a conformational rearrangement that leads to the activation of the corresponding G proteins that differentially affect downstream signaling pathways (Table 1) [6,7].

Table 1.
Distribution and functions of ARs.
ARs have a wide distribution and affect numerous functions [6,7], as summarized in Table 1. Agonists and antagonists of ARs are clinically used in the treatment of various diseases, mostly of the cardiovascular and the respiratory systems. The most recent findings that are discussed in the following paragraphs suggest their involvement in the regulation of angiogenesis in health and disease.
2. Search Strategy
We searched the MEDLINE/PubMed and the Scopus databases focusing on areas of relevance to angiogenesis and the role of adrenergic receptors in new blood vessel formation. We excluded all items that were not in research journals. As keywords we used different combinations of the terms: “angiogenesis”, “adrenergic receptors”, “endothelial cells”, and “adrenergic signaling”. To be more focused, we used the criterion that the two terms coexist in the title and/or the abstract of the articles. When we used the combination “angiogenesis” and “adrenergic receptors”, the search yielded 65 results, 25 of which were published during the last five years. The combination “angiogenesis” and “adrenergic signaling” yielded 35 results, 15 of which were published during the last five years. The combination “endothelial cells” and “adrenergic signaling” led to 21 results, among which 10 were published in the last five years. Finally, the combination “endothelial cells” and “adrenergic receptors” led to 120 results, among which 26 were published in the last five years. Many of the publications appeared in more than one of the above-mentioned combinations and we finally chose 73 publications that were most relevant to the scope of this review, including all the relevant publications of the last five years (31 papers). We also included seven review papers in the Introduction of this review, in which the readers can look for more information on mechanisms of angiogenesis and on the physiology and pharmacology of the adrenergic receptors.
3. Expression of ARs in Endothelial Cells
ARs subtypes expression differs among endothelial cells of different origin. Human coronary artery endothelial cells express α1 ARs, with the α1B subtype being predominant [8]. Bovine aortic and bovine pulmonary artery endothelial cells [9], as well as human pulmonary artery and human umbilical vein endothelial cells (HUVEC) [10] express functional β ARs that are downregulated by hypoxia [10] and may belong to the β2 subtype [11]. Cultured endothelial cells from the human iliac vein, human skin, and bovine fetal aorta also express β2 ARs and respond to β2 agonists [12,13]. Bovine brain capillary endothelial cells express both β1 and β2 ARs (relative proportion 42% and 58% respectively) [14]. Endothelial progenitor cells (EPCs) express β2 ARs [15]. Human dermal microvascular endothelial cells express all subtypes of β ARs [13], while human retinal endothelial cells express both β1 and β3 but not β2 ARs [16].
4. ARs and Physiological Angiogenesis
4.1. The Evidence on the Role of α ARs in Physiological Angiogenesis Is Limited and Contradictory
The α1 AR agonist phenylephrine at very low doses that do not affect vascular contraction has been shown to stimulate α1D ARs and promote NO-dependent endothelial cell migration and proliferation in vitro, an effect that was significantly enhanced under hypoxic conditions [17]. In the same line, the nonselective reversible antagonist of α1 and α2 ARs, phentolamine, suppresses endothelial cell proliferation, migration, and tube formation in vitro. Phentolamine decreases expression of VEGF receptor 2 (VEGFR2) and angiopoietins 1 and 2, without affecting the expression of VEGFA [18]. It also inhibits proliferation, migration, and secretion of VEGFA and angiopoietin 1 by pericytes in vitro [19]. The potent and selective α1 AR antagonist doxazosin hinders the VEGFA-mediated proliferation and migration of HUVEC in vitro, prevents attachment on fibronectin and activates caspase-3-dependent apoptosis. It also hinders the action of fibroblast growth factor 2 (FGF2) on endothelial cells [20]. In the same line, the selective α1 AR antagonist terazosin inhibits HUVEC proliferation, migration, and tube formation in vitro and blocks the stimulatory effects of VEGFA [21].
However, in another study, doxazosin stimulates, while phenylephrine inhibits proliferation, migration, and tube formation by Wistar-Kyoto rat aortic endothelial cells in vitro [22]. This discrepancy may be due to the different origin of the endothelial cells studied but needs to be further researched.
During the vascular development of the labyrinth of fetal and maternal vessels, α2B ARs hinder the expression of the anti-angiogenic soluble VEFG receptor 1 (VEGFR1). Targeted deletion of the α2B AR gene leads to upregulation of soluble VEGFR1 and inhibition of embryonic vasculogenesis. Neutralization of soluble VEGFR1 by a specific antibody restored the number of vessels in α2B-deficient placentae in vivo at embryonic day 10.5 [23].
In chick and quail models, it has been shown that sympathetic innervation promotes the differentiation and the arterial fate of endothelial cells through ERK1/2 activation downstream of both α1 and α2 ARs. The α1 ARs stimulate PLC that activates the ERK pathway, while the α2 ARs inhibit the AC/protein kinase A (PKA) pathway and thus also activate ERK1/2 [24,25].
4.2. The Role of β ARs in Angiogenesis Regulation Is Better Evidenced Compared to That of α ARs but Contradicting Data Still Exist
It has been demonstrated that the β ARs in endothelial cells are highly associated with angiogenesis. Isoproterenol, a non-selective β AR agonist, as well as overexpression of β2 ARs in human endothelial cells, increase endothelial cell proliferation, migration, and tube formation in vitro through ERK1/2 activation [26]. Activation of β2 ARs in HUVEC activates both the cAMP/PKA and the cAMP/exchange proteins activated by cyclic AMP (Epac1) pathways. The former enhances VEGFA expression and secretion, and the latter increases VEGFR2 expression, thus upregulating a significant pro-angiogenic pathway [27]. Deletion of β2 ARs in mouse aortic endothelial cells inhibits VEGFA expression and tube formation in vitro, an effect attributed to the nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) pathway inactivation downstream of the impaired cAMP-response element binding (CREB) phosphorylation [28]. The selective β2 AR agonist terbutaline has been shown to stimulate bEnd.3 microvascular endothelial cells tube formation without affecting their proliferation rate. This effect is inhibited using specific AKT and ERK1/2 kinase inhibitors, suggesting that both kinases act downstream of the β2 ARs. In vivo, terbutaline stimulates angiogenesis in the chick embryo chorioallantoic membrane assay [29]. In the immortalized human dermal microvascular endothelial cell line HMEC-1 and in primary human dermal endothelial cells, β2 AR activation has been shown to increase IL-6 production [30], which may then stimulate angiogenesis [31].
EPCs express β2 ARs and isoproterenol or overexpression of β2 ARs stimulates EPCs proliferation, migration, VEGFA production and tube formation in vitro [32]. Activation of β2 ARs in EPCs leads to activation of the Akt/endothelial nitric oxide (NO) synthase pathway to promote VEGFA secretion and enhance the reendothelialization capacity of EPCs in vivo [15]. Similarly, overexpression of β2 ARs in infused peripheral blood-derived EPCs has been shown to increase their proliferation, migration, and NO production in vitro and in vivo, enhancing restoration of endothelium-related vascular injuries [33].
Enhancement of NO production downstream of β2 ARs in mouse pulmonary artery endothelial cells has been shown to involve a Gi/o protein, c-Src kinase, phosphoinositide 3-kinase and Akt kinase but to be independent of cAMP/PKA, ERK1/2, and 5′ AMP-activated protein kinase [34], thus questioning the involvement of ERK1/2 downstream of β2 ARs in the regulation of angiogenesis. Involvement of ERK1/2 kinase in the regulation of angiogenesis by the β2 ARs has been also questioned by a study showing that in rat aorta endothelial cells in vitro, the non-selective β AR agonist isoprenaline decreases and the non-selective β AR antagonist propranolol increases ERK1/2 activity [35].
In contrast to positive regulation of angiogenesis by β2 ARs, it has been reported that activation of β2 ARs that are highly expressed in human dermal microvascular endothelial cells (HDMEC) delays HDMEC migration and tube formation and decreases secretion of the pro-angiogenic growth factors FGF2 and VEGFA in vitro. Moreover, β2 AR activation decreases angiogenesis in murine skin wounds in vivo. These effects have been attributed to a cAMP/EPAC1-dependent mechanism [13]. In the same line, antagonism, or gene deletion of the β2 AR can increase VEGFA secretion from keratinocytes in vitro and angiogenesis in vivo [36]. Moreover, the selective β2 AR agonist salbutamol inhibits angiogenesis in the in vivo chick embryo chorioallantoic membrane assay and decreases the number of vessels in a rat model of skin wound healing [37]. There are also data showing that norepinephrine via β2 ARs [38] and cAMP via PKA [39,40,41] induce endothelial cell apoptosis and inhibit angiogenesis.
Another function of endothelial cells that may be related to angiogenesis initiation and seems to be affected by β2 ARs is endothelial cell layer permeability. It has been shown that the selective β2 agonist formoterol decreases vascular permeability in the rat trachea by inhibiting endothelial gap formation [42]. Isoproterenol and formoterol also decrease endothelial cell permeability in cultures of bovine aortic and retina endothelial cells, possibly via cAMP production [43].
The first data that supported an (indirect) role of β3 ARs in angiogenesis were obtained in cultures of brown adipocytes that synthesize and release VEGFA in response to catecholamines [44]. It was later shown that the selective β blocker nebivolol stimulates endothelial NO release through the β₃ AR expressed in HUVEC [45] and that activation of β3 ARs by catecholamines in bovine aortic endothelial cells may lead to an increase in NO release through the small G protein Rac1 that is upstream of PKA and Akt kinase activation [46].
An interesting observation in rat pulmonary microvascular endothelial cells is that the effect of β ARs could be manipulated by regulating their transport from the endoplasmic reticulum to the Golgi, mediated by the Ras-like GTPase Rab1, thus affecting their cell surface expression. Decreased cell surface expression of β ARs coincides with increased endothelial cell permeability following stimulation of cells with lipopolysaccharide [47]. In the same line, it has been shown that β2 ARs can form oligomeric complexes with VEGFR2 on the surface and in intracellular endosomes of endothelial cells. These complexes can co-internalize and increase in number in response to both β2 AR and VEGFR2 agonists. Treatment of endothelial cells with VEGFA can also prolong the β2 AR agonist-stimulated association between the β2 AR and β-arrestin 2, thus potentially affecting their subcellular distribution and signaling [48].
5. Role of ARs in Non-Neoplastic Pathological Angiogenesis
5.1. The Role of α1 ARs in Heart Failure and Ischemia and Their Correlation to Angiogenesis
Induction of angiogenesis may prove beneficial in cases such as heart failure caused by chronic myocardial infarction. Stimulation of α1 ARs has promising results in heart failure, not only due to their increased inotropic properties, but also due to the induction of a paracrine mechanism that results in angiogenesis stimulation and inhibition of cardiac remodeling. Overexpression of α1A ARs in cardiomyocytes in a transgenic mouse model led to reduced cardiac remodeling after chronic myocardial infarction that has been attributed to the induction of angiogenesis. The latter is the result of crosstalk signaling between the cardiomyocyte and the endothelial cell. Cardiomyocytes that overexpress the α1A ARs secrete VEGFA downstream of the MEK/ERK1/2 pathway. VEGFA then acts on endothelial cells resulting in their proliferation, migration, and activation of the angiogenic process [49].
On the other hand, the density of α1 ARs has been shown to increase in a model of hindlimb ischemia in Wistar-Kyoto rats and treatment of the rats with doxazosin seems to promote neo-angiogenesis without affecting systemic blood pressure, suggesting an anti-angiogenic effect of α1 ARs stimulation [22].
5.2. Involvement of β ARs in Angiogenesis-Related Pathologies—Evidence Supports a Positive Regulation of Angiogenesis by β ARs Stimulation
Based on the observations that β2 AR overexpression or activation enhances angiogenic activities of EPCs in vitro [15,32,33], in a mouse model of induced ischemia, injection of isoproterenol-treated wildtype EPCs has been shown to significantly improve blood density and blood flow compared to non-treated or β2 AR knockout EPC-treated mice [32].
Chronic stress has been implicated in the development of endometriosis through activation of the β2 ARs, expressed in ectopic endometrium, by the released catecholamines. The downstream cAMP/PKA signaling pathway, followed by the activation of CREB, seem to positively affect angiogenesis, thus accelerating endometriosis [50]. In a rat model of surgically induced endometriosis, propranolol has been shown to decrease expression of VEGFA, MMP2 and MMP9 and suppress endometrial tissue development [51].
Chronic ischemia in Wistar Kyoto Rats leads to decreased vascular density within the ischemic hindlimb. Overexpression of β2 ARs restores capillary density and improves hindlimb perfusion. Similarly, the impaired angiogenesis observed in the spontaneously hypertensive rat model was significantly ameliorated following overexpression of β2 ARs [26].
Despite the observation that terbutaline promotes physiological angiogenesis in vitro and in vivo [29], it has no effect on revascularization following spinal cord injury in a mouse model [29]. It needs to be clarified by further studies whether stimulation of the β2 ARs may prove beneficial in central nervous system injuries that would benefit from angiogenesis stimulation.
Antagonism of β ARs has shown beneficial effects in several pathologies that may benefit from angiogenesis inhibition, as evidenced by several studies that have used propranolol. First, propranolol has been suggested as a treatment for infantile hemangiomas, significantly reducing their extent. Propranolol treatment decreases the anti-apoptotic Bcl-2 protein expression and increases expression of the pro-apoptotic Bax protein in infantile hemangioma tissues in vivo. Propranolol also hinders the expression of PDGF-BB in vivo and downregulates expression of the angiopoietin-like protein 4 in endothelial cells [52,53,54]. Hemangioma-derived endothelial cells express β1 and β2 ARs. Isoprenaline increases intracellular cAMP levels leading to increased VEGFA expression and VEGFR2 phosphorylation. Activation of VEGFR2 leads to ERK1/2 phosphorylation and cell proliferation through activation of several cell cycle regulators. The stimulatory effects of isoprenaline are VEGFR2-dependent and are inhibited by both β1 and β2 AR antagonists, with the effects of the β2 AR antagonists being more prominent [55]. Many β blockers, such as propranolol, have high affinities for β3 ARs and accumulating evidence suggests that the β3 ARs may play a major role in the pathophysiology of infantile hemangioma [56].
Propranolol has been also used in the therapy of choroidal neovascularization (CNV), which is an ischemic complication of various eye diseases such as Stargardt disease, degenerative myopia, and choroidal hemangioma. CNV occurs in the choroid that forms the middle layer of the eyeball and is characterized by loss of homeostasis between the expression of angiogenic factors, such as VEGFA, FGF2 and PDGF, and anti-angiogenic factors such as pigment epithelium-derived factor (PEDF) and thrombospondin-1 (TSP1). The enhanced angiogenesis in the choroidal space gradually leads to blindness [57]. Mononuclear macrophages that express all three types of β ARs appear to be the major inflammatory component of this condition, releasing pro- and anti-angiogenic agents depending on their polarization [58]. Propranolol seems to limit CNV by decreasing the expression of the inflammatory mediators IL-6 and TNFα and increasing the expression of the anti-inflammatory IL-10 and the anti-angiogenic PEDF by the mononuclear macrophages [59].
Propranolol has been also studied in retinopathy of prematurity (ROP), an eye disease that leads to blindness caused by delayed vascular growth, retinal ischemia, and abnormal angiogenesis. In oxygen induced ROP models, propranolol decreases VEGFA expression and retinal angiogenesis, while it enhances pericyte apoptosis via the inhibition of the Akt signaling pathway [60]. The effect seems to be mainly due to β2 but not β1 or β3 AR antagonism [61].
Propranolol can be also beneficial in diabetic foot ulcers, as evidenced in mouse models, in which topical use of propranolol in the wounded area promotes keratinocyte proliferation and migration. Dermal and epidermal regeneration is enhanced via the increased levels of epidermal growth factor and MMP9, while the expression of VEGFA and endothelial cell proliferation are decreased. Pericytes and mural cell proliferation is enhanced, and this may explain the increased density of functional blood vessels in diabetic wounds following propranolol treatment [62]. Like the effects of topical application, in an in vivo model of cutaneous wound healing in streptozotocin-induced diabetic rats, oral administration of propranolol improves wound healing by enhancing cell proliferation, collagen deposition, NO levels and blood vessel density [63].
6. Role of ARs in Cancer Angiogenesis
The induction of angiogenesis has been characterized as one of the most important hallmarks of cancer and is a crucial process for tumor growth. As tumors increase in size, tumor parenchyma due to insufficient blood supply becomes hypoxic, secretes pro-angiogenic growth factors, such as VEGFA, and stimulates angiogenesis. In contrast to physiological angiogenesis, the cancer blood vessels are usually abnormal, leaky, and misshapen, characterized by a lack of auxiliary pericytes and high permeability [64,65].
Besides a direct effect of ARs on endothelial cells as described in previous paragraphs, tumor angiogenesis may be regulated indirectly, following activation or inhibition of ARs expressed in cancer cells. Human lung adenocarcinoma A549 cells [66], human breast cancer MDA-MB-231 cells [67], human colon cancer HCT116 cells, human ovarian carcinoma OVCAR8 cells [68] and epidermoid carcinoma cell line A-431 [69] are examples of cancer cell lines that express functional β ARs. To the best of our knowledge, there are limited data on the involvement of the α ARs in the regulation of cancer angiogenesis.
In line with an indirect role of β ARs in the stimulation of cancer angiogenesis are data showing that nebivolol suppresses mitochondrial respiration and ATP synthase in colon and breast cancer cell lines. Even though treatment with nebivolol does not affect cancer cell proliferation and apoptosis in vitro, in mice carrying colon carcinomas, treatment with nebivolol results in delayed tumor growth and decreased angiogenesis with less well-structured basal lamina and fewer pericytes. In addition, although VEGFA levels remain unaffected, nebivolol significantly decreases VEGFR2 expression and impedes normal endothelial cell proliferation [68].
A mechanism through which ARs in cancer cells may enhance cancer angiogenesis involves the peroxisome proliferator-activated receptor gamma (PPARγ). In animal models of breast cancer, activation of β ARs by chronic stress-produced catecholamines suppresses PPARγ expression and enhances the production of VEGFA and FGF2 by cancer cells, resulting in highly vascularized tumors. Treatment with the PPARγ synthetic agonist pioglitazone results in almost avascular tumors and abolishes the effects of chronic stress. The effect of chronic stress and norepinephrine on PPARγ downregulation and angiogenesis stimulation is mediated by the β2 ARs [70].
The β2 AR seems to also affect breast cancer metastasis to the bone. Isoproterenol or chronic stress following injection of an osteotropic variant of MDA-MB-231 breast cancer cells in mice increases VEGFA-positive osteoblasts, bone vessel density and incidence of bone metastatic lesions, effects that are abrogated by global or osteoblast-specific genetic loss of β2 ARs [67]. The effect of isoproterenol on the VEGFA expression by osteoblasts is independent of the presence of cancer cells and can be inhibited by blocking the interaction of VEGFA with VEGFR2 [67], similarly to what has been observed in hemangioma-derived endothelial cells [55].
Treatment of human lung adenocarcinoma A549 cells that express β2 ARs with isoproterenol results in increased phosphorylation of ERK1/2 and CREB, and increased mRNA levels of MMP2, MMP9 and VEGFA. Gene silencing of CREB inhibits isoproterenol-induced production of MMP2, MMP9 and VEGFA [66]. Similar results are obtained from an in vivo model of repeated social defeat stress-induced lung cancer, where it has been shown that stress exposure enhances expression of VEGFA, VEGFR2, MMP2 and MMP9, thus enabling tumor vasculature formation and cancer progression [71]. The mammalian C-terminal Eps15-homology domain 1-containing protein (EHD1), which regulates β2 AR recycling from the endocytic compartment to the plasma membrane, has recently been shown to promote non-small-cell lung cancer progression by positively regulating angiogenesis through β2 AR signaling in lung cancer cell lines [72].
Ultraviolet B (UVB)-irradiated murine skin keratinocytes express β2 ARs. Angiogenesis, as well as the number of skin tumors and the tumor burden, are significantly decreased in UVB-irradiated mice following treatment with two selective β2 AR antagonists that decrease VEGFA expression through inhibition of CREB phosphorylation [69]. In the same line, in prostate cancer cells in vitro and in a prostate cancer xenograft model in vivo, isoproterenol and chronic stress respectively, through β adrenergic signaling, enhance the phosphorylation of CREB, which induces the expression of histone deacetylase C2 (HDAC2) and suppresses TSP1 production by epigenetic modification [73]. Signaling through β2 ARs in prostate cancer endothelial cells enhances endothelial oxidative phosphorylation and prostate cancer angiogenesis; targeting this pathway may improve prostate cancer therapeutic approaches [74]. In prostate cancer, selective α1 AR antagonists may have both anticancer and anti-angiogenic effects. This is based on the observation that terazosin not only directly inhibits prostate cancer cell proliferation, but also inhibits angiogenic functions of endothelial cells in vitro and VEGFA-induced angiogenesis in nude mice in vivo [21].
In addition to an effect on tumor cells, catecholamines may indirectly induce angiogenesis and neoplasia progression by activating ARs in tumor stroma cells, such as macrophages. In in vivo mouse models of lung cancer, chemical depletion of norepinephrine delays tumor growth, which seems to result from a decrease in the percentage of polarized M2 macrophages compared to M1 macrophages, as well as a decrease in the concentration of VEGFA in the tumors. Following stimulation with epinephrine or norepinephrine, M2 polarized macrophages secrete VEGFA and enhance angiogenesis in vitro, effects that are prevented by their pretreatment with propranolol [75]. Similarly, in a mouse model of breast cancer, stress-induced epinephrine and norepinephrine lead to the polarization of macrophages to the M2 phenotype and increased tumor weight and volume. The effect of epinephrine on the polarization of macrophages towards the M2 phenotype has been verified in vitro and occurs through β2 ARs [76]. In an ovarian cancer cells/macrophages co-culture system in vitro, long-term exposure to epinephrine and norepinephrine increases the expression of numerous angiogenic molecules, including VEGFA, PDGF-AA and angiogenin. In an orthotopic ovarian cancer model in vivo, stress conditions also lead to enhanced PDGF-AA production, infiltration of CD68+ macrophages in tumors, and increased ovarian cancer growth [77].
7. Conclusions and Future Directions
It is evident that both α and β ARs modulate angiogenesis (Figure 1) and their many agonists and antagonists, which are already in clinical use, can be exploited for their use in the control of angiogenesis. This has already been the case in pathologies, such as in infantile hemangiomas, where the use of propranolol is already established [78,79]. There are, however, many unanswered questions that, so far, have led to unclear and sometimes conflicting data, as mentioned in this review, and need to be clarified by future studies. The AR subtype(s) expressed by different endothelial cells should be clearly identified and verified by both in vitro and in vivo studies. The exact pathways downstream of each subtype in each case should be also elucidated. This task is very difficult due to homologies of these receptors at the level of effector binding sites and the common downstream signaling pathways. An example that is indicative of this complexity is the accumulating evidence suggesting that many β (especially selective β1) AR antagonists are linked to β3 AR activation [80], and some of their effects may not be due to β (β1) antagonism, but rather result from β3 activation, as described in this review for the pro-angiogenic effects of nebivolol. Future studies will clarify the missing points and will help expand the therapeutic indications of AR agonists and antagonists to regulate angiogenesis-related pathologies.
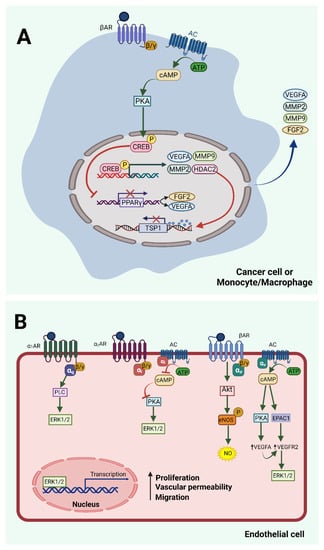
Figure 1.
Adrenergic signaling in endothelial and other cells that regulate angiogenesis. (A) Indirect regulation. Norepinephrine and/or epinephrine reach the microenvironment of cancer or blood or other cells through the local sympathetic nerves or the bloodstream and bind to β ARs, thus activating the AC/cAMP/PKA pathway. PKA phosphorylates the transcription factor CREB, which induces the transcription of multiple pro-angiogenic factors, such as MMP2 and MMP9, VEGFA, and HDAC2. Secreted MMPs destabilize the extracellular matrix, releasing growth factors and allowing cancer cell metastasis and angiogenesis. Secreted VEGFA binds to VEGFR2 on neighboring endothelial cells and enhances their pro-angiogenic phenotype. Similarly, FGF2 and other secreted proangiogenic growth factors stimulate endothelial cells through their specific receptors. HDAC2 epigenetically inhibits the production of the antiangiogenic TSP1. The CREB transcription factor also inhibits PPARγ, leading to further enhancement of VEGFA and FGF2 production. (B) Direct regulation. Endothelial cells express α and β ARs that affect the process of angiogenesis. Activation of α1 and α2 ARs triggers angiogenesis through ERK1/2 activation by different pathways. However, there is limited evidence and conflicting data related to the role of α ARs, as discussed in the text. Activation of β ARs leads to activation of AC/cAMP/PKA or EPAC1-dependent pathways that seem to transactivate the VEGFA/VEGFR2 pathway, also leading to ERK1/2 and endothelial cell activation. The Akt/eNOS pathway which leads to release of NO has been described downstream of β3 ARs, and in the case of EPCs downstream of β2 ARs, stimulating angiogenic properties of endothelial cells. The figure was created with BioRender.com (accessed on 29 November 2021).
Author Contributions
Conceptualization, E.P.; Methodology, all; Software, all; Investigation, all; Resources, E.P.; Writing—Original Draft Preparation, A.X., I.D. and S.F.; Writing—Review & Editing, E.P.; Supervision, E.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef]
- Sweeney, M.; Foldes, G. It Takes Two: Endothelial-Perivascular Cell Cross-Talk in Vascular Development and Disease. Front. Cardiovasc. Med. 2018, 5, 154. [Google Scholar] [CrossRef]
- Jaipersad, A.S.; Lip, G.Y.; Silverman, S.; Shantsila, E. The Role of Monocytes in Angiogenesis and Atherosclerosis. J. Am. Coll. Cardiol. 2014, 63, A22. [Google Scholar] [CrossRef] [PubMed]
- Majewska, A.; Wilkus, K.; Brodaczewska, K.; Kieda, C. Endothelial Cells as Tools to Model Tissue Microenvironment in Hypoxia-Dependent Pathologies. Int. J. Mol. Sci. 2021, 22, 520. [Google Scholar] [CrossRef] [PubMed]
- Wehrwein, E.A.; Orer, H.S.; Barman, S.M. Overview of the Anatomy, Physiology, and Pharmacology of the Autonomic Nervous System. Compr. Physiol. 2016, 6, 1239–1278. [Google Scholar] [CrossRef] [PubMed]
- Schena, G.; Caplan, M.J. Everything You Always Wanted to Know about β3-AR * (* But Were Afraid to Ask). Cells 2019, 8, 357. [Google Scholar] [CrossRef]
- Jensen, B.C.; Swigart, P.M.; Montgomery, M.D.; Simpson, P.C. Functional alpha-1B adrenergic receptors on human epicardial coronary artery endothelial cells. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2010, 382, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Graf, K.; Gräfe, M.; Dümmler, U.; O’Connor, A.; Regitz-Zagrosek, V.; Kunkel, G.; Auch-Schwelk, W.; Fleck, E. Regulation of beta-adrenergic receptors on endothelial cells in culture. Eur. Heart J. 1993, 14, 173–176. [Google Scholar]
- Tkachuk, V.; Buravkova, L.; Resink, T.J.; Mirzopoiazova, T.Y.; Grigorian, G.Y. Molecular mechanism of the desensitization of beta-adrenergic receptors and adenilate cyclase by hypoxia in human endothelial cells. Rossiiskii Fiziologicheskii Zhurnal Imeni IM Sechenova 1997, 83, 94–118. [Google Scholar]
- Grigorian, G.I.; Mirzopoiazova, T.I.; Nikashin, A.V.; Goncharov, N.V.; Danilov, S.M. Identification and characteristics of the beta-adrenergic receptors in the membranes of cultured endothelial cells of the human pulmonary artery. Probl. Endocrinol. 1989, 35, 37–40. [Google Scholar]
- Howell, R.E.; Albelda, S.M.; Daise, M.L.; Levine, E.M. Characterization of beta-adrenergic receptors in cultured human and bovine endothelial cells. J. Appl. Physiol. 1988, 65, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, A.P.; Fox, J.M.; Pullar, C.E. Beta-Adrenoceptor Activation Reduces Both Dermal Microvascular Endothelial Cell Migration via a cAMP-Dependent Mechanism and Wound Angiogenesis. J. Cell. Physiol. 2015, 230, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Durieu-Trautmann, O.; Foignant, N.; Strosberg, A.D.; Couraud, P.O. Coexpression of ß1- and ß2-Adrenergic Receptors on Bovine Brain Capillary Endothelial Cells in Culture. J. Neurochem. 1991, 56, 775–781. [Google Scholar] [CrossRef]
- Safi, S.Z.; Qvist, R.; Yan, G.O.S.; Bin Ismail, I.S. Differential expression and role of hyperglycemia induced oxidative stress in epigenetic regulation of β1, β2 and β3-adrenergic receptors in retinal endothelial cells. BMC Med. Genom. 2014, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Vinci, M.C.; Bellik, L.; Filippi, S.; Ledda, F.; Parenti, A. Trophic effects induced by α1D-adrenoceptors on endothelial cells are potentiated by hypoxia. Am. J. Physiol. Circ. Physiol. 2007, 293, H2140–H2147. [Google Scholar] [CrossRef]
- Pan, L.; Liu, C.; Kong, Y.; Piao, Z.; Cheng, B. Phentolamine inhibits angiogenesis in vitro: Suppression of proliferation migration and differentiation of human endothelial cells. Clin. Hemorheol. Microcirc. 2017, 65, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Pang, M.; Lei, X.; Yao, Z.; Chen, C.; Cheng, B. Nonselective alpha-/beta- AR antagonists can inhibit pericyte proliferation, migration, and secretion in vitro. Clin. Hemorheol. Microcirc. 2020, 75, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Keledjian, K.; Garrison, J.B.; Kyprianou, N. Doxazosin inhibits human vascular endothelial cell adhesion, migration, and invasion. J. Cell. Biochem. 2005, 94, 374–388. [Google Scholar] [CrossRef] [PubMed]
- Ciccarelli, M.; Santulli, G.; Campanile, A.; Galasso, G.; Cervero, P.; Altobelli, G.G.; Cimini, V.; Pastore, L.; Piscione, F.; Trimarco, B.; et al. Endothelial α1 -adrenoceptors regulate neo-angiogenesis. Br. J. Pharmacol. 2008, 153, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.-L.; Guh, J.-H.; Huang, Y.-W.; Chern, J.-W.; Chou, J.-Y.; Teng, C.-M. Identification of apoptotic and antiangiogenic activities of terazosin in human prostate cancer and endothelial cells. J. Urol. 2003, 169, 724–729. [Google Scholar] [CrossRef]
- Muthig, V.; Gilsbach, R.; Haubold, M.; Philipp, M.; Ivacevic, T.; Gessler, M.; Hein, L. Upregulation of Soluble Vascular Endothelial Growth Factor Receptor 1 Contributes to Angiogenesis Defects in the Placenta of α 2B -Adrenoceptor–Deficient Mice. Circ. Res. 2007, 101, 682–691. [Google Scholar] [CrossRef]
- Roskoski, R. A historical overview of protein kinases and their targeted small molecule inhibitors. Pharmacol. Res. 2015, 100, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Pardanaud, L.; Pibouin-Fragner, L.; Dubrac, A.; Mathivet, T.; English, I.; Brunet, I.; Simons, M.; Eichmann, A. Sympathetic Innervation Promotes Arterial Fate by Enhancing Endothelial ERK Activity. Circ. Res. 2016, 119, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Iaccarino, G.; Ciccarelli, M.; Sorriento, D.; Galasso, G.; Campanile, A.; Santulli, G.; Cipolletta, E.; Cerullo, V.; Cimini, V.; Altobelli, G.G.; et al. Ischemic Neoangiogenesis Enhanced by β2-Adrenergic Receptor Overexpression. Circ. Res. 2005, 97, 1182–1189. [Google Scholar] [CrossRef] [PubMed]
- Garg, J.; Feng, Y.-X.; Jansen, S.R.; Friedrich, J.; Lezoualc’H, F.; Schmidt, M.; Wieland, T. Catecholamines facilitate VEGF-dependent angiogenesis via β2-adrenoceptor-induced Epac1 and PKA activation. Oncotarget 2017, 8, 44732–44748. [Google Scholar] [CrossRef] [PubMed]
- Galasso, G.; De Rosa, R.; Ciccarelli, M.; Sorriento, D.; Del Giudice, C.; Strisciuglio, T.; De Biase, C.; Luciano, R.; Piccolo, R.; Pierri, A.; et al. β 2 -Adrenergic Receptor Stimulation Improves Endothelial Progenitor Cell–Mediated Ischemic Neoangiogenesis. Circ. Res. 2013, 112, 1026–1034. [Google Scholar] [CrossRef]
- Ciccarelli, M.; Sorriento, D.; Cipolletta, E.; Santulli, G.; Fusco, A.; Zhou, R.-H.; Eckhart, A.D.; Peppel, K.; Koch, W.J.; Trimarco, B.; et al. Impaired neoangiogenesis in β2-adrenoceptor gene-deficient mice: Restoration by intravascular human β2-adrenoceptor gene transfer and role of NFκB and CREB transcription factors. Br. J. Pharmacol. 2011, 162, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, S.; Kusters, L.; Bronckaers, A.; Geurts, N.; Hendrix, S. The β2-Adrenoceptor Agonist Terbutaline Stimulates Angiogenesis via Akt and ERK Signaling. J. Cell Physiol. 2017, 232, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Shu, X.-R.; Wu, F.; Hu, Q.-S.; Deng, B.-Q.; Wang, J.-F.; Nie, R.-Q. Overexpression of the β2AR gene improves function and re-endothelialization capacity of EPCs after arterial injury in nude mice. Stem. Cell Res. Ther. 2016, 7, 73. [Google Scholar] [CrossRef]
- Banquet, S.; Delannoy, E.; Agouni, A.; Dessy, C.; Lacomme, S.; Hubert, F.; Richard, V.; Muller, B.; Leblais, V. Role of Gi/o-Src kinase-PI3K/Akt pathway and caveolin-1 in β2-adrenoceptor coupling to endothelial NO synthase in mouse pulmonary artery. Cell Signal 2011, 23, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Perez-Aso, M.; Flacco, N.; Carpena, N.; Montesinos, M.C.; D’Ocon, P.; Ivorra, M.D. β-Adrenoceptors differentially regulate vascular tone and angiogenesis of rat aorta via ERK1/2 and p38. Vasc. Pharmacol. 2014, 61, 80–89. [Google Scholar] [CrossRef]
- Stohl, L.L.; Zang, J.B.; Ding, W.; Manni, M.; Zhou, X.K.; Granstein, R.D. Norepinephrine and adenosine-5′-triphosphate synergize in inducing IL-6 production by human dermal microvascular endothelial cells. Cytokine 2013, 64, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Gopinathan, G.; Milagre, C.; Pearce, O.; Reynolds, L.E.; Hodivala-Dilke, K.; Leinster, D.A.; Zhong, H.; Hollingsworth, R.E.; Thompson, R.G.; Whiteford, J.R.; et al. Interleukin-6 Stimulates Defective Angiogenesis. Cancer Res. 2015, 75, 3098–3107. [Google Scholar] [CrossRef] [PubMed]
- Pullar, C.E.; Le Provost, G.S.; O’Leary, A.P.; Evans, S.E.; Baier, B.S.; Isseroff, R.R. β2AR Antagonists and β2AR Gene Deletion Both Promote Skin Wound Repair Processes. J. Investig. Dermatol. 2012, 132, 2076–2084. [Google Scholar] [CrossRef] [PubMed]
- Le Provost, G.S.; Pullar, C.E. β2-Adrenoceptor Activation Modulates Skin Wound Healing Processes to Reduce Scarring. J. Investig. Dermatol. 2015, 135, 279–288. [Google Scholar] [CrossRef]
- Fu, Y.-C.; Chi, C.-S.; Yin, S.-C.; Hwang, B.; Chiu, Y.-T.; Hsu, S.-L. Norepinephrine induces apoptosis in neonatal rat endothelial cells via down-regulation of Bcl-2 and activation of β-adrenergic and caspase-2 pathways. Cardiovasc. Res. 2004, 61, 143–151. [Google Scholar] [CrossRef]
- Tsopanoglou, N.E.; Haralabopoulos, G.C.; Maragoudakis, M.E. Opposing Effects on Modulation of Angiogenesis by Protein Kinase C and cAMP-Mediated Pathways. J. Vasc. Res. 1994, 31, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Favot, L.; Keravis, T.; Holl, V.; Le Bec, A.; Lugnier, C. VEGF-induced HUVEC migration and proliferation are decreased by PDE2 and PDE4 inhibitors. Thromb. Haemost. 2003, 90, 334–343. [Google Scholar] [CrossRef]
- Kim, S.; Bakre, M.; Yin, H.; Varner, J.A. Inhibition of endothelial cell survival and angiogenesis by protein kinase A. J. Clin. Investig. 2002, 110, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Baluk, P.; McDonald, D.M. The beta 2-adrenergic receptor agonist formoterol reduces microvascular leakage by inhibiting endothelial gap formation. Am. J. Physiol. Cell. Mol. Physiol. 1994, 266, L461–L468. [Google Scholar] [CrossRef] [PubMed]
- Zink, S.; Rösen, P.; Lemoine, H. Micro- and macrovascular endothelial cells in beta-adrenergic regulation of transendothelial permeability. Am. J. Physiol. Physiol. 1995, 269, C1209–C1218. [Google Scholar] [CrossRef]
- Tonello, C.; Giordano, A.; Cozzi, V.; Cinti, S.; Stock, M.J.; Carruba, M.O.; Nisoli, E. Role of sympathetic activity in controlling the expression of vascular endothelial growth factor in brown fat cells of lean and genetically obese rats. FEBS Lett. 1999, 442, 167–172. [Google Scholar] [CrossRef]
- Mason, R.P.; Jacob, R.F.; Corbalan, J.J.; Szczesny, D.; Matysiak, K.; Malinski, T. The favorable kinetics and balance of nebivolol-stimulated nitric oxide and peroxynitrite release in human endothelial cells. BMC Pharmacol. Toxicol. 2013, 14, 48. [Google Scholar] [CrossRef]
- Kou, R.; Michel, T. Epinephrine Regulation of the Endothelial Nitric-oxide Synthase. J. Biol. Chem. 2007, 282, 32719–32729. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, G.; Lin, K.; Yin, H.; Zhou, C.; Liu, T.; Wu, G.; Qian, G. Rab1 GTPase promotes expression of β-adrenergic receptors in rat pulmonary microvascular endothelial cells. Int. J. Biochem. Cell Biol. 2010, 42, 1201–1209. [Google Scholar] [CrossRef][Green Version]
- Kilpatrick, L.E.; Alcobia, D.C.; White, C.; Peach, C.; Glenn, J.R.; Zimmerman, K.; Kondrashov, A.; Pfleger, K.; Ohana, R.F.; Robers, M.B.; et al. Complex Formation between VEGFR2 and the β2-Adrenoceptor. Cell Chem. Biol. 2019, 26, 830–841.e9. [Google Scholar] [CrossRef]
- Zhao, X.; Balaji, P.; Pachon, R.; Beniamen, D.M.; Vatner, D.E.; Graham, R.M.; Vatner, S.F. Overexpression of Cardiomyocyte α 1A -Adrenergic Receptors Attenuates Postinfarct Remodeling by Inducing Angiogenesis Through Heterocellular Signaling. Arter. Thromb. Vasc. Biol. 2015, 35, 2451–2459. [Google Scholar] [CrossRef] [PubMed]
- Wnęk, A.; Andrzejewska, E.; Kobos, J.; Taran, K.; Przewratil, P. Molecular and immunohistochemical expression of apoptotic proteins Bax, Bcl-2 and Caspase 3 in infantile hemangioma tissues as an effect of propranolol treatment. Immunol. Lett. 2017, 185, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Tani, S.; Kunimoto, K.; Inaba, Y.; Mikita, N.; Kaminaka, C.; Kanazawa, N.; Yamamoto, Y.; Kakimoto, N.; Suenaga, T.; Takeuchi, T.; et al. Change of serum cytokine profiles by propranolol treatment in patients with infantile hemangioma. Drug Discov. Ther. 2020, 14, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; North, P.E.; Elsey, J.; Bubley, J.; Rao, S.; Jung, Y.; Wu, S.; Zou, M.-H.; Pollack, B.P.; Kumar, J.; et al. Propranolol exhibits activity against hemangiomas independent of beta blockade. NPJ Precis. Oncol. 2019, 3, 27. [Google Scholar] [CrossRef]
- Ji, Y.; Chen, S.; Li, K.; Xiao, X.; Zheng, S.; Xu, T. The role of β-adrenergic receptor signaling in the proliferation of hemangioma-derived endothelial cells. Cell Div. 2013, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- González, C.E.E.; Hernández, A.G.; Villalón, C.M.; Rodríguez, M.G.; Cancino, B.A.M. β-Adrenoceptor Blockade for Infantile Hemangioma Therapy: Do β3-Adrenoceptors Play a Role? J. Vasc. Res. 2018, 55, 159–168. [Google Scholar] [CrossRef]
- Bhutto, I.A.; McLeod, D.S.; Hasegawa, T.; Kim, S.Y.; Merges, C.; Tong, P.; Lutty, G.A. Pigment epithelium-derived factor (PEDF) and vascular endothelial growth factor (VEGF) in aged human choroid and eyes with age-related macular degeneration. Exp. Eye Res. 2006, 82, 99–110. [Google Scholar] [CrossRef]
- Tahiri, H.; Omri, S.; Yang, C.; Duhamel, F.; Samarani, S.; Ahmad, A.; Vezina, M.; Bussières, M.; Vaucher, E.; Sapieha, P.; et al. Lymphocytic Microparticles Modulate Angiogenic Properties of Macrophages in Laser-induced Choroidal Neovascularization. Sci. Rep. 2016, 6, 37391. [Google Scholar] [CrossRef]
- Omri, S.; Tahiri, H.; Pierre, W.C.; DesJarlais, M.; Lahaie, I.; Loiselle, S.-E.; Rezende, F.; Lodygensky, G.; Hebert, T.E.; Ong, H.; et al. Propranolol Attenuates Proangiogenic Activity of Mononuclear Phagocytes: Implication in Choroidal Neovascularization. Investig. Opthalmology Vis. Sci. 2019, 60, 4632–4642. [Google Scholar] [CrossRef]
- Yun, J.-H.; Koh, Y.J.; Jeong, H.-S.; Lee, D.-H.; Lee, E.H.; Cho, C.-H. Propranolol increases vascular permeability through pericyte apoptosis and exacerbates oxygen-induced retinopathy. Biochem. Biophys. Res. Commun. 2018, 503, 2792–2799. [Google Scholar] [CrossRef]
- Martini, D.; Monte, M.D.; Ristori, C.; Cupisti, E.; Mei, S.; Fiorini, P.; Filippi, L.; Bagnoli, P. Antiangiogenic effects of β2-adrenergic receptor blockade in a mouse model of oxygen-induced retinopathy. J. Neurochem. 2011, 119, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Liu, Y.; Yang, Y.; Tang, J.; Cheng, B. Topical 1% propranolol cream promotes cutaneous wound healing in spontaneously diabetic mice. Wound Repair Regen. 2017, 25, 389–397. [Google Scholar] [CrossRef]
- Romana-Souza, B.; Nascimento, A.P.; Monte-Alto-Costa, A. Propranolol improves cutaneous wound healing in streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 2009, 611, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, R.I.; Chircov, C.; Grumezescu, A.M.; Teleanu, D.M. Tumor Angiogenesis and Anti-Angiogenic Strategies for Cancer Treatment. J. Clin. Med. 2019, 9, 84. [Google Scholar] [CrossRef]
- Zuazo-Gaztelu, I.; Casanovas, O. Unraveling the Role of Angiogenesis in Cancer Ecosystems. Front. Oncol. 2018, 8, 248. [Google Scholar] [CrossRef]
- Hu, P.; He, J.; Liu, S.; Wang, M.; Pan, B.; Zhang, W. β2-adrenergic receptor activation promotes the proliferation of A549 lung cancer cells via the ERK1/2/CREB pathway. Oncol. Rep. 2016, 36, 1757–1763. [Google Scholar] [CrossRef] [PubMed]
- Mulcrone, P.L.; Campbell, J.P.; Clément-Demange, L.; Anbinder, A.L.; Merkel, A.; Brekken, R.A.; Sterling, J.A.; Elefteriou, F. Skeletal Colonization by Breast Cancer Cells Is Stimulated by an Osteoblast and β2AR-Dependent Neo-Angiogenic Switch. J. Bone Miner. Res. 2017, 32, 1442–1454. [Google Scholar] [CrossRef]
- Nuevo-Tapioles, C.; Santacatterina, F.; Stamatakis, K.; De Arenas, C.N.; De Cedrón, M.G.; Formentini, L.; Cuezva, J.M. Coordinate β-adrenergic inhibition of mitochondrial activity and angiogenesis arrest tumor growth. Nat. Commun. 2020, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Bhat, M.; Peters, S.; Mitra, R.; Oberyszyn, T.; Basu, S. Suppression of beta 2 adrenergic receptor actions prevent UVB mediated cutaneous squamous cell tumorigenesis through inhibition of VEGF-A induced angiogenesis. Mol. Carcinog. 2021, 60, 172–178. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Z.; Zhang, L.; Hu, X.; Wang, Z.; Ni, H.; Wang, Y.; Qin, J. Activation of β2-Adrenergic Receptor Promotes Growth and Angiogenesis in Breast Cancer by Down-regulating PPARγ. Cancer Res. Treat. 2020, 52, 830–847. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, B.-J.; Ji, S.; Wu, J.-F.; Xu, C.-Q.; Du, Y.-J.; You, X.-F.; Li, B.; Le, J.-J.; Xu, H.-L.; et al. Social defeat stress promotes tumor growth and angiogenesis by upregulating vascular endothelial growth factor/extracellular signal-regulated kinase/matrix metalloproteinase signaling in a mouse model of lung carcinoma. Mol. Med. Rep. 2012, 12, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xing, Y.; Meng, Q.; Lu, H.; Liu, W.; Yan, S.; Song, Y.; Xu, X.; Huang, J.; Cui, Y.; et al. Mammalian Eps15 homology domain 1 potentiates angiogenesis of non-small cell lung cancer by regulating β2AR signaling. J. Exp. Clin. Cancer Res. 2019, 38, 174. [Google Scholar] [CrossRef] [PubMed]
- Hulsurkar, M.; Li, Z.; Zhang, Y.; Li, X.; Zheng, D.; Li, W. Beta-adrenergic signaling promotes tumor angiogenesis and prostate cancer progression through HDAC2-mediated suppression of thrombospondin-1. Oncogene 2017, 36, 1525–1536. [Google Scholar] [CrossRef]
- Zahalka, A.H.; Arnal-Estapé, A.; Maryanovich, M.; Nakahara, F.; Cruz, C.D.; Finley, L.W.S.; Frenette, P.S. Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Science 2017, 358, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Wei, Y.; Li, Z.-Y.; Cai, X.-Y.; Zhang, L.-L.; Dong, X.-R.; Zhang, S.; Zhang, R.-G.; Meng, R.; Zhu, F.; et al. Catecholamines contribute to the neovascularization of lung cancer via tumor-associated macrophages. Brain Behav. Immun. 2019, 81, 111–121. [Google Scholar] [CrossRef]
- Qin, J.-F.; Jin, F.-J.; Li, N.; Guan, H.-T.; Lan, L.; Ni, H.; Wang, Y. Adrenergic receptor β2 activation by stress promotes breast cancer progression through macrophages M2 polarization in tumor microenvironment. BMB Rep. 2015, 48, 295–300. [Google Scholar] [CrossRef]
- Colon-Echevarria, C.B.; Ortiz, T.; Maldonado, L.; Hidalgo-Vargas, M.J.; Pérez-Morales, J.; Aquino-Acevedo, A.N.; Herrera-Noriega, R.; Bonilla-Claudio, M.; Castro, E.M.; Armaiz-Pena, G.N. Zoledronic Acid Abrogates Restraint Stress-Induced Macrophage Infiltration, PDGF-AA Expression, and Ovarian Cancer Growth. Cancers 2020, 12, 2671. [Google Scholar] [CrossRef]
- Léauté-Labrèze, C.; De La Roque, E.D.; Hubiche, T.; Boralevi, F.; Thambo, J.-B.; Taïeb, A. Propranolol for Severe Hemangiomas of Infancy. N. Engl. J. Med. 2008, 358, 2649–2651. [Google Scholar] [CrossRef]
- Saerens, J.; De Leye, H.; Janmohamed, S.R. News on infantile haemangioma. Part 2: Therapy and evaluation. Clin. Exp. Dermatol. 2021, 46, 480–486. [Google Scholar] [CrossRef]
- Cannavo, A.; Koch, W.J. Targeting β3-Adrenergic Receptors in the Heart: Selective Agonism and β-Blockade. J. Cardiovasc. Pharmacol. 2017, 69, 71–78. [Google Scholar] [CrossRef]
- Wong, B.W.; Marsch, E.; Treps, L.; Baes, M.; Carmeliet, P. Endothelial cell metabolism in health and disease: Impact of hypoxia. EMBO J. 2017, 36, 2187–2203. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, T.; Li, Y.; Feng, J.; Nie, R.; Wang, X.; Peng, C.; Ke, X. β2AR-dependent signaling contributes to in-vivo reendothelialization capacity of endothelial progenitor cells by shear stress. J. Hypertens. 2020, 38, 82–94. [Google Scholar] [CrossRef]
- Long, Q.; Liu, X.; Qi, Q.; Guo, S.-W. Chronic stress accelerates the development of endometriosis in mouse through adrenergic receptor β2. Hum. Reprod. 2016, 31, 2506–2519. [Google Scholar] [CrossRef]
- Uzunlar, O.; Ozyer, S.; Engin-Ustun, Y.; Moraloglu, O.; Gulerman, H.C.; Caydere, M.; Keskin, S.M.; Mollamahmutoglu, L. Effects of repeated propranolol administration in a rat model of surgically induced endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 182, 167–171. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).