Nanomedicine for Treating Diabetic Retinopathy Vascular Degeneration
Abstract
:1. Introduction
2. Clinical Management of DR
3. Ocular Biological Barriers and Investigation Models
4. Nanomedicine Application in DR
4.1. Natural and Synthetic Polymers
4.2. Albumin Nanoparticles
4.3. Inorganic Nanoparticles
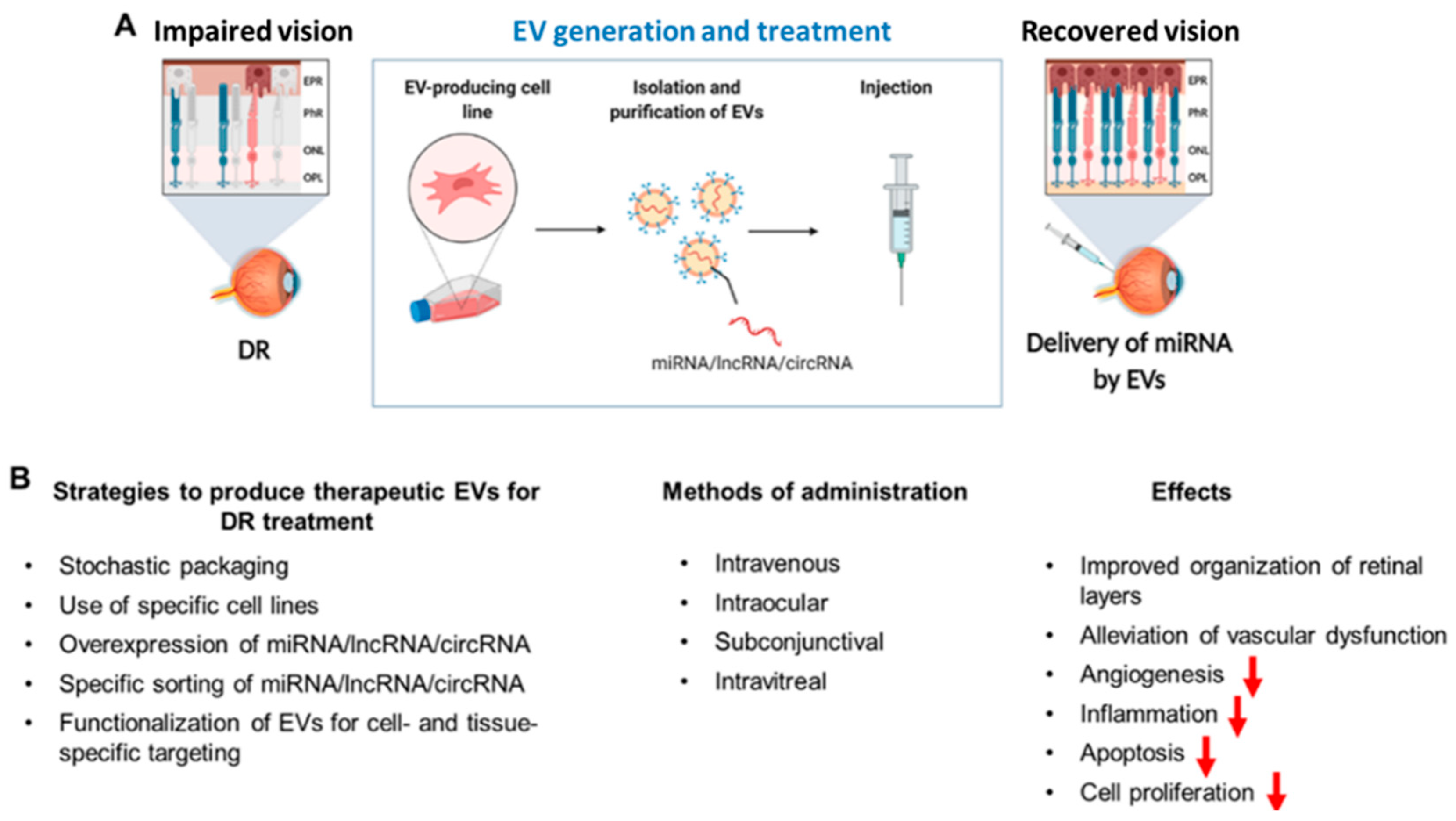
4.4. Extracellular Vesicles for RNA Delivery
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Center for Disease and Control Prevention. Diabetes. Available online: https://www.cdc.gov/diabetes/basics/diabetes.html (accessed on 11 October 2021).
- DiMeglio, L.A.; Evans-Molina, C.; Oram, R.A. Type 1 diabetes. Lancet 2018, 391, 2449–2462. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I. Type 2 diabetes mellitus. Nat. Rev. Dis. Primers 2015, 1, 1–22. [Google Scholar] [CrossRef]
- McIntyre, H.D.; Catalano, P.; Zhang, C.; Desoye, G.; Mathiesen, E.R.; Damm, P. Gestational diabetes mellitus. Nat. Rev. Dis. Primers 2019, 5, 1–19. [Google Scholar] [CrossRef]
- Ingelfinger, J.R.; Jarcho, J.A. Increase in the incidence of diabetes and its implications. N. Engl. J. Med. 2017, 376, 1473–1474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 11 October 2021).
- Liu, Y.; Wu, N. Progress of Nanotechnology in Diabetic Retinopathy Treatment. Int. J. Nanomed. 2021, 16, 1391. [Google Scholar] [CrossRef]
- Metsker, O.; Magoev, K.; Yakovlev, A.; Yanishevskiy, S.; Kopanitsa, G.; Kovalchuk, S.; Krzhizhanovskaya, V.V. Identification of risk factors for patients with diabetes: Diabetic polyneuropathy case study. BMC Med. Inform. Decis. Mak. 2020, 20, 1–15. [Google Scholar] [CrossRef]
- Frolov, D.V.; Kryukov, E.V.; Gerasimenko, M.Y.; Kulikov, A.G. Combined physical therapy for diabetic angiopathy. Russ. J. Physiother. Balneol. Rehabil. 2020, 19, 25–31. [Google Scholar] [CrossRef]
- Unnikrishnan, R.; Misra, A. Infections and diabetes: Risks and mitigation with reference to India. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 1889–1894. [Google Scholar] [CrossRef]
- Biessels, G.J.; Despa, F. Cognitive decline and dementia in diabetes mellitus: Mechanisms and clinical implications. Nat. Rev. Endocrinol. 2018, 14, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Nusca, A.; Tuccinardi, D.; Albano, M.; Cavallaro, C.; Ricottini, E.; Manfrini, S.; Pozzilli, P.; Di Sciascio, G. Glycemic variability in the development of cardiovascular complications in diabetes. Diabetes/Metab. Res. Rev. 2018, 34, e3047. [Google Scholar] [CrossRef] [PubMed]
- Shatnawi, N.J.; Al-Zoubi, N.A.; Hawamdeh, H.M.; Khader, Y.S.; Garaibeh, K.; Heis, H.A. Predictors of major lower limb amputation in type 2 diabetic patients referred for hospital care with diabetic foot syndrome. Diabetes Metab. Syndr. Obes. Targets Ther. 2018, 11, 313. [Google Scholar] [CrossRef] [Green Version]
- Maseko, S.; van Staden, D.; Mhlongo, E. The Rising Burden of Diabetes-Related Blindness: A Case for Integration of Primary Eye Care into Primary Health Care in Eswatini. Healthcare 2021, 9, 835. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.Y.; Ng, D.S.; Lam, A.; Luk, F.; Wong, R.; Chan, C.; Mohamed, S.; Fong, A.; Lok, J.; Tso, T. Determinants of quantitative optical coherence tomography angiography metrics in patients with diabetes. Sci. Rep. 2017, 7, 1–10. [Google Scholar]
- Semeraro, F.; Morescalchi, F.; Cancarini, A.; Russo, A.; Rezzola, S.; Costagliola, C. Diabetic retinopathy, a vascular and inflammatory disease: Therapeutic implications. Diabetes Metab. 2019, 45, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Alghadyan, A.A. Diabetic retinopathy–An update. Saudi J. Ophthalmol. 2011, 25, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Tarr, J.M.; Kaul, K.; Wolanska, K.; Kohner, E.M.; Chibber, R. Retinopathy in diabetes. Diabetes 2013, 88–106. [Google Scholar]
- Chopdar, A.; Chakravarthy, U.; Verma, D. Age related macular degeneration. BMJ 2003, 326, 485–488. [Google Scholar] [CrossRef]
- Pollinger, K.; Hennig, R.; Ohlmann, A.; Fuchshofer, R.; Wenzel, R.; Breunig, M.; Tessmar, J.; Tamm, E.R.; Goepferich, A. Ligand-functionalized nanoparticles target endothelial cells in retinal capillaries after systemic application. Proc. Natl. Acad. Sci. USA 2013, 110, 6115–6120. [Google Scholar] [CrossRef] [Green Version]
- Bhagat, N.; Zarbin, M.A. Epidemiology, risk factors, and pathophysiology of diabetic retinopathy. In Clinical Strategies in the Management of Diabetic Retinopathy; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–19. [Google Scholar]
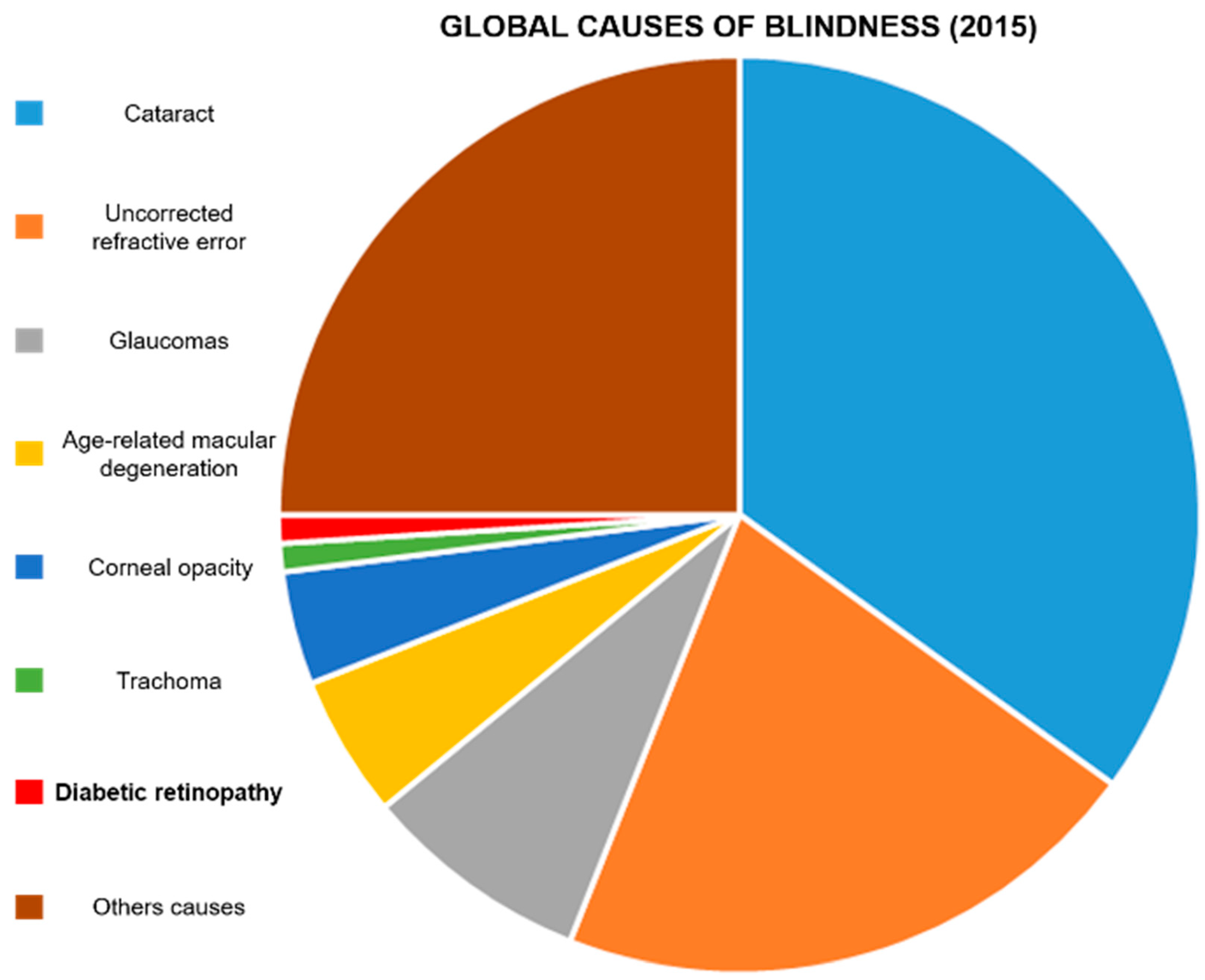
- Flaxman, S.R.; Bourne, R.R.; Resnikoff, S.; Ackland, P.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H. Global causes of blindness and distance vision impairment 1990–2020: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e1221–e1234. [Google Scholar] [CrossRef] [Green Version]
- Ackland, P.; Resnikoff, S.; Bourne, R. World blindness and visual impairment: Despite many successes, the problem is growing. Community Eye Health 2017, 30, 71. [Google Scholar]
- Mahaling, B.; Srinivasarao, D.A.; Raghu, G.; Kasam, R.K.; Reddy, G.B.; Katti, D.S. A non-invasive nanoparticle mediated delivery of triamcinolone acetonide ameliorates diabetic retinopathy in rats. Nanoscale 2018, 10, 16485–16498. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, L.-J.; Yu, J.; Wang, H.-J.; Zhang, F.; Liu, Q.; Wu, J. Involvement of advanced glycation end products in the pathogenesis of diabetic retinopathy. Cell. Physiol. Biochem. 2018, 48, 705–717. [Google Scholar] [CrossRef] [PubMed]
- WWu, M.-Y.; Yiang, G.-T.; Lai, T.-T.; Li, C.-J. The Oxidative Stress and Mitochondrial Dysfunction during the Pathogenesis of Diabetic Retinopathy. Oxid. Med. Cell. Longev. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Goldin, A.; Beckman, J.A.; Schmidt, A.M.; Creager, M.A. Advanced glycation end products: Sparking the development of diabetic vascular injury. Circulation 2006, 114, 597–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Kharashi, A.S. Role of oxidative stress, inflammation, hypoxia and angiogenesis in the development of diabetic retinopathy. Saudi J. Ophthalmol. 2018, 32, 318–323. [Google Scholar] [CrossRef]
- Lange, J.; Hadziahmetovic, M.; Zhang, J.; Li, W. Region-specific ischemia, neovascularization and macular oedema in treatment-naïve proliferative diabetic retinopathy. Clin. Exp. Ophthalmol. 2018, 46, 757–766. [Google Scholar] [CrossRef]
- Saravia, M.; Zeman, L.; Ingolotti, M.; Schlaen, A. The VEGF paradox: Does diabetic retinopathy protect from age related macular degeneration? Med. Hypotheses 2017, 109, 156–161. [Google Scholar] [CrossRef]
- Barber, A.J.; Gardner, T.W.; Abcouwer, S.F. The significance of vascular and neural apoptosis to the pathology of diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1156–1163. [Google Scholar] [CrossRef]
- Pan, H.-Z.; Zhang, H.; Chang, D.; Li, H.; Sui, H. The change of oxidative stress products in diabetes mellitus and diabetic retinopathy. Br. J. Ophthalmol. 2008, 92, 548–551. [Google Scholar] [CrossRef]
- Yamagishi, S.-i.; Matsui, T. Advanced glycation end products (AGEs), oxidative stress and diabetic retinopathy. Curr. Pharm. Biotechnol. 2011, 12, 362–368. [Google Scholar] [CrossRef]
- Dejana, E.; Tournier-Lasserve, E.; Weinstein, B.M. The control of vascular integrity by endothelial cell junctions: Molecular basis and pathological implications. Dev. Cell 2009, 16, 209–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouchi, M.; West, K.; Crabb, J.W.; Kinoshita, S.; Kamei, M. Proteomic analysis of vitreous from diabetic macular edema. Exp. Eye Res. 2005, 81, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Lamy, R.; Ma, D.; Laotaweerungsawat, S.; Chen, Y.; Zhao, T.; Ma, W.; Zhang, F.; Psaras, C.; Stewart, J.M. Correlation of aqueous, vitreous, and plasma cytokine levels in patients with proliferative diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2020, 61, 26. [Google Scholar] [CrossRef] [Green Version]
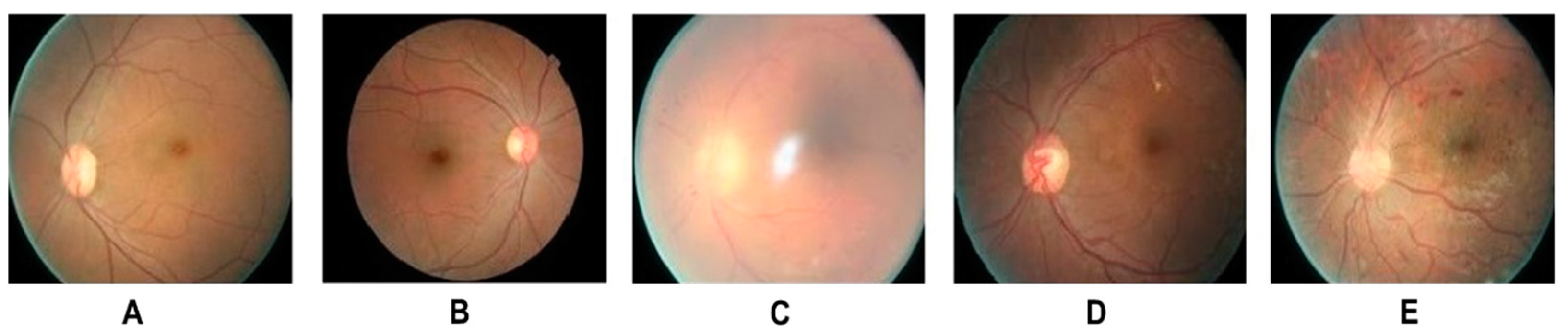
- Wilkinson, C.; Ferris III, F.L.; Klein, R.E.; Lee, P.P.; Agardh, C.D.; Davis, M.; Dills, D.; Kampik, A.; Pararajasegaram, R.; Verdaguer, J.T. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003, 110, 1677–1682. [Google Scholar] [CrossRef]
- Kandhasamy, J.P.; Balamurali, S.; Kadry, S.; Ramasamy, L.K. Diagnosis of diabetic retinopathy using multi level set segmentation algorithm with feature extraction using svm with selective features. Multimed. Tools Appl. 2020, 79, 10581–10596. [Google Scholar] [CrossRef]
- Hwang, H.B.; Jee, D.; Kwon, J.-W. Characteristics of diabetic macular edema patients with serous retinal detachment. Medicine 2019, 98, e18333. [Google Scholar] [CrossRef] [PubMed]
- Shankar, K.; Perumal, E.; Elhoseny, M.; Nguyen, P.T. An iot-cloud based intelligent computer-aided diagnosis of diabetic retinopathy stage classification using deep learning approach. CMC—Comput. Mater. Contin. 2021, 66, 1665–1680. [Google Scholar] [CrossRef]
- Tang, J.; Kern, T.S. Inflammation in diabetic retinopathy. Prog. Retin. Eye Res. 2011, 30, 343–358. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Alam, M.N.; Le, D.; Toslak, D. Quantitative optical coherence tomography angiography: A review. Exp. Biol. Med. 2020, 245, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Moutray, T.; Evans, J.R.; Lois, N.; Armstrong, D.J.; Peto, T.; Azuara-Blanco, A. Different lasers and techniques for proliferative diabetic retinopathy. Cochrane Database Syst. Rev. 2018, 1–84. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.; Bu, S.; Zhang, X.; Jiang, Y.; Tan, L.; Zhang, H.; Li, X. Safety and efficacy of intravitreal conbercept injection after vitrectomy for the treatment of proliferative diabetic retinopathy. Eye 2019, 33, 1177–1183. [Google Scholar] [CrossRef]
- Elkjaer, A.S.; Lynge, S.K.; Grauslund, J. Evidence and indications for systemic treatment in diabetic retinopathy: A systematic review. Acta Ophthalmol. 2020, 98, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Aiello, L.P.; DCCT/EDIC Research Group. Diabetic retinopathy and other ocular findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care 2014, 37, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Shi, R.; Zhao, L.; Wang, F.; Liu, F.; Chen, Z.; Li, R.; Liu, Y.; Lin, R. Effects of lipid-lowering agents on diabetic retinopathy: A meta-analysis and systematic review. Int. J. Ophthalmol. 2018, 11, 287. [Google Scholar] [PubMed]
- Knickelbein, J.E.; Abbott, A.B.; Chew, E.Y. Fenofibrate and diabetic retinopathy. Curr. Diabetes Rep. 2016, 16, 1–6. [Google Scholar] [CrossRef]
- Maniadakis, N.; Konstantakopoulou, E. Cost effectiveness of treatments for diabetic retinopathy: A systematic literature review. Pharmacoeconomics 2019, 37, 995–1010. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Quang, N.D.; Banu, R.; Kumar, H.; Tham, Y.-C.; Cheng, C.-Y.; Wong, T.Y.; Sabanayagam, C. Hypertension, blood pressure control and diabetic retinopathy in a large population-based study. PLoS ONE 2020, 15, e0229665. [Google Scholar] [CrossRef]
- Hu, H.; Liu, J.; Wang, D.; Qiu, S.; Yuan, Y.; Wang, F.; Wen, L.; Song, Q.; Sun, Z.-L. Efficacy of calcium dobesilate in treating Chinese patients with mild-to-moderate non-proliferative diabetic retinopathy (CALM-DR): Protocol for a single-blind, multicentre, 24-armed cluster-randomised, controlled trial. BMJ Open 2021, 11, e045256. [Google Scholar] [CrossRef]
- Hernández, C.; Simó-Servat, A.; Bogdanov, P.; Simó, R. Diabetic retinopathy: New therapeutic perspectives based on pathogenic mechanisms. J. Endocrinol. Investig. 2017, 40, 925–935. [Google Scholar] [CrossRef]
- Iglicki, M.; Zur, D.; Busch, C.; Okada, M.; Loewenstein, A. Progression of diabetic retinopathy severity after treatment with dexamethasone implant: A 24-month cohort study the ‘DR-Pro-DEX Study’. Acta Diabetol. 2018, 55, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lo, A.C. Diabetic retinopathy: Pathophysiology and treatments. Int. J. Mol. Sci. 2018, 19, 1816. [Google Scholar] [CrossRef] [Green Version]
- Wroblewski, J.J.; Hu, A.Y. Topical squalamine 0.2% and intravitreal ranibizumab 0.5 mg as combination therapy for macular edema due to branch and central retinal vein occlusion: An open-label, randomized study. Ophthalmic Surg. Lasers Imaging Retin. 2016, 47, 914–923. [Google Scholar] [CrossRef]
- Starita, C.; Patel, M.; Katz, B.; Adamis, A.P. Vascular endothelial growth factor and the potential therapeutic use of pegaptanib (Macugen®) in diabetic retinopathy. Diabet. Retin. 2007, 39, 122–148. [Google Scholar]
- Rodrigues, E.B.; Farah, M.E.; Maia, M.; Penha, F.M.; Regatieri, C.; Melo, G.B.; Pinheiro, M.M.; Zanetti, C.R. Therapeutic monoclonal antibodies in ophthalmology. Prog. Retin. Eye Res. 2009, 28, 117–144. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, B.; Zheng, Y. Exploring the Mechanism of Action Compound-Xueshuantong Capsule in Diabetic Retinopathy Treatment Based on Network Pharmacology. Evid.-Based Complement. Altern. Med. 2020, 2020, 1–12. [Google Scholar] [CrossRef]
- Wei, X.; Balne, P.K.; Meissner, K.E.; Barathi, V.A.; Schmetterer, L.; Agrawal, R. Assessment of flow dynamics in retinal and choroidal microcirculation. Surv. Ophthalmol. 2018, 63, 646–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laddha, U.D.; Kshirsagar, S.J. Ppar Receptor Modulation And Its Implications In Diabetic Retinopathy: An Overview. J. Crit. Rev. 2020, 7, 3614–3625. [Google Scholar]
- Jager, R.D.; Mieler, W.F.; Miller, J.W. Age-related macular degeneration. N. Engl. J. Med. 2008, 358, 2606–2617. [Google Scholar] [CrossRef] [Green Version]
- Nishijima, K.; Ng, Y.-S.; Zhong, L.; Bradley, J.; Schubert, W.; Jo, N.; Akita, J.; Samuelsson, S.J.; Robinson, G.S.; Adamis, A.P. Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am. J. Pathol. 2007, 171, 53–67. [Google Scholar] [CrossRef] [Green Version]
- Tolentino, M. Systemic and ocular safety of intravitreal anti-VEGF therapies for ocular neovascular disease. Surv. Ophthalmol. 2011, 56, 95–113. [Google Scholar] [CrossRef]
- Deng, G.; Moran, E.P.; Cheng, R.; Matlock, G.; Zhou, K.; Moran, D.; Chen, D.; Yu, Q.; Ma, J.-X. Therapeutic effects of a novel agonist of peroxisome proliferator-activated receptor alpha for the treatment of diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5030–5042. [Google Scholar] [CrossRef]
- Amato, R.; Dal Monte, M.; Lulli, M.; Raffa, V.; Casini, G. Nanoparticle-mediated delivery of neuroprotective substances for the treatment of diabetic retinopathy. Current neuropharmacology 2018, 16, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Rong, X.; Ji, Y.; Zhu, X.; Yang, J.; Qian, D.; Mo, X.; Lu, Y. Neuroprotective effect of insulin-loaded chitosan nanoparticles/PLGA-PEG-PLGA hydrogel on diabetic retinopathy in rats. Int. J. Nanomed. 2019, 14, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bessone, C.D.; Martinez, S.M.; Luna, J.D.; Marquez, M.A.; Ramírez, M.L.; Allemandi, D.A.; Carpentieri, Á.R.; Quinteros, D.A. Neuroprotective effect of melatonin loaded in ethylcellulose nanoparticles applied topically in a retinal degeneration model in rabbits. Experimental Eye Research 2020, 200, 108222. [Google Scholar] [CrossRef]
- Laradji, A.M.; Kolesnikov, A.V.; Karakoçak, B.B.; Kefalov, V.J.; Ravi, N. Redox-Responsive Hyaluronic Acid-Based Nanogels for the Topical Delivery of the Visual Chromophore to Retinal Photoreceptors. ACS Omega 2021, 6, 6172–6184. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.R.; Truong, Y.B.; O’Brien, C.M.; Glattauer, V. Bio-inspired human in vitro outer retinal models: Bruch’s membrane and its cellular interactions. Acta Biomater. 2020, 104, 1–16. [Google Scholar] [CrossRef]
- Koo, H.; Moon, H.; Han, H.; Na, J.H.; Huh, M.S.; Park, J.H.; Woo, S.J.; Park, K.H.; Kwon, I.C.; Kim, K. The movement of self-assembled amphiphilic polymeric nanoparticles in the vitreous and retina after intravitreal injection. Biomaterials 2012, 33, 3485–3493. [Google Scholar] [CrossRef] [PubMed]
- Radwan, S.E.-S.; El-Kamel, A.; Zaki, E.I.; Burgalassi, S.; Zucchetti, E.; El-Moslemany, R.M. Hyaluronic-coated albumin nanoparticles for the non-invasive delivery of apatinib in diabetic retinopathy. Int. J. Nanomed. 2021, 16, 4481. [Google Scholar] [CrossRef]
- Baudouin, C.; Labbé, A.; Liang, H.; Pauly, A.; Brignole-Baudouin, F. Preservatives in eyedrops: The good, the bad and the ugly. Prog. Retin. Eye Res. 2010, 29, 312–334. [Google Scholar] [CrossRef]
- Adelman, R.A.; Zheng, Q.; Mayer, H.R. Persistent ocular hypertension following intravitreal bevacizumab and ranibizumab injections. J. Ocul. Pharmacol. Ther. 2010, 26, 105–110. [Google Scholar] [CrossRef]
- Krishnan, R.; Goverdhan, S.; Lochhead, J. Submacular haemorrhage after intravitreal bevacizumab compared with intravitreal ranibizumab in large occult choroidal neovascularization. Clin. Exp. Ophthalmol. 2009, 37, 384–388. [Google Scholar] [CrossRef]
- Cabrera, F.J.; Wang, D.C.; Reddy, K.; Acharya, G.; Shin, C.S. Challenges and opportunities for drug delivery to the posterior of the eye. Drug Discov. Today 2019, 24, 1679–1684. [Google Scholar] [CrossRef]
- Koyama, R.; Nakanishi, T.; Ikeda, T.; Shimizu, A. Catalogue of soluble proteins in human vitreous humor by one-dimensional sodium dodecyl sulfate–polyacrylamide gel electrophoresis and electrospray ionization mass spectrometry including seven angiogenesis-regulating factors. J. Chromatogr. B 2003, 792, 5–21. [Google Scholar] [CrossRef]
- Mandal, A.; Pal, D.; Agrahari, V.; Trinh, H.M.; Joseph, M.; Mitra, A.K. Ocular delivery of proteins and peptides: Challenges and novel formulation approaches. Adv. Drug Deliv. Rev. 2018, 126, 67–95. [Google Scholar] [CrossRef] [PubMed]
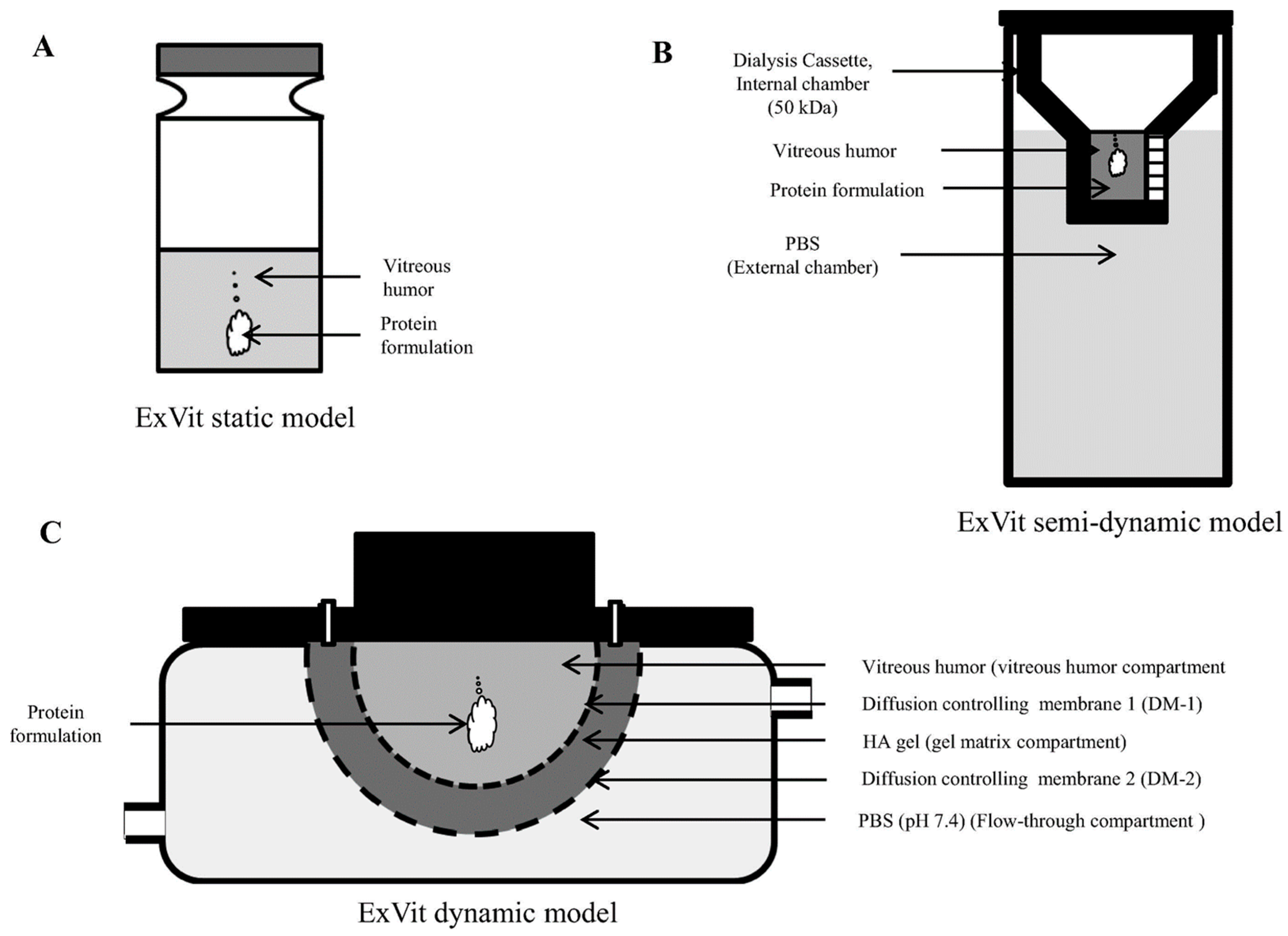
- Patel, S.; Müller, G.; Stracke, J.O.; Altenburger, U.; Mahler, H.-C.; Jere, D. Evaluation of protein drug stability with vitreous humor in a novel ex-vivo intraocular model. Eur. J. Pharm. Biopharm. 2015, 95, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Lu, T.; Tuomi, L.; Jumbe, N.; Lu, J.; Eppler, S.; Kuebler, P.; Damico-Beyer, L.A.; Joshi, A. Pharmacokinetics of ranibizumab in patients with neovascular age-related macular degeneration: A population approach. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1616–1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shafaie, S.; Hutter, V.; Brown, M.B.; Cook, M.T.; Chau, D.Y. Diffusion through the ex vivo vitreal body–Bovine, porcine, and ovine models are poor surrogates for the human vitreous. Int. J. Pharm. 2018, 550, 207–215. [Google Scholar] [CrossRef]
- Le Goff, M.; Bishop, P. Adult vitreous structure and postnatal changes. Eye 2008, 22, 1214–1222. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Feng, L.; Wang, S. Conjugated polymer nanoparticles for imaging, cell activity regulation, and therapy. Adv. Funct. Mater. 2019, 29, 1806818. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, N.; Huang, X.; Cheng, J.-W.; Li, F.-Q.; Wei, R.-L.; Cai, J.-P. Effect of intravitreal injection of bevacizumab-chitosan nanoparticles on retina of diabetic rats. Int. J. Ophthalmol. 2014, 7, 1. [Google Scholar] [PubMed]
- Oh, E.J.; Choi, J.-S.; Kim, H.; Joo, C.-K.; Hahn, S.K. Anti-Flt1 peptide–hyaluronate conjugate for the treatment of retinal neovascularization and diabetic retinopathy. Biomaterials 2011, 32, 3115–3123. [Google Scholar] [CrossRef]
- Lapcık Jr, L.; Lapcık, L.; De Smedt, S.; Demeester, J.; Chabrecek, P. Hyaluronan: Preparation, structure, properties, and applications. Chem. Rev. 1998, 98, 2663–2684. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Meng, T.; Chen, Q.; Zhou, K.; Shao, Y.; Matlock, G.; Ma, X.; Wu, W.; Du, Y.; Wang, X. Fenofibrate-loaded biodegradable nanoparticles for the treatment of experimental diabetic retinopathy and neovascular age-related macular degeneration. Mol. Pharm. 2019, 16, 1958–1970. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.R.; Besson, V.C.; Palmier, B.; Garcia, Y.; Plotkine, M.; Marchand-Leroux, C. Neurological recovery-promoting, anti-inflammatory, and anti-oxidative effects afforded by fenofibrate, a PPAR alpha agonist, in traumatic brain injury. J. Neurotrauma 2007, 24, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Laddha, U.D.; Kshirsagar, S.J. Formulation of PPAR-gamma agonist as surface modified PLGA nanoparticles for non-invasive treatment of diabetic retinopathy: In vitro and in vivo evidences. Heliyon 2020, 6, e04589. [Google Scholar] [CrossRef]
- Mahaling, B.; Katti, D.S. Physicochemical properties of core–shell type nanoparticles govern their spatiotemporal biodistribution in the eye. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 2149–2160. [Google Scholar] [CrossRef]
- Parodi, A.; Miao, J.; Soond, S.M.; Rudzińska, M.; Zamyatnin, A.A. Albumin nanovectors in cancer therapy and imaging. Biomolecules 2019, 9, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, J.H.; Nguyen, H.K.; Lee, J.E.; Suh, W. Therapeutic effect of apatinib-loaded nanoparticles on diabetes-induced retinal vascular leakage. Int. J. Nanomed. 2016, 11, 3101. [Google Scholar]
- Huang, D.; Chen, Y.-S.; Thakur, S.S.; Rupenthal, I.D. Ultrasound-mediated nanoparticle delivery across ex vivo bovine retina after intravitreal injection. Eur. J. Pharm. Biopharm. 2017, 119, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Parodi, A.; Buzaeva, P.; Nigovora, D.; Baldin, A.; Kostyushev, D.; Chulanov, V.; Savvateeva, L.V.; Zamyatnin, A.A. Nanomedicine for increasing the oral bioavailability of cancer treatments. J. Nanobiotechnol. 2021, 19, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Park, J.-H.; Jeong, H.; Hong, J.; Choi, W.S.; Lee, B.-H.; Park, C.Y. An evaluation of the in vivo safety of nonporous silica nanoparticles: Ocular topical administration versus oral administration. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
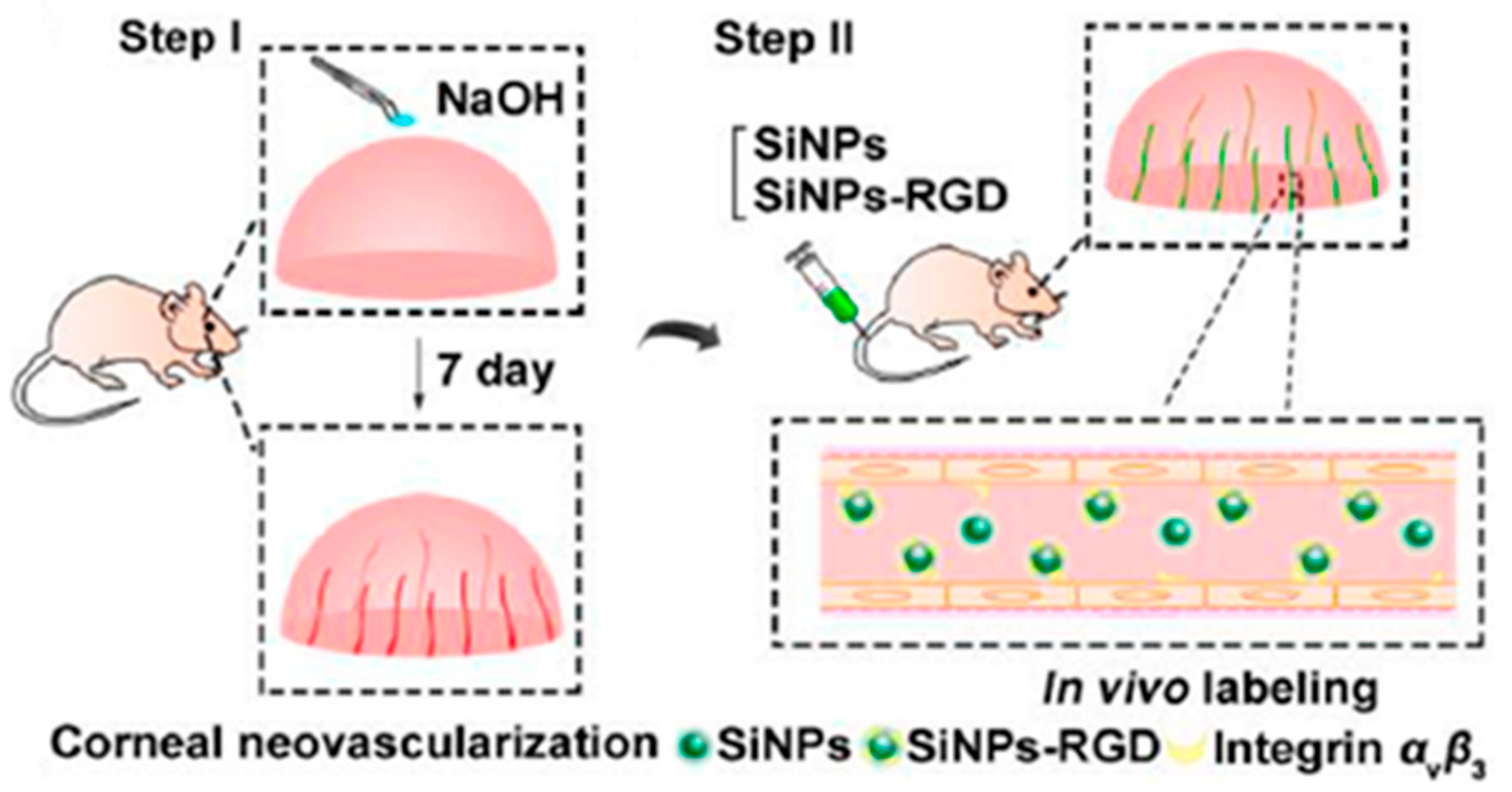
- Jo, D.H.; Kim, J.H.; Yu, Y.S.; Lee, T.G.; Kim, J.H. Antiangiogenic effect of silicate nanoparticle on retinal neovascularization induced by vascular endothelial growth factor. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Paiva, M.R.B.; Andrade, G.F.; Dourado, L.F.N.; Castro, B.F.M.; Fialho, S.L.; Sousa, E.M.B.; Silva-Cunha, A. Surface functionalized mesoporous silica nanoparticles for intravitreal application of tacrolimus. J. Biomater. Appl. 2021, 35, 1019–1033. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Ji, X.; Xu, H.; Zhang, L.; Jiang, A.; Song, B.; Su, Y.; He, Y. Photostable and biocompatible fluorescent silicon nanoparticles-based theranostic probes for simultaneous imaging and treatment of ocular neovascularization. Anal. Chem. 2018, 90, 8188–8195. [Google Scholar] [CrossRef]
- BarathManiKanth, S.; Kalishwaralal, K.; Sriram, M.; Pandian, S.R.K.; Youn, H.-S.; Eom, S.; Gurunathan, S. Anti-oxidant effect of gold nanoparticles restrains hyperglycemic conditions in diabetic mice. J. Nanobiotechnol. 2010, 8, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Karthikeyan, B.; Kalishwaralal, K.; Sheikpranbabu, S.; Deepak, V.; Haribalaganesh, R.; Gurunathan, S. Gold nanoparticles downregulate VEGF-and IL-1β-induced cell proliferation through Src kinase in retinal pigment epithelial cells. Exp. Eye Res. 2010, 91, 769–778. [Google Scholar] [CrossRef]
- Shen, N.; Zhang, R.; Zhang, H.-R.; Luo, H.-Y.; Shen, W.; Gao, X.; Guo, D.-Z.; Shen, J. Inhibition of retinal angiogenesis by gold nanoparticles via inducing autophagy. Int. J. Ophthalmol. 2018, 11, 1269. [Google Scholar]
- Dong, Y.; Wan, G.; Yan, P.; Qian, C.; Li, F.; Peng, G. Fabrication of resveratrol coated gold nanoparticles and investigation of their effect on diabetic retinopathy in streptozotocin induced diabetic rats. J. Photochem. Photobiol. B Biol. 2019, 195, 51–57. [Google Scholar] [CrossRef]
- Apaolaza, P.; Busch, M.; Asin-Prieto, E.; Peynshaert, K.; Rathod, R.; Remaut, K.; Dünker, N.; Göpferich, A. Hyaluronic acid coating of gold nanoparticles for intraocular drug delivery: Evaluation of the surface properties and effect on their distribution. Exp. Eye Res. 2020, 198, 108151. [Google Scholar] [CrossRef]
- Dave, V.; Sharma, R.; Gupta, C.; Sur, S. Folic acid modified gold nanoparticle for targeted delivery of Sorafenib tosylate towards the treatment of diabetic retinopathy. Colloids Surf. B Biointerfaces 2020, 194, 111151. [Google Scholar] [CrossRef]
- Sheikpranbabu, S.; Kalishwaralal, K.; Venkataraman, D.; Eom, S.H.; Park, J.; Gurunathan, S. Silver nanoparticles inhibit VEGF-and IL-1β-induced vascular permeability via Src dependent pathway in porcine retinal endothelial cells. J. Nanobiotechnol. 2009, 7, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amato, R.; Giannaccini, M.; Dal Monte, M.; Cammalleri, M.; Pini, A.; Raffa, V.; Lulli, M.; Casini, G. Association of the somatostatin analog octreotide with magnetic nanoparticles for intraocular delivery: A possible approach for the treatment of diabetic retinopathy. Front. Bioeng. Biotechnol. 2020, 8, 144. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef]
- Kostyushev, D.; Kostyusheva, A.; Brezgin, S.; Smirnov, V.; Volchkova, E.; Lukashev, A.; Chulanov, V. Gene editing by extracellular vesicles. Int. J. Mol. Sci. 2020, 21, 7362. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Takahashi, Y.; Takakura, Y. Possibility of exosome-based therapeutics and challenges in production of exosomes eligible for therapeutic application. Biol. Pharm. Bull. 2018, 41, 835–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Gu, N.; Zhang, X.E.; Wang, D.B. Light-Inducible Exosome-Based Vehicle for Endogenous RNA Loading and Delivery to Leukemia Cells. Adv. Funct. Mater. 2019, 29, 1807189. [Google Scholar] [CrossRef]
- Yim, N.; Ryu, S.-W.; Choi, K.; Lee, K.R.; Lee, S.; Choi, H.; Kim, J.; Shaker, M.R.; Sun, W.; Park, J.-H. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein–protein interaction module. Nat. Commun. 2016, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Stickney, Z.; Losacco, J.; McDevitt, S.; Zhang, Z.; Lu, B. Development of exosome surface display technology in living human cells. Biochem. Biophys. Res. Commun. 2016, 472, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.; Losacco, J.; Stickney, Z.; Li, L.; Marriott, G.; Lu, B. Pseudotyping exosomes for enhanced protein delivery in mammalian cells. Int. J. Nanomed. 2017, 12, 3153. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef]
- Dooley, K.; McConnell, R.E.; Xu, K.; Lewis, N.D.; Haupt, S.; Youniss, M.R.; Martin, S.; Sia, C.L.; McCoy, C.; Moniz, R.J. A versatile platform for generating engineered extracellular vesicles with defined therapeutic properties. Mol. Ther. 2021, 29, 1729–1743. [Google Scholar] [CrossRef] [PubMed]
- Zeelenberg, I.S.; Ostrowski, M.; Krumeich, S.; Bobrie, A.; Jancic, C.; Boissonnas, A.; Delcayre, A.; Le Pecq, J.-B.; Combadière, B.; Amigorena, S. Targeting tumor antigens to secreted membrane vesicles in vivo induces efficient antitumor immune responses. Cancer Res. 2008, 68, 1228–1235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Yu, J.; Kadungure, T.; Beyene, J.; Zhang, H.; Lu, Q. ARMMs as a versatile platform for intracellular delivery of macromolecules. Nat. Commun. 2018, 9, 1–7. [Google Scholar] [CrossRef]
- Statello, L.; Maugeri, M.; Garre, E.; Nawaz, M.; Wahlgren, J.; Papadimitriou, A.; Lundqvist, C.; Lindfors, L.; Collen, A.; Sunnerhagen, P. Identification of RNA-binding proteins in exosomes capable of interacting with different types of RNA: RBP-facilitated transport of RNAs into exosomes. PLoS ONE 2018, 13, e0195969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reshke, R.; Taylor, J.A.; Savard, A.; Guo, H.; Rhym, L.H.; Kowalski, P.S.; Trung, M.T.; Campbell, C.; Little, W.; Anderson, D.G. Reduction of the therapeutic dose of silencing RNA by packaging it in extracellular vesicles via a pre-microRNA backbone. Nat. Biomed. Eng. 2020, 4, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Platania, C.B.M.; Maisto, R.; Trotta, M.C.; D’Amico, M.; Rossi, S.; Gesualdo, C.; D’Amico, G.; Balta, C.; Herman, H.; Hermenean, A. Retinal and circulating mi RNA expression patterns in diabetic retinopathy: An in silico and in vivo approach. Br. J. Pharmacol. 2019, 176, 2179–2194. [Google Scholar] [PubMed]
- Afarid, M.; Namvar, E.; Sanie-Jahromi, F. Diabetic Retinopathy and BDNF: A Review on Its Molecular Basis and Clinical Applications. J. Ophthalmol. 2020, 2020, 1–7. [Google Scholar] [CrossRef]
- Li, W.; Jin, L.; Cui, Y.; Nie, A.; Xie, N.; Liang, G. Bone marrow mesenchymal stem cells-induced exosomal microRNA-486-3p protects against diabetic retinopathy through TLR4/NF-κB axis repression. J. Endocrinol. Investig. 2021, 44, 1193–1207. [Google Scholar] [CrossRef] [PubMed]
- Safwat, A.; Sabry, D.; Ragiae, A.; Amer, E.; Mahmoud, R.; Shamardan, R. Adipose mesenchymal stem cells–derived exosomes attenuate retina degeneration of streptozotocin-induced diabetes in rabbits. J. Circ. Biomark. 2018, 7, 1849454418807827. [Google Scholar] [CrossRef] [Green Version]
- Maisto, R.; Trotta, M.C.; Petrillo, F.; Izzo, S.; Cuomo, G.; Alfano, R.; Hermenean, A.; Barcia, J.M.; Galdiero, M.; Platania, C.B.M. Resolvin D1 modulates the intracellular VEGF-related miRNAs of retinal photoreceptors challenged with high glucose. Front. Pharmacol. 2020, 11, 235. [Google Scholar] [CrossRef] [Green Version]
- Lazzara, F.; Trotta, M.C.; Platania, C.B.M.; D’Amico, M.; Petrillo, F.; Galdiero, M.; Gesualdo, C.; Rossi, S.; Drago, F.; Bucolo, C. Stabilization of HIF-1α in human retinal endothelial cells modulates expression of miRNAs and proangiogenic growth factors. Front. Pharmacol. 2020, 11, 1063. [Google Scholar] [CrossRef]
- Liu, C.; Ge, H.-M.; Liu, B.-H.; Dong, R.; Shan, K.; Chen, X.; Yao, M.-D.; Li, X.-M.; Yao, J.; Zhou, R.-M. Targeting pericyte–endothelial cell crosstalk by circular RNA-cPWWP2A inhibition aggravates diabetes-induced microvascular dysfunction. Proc. Natl. Acad. Sci. USA 2019, 116, 7455–7464. [Google Scholar] [CrossRef] [Green Version]
- Gu, S.; Liu, Y.; Zou, J.; Wang, W.; Wei, T.; Wang, X.; Zhu, L.; Zhang, M.; Zhu, J.; Xie, T. Retinal pigment epithelial cells secrete miR-202-5p-containing exosomes to protect against proliferative diabetic retinopathy. Exp. Eye Res. 2020, 201, 108271. [Google Scholar] [CrossRef]
- Gu, C.; Zhang, H.; Gao, Y. Adipose mesenchymal stem cells-secreted extracellular vesicles containing microRNA-192 delays diabetic retinopathy by targeting ITGA1. J. Cell. Physiol. 2021, 236, 5036–5051. [Google Scholar] [CrossRef]
- Deng, C.-L.; Hu, C.-B.; Ling, S.-T.; Zhao, N.; Bao, L.-H.; Zhou, F.; Xiong, Y.-C.; Chen, T.; Sui, B.-D.; Yu, X.-R. Photoreceptor protection by mesenchymal stem cell transplantation identifies exosomal MiR-21 as a therapeutic for retinal degeneration. Cell Death Differ. 2021, 28, 1041–1061. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y.; Kong, Y. Exosomes derived from mesenchymal stem cells modulate miR-126 to ameliorate hyperglycemia-induced retinal inflammation via targeting HMGB1. Investig. Ophthalmol. Vis. Sci. 2019, 60, 294–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamalden, T.A.; Macgregor-Das, A.M.; Kannan, S.M.; Dunkerly-Eyring, B.; Khaliddin, N.; Xu, Z.; Fusco, A.P.; Yazib, S.A.; Chow, R.C.; Duh, E.J. Exosomal microRNA-15a transfer from the pancreas augments diabetic complications by inducing oxidative stress. Antioxid. Redox Signal. 2017, 27, 913–930. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Fisher, K.P.; Hammer, S.S.; Navitskaya, S.; Blanchard, G.J.; Busik, J.V. Plasma exosomes contribute to microvascular damage in diabetic retinopathy by activating the classical complement pathway. Diabetes 2018, 67, 1639–1649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Chen, C.; McLaughlin, T.; Wang, Y.; Le, Y.-Z.; Wang, J.J.; Zhang, S.X. Loss of X-box binding protein 1 in Müller cells augments retinal inflammation in a mouse model of diabetes. Diabetologia 2019, 62, 531–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, X.; Xue, L.-D.; Di, Y.; Li, T.; Tian, Y.-J.; Song, Y. MSC-derived exosomal lncRNA SNHG7 suppresses endothelial-mesenchymal transition and tube formation in diabetic retinopathy via miR-34a-5p/XBP1 axis. Life Sci. 2021, 272, 119232. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.J.; Zhang, S.X. Preconditioning with endoplasmic reticulum stress mitigates retinal endothelial inflammation via activation of X-box binding protein 1. J. Biol. Chem. 2011, 286, 4912–4921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ke, N.; Pi, L.-H.; Liu, Q.; Chen, L. Long noncoding RNA SNHG7 inhibits high glucose-induced human retinal endothelial cells angiogenesis by regulating miR-543/SIRT1 axis. Biochem. Biophys. Res. Commun. 2019, 514, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.A.; Carlos, D.; Ferreira, N.S.; Silva, J.F.; Zanotto, C.Z.; Zamboni, D.S.; Garcia, V.D.; Ventura, D.F.; Silva, J.S.; Tostes, R.C. Mitochondrial DNA promotes NLRP3 inflammasome activation and contributes to endothelial dysfunction and inflammation in type 1 diabetes. Front. Physiol. 2020, 10, 1557. [Google Scholar] [CrossRef] [Green Version]
- Ye, L.; Guo, H.; Wang, Y.; Peng, Y.; Zhang, Y.; Li, S.; Yang, M.; Wang, L. Exosomal circEhmt1 Released from Hypoxia-Pretreated Pericytes Regulates High Glucose-Induced Microvascular Dysfunction via the NFIA/NLRP3 Pathway. Oxidative Med. Cell. Longev. 2021, 2021, 1–13. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borodina, T.; Kostyushev, D.; Zamyatnin, A.A., Jr.; Parodi, A. Nanomedicine for Treating Diabetic Retinopathy Vascular Degeneration. Int. J. Transl. Med. 2021, 1, 306-322. https://doi.org/10.3390/ijtm1030018
Borodina T, Kostyushev D, Zamyatnin AA Jr., Parodi A. Nanomedicine for Treating Diabetic Retinopathy Vascular Degeneration. International Journal of Translational Medicine. 2021; 1(3):306-322. https://doi.org/10.3390/ijtm1030018
Chicago/Turabian StyleBorodina, Tatiana, Dmitry Kostyushev, Andrey A. Zamyatnin, Jr., and Alessandro Parodi. 2021. "Nanomedicine for Treating Diabetic Retinopathy Vascular Degeneration" International Journal of Translational Medicine 1, no. 3: 306-322. https://doi.org/10.3390/ijtm1030018
APA StyleBorodina, T., Kostyushev, D., Zamyatnin, A. A., Jr., & Parodi, A. (2021). Nanomedicine for Treating Diabetic Retinopathy Vascular Degeneration. International Journal of Translational Medicine, 1(3), 306-322. https://doi.org/10.3390/ijtm1030018








