Making Fluorescent Nylon, Polypropylene, and Polystyrene Microplastics for In Vivo and In Vitro Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Aims
2.2. Plastics
2.3. Rhodamine 6G Staining and Microplastic Preparation
2.4. IRDye Conjugation with Polystyrene
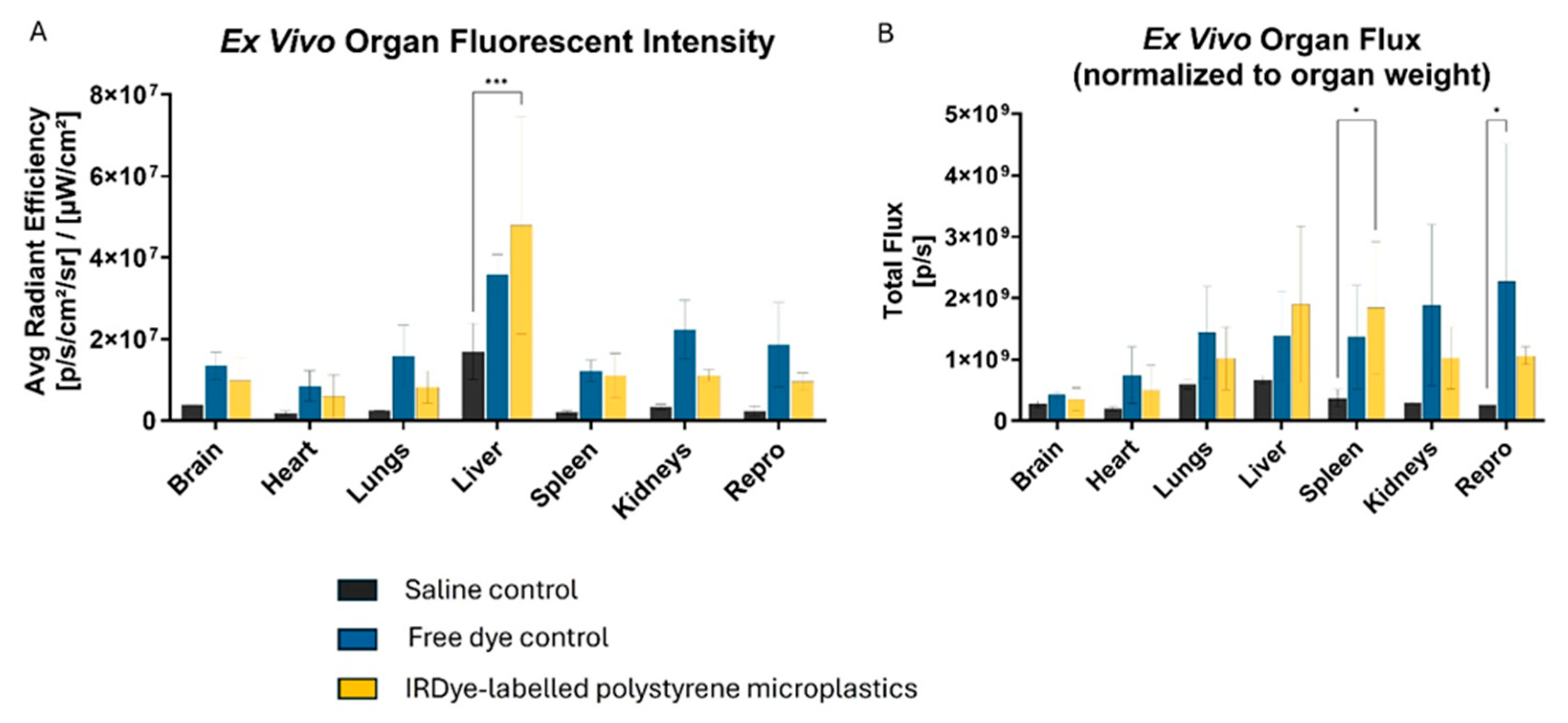
2.5. Visualizing MPs Using In Vivo Imaging System
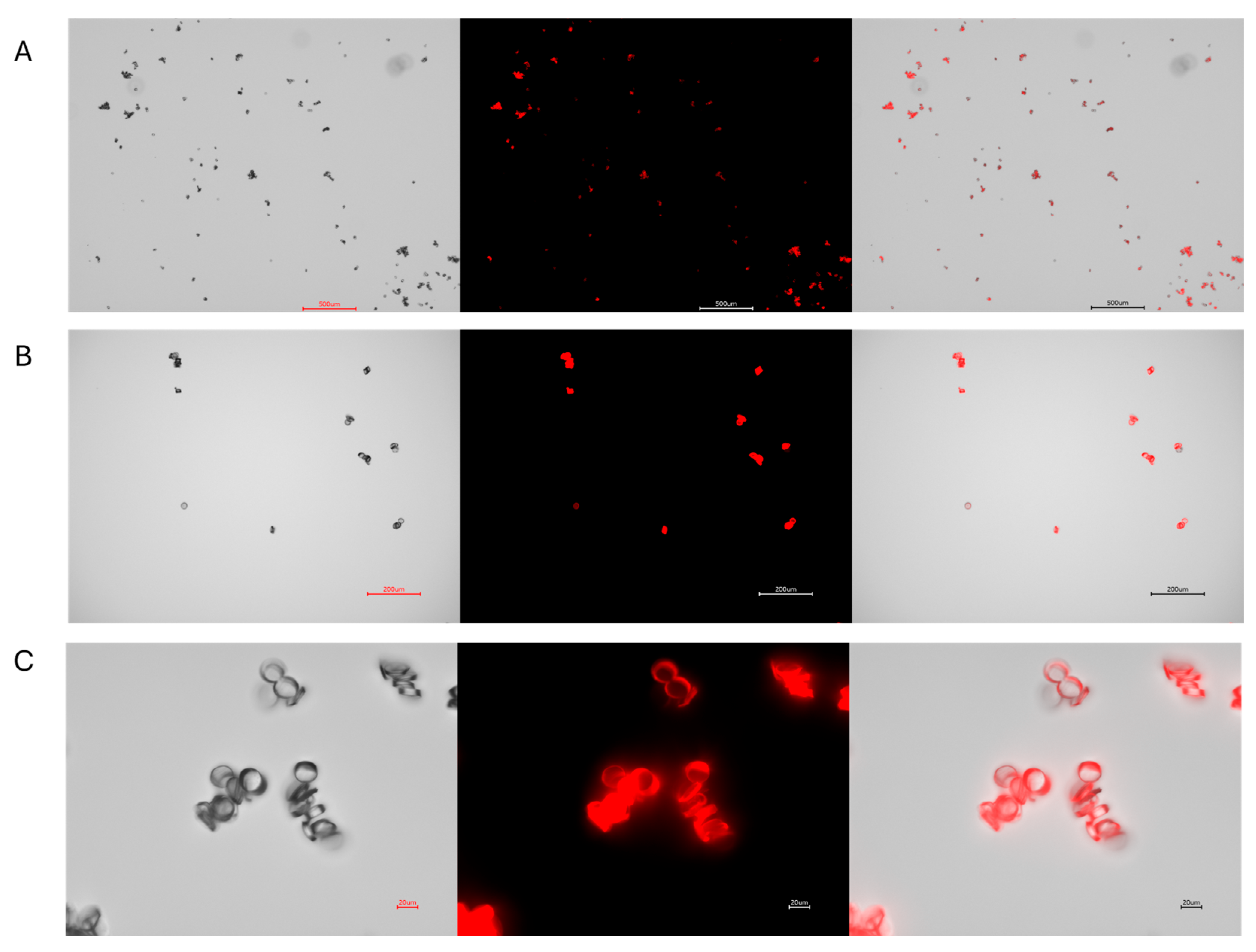
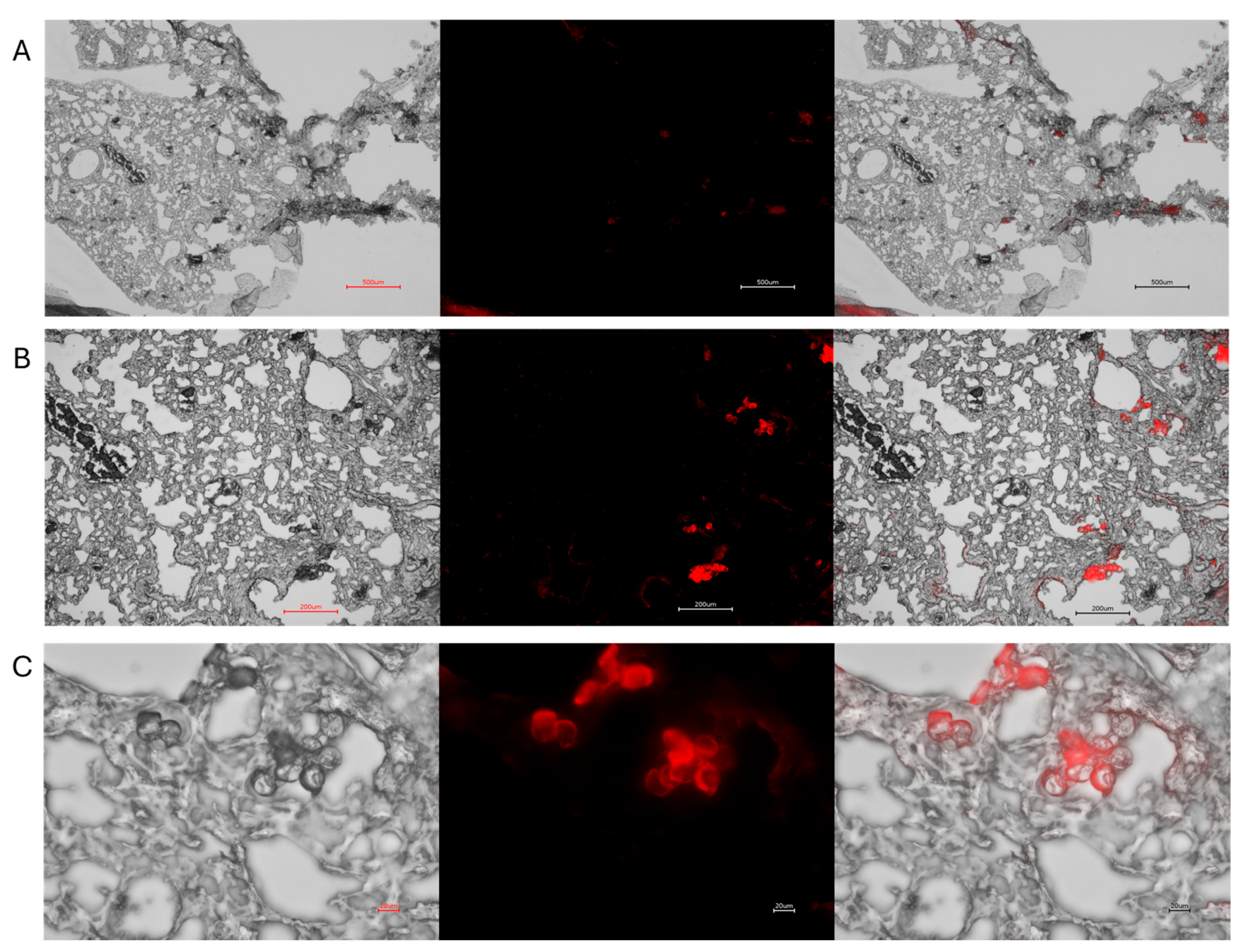
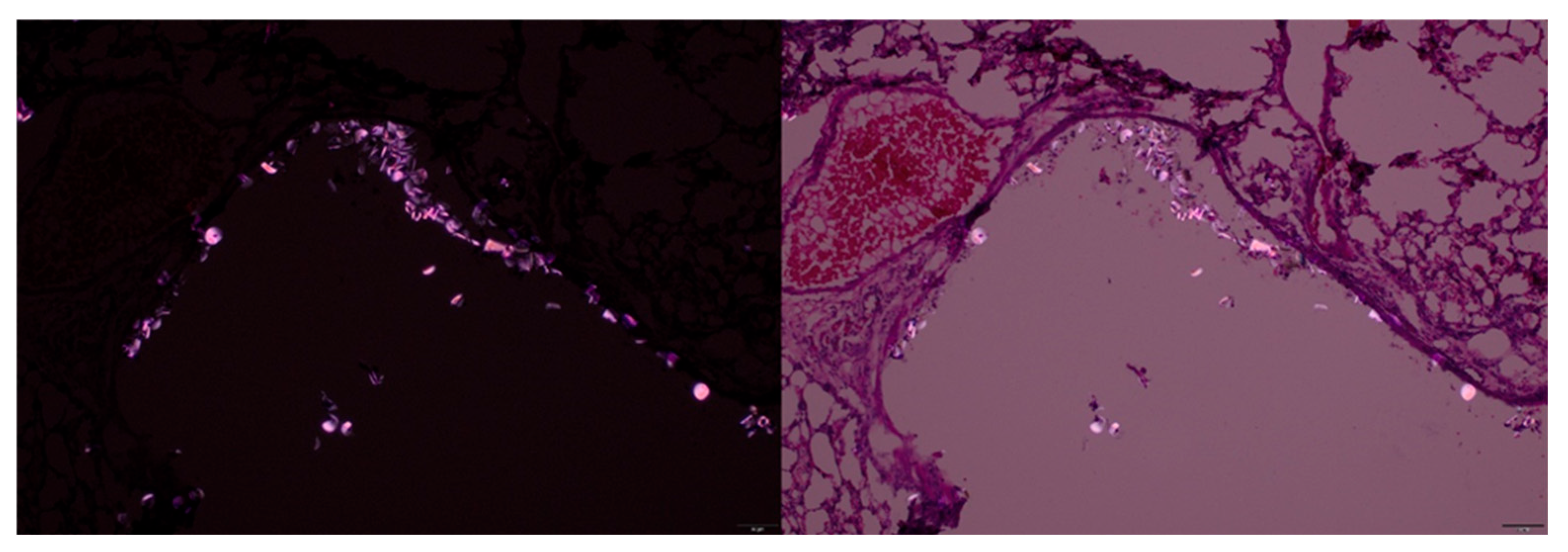
2.6. Visualizing MPs Using Microscopy
2.7. Flow Cytometry
3. Results
3.1. Nylon and Polypropylene with Rhodamine 6G
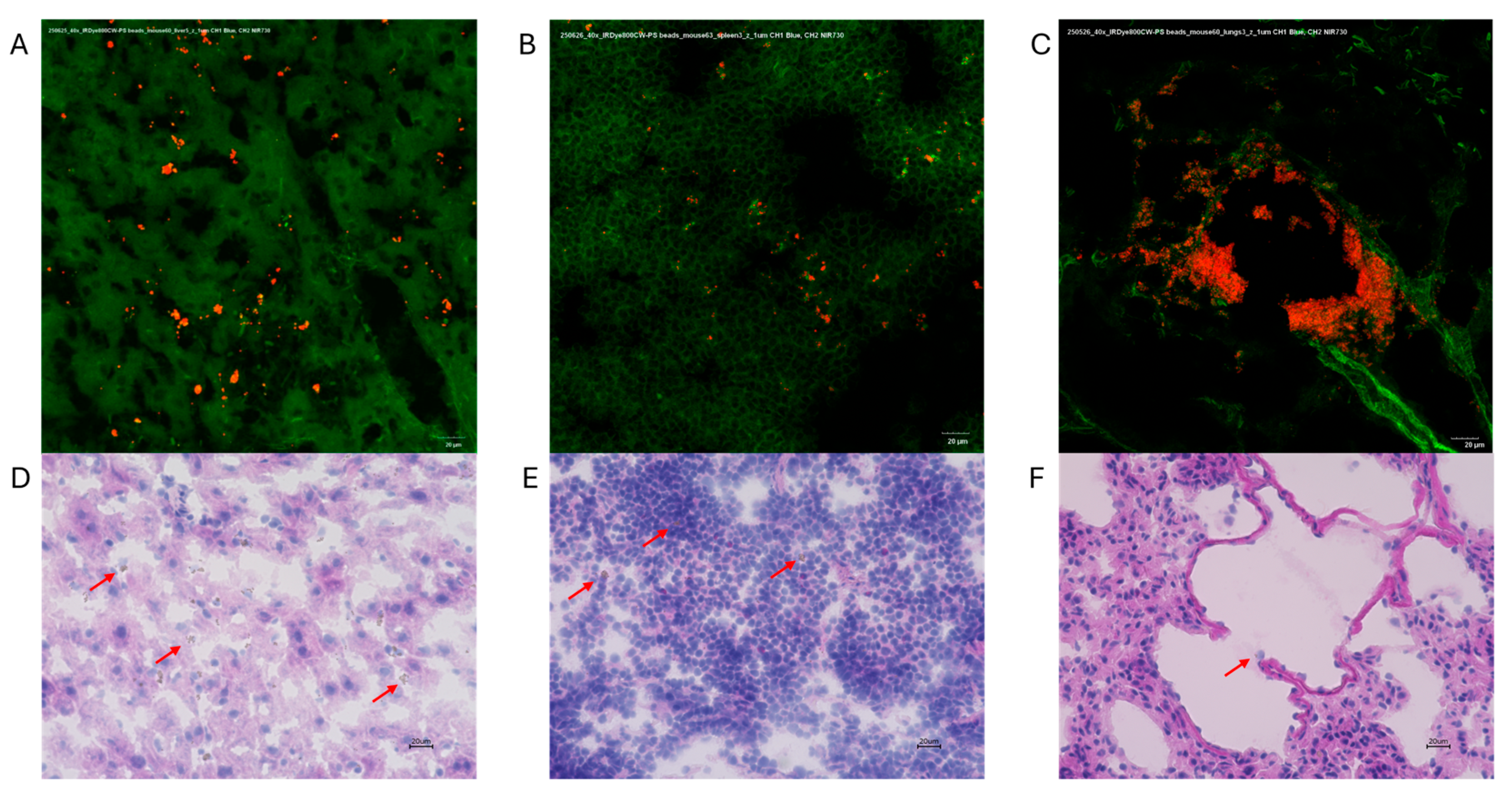
3.2. Polystyrene with IRDye 800CW
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IVIS | In vivo imaging system |
| MP | Microplastic |
| PS | Polystyrene |
| OCT | Optimal cutting temperature |
| SEM | Scanning Electron Microscope |
References
- US EPA. Microplastics Research. Available online: https://www.epa.gov/water-research/microplastics-research (accessed on 3 April 2025).
- Du, F.; Cai, H.; Zhang, Q.; Chen, Q.; Shi, H. Microplastics in Take-out Food Containers. J. Hazard. Mater. 2020, 399, 122969. [Google Scholar] [CrossRef]
- Fang, C.; Awoyemi, O.S.; Saianand, G.; Xu, L.; Niu, J.; Naidu, R. Characterising Microplastics in Indoor Air: Insights from Raman Imaging Analysis of Air Filter Samples. J. Hazard. Mater. 2024, 464, 132969. [Google Scholar] [CrossRef]
- Li, C.; Busquets, R.; Campos, L.C. Assessment of Microplastics in Freshwater Systems: A Review. Sci. Total Environ. 2020, 707, 135578. [Google Scholar] [CrossRef]
- Pivokonsky, M.; Cermakova, L.; Novotna, K.; Peer, P.; Cajthaml, T.; Janda, V. Occurrence of Microplastics in Raw and Treated Drinking Water. Sci. Total Environ. 2018, 643, 1644–1651. [Google Scholar] [CrossRef]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Torres-Agullo, A.; Karanasiou, A.; Moreno, T.; Lacorte, S. Airborne Microplastic Particle Concentrations and Characterization in Indoor Urban Microenvironments. Environ. Pollut. 2022, 308, 119707. [Google Scholar] [CrossRef] [PubMed]
- Young, N. Microplastics Are in the Air We Breathe and in Earth’s Atmosphere, and They Affect the Climate. Available online: https://www.greenpeace.org/aotearoa/story/microplastics-are-in-the-air-we-breathe-and-in-earths-atmosphere-and-they-affect-the-climate/ (accessed on 28 March 2025).
- Xiao, S.; Cui, Y.; Brahney, J.; Mahowald, N.M.; Li, Q. Long-Distance Atmospheric Transport of Microplastic Fibres Influenced by Their Shapes. Nat. Geosci. 2023, 16, 863–870. [Google Scholar] [CrossRef]
- World Health Organization. Dietary and Inhalation Exposure to Nano-and Microplastic Particles and Potential Implications for Human Health; World Health Organization: Geneva, Switzerland, 2022; p. 154.
- Khalid Ageel, H.; Harrad, S.; Abou-Elwafa Abdallah, M. Occurrence, Human Exposure, and Risk of Microplastics in the Indoor Environment. Environ. Sci. Process. Impacts 2022, 24, 17–31. [Google Scholar] [CrossRef]
- O’Brien, S.; Rauert, C.; Ribeiro, F.; Okoffo, E.D.; Burrows, S.D.; O’Brien, J.W.; Wang, X.; Wright, S.L.; Thomas, K.V. There’s Something in the Air: A Review of Sources, Prevalence and Behaviour of Microplastics in the Atmosphere. Sci. Total Environ. 2023, 874, 162193. [Google Scholar] [CrossRef]
- Zulauf, N.; Dröge, J.; Klingelhöfer, D.; Braun, M.; Oremek, G.M.; Groneberg, D.A. Indoor Air Pollution in Cars: An Update on Novel Insights. Int. J. Environ. Res. Public. Health 2019, 16, 2441. [Google Scholar] [CrossRef] [PubMed]
- Amato-Lourenço, L.F.; Carvalho-Oliveira, R.; Júnior, G.R.; dos Santos Galvão, L.; Ando, R.A.; Mauad, T. Presence of Airborne Microplastics in Human Lung Tissue. J. Hazard. Mater. 2021, 416, 126124. [Google Scholar] [CrossRef]
- Jenner, L.C.; Rotchell, J.M.; Bennett, R.T.; Cowen, M.; Tentzeris, V.; Sadofsky, L.R. Detection of Microplastics in Human Lung Tissue Using μFTIR Spectroscopy. Sci. Total Environ. 2022, 831, 154907. [Google Scholar] [CrossRef]
- Xu, J.-L.; Thomas, K.V.; Luo, Z.; Gowen, A.A. FTIR and Raman Imaging for Microplastics Analysis: State of the Art, Challenges and Prospects. TrAC Trends Anal. Chem. 2019, 119, 115629. [Google Scholar] [CrossRef]
- Dawson, A.L.; Kawaguchi, S.; King, C.K.; Townsend, K.A.; King, R.; Huston, W.M.; Bengtson Nash, S.M. Turning Microplastics into Nanoplastics through Digestive Fragmentation by Antarctic Krill. Nat. Commun. 2018, 9, 1001. [Google Scholar] [CrossRef]
- Greenspan, P.; Mayer, E.P.; Fowler, S.D. Nile Red: A Selective Fluorescent Stain for Intracellular Lipid Droplets. J. Cell Biol. 1985, 100, 965–973. [Google Scholar] [CrossRef]
- Shim, W.J.; Song, Y.K.; Hong, S.H.; Jang, M. Identification and Quantification of Microplastics Using Nile Red Staining. Mar. Pollut. Bull. 2016, 113, 469–476. [Google Scholar] [CrossRef]
- Gao, Z.; Wontor, K.; Cizdziel, J.V.; Gao, Z.; Wontor, K.; Cizdziel, J.V. Labeling Microplastics with Fluorescent Dyes for Detection, Recovery, and Degradation Experiments. Molecules 2022, 27, 7415. [Google Scholar] [CrossRef] [PubMed]
- Karakolis, E.G.; Nguyen, B.; You, J.B.; Rochman, C.M.; Sinton, D. Fluorescent Dyes for Visualizing Microplastic Particles and Fibers in Laboratory-Based Studies. Environ. Sci. Technol. Lett. 2019, 6, 334–340. [Google Scholar] [CrossRef]
- Keinänen, O.; Dayts, E.J.; Rodriguez, C.; Sarrett, S.M.; Brennan, J.M.; Sarparanta, M.; Zeglis, B.M. Harnessing PET to Track Micro- and Nanoplastics in Vivo. Sci. Rep. 2021, 11, 11463. [Google Scholar] [CrossRef]
- Im, C.; Kim, H.; Zaheer, J.; Kim, J.Y.; Lee, Y.-J.; Kang, C.M.; Kim, J.S. PET Tracing of Biodistribution for Orally Administered 64Cu-Labeled Polystyrene in Mice. J. Nucl. Med. 2022, 63, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.A. Indoor Microplastics: A Comprehensive Review and Bibliometric Analysis. Environ. Sci. Pollut. Res. 2023, 30, 121269–121291. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Jiang, Q.; Zhong, X.; Hu, X. Rhodamine B Dye Staining for Visualizing Microplastics in Laboratory-Based Studies. Environ. Sci. Pollut. Res. 2021, 28, 4209–4215. [Google Scholar] [CrossRef] [PubMed]
- Bucevičius, J.; Kostiuk, G.; Gerasimaitė, R.; Gilat, T.; Lukinavičius, G. Enhancing the Biocompatibility of Rhodamine Fluorescent Probes by a Neighbouring Group Effect. Chem. Sci. 2020, 11, 7313–7323. [Google Scholar] [CrossRef]
- Cole, M. A Novel Method for Preparing Microplastic Fibers. Sci. Rep. 2016, 6, 34519. [Google Scholar] [CrossRef]
- Song, S.; van Dijk, F.; Vasse, G.F.; Liu, Q.; Gosselink, I.F.; Weltjens, E.; Remels, A.H.V.; de Jager, M.H.; Bos, S.; Li, C.; et al. Inhalable Textile Microplastic Fibers Impair Airway Epithelial Differentiation. Am. J. Respir. Crit. Care Med. 2024, 209, 427–443. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, A.; Villalobos Santeli, A.; Nannu Shankar, S.; Shirkhani, A.; Baker, T.R.; Wu, C.-Y.; Mehrad, B.; Ferguson, P.L.; Sabo-Attwood, T. Toxicity of Microplastic Fibers Containing Azobenzene Disperse Dyes to Human Lung Epithelial Cells Cultured at an Air-Liquid Interface. J. Hazard. Mater. 2024, 480, 136280. [Google Scholar] [CrossRef]
- Paplińska-Goryca, M.; Misiukiewicz-Stępień, P.; Wróbel, M.; Mycroft-Rzeszotarska, K.; Adamska, D.; Rachowka, J.; Królikowska, M.; Goryca, K.; Krenke, R. The Impaired Response of Nasal Epithelial Cells to Microplastic Stimulation in Asthma and COPD. Sci. Rep. 2025, 15, 4242. [Google Scholar] [CrossRef]
- Pei, J.; Juniper, G.; van den Berg, N.S.; Nisho, N.; Broadt, T.; Welch, A.R.; Yi, G.S.; Raymundo, R.C.; Chirita, S.U.; Lu, G.; et al. Safety and Stability of Antibody-Dye Conjugate in Optical Molecular Imaging. Mol. Imaging Biol. 2021, 23, 109–116. [Google Scholar] [CrossRef]
- Huizinga, H.K.; Hooghiemstra, W.T.R.; Linssen, M.D.; Allersma, D.P.; Gareb, B.; Dekkers, B.G.J.; Nagengast, W.B.; Hooge, M.N.L.; Huizinga, H.K.; Hooghiemstra, W.T.R.; et al. Development of Clinical-Grade Durvalumab-680LT and Nivolumab-800CW for Multispectral Fluorescent Imaging of the PD-1/PD-L1 Axis of the Immune Checkpoint Pathway. Pharmaceuticals 2025, 18, 1501. [Google Scholar] [CrossRef]
- Würth, C.; González, M.G.; Niessner, R.; Panne, U.; Haisch, C.; Genger, U.R. Determination of the Absolute Fluorescence Quantum Yield of Rhodamine 6G with Optical and Photoacoustic Methods—Providing the Basis for Fluorescence Quantum Yield Standards. Talanta 2012, 90, 30–37. [Google Scholar] [CrossRef]
- Savarese, M.; Aliberti, A.; De Santo, I.; Battista, E.; Causa, F.; Netti, P.A.; Rega, N. Fluorescence Lifetimes and Quantum Yields of Rhodamine Derivatives: New Insights from Theory and Experiment. J. Phys. Chem. A 2012, 116, 7491–7497. [Google Scholar] [CrossRef] [PubMed]
- Würth, C.; Grabolle, M.; Pauli, J.; Spieles, M.; Resch-Genger, U. Relative and Absolute Determination of Fluorescence Quantum Yields of Transparent Samples. Nat. Protoc. 2013, 8, 1535–1550. [Google Scholar] [CrossRef]
- Samsonova, L.G.; Selivanov, N.I.; Kopylova, T.N. Spectral Properties of Nile Red in Solutions and Thin Films. Opt. Spectrosc. 2014, 116, 72–76. [Google Scholar] [CrossRef]
- Gear, A.R.L. Rhodamine 6G: A potent inhibitor of mitochondrial oxidative phosphorylation. J. Biol. Chem. 1974, 249, 3628–3637. [Google Scholar] [CrossRef]
- Malafaia, G.; da Luz, T.M.; Ahmed, M.A.I.; Karthi, S.; Araújo, A.P. da C. When Toxicity of Plastic Particles Comes from Their Fluorescent Dye: A Preliminary Study Involving Neotropical Physalaemus Cuvieri Tadpoles and Polyethylene Microplastics. J. Hazard. Mater. Adv. 2022, 6, 100054. [Google Scholar] [CrossRef]
- Gaylarde, C.C.; Neto, J.A.B.; da Fonseca, E.M.; Gaylarde, C.C.; Neto, J.A.B.; Fonseca, E.M. da Indoor Airborne Microplastics: Human Health Importance and Effects of Air Filtration and Turbulence. Microplastics 2024, 3, 653–670. [Google Scholar] [CrossRef]
- Sierra, I.; Chialanza, M.R.; Faccio, R.; Carrizo, D.; Fornaro, L.; Pérez-Parada, A. Identification of Microplastics in Wastewater Samples by Means of Polarized Light Optical Microscopy. Environ. Sci. Pollut. Res. 2020, 27, 7409–7419. [Google Scholar] [CrossRef] [PubMed]
- Labbe, A.B.; Bagshaw, C.R.; Uttal, L. Inexpensive Adaptations of Basic Microscopes for the Identification of Microplastic Contamination Using Polarization and Nile Red Fluorescence Detection. J. Chem. Educ. 2020, 97, 4026–4032. [Google Scholar] [CrossRef]
- Deng, Y.; Yan, Z.; Shen, R.; Wang, M.; Huang, Y.; Ren, H.; Zhang, Y.; Lemos, B. Microplastics Release Phthalate Esters and Cause Aggravated Adverse Effects in the Mouse Gut. Environ. Int. 2020, 143, 105916. [Google Scholar] [CrossRef]
- Tomonaga, T.; Higashi, H.; Izumi, H.; Nishida, C.; Kawai, N.; Sato, K.; Morimoto, T.; Higashi, Y.; Yatera, K.; Morimoto, Y. Investigation of Pulmonary Inflammatory Responses Following Intratracheal Instillation of and Inhalation Exposure to Polypropylene Microplastics. Part. Fibre Toxicol. 2024, 21, 29. [Google Scholar] [CrossRef]
- Bianco, A.; Carena, L.; Peitsaro, N.; Sordello, F.; Vione, D.; Passananti, M. Rapid Detection of Nanoplastics and Small Microplastics by Nile-Red Staining and Flow Cytometry. Environ. Chem. Lett. 2023, 21, 647–653. [Google Scholar] [CrossRef]
- Ainé, L.; Jacquin, J.; Breysse, C.; Colin, C.; Andanson, J.-M.; Delor-Jestin, F. Microplastics and Nanoplastics Detection Using Flow Cytometry: Challenges and Methodological Advances with Fluorescent Dye Application. MethodsX 2025, 14, 103200. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhong, X.; Dennis, A.M. Minimizing Near-Infrared Autofluorescence in Preclinical Imaging with Diet and Wavelength Selection. J. Biomed. Opt. 2023, 28, 094805. [Google Scholar] [CrossRef] [PubMed]
- Frangioni, J.V. In Vivo near-Infrared Fluorescence Imaging. Curr. Opin. Chem. Biol. 2003, 7, 626–634. [Google Scholar] [CrossRef]
- Kovar, J.L.; Simpson, M.A.; Schutz-Geschwender, A.; Olive, D.M. A Systematic Approach to the Development of Fluorescent Contrast Agents for Optical Imaging of Mouse Cancer Models. Anal. Biochem. 2007, 367, 1–12. [Google Scholar] [CrossRef]
- Josserand, V.; Bernard, C.; Michy, T.; Guidetti, M.; Vollaire, J.; Coll, J.-L.; Hurbin, A.; Josserand, V.; Bernard, C.; Michy, T.; et al. Tumor-Specific Imaging with Angiostamp800 or Bevacizumab-IRDye 800CW Improves Fluorescence-Guided Surgery over Indocyanine Green in Peritoneal Carcinomatosis. Biomedicines 2022, 10, 1059. [Google Scholar] [CrossRef]
- Marshall, M.V.; Draney, D.; Sevick-Muraca, E.M.; Olive, D.M. Single-Dose Intravenous Toxicity Study of IRDye 800CW in Sprague-Dawley Rats. Mol. Imaging Biol. 2010, 12, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Du, Y.; Liu, X.; Zhang, Q.; Jing, L.; Liang, X.; Chi, C.; Dai, Z.; Tian, J. Monitoring Tumor Targeting and Treatment Effects of IRDye 800CW and GX1-Conjugated Polylactic Acid Nanoparticles Encapsulating Endostar on Glioma by Optical Molecular Imaging. Mol. Imaging 2015, 14, 7290.2015.00014. [Google Scholar] [CrossRef]




| Microtome Setting | 10 µm | 5 µm | 1 µm |
|---|---|---|---|
| Minimum (μm) | 8.66 | 4.06 | 1.12 |
| 25% percentile (μm) | 10.93 | 6.13 | 2.28 |
| Median (μm) | 14.16 | 7.16 | 2.95 |
| 75% percentile (μm) | 16.77 | 8.68 | 4.04 |
| Maximum (μm) | 26.62 | 13.68 | 9.12 |
| Range (μm) | 17.97 | 9.61 | 8.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bardawil, C.E.; Dobbins, J.; Lankford, S.; Chowdrey, S.; Shumway, J.; Balamayooran, G.; Schaack, C.; Dhupar, R. Making Fluorescent Nylon, Polypropylene, and Polystyrene Microplastics for In Vivo and In Vitro Imaging. Microplastics 2025, 4, 84. https://doi.org/10.3390/microplastics4040084
Bardawil CE, Dobbins J, Lankford S, Chowdrey S, Shumway J, Balamayooran G, Schaack C, Dhupar R. Making Fluorescent Nylon, Polypropylene, and Polystyrene Microplastics for In Vivo and In Vitro Imaging. Microplastics. 2025; 4(4):84. https://doi.org/10.3390/microplastics4040084
Chicago/Turabian StyleBardawil, Charles E., Jarrett Dobbins, Shannon Lankford, Saif Chowdrey, Jack Shumway, Gayathriy Balamayooran, Cedric Schaack, and Rajeev Dhupar. 2025. "Making Fluorescent Nylon, Polypropylene, and Polystyrene Microplastics for In Vivo and In Vitro Imaging" Microplastics 4, no. 4: 84. https://doi.org/10.3390/microplastics4040084
APA StyleBardawil, C. E., Dobbins, J., Lankford, S., Chowdrey, S., Shumway, J., Balamayooran, G., Schaack, C., & Dhupar, R. (2025). Making Fluorescent Nylon, Polypropylene, and Polystyrene Microplastics for In Vivo and In Vitro Imaging. Microplastics, 4(4), 84. https://doi.org/10.3390/microplastics4040084







