From Environment to Endothelium: The Role of Microplastics in Vascular Aging
Abstract
1. Introduction
Literature Search
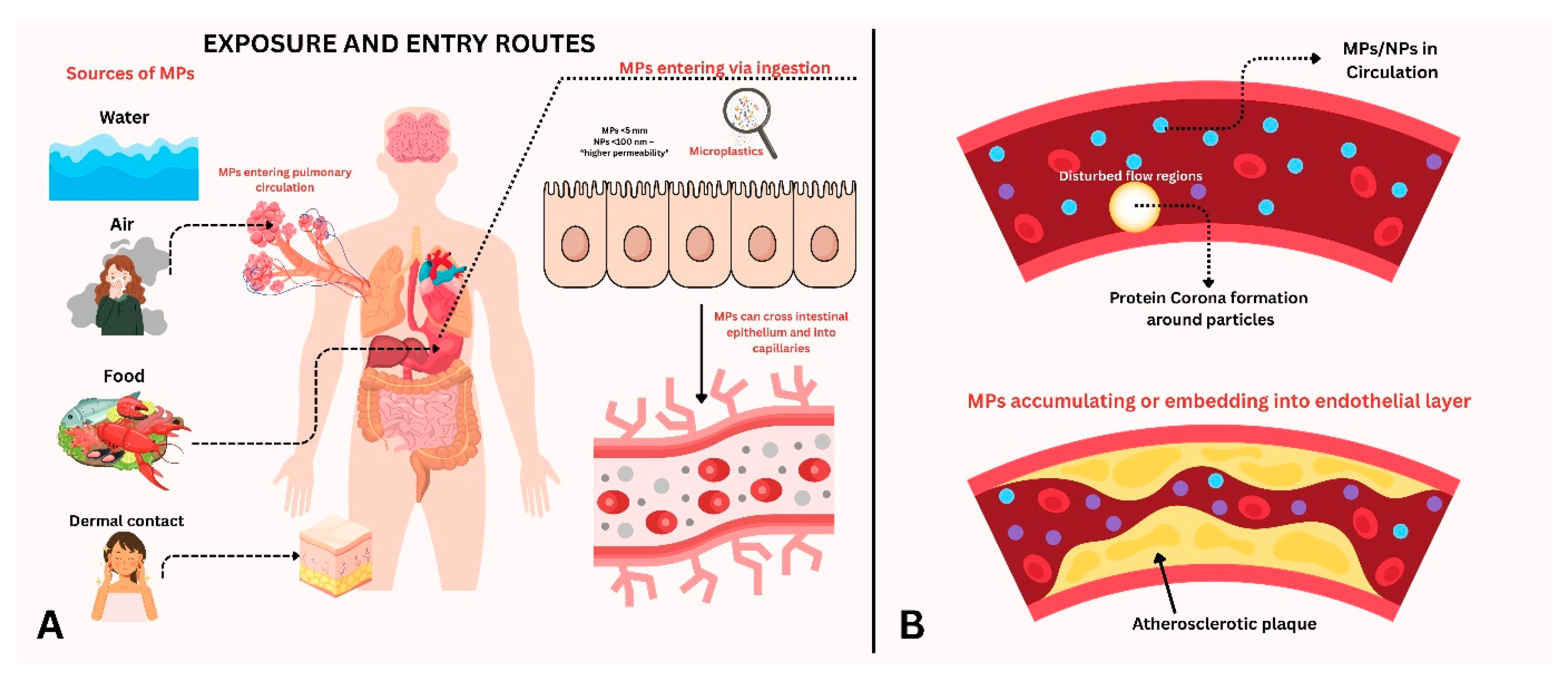
2. Source and Exposure
2.1. Exposure of Microplastics into the Human Body
2.2. Microplastic Exposure and Detection in Humans
3. Negative Impacts on Vascular Health
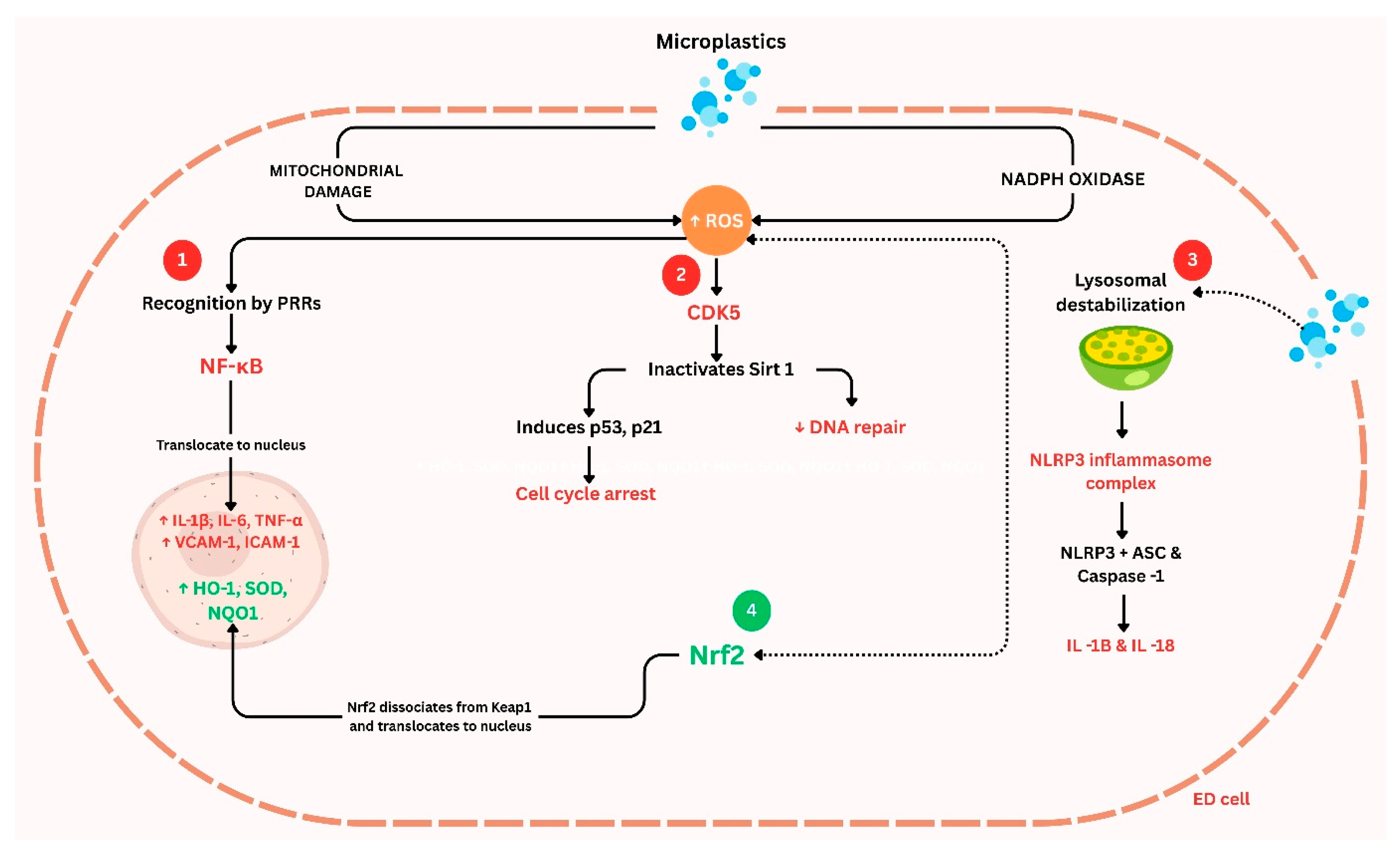
3.1. Oxidative Stress and Inflammation
3.2. Acceleration of Vascular Aging
3.3. Type- and Size-Specific Microplastics and Vascular Aging
3.4. Experimental and Human Evidence of Microplastic-Induced Vascular Effects
4. Molecular Insights: Signaling Pathways Affected by Microplastics in Vascular Cells
4.1. Inflammatory Activation and Immune Signaling
4.2. Endothelial Dysfunction and Barrier Integrity
4.3. Vascular Smooth Muscle Cell and Foam Cell Pathways
4.4. Cell Death and Stress Pathways
4.5. Acute vs. Chronic Exposure: Differences in Pathway Activation
5. Evidence of Adaptation to Microplastic Exposure
6. Future Therapeutic and Policy Interventions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MPs | Microplastic |
| NPs | Nanoplastic |
| ROS | Reactive Oxygen Species |
| NO | Nitric Oxide |
| IL-6 | Interleukin-6 |
| TNF-α | Tumor Necrosis Factor-alpha |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| NLRP3 | NOD-like receptor family pyrin domain containing 3 |
| ASC | Apoptosis-associated speck-like protein containing a CARD |
| VCAM-1 | Vascular Cell Adhesion Molecule-1 |
| ICAM-1 | Intercellular Adhesion Molecule-1 |
| PSMPs | Polystyrene Microplastic Particles |
| SOD | Superoxide Dismutase |
| GPx | Glutathione Peroxidase |
| MDA | Malondialdehyde |
| 4-HNE | 4-Hydroxynonenal |
| HUVECs | Human Umbilical Vein Endothelial Cells |
| VSMCs | Vascular Smooth Muscle Cells |
| MACE | Major Adverse Cardiovascular Events |
| SA-β-gal | Senescence-Associated β-Galactosidase |
| eNOS | Endothelial Nitric Oxide Synthase |
| FTIR | Fourier-Transform Infrared Spectroscopy |
| LDIR | Laser Direct Infrared |
| Py-GC/MS | Pyrolysis–Gas Chromatography/Mass Spectrometry |
| KIF15 | Kinesin Family Member 15 |
| ER | Endoplasmic Reticulum |
| TGF-β1 | Transforming Growth Factor Beta 1 |
| MAPK | Mitogen-Activated Protein Kinase |
| SGLT2 | Sodium-Glucose Cotransporter-2 |
| RIPK3 | Receptor-interacting serine/threonine-protein kinase 3 |
| MLKL | Mixed Lineage Kinase Domain-like Protein |
| GSDMD | Gasdermin D |
| CK-MB | Creatine Kinase-MB |
| VEGF | Vascular Endothelial Growth Factor |
| LDL | Low-Density Lipoprotein |
| HDL | High-Density Lipoprotein |
| MARCO | Macrophage Receptor with Collagenous Structure |
| CD36 | Cluster of Differentiation 36 |
| ACS | Acute Coronary Syndrome |
| BP | Blood Pressure |
| CRP | C-Reactive Protein |
| aPTT | Activated Partial Thromboplastin Time |
| NOX | NADPH Oxidase |
| Nrf2 | Nuclear factor erythroid 2–related factor 2 |
References
- United Nations Conference on Trade and Development. Plastic Pollution: The Pressing Case for Natural and Environmentally Friendly Substitutes to Plastics; UN: New York, NY, USA, 2023; pp. viii–ix. [Google Scholar] [CrossRef]
- Pratap, B.; Qureshi, A.; Akanksha, G.; Kumar, V.; Manjul, G. Global Scenario of Plastic Production, Consumption, and Waste Generation and Their Impacts on Environment and Human Health; Springer: Cham, Switzerland, 2024; pp. 1–34. [Google Scholar] [CrossRef]
- Walker, T.R. Consuming Plastics; Routledge: Milton Park, UK, 2024; pp. 105–117. [Google Scholar] [CrossRef]
- Kubíková, L.; Rudý, S. The Current Global Situation of Plastics and Forecast of Plastic Waste; University of Economics in Bratislava: Petržalka, Slovakia, 2024. [Google Scholar] [CrossRef]
- Walker, T.R.; Fequet, L. Current trends of unsustainable plastic production and micro(nano)plastic pollution. TrAC Trends Anal. Chem. 2023, 160, 116984. [Google Scholar] [CrossRef]
- Yu, R.F.; Yang, Y.; Singh, S. Global analysis of marine plastics and implications of control measure strategies. Front. Mar. Sci. 2023, 10, 1305091. [Google Scholar] [CrossRef]
- Samuels, F. A macroscopic view of microplastic formation. CEN Glob. Enterp. 2024, 102, 16–17. [Google Scholar] [CrossRef]
- Khalaf Amrullah, M.; Putri, E.; Yosephin Nababan, W.; Marolop, A. Microplastic: Characteristics, exposure pathways, toxicity, and implication for human health. J. Prima Med. Sains 2024, 6, 87–92. [Google Scholar] [CrossRef]
- Anas Ahmed, K.; Bose, K. Microplastics: An Overview on Ecosystem and Human Health. Int. J. Rese. Publ. Rev. 2024, 5, 3691–3702. [Google Scholar] [CrossRef]
- Law, K.L.; Thompson, R.C. Microplastics in the seas. Science 2014, 345, 144–145. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, S.; Allen, S.; Allen, D.; Gao, T.; Sillanpää, M. Atmospheric microplastics: A review on current status and perspectives. Earth-Sci. Rev. 2020, 203, 103118. [Google Scholar] [CrossRef]
- Watteau, F.; Dignac, M.F.; Bouchard, A.; Revallier, A.; Houot, S. Microplastic Detection in Soil Amended with Municipal Solid Waste Composts as Revealed by Transmission Electronic Microscopy and Pyrolysis/GC/MS. Front. Sustain. Food Syst. 2018, 2, 81. [Google Scholar] [CrossRef]
- John, K.I.; Omorogie, M.O.; Bayode, A.A.; Adeleye, A.T.; Helmreich, B. Environmental microplastics and their additives—A critical review on advanced oxidative techniques for their removal. Chem. Pap. 2022, 77, 657–676. [Google Scholar] [CrossRef]
- Do ATNgoc Ha, Y.; Kwon, J.H. Leaching of microplastic-associated additives in aquatic environments: A critical review. Environ. Pollut. 2022, 305, 119258. [Google Scholar] [CrossRef]
- Nam, H.; Gomez-Flores, A.; Kim, H. Combining size distribution and shape of plastic and oxide particles to evaluate physicochemical interactions: Aggregation and attachment. J. Hazard. Mater. 2025, 488, 137385. [Google Scholar] [CrossRef]
- Liu, X.; Wei, H.; Ahmad, S.; Wang, R.; Gao, P.; Chen, J.; Song, Y.; Liu, C.; Ding, N.; Tang, J. Effects and mechanism of microplastics on abundance and transfer of antibiotic resistance genes in the environment—A critical review. Crit. Rev. Environ. Sci. Technol. 2024, 54, 1852–1874. [Google Scholar] [CrossRef]
- Lotz, T.; Chen, W.; Su, S.; Chifflard, P. The Evolution of Microplastics Research: Global Trends and Agricultural Implications. Land Degrad. Dev. 2024, 36, 689–705. [Google Scholar] [CrossRef]
- Bao, Y.; Fang, X.; Cheng, Y.; Cui, D.; Liu, H.; Xiang, P. An often-overlooked health issue: The potential adverse effects of microplastics on the human ocular health. Crit. Rev. Environ. Sci. Technol. 2024, 55, 1–21. [Google Scholar] [CrossRef]
- Grzelak, A. From Environment to Body: Microplastics’ Sources, Pathways, and Health Repercussions. J. Educ. Health Sport 2024, 75, 56606. [Google Scholar] [CrossRef]
- Marszalek, A.; Marzec, W.Z.; Łakoma, A.; Marzec, M.T.; Choiński, M.; Wasiewicz-Ciach, P.; Kuczyński, P.; Wydra-rojek, A.; Kutyła, K.; Mokot, W.J. Impact of microplastics on human health: Exposure mechanisms and potential health implications. Qual. Sport 2024, 19, 54024. [Google Scholar] [CrossRef]
- Li, A.; Yan, J.; Zhao, Y.; Yu, Z.; Tian, S.; Khan, A.H.; Zhu, Y.; Wu, A.; Zhang, C.; Tian, X. Vascular Aging: Assessment and Intervention. Clin. Interv. Aging 2023, 18, 1373–1395. [Google Scholar] [CrossRef]
- Climie, R.E.; Alastruey, J.; Mayer, C.C.; Schwarz, A.; Laucyte-Cibulskiene, A.; Voicehovska, J.; Bianchini, E.; Bruno, R.M.; Charlton, P.H.; Grillo, A.; et al. Vascular ageing: Moving from bench towards bedside. Eur. J. Prev. Cardiol. 2023, 30, 1101–1117, Correction in Eur. J. Prev. Cardiol. 2023, 30, 1165. [Google Scholar] [CrossRef]
- Zheng, H.; Vidili, G.; Casu, G.; Navarese, E.P.; Sechi, L.A.; Chen, Y. Microplastics and nanoplastics in cardiovascular disease—A narrative review with worrying links. Front. Toxicol. 2024, 6, 1479292. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, F.; Jiang, Z.; Du, Z.; Liu, S.; Zhang, M.; Jin, Y.; Qin, Y.; Yang, X.; Wang, C.; et al. Microplastics are associated with elevated atherosclerotic risk and increased vascular complexity in acute coronary syndrome patients. Part. Fibre Toxicol. 2024, 21, 34. [Google Scholar] [CrossRef]
- Blackburn, K.; Green, D. The Potential Effects of Microplastics on Human health: What Is Known and What Is Unknown. Ambio 2021, 51, 518–530. [Google Scholar] [CrossRef]
- Liu, S.; Liu, S.; Xiao, X.; Liu, L.; Peijnenburg, W.; Xu, Y.; Wang, Y.; Yu, Y.; Li, L.; She, X. Fibrous microplastics in the environment: Sources, occurrence, impacts, and mitigation strategies. Aquat. Toxicol. 2024, 276, 107119. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Singh, P.K.; Kulshreshtha, U.C. Microplastics: An Invisible Air Pollutant of New Era. In Advances in Environmental Engineering and Green Technologies Book Series; IGI Global: Hershey, PA, USA, 2024; pp. 27–42. [Google Scholar] [CrossRef]
- Jaafarzadeh, N.; Reshadatian, N.; Feyzi Kamareh, T.; Sabaghan, M.; Feizi, R.; Jorfi, S. Study of the litter in the urban environment as primary and secondary microplastics sources. Sci. Rep. 2024, 14, 31645. [Google Scholar] [CrossRef] [PubMed]
- Holloway, N.; Adams, H.; Espindola, C.A.; Ikehata, K. Microplastics and the Water Industry. J. AWWA 2024, 116, 36–44. [Google Scholar] [CrossRef]
- Kobzar, V.; Gasanov, R.; Suyunbekkyzy, A.; Peresadin, N. Microplastics in Water and Food: [Not]Awareness. Bull. Sci. Pract. 2024, 10, 108–118. [Google Scholar] [CrossRef]
- Chandra, S.; Walsh, K.B. Microplastics in water: Occurrence, fate and removal. J. Contam. Hydrol. 2024, 264, 104360. [Google Scholar] [CrossRef]
- Zhu, Y.; Che, R.; Zong, X.; Wang, J.; Li, J.; Zhang, C.; Wang, F. A comprehensive review on the source, ingestion route, attachment and toxicity of microplastics/nanoplastics in human systems. J. Environ. Manag. 2024, 352, 120039. [Google Scholar] [CrossRef]
- Amado Enrique, N.F.; Marelis, P.L.; Paula Montserrat, C.B. Human skin and micro- and nanoplastics: A mini-review. MOJ Ecol. Environ. Sci. 2024, 9, 122–125. [Google Scholar] [CrossRef]
- Yang, S. Global Modeling of Microplastics in the Atmosphere. In Proceedings of the 25th EGU General Assembly, Vienna, Austria, 23–28 April 2023. [Google Scholar] [CrossRef]
- Chen, C.; Pagsuyoin, S.A.; Tim, H.M.; Xu, Y.; He, Y.; Guo, Z.; Liu, D.; Xu, Y. Significant regional disparities in riverine microplastics. J. Hazard. Mater. 2024, 472, 134571. [Google Scholar] [CrossRef]
- Bosker, T.; Behrens, P.; Vijver, M.G. Determining global distribution of microplastics by combining citizen science and in-depth case studies. Integr. Environ. Assess. Manag. 2017, 13, 536–541. [Google Scholar] [CrossRef]
- Zuri, G.; Angeliki Karanasiou Lacorte, S. Microplastics: Human exposure assessment through air, water, and food. Environ. Int. 2023, 179, 108150. [Google Scholar] [CrossRef]
- Mohamed Nor, N.H.; Kooi, M.; Diepens, N.J.; Koelmans, A.A. Lifetime Accumulation of Microplastic in Children and Adults. Environ. Sci. Technol. 2021, 55, 5084–5096. [Google Scholar] [CrossRef]
- Roslan, N.S.; Lee, Y.Y.; Ibrahim, Y.S.; Anuar, S.T.; Yusof, K.M.K.K.; Lai, L.A.; Brentnall, T. Detection of microplastics in human tissues and organs: A scoping review. J. Glob. Health 2024, 14, 04179. [Google Scholar] [CrossRef]
- Leslie, H.A.; JMvan Velzen, M.; Brandsma, S.H.; Vethaak, D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Du, S.; Liu, Z.; Wu, L.; Tao, F. Identification and characterization of microplastics released during the actual use of disposable cups using laser direct infrared imaging. Analyst 2025, 150, 989–997. [Google Scholar] [CrossRef]
- Prattichizzo, F.; Ceriello, A.; Pellegrini, V.; La Grotta, R.; Graciotti, L.; Olivieri, F.; Paolisso, P.; D’Agostino, B.; Iovino, P.; Balestrieri, M.L.; et al. Micro-nanoplastics and cardiovascular diseases: Evidence and perspectives. Eur. Heart J. 2024, 45, 4099–4110. [Google Scholar] [CrossRef]
- Persiani, E.; Cecchettini, A.; Ceccherini, E.; Gisone, I.; Morales, M.A.; Vozzi, F. Microplastics: A Matter of the Heart (and Vascular System). Biomedicines 2023, 11, 264. [Google Scholar] [CrossRef]
- Rezabakhsh, A.; Rahbarghazi, R.; Khalilzadeh, B.; Mahdipour, M. Toxic effects of plastic particles on cardiovascular system and angiogenesis potential: A major challenge of the current century. Med. J. Tabriz Univ. Med. Sci. 2024, 46, 475–489. [Google Scholar] [CrossRef]
- Lerchner, T.; Jedlička, J.; Kripnerová, M.; Dejmek, J.; Kuncová, J. Influence of Micro- and Nanoplastics on Mitochondrial Function in the Cardiovascular System: A Review of the Current Literature. Physiol. Res. 2024, 73 (Suppl. 3), S685–S695. [Google Scholar] [CrossRef] [PubMed]
- Simmons, T.; Vandergon, T. Nanoplastic effects on human vascular endothelial cells: A comparison of primary cells (HUVEC) and immortalized cells (hy926) after exposure to polystyrene nanoplastics. FASEB J. 2022, 36, r3747. [Google Scholar] [CrossRef]
- Xie, X.; Wang, K.; Shen, X.; Wang, S.; Yuan, S.; Li, B.; Wang, Z. Potential mechanisms of aortic medial degeneration promoted by co-exposure to microplastics and lead. J. Hazard. Mater. 2024, 475, 134854. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, Y.; Lemos, B.; Ren, H. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci. Rep. 2017, 7, 46687. [Google Scholar] [CrossRef]
- Xiao, Y.; Hu, L.; Duan, J.; Che, H.; Wang, W.; Yuan, Y.; Xu, J.; Chen, D.; Zhao, S. Polystyrene microplastics enhance microcystin-LR-induced cardiovascular toxicity and oxidative stress in zebrafish embryos. Environ. Pollut. 2024, 352, 124022. [Google Scholar] [CrossRef]
- Vlacil, A.-K.; Bänfer, S.; Jacob, R.; Trippel, N.; Kuzu, I.; Schieffer, B.; Grote, K.; Cao, Y. Polystyrene microplastic particles induce endothelial activation. PLoS ONE 2021, 16, e0260181. [Google Scholar] [CrossRef]
- Song, Z.; Wu, H.; Fang, X.; Feng, X.; Zhou, L. The cardiovascular toxicity of polystyrene microplastics in rats: Based on untargeted metabolomics analysis. Front. Pharmacol. 2024, 15, 1336369. [Google Scholar] [CrossRef]
- Lomonaco, T.; Persiani, E.; Biagini, D.; Gisone, I.; Ceccherini, E.; Cecchettini, A.; Corti, A.; Ghimenti, S.; Di Francesco, F.; Castelvetro, V.; et al. Type-specific inflammatory responses of vascular cells activated by interaction with virgin and aged microplastics. Ecotoxicol. Environ. Saf. 2024, 282, 116695. [Google Scholar] [CrossRef]
- Guzik, T.J.; Touyz, R.M. Oxidative Stress, Inflammation, and Vascular Aging in Hypertension. Hypertension 2017, 70, 660–667. [Google Scholar] [CrossRef]
- Lu, T.; Yuan, X.; Sui, C.; Yang, C.; Li, D.; Liu, H.; Zhang, G.; Li, G.; Li, S.; Zhang, J.; et al. Exposure to Polypropylene Microplastics Causes Cardiomyocyte Apoptosis Through Oxidative Stress and Activation of the MAPK-Nrf2 Signaling Pathway. Environ. Toxicol. 2024, 39, 5371–5381. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, F.; Sarker, D.B.; Jocelyn, J.A.; Sang, Q.X.A. Molecular and Cellular Effects of Microplastics and Nanoplastics: Focus on Inflammation and Senescence. Cells 2024, 13, 1788. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Pan, Y.; He, J.; Pang, X.; Shao, W.; Wang, C.; Wang, R.; He, Y.; Zhang, M.; Ye, J.; et al. Toxic vascular effects of polystyrene microplastic exposure. Sci. Total Environ. 2023, 905, 167215. [Google Scholar] [CrossRef]
- Zhang, M.; Shi, J.; Huang, Q.; Xie, Y.; Wu, R.; Zhong, J.; Deng, H. Multi-omics analysis reveals size-dependent toxicity and vascular endothelial cell injury induced by microplastic exposure in vivo and in vitro. Environ. Sci. Nano 2022, 9, 663–683. [Google Scholar] [CrossRef]
- Wang, K.; Du, Y.; Li, P.; Guan, C.; Zhou, M.; Wu, L.; Liu, Z.; Huang, Z. Microplastics accelerates the premature aging of blood vessels though ROS-mediated CDK5 signaling pathway. 2023. Preprint 2023. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Dey, A.; Mondal, S.; Gautam, M.K. Environmental microplastics and nanoplastics: Effects on cardiovascular system. Toxicol. Anal. Et Clin. 2024, 36, 145–157. [Google Scholar] [CrossRef]
- Zhang, M.; Shi, J.; Zhu, Y.; Pan, H.; Song, L.; Deng, H. Polystyrene nanoplastics induce vascular stenosis via regulation of the PIWI-interacting RNA expression profile. Environ. Pollut. 2024, 345, 123441. [Google Scholar] [CrossRef]
- Zhong, Y.; Feng, Y.; Huang, Y.; Wang, B.; Shi, W.; Liang, B.; Li, Z.; Zhang, B.; Du, J.; Xiu, J.; et al. Polystyrene nanoplastics accelerate atherosclerosis: Unraveling the impact on smooth muscle cells through KIF15-mediated migration. Ecotoxicol. Environ. Saf. 2024, 284, 116983. [Google Scholar] [CrossRef]
- Wen, J.; Sun, H.; Yang, B.; Song, E.; Song, Y. Long-term polystyrene nanoplastic exposure disrupt hepatic lipid metabolism and cause atherosclerosis in ApoE−/− mice. J. Hazard. Mater. 2024, 466, 133583. [Google Scholar] [CrossRef]
- Zhao, J.; Gomes, D.C.; Conklin, D.; O’Toole, T.E. Abstract 9880: Microplastics Exposure Promotes Cardiovascular Disease Risk in Mice. Circulation 2021, 144 (Suppl. S1), 9880. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, Q.; Li, Y.; Feng, Y.; Wang, Y.; Cheng, W. Low-dose of polystyrene microplastics induce cardiotoxicity in mice and human-originated cardiac organoids. Environ. Int. 2023, 179, 108171. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jia, Z.; Zhou, X.; Su, L.; Wang, M.; Wang, T.; Zhang, H. Nanoplastic-induced vascular endothelial injury and coagulation dysfunction in mice. Sci. Total Environ. 2023, 865, 161271. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liang, B.; Huang, Y.; Li, Z.; Zhang, B.; Du, J.; Ye, R.; Xian, H.; Deng, Y.; Xiu, J.; et al. Long-Chain Acyl Carnitines Aggravate Polystyrene Nanoplastics-Induced Atherosclerosis by Upregulating MARCO. Adv. Sci. 2023, 10, e2205876. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, S.; Liu, Q.; Wei, J.; Jin, Y.; Wang, X.; Zhang, L. Polystyrene microplastics cause cardiac fibrosis by activating Wnt/β-catenin signaling pathway and promoting cardiomyocyte apoptosis in rats. Environ. Pollut. 2020, 265, 115025. [Google Scholar] [CrossRef]
- Zhao, J.; Gomes, D.; Jin, L.; Mathis, S.P.; Li, X.; Rouchka, E.C.; Bodduluri, H.; Conklin, D.J.; O’tOole, T.E. Polystyrene bead ingestion promotes adiposity and cardiometabolic disease in mice. Ecotoxicol. Environ. Saf. 2022, 232, 113239. [Google Scholar] [CrossRef]
- Lin, P.; Tong, X.; Xue, F.; Qianru, C.; Xinyu, T.; Zhe, L.; Zhikun, B.; Shu, L. Polystyrene nanoplastics exacerbate lipopolysaccharide-induced myocardial fibrosis and autophagy in mice via ROS/TGF-β1/Smad. Toxicology 2022, 480, 153338. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Lu, H.; Zhang, Y.; Hou, L.; Meng, X.; Li, J.; Zhao, H.; Xing, M. Secondary brain injury after polystyrene microplastic-induced intracerebral hemorrhage is associated with inflammation and pyroptosis. Chem. Biol. Interact. 2022, 367, 110180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yin, K.; Wang, D.; Wang, Y.; Lu, H.; Zhao, H.; Xing, M. Polystyrene microplastics-induced cardiotoxicity in chickens via the ROS-driven NF-κB-NLRP3-GSDMD and AMPK-PGC-1α axes. Sci. Total Environ. 2022, 840, 156727. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, D.; Yin, K.; Zhao, H.; Lu, H.; Meng, X.; Hou, L.; Li, J.; Xing, M. Endoplasmic reticulum stress-controlled autophagic pathway promotes polystyrene microplastics-induced myocardial dysplasia in birds. Environ. Pollut. 2022, 311, 119963. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, S.; Ge, Y.; Wan, X.; Zhu, Y.; Yang, F.; Li, J.; Gong, S.; Cheng, Y.; Hu, C.; et al. Multi-dimensional evaluation of cardiotoxicity in mice following respiratory exposure to polystyrene nanoplastics. Part. Fibre Toxicol. 2023, 20, 46. [Google Scholar] [CrossRef]
- Shiwakoti, S.; Ko, J.-Y.; Gong, D.; Dhakal, B.; Lee, J.-H.; Adhikari, R.; Gwak, Y.; Park, S.-H.; Choi, I.J.; Schini-Kerth, V.B.; et al. Effects of polystyrene nanoplastics on endothelium senescence and its underlying mechanism. Environ. Int. 2022, 164, 107248. [Google Scholar] [CrossRef]
- Dhakal, B.; Shiwakoti, S.; Park, E.-Y.; Kang, K.-W.; Schini-Kerth, V.B.; Park, S.-H.; Ji, H.-Y.; Park, J.S.; Ko, J.-Y.; Oak, M.-H. SGLT2 inhibition ameliorates nano plastics-induced premature endothelial senescence and dysfunction. Sci. Rep. 2023, 13, 6256. [Google Scholar] [CrossRef]
- Basini, G.; Grolli, S.; Bertini, S.; Bussolati, S.; Berni, M.; Berni, P.; Ramoni, R.; Scaltriti, E.; Quintavalla, F.; Grasselli, F. Nanoplastics induced oxidative stress and VEGF production in aortic endothelial cells. Environ. Toxicol. Pharmacol. 2023, 104, 104294. [Google Scholar] [CrossRef]
- Roshanzadeh, A.; Oyunbaatar, N.-E.; Ganjbakhsh, S.E.; Park, S.; Kim, D.-S.; Kanade, P.P.; Lee, S.; Lee, D.-W.; Kim, E.-S. Exposure to nanoplastics impairs collective contractility of neonatal cardiomyocytes under electrical synchronization. Biomaterials 2021, 278, 121175. [Google Scholar] [CrossRef]
- Barshtein, G.; Livshits, L.; Shvartsman, L.D.; Shlomai, N.O.; Yedgar, S.; Arbell, D. Polystyrene Nanoparticles Activate Erythrocyte Aggregation and Adhesion to Endothelial Cells. Cell Biochem. Biophys. 2015, 74, 19–27. [Google Scholar] [CrossRef]
- Florance, I.; Chandrasekaran, N.; Gopinath, P.M.; Mukherjee, A. Exposure to polystyrene nanoplastics impairs lipid metabolism in human and murine macrophages in vitro. Ecotoxicol. Environ. Saf. 2022, 238, 113612. [Google Scholar] [CrossRef] [PubMed]
- Marfella, R.; Prattichizzo, F.; Sardu, C.; Fulgenzi, G.; Graciotti, L.; Spadoni, T.; D’oNofrio, N.; Scisciola, L.; La Grotta, R.; Frigé, C.; et al. Microplastics and Nanoplastics in Atheromas and Cardiovascular Events. N. Engl. J. Med. 2024, 390, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yan, K.; Dong, Y.; Chen, Y.; Song, J.; Chen, Y.; Liu, X.; Qi, R.; Zhou, X.; Zhong, J.; et al. The influence of microplastics on hypertension-associated cardiovascular injury via the modulation of gut microbiota. Environ. Pollut. 2025, 368, 125760. [Google Scholar] [CrossRef] [PubMed]
- Geppner, L.; Grammatidis, S.; Wilfing, H.; Henjakovic, M. First Evidence of the Possible Influence of Avoiding Daily Liquid Intake from Plastic and Glass Beverage Bottles on Blood Pressure in Healthy Volunteers. Microplastics 2024, 3, 419–432. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, E.; Du, Z.; Peng, Z.; Han, Z.; Li, L.; Zhao, R.; Qin, Y.; Xue, M.; Li, F.; et al. Detection of Various Microplastics in Patients Undergoing Cardiac Surgery. Environ. Sci. Technol. 2023, 57, 2c07179. [Google Scholar] [CrossRef]
- Wang, T.; Yi, Z.; Liu, X.; Cai, Y.; Huang, X.; Fang, J.; Shen, R.; Lu, W.; Xiao, Y.; Zhuang, W.; et al. Multimodal detection and analysis of microplastics in human thrombi from multiple anatomically distinct sites. EBioMedicine 2024, 103, 105118. [Google Scholar] [CrossRef]
- Lee, D.-W.; Jung, J.; Park, S.-A.; Lee, Y.; Kim, J.; Han, C.; Kim, H.-C.; Lee, J.H.; Hong, Y.-C. Microplastic particles in human blood and their association with coagulation markers. Sci. Rep. 2024, 14, 30419. [Google Scholar] [CrossRef]
- Lee, S.E.; Yoon, H.K.; Kim, D.Y.; Jeong, T.S.; Park, Y.S. An Emerging Role of Micro- and Nanoplastics in Vascular Diseases. Life 2024, 14, 255. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liang, S.; Zhang, J.; Ning, R.; Du, Z.; Liu, J.; Batibawa, J.W.; Duan, J.; Sun, Z. The critical role of endothelial function in fine particulate matter-induced atherosclerosis. Part Fibre Toxicol. 2020, 17, 61. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Y.C.; Chen, K.F.; Andrew Lin, K.Y.; Su, H.P.; Wu, D.N.; Lin, C.H. Evaluation of toxicity of polystyrene microplastics under realistic exposure levels in human vascular endothelial EA.hy926 cells. Chemosphere 2023, 313, 137582. [Google Scholar] [CrossRef]
- Fu, Y.; Fan, M.; Xu, L.; Wang, H.; Hu, Q.; Jin, Y. Amino-Functionalized Polystyrene Nano-Plastics Induce Mitochondria Damage in Human Umbilical Vein Endothelial Cells. Toxics 2022, 10, 215. [Google Scholar] [CrossRef]
- Wu, S.; Wu, M.; Tian, D.; Qiu, L.; Li, T. Effects of polystyrene microbeads on cytotoxicity and transcriptomic profiles in human Caco-2 cells. Environ. Toxicol. 2020, 35, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Kadac-Czapska, K.; Ośko, J.; Knez, E.; Grembecka, M. Microplastics and Oxidative Stress-Current Problems and Prospects. Antioxidants 2024, 13, 579. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.; Zhang, Y.; Zhao, H.; Zeng, T.; Zhao, X. Polystyrene nanoplastics penetrate across the blood-brain barrier and induce activation of microglia in the brain of mice. Chemosphere 2022, 298, 134261. [Google Scholar] [CrossRef]
- Gangwar, R.S.; Bevan, G.H.; Palanivel, R.; Das, L.; Rajagopalan, S. Oxidative stress pathways of air pollution mediated toxicity: Recent insights. Redox Biol. 2020, 34, 101545. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ding, R.; Huang, L.; Yan, K.; Sun, Z.; Duan, J. New insight into air pollution-related cardiovascular disease: An adverse outcome pathway framework of PM2.5-associated vascular calcification. Cardiovasc. Res. 2024, 120, 699–707. [Google Scholar] [CrossRef]
- Cary, C.M.; Seymore, T.N.; Singh, D.; Vayas, K.N.; Goedken, M.J.; Adams, S.; Polunas, M.; Sunil, V.R.; Laskin, D.L.; Demokritou, P.; et al. Single inhalation exposure to polyamide micro and nanoplastic particles impairs vascular dilation without generating pulmonary inflammation in virgin female Sprague Dawley rats. Part. Fibre Toxicol. 2023, 20, 16. [Google Scholar] [CrossRef]
- Zhang, M.; Shi, J.; Pan, H.; Zhu, J.; Wang, X.; Song, L.; Deng, H. A novel tiRNA-Glu-CTC induces nanoplastics accelerated vascular smooth muscle cell phenotypic switching and vascular injury through mitochondrial damage. Sci. Total Environ. 2024, 912, 169515. [Google Scholar] [CrossRef] [PubMed]
- Florance, I.; Ramasubbu, S.; Mukherjee, A.; Chandrasekaran, N. Polystyrene nanoplastics dysregulate lipid metabolism in murine macrophages in vitro. Toxicology 2021, 458, 152850. [Google Scholar] [CrossRef] [PubMed]
- Bäck, M.; Yurdagul, A.; Tabas, I.; Öörni, K.; Kovanen, P.T. Inflammation and its resolution in atherosclerosis: Mediators and therapeutic opportunities. Nat. Rev. Cardiol. 2019, 16, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Nnoruka, U.C.; Okonkwo, C.J.; Ilechukwu, I.; Okonkwo, C.J.; Belonwu, D.C. Impact of polystyrene microplastic exposure on lipid profile and oxidative stress status of male and female Wistar rats. Environ. Anal Health Toxicol. 2022, 37, e2022024-0. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wei, J.; Wang, X.; Liu, Q.; Zhou, N.; Zhu, S.; Li, Z.; Li, X.; Yao, J.; Zhang, L. The impact of polystyrene microplastics on cardiomyocytes pyroptosis through NLRP3 /Caspase-1 signaling pathway and oxidative stress in Wistar rats. Environ. Toxicol. 2021, 36, 935–944. [Google Scholar] [CrossRef]
- Lu, Y.-Y.; Li, H.; Ren, H.; Zhang, X.; Huang, F.; Zhang, D.; Huang, Q.; Zhang, X. Size-dependent effects of polystyrene nanoplastics on autophagy response in human umbilical vein endothelial cells. J. Hazard. Mater. 2022, 421, 126770. [Google Scholar] [CrossRef]
- Lee, H.S.; Amarakoon, D.; Wei, C.; Choi, K.Y.; Smolensky, D.; Lee, S.H. Adverse effect of polystyrene microplastics (PS-MPs) on tube formation and viability of human umbilical vein endothelial cells. Food Chem. Toxicol. 2021, 154, 112356. [Google Scholar] [CrossRef]
- Lu, Y.; Cao, M.; Tian, M.; Huang, Q. Internalization and cytotoxicity of polystyrene microplastics in human umbilical vein endothelial cells. J. Appl. Toxicol. 2022, 43, 262–271. [Google Scholar] [CrossRef]
- Yu, P.; Liu, Z.; Wu, D.; Chen, M.; Lv, W.; Zhao, Y. Accumulation of polystyrene microplastics in juvenile Eriocheir sinensis and oxidative stress effects in the liver. Aquat. Toxicol. 2018, 200, 28–36. [Google Scholar] [CrossRef]
- Nugrahapraja, H.; Sugiyo, P.W.W.; Putri, B.Q.; Ni’matuzahroh; Fatimah; Huang, L.; Hafza, N.; Götz, F.; Santoso, H.; Wibowo, A.T.; et al. Effects of Microplastic on Human Gut Microbiome: Detection of Plastic-Degrading Genes in Human Gut Exposed to Microplastics—Preliminary Study. Environments 2022, 9, 140. [Google Scholar] [CrossRef]
- Fröhlich, E. Local and systemic effects of microplastic particles through cell damage, release of chemicals and drugs, dysbiosis, and interference with the absorption of nutrients. J. Toxicol. Environ. Health Part B 2024, 27, 315–344. [Google Scholar] [CrossRef]
- Ma, M.D.; Zhao, Y.C.; Zhu, L.; Wang, W.P.; Kang, Y.L.; An, L.H. Research Progress on Characteristics of Human Microplastic Pollution and Health Risks. PubMed 2024, 45, 459–469. [Google Scholar] [CrossRef]
- Poljsak, B.; Milisav, I. NAD+ as the Link Between Oxidative Stress, Inflammation, Caloric Restriction, Exercise, DNA Repair, Longevity, and Health Span. Rejuvenation Res. 2016, 19, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Trushina, E.N.; Riger, N.A.; Mustaphina, O.K.; Timonin, A.N. Effect of micro- and nanoplastics as food contaminants on the immune system. Probl. Nutr. 2023, 92, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Zhi, L.; Li, Z.; Su, Z.; Wang, J. Immunotoxicity of microplastics: Carrying pathogens and destroying the immune system. TrAC Trends Anal. Chem. 2024, 177, 117817. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, M.; Wang, L.; Gu, W.; Li, X.; Han, Z.; Fu, X.; Wang, X.; Li, X.; Su, Z. Continuous oral exposure to micro- and nanoplastics induced gut microbiota dysbiosis, intestinal barrier and immune dysfunction in adult mice. Environ. Int. 2023, 182, 108353. [Google Scholar] [CrossRef]
- Sayed, H.; Hana, M.N.; Hamed, M.; Hany Lee, J.S.; Soliman, H.A.M. Protective efficacy of dietary natural antioxidants on microplastic particles-induced histopathological lesions in African catfish (Clarias gariepinus). Environ. Sci. Pollut. Res. 2022, 30, 24424–24440. [Google Scholar] [CrossRef]
- Roman, J.; Gondko, D.; Patrycja Dębiec Pietrzak, N. The Hidden Health Crisis: Microplastics and Their Medical Consequences. J. Educ. Health Sport 2024, 72, 51690. [Google Scholar] [CrossRef]
- Bora, S.S.; Gogoi, R.; Sharma, M.R.; Anshu; Borah, M.P.; Deka, P.; Bora, J.; Naorem, R.S.; Das, J.; Teli, A.B. Microplastics and human health: Unveiling the gut microbiome disruption and chronic disease risks. Front. Cell. Infect. Microbiol. 2024, 14, 1492759. [Google Scholar] [CrossRef]
- Kravitz, R.L.; Ritley, D.; Padilla, K.; Woodruff, T.; Chartres, N.; Cooper, C.; Bland, G.; Woodley, D.; Hoffman, E. Microplastics Occurrence, Health Effects, and Mitigation Policies; CalSPEC: Sacramento, CA, USA, 2023. [Google Scholar] [CrossRef]

| Author(s) | Model | Microplastic Type, Size, Dose | Exposure Duration | Vascular End Points | Key Findings |
|---|---|---|---|---|---|
| Animal models (in vivo) | |||||
| Wang X, Jia Z, Zhou X et al. [65] | Mouse (C57BL/6) | Polystyrene (PS) nanoplastics, ~50 nm; surface variants; 0.05–20 mg/kg (oral) | 14 days (short-term) | Endothelial integrity, inflammation markers, and coagulation parameters | Exposure caused structural damage to the vascular endothelium and an inflammatory response in mice. Notably, pro-thrombotic effects were observed—nanoplastics induced a prothrombotic state with coagulation dysfunction via activation of the JAK1/STAT3/Tissue Factor pathway. |
| Wang B, Liang B, Huang Y et al. [66] | Mouse (ApoE−/−) | PS nanoplastics, ~50 nm; 2.5, 25, 250 mg/kg (oral gavage) + high-fat diet | 19 weeks (chronic) | Arterial stiffness, atherosclerotic plaque burden, macrophage activation, serum lipid metabolism | Chronic low-dose PS nanoparticle ingestion significantly increased arterial stiffness and promoted atherosclerotic plaque formation in ApoE−/− mice. Treated mice showed enhanced M1 macrophage uptake in lesions and disrupted lipid metabolism, suggesting microplastics exacerbate plaque development via metabolic and inflammatory mechanisms. |
| Zhou Y, Wu Q, Li Y et al. [64] | Mouse (C57BL/6) | PS microplastics; low human-equivalent dose. | 4 weeks (chronic) | Cardiac hypertrophy index, cardiac function | Even at low, human-relevant doses, chronic PS microplastic ingestion induced myocardial hypertrophy and reduced cardiac output in mice. Notably, the same study also showed similar cardiotoxic effects in human cardiac organoids. |
| Li Z, Zhu S, Liu Q et al. [67] | Rat (Wistar) | PS microplastics, ~0.5 μm; dose not reported (oral) | 8 weeks (chronic) | Myocardial histopathology, cardiomyocyte apoptosis, cardiac injury biomarkers (troponin I, CK-MB) | PS microplastic exposure activated pro-fibrotic Wnt/β-catenin signaling in the rat heart, leading to interstitial cardiac fibrosis and increased cardiomyocyte apoptosis. Treated rats exhibited elevated cardiac injury biomarkers and oxidative stress, implicating microplastics as a trigger for adverse cardiac remodeling. |
| Zhao J, Gomes D, Jin L et al. [68] | Mouse (ICR) | PS nanoplastics; size not specified (nanoscale); 0.5 and 5 μm aggregates; 0.1 and 1.0 μg/mL in drinking water | 5 months (chronic) | Metabolic indices, fasting blood glucose, and insulin sensitivity | Chronic ingestion of low-dose PS micro/nanoplastics caused increased adiposity and weight gain in mice, along with elevated blood glucose. These changes suggest microplastic exposure can promote a cardiometabolic profile that may accelerate vascular aging and elevate cardiovascular risk. |
| Lin P, Tong X, Xue F et al. [69] | Mouse (Kunming) | PS nanoplastics; ~100 nm; 5 mg/kg (oral) | 60 days (chronic) | Myocardial ultrastructure, ROS levels in the heart, fibrosis markers, TGF-β1/Smad pathway activation | Oral PS nanoplastics led to myocardial structural abnormalities with elevated ROS in cardiac tissue. Mice showed activation of TGF-β1/Smad signaling and increased collagen deposition in the myocardium, culminating in fibrotic cardiac remodeling. This indicates microplastics can provoke oxidative-stress–driven cardiac fibrosis and impair heart function. |
| Yin K, Lu H, Zhang Y et al. [70] | Chicken (Gallus domesticus) | PS microplastics, ~5 μm; 1, 10, 100 mg/L in drinking water | 4 weeks (chronic) | Cerebrovascular injury (intracerebral hemorrhage incidence), brain inflammation (IL-1β), neuronal pyroptosis (caspase-1, GSDMD) | Oral microplastic exposure in chickens precipitated intracerebral hemorrhages accompanied by mitochondrial dysfunction in brain tissue. Affected birds exhibited intense neuroinflammation and pyroptotic cell death in the brain, linking microplastics to cerebrovascular injury and stroke risk. |
| Zhang Y, Yin K, Wang D et al. [71] | Chicken (Gallus domesticus) | PS microplastics, ~5 μm; 1, 10, 100 mg/L in drinking water | 4 weeks (chronic) | Cardiac inflammation (myocardial IL-6, TNFα), pyroptosis in heart (NLRP3 inflammasome activation, caspase-1) | Chronic PS microplastic intake induced cardiotoxic effects in chickens, including myocarditis and cardiomyocyte pyroptosis. Hearts showed increased pro-inflammatory cytokines and NLRP3 inflammasome activation, leading to pyroptotic cell death. This ROS-driven inflammatory injury to the heart highlights microplastics as a trigger for pathological cardiac inflammation. |
| Zhang Y, Wang D, Yin K et al. [72] | Bird (Domestic fowl) | PS microplastics, ~5 μm; 1, 10, 100 mg/L in drinking water | 4 weeks (chronic) | Cardiac development, ER stress markers (GRP78, CHOP), and autophagy flux in myocardium | Microplastic exposure in developing birds led to myocardial dysplasia and structural abnormalities in the heart. The mechanism involved pronounced endoplasmic reticulum stress and altered autophagic pathways in cardiac tissue, suggesting that microplastics impair protein homeostasis in cardiomyocytes and disrupt normal heart development. |
| Zhang T, Yang S, Ge Y et al. [73] | Mouse (ICR) | PS nanoplastics, ~40 nm; inhalation at ~16, 40, 100 µg/day | 1, 4, or 12 weeks (acute to subchronic) | Cardiac function, myocardial injury biomarkers (LDH, CK-MB), oxidative stress (MDA, SOD levels), cardiac histopathology (inflammation, fibrosis) | Respiratory exposure to nano-PS caused dose- and time-dependent cardiotoxic effects. Acute exposure provoked intense cardiac inflammation and immune cell infiltration, while subacute/subchronic exposure led to reduced systolic function and structural heart damage. Mice showed elevated cardiac injury enzymes and oxidative stress, with evidence of mitochondrial damage in cardiomyocytes. Overall, inhaled nanoplastics induced myocardial injury and accelerated cardiac tissue aging changes. |
| Cell Line (in vitro) | |||||
| Saugat Shiwakoti, Ko JY, Gong DS et al. [74] | Endothelial cells (PCAEC, porcine coronary artery) | PS nanoplastics, ~25 nm | 0.1, 1, 10 µg/mL; 24 h exposure | Cellular senescence markers (SA-β-gal activity, p16, p21, p53 levels), nitric oxide bioavailability | PS nanoparticle exposure caused endothelial cells to acquire aging features. Treated PCAECs showed increased senescence-associated β-galactosidase staining and upregulated cyclin-dependent kinase inhibitors p16, p21, and p53. Endothelial NO production was impaired, indicating functional endothelial dysfunction alongside the senescence phenotype. |
| Bikalpa Dhakal, Saugat Shiwakoti, Park EY et al. [75] | Endothelial cells + SGLT2 inhibitor (porcine coronary artery) | PS nanoplastics, ~25 nm | 5 and 10 µg/mL; 24 h (± empagliflozin) | Endothelial senescence (SA-β-gal), ROS generation, eNOS expression, and cell viability | Low-dose PS NPs induced marked premature senescence and dysfunction in endothelial cells. Co-treatment with an SGLT2 inhibitor blunted these effects, reducing oxidative stress and senescent cell burden. This demonstrates that NP-induced endothelial aging is mechanistically linked to SGLT2, and blocking this pathway can ameliorate microplastic-induced vascular aging. |
| Basini G, Stefano Grolli, Bertini S et al. [76] | Endothelial cells (AOC line, porcine aorta) | PS nanoplastics, ~100 nm | 5, 25, 75 µg/mL; 24 h exposure | Cell metabolic activity (MTT assay), redox status (intracellular ROS), and angiogenic factor (VEGF) secretion | High concentrations of PS nanoplastics led to dose-dependent cytotoxicity in aortic endothelial cells. Nanoplastic-treated cells showed reduced metabolic activity and a significant increase in oxidative stress. Secretion of VEGF was altered, indicating that microplastics can impair endothelial angiogenic signaling and viability at higher doses. |
| Vlacil AK, Bänfer S, Jacob R et al. [50] | Microvascular endothelial cells (murine heart) | PS microplastics, ~1 µm | 0.54 ng/mL, 54 ng/mL, 5.4 µg/mL; 6 h + 18 h (24 h total) | Endothelial activation, monocyte adhesion assay (THP-1 binding) | Exposure to 1 µm PS particles activated mouse cardiac endothelial cells, evidenced by upregulation of adhesion molecules and a pro-inflammatory phenotype. Treated endothelial monolayers showed significantly increased adhesion of monocytes, indicating microplastics promote endothelial dysfunction that favors leukocyte recruitment—an early step in vascular inflammation and atherogenesis. |
| Roshanzadeh A, Oyunbaatar NE, Ganjbakhsh SE et al. [77] | Cardiomyocytes (neonatal rat heart cells) | PS nanoplastics, ~50 nm | 25 μg/mL | Contractile function, calcium handling (L-type Ca2+ channel current) | Neonatal cardiomyocytes exposed to PS nanoplastics exhibited weakened, synchronized contractions. Nanoplastics readily internalized into cardiomyocytes and inhibited L-type Ca2+ channels, leading to reduced calcium influx and contraction force. This suggests microplastics can directly disturb cardiac electrophysiology and muscle function, potentially contributing to arrhythmias or heart failure with prolonged exposure. |
| Barshtein G, Livshits L, Shvartsman LD et al. [78] | Red blood cells + endothelium (human) | PS nanoplastics, 50–250 nm | 50 and 100 µg/mL; 2 h co-incubation | Erythrocyte aggregation index, RBC–endothelial adhesion | Polystyrene nanoparticles promoted abnormal clumping of human erythrocytes and their adhesion to endothelial cell layers. Smaller nanoplastics had a more pronounced effect than larger ones, causing up to a 2.5-fold increase in RBC aggregation. These findings highlight a potential mechanism for microplastics to induce microvascular occlusions or thrombosis by enhancing red cell aggregation and vascular sticking. |
| Florance I, Chandrasekaran N et al. [79] | Monocyte-macrophage cells (THP-1, human) | Mixed PS micro/nanoplastics (20 nm–10 µm) | 10–100 µg/mL; 24 h exposure | Inflammasome activation, IL-1β and IL-18 cytokine release | Across a panel of plastic particles, THP-1 macrophage-like cells responded to micro/nanoplastics with significant NLRP3 inflammasome activation. This led to the release of IL-1β, IL-18, and other inflammatory cytokines, indicating that microplastics can directly trigger innate immune pathways. Such macrophage activation within vessels can exacerbate endothelial inflammation and contribute to vascular aging and plaque instability. |
| Author(s) | Study Design | Population | MP Exposure | Cardiovascular Outcomes | Vascular Aging Indicators | Key Findings |
|---|---|---|---|---|---|---|
| Marfella R., Prattichizzo F., Sardu C. et al. [80] | Prospective cohort | Patients with carotid atherosclerotic plaques (n = 257) | Carotid plaque (pyrolysis-GC/MS, mostly polyethylene) | 3-year incidence of MI, stroke, and death | Carotid plaque burden (atherosclerosis) | Microplastics were found in 58% of plaques. A 4.5-fold higher CV event risk |
| Yang Y., Zhang F., Jiang Z. et al. [24] | Cross-sectional | ACS patients vs. controls (n = 101) | Blood MPs via pyrolysis-GC/MS (PE, PVC, PS) | ACS severity, SYNTAX score | Coronary complexity (indirect) | Higher MP levels were seen in myocardial infarction patients than with unstable angina and controls. Levels of MPs were correlated with inflammation. |
| Wang S., Yan K., Dong Y. et al. [81] | Cross-sectional + animal study | Hypertensive vs. normotensive adults | MPs in feces | Hypertension, BP | None in humans | Higher MP levels were seen in hypertensive patients. The animal model confirmed CV remodeling. |
| Geppner L., Grammatidis S., Wilfing H. et al. [82] | Single-arm intervention | Healthy adults (n = 8) | Plastic-free diet (water intake from tap) | Brachial BP (pre vs. post) | None | Reduced systolic/diastolic BP after plastic-reduction, especially in females. |
| Hua K., Yang X. et al. [83] | Pilot tissue analysis | Cardiac surgery patients (n = 15) | Heart tissue and blood MPs (laser IR imaging) | None directly measured | None | MPs were detected in most heart tissues and circulating blood pre-/post-op |
| Wang T., Yi Z., Liu X. et al. [84] | Cross-sectional | Thrombotic event patients (n = 30) | Excised thrombi (10 polymer types) | Stroke severity, D-dimer levels | Thrombotic burden (indirect) | 80% of thrombi had MPs. Higher MP levels were linked to severe stroke, coagulopathy. |
| Lee D-W., Jung J., Park S. et al. [85] | Cross-sectional | Healthy adults (n = 36) | Blood MPs via μ-FTIR (4.2 particles/mL avg) | aPTT, fibrinogen, CRP, platelets | None | Higher MPs are linked to altered coagulation and CRP. MP levels were also linked to plastic food use. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivakumar, R.; Senghor Kadalangudi Aravaanan, A.; Vellore Mohanakrishnan, V.; Kumar, J. From Environment to Endothelium: The Role of Microplastics in Vascular Aging. Microplastics 2025, 4, 52. https://doi.org/10.3390/microplastics4030052
Sivakumar R, Senghor Kadalangudi Aravaanan A, Vellore Mohanakrishnan V, Kumar J. From Environment to Endothelium: The Role of Microplastics in Vascular Aging. Microplastics. 2025; 4(3):52. https://doi.org/10.3390/microplastics4030052
Chicago/Turabian StyleSivakumar, Rooban, Arul Senghor Kadalangudi Aravaanan, Vinodhini Vellore Mohanakrishnan, and Janardhanan Kumar. 2025. "From Environment to Endothelium: The Role of Microplastics in Vascular Aging" Microplastics 4, no. 3: 52. https://doi.org/10.3390/microplastics4030052
APA StyleSivakumar, R., Senghor Kadalangudi Aravaanan, A., Vellore Mohanakrishnan, V., & Kumar, J. (2025). From Environment to Endothelium: The Role of Microplastics in Vascular Aging. Microplastics, 4(3), 52. https://doi.org/10.3390/microplastics4030052






