Effects of Commercially Available Plastics on Estuarine Sediment Dweller Polychaeta Hediste diversicolor
Abstract
1. Introduction
2. Materials and Methods
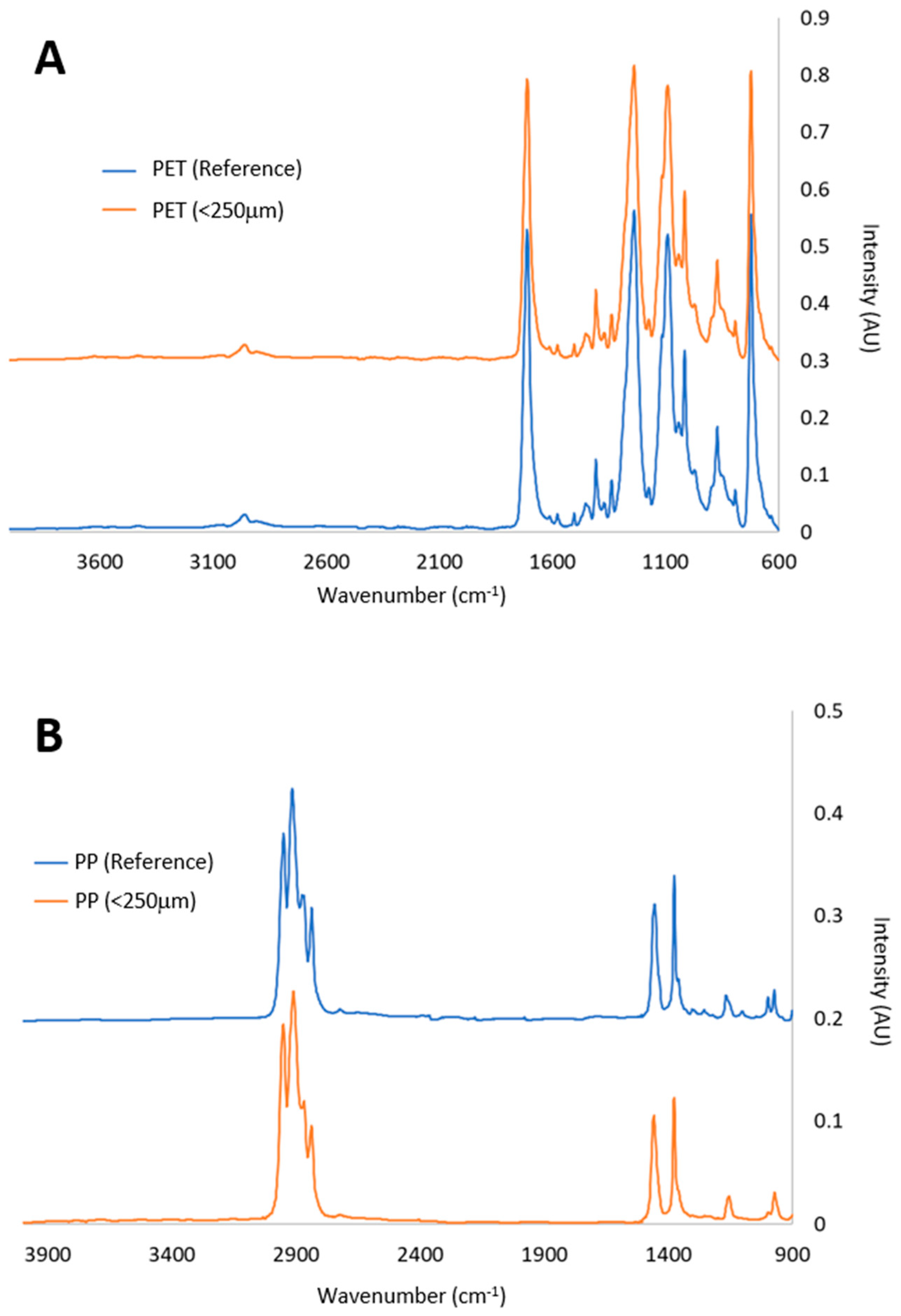
2.1. Plastics
2.2. Animal Collection
2.3. Acute and Chronic Exposure
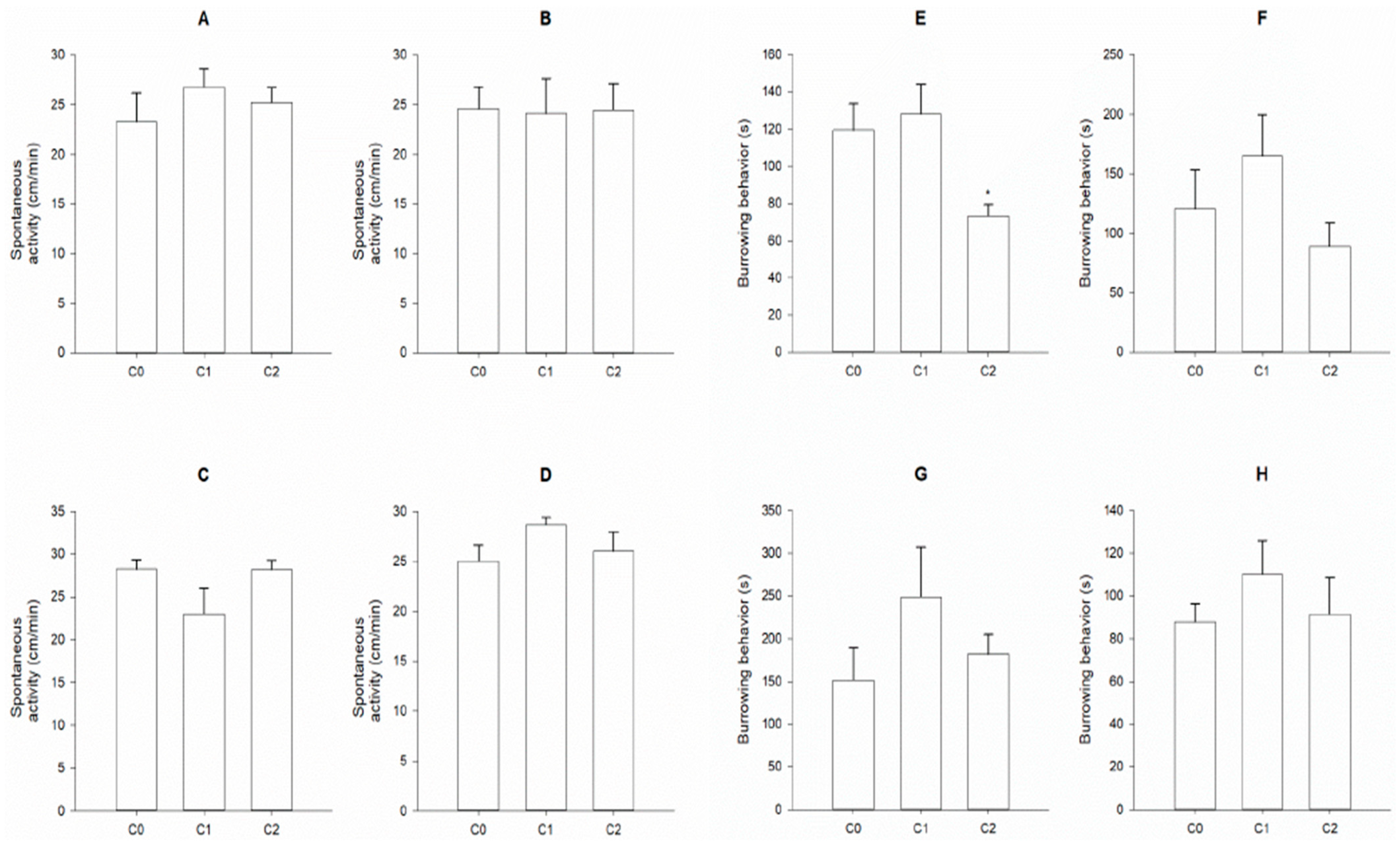
2.4. Behavioral Endpoints
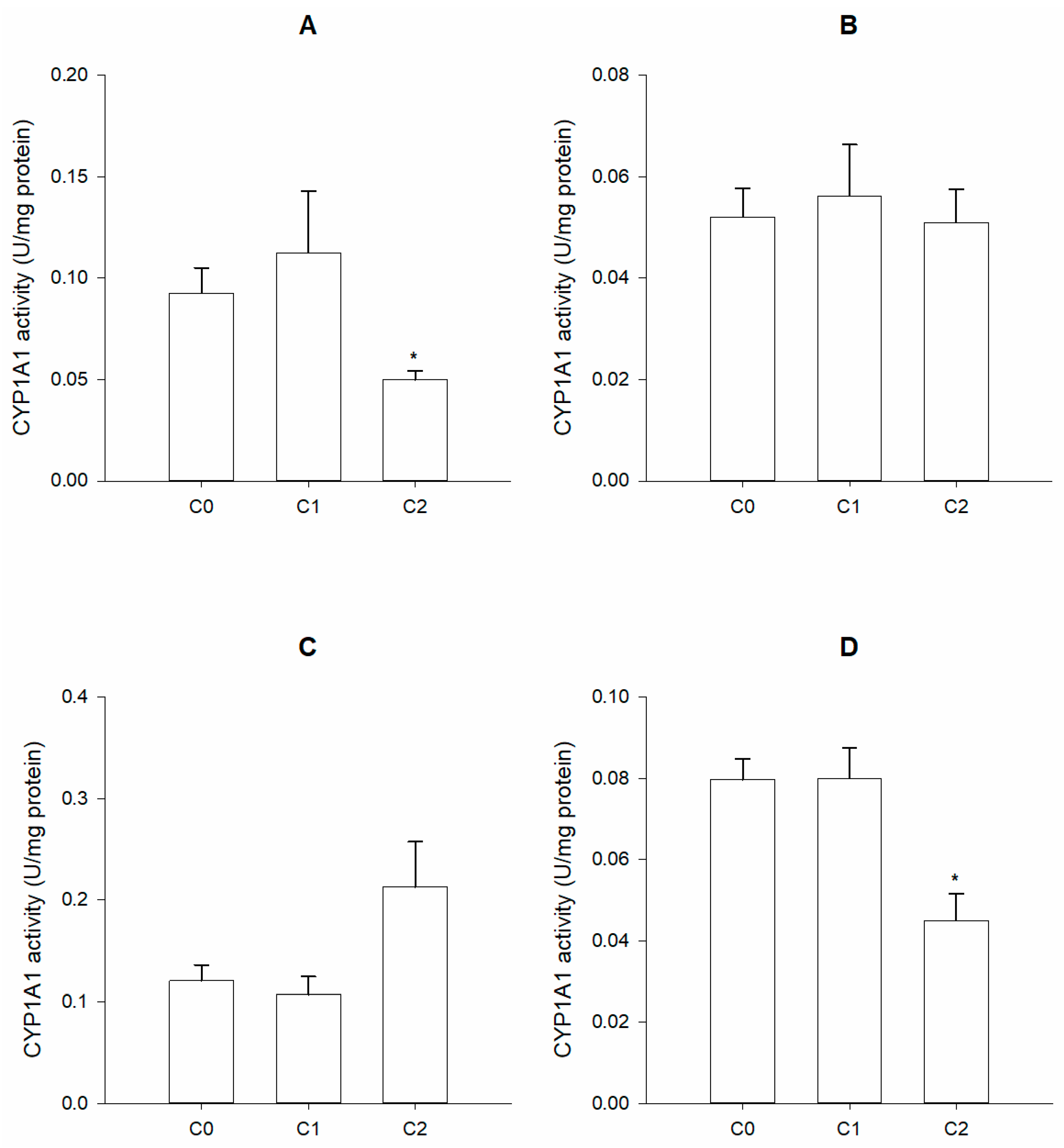
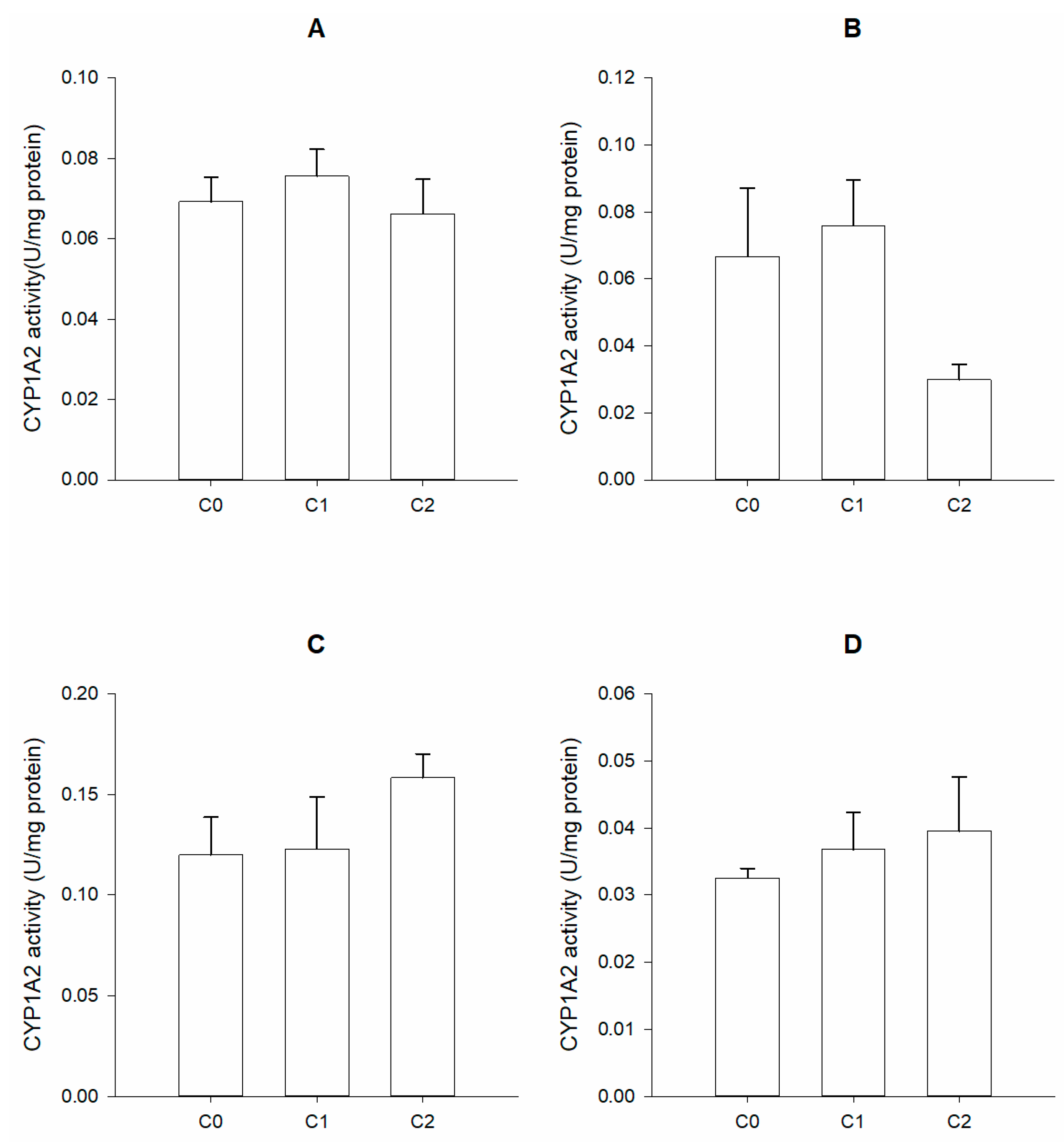
2.5. Phase I Biomarkers
2.5.1. Preparation of Microsomal Fractions
2.5.2. Enzymatic Activity Determination
2.6. Conjugation and Antioxidant Parameters
2.7. Quantification of Soluble Protein
2.8. Statistical Analysis
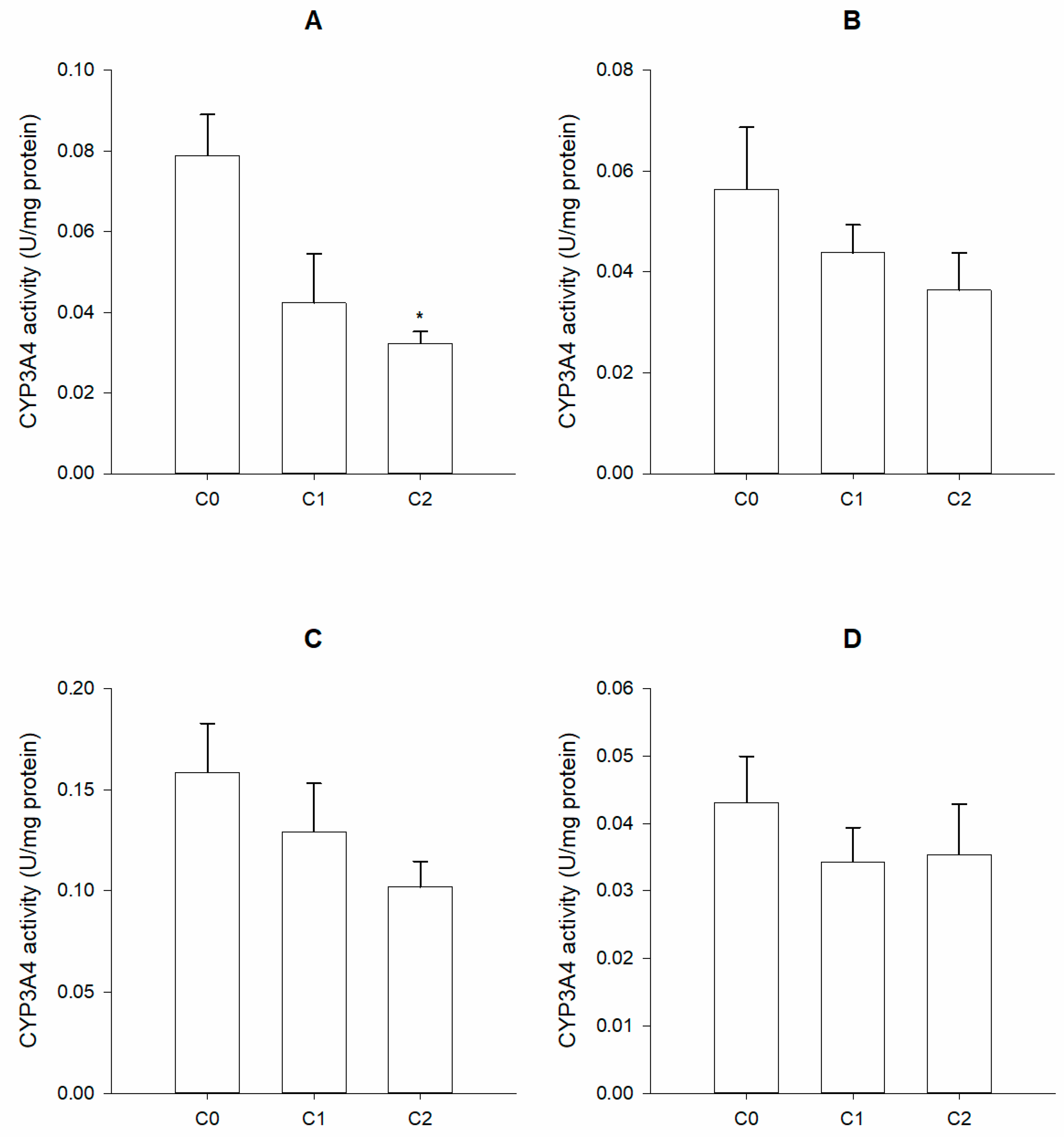
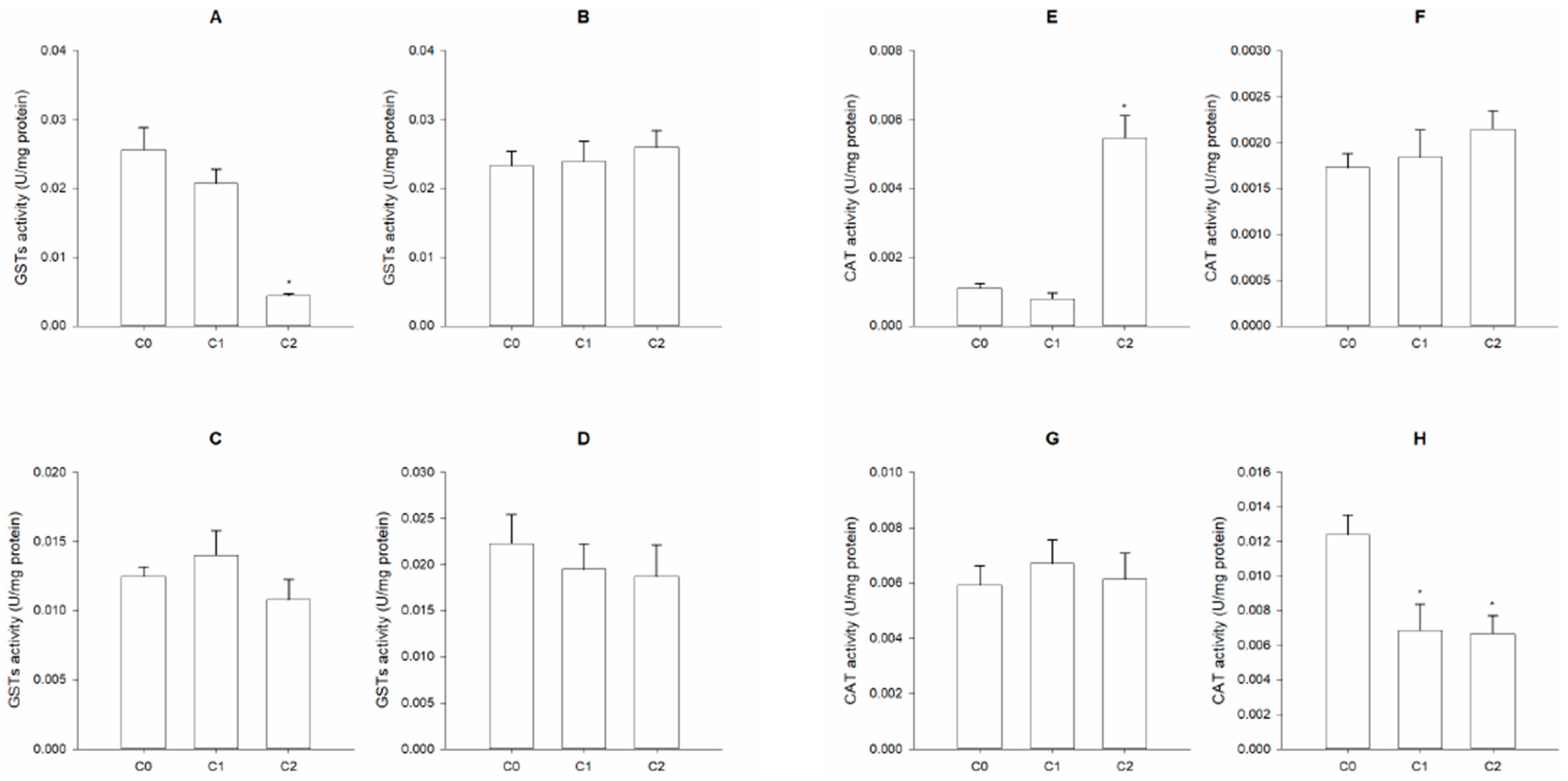
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Koshti, R.; Mehta, L.B.; Samarth, N. Biological Recycling of Polyethylene Terephthalate: A Mini-Review. J. Polym. Environ. 2018, 26, 3520–3529. [Google Scholar] [CrossRef]
- Maurya, A.; Bhattacharya, A.; Khare, S.K. Enzymatic Remediation of Polyethylene Terephthalate (PET)–Based Polymers for Effective Management of Plastic Wastes: An Overview. Front. Bioeng. Biotechnol. 2020, 8, 602325. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef] [PubMed]
- Wayman, C.; Niemann, H. The fate of plastic in the ocean environment—A minireview. Environ. Sci. Process. Impacts 2021, 23, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, X.; Su, M.; Zou, X.; Duan, L.; Zhang, H. Characteristics of Plastic Pollution in the Environment: A Review. Bull. Environ. Contam. Toxicol. 2021, 107, 577–584. [Google Scholar] [CrossRef]
- Ali, S.S.; Elsamahy, T.; Al-Tohamy, R.; Zhu, D.; Mahmoud, Y.A.; Koutra, E.; Metwally, M.A.; Kornaros, M.; Sun, J. Plastic wastes biodegradation: Mechanisms, challenges and future prospects. Sci. Total Environ. 2021, 780, 146590. [Google Scholar] [CrossRef]
- Frias, J.P.L.; Nash, R. Microplastics: Finding a consensus on the definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef]
- GESAMP. Sources, Fate and Effects of Microplastics in the Marine Environment (Part 1)|GESAMP. 2015. Available online: http://www.gesamp.org/publications/reports-and-studies-no-90 (accessed on 18 February 2022).
- GESAMP. Sources, Fate and Effects of Microplastics in the Marine Environment (Part 2)|GESAMP. 2016. Available online: http://www.gesamp.org/publications/microplastics-in-the-marine-environment-part-2 (accessed on 18 February 2022).
- Alimi, O.S.; Farner Budarz, J.; Hernandez, L.M.; Tufenkji, N. Microplastics and Nanoplastics in Aquatic Environments: Aggregation, Deposition, and Enhanced Contaminant Transport. Environ. Sci. Technol. 2018, 52, 1704–1724. [Google Scholar] [CrossRef]
- Picó, Y.; Barceló, D. Analysis and Prevention of Microplastics Pollution in Water: Current Perspectives and Future Directions. ACS Omega 2019, 4, 6709–6719. [Google Scholar] [CrossRef]
- Yan, M.; Yang, J.; Sun, H.; Liu, C.; Wang, L. Occurrence and distribution of microplastics in sediments of a man-made lake receiving reclaimed water. Sci. Total Environ. 2022, 813, 152430. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Kannan, K. Quantitative analysis of polyethylene terephthalate and polycarbonate microplastics in sediment collected from South Korea, Japan and the USA. Chemosphere 2021, 279, 130551. [Google Scholar] [CrossRef]
- Huang, W.; Song, B.; Liang, J.; Niu, Q.; Zeng, G.; Shen, M.; Deng, J.; Luo, Y.; Wen, X.; Zhang, Y. Microplastics and associated contaminants in the aquatic environment: A review on their ecotoxicological effects, trophic transfer, and potential impacts to human health. J. Hazard. Mater. 2021, 405, 124187. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Nakaoka, M. Trophic transfer of microplastics from mysids to fish greatly exceeds direct ingestion from the water column. Environ. Pollut. 2021, 273, 116468. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Pu, Q.; Xu, Y.; Yang, H.; Wu, Y.; Wang, W.; Li, Y. The masking phenomenon of microplastics additives on oxidative stress responses in freshwater food chains. Sci. Total Environ. 2024, 927, 172156. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T.; Mizukawa, K.; Yeo, B.G.; Sekioka, T.; Takada, H.; Nakaoka, M. The significance of trophic transfer of microplastics in the accumulation of plastic additives in fish: An experimental study using brominated flame retardants and UV stabilizers. Mar. Pollut. Bull. 2022, 185, 114343. [Google Scholar] [CrossRef]
- Smith, M.; Love, D.C.; Rochman, C.M.; Neff, R.A. Microplastics in Seafood and the Implications for Human Health. Curr. Environ. Health Rep. 2018, 5, 375–386. [Google Scholar] [CrossRef]
- Nair, H.T.; Perumal, S. Trophic Transfer and Accumulation of Microplastics in Freshwater Ecosystem: Risk to Food Security and Human Health. Int. J. Ecol. 2022, 2022, 1234078. [Google Scholar] [CrossRef]
- Carbery, M.; O’COnnor, W.; Palanisami, T. Trophic transfer of microplastics and mixed contaminants in the marine food web and implications for human health. Environ. Int. 2018, 115, 400–409. [Google Scholar] [CrossRef]
- Gambino, I.; Bagordo, F.; Grassi, T.; Panico, A.; De Donno, A. Occurrence of Microplastics in Tap and Bottled Water: Current Knowledge. Int. J. Environ. Res. Public Health 2022, 19, 5283. [Google Scholar] [CrossRef]
- Tong, H.; Jiang, Q.; Hu, X.; Zhong, X. Occurrence and identification of microplastics in tap water from China. Chemosphere 2020, 252, 126493. [Google Scholar] [CrossRef]
- Van Cauwenberghe, L.; Claessens, M.; Vandegehuchte, M.B.; Janssen, C.R. Microplastics are taken up by mussels (Mytilus edulis) and lugworms (Arenicola marina) living in natural habitats. Environ. Pollut. 2015, 199, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zhang, S.; Razanajatovo, R.M.; Zou, H.; Zhu, W. Accumulation, tissue distribution, and biochemical effects of polystyrene microplastics in the freshwater fish red tilapia (Oreochromis niloticus). Environ. Pollut. 2018, 238, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Solomando, A.; Capó, X.; Alomar, C.; Álvarez, E.; Compa, M.; Valencia, J.M.; Pinya, S.; Deudero, S.; Sureda, A. Long-term exposure to microplastics induces oxidative stress and a pro-inflammatory response in the gut of Sparus aurata Linnaeus, 1758. Environ. Pollut. 2020, 266, 115295. [Google Scholar] [CrossRef] [PubMed]
- Gardon, T.; Morvan, L.; Huvet, A.; Quillien, V.; Soyez, C.; Le Moullac, G.; Le Luyer, J. Microplastics induce dose-specific transcriptomic disruptions in energy metabolism and immunity of the pearl oyster Pinctada margaritifera. Environ. Pollut. 2020, 266, 115180. [Google Scholar] [CrossRef]
- Jeong, C.B.; Won, E.J.; Kang, H.M.; Lee, M.C.; Hwang, D.S.; Hwang, U.K.; Zhou, B.; Souissi, S.; Lee, S.J.; Lee, J.S. Microplastic Size-Dependent Toxicity, Oxidative Stress Induction, and p-JNK and p-p38 Activation in the Monogonont Rotifer (Brachionus koreanus). Environ. Sci. Technol. 2016, 50, 8849–8857. [Google Scholar] [CrossRef]
- Hu, M.; Palić, D. Micro- and nano-plastics activation of oxidative and inflammatory adverse outcome pathways. Redox Biol. 2020, 37, 101620. [Google Scholar] [CrossRef]
- Sharifian, S.; Homaei, A.; Kamrani, E.; Etzerodt, T.; Patel, S. New insights on the marine cytochrome P450 enzymes and their biotechnological importance. Int. J. Biol. Macromol. 2020, 142, 811–821. [Google Scholar] [CrossRef]
- Ribeiro, F.; Garcia, A.R.; Pereira, B.P.; Fonseca, M.; Mestre, N.C.; Fonseca, T.G.; Ilharco, L.M.; Bebianno, M.J. Microplastics effects in Scrobicularia plana. Mar. Pollut. Bull. 2017, 122, 379–391. [Google Scholar] [CrossRef]
- de Sá, L.C.; Oliveira, M.; Ribeiro, F.; Rocha, T.L.; Futter, M.N. Studies of the effects of microplastics on aquatic organisms: What do we know and where should we focus our efforts in the future? Sci. Total Environ. 2018, 645, 1029–1039. [Google Scholar] [CrossRef]
- Dovidat, L.C.; Brinkmann, B.W.; Vijver, M.G.; Bosker, T. Plastic particles adsorb to the roots of freshwater vascular plant Spirodela polyrhiza but do not impair growth. Limnol. Oceanogr. Lett. 2020, 5, 37–45. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Lin, S.; Turner, J.P.; Ke, P.C. Physical adsorption of charged plastic nanoparticles affects algal photosynthesis. J. Phys. Chem. C 2010, 114, 16556–16561. [Google Scholar] [CrossRef]
- Limonta, G.; Mancia, A.; Benkhalqui, A.; Bertolucci, C.; Abelli, L.; Fossi, M.C.; Panti, C. Microplastics induce transcriptional changes, immune response and behavioral alterations in adult zebrafish. Sci. Rep. 2019, 9, 15775. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.; Valente, P.; Figueira, E.; Oliveira, M.; Pires, A.; Silva, M. Behavior and biochemical responses of the polychaeta Hediste diversicolor to polystyrene nanoplastics. Sci. Total Environ. 2020, 707, 134434. [Google Scholar] [CrossRef]
- Green, D.S.; Boots, B.; O’cOnnor, N.E.; Thompson, R. Microplastics Affect the Ecological Functioning of an Important Biogenic Habitat. Environ. Sci. Technol. 2017, 51, 68–77. [Google Scholar] [CrossRef]
- Mazurais, D.; Ernande, B.; Quazuguel, P.; Severe, A.; Huelvan, C.; Madec, L.; Mouchel, O.; Soudant, P.; Robbens, J.; Huvet, A.; et al. Evaluation of the impact of polyethylene microbeads ingestion in European sea bass (Dicentrarchus labrax) larvae. Mar. Environ. Res. 2015, 112, 78–85. [Google Scholar] [CrossRef]
- Hitchcock, J.N.; Mitrovic, S.M. Microplastic pollution in estuaries across a gradient of human impact. Environ. Pollut. 2019, 247, 457–466. [Google Scholar] [CrossRef]
- Rudén, C.; Adams, J.; Ågerstrand, M.; Brock, T.C.; Poulsen, V.; Schlekat, C.E.; Wheeler, J.R.; Henry, T.R. Assessing the relevance of ecotoxicological studies for regulatory decision making. Integr. Environ. Assess. Manag. 2017, 13, 652–663. [Google Scholar] [CrossRef]
- Fonseca, L.C.; e Costa, P.F.; Gil, J.; Passos, A.M.; Pereira, P.; Melo, P.; Batista, F. The market features of imported non-indigenous polychaetes in Portugal and consequent ecological concerns. Sci. Mar. 2006, 70 (Suppl. 3), 287–292. [Google Scholar] [CrossRef]
- Thit, A.; Dybowska, A.; Købler, C.; Kennaway, G.; Selck, H. Influence of copper oxide nanoparticle shape on bioaccumulation, cellular internalization and effects in the estuarine sediment-dwelling polychaete, Nereis diversicolor. Mar. Environ. Res. 2015, 111, 89–98. [Google Scholar] [CrossRef]
- Scaps, P. A review of the biology, ecology and potential use of the common ragworm Hediste diversicolor (O.F. Müller) (Annelida: Polychaeta). Hydrobiologia 2002, 470, 203–218. [Google Scholar] [CrossRef]
- Nogueira, A.F.; Nunes, B. Effects of paracetamol on the polychaete Hediste diversicolor: Occurrence of oxidative stress, cyclooxygenase inhibition and behavioural alterations. Environ. Sci. Pollut. Res. 2021, 28, 26772–26783. [Google Scholar] [CrossRef]
- Nunes, B.; Daniel, D.; Correia, A.T. Toxic effects of environmentally realistic concentrations of diclofenac in organisms from two distinct trophic levels, Hediste diversicolor and Solea senegalensis. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 231, 108722. [Google Scholar] [CrossRef] [PubMed]
- da Fonseca, T.G.; Abessa, D.M.; Bebianno, M.J. Effects of mixtures of anticancer drugs in the benthic polychaete Nereis diversicolor. Environ. Pollut. 2019, 252, 1180–1192. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, T.; Morais, M.; Rocha, T.; Abessa, D.; Aureliano, M.; Bebianno, M. Ecotoxicological assessment of the anticancer drug cisplatin in the polychaete Nereis diversicolor. Sci. Total Environ. 2017, 575, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, A.F.; Nunes, B. Effects of low levels of the antibiotic ciprofloxacin on the polychaete Hediste diversicolor: Biochemical and behavioural effects. Environ. Toxicol. Pharmacol. 2020, 80, 103505. [Google Scholar] [CrossRef]
- Daniel, D.; Barros, L.; da Costa, J.P.; Girão, A.V.; Nunes, B. Using marine mussels to assess the potential ecotoxicological effects of two different commercial microplastics. Mar. Pollut. Bull. 2024, 203, 116441. [Google Scholar] [CrossRef]
- Ghribi, R.; Correia, A.T.; Elleuch, B.; Nunes, B. Toxicity Assessment of Impacted Sediments from Southeast Coast of Tunisia Using a Biomarker Approach with the Polychaete Hediste diversicolor. Arch. Environ. Contam. Toxicol. 2019, 76, 678–691. [Google Scholar] [CrossRef]
- Daniel, D.; Nunes, B.; Pinto, E.; Ferreira, I.M.P.L.V.O.; Correia, A.T. Assessment of Paracetamol Toxic Effects under Varying Seawater pH Conditions on the Marine Polychaete Hediste diversicolor Using Biochemical Endpoints. Biology 2022, 11, 581. [Google Scholar] [CrossRef]
- Burke, M.D.; Mayer, R.T. Ethoxyresorufin: Direct fluorimetric assay of a microsomal o-dealkylation which is preferentially inducible by 3-methylcholanthrene. Drug Metab. Dispos. 1974, 2, 583–588. [Google Scholar] [CrossRef]
- MattSiebert, M.N.; Mattos, J.J.; Piazza, C.E.; de Lima, D.; Gomes, C.H.A.; de Melo, C.M.; Bainy, A.C. Characterization of ethoxyresorufin O-deethylase activity (EROD) in oyster Crassostrea brasiliana. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2017, 203, 115–121. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kuptsov, A.; Zhizhin, G. Handbook of Fourier Transform Raman and Infrared Spectra of Polymers; Elsevier Science: Amsterdam, The Netherlands, 1998; 581p. [Google Scholar]
- Beiras, R.; Schönemann, A.M. Currently monitored microplastics pose negligible ecological risk to the global ocean. Sci. Rep. 2020, 10, 22281. [Google Scholar] [CrossRef] [PubMed]
- Mehinto, A.C.; Coffin, S.; Koelmans, A.A.; Brander, S.M.; Wagner, M.; Thornton Hampton, L.M.; Burton, A.G.; Miller, E.; Gouin, T.; Weisberg, S.B.; et al. Risk-based management framework for microplastics in aquatic ecosystems. Microplast. Nanoplast. 2022, 2, 17. [Google Scholar] [CrossRef]
- Leusch, F.D.; Ziajahromi, S. Converting mg/L to Particles/L: Reconciling the Occurrence and Toxicity Literature on Microplastics. Environ. Sci. Technol. 2021, 55, 11470–11472. [Google Scholar] [CrossRef]
- Porter, A.; Barber, D.; Hobbs, C.; Love, J.; Power, A.L.; Bakir, A.; Galloway, T.S.; Lewis, C. Uptake of microplastics by marine worms depends on feeding mode and particle shape but not exposure time. Sci. Total Environ. 2023, 857, 159287. [Google Scholar] [CrossRef] [PubMed]
- Vecchi, S.; Bianchi, J.; Scalici, M.; Fabroni, F.; Tomassetti, P. Field evidence for microplastic interactions in marine benthic invertebrates. Sci. Rep. 2021, 11, 20900. [Google Scholar] [CrossRef]
- Taghon, G.L. Optimal foraging by deposit-feeding invertebrates: Roles of particle size and organic coating. Oecologia 1982, 52, 295–304. [Google Scholar] [CrossRef]
- Esteves, F.; Rueff, J.; Kranendonk, M. The Central Role of Cytochrome P450 in Xenobiotic Metabolism—A Brief Review on a Fascinating Enzyme Family. J. Xenobiotics 2021, 11, 94–114. [Google Scholar] [CrossRef]
- O’Donovan, S.; Mestre, N.C.; Abel, S.; Fonseca, T.G.; Carteny, C.C.; Cormier, B.; Keiter, S.H.; Bebianno, M.J. Ecotoxicological effects of chemical contaminants adsorbed to microplastics in the Clam Scrobicularia plana. Front. Mar. Sci. 2018, 5, 143. [Google Scholar] [CrossRef]
- Żwierełło, W.; Maruszewska, A.; Skórka-Majewicz, M.; Goschorska, M.; Baranowska-Bosiacka, I.; Dec, K.; Styburski, D.; Nowakowska, A.; Gutowska, I. The influence of polyphenols on metabolic disorders caused by compounds released from plastics—Review. Chemosphere 2020, 240, 124901. [Google Scholar] [CrossRef]
- Cooper, B.W.; Cho, T.M.; Thompson, P.M.; Wallace, A.D. Phthalate induction of CYP3A4 is dependent on glucocorticoid regulation of PXR expression. Toxicol. Sci. 2008, 103, 268–277. [Google Scholar] [CrossRef]
- Anbumani, S.; Kakkar, P. Ecotoxicological effects of microplastics on biota: A review. Environ. Sci. Pollut. Res. 2018, 25, 14373–14396. [Google Scholar] [CrossRef]
- Cheng, W.; Li, X.; Zhou, Y.; Yu, H.; Xie, Y.; Guo, H.; Wang, H.; Li, Y.; Feng, Y.; Wang, Y. Polystyrene microplastics induce hepatotoxicity and disrupt lipid metabolism in the liver organoids. Sci. Total Environ. 2022, 806, 150328. [Google Scholar] [CrossRef]
- Zimmermann, L.; Bartosova, Z.; Braun, K.; Oehlmann, J.; Völker, C.; Wagner, M. Plastic Products Leach Chemicals That Induce In Vitro Toxicity under Realistic Use Conditions. Environ. Sci. Technol. 2021, 55, 11814–11823. [Google Scholar] [CrossRef]
- Goh, J.J.; Behn, J.; Chong, C.S.; Zhong, G.; Maurer-Stroh, S.; Fan, H.; Loo, L.H. Structure-based virtual screening of CYP1A1 inhibitors: Towards rapid tier-one assessment of potential developmental toxicants. Arch. Toxicol. 2021, 95, 3031–3048. [Google Scholar] [CrossRef]
- Devito, M.; Ma, X.; Babish, J.; Menache, M.; Birnbaum, L. Dose-Response Relationships in Mice Following Subchronic Exposure to 2,3,7,8-Tetrachlorodibenzo-p-dioxin: CYP1A1, CYP1A2, Estrogen Receptor, and Protein Tyrosine Phosphorylation. Toxicol. Appl. Pharmacol. 1994, 124, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Lambert, G.H.; Needham, L.L.; Turner, W.; Lai, T.J.; Patterson, D.G.; Guo, Y.L. Induced CYP1A2 Activity as a Phenotypic Biomarker in Humans Highly Exposed to Certain PCBs/PCDFs. Environ. Sci. Technol. 2006, 40, 6176–6180. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, A.P.; Kaminski, D.L.; Ruh, M.F. 2,3,7,8 Tetrachlorodibenzo-p-dioxin induction of cytochrome P4501A in cultured rat and human hepatocytes. Chem. Interact. 2000, 124, 173–189. [Google Scholar] [CrossRef] [PubMed]
- Hirt, N.; Body-Malapel, M. Immunotoxicity and intestinal effects of nano- and microplastics: A review of the literature. Part. Fibre Toxicol. 2020, 17, 57. [Google Scholar] [CrossRef]
- Santos, L.H.; Rodríguez-Mozaz, S.; Barceló, D. Microplastics as vectors of pharmaceuticals in aquatic organisms—An overview of their environmental implications. Case Stud. Chem. Environ. Eng. 2021, 3, 100079. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, X.; Shu, X.; Gong, J.; Yang, J.; Li, B.; Lin, J.; Chai, Y.; Liu, J. The effects of polyethylene microplastics on the growth, reproduction, metabolic enzymes, and metabolomics of earthworms Eisenia fetida. Ecotoxicol. Environ. Saf. 2023, 263, 115390. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E.; Kueznik, T.; Samberger, C.; Roblegg, E.; Wrighton, C.; Pieber, T.R. Size-dependent effects of nanoparticles on the activity of cytochrome P450 isoenzymes. Toxicol. Appl. Pharmacol. 2010, 242, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Rosellini, M.; Omer, E.A.; Schulze, A.; Ali, N.T.; Boulos, J.C.; Marini, F.; Küpper, J.H.; Efferth, T. Impact of plastic-related compounds on the gene expression signature of HepG2 cells transfected with CYP3A4. Arch. Toxicol. 2024, 98, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Kılıç, Ö.; Belivermiş, M.; Sıkdokur, E.; Sezer, N.; Aksüt, Y.; Pekmez, M.; Kösesakal, T.; Gerçek, Y.C. The combined effects of polyethylene microplastics and benzoanthracene on Manila clam Ruditapes philippinarum. Chemosphere 2023, 329, 138664. [Google Scholar] [CrossRef]
- Ward, J.E.; Rosa, M.; Shumway, S.E. Capture, ingestion, and egestion of microplastics by suspension-feeding bivalves: A 40-year history. Anthr. Coasts 2019, 2, 39–49. [Google Scholar] [CrossRef]
- Wright, S.L.; Rowe, D.; Thompson, R.C.; Galloway, T.S. Microplastic ingestion decreases energy reserves in marine worms. Curr. Biol. 2013, 23, R1031–R1033. [Google Scholar] [CrossRef]
- Trestrail, C.; Walpitagama, M.; Miranda, A.; Nugegoda, D.; Shimeta, J. Microplastics alter digestive enzyme activities in the marine bivalve, Mytilus galloprovincialis. Sci. Total Environ. 2021, 779, 146418. [Google Scholar] [CrossRef]
- Weis, J.S. Aquatic Microplastic Research—A Critique and Suggestions for the Future. Water 2020, 12, 1475. [Google Scholar] [CrossRef]
- Eltemsah, Y.S.; Bøhn, T. Acute and chronic effects of polystyrene microplastics on juvenile and adult Daphnia magna. Environ. Pollut. 2019, 254, 112919. [Google Scholar] [CrossRef]
- Shore, L.J.; Mogilevsky, W.S.; Smith, P.B.; Fenselau, C.; Odell, G.B. In vitro formation of glutathione conjugates of the dimethylester of bilirubin. Biochem. Pharmacol. 1991, 42, 1969–1976. [Google Scholar] [CrossRef] [PubMed]
- Testa, B.; Clement, B. Biotransformation Reactions and Their Enzymes. In The Practice of Medicinal Chemistry, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 561–584. [Google Scholar] [CrossRef]
- Zitouni, N.; Bousserrhine, N.; Missawi, O.; Boughattas, I.; Chèvre, N.; Santos, R.; Belbekhouche, S.; Alphonse, V.; Tisserand, F.; Balmassiere, L.; et al. Uptake, tissue distribution and toxicological effects of environmental microplastics in early juvenile fish Dicentrarchus labrax. J. Hazard. Mater. 2021, 403, 124055. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Liu, Z.; Wu, D.; Chen, M.; Lv, W.; Zhao, Y. Accumulation of polystyrene microplastics in juvenile Eriocheir sinensis and oxidative stress effects in the liver. Aquat. Toxicol. 2018, 200, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Salinas, A.E.; Wong, M.G. Glutathione S-Transferases—A Review. Curr. Med. Chem. 1999, 6, 279–309. [Google Scholar] [CrossRef]
- Wang, Y.; Han, Q.; Wei, Z.; Wang, S.; Xie, J.; Chen, M. Polystyrene microplastics affect learning and memory in mice by inducing oxidative stress and decreasing the level of acetylcholine. Food Chem. Toxicol. 2022, 162, 112904. [Google Scholar] [CrossRef]
- Letelier, M.; Martínez, M.; González-Lira, V.; Faúndez, M.; Aracena-Parks, P. Inhibition of cytosolic glutathione S-transferase activity from rat liver by copper. Chem. Interact. 2006, 164, 39–48. [Google Scholar] [CrossRef]
- Revel, M.; Lagarde, F.; Perrein-Ettajani, H.; Bruneau, M.; Akcha, F.; Sussarellu, R.; Rouxel, J.; Costil, K.; Decottignies, P.; Cognie, B.; et al. Tissue-specific biomarker responses in the blue mussel Mytilus spp. exposed to a mixture of microplastics at environmentally relevant concentrations. Front. Environ. Sci. 2019, 7, 33. [Google Scholar] [CrossRef]
- Parolini, M.; De Felice, B.; Gazzotti, S.; Annunziata, L.; Sugni, M.; Bacchetta, R.; Ortenzi, M.A. Oxidative stress-related effects induced by micronized polyethylene terephthalate microparticles in the Manila clam. J. Toxicol. Environ. Health Part A 2020, 83, 168–179. [Google Scholar] [CrossRef]
- Telford, W.M.; Geldart, L.P.; Sheriff, R.E. Applied Geophysics, 2nd ed.; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Wang, F.; Salvati, A.; Boya, P. Lysosome-dependent cell death and deregulated autophagy induced by amine-modified polystyrene nanoparticles. Open Biol. 2018, 8, 170271. [Google Scholar] [CrossRef]
- Nandi, A.; Yan, L.-J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxidative Med. Cell. Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Deng, Y.; Jiang, W.; Zhao, Y.; Geng, J.; Ding, L.; Ren, H. Uptake and Accumulation of Polystyrene Microplastics in Zebrafish (Danio rerio) and Toxic Effects in Liver. Environ. Sci. Technol. 2016, 50, 4054–4060. [Google Scholar] [CrossRef]
- Zhang, P.; Yan, Z.; Lu, G.; Ji, Y. Single and combined effects of microplastics and roxithromycin on Daphnia magna. Environ. Sci. Pollut. Res. 2019, 26, 17010–17020. [Google Scholar] [CrossRef]
- Paul-Pont, I.; Lacroix, C.; Fernández, C.G.; Hégaret, H.; Lambert, C.; Le Goïc, N.; Frère, L.; Cassone, A.L.; Sussarellu, R.; Fabioux, C.; et al. Exposure of marine mussels Mytilus spp. to polystyrene microplastics: Toxicity and influence on fluoranthene bioaccumulation. Environ. Pollut. 2016, 216, 724–737. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daniel, D.; da Costa, J.P.; Girão, A.V.; Nunes, B. Effects of Commercially Available Plastics on Estuarine Sediment Dweller Polychaeta Hediste diversicolor. Microplastics 2025, 4, 46. https://doi.org/10.3390/microplastics4030046
Daniel D, da Costa JP, Girão AV, Nunes B. Effects of Commercially Available Plastics on Estuarine Sediment Dweller Polychaeta Hediste diversicolor. Microplastics. 2025; 4(3):46. https://doi.org/10.3390/microplastics4030046
Chicago/Turabian StyleDaniel, David, João Pinto da Costa, Ana Violeta Girão, and Bruno Nunes. 2025. "Effects of Commercially Available Plastics on Estuarine Sediment Dweller Polychaeta Hediste diversicolor" Microplastics 4, no. 3: 46. https://doi.org/10.3390/microplastics4030046
APA StyleDaniel, D., da Costa, J. P., Girão, A. V., & Nunes, B. (2025). Effects of Commercially Available Plastics on Estuarine Sediment Dweller Polychaeta Hediste diversicolor. Microplastics, 4(3), 46. https://doi.org/10.3390/microplastics4030046






