Fate of Microplastic Pollution Along the Water and Sludge Lines in Municipal Wastewater Treatment Plants
Abstract
1. Introduction
2. Materials and Methods
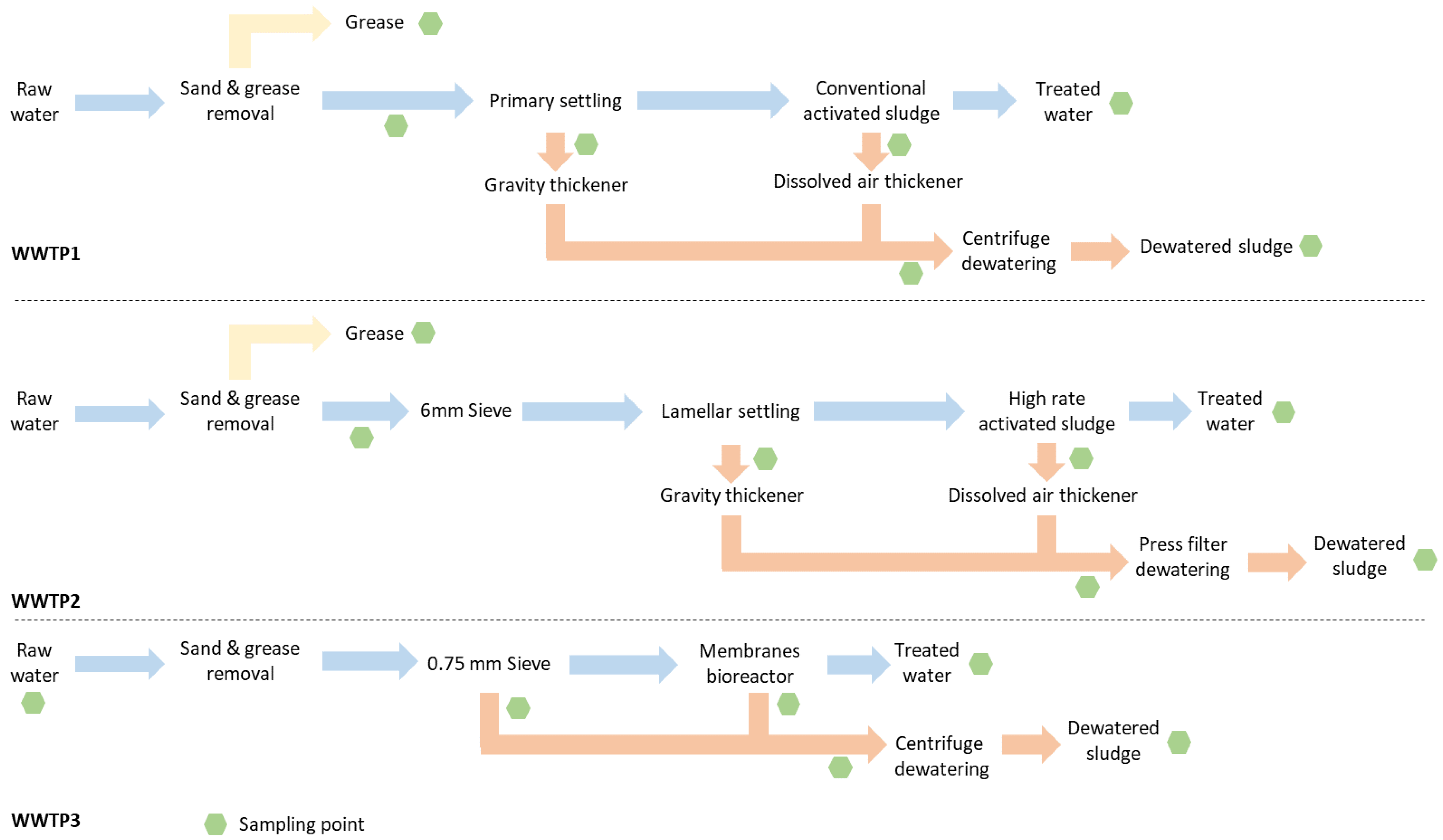
2.1. Description of WWTPs
2.2. Sampling and Analytical Procedure
2.3. Data Analysis
3. Results and Discussion
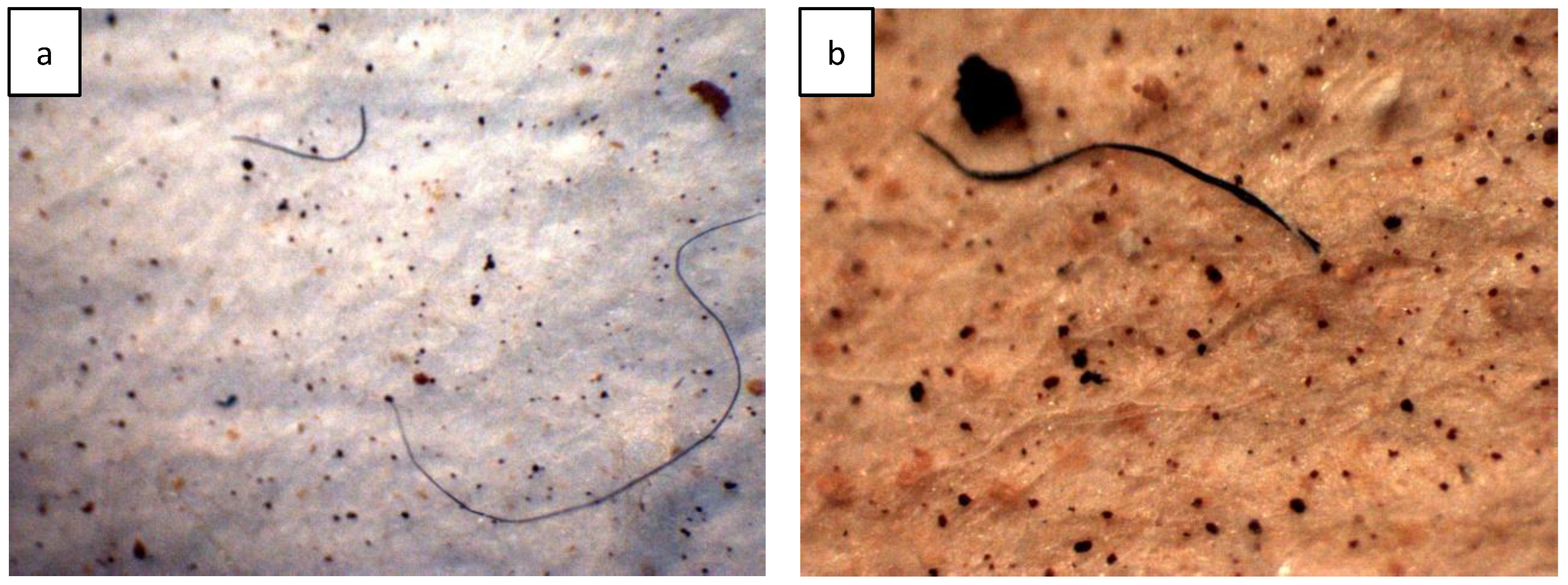
3.1. MP Contamination in WWTPs
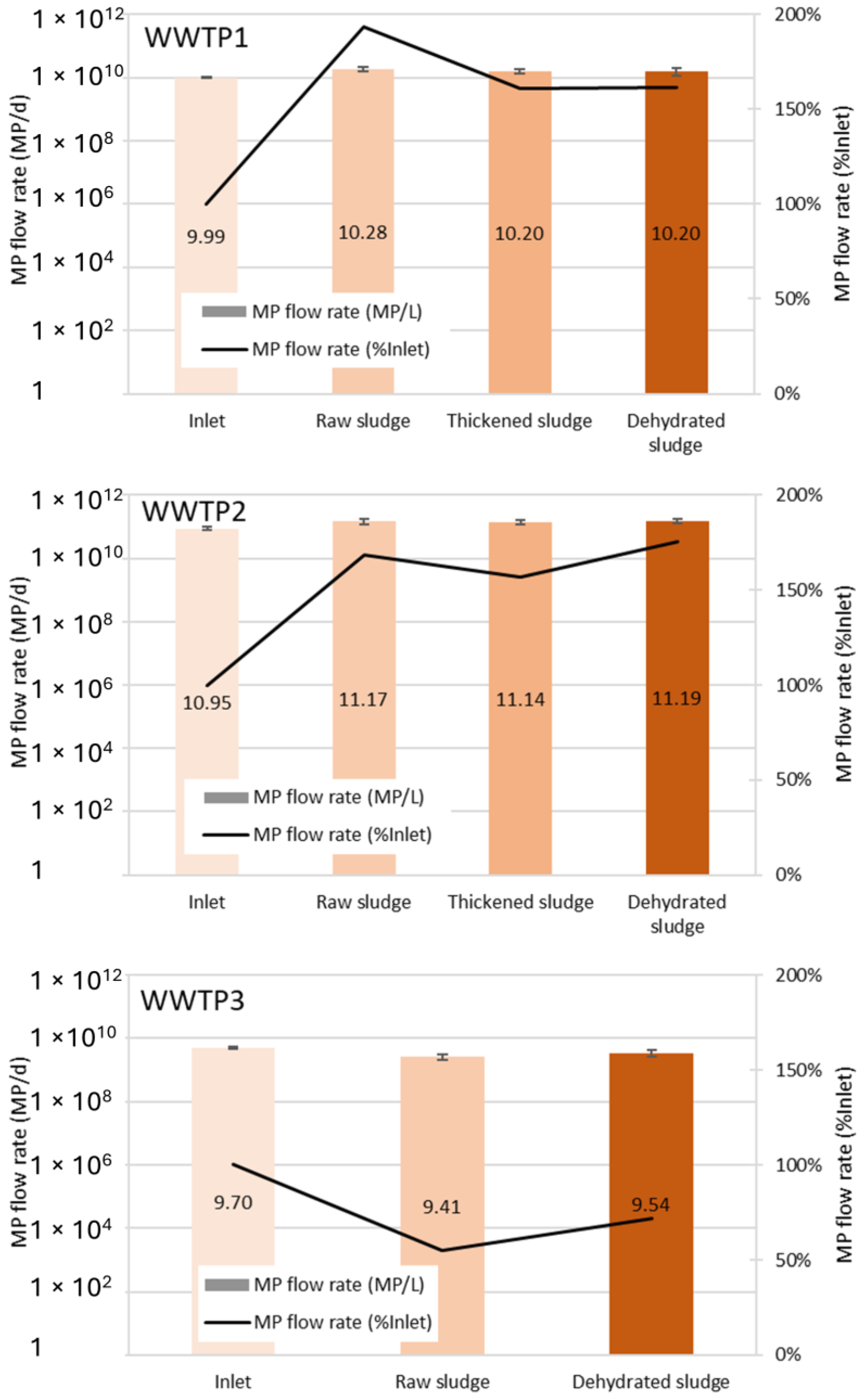
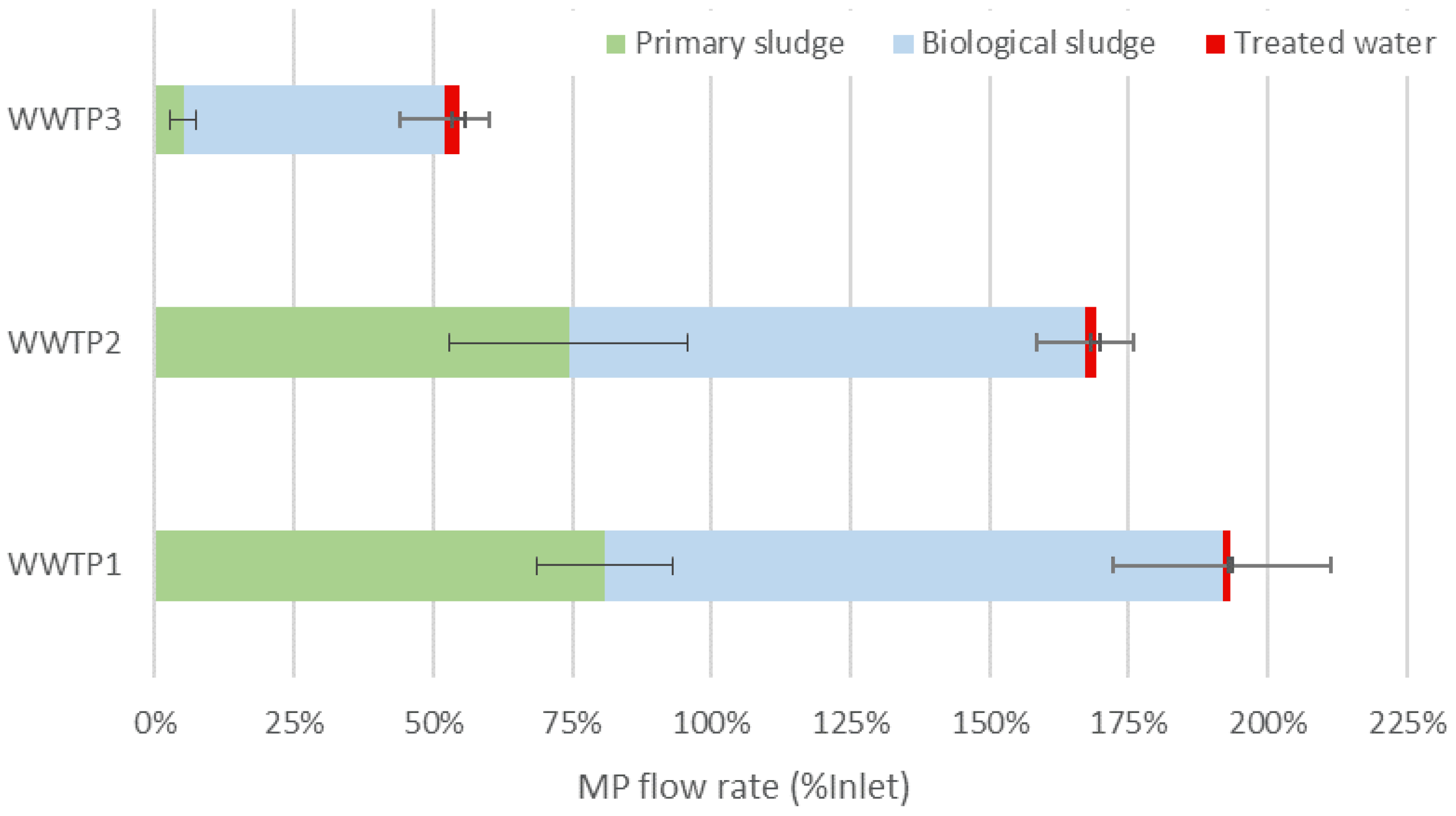
3.2. MP Flow Rate and Pollution Balance of the Sludge Line Processes
3.3. Impact of the Sludge Line Processes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Janssens, V. Plastics—The Facts 2022. Plast. Eur. 2022, 2022, 1–81. [Google Scholar]
- Rochman, C.M.; Browne, M.A.; Halpern, B.S.; Hentschel, B.T.; Hoh, E.; Karapanagioti, H.K.; Rios-Mendoza, L.M.; Takada, H.; Teh, S.; Thompson, R.C. Classify Plastic Waste as Hazardous. Nature 2013, 494, 169–171. [Google Scholar] [CrossRef] [PubMed]
- OECD. Global Plastics Outlook: Economic Drivers, Environmental Impacts and Policy Options; OECD: Paris, France, 2022; ISBN 9789264654945. [Google Scholar]
- Gérigny, O.; Pedrotti, M.-L.; El Rakwe, M.; Brun, M.; Pavec, M.; Henry, M.; Mazeas, F.; Maury, J.; Garreau, P.; Galgani, F. Characterization of Floating Microplastic Contamination in the Bay of Marseille (French Mediterranean Sea) and Its Impact on Zooplankton and Mussels. Mar. Pollut. Bull. 2022, 175, 113353. [Google Scholar] [CrossRef]
- Seoane, M.; González-Fernández, C.; Soudant, P.; Huvet, A.; Esperanza, M.; Cid, Á.; Paul-Pont, I. Polystyrene Microbeads Modulate the Energy Metabolism of the Marine Diatom Chaetoceros Neogracile. Environ. Pollut. 2019, 251, 363–371. [Google Scholar] [CrossRef] [PubMed]
- SAPEA. Science Advice for Policy by European Academies: A Scientific Perspective on Microplastics in Nature and Society; SAPEA: Berlin, Germany, 2019. [Google Scholar]
- Acarer Arat, S. A Review of Microplastics in Wastewater Treatment Plants in Türkiye: Characteristics, Removal Efficiency, Mitigation Strategies for Microplastic Pollution and Future Perspective. Water Sci. Technol. 2024, 89, 1771–1786. [Google Scholar] [CrossRef]
- Talvitie, J.; Heinonen, M.; Pääkkönen, J.-P.; Vahtera, E.; Mikola, A.; Setälä, O.; Vahala, R. Do Wastewater Treatment Plants Act as a Potential Point Source of Microplastics? Preliminary Study in the Coastal Gulf of Finland, Baltic Sea. Water Sci. Technol. 2015, 72, 1495–1504. [Google Scholar] [CrossRef]
- Nie, H.; Wang, J.; Xu, K.; Huang, Y.; Yan, M. Microplastic Pollution in Water and Fish Samples around Nanxun Reef in Nansha Islands, South China Sea. Sci. Total Environ. 2019, 696, 134022. [Google Scholar] [CrossRef]
- Geilfus, N.-X.; Munson, K.M.; Sousa, J.; Germanov, Y.; Bhugaloo, S.; Babb, D.; Wang, F. Distribution and Impacts of Microplastic Incorporation within Sea Ice. Mar. Pollut. Bull. 2019, 145, 463–473. [Google Scholar] [CrossRef]
- Xiong, X.; Wu, C.; Elser, J.J.; Mei, Z.; Hao, Y. Occurrence and Fate of Microplastic Debris in Middle and Lower Reaches of the Yangtze River—From Inland to the Sea. Sci. Total Environ. 2019, 659, 66–73. [Google Scholar] [CrossRef]
- Boucher, J.; Friot, D. Primary Microplastics in the Oceans: A Global Evaluation of Sources; IUCN: Gland, Switzerland, 2017. [Google Scholar]
- Acarer, S. Microplastics in Wastewater Treatment Plants: Sources, Properties, Removal Efficiency, Removal Mechanisms, and Interactions with Pollutants. Water Sci. Technol. 2023, 87, 685–710. [Google Scholar] [CrossRef]
- Frère, L.; Paul-Pont, I.; Rinnert, E.; Petton, S.; Jaffré, J.; Bihannic, I.; Soudant, P.; Lambert, C.; Huvet, A. Influence of Environmental and Anthropogenic Factors on the Composition, Concentration and Spatial Distribution of Microplastics: A Case Study of the Bay of Brest (Brittany, France). Environ. Pollut. 2017, 225, 211–222. [Google Scholar] [CrossRef]
- Sun, J.; Dai, X.; Wang, Q.; van Loosdrecht, M.C.M.; Ni, B.-J. Microplastics in Wastewater Treatment Plants: Detection, Occurrence and Removal. Water Res. 2019, 152, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, L.; Mei, Q.; Dong, B.; Dai, X.; Ding, G.; Zeng, E.Y. Microplastics in Sewage Sludge from the Wastewater Treatment Plants in China. Water Res. 2018, 142, 75–85. [Google Scholar] [CrossRef]
- Salmi, P.; Ryymin, K.; Karjalainen, A.K.; Mikola, A.; Uurasjärvi, E.; Talvitie, J. Particle Balance and Return Loops for Microplastics in a Tertiary-Level Wastewater Treatment Plant. Water Sci. Technol. 2021, 84, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Mintenig, S.M.; Int-Veen, I.; Löder, M.G.J.; Primpke, S.; Gerdts, G. Identification of Microplastic in Effluents of Waste Water Treatment Plants Using Focal Plane Array-Based Micro-Fourier-Transform Infrared Imaging. Water Res. 2017, 108, 365–372. [Google Scholar] [CrossRef]
- Sujathan, S.; Kniggendorf, A.-K.; Kumar, A.; Roth, B.; Rosenwinkel, K.-H.; Nogueira, R. Heat and Bleach: A Cost-Efficient Method for Extracting Microplastics from Return Activated Sludge. Arch. Environ. Contam. Toxicol. 2017, 73, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Alavian Petroody, S.S.; Hashemi, S.H.; van Gestel, C.A.M. Transport and Accumulation of Microplastics through Wastewater Treatment Sludge Processes. Chemosphere 2021, 278, 130471. [Google Scholar] [CrossRef]
- Li, Q.; Wu, J.; Zhao, X.; Gu, X.; Ji, R. Separation and Identification of Microplastics from Soil and Sewage Sludge. Environ. Pollut. 2019, 254, 113076. [Google Scholar] [CrossRef]
- Hatinoğlu, M.D.; Sanin, F.D. Sewage Sludge as a Source of Microplastics in the Environment: A Review of Occurrence and Fate during Sludge Treatment. J. Environ. Manag. 2021, 295, 113028. [Google Scholar] [CrossRef]
- Bäuerlein, P.S.; Pieke, E.N.; Oesterholt, F.I.H.M.; ter Laak, T.; Kools, S.A.E. Microplastic Discharge from a Wastewater Treatment Plant: Long Term Monitoring to Compare Two Analytical Techniques, LDIR and Optical Microscopy While Also Assessing the Removal Efficiency of a Bubble Curtain. Water Sci. Technol. 2023, 87, 39–56. [Google Scholar] [CrossRef]
- ISO 5667-12:2011; Water Quality—Sampling—Part 13: Guidance on Sampling of Sludges. International Organization for Standardization: Geneva, Switzerland, 2011.
- Müller, Y.K.; Wernicke, T.; Pittroff, M.; Witzig, C.S.; Storck, F.R.; Klinger, J.; Zumbülte, N. Microplastic Analysis—Are We Measuring the Same? Results on the First Global Comparative Study for Microplastic Analysis in a Water Sample. Anal. Bioanal. Chem. 2020, 412, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Mahon, A.M.; O’Connell, B.; Healy, M.G.; O’Connor, I.; Officer, R.; Nash, R.; Morrison, L. Microplastics in Sewage Sludge: Effects of Treatment. Environ. Sci. Technol. 2017, 51, 810–818. [Google Scholar] [CrossRef]
- ISO 11923:1997; Water Quality—Determination of Suspended Solids by Filtration Through Glass-Fibre Filters. International Organization for Standardization: Geneva, Switzerland, 1997.
- NF T90-105-2; Qualité de L’eau—Dosage des Matières en Suspension—Méthode par Centrifugation. Association Française de Normalisation: La Plaine Saint-Denis, France, 1997.
- Pedrotti, M.L.; Petit, S.; Eyheraguibel, B.; Kerros, M.E.; Elineau, A.; Ghiglione, J.F.; Loret, J.F.; Rostan, A.; Gorsky, G. Pollution by Anthropogenic Microfibers in North-West Mediterranean Sea and Efficiency of Microfiber Removal by a Wastewater Treatment Plant. Sci. Total Environ. 2021, 758, 144195. [Google Scholar] [CrossRef]
- Hann, S.; Sherrington, C.; Jamieson, O.; Hickman, M.; Kershaw, P.; Bapasola, A.; Cole, G. Investigating Options for Reducing Releases in the Aquatic Environment of Microplastics Emitted by (but Not Intentionally Added in) Products—Interim Report. Rep. DG Environ. Eur. Comm. 2018, 62, 1596–1605. [Google Scholar]
- Murphy, F.; Ewins, C.; Carbonnier, F.; Quinn, B. Wastewater Treatment Works (WwTW) as a Source of Microplastics in the Aquatic Environment. Environ. Sci. Technol. 2016, 50, 5800–5808. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Liu, H.; Guo, X.; Zhang, X.; Yao, X.; Cao, Z.; Zhang, T. A Review of the Removal of Microplastics in Global Wastewater Treatment Plants: Characteristics and Mechanisms. Environ. Int. 2021, 146, 106277. [Google Scholar] [CrossRef] [PubMed]
- Koutnik, V.S.; Alkidim, S.; Leonard, J.; DePrima, F.; Cao, S.; Hoek, E.M.V.; Mohanty, S.K. Unaccounted Microplastics in Wastewater Sludge: Where Do They Go? ACS ES&T Water 2021, 1, 1086–1097. [Google Scholar] [CrossRef]
- Rasmussen, L.A.; Iordachescu, L.; Tumlin, S.; Vollertsen, J. A Complete Mass Balance for Plastics in a Wastewater Treatment Plant—Macroplastics Contributes More than Microplastics. Water Res. 2021, 201, 117307. [Google Scholar] [CrossRef]
- Krishnan, R.Y.; Manikandan, S.; Subbaiya, R.; Karmegam, N.; Kim, W.; Govarthanan, M. Recent Approaches and Advanced Wastewater Treatment Technologies for Mitigating Emerging Microplastics Contamination—A Critical Review. Sci. Total Environ. 2023, 858, 159681. [Google Scholar] [CrossRef]
- Talvitie, J.; Mikola, A.; Koistinen, A.; Setälä, O. Solutions to Microplastic Pollution—Removal of Microplastics from Wastewater Effluent with Advanced Wastewater Treatment Technologies. Water Res. 2017, 123, 401–407. [Google Scholar] [CrossRef]
- Jimenez, J.; Miller, M.; Bott, C.; Murthy, S.; De Clippeleir, H.; Wett, B. High-Rate Activated Sludge System for Carbon Management—Evaluation of Crucial Process Mechanisms and Design Parameters. Water Res. 2015, 87, 476–482. [Google Scholar] [CrossRef]
- Enfrin, M.; Dumée, L.F.; Lee, J. Nano/Microplastics in Water and Wastewater Treatment Processes—Origin, Impact and Potential Solutions. Water Res. 2019, 161, 621–638. [Google Scholar] [CrossRef]
- Mohanan, N.; Montazer, Z.; Sharma, P.K.; Levin, D.B. Microbial and Enzymatic Degradation of Synthetic Plastics. Front. Microbiol. 2020, 11, 580709. [Google Scholar] [CrossRef] [PubMed]
- Monira, S.; Roychand, R.; Hai, F.I.; Bhuiyan, M.; Dhar, B.R.; Pramanik, B.K. Nano and Microplastics Occurrence in Wastewater Treatment Plants: A Comprehensive Understanding of Microplastics Fragmentation and Their Removal. Chemosphere 2023, 334, 139011. [Google Scholar] [CrossRef] [PubMed]
- Wilkes, R.A.; Aristilde, L. Degradation and Metabolism of Synthetic Plastics and Associated Products by Pseudomonas sp.: Capabilities and Challenges. J. Appl. Microbiol. 2017, 123, 582–593. [Google Scholar] [CrossRef]
- Bilgin, M.; Yurtsever, M.; Karadagli, F. Microplastic Removal by Aerated Grit Chambers versus Settling Tanks of a Municipal Wastewater Treatment Plant. J. Water Process Eng. 2020, 38, 101604. [Google Scholar] [CrossRef]
- Nakao, S.; Akita, K.; Ozaki, A.; Masumoto, K.; Okuda, T. Circulation of Fibrous Microplastic (Microfiber) in Sewage and Sewage Sludge Treatment Processes. Sci. Total Environ. 2021, 795, 148873. [Google Scholar] [CrossRef]
- Talvitie, J.; Mikola, A.; Setala, O.; Heinonen, M.; Koistinen, A.; Setälä, O.; Heinonen, M.; Koistinen, A. How Well Is Microlitter Purified from Wastewater?—A Detailed Study on the Stepwise Removal of Microlitter in a Tertiary Level Wastewater Treatment Plant. Water Res. 2017, 109, 164–172. [Google Scholar] [CrossRef]
- Carnevale Miino, M.; Galafassi, S.; Zullo, R.; Torretta, V.; Rada, E.C. Microplastics Removal in Wastewater Treatment Plants: A Review of the Different Approaches to Limit Their Release in the Environment. Sci. Total Environ. 2024, 930, 172675. [Google Scholar] [CrossRef]
- Edo, C.; González-Pleiter, M.; Leganés, F.; Fernández-Piñas, F.; Rosal, R. Fate of Microplastics in Wastewater Treatment Plants and Their Environmental Dispersion with Effluent and Sludge. Environ. Pollut. 2020, 259, 113837. [Google Scholar] [CrossRef]
- Layer, M.; Bock, K.; Ranzinger, F.; Horn, H.; Morgenroth, E.; Derlon, N. Particulate Substrate Retention in Plug-Flow and Fully-Mixed Conditions during Operation of Aerobic Granular Sludge Systems. Water Res. X 2020, 9, 100075. [Google Scholar] [CrossRef] [PubMed]
- Carr, S.A.; Liu, J.; Tesoro, A.G. Transport and Fate of Microplastic Particles in Wastewater Treatment Plants. Water Res. 2016, 91, 174–182. [Google Scholar] [CrossRef] [PubMed]
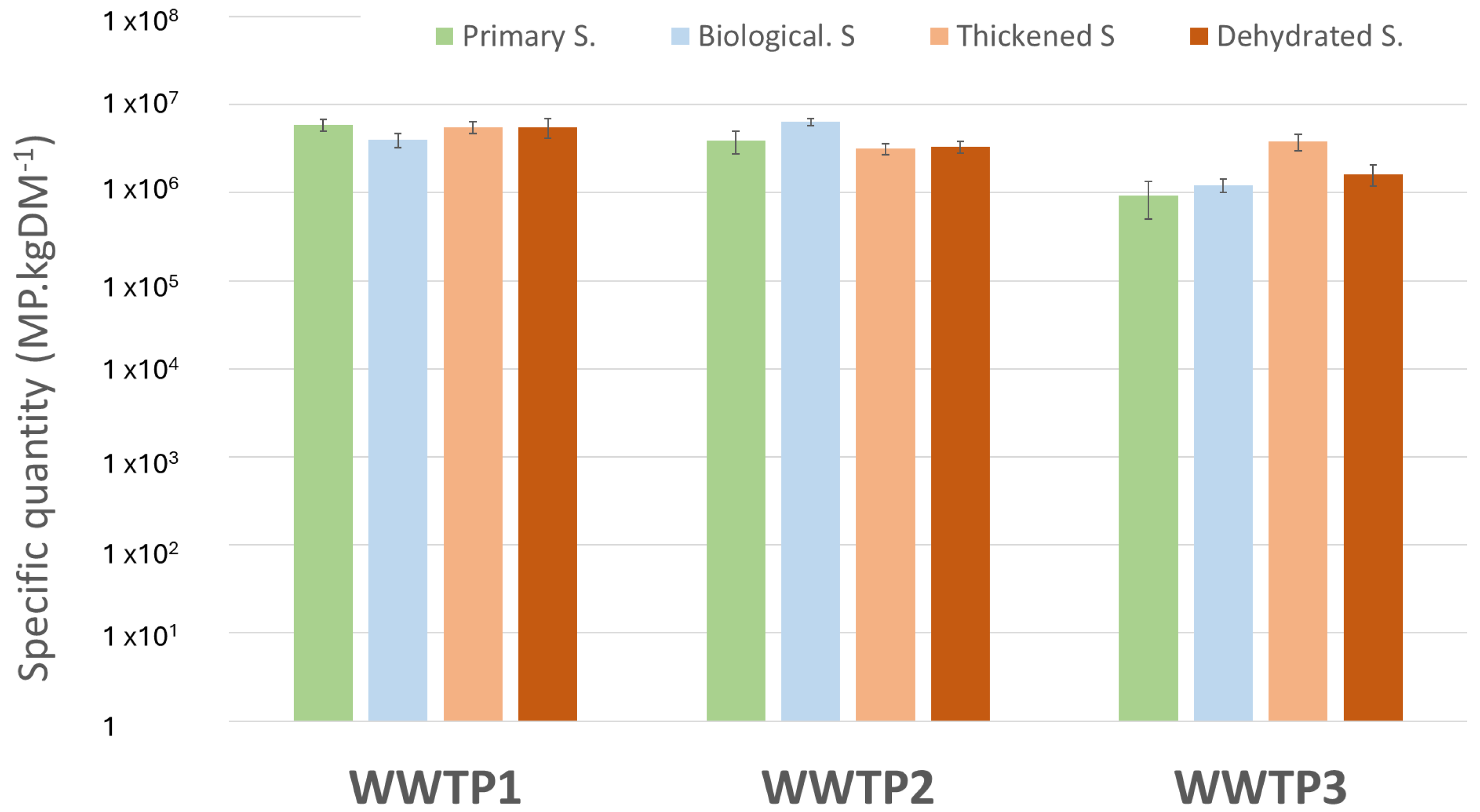
| Inlet Water | Treated Water | Primary Sludge | Biological Sludge | Thickened Sludge | Dewatered Sludge | ||
|---|---|---|---|---|---|---|---|
| WWTP1 | Concentration | 1000 ± 50 | 15 ± 2 | 19,048 ± 2862 | 12,914 ± 2283 | − | − |
| Specific quantity | − | − | 5.9 ± 0.9 | 4.0 ± 0.7 | 5.5 ± 0.9 | 5.6 ± 1.4 | |
| Sludge TSS | − | − | 3.23 | 3.26 | − | − | |
| Sludge dryness | − | − | − | − | 4.68 | 17.42 | |
| Removal rate | 98.5 | ||||||
| MP content (DM) | 0.56 | ||||||
| WWTP2 | Concentration | 808 ± 101 | 15 ± 6 | 16,234 ± 4685 | 13,943 ± 1295 | − | − |
| Specific quantity | − | − | 3.9 ± 1.1 | 6.3 ± 0.6 | 3.2 ± 0.5 | 3.3 ± 0.5 | |
| Sludge TSS | − | − | 4.16 | 2.20 | - | − | |
| Sludge dryness | − | − | − | - | 4.16 | 37.03 | |
| Removal rate | 98.2 | ||||||
| MP content (DM) | 0.33 | ||||||
| WWTP3 | Concentration | 567 ± 38 | 14 ± 6 | − | 6971 ± 1197 | − | − |
| Specific quantity | − | − | 0.9 ± 0.4 | 1.2 ± 0.2 | − | 1.6 ± 0.4 | |
| Sludge TSS | − | − | − | 5.72 | − | − | |
| Sludge dryness | − | − | 11.59 | − | − | 23.8 | |
| Removal rate | 97.5 | ||||||
| MP content (DM) | 0.16 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saur, T.; Paillet, F.; Robert, S.; Alibar, J.-C.; Loret, J.-F.; Barillon, B. Fate of Microplastic Pollution Along the Water and Sludge Lines in Municipal Wastewater Treatment Plants. Microplastics 2025, 4, 19. https://doi.org/10.3390/microplastics4020019
Saur T, Paillet F, Robert S, Alibar J-C, Loret J-F, Barillon B. Fate of Microplastic Pollution Along the Water and Sludge Lines in Municipal Wastewater Treatment Plants. Microplastics. 2025; 4(2):19. https://doi.org/10.3390/microplastics4020019
Chicago/Turabian StyleSaur, Thibaut, Florian Paillet, Samuel Robert, Jean-Claude Alibar, Jean-François Loret, and Bruno Barillon. 2025. "Fate of Microplastic Pollution Along the Water and Sludge Lines in Municipal Wastewater Treatment Plants" Microplastics 4, no. 2: 19. https://doi.org/10.3390/microplastics4020019
APA StyleSaur, T., Paillet, F., Robert, S., Alibar, J.-C., Loret, J.-F., & Barillon, B. (2025). Fate of Microplastic Pollution Along the Water and Sludge Lines in Municipal Wastewater Treatment Plants. Microplastics, 4(2), 19. https://doi.org/10.3390/microplastics4020019







