Abstract
Detecting microplastics (MPs) in marine organisms is vital for understanding the ecological impact of MP pollution. Free-living marine nematodes, key players in benthic ecosystems, are often employed as bioindicators because of their sensitivity to environmental changes and thus hold promise as bioindicators for MP pollution too. This study investigated the detection of MPs in nematodes using µ-Raman spectroscopy combined with a tailored digestion protocol, targeting MPs in size ranges between 1 and 15 µm. While this is the first documented attempt to detect MPs in field-collected nematodes, significant challenges were identified. Contamination, particularly from airborne MPs and plastic-based laboratory materials, posed a major obstacle. We found higher numbers of <5 µm particles of polypropylene (PP), polyethylene terephthalate (PET), polylactic acid (PLA), polymethyl methacrylate (PMMA), and polystyrene (PS) in a natural community of nematodes compared to blank controls, suggesting the potential ingestion of small-sized MPs by nematodes in the real world. However, small MPs exhibited greater contamination challenges, underscoring the need for improved contamination control measures, such as open-air filters and plastic-free workflows. Despite these challenges, this study highlights the potential of µ-Raman spectroscopy as a valuable tool for detecting small-sized MPs in field-collected marine invertebrates, provided contamination risks are minimized. The likelihood of nematodes encountering MPs in marine sediments is high, but whether this translates to significant ingestion remains uncertain pending on the analysis of more field samples and the application of efficient measures of contamination reduction.
1. Introduction
Free-living marine nematodes constitute approximately 80% of the total biomass of marine sediments worldwide and act as both primary and secondary consumers. Their ubiquity and abundance in marine sediments globally and their role in regulating biogeochemical cycles make them excellent bioindicators for assessing ecosystem health and functioning [1,2]. Nematodes range from 20 to 3000 µm in size and have buccal cavity dimensions typically between 1 and 10 µm. Laboratory studies have shown that nematodes ingest microplastic particles (MPs) [3,4], a well-documented pollutant in the marine environment. The size of the ingested MPs is correlated with the size of the nematodes’ buccal cavity and depends on species-specific feeding habits, with bactivorous species ingesting mostly 1 µm particles, while epistrate and deposit feeders have buccal structures capable of ingesting larger MPs [3,5]. Laboratory studies in which nematodes were exposed to 1 µm polystyrene (PS) beads have shown the potential for ingestion, reduced feeding efficiency, impaired reproduction, and altered physiological states, potentially leading to broader ecological implications [6,7]. Due to their crucial role in benthic food webs, shifts in nematode species composition can have cascading effects on ecosystem functioning, as different species contribute differently to nutrient cycling and energy transfer within sediments. Additionally, there is potential for the bioaccumulation and/or biomagnification of ingested MPs up the food chain. MPs consumed by meiofauna may transfer to higher trophic levels through predation, potentially leading to their accumulation in larger organisms, including those consumed by humans [8]. This not only poses possible risks to human health but may also disrupt the delicate balance of benthic ecosystems, ultimately altering food web dynamics and the stability of marine habitats.
Despite these considerations, studies exploring the ingestion of microplastics by natural nematodes in the field are notably absent, and, consequently, potential ecological implications in the real world are not understood. This knowledge gap is primarily due to technological limitations in detecting small particles and the complexities involved in sampling and analyzing such tiny organisms, along with the MPs they ingest. Firstly, the most commonly used spectroscopic method for the detection of MPs, Fourier-transform infrared spectroscopy (FTIR), has a lower size detection limit of about 10–20 µm [9,10], which is above buccal cavity dimensions for the majority of nematodes in marine sediments. To detect smaller particles, techniques such as micro-Raman spectroscopy (µ-Raman) are therefore essential, as they allow one to analyze particles down to 1 µm in size [10,11,12], offering a more precise method for studying microplastic ingestion by nematodes. In fact, particles within the lowest MP size fraction (1–5 µm) are expected to be highly abundant in marine sediments because of fragmentation [13], although evidence is limited because of inevitable technical drawbacks. Furthermore, MP extraction protocols from biota typically involve the digestion of organic matter to isolate the MPs for spectroscopic analysis [14]. While this is usually effective for soft-bodied organisms, sturdier structures (e.g., zooplankton exoskeleton) are more challenging to dissolve completely. In marine nematodes, this process is further complicated by the particularly strong cuticle, a highly structured extra-cellular matrix (ECM), mainly composed of collagens, highly cross-linked insoluble proteins (i.e., cuticlins), and the associated glycoproteins and lipids [15]. Here, we applied a tailored digestion protocol based on MP research on zooplanktonic organisms [16] and nematode DNA extraction methods [17,18] to examine the possible ingestion of MPs by nematodes from a subtidal community from the English Channel. Our approach aimed to efficiently dissolve nematodes while preserving potentially ingested MPs for subsequent identification to support a qualitative assessment of the possible ingestion of MPs by natural nematode communities. With our approach, we also want to validate µ-Raman spectroscopy as a method to distinguish small-sized MPs from marine biota in general.
2. Materials and Methods
2.1. Sampling of Natural Nematode Communities
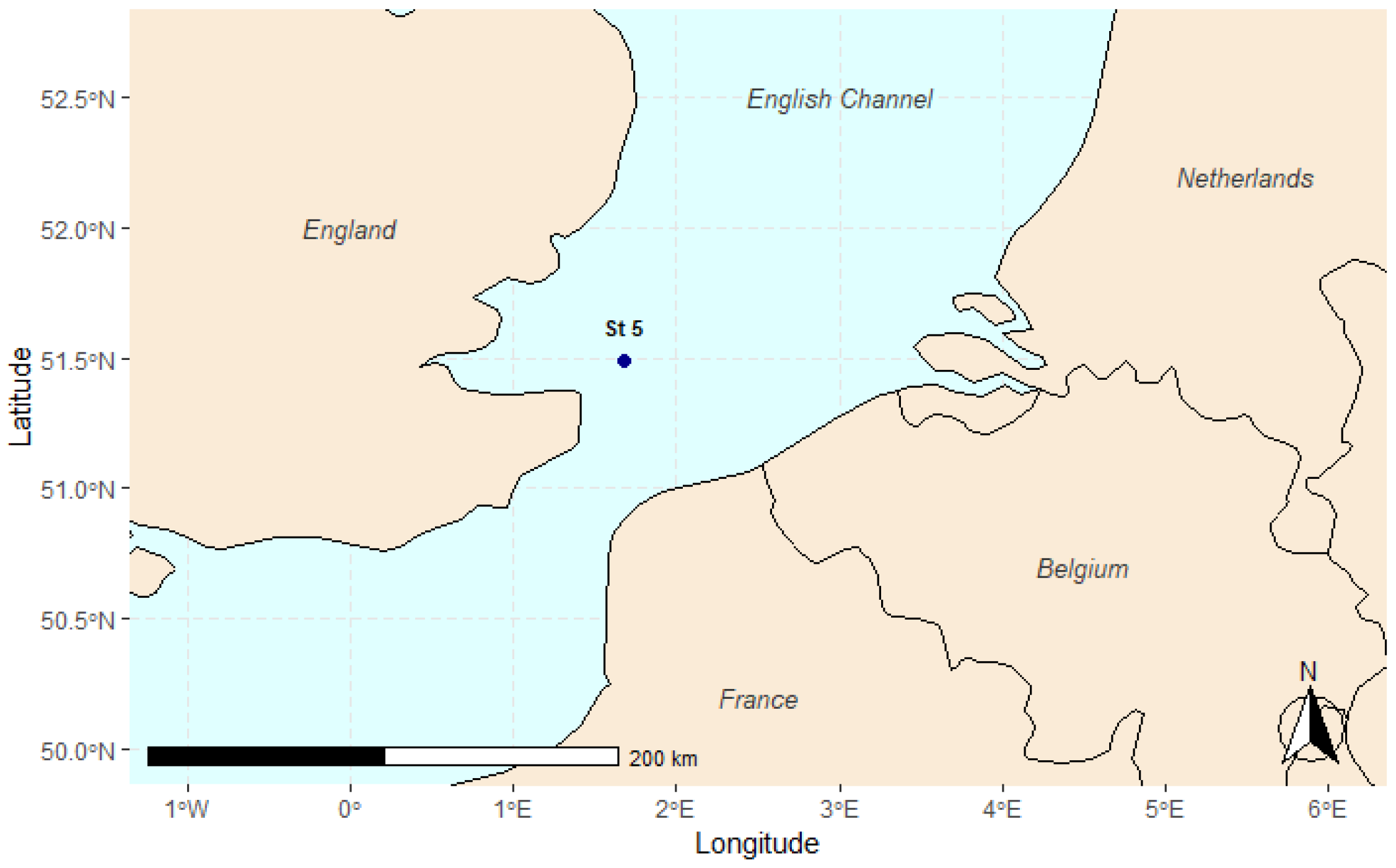
The sediment samples used in this study were collected during the research cruise AL 534/2, on board of the RV Alkor. The samples were collected in March 2020 at one station in the English Channel at 32 m depth (coordinates: 51°29′21.9″ N 1°40′43.2″ E, Figure 1), approximately 20 km off the English coast. Of the 10 stations sampled during the campaign, station 5 was selected because it provided a sufficiently high number of nematodes (almost 5000 individuals), making it well suited for this study. Additionally, based on the microplastics identified in our previous study, we expected this station to exhibit a diverse range of polymer types, if present.
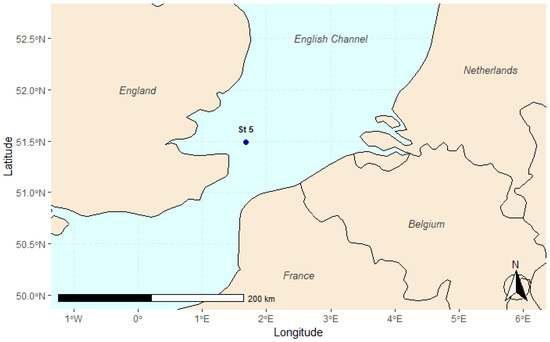
Figure 1.
The map shows the location of station 5, situated in the English Channel, approximately 20 km off the English coast. Station 5 was one of the stations sampled during the AL 534/2 research cruise.
A Mini Multicorer was used to collect intact sediment samples from three separate deployments (replicates). These samples were successively subsampled using plexi tubes (d = 10 cm, H = 15 cm) that were rinsed prior to sampling to limit plastic contamination from the device. Subsequently, the top 0–1 cm slice of each sample was transferred onto aluminum foil. As a further subsampling step, a 4 cm diameter sediment disc was obtained by inserting a metal ring (previously rinsed) in each slice and then stored in aluminum trays at −20 °C. This subsample was taken from the middle area of each slice to avoid contamination with the outer sediment (in contact with the plastic core during deployment) and was used for meiofauna community analysis and nematode digestion (see Section 2.2). Meiofauna was extracted from the sediments via centrifugation with Ludox [19] and stored in distilled water at −20 °C. Nematode density was assessed by counting individuals under a stereomicroscope (mean of 3 replicates) and standardizing the count to 10 cm2. Subsequently, a total of 150 nematodes were randomly picked from the three replicates (50 specimens per replicate) and mounted on glass slides for taxonomical classification, carried out following the online key for free-living marine nematodes (NeMysKey©) [20] and the pictorial key from Warwick et al. (1998) [21] based on relevant morphological traits (buccal cavity, tail, amphid, and reproductive organs). The nematodes were then divided into trophic guilds following the subdivision suggested by Wieser (1953) [22], considering the structure of their buccal cavities: selective deposit feeders (1A) with small buccal cavities able to mainly feed on bacteria; non-selective deposit feeders (1B), characterized by a larger unarmed buccal structure and expected to feed on detritus and particulate organic matter; epistrate feeders (2A), with one or more small teeth to perforate the outer shell of unicellular organisms such as microalgae; and predators/omnivores (2B), usually larger nematodes with cuticularized teeth (and possibly strong mandible structures) which predate on other meiofauna organisms and/or ingest detritus particles.
2.2. Digestion of Nematodes
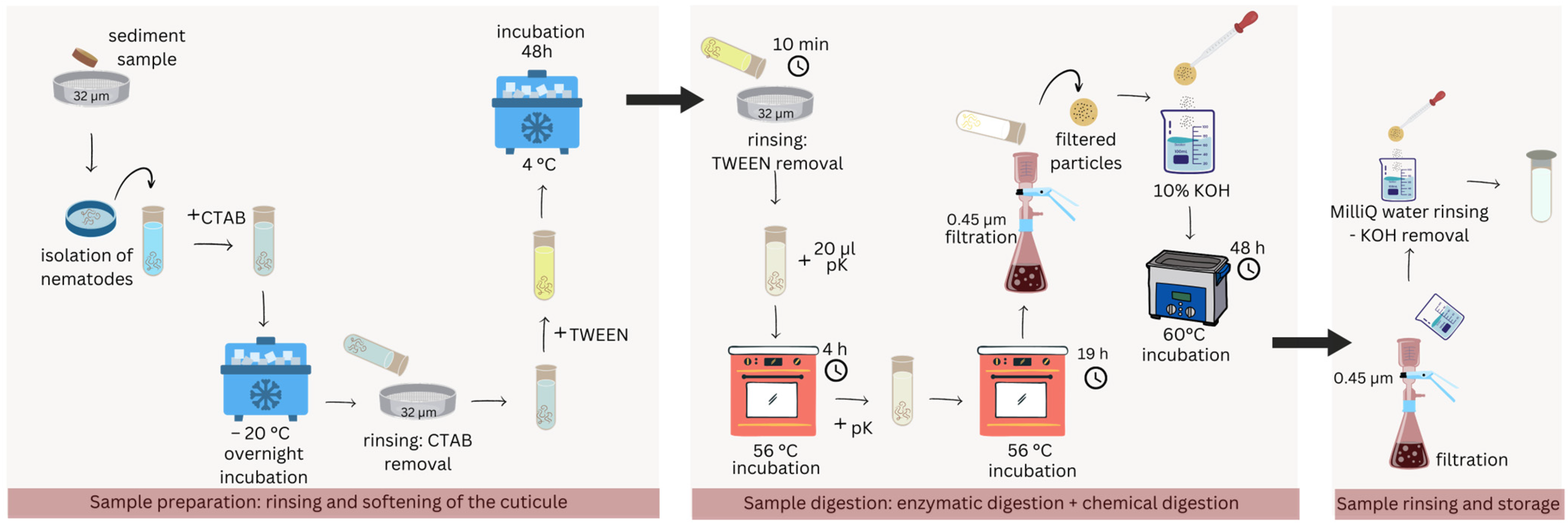
A total of 2000 nematode specimens (pooled from the three replicates) underwent several steps to ensure digestion of the organic matter and the isolation of potentially ingested MPs (Figure 2).
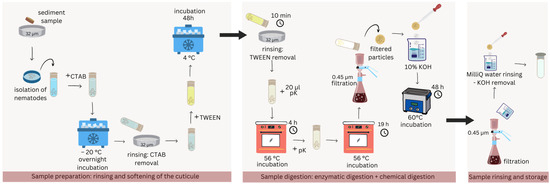
Figure 2.
Schematic representation of the digestion protocol in detail. Three main sections can be identified: sample preparation, digestion, and final rinsing.
Thawed samples were rinsed on a 32 µm sieve and transferred to a glass vial, to which 2 mL of cetyltrimethylammonium bromide (CTAB) was added before freezing overnight at −20 °C. After thawing, the samples were washed again and TWEEN 40 (Sigma-Aldrich®, Saint Louis, MO, USA) was added before incubation for 48 h at 4 °C. Residual TWEEN was thoroughly removed during a 10 min washing step in which the samples were stirred, sieved, and flushed with clean MilliQ water for approximately 5 times until no soapiness was left. Subsequently, 20 µL of proteinase K (Merck KGa, Darmstadt, Germany) (pK in Figure 2) was added to the samples and incubated for 5 h at 56 °C. Subsequently, an additional 20 µL of proteinase K was added, and the samples were incubated again at 56 °C for 19 h. The samples underwent another wash over a 0.45 µm cellulose acetate membrane filter and were incubated in 10% KOH (Sigma-Aldrich®, Saint Louis, MO, USA) in a 60 °C water bath for 48 h. Finally, the samples were rinsed again over a new 0.45 µm cellulose acetate filter to remove KOH. The filter was then rinsed in clean vials with MilliQ water and stored at 4 °C awaiting MP detection analysis. The samples will be referred to as “field samples” from here onwards. All washing steps were performed with MilliQ water.
2.3. MP Identification and Quantification
2.3.1. Filtration
The aqueous samples were filtered under vacuum (δp = 250 mbar) on Au-coated track-etched polycarbonate filters (pore size 0.8 μm, Analytische Produktions-, Steuerungs- and Controllgeräte GmbH, Germany) for a few minutes, using a Sartorius filtration apparatus 16306 (25 mm glass vacuum filter holder, glass frit filter support, Sartorius, Germany). The filtration was carried out in a laminar flow box (ENVAIR eco, ISO 3 standard, ENVAIR GmbH, Germany). Thereafter, the apparatus and the sample flask were rinsed with MilliQ water (3 × 50 mL), which had been additionally filtered over a cellulose acetate filter (pore size 0.45 μm). An additional 50 mL was used to rinse the filtration apparatus only.
2.3.2. µ-Raman Measurements
The particles were quantified through static image analysis followed by Raman measurements for chemical identification within a fully automated procedure using the open-source software TUM-ParticleTyper 2 (random window sampling mode, 0.1% analyzed proportion of the entire filter area for the samples, 0.5% for the digestion blanks, followed by a corresponding extrapolation to the whole sample, respectively)) [23]. A computer-controlled Raman microscope was used (WITec apyron, WITec GmbH, Germany, laser wavelength 532 nm, laser power 3.5 mW, maximum spectral acquisition time 4 × 0.5 s, minimum required signal-to-noise ratio (SNR) 12, spectral autofocus ranged from −10 μm to 15 μm, dark-field objective with 100× magnification, N.A. = 0.9, working distance (WD) = 1 mm, Zeiss EC Epiplan-Neofluar). The spectral range of 590 cm−1 to 1770 cm−1 and 2800 cm−1 to 3200 cm−1 (Raman shift) was used for comparison with the reference spectra (database matching). For database matching, WITec TrueMatch was used, and a custom-made database was applied (including spectra of the polymer types polyethylene (PE), polyethersulfone (PES), polyethylene terephthalate (PET), polylactic acid (PLA), polymethyl methacrylate (PMMA), polysiloxane, polyoxymethylene (POM), polypropylene (PP), poly-p-phenylene terephthalamide (PPTA), polystyrene (PS), polytetrafluoroethylene (PTFE), and polyvinyl chloride (PVC). The band positions of the Raman system were calibrated to an internal standard (diode lamp). The Raman signal intensity was checked regularly with the Si band at 520 cm−1 [23].
2.4. Contamination Prevention and Blank Controls
To prevent sample contamination, stringent field and laboratory practices were followed consistently. These included wearing 100% cotton lab coats and clothes, avoiding the use of laboratory gloves to minimize contamination risk, washing hands often, and utilizing dust-free, thoroughly rinsed laboratory and field sampling equipment. Nematode extraction from sediment was performed under a laminar flow. Metal and glass tools were preferred over plastic equipment, which was thoroughly rinsed when impossible to avoid (i.e., nematode extraction procedure). Concurrent with the nematode digestion, three blank samples (1 mL MilliQ water without nematodes) were processed identically to test for MP contamination during the digestion process, referred to as “digestion blanks”. Secondly, another laboratory blank sample was collected immediately before filtration of the digested samples (i.e., field sample, lab culture samples, digestion blanks) awaiting µ-Raman analysis. Here, only 200 mL of water (corresponding to the 4 × 50 mL rinsing portions described above) was used (MilliQ, additional filtration over cellulose acetate filter, pore size 0.45 μm, from the same source as the one used for rinsing the sample flasks and the filtration system). After collecting this sample, the filtration apparatus remained unchanged. This contamination check revealed the presence of maximal 480 PP particles in the 5–10 µm size range only; filtration contamination, thus, did not compromise interpretation of the results for the other polymers and other size fractions of PP.
The range of microplastic concentrations potentially attributable to contamination was determined by calculating the mean concentration of MPs in three blank samples, computing the standard deviation (SD) of this mean and multiplying it by 3 (+3 SD) to ensure 90% confidence. The mean increased by the threefold SD represented the detection limit, i.e., the minimum concentration of MPs which could reasonably be distinguished from potential contamination during sample processing. This method provided a robust way to differentiate between true environmental MPs and possible contamination in the samples [24].
2.5. Post Hoc PE Contamination Assessment
The µ-Raman analysis showed a suspiciously high amount of PE particles in the field sample (1.4 × 106 particles), suggesting the possible presence of plastic contamination or false-positive material identification (see Section 3, Table 1). Two possible sources were identified: (1) a low-density PE squeezing bottle used for a washing step in the digestion protocol or (2) CTAB, a compound which, due to its chemical structure, gives a spectroscopic signal very similar to that of PE. Although CTAB was thoroughly rinsed off from the nematodes during the protocol, we suspected it might have been retained in some remaining organic matter and could thus be detected during the spectroscopic analysis. For this reason, we tested the protocol on laboratory nematodes cultures, with and without CTAB and the PE rinsing bottle, to investigate possible contamination sources during the extraction of this polymer from the nematodes. Contamination assessments were conducted on nematodes grown in plastic-free laboratory cultures, using glass materials at all times. Monospecific cultures of Litoditis marina Pm IV were raised from a single gravid female obtained from Lake Grevelingen, the Netherlands (51°440 N, 3°570 E; salinity: 32). Cultures were maintained on sloppy (1%) nutrient–bacto agar (ratio of 1:4) media [25] and incubated in the dark under standardized conditions (temperature: 20 °C; salinity: 25) for many generations prior to harvesting. After picking, nematodes were placed in MilliQ water and stored at −20 °C before harvesting. A total of 6000 nematodes were harvested, 2000 for each treatment: treatment 1 (T1) contained CTAB but the PE bottle was not used; in treatment 2 (T2), the PE bottle was used but CTAB was removed; in treatment 3 (T3), neither CTAB nor the PE bottle were used. The frozen nematodes were processed following the digestion protocol described in Section 2.2, and the MP identification analysis followed the same steps presented in Section 2.3.

Table 1.
The table shows the number of particles detected in the field sample (Sample) and in the digestion blanks for three size ranges (1–5 µm, 5–10 µm, and 10–15 µm). The detection limit (+3 SD) is also shown.
3. Results and Discussion
3.1. Nematode Community
The meiofauna community comprised 12 taxa, of which nematodes constituted ~ 94% (621.11 individuals/10 cm2) of all meiobenthic organisms. The nematode community included 35 genera, with Chromadorita sp. (29%), Sabatieria sp. (13%), and Molgolaimus sp. (10%) being the most abundant ones (Supplementary Material, Table S1). The majority of organisms detected were epistrate feeders (43%), followed by bactivorous species and omnivores in equal parts (20%). Based on their feeding habit and buccal cavity structure, the investigated nematode community primarily ingested particles between 1 and 10 µm [5].
3.2. Microplastics
Approximately 90% of all MPs detected by the spectroscopic analysis ranged between 1 and 5 µm in size (Table 1). This result is in line with previous studies showing that MP abundance is highest in the smaller size classes [26]. In fact, Leusch et al. (2023) [13] recently demonstrated a strong negative relationship between MP size and concentration, regardless of the matrix they occupy. The most abundant polymer recorded was PE, with 1.2 × 106 particles detected in the field sample. PE was also the only polymer identified in the field sample for the size fractions > 5 µm. However, the extremely high number of particles in the 1–5 µm size range and an average particle count of 3593.33 in the digestion blanks raised suspicions regarding the reliability of the detection and suggested a contamination which at least spectroscopically imitated PE, resulting in numerous false-positive assignments for the low signal-to-noise ratios of those spectra.
The second most abundant polymers detected in the field sample were polypropylene (PP) and poly-p-phenylene terephthalamide (PPTA). However, the high concentration of particles within the digestion blanks complicated interpretation and indicated a heightened risk of contamination. In contrast, the detection of PET, PLA, PMMA, and possibly PS was more likely to reflect real environmental conditions. These polymers were either exclusively found in the field sample (PLA, PET, and PMMA) or present in numbers above the detection limit (PS, Table 1). Additionally, with the exception of PET, these polymers were not detected in larger particle size classes, suggesting that ingestion was limited by size or that contamination was inversely proportional to MP size. For a more detailed analysis of particle detection related to these polymers, refer to Section 3.3.
While PLA and PMMA are relatively uncommon polymers, PET and PS are more prevalent in sediments, increasing the likelihood of nematode exposure to these particles [27]. Notably, both polymers were detected in sediment samples at the time of sampling at the same location. Although the available data on sediment MP pollution levels refer to larger particles (80–400 µm) [28], the potential for fragmentation suggests that smaller particles of PET and PS may have also been present in the nematode habitat at the time of sampling.
3.3. Blank Samples and Interpretation
One of the biggest concerns in microplastics’ research is the presence of airborne contamination in the field and during laboratory investigations [29,30]. Although good laboratory practices were followed at all times, microplastic contamination is incredibly difficult to avoid and brings questions regarding the reliability of the data presented. How studies deal with contamination varies largely, with some studies removing entire categories found in blanks to more conservative practices where the amount of contamination is considered more important for the interpretation of results than its presence/absence. In our study, interpretation of the ingested particles (i.e., field sample) also needed to be carried out with extreme caution. For example, whereas the absence of PET, PLA, and PMMA in the 1–5 µm range of the digestion blanks suggested evidence for ingestion of these polymers by natural nematodes, the estimated concentrations in the fields sample were based on one single detection analysis for each polymer spectrum, highlighting the need for more field replicates to increase support for this conclusion. Further, considering that the sample contained 2000 individual nematodes, these findings indicate that, while nematodes may ingest microplastics in natural environments, they likely perform so only at relatively low levels, and there is no evidence for accumulation in the body.
3.4. PE Contamination Origin Check
Our analysis of nematode samples from lab cultures raised uncertainties on the possible source(s) of contamination. Our first hypothesis that the high amount of PE particles in all size ranges of the field sample was associated with either CTAB or a squeezing bottle was disproved when high amounts of PE were detected in samples where neither were present (T3, Table 2). The total number of detected PE particles was instead lower when either elements were used (T1 and T2), suggesting that PE contamination was indiscriminately not dependent on these two factors. Our second hypothesis assumed that the field sample would contain more particles compared to our initial three digestion blanks (blanks 1, 2, and 3, Table 1) due to their tendency to adhere to residual organic matter. However, our post hoc contamination check also disproved this hypothesis, as the number of detected particles remained much lower than in the field sample and was, in fact, comparable to the digestion blanks (Table 1). Other plastic types were also detected in the post hoc check samples, although mostly in minor quantities compared to the field sample (Table 2).

Table 2.
The table shows a comparison between all the biotic samples analyzed: the nematode community from the English Channel (field sample) and the three post hoc contamination treatments with nematode laboratory cultures (T1, T2, and T3).
Considering that the nematodes used in the post hoc contamination tests were cultivated in a laboratory environment with minimized plastic exposure, we assumed that any microplastics detected in the T1, T2, and T3 samples were not ingested particles and thus originated from contamination during sample processing. However, pinpointing the exact sources of contamination remains challenging. Even though no plastic tools were used at any stage, microplastics could have originated from containers (such as those used to store chemicals like KOH pellets, typically made of PP) or from exposure to the air. Because the same procedures and materials were followed for the digestion of all samples, the contamination of PET, PLA, and PMMA in the lab culture samples (T1–T3) must have been related to airborne contamination at the moment of processing these samples. Alternatively, the discrepancy in contamination between the digestion blanks and lab culture samples may simply illustrate that more blanks are needed to increase the likelihood of detecting polymers’ presence from contamination. This issue becomes even more pronounced as the particle size of interest decreases.
4. Conclusions and Future Recommendations
In this study, we explored the detection of microplastics (1–5 µm, 5–10 µm, and 10–15 µm size ranges) in free-living marine nematodes, which play a critical role in benthic ecosystems. While this represents the first documented attempt of detecting microplastics’ presence in marine nematodes from the field, the interpretation of our findings requires careful consideration, as our study underscores the necessity for refining existing protocols. First and foremost, research of this nature must be conducted in laboratory environments as free from microplastic contamination as possible. Despite best efforts, some level of contamination is inevitable due to the use of plastic containers for the chemicals involved in the digestion protocol, the extraction of organisms, and even Milli-Q water dispensers. Moreover, although filtering all reagents and cleaning materials can reduce contamination, preventing airborne microplastics remains a significant challenge, especially when dealing with small particles.
Our results indicate that the field samples contained higher numbers of particles compared to all blank control and most samples from the lab cultures. However, additional field samples are required to confirm whether the detected polymers were ingested by nematodes or were due to contamination. This is particularly true for PE, which showed exceptionally high concentrations. Given the prevalence of PE in the environment, ingestion is plausible, but current contamination levels make interpretation difficult. Moreover, elevated amounts of PE were also detected in other size classes, suggesting that contamination of this polymer may be higher than for others. Future studies should consider using air filters to reduce the influence of airborne microplastics.
The possibility of nematodes encountering small-sized MPs in marine sediments is extremely high. Whether such probability is reflected in MP ingestion in the field, however, is still questionable. Although the reliability of our results at the time being is too uncertain to draw confident conclusions on the ecotoxicology of MPs for marine free-living nematodes, this study indicates the potential of µ-Raman spectroscopy as a valuable method for detecting MPs in samples of digested biota, provided that contamination is effectively controlled throughout sample processing.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/microplastics4020020/s1, Table S1: The table shows all the nematodes genera identified in the digested field sample (D1), their feeding type, and the number of individuals (N). The feeding types are categorized as follow: selective deposit feeders (1A); non-selective deposit feeders (1B); epistrate feeders (2A); and predators/omnivores (2B).
Author Contributions
Conceptualization, C.V.C., A.V. and G.P.; methodology, C.V.C., G.P., O.J. and N.P.I.; formal analysis, G.P. and O.J.; investigation, O.J.; data curation, G.P. and O.J.; writing—original draft preparation, G.P.; writing—review and editing, C.V.C., A.V., G.P., O.J. and N.P.I.; supervision, C.V.C., A.V. and N.P.I.; funding acquisition, A.V. and N.P.I. All authors have read and agreed to the published version of the manuscript.
Funding
This study contributed to project B2/191/p1/HOTMIC of the Belgian Science Policy (BELSPO/BRAIN). The scientific research was carried out with infrastructure funded by EMBRC Belgium—FWO international research infrastructure I001621N. Oliver Jacob and Natalia P. Ivleva would like to thank the Federal Ministry of Education and Research, Germany (BMBF), for the financial support (JPI Oceans project HOTMIC, grant number: 03F0851B).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Dataset available on request from the authors.
Acknowledgments
The authors wish to thank the crew members of the RV Alkor and the scientific crew on board for the logistic support received during the sampling campaign. We also wish to thank Tom Moens, Marie Cours, and Annelien Rigaux from the Marine Biology Research lab for providing the L. marina cultures and for their assistance during nematode picking, together with Annick Van Kenhove and Yen Nguyen Thi My. Finally, we also thank Rajeshwari Paul for her help in developing the digestion protocol.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alfonso, M.B.; Takashima, K.; Yamaguchi, S.; Tanaka, M.; Isobe, A. Microplastics on plankton samples: Multiple digestion techniques assessment based on weight, size, and FTIR spectroscopy analyses. Mar. Pollut. Bull. 2021, 173, 113027. [Google Scholar] [CrossRef] [PubMed]
- Araujo, C.F.; Nolasco, M.M.; Ribeiro, A.M.P.; Ribeiro-claro, P.J.A. Identi fi cation of microplastics using Raman spectroscopy: Latest developments and future prospects. Water Res. 2018, 142, 426–440. [Google Scholar] [CrossRef] [PubMed]
- de França, F.J.L.; Moens, T.; da Silva, R.B.; Pessoa, G.L.; França, D.A.A.; Dos Santos, G.A.P. Short-term microplastic effects on marine meiofauna abundance, diversity and community composition. PeerJ 2024, 12, e17641. [Google Scholar] [CrossRef] [PubMed]
- Eisendle-Flöckner, U.; Bezerra, T.N.; Decraemer, W.; Hodda, M.; Holovachov, O.; Leduc, D.; Miljutin, D.; Mokievsky, V.; Peña Santiago, R.; Sharma, J.; et al. Nemys: World Database of Nematodes; University of Salzburg: Salzburg, Austria, 2019. [Google Scholar]
- Fueser, H.; Mueller, M.T.; Traunspurger, W. Ingestion of microplastics by meiobenthic communities in small-scale microcosm experiments. Sci. Total Environ. 2020, 746, 141276. [Google Scholar] [CrossRef]
- Fueser, H.; Mueller, M.T.; Weiss, L.; Höss, S.; Traunspurger, W. Ingestion of microplastics by nematodes depends on feeding strategy and buccal cavity size. Environ. Pollut. 2019, 255, 113227. [Google Scholar] [CrossRef]
- Heip, C.; Vincx, M.; Vranken, G. The ecology of marine nematodes. Oceanogr. Mar. Biol. Annu. Rev. 1985, 23, 399–489. [Google Scholar]
- Ivleva, N.P. Chemical analysis of microplastics and nanoplastics: Challenges, advanced methods, and perspectives. Chem. Rev. 2021, 121, 11886–11936. [Google Scholar] [CrossRef]
- Jacob, O.; Ramírez-Piñero, A.; Elsner, M.; Ivleva, N.P. TUM-ParticleTyper 2: Automated quantitative analysis of (microplastic) particles and fibers down to 1 μm by Raman microspectroscopy. Anal. Bioanal. Chem. 2023, 415, 2947–2961. [Google Scholar] [CrossRef]
- Kaandorp, M.L.A.; Dijkstra, H.A.; van Sebille, E. Modelling size distributions of marine plastics under the influence of continuous cascading fragmentation. Environ. Res. Lett. 2021, 16, 054075. [Google Scholar] [CrossRef]
- Kang, T.; Kim, D.; Oh, J.H. Ingestion of microplastics by free-living marine nematodes, especially enoplolaimus spp., in mallipo beach, south korea. Plankt. Benthos Res. 2021, 16, 109–117. [Google Scholar] [CrossRef]
- Leusch, F.D.; Lu, H.C.; Perera, K.; Neale, P.A.; Ziajahromi, S. Analysis of the literature shows a remarkably consistent relationship between size and abundance of microplastics across different environmental matrices. Environ. Pollut. 2023, 319, 120984. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Pang, X.; Chen, H.; Jiang, L. Visual detection of microplastics using Raman spectroscopic imaging. Analyst 2024, 149, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Macheriotou, L.; Guilini, K.; Bezerra, T.N.; Tytgat, B.; Nguyen, D.T.; Phuong Nguyen, T.X.; Noppe, F.; Armenteros, M.; Boufahja, F.; Rigaux, A.; et al. Metabarcoding free-living marine nematodes using curated 18S and CO1 reference sequence databases for species-level taxonomic assignments. Ecol. Evol. 2019, 9, 1211–1226. [Google Scholar] [CrossRef] [PubMed]
- Moens, T.; Vincx, M. On the cultivation of free-living marine and estuarine nematodes. Helgoländer Meeresunters. 1998, 139, 115–139. [Google Scholar] [CrossRef]
- Mueller, M.T.; Fueser, H.; Höss, S.; Traunspurger, W. Species-specific effects of long-term microplastic exposure on the population growth of nematodes, with a focus on microplastic ingestion. Ecol. Indic. 2020, 118, 106698. [Google Scholar] [CrossRef]
- Miller, M.E.; Hamann, M.; Kroon, F.J. Bioaccumulation and biomagnification of microplastics in marine organisms: A review and meta-analysis of current data. PLoS ONE 2020, 15, e0240792. [Google Scholar] [CrossRef]
- Pantó, G.; Pasotti, F.; Macheriotou, L.; Vanreusel, A. Combining Traditional Taxonomy and Metabarcoding: Assemblage Structure of Nematodes in the Shelf Sediments of the Eastern Antarctic Peninsula. Front. Mar. Sci. 2021, 8, 629706. [Google Scholar] [CrossRef]
- Pantó, G.; Vanreusel, A.; Vercauteren, M.; Asselman, J.; Van Colen, C. Seabed microplastics in the European continental shelf: Unravelling physical and biological transport pathways and reciprocal fauna–Polymer relationships. Environ. Pollut. 2025, 365, 125392. [Google Scholar] [CrossRef]
- Phuong, N.N.; Fauvelle, V.; Grenz, C.; Ourgaud, M.; Schmidt, N.; Strady, E.; Sempéré, R. Highlights from a review of microplastics in marine sediments. Sci. Total Environ. 2021, 777, 146225. [Google Scholar] [CrossRef]
- Podbielski, I.; Hamm, T.; Lenz, M. Customized digestion protocols for copepods, euphausiids, chaetognaths and fish larvae facilitate the isolation of ingested microplastics. Sci. Rep. 2024, 14, 19985. [Google Scholar] [CrossRef]
- Prata, J.C.; Reis, V.; Mouneyrac, C.; Duarte, C.; Rocha-santos, T. Contamination issues as a challenge in quality control and quality assurance in microplastics analytics. J. Hazard. Mater. 2021, 403, 123660. [Google Scholar] [CrossRef] [PubMed]
- Ridall, A.; Ingels, J. Suitability of Free-Living Marine Nematodes as Bioindicators: Status and Future Considerations. Front. Mar. Sci. 2021, 8, 685327. [Google Scholar] [CrossRef]
- Schymanski, D.; Oßmann, B.E.; Benismail, N.; Boukerma, K.; Dallmann, G.; von der Esch, E.; Fischer, D.; Fischer, F.; Gilliland, D.; Glas, K.; et al. Analysis of microplastics in drinking water and other clean water samples with micro-Raman and micro-infrared spectroscopy: Minimum requirements and best practice guidelines. Anal. Bioanal. Chem. 2021, 413, 5969–5994. [Google Scholar] [CrossRef]
- Semprucci, F.; Frontalini, F.; Sbrocca, C. Meiobenthos and free-living nematodes as tools for biomonitoring environments affected by riverine impact. Environ. Monit. Assess. 2015, 187, 251. [Google Scholar] [CrossRef]
- Stock, F.; Vinay, V.K.; Scherer, C.; Löder, M.G.J.; Brennholt, N.; Laforsch, C.; Reifferscheid, G. Pitfalls and Limitations in Microplastic Analyses. Handb. Environ. Chem. 2022, 111, 13–42. [Google Scholar] [CrossRef]
- Warwick, R.M.; Platt, H.M.; Somerfield, P.J. Monhysterids: Pictorial Key to World Genera and Notes for the Identification of British Species; The Linnean Society of London: London, UK, 1998. [Google Scholar]
- Wetzel, M.A.; Weber, A.; Giere, O. Re-colonization of anoxic/sulfidic sediments by marine nematodes after experimental removal of macroalgal cover. Mar. Biol. 2002, 141, 679–689. [Google Scholar] [CrossRef]
- Wieser, W. Die Beziehung zwischen Mundhohlengestalt, Ernahrungsweise und Vorkommen bei freilebenden marinen Nematoden. Ark. Zool. 1953, 4, 439–484. [Google Scholar]
- Yao, P.; Zhou, B.; Lu, Y.H.; Yin, Y.; Zong, Y.Q.; Chen, M.T.; O’Donnell, Z. A review of microplastics in sediments: Spatial and temporal occurrences, biological effects, and analytic methods. Quat. Int. 2019, 519, 274–281. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).