The Need for Properly Designed Synthesized Micro- and Nanoplastics with Core–Shell Structure
Abstract
1. Introduction
2. The Motivation for the Appropriate Design of Synthesized MNPs
2.1. The Availability of Reference MNP Particles from Certification Bodies and Other Providers
2.2. The Preparation of MNP Particles through a Mechanical Degradation Process (Also Known as “Top-Down”)
3. The Preparation of MNP Particles through a Chemical Synthesis (Also Known as “Bottom-Up”)
3.1. The Theoretical Background to Polymer Particle Synthesis
3.2. Examples of the Design of Synthesized MNP Particles
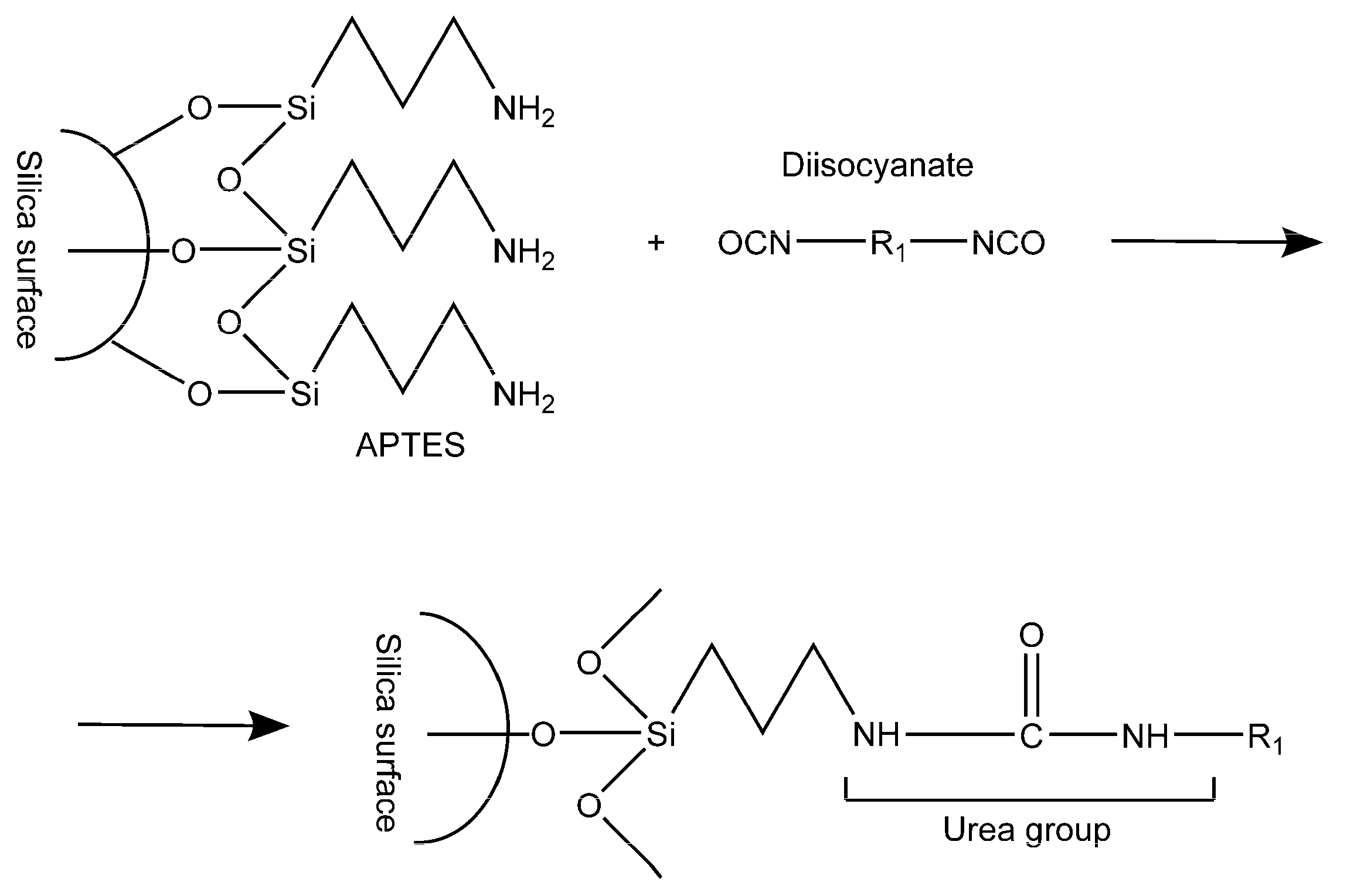
3.2.1. Polyurethane (PUR)
3.2.2. Polymethyl Methacrylate (PMMA)
3.2.3. Polyvinyl Chloride (PVC)
3.2.4. Polystyrene (PS)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Schubert, U.S. From polymers or colloids to polymers and colloids. Colloid Polym. Sci. 2020, 298, 1609–1610. [Google Scholar] [CrossRef]
- Mitrano, D.M.; Wick, P.; Nowack, B. Placing nanoplastics in the context of global plastic pollution. Nat. Nanotechnol. 2021, 16, 491–500. [Google Scholar] [CrossRef] [PubMed]
- ISO 4484-2:2023; Textiles and Textile Products—Microplastics from Textile Sources—Part 2: Qualitative and Quantitative Analysis of Microplastics. International Organization for Standardization: Geneva, Switzerland, 2023.
- ISO/TR 21960:2020; Plastics—Environmental Aspects—State of Knowledge and Methodologies. International Organization for Standardization: Geneva, Switzerland, 2020.
- Gigault, J.; Halle, A.T.; Baudrimont, M.; Pascal, P.-Y.; Gauffre, F.; Phi, T.-L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current opinion: What is a nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, N.B.; Hüffer, T.; Thompson, R.C.; Hassellöv, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.; Brennholt, N.; Cole, M.; et al. Are We Speaking the Same Language? Recommendations for a Definition and Categorization Framework for Plastic Debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Gigault, J.; El Hadri, H.; Nguyen, B.; Grassl, B.; Rowenczyk, L.; Tufenkji, N.; Feng, S.; Wiesner, M. Nanoplastics are neither microplastics nor engineered nanoparticles. Nat. Nanotechnol. 2021, 16, 501–507. [Google Scholar] [CrossRef]
- Cai, H.; Xu, E.G.; Du, F.; Li, R.; Liu, J.; Shi, H. Analysis of environmental nanoplastics: Progress and challenges. Chem. Eng. J. 2021, 410, 128208. [Google Scholar] [CrossRef]
- Gruber, E.S.; Stadlbauer, V.; Pichler, V.; Resch-Fauster, K.; Todorovic, A.; Meisel, T.C.; Trawoeger, S.; Hollóczki, O.; Turner, S.D.; Wadsak, W.; et al. To Waste or Not to Waste: Questioning Potential Health Risks of Micro- and Nanoplastics with a Focus on Their Ingestion and Potential Carcinogenicity. Expo. Health 2023, 15, 33–51. [Google Scholar] [CrossRef]
- Mortula, M.M.; Atabay, S.; Fattah, K.P.; Madbuly, A. Leachability of microplastic from different plastic materials. J. Environ. Manag. 2021, 294, 112995. [Google Scholar] [CrossRef]
- Zhou, X.-X.; Liu, R.; Hao, L.-T.; Liu, J.-F. Identification of polystyrene nanoplastics using surface enhanced Raman spectroscopy. Talanta 2021, 221, 121552. [Google Scholar] [CrossRef]
- Fang, C.; Sobhani, Z.; Zhang, X.; McCourt, L.; Routley, B.; Gibson, C.T.; Naidu, R. Identification and visualisation of microplastics / nanoplastics by Raman imaging (iii): Algorithm to cross-check multi-images. Water Res. 2021, 194, 116913. [Google Scholar] [CrossRef]
- Dong, S.; Cai, W.; Xia, J.; Sheng, L.; Wang, W.; Liu, H. Aggregation kinetics of fragmental PET nanoplastics in aqueous environment: Complex roles of electrolytes, pH and humic acid. Environ. Pollut. 2021, 268, 115828. [Google Scholar] [CrossRef]
- Mitrano, D.M.; Beltzung, A.; Frehland, S.; Schmiedgruber, M.; Cingolani, A.; Schmidt, F. Synthesis of metal-doped nanoplastics and their utility to investigate fate and behaviour in complex environmental systems. Nat. Nanotechnol. 2019, 14, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Senfter, T.; Walter, A.; Dür, L.; Alber, F.; Pillei, M. Do We Speak the Same Language for Reference Particles in Microplastic Research? Microplastics 2022, 1, 221–228. [Google Scholar] [CrossRef]
- Parker, L.A.; Höppener, E.M.; Van Amelrooij, E.F.; Henke, S.; Kooter, I.M.; Grigoriadi, K.; Nooijens, M.G.A.; Brunner, A.M.; Boersma, A. Protocol for the production of micro- and nanoplastic test materials. Microplast. Nanoplast. 2023, 3, 10. [Google Scholar] [CrossRef]
- Mitrano, D.M.; Diamond, M.L.; Kim, J.-H.; Tam, K.C.; Yang, M.; Wang, Z. Balancing New Approaches and Harmonized Techniques in Nano- and Microplastics Research. ACS Sustain. Chem. Eng. 2023, 11, 8702–8705. [Google Scholar] [CrossRef]
- Mitrano, D.M.; Wohlleben, W. Microplastic regulation should be more precise to incentivize both innovation and environmental safety. Nat. Commun. 2020, 11, 5324. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Yang, T.; Mitrano, D.M.; Heuberger, M.; Hufenus, R.; Nowack, B. Systematic Study of Microplastic Fiber Release from 12 Different Polyester Textiles during Washing. Environ. Sci. Technol. 2020, 54, 4847–4855. [Google Scholar] [CrossRef]
- Chen, Q.; Shi, G.; Revell, L.E.; Zhang, J.; Zuo, C.; Wang, D.; Le Ru, E.C.; Wu, G.; Mitrano, D.M. Long-range atmospheric transport of microplastics across the southern hemisphere. Nat. Commun. 2023, 14, 7898. [Google Scholar] [CrossRef]
- Rahman, A.; Sarkar, A.; Yadav, O.P.; Achari, G.; Slobodnik, J. Potential human health risks due to environmental exposure to nano- and microplastics and knowledge gaps: A scoping review. Sci. Total Environ. 2021, 757, 143872. [Google Scholar] [CrossRef]
- Daniel, D.B.; Ashraf, P.M.; Thomas, S.N. Abundance, characteristics and seasonal variation of microplastics in Indian white shrimps (Fenneropenaeus indicus) from coastal waters off Cochin, Kerala, India. Sci. Total Environ. 2020, 737, 139839. [Google Scholar] [CrossRef]
- Kutralam-Muniasamy, G.; Pérez-Guevara, F.; Elizalde-Martínez, I.; Shruti, V.C. Branded milks—Are they immune from microplastics contamination? Sci. Total Environ. 2020, 714, 136823. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.; Rahman, M.M.; Hossain, M.N.; Uddin, M.K.; Malafaia, G. Are there plastic particles in my sugar? A pioneering study on the characterization of microplastics in commercial sugars and risk assessment. Sci. Total Environ. 2022, 837, 155849. [Google Scholar] [CrossRef] [PubMed]
- Oßmann, B.E. Microplastics in drinking water? Present state of knowledge and open questions. Curr. Opin. Food Sci. 2021, 41, 44–51. [Google Scholar] [CrossRef]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool: A Prospective Case Series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef]
- Mastad, O.C.L. Microplastic Studies in Humans: Evidence from Feces and Effects in Blood. Master’s Thesis, University of Bergen, Bergen, Norway, 2022. [Google Scholar]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.; Ehrlich, L.; Henrich, W.; Koeppel, S.; Lomako, I.; Schwabl, P.; Liebmann, B. Detection of Microplastic in Human Placenta and Meconium in a Clinical Setting. Pharmaceutics 2021, 13, 921. [Google Scholar] [CrossRef] [PubMed]
- Amato-Lourenço, L.F.; Carvalho-Oliveira, R.; Júnior, G.R.; Dos Santos Galvão, L.; Ando, R.A.; Mauad, T. Presence of airborne microplastics in human lung tissue. J. Hazard. Mater. 2021, 416, 126124. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Gao, J.; Yu, H.; Su, H.; Yang, Y.; Cao, Y.; Zhang, Q.; Ren, Y.; Hollert, H.; Shi, H.; et al. An emerging role of microplastics in the etiology of lung ground glass nodules. Environ. Sci. Eur. 2022, 34, 25. [Google Scholar] [CrossRef]
- Leslie, H.A.; Van Velzen, M.J.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Ragusa, A.; Notarstefano, V.; Svelato, A.; Belloni, A.; Gioacchini, G.; Blondeel, C.; Zucchelli, E.; De Luca, C.; D’Avino, S.; Gulotta, A.; et al. Raman Microspectroscopy Detection and Characterisation of Microplastics in Human Breastmilk. Polymers 2022, 14, 2700. [Google Scholar] [CrossRef]
- Hendriks, L.; Mitrano, D.M. Direct Measurement of Microplastics by Carbon Detection via Single Particle ICP-TOFMS in Complex Aqueous Suspensions. Environ. Sci. Technol. 2023, 57, 7263–7272. [Google Scholar] [CrossRef] [PubMed]
- Reference Material BAM-P201 Artificially Aged Polyethylene (PE); The Federal Institute for Materials Research and Testing (BAM): Berlin, Germany, 2019; p. 4.
- Reference Material BAM-P202 Polystyrene (PS); The Federal Institute for Materials Research and Testing (BAM): Berlin, Germany, 2021; p. 4.
- Reference Material BAM-P206 Polyethylene Terephthalate (Microplastic Powder); The Federal Institute for Materials Research and Testing (BAM): Berlin, Germany, 2023; p. 5.
- Standard Reference Material 1690 Polystyrene Spheres 1 µm; National Institute of Standards and Technology (NIST): Gaithersburg, MD, USA, 2016; p. 4.
- Standard Reference Material 1691 Polystyrene Spheres 0.3 µm; National Institute of Standards and Technology (NIST): Gaithersburg, MD, USA, 2016; p. 3.
- Standard Reference Material 1961 Polystyrene Spheres 30 µm; National Institute of Standards and Technology (NIST): Gaithersburg, MD, USA, 1987; p. 2.
- Standard Reference Material 1963a Polystyrene Spheres 100 nm; National Institute of Standards and Technology (NIST): Gaithersburg, MD, USA, 2019; p. 5.
- Reference Material 8017 Polyvinylpyrrolidone Coated Silver Nanoparticles 75 nm; National Institute of Standards and Technology (NIST): Gaithersburg, MD, USA, 2015; p. 10.
- Ramsperger, A.F.R.M.; Jasinski, J.; Völkl, M.; Witzmann, T.; Meinhart, M.; Jérôme, V.; Kretschmer, W.P.; Freitag, R.; Senker, J.; Fery, A.; et al. Supposedly identical microplastic particles substantially differ in their material properties influencing particle-cell interactions and cellular responses. J. Hazard. Mater. 2022, 425, 127961. [Google Scholar] [CrossRef] [PubMed]
- Biale, G.; La Nasa, J.; Mattonai, M.; Corti, A.; Castelvetro, V.; Modugno, F. Seeping plastics: Potentially harmful molecular fragments leaching out from microplastics during accelerated ageing in seawater. Water Res. 2022, 219, 118521. [Google Scholar] [CrossRef] [PubMed]
- Wieland, S.; Balmes, A.; Bender, J.; Kitzinger, J.; Meyer, F.; Ramsperger, A.F.; Roeder, F.; Tengelmann, C.; Wimmer, B.H.; Laforsch, C.; et al. From properties to toxicity: Comparing microplastics to other airborne microparticles. J. Hazard. Mater. 2022, 428, 128151. [Google Scholar] [CrossRef] [PubMed]
- Magrì, D.; Sánchez-Moreno, P.; Caputo, G.; Gatto, F.; Veronesi, M.; Bardi, G.; Catelani, T.; Guarnieri, D.; Athanassiou, A.; Pompa, P.P.; et al. Laser Ablation as a Versatile Tool To Mimic Polyethylene Terephthalate Nanoplastic Pollutants: Characterization and Toxicology Assessment. ACS Nano 2018, 12, 7690–7700. [Google Scholar] [CrossRef] [PubMed]
- Kanehara, I.; Yamashita, H.; Fujii, S.; Kimura, T.; Yamamoto, M.; Tanabe, T. Nano-Sized Polyethylene Particles Produced by Nano-Second UV Laser Ablation. Lasers Manuf. Mater. Process. 2023, 10, 389–399. [Google Scholar] [CrossRef]
- Dong, H.; Huang, X.; Wu, Z.; Li, P.; Silvain, J.-F.; Hussain, K.A.; Cui, B.; Li, Y.; Lu, Y. Generation of Nano-to-Microplastics from Polypropylene Surfaces via Femtosecond Laser Ablation in Liquids with Different Viscosities. Appl. Surf. Sci. 2024, 670, 160661. [Google Scholar] [CrossRef]
- González-Pleiter, M.; Novak, P.J.; Vikesland, P.J. Secondary nanoplastics released from a biodegradable microplastic severely impact freshwater environments. Environ. Sci. Process. Impacts 2020, 22, 860–862. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Chen, B.; He, B.; Wu, H.; Liu, A. Microplastic degradations in simulated UV light, natural light and natural water body: A comparison investigation. Emerg. Contam. 2024, 10, 100306. [Google Scholar] [CrossRef]
- Lozano, Y.M.; Gordillo-Rocha, H.; Waldman, W.R.; Rillig, M.C. Photodegradation modifies microplastic effects on soil properties and plant performance. J. Appl. Ecol. 2024, 61, 13–24. [Google Scholar] [CrossRef]
- Baudrimont, M.; Arini, A.; Guégan, C.; Venel, Z.; Gigault, J.; Pedrono, B.; Prunier, J.; Maurice, L.; Ter Halle, A.; Feurtet-Mazel, A. Ecotoxicity of polyethylene nanoplastics from the North Atlantic oceanic gyre on freshwater and marine organisms (microalgae and filter-feeding bivalves). Environ. Sci. Pollut. Res. 2020, 27, 3746–3755. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Brito, W.A.; Ravandeh, M.; Saadati, F.; Singer, D.; Dorsch, A.D.; Schmidt, A.; Cecchini, A.L.; Wende, K.; Bekeschus, S. Sonicated polyethylene terephthalate nano- and micro-plastic-induced inflammation, oxidative stress, and autophagy in vitro. Chemosphere 2024, 355, 141813. [Google Scholar] [CrossRef]
- El Hadri, H.; Gigault, J.; Maxit, B.; Grassl, B.; Reynaud, S. Nanoplastic from mechanically degraded primary and secondary microplastics for environmental assessments. NanoImpact 2020, 17, 100206. [Google Scholar] [CrossRef]
- Kalogerakis, N.; Karkanorachaki, K.; Kalogerakis, G.C.; Triantafyllidi, E.I.; Gotsis, A.D.; Partsinevelos, P.; Fava, F. Microplastics Generation: Onset of Fragmentation of Polyethylene Films in Marine Environment Mesocosms. Front. Mar. Sci. 2017, 4, 84. [Google Scholar] [CrossRef]
- Ducoli, S.; Federici, S.; Nicsanu, R.; Zendrini, A.; Marchesi, C.; Paolini, L.; Radeghieri, A.; Bergese, P.; Depero, L.E. A different protein corona cloaks “true-to-life” nanoplastics with respect to synthetic polystyrene nanobeads. Environ. Sci. Nano 2022, 9, 1414–1426. [Google Scholar] [CrossRef]
- Ducoli, S.; Federici, S.; Cocca, M.; Gentile, G.; Zendrini, A.; Bergese, P.; Depero, L.E. Characterization of polyethylene terephthalate (PET) and polyamide (PA) true-to-life nanoplastics and their biological interactions. Environ. Pollut. 2024, 343, 123150. [Google Scholar] [CrossRef] [PubMed]
- De Macêdo Neto, J.C.; De Freitas, B.M.; De Miranda, A.G.; De Almeida Rodrigues, R.; Del Pino, G.G.; Kieling, A.C.; Dos Santos, M.D.; Duvoisin Junior, S.; Sanches, A.E.; Gondres Torné, I.; et al. The Stability and Properties of Polystyrene/Kaolinite Nanocomposites during Synthesis via Emulsion Polymerization. Polymers 2023, 15, 2094. [Google Scholar] [CrossRef]
- Beck, S.; Narain, R. Polymer synthesis. In Polymer Science and Nanotechnology; Elsevier: North York, ON, Canada, 2020; pp. 21–85. [Google Scholar] [CrossRef]
- Yuan, H.G.; Kalfas, G.; Ray, W.H. Suspension Polymerization. J. Macromol. Sci. Part C Polym. Rev. 1991, 31, 215–299. [Google Scholar] [CrossRef]
- Tu, X.; Chen, W. A Review on the Recent Progress in Matrix Solid Phase Dispersion. Molecules 2018, 23, 2767. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Rist, S.; Bodin, J.; Jensen, L.H.; Schmidt, S.N.; Mayer, P.; Meibom, A.; Baun, A. Microplastics as vectors for environmental contaminants: Exploring sorption, desorption, and transfer to biota. Integr. Environ. Assess. Manag. 2017, 13, 488–493. [Google Scholar] [CrossRef]
- Cai, L.; Hu, L.; Shi, H.; Ye, J.; Zhang, Y.; Kim, H. Effects of inorganic ions and natural organic matter on the aggregation of nanoplastics. Chemosphere 2018, 197, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Oriekhova, O.; Stoll, S. Heteroaggregation of nanoplastic particles in the presence of inorganic colloids and natural organic matter. Environ. Sci. Nano 2018, 5, 792–799. [Google Scholar] [CrossRef]
- Wu, J.; Jiang, R.; Lin, W.; Ouyang, G. Effect of salinity and humic acid on the aggregation and toxicity of polystyrene nanoplastics with different functional groups and charges. Environ. Pollut. 2019, 245, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Reynaud, S.; Aynard, A.; Grassl, B.; Gigault, J. Nanoplastics: From model materials to colloidal fate. Curr. Opin. Colloid Interface Sci. 2022, 57, 101528. [Google Scholar] [CrossRef]
- Lasareva, E.V.; Parfenova, A.M.; Demina, T.S.; Romanova, N.D.; Belyaev, N.A.; Romankevich, E.A. Transport of the colloid matter of riverine runoff through estuaries. Oceanology 2017, 57, 520–529. [Google Scholar] [CrossRef]
- Kokalj, A.J.; Hartmann, N.B.; Drobne, D.; Potthoff, A.; Kühnel, D. Quality of nanoplastics and microplastics ecotoxicity studies: Refining quality criteria for nanomaterial studies. J. Hazard. Mater. 2021, 415, 125751. [Google Scholar] [CrossRef]
- Hernandez, L.M.; Yousefi, N.; Tufenkji, N. Are There Nanoplastics in Your Personal Care Products? Environ. Sci. Technol. Lett. 2017, 4, 280–285. [Google Scholar] [CrossRef]
- Hernandez, L.M.; Xu, E.G.; Larsson, H.C.E.; Tahara, R.; Maisuria, V.B.; Tufenkji, N. Plastic Teabags Release Billions of Microparticles and Nanoparticles into Tea. Environ. Sci. Technol. 2019, 53, 12300–12310. [Google Scholar] [CrossRef]
- Sobhani, Z.; Zhang, X.; Gibson, C.; Naidu, R.; Megharaj, M.; Fang, C. Identification and visualisation of microplastics/nanoplastics by Raman imaging (i): Down to 100 nm. Water Res. 2020, 174, 115658. [Google Scholar] [CrossRef]
- Ivleva, N.P.; Wiesheu, A.C.; Niessner, R. Microplastic in Aquatic Ecosystems. Angew. Chem. Int. Ed. 2017, 56, 1720–1739. [Google Scholar] [CrossRef]
- Primpke, S.; Christiansen, S.H.; Cowger, W.; De Frond, H.; Deshpande, A.; Fischer, M.; Holland, E.B.; Meyns, M.; O’Donnell, B.A.; Ossmann, B.E.; et al. Critical Assessment of Analytical Methods for the Harmonized and Cost-Efficient Analysis of Microplastics. Appl. Spectrosc. 2020, 74, 1012–1047. [Google Scholar] [CrossRef] [PubMed]
- Cowger, W.; Gray, A.; Christiansen, S.H.; DeFrond, H.; Deshpande, A.D.; Hemabessiere, L.; Lee, E.; Mill, L.; Munno, K.; Ossmann, B.E.; et al. Critical Review of Processing and Classification Techniques for Images and Spectra in Microplastic Research. Appl. Spectrosc. 2020, 74, 989–1010. [Google Scholar] [CrossRef]
- Lu, S.; Zhu, K.; Song, W.; Song, G.; Chen, D.; Hayat, T.; Alharbi, N.S.; Chen, C.; Sun, Y. Impact of water chemistry on surface charge and aggregation of polystyrene microspheres suspensions. Sci. Total Environ. 2018, 630, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, L.; Mei, Q.; Dong, B.; Dai, X.; Ding, G.; Zeng, E.Y. Microplastics in sewage sludge from the wastewater treatment plants in China. Water Res. 2018, 142, 75–85. [Google Scholar] [CrossRef]
- Kusch, P. Application of Pyrolysis-Gas Chromatography/Mass Spectrometry (Py-GC/MS). In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2017; Volume 75, pp. 169–207. [Google Scholar] [CrossRef]
- Keller, A.S.; Jimenez-Martinez, J.; Mitrano, D.M. Transport of Nano- and Microplastic through Unsaturated Porous Media from Sewage Sludge Application. Environ. Sci. Technol. 2020, 54, 911–920. [Google Scholar] [CrossRef]
- Dümichen, E.; Eisentraut, P.; Bannick, C.G.; Barthel, A.-K.; Senz, R.; Braun, U. Fast identification of microplastics in complex environmental samples by a thermal degradation method. Chemosphere 2017, 174, 572–584. [Google Scholar] [CrossRef]
- Blum, Y.D.; Kambe, N.; Brent MacQueen, D.; Kumar, S.; Chiruvolu, S.; Chaloner-Gill, B. Nanocomposites by Covalent Bonding between Inorganic Nanoparticles and Polymers. MRS Proc. 2001, 676, Y1.8. [Google Scholar] [CrossRef]
- Boven, G. Grafting kinetics of poly(methyl methacrylate) on microparticulate silica. Polymer 1990, 31, 2377–2383. [Google Scholar] [CrossRef]
- Radhakrishnan, B.; Cloutet, E.; Cramail, H. Synthesis of uniform polyurethane particles by step growth polymerization in a dispersed medium. Colloid Polym. Sci. 2002, 280, 1122–1130. [Google Scholar] [CrossRef]
- Ziegler, W. Polyurethan-Nanocomposite als Werkstoffe zur Schwingungsisolierung. Ph.D. Thesis, Montanuniversität Leoben, Leoben, Austria, 2019. [Google Scholar]
- Alves, P.; Ferreira, P.; Gil, M.H. Biomedical Polyurethanes-Based Materials; Nova Science Publishers, Inc.: Porto, Portugal, 2012. [Google Scholar]
- Xiu, Z.; Yang, M.; Wu, R.; Lei, C.; Ren, H.-M.; Yu, B.; Gao, S.; Duan, S.; Wu, D.; Xu, F.-J. Scalable anti-infection polyurethane catheters with long-acting and autoclavable properties. Chem. Eng. J. 2023, 451, 138495. [Google Scholar] [CrossRef]
- Curtis, T.; Taylor, A.K.; Alden, S.E.; Swanson, C.; Lo, J.; Knight, L.; Silva, A.; Gates, B.D.; Emory, S.R.; Rider, D.A. Synthesis and Characterization of Tunable, pH-Responsive Nanoparticle–Microgel Composites for Surface-Enhanced Raman Scattering Detection. ACS Omega 2018, 3, 10572–10588. [Google Scholar] [CrossRef] [PubMed]
- Li, W.S.J.; Ladmiral, V.; Takeshima, H.; Satoh, K.; Kamigaito, M.; Semsarilar, M.; Negrell, C.; Lacroix-Desmazes, P.; Caillol, S. Ferulic acid-based reactive core–shell latex by seeded emulsion polymerization. Polym. Chem. 2019, 10, 3116–3126. [Google Scholar] [CrossRef]
- Rauschendorfer, R.J.; Whitham, K.M.; Summer, S.; Patrick, S.A.; Pierce, A.E.; Sefi-Cyr, H.; Tadjiki, S.; Kraft, M.D.; Emory, S.R.; Rider, D.A.; et al. Development and Application of Nanoparticle-Nanopolymer Composite Spheres for the Study of Environmental Processes. Front. Toxicol. 2021, 3, 752296. [Google Scholar] [CrossRef] [PubMed]
- Kaltenegger-Uray, A.; Rieß, G.; Lucyshyn, T.; Holzer, C.; Kern, W. Physical Foaming and Crosslinking of Polyethylene with Modified Talcum. Polymers 2019, 11, 1472. [Google Scholar] [CrossRef] [PubMed]
- Revel, M.; Lagarde, F.; Perrein-Ettajani, H.; Bruneau, M.; Akcha, F.; Sussarellu, R.; Rouxel, J.; Costil, K.; Decottignies, P.; Cognie, B.; et al. Tissue-Specific Biomarker Responses in the Blue Mussel mytilus spp. Exposed to a Mixture of Microplastics at Environmentally Relevant Concentrations. Front. Environ. Sci. 2019, 7, 33. [Google Scholar] [CrossRef]
- Facchetti, S.V.; La Spina, R.; Fumagalli, F.; Riccardi, N.; Gilliland, D.; Ponti, J. Detection of Metal-Doped Fluorescent PVC Microplastics in Freshwater Mussels. Nanomaterials 2020, 10, 2363. [Google Scholar] [CrossRef] [PubMed]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase Liquid–Liquid system. J. Chem. Soc. Chem. Commun. 1994, 7, 801–802. [Google Scholar] [CrossRef]
- Cassano, D.; Bogni, A.; La Spina, R.; Gilliland, D.; Ponti, J. Investigating the Cellular Uptake of Model Nanoplastics by Single-Cell ICP-MS. Nanomaterials 2023, 13, 594. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, B.; Ranjan, R.; Brittain, W.J. Surface initiated polymerizations from silica nanoparticles. Soft Matter 2006, 2, 386. [Google Scholar] [CrossRef]
- Prucker, O.; Rühe, J. Prucker Mechanism of Radical Chain Polymerizations Initiated by Azo Compounds Covalently Bound to the Surface of Spherical Particles. Macromolecules 1998, 31, 602–613. [Google Scholar] [CrossRef]
- Prucker, O.; Rühe, J. Prucker Synthesis of Poly(styrene) Monolayers Attached to High Surface Area Silica Gels through Self-Assembled Monolayers of Azo Initiators. Macromolecules 1998, 31, 592–601. [Google Scholar] [CrossRef]
- Galakhova, A. Polyols Bearing Azosulphonate Units as Novel Additives for Polyurethane Foams. Ph.D. Thesis, Montanuniversität Leoben, Leoben, Austria, 2019. [Google Scholar]
- Nothdurft, P.; Schauberger, J.; Riess, G.; Kern, W. Preparation of a Water-Based Photoreactive Azosulphonate-Doped Poly(Vinyl Alcohol) and the Investigation of Its UV Response. Polymers 2019, 11, 169. [Google Scholar] [CrossRef] [PubMed]
- Al-Sid-Cheikh, M.; Rowland, S.J.; Kaegi, R.; Henry, T.B.; Cormier, M.-A.; Thompson, R.C. Synthesis of 14C-labelled polystyrene nanoplastics for environmental studies. Commun. Mater. 2020, 1, 97. [Google Scholar] [CrossRef]
- Lu, S.; Qu, R.; Forcada, J. Preparation of magnetic polymeric composite nanoparticles by seeded emulsion polymerization. Mater. Lett. 2009, 63, 770–772. [Google Scholar] [CrossRef]
- Vicentini, D.S.; Nogueira, D.J.; Melegari, S.P.; Arl, M.; Köerich, J.S.; Cruz, L.; Justino, N.M.; Oscar, B.V.; Puerari, R.C.; Da Silva, M.L.N.; et al. Toxicological Evaluation and Quantification of Ingested Metal-Core Nanoplastic by Daphnia magna through Fluorescence and Inductively Coupled Plasma-Mass Spectrometric Methods. Environ. Toxic Chem. 2019, 38, 2101–2110. [Google Scholar] [CrossRef] [PubMed]
- Burton, R.A.; Gidley, M.J.; Fincher, G.B. Heterogeneity in the chemistry, structure and function of plant cell walls. Nat. Chem. Biol. 2010, 6, 724–732. [Google Scholar] [CrossRef]
- Luo, Y.; Li, L.; Feng, Y.; Li, R.; Yang, J.; Peijnenburg, W.J.G.M.; Tu, C. Quantitative tracing of uptake and transport of submicrometre plastics in crop plants using lanthanide chelates as a dual-functional tracer. Nat. Nanotechnol. 2022, 17, 424–431. [Google Scholar] [CrossRef] [PubMed]
- PlasticsEurope. Plastics—The Facts 2022; PlasticsEurope: Brussels, Belgium, 2022; p. 80. [Google Scholar]
- Ziani, K.; Ioniță-Mîndrican, C.-B.; Mititelu, M.; Neacșu, S.M.; Negrei, C.; Moroșan, E.; Drăgănescu, D.; Preda, O.-T. Microplastics: A Real Global Threat for Environment and Food Safety: A State of the Art Review. Nutrients 2023, 15, 617. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, L.; Gerace, M.H.; Booth, A.M. Small micro- and nanoplastic test and reference materials for research: Current status and future needs. Camb. Prism. Plast. 2024, 2, e13. [Google Scholar] [CrossRef]

| Plastic Type | Index | Certificate Details | Particle Size | Particle Use |
|---|---|---|---|---|
| PE | BAM-P201 | Artificially aged polyethylene (PE). Particle size distribution (by laser diffraction), carbonyl index (by FTIR), and enthalpy (by DSC). | 17.9 µm 61.2 µm 156.6 µm | Validation of sampling, sample preparation and detection of microplastics in the field of ecotoxicology or human toxicology, and pollutant transport and agglomeration behavior. |
| PS | BAM-P202 | Polystyrene (PS) powder. Particle size distribution (by laser diffraction), and average value spectrum (by FTIR and DSC). | 91 µm 206 µm 311 µm | |
| PET | BAM-P206 | Polyethylene terephthalate (PET) powder. Equivalent particle diameter with standard deviation (by laser diffraction), SEM image of particles, and ATR-FTIR spectrum. DSC spectrum. Average values for glass transition and melting temperatures. | 30.5 µm 62.6 µm 107 µm | |
| PS | SRM 1691 | Number average particle determined by laser diffraction. | 0.3 µm | Primary particle size reference standard for the calibration of particle-size-measuring instruments including electron microscopes. |
| PS | SRM 1961 | Number of average particles measured in air by center distance finding, and optical technique related to array sizing. | 30 µm | Primary particle size reference standard for the calibration of particle-measuring instruments including flow-through counters, and optical and electron microscopes. |
| PS | SRM 1963a | Size probability distribution. | 100 nm | Calibration/validation of particle-sizing instruments, including electron microscopes, differential mobility analyzers, scanning surface inspection systems, and other light scattering instruments. |
| PVP | RM 8017 | Polyvinylpyrrolidone (PVP)-coated silver nanoparticles. Particle size (by dynamic light scattering, ultra-small-angle X-ray scattering, and transmission electron and atomic-force microscopy (TEM and AFM)). Ultraviolet–visible absorbance spectrum. Values from isotope dilution mass spectrometry, asymmetric-flow field flow fractionation, and zeta potential and pH of suspension. | 75 nm | Benchmark and investigative tool for the evaluation of potential environmental, health, and safety risks that may be associated with manufactured nanomaterials during their product lifetime. |
| MNP Type | MNP Size | Positive Assessment of the Study |
|---|---|---|
| PUR MNP | 0.2–5 µm | Narrow size distribution [83,84] |
| PS NP | 21.5 nm | High-molecular-weight polymer brushes with high graft density were successfully produced. Kinetic studies of the polymerization process showed that the initiation and propagation of the polymer at low conversion was similar to that of solution polymerization [95,96,97]. |
| PS NP Radio–labeled PS NP | 20 nm | One-step surfactant-free polymerization process. Radio-labeling approach provides valuable procedure. Behavior of synthesized polystyrene nanoplastic (PS NP) using a common initiator represents the behavior of PS NP found in the environment [100]. |
| Metal-doped PS NP | 150–170 nm | The chemical bond between acrylonitrile and palladium and the core–shell structure ensure that there is minimal leaching of metal from the particle (eight weeks of stability). The shell could be modified independently of the core (i.e., different styrene morphologies and/or different polymers could be added to the shell). The metal-doping tracer in NP has been designed for detection in complex matrices by inductively coupled plasma–mass spectrometry (ICP–MS) [14,79]. |
| Fluorescent-labeled PS NP | 94.5 nm | Two methods are proposed for the quantification of NP in organisms: the addition of fluorescent monomer to NP for fluorescence light microscopy and the addition of aluminum as a metal core of NP for ICP–MS. NP was functionalized with palmitic acid to simulate environmental conditions [101,102]. |
| Metal-doped PS MNP Metal-doped PMMA MNP | 300–500 nm | Photoreduction resulted in solid gold particles growing within the core of the NP, ensuring that the gold does not leach out into solution. Metal-doping tracers were developed with recognizable isotopic, metallic, fluorescent signatures for detection in complex matrices by ICP–MS: a single-particle ICP–MS. Synthetic approach produces well-defined and monodisperse materials and provides potential for multiplexing. Core–shell structures allow the environmental matrix to interact only with a shell polymer composition (besides PS and polymethyl methacrylate (PMMA), other vinyl-based polymers such as butyl rubber, natural rubber, and vinyl chloride can be used as shells). The ability to adjust the hydrophilicity of the cores by adjusting the pH of the solution may allow the inclusion of more hydrophilic polymers as the shell material [82,87,89]. |
| Metal-doped PS MNP | 244 nm | The synthesis is not complicated. In addition to PS, the method can be extended to other types of NPs found in the environment. The labeling technology used in this study could also be applied in microcosm or mesocosm experiments to increase the sensitivity of NP detection. Indirect quantification and visualization of NPs in plant tissues were performed by fluorescence of europium (Eu) element doping. Due to the time-dependent luminescence of Eu chelates, Eu chelate doping also allows background-free fluorescence imaging. PS–Eu particles prepared with Eu chelate were chosen as luminophore because of their high luminescence quantum yield, stability, and solubility in aqueous buffers [101,103,104]. |
| Fluorescent metal-doped PVC MP | 100 µm | This provides a benefit to the scientific community as polyvinyl chloride microplastic (PVC MP) is in the minority of particles studied compared to other types of plastics. Synthesis of a new MP tracer for detection in complex matrices (optical method, ICP–MS, and total reflection X-rays fluorescence). An important advantage is that the fluorescence of MP is not limited to a single excitation band (like most MPs used in the literature), but to two (blue and green light). This makes them an ideal material for identification studies of microplastics in natural auto-fluorescent samples, as they are perfectly distinguishable from any other endogenous or xenobiotic particles often present in the matrix (such as algae). Platinum octaethylporphyrin is a tracer suitable for following the bioaccumulation of plastics in organisms. The release of platinum octaethylporphyrin and platinum during analysis can be largely excluded as the dye is insoluble in aqueous media [92]. |
| Metal-doped PVC NP Metal-doped PE NP | 50–350 nm | Synthesized environmentally relevant NP. Gold nanoparticles were chosen as the dopant because they are considered to be chemically stable, relatively easy to obtain, interference-free for elemental analysis; and suitable for bio-applications and as a tracer for detection by ICP–MS. Successful metal doping distributed throughout the volume of the NP. Narrow size distribution. The presence of metal doping did not alter the Raman spectra of polyethylene (PE) and PVC due to the small size of the metal-doping particles. Metal-doping content in NP was sufficient to produce a detectable individual by single-cell ICP–MS [93,94]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galakhova, A.; Meisel, T.C.; Riess, G. The Need for Properly Designed Synthesized Micro- and Nanoplastics with Core–Shell Structure. Microplastics 2024, 3, 433-448. https://doi.org/10.3390/microplastics3030027
Galakhova A, Meisel TC, Riess G. The Need for Properly Designed Synthesized Micro- and Nanoplastics with Core–Shell Structure. Microplastics. 2024; 3(3):433-448. https://doi.org/10.3390/microplastics3030027
Chicago/Turabian StyleGalakhova, Anastasiia, Thomas C. Meisel, and Gisbert Riess. 2024. "The Need for Properly Designed Synthesized Micro- and Nanoplastics with Core–Shell Structure" Microplastics 3, no. 3: 433-448. https://doi.org/10.3390/microplastics3030027
APA StyleGalakhova, A., Meisel, T. C., & Riess, G. (2024). The Need for Properly Designed Synthesized Micro- and Nanoplastics with Core–Shell Structure. Microplastics, 3(3), 433-448. https://doi.org/10.3390/microplastics3030027








