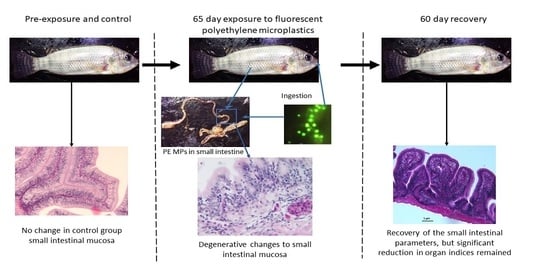
Histomorphological Damage in the Small Intestine of Wami Tilapia (Oreochromis urolepis) (Norman, 1922) Exposed to Microplastics Remain Long after Depuration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish Husbandry
2.2. PE MPs Characteristics and Preparation
2.3. Fish Exposure to and Recovery from PE MPs
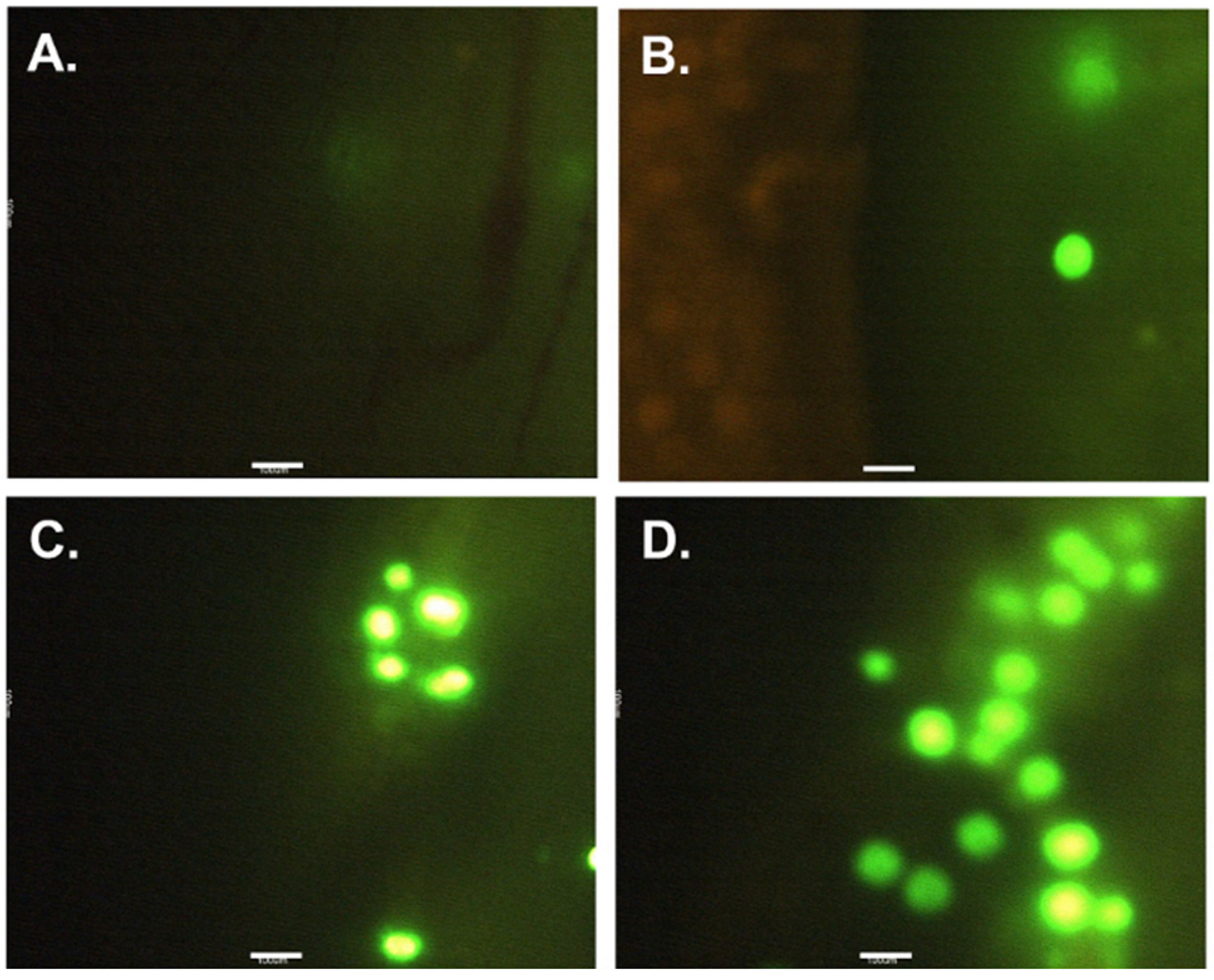
2.4. Examination of PE MPs Ingestion and Retention
2.5. Intestinal Histomorphological Change Examination
2.6. Statistical Analyses
3. Results
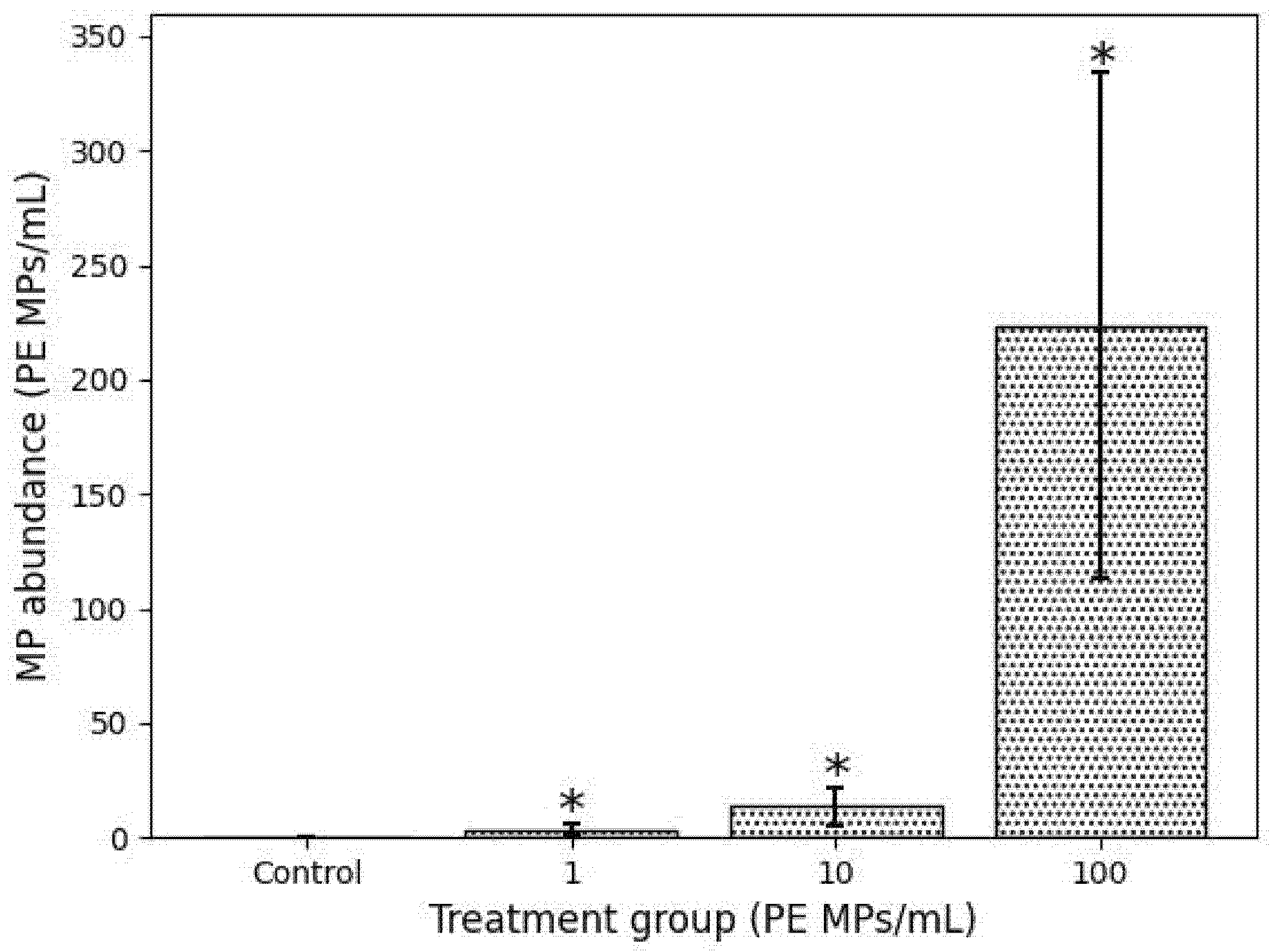
3.1. Ingestion and Retention of PE MPs after Exposure and Recovery Phase
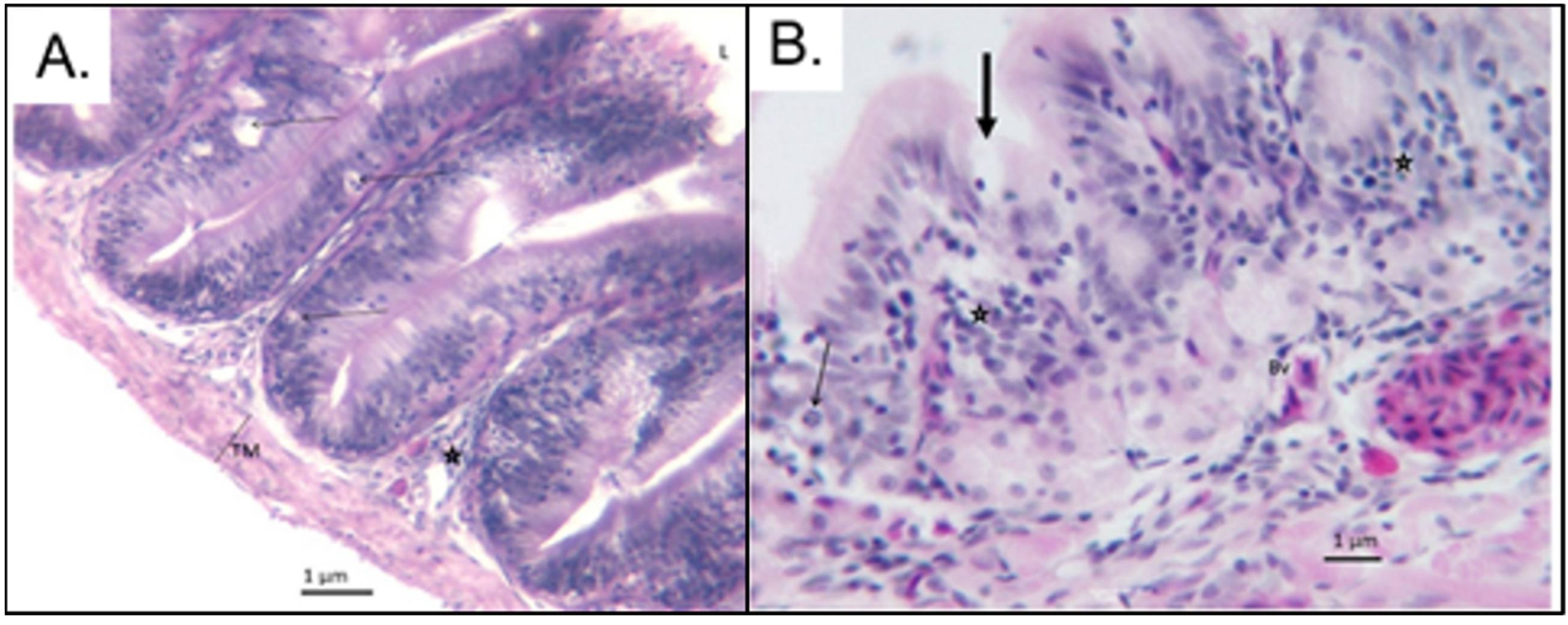
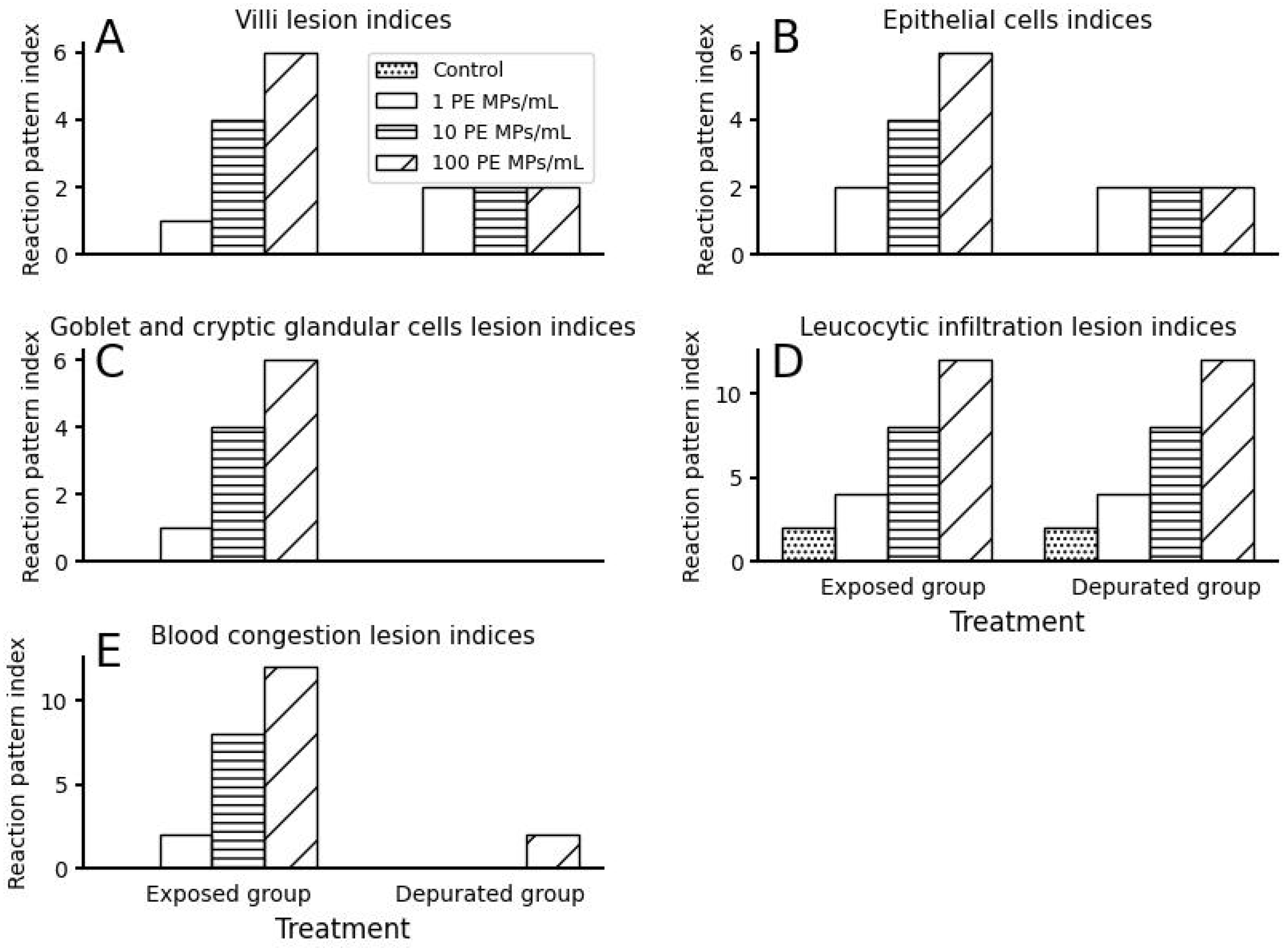
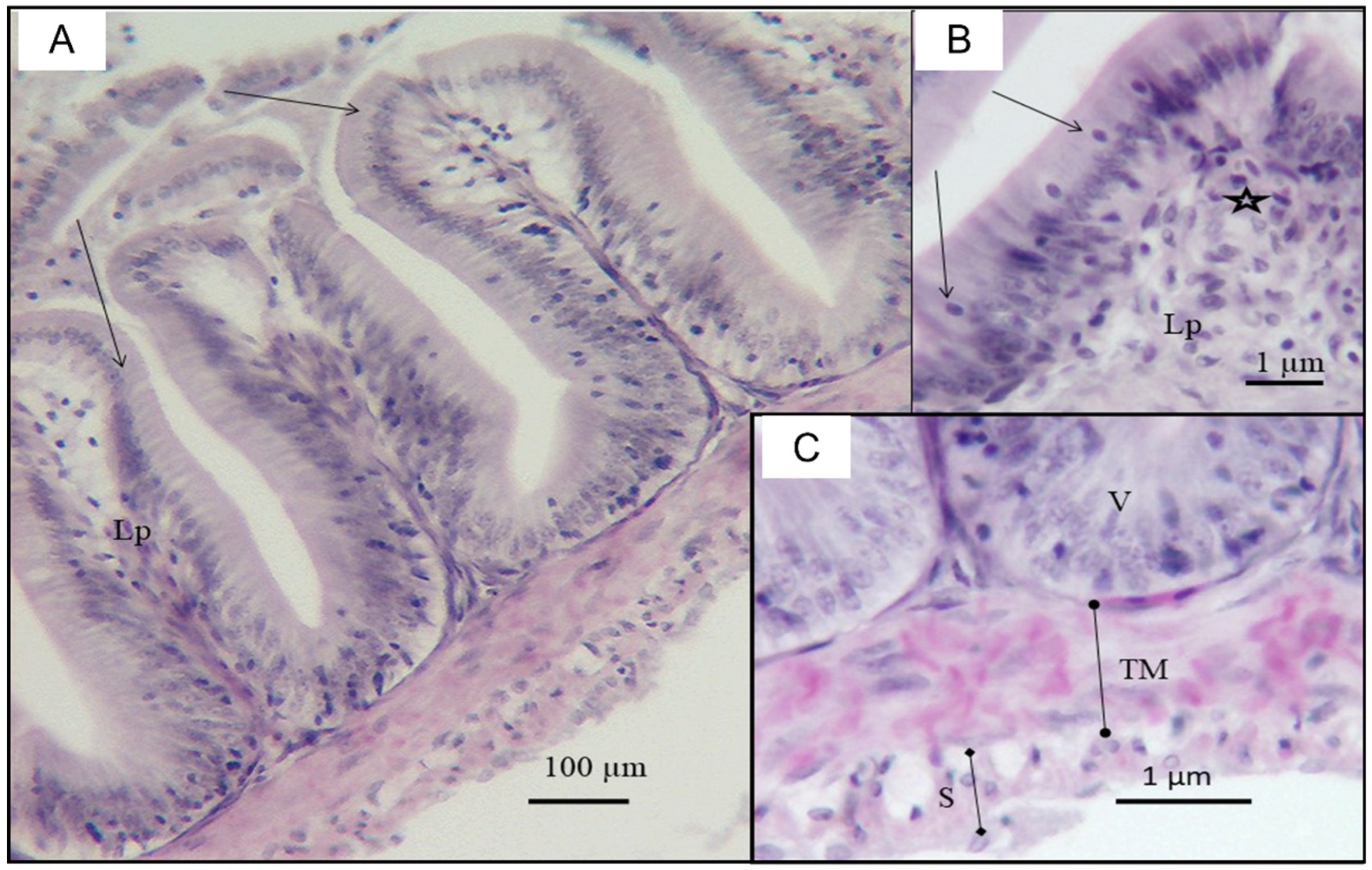
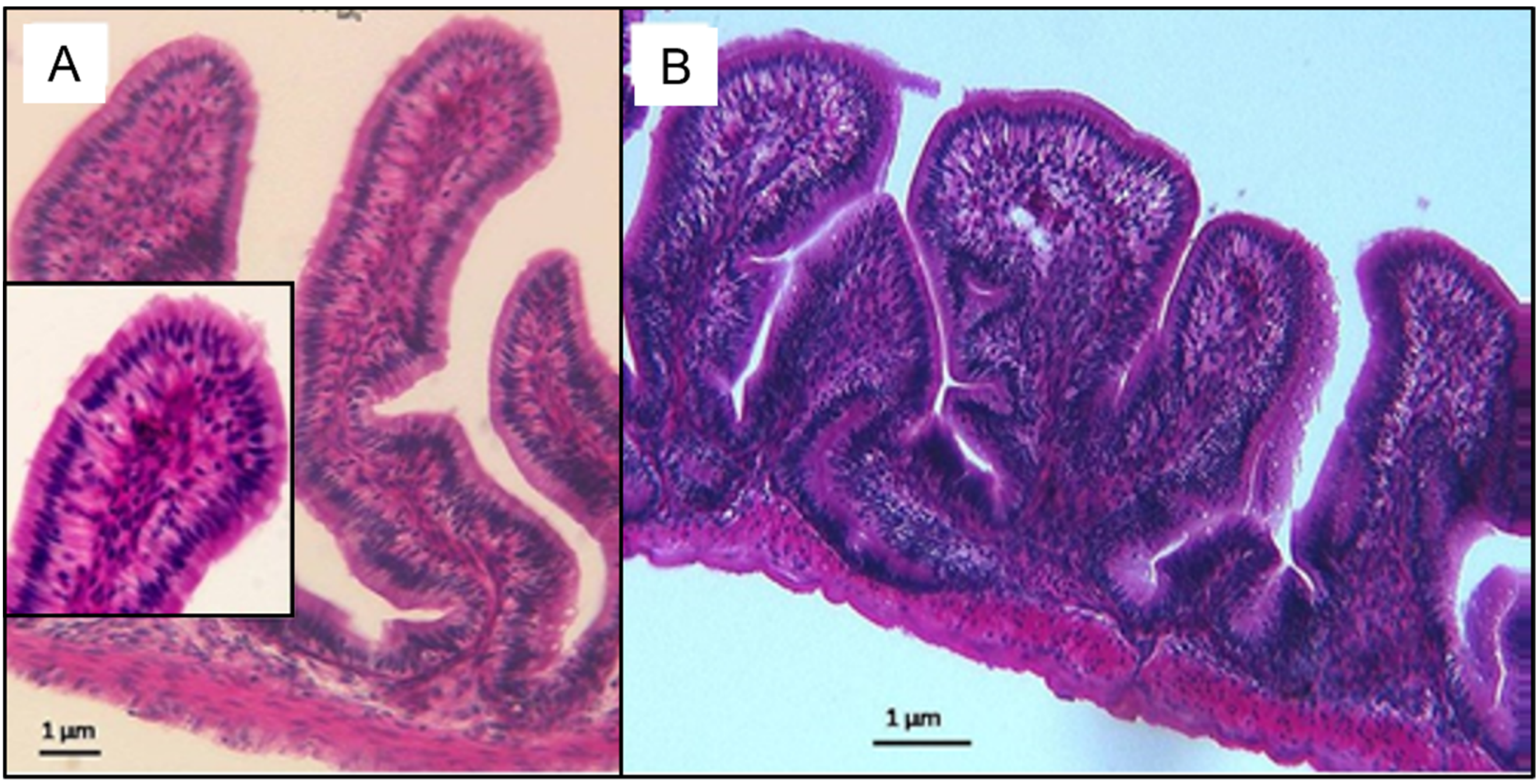
3.2. Small Intestinal Histomorphological Changes after Exposure and Depuration Phase
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, F.R.; Mayoma, B.S.; Biginagwa, F.J.; Syberg, K. Microplastics in inland African waters: Presence, sources, and fate. Freshw. Microplastics 2018, 58, 101–124. [Google Scholar]
- Shilla, D. Status updates on plastics pollution in aquatic environment of Tanzania: Data Availability, Current Challenges and Future Research Needs. Tanzan. J. Sci. 2019, 45, 101–113. [Google Scholar]
- Onyango, P. Socio-economic characteristics of the Lake Victoria Fisheries. In Lake Victoria Fisheries Resources; Springer: Berlin/Heidelberg, Germany, 2017; Volume 93, pp. 161–184. [Google Scholar]
- Biginagwa, F.J.; Mayoma, B.S.; Shashoua, Y.; Syberg, K.; Khan, F.R. First evidence of microplastics in the African Great Lakes: Recovery from Lake Victoria Nile perch and Nile tilapia. J. Great Lakes Res. 2016, 42, 146–149. [Google Scholar] [CrossRef]
- Khan, F.R.; Shashoua, Y.; Crawford, A.; Drury, A.; Sheppard, K.; Stewart, K.; Sculthorp, T. ‘The Plastic Nile’: First Evidence of Microplastic Contamination in Fish from the Nile River (Cairo, Egypt). Toxics 2020, 8, 22. [Google Scholar] [CrossRef] [Green Version]
- Merga, L.B.; Redondo-Hasselerharm, P.E.; Van den Brink, P.J.; Koelmans, A.A. Distribution of microplastic and small macroplastic particles across four fish species and sediment in an African lake. Sci. Total Environ. 2020, 741, 140527. [Google Scholar] [CrossRef]
- Rochman, C.M.; Hoh, E.; Kurobe, T.; Teh, S.J. Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress. Sci. Rep. 2013, 3, 3263. [Google Scholar] [CrossRef]
- Kashiwada, S. Distribution of nanoparticles in the see-through medaka (Oryzias latipes). Environ. Health Perspect. 2006, 114, 1697–1702. [Google Scholar] [CrossRef] [Green Version]
- Pedà, C.; Caccamo, L.; Fossi, M.C.; Gai, F.; Andaloro, F.; Genovese, L.; Perdichizzi, A.; Romeo, T.; Maricchiolo, G. Intestinal alterations in European sea bass Dicentrarchus labrax (Linnaeus, 1758) exposed to microplastics: Preliminary results. Environ. Pollut. 2016, 212, 251–256. [Google Scholar] [CrossRef]
- Ahrendt, C.; Perez-Venegas, D.J.; Urbina, M.; Gonzalez, C.; Echeveste, P.; Aldana, M.; Pulgar, J.; Galban-Malagon, C. Microplastic ingestion cause intestinal lesions in the intertidal fish Girella laevifrons. Mar. Pollut. Bull. 2020, 151, 110795. [Google Scholar] [CrossRef]
- Lei, L.; Wu, S.; Lu, S.; Liu, M.; Song, Y.; Fu, Z.; Shi, H.; Raley-Susman, K.M.; He, D. Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Sci. Total Environ. 2018, 619–620, 1–8. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, J.; Pan, Z.; Wang, S.; Zhang, L.; Wang, Q.; Ye, Q.; Zhou, A.; Xie, S.; Zeng, F.; et al. A dosage-effect assessment of acute toxicology tests of microplastic exposure in filter-feeding fish. Fish Shellfish Immunol. 2021, 113, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Solomando, A.; Capo, X.; Alomar, C.; Alvarez, E.; Compa, M.; Valencia, J.M.; Pinya, S.; Deudero, S.; Sureda, A. Long-term exposure to microplastics induces oxidative stress and a pro-inflammatory response in the gut of Sparus aurata Linnaeus, 1758. Environ. Pollut. 2020, 266 Pt 1, 115295. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, M.Y.; Feng, X.S.; Li, X.X.; Wen, B.; Liu, J.H.; Huang, J.N.; Gao, J.Z.; Chen, Z.Z. Microplastics intake and excretion: Resilience of the intestinal microbiota but residual growth inhibition in common carp. Chemosphere 2021, 276, 130144. [Google Scholar] [CrossRef]
- Calil, J.; Gutiérrez-Graudiņš, M.; Munguía, S.; Chin, C. Neglected: Environmental Justice Impacts of Marine Litter and Plastic Pollution; Technical Report; UN Environment (UNEP): Nairobi, Kenya, 7 April 2021. [Google Scholar]
- Alessi, E.; Di Carlo, G.; Di Carlo, E.B.; Campogianni, S. Out of the Plastic Trap: Saving the Mediterranean from Plastic Pollution; WWF Report: Rome, Italy, 2018. [Google Scholar]
- URT. Regulation on Prohibition of Plastics Carrier Bags; Government of United Republic of Tanzania Mainland: Dodoma, Tanzaniam, 2019; Volume 394. [Google Scholar]
- Directive, E. Reduction of the impact of certain plastic products on the environment. Off. J. Eur. Union 2019, L155. Available online: https://www.eumonitor.eu/9353000/1/j9vvik7m1c3gyxp/vkzb7kgzwlzz (accessed on 12 June 2019).
- Maione, C. Quantifying plastics waste accumulations on coastal tourism sites in Zanzibar, Tanzania. Mar. Pollut. Bull. 2021, 168, 112418. [Google Scholar] [CrossRef] [PubMed]
- Chale, F. Studies on the fisheries and biology of Oreochromis urolepis (pisces: Cichlidae) in the Mtera reservoir (Tanzania). Tanzan. J. Sci. 2004, 30, 33–39. [Google Scholar]
- Mapenzi, L.L.; Mmochi, A.J. Effect of stocking density on growth performance of hybrid of Oreochromis niloticus and Oreochromis urolepis urolepis in saline water. WIO J. Mar. Sci. 2016, 15, 67–74. [Google Scholar]
- Zhang, K.; Xiong, X.; Hu, H.; Wu, C.; Bi, Y.; Wu, Y.; Zhou, B.; Lam, P.K.; Liu, J. Occurrence and Characteristics of Microplastic Pollution in Xiangxi Bay of Three Gorges Reservoir, China. Environ. Sci. Technol. 2017, 51, 3794–3801. [Google Scholar] [CrossRef]
- Popma, T.; Masser, M. Tilapia Life History and Biology. South. Reg. Aquac. Cent. 1990, 283, 4. [Google Scholar]
- Khan, F.R.; Syberg, K.; Shashoua, Y.; Bury, N.R. Influence of polyethylene microplastic beads on the uptake and localization of silver in zebrafish (Danio rerio). Environ. Pollut. 2015, 206, 73–79. [Google Scholar] [CrossRef]
- Wang, W.; Gao, H.; Jin, S.; Li, R.; Na, G. The ecotoxicological effects of microplastics on aquatic food web, from primary producer to human: A review. Ecotoxicol. Environ. Saf. 2019, 173, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Karami, A.; Golieskardi, A.; Choo, C.K.; Romano, N.; Ho, Y.B.; Salamatinia, B. A high-performance protocol for extraction of microplastics in fish. Sci. Total Environ. 2017, 578, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Bernet, D.; Schmidt, H.; Meier, W.; Burkhardt-Hom, P.; Wahhli, T. Histopathology in fish: Proposal for a protocol to assess aquatic pollution. Fish Dis. 1999, 22, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Ding, J.; Zhang, S.; Razanajatovo, R.M.; Zou, H.; Zhu, W. Accumulation, tissue distribution, and biochemical effects of polystyrene microplastics in the freshwater fish red tilapia (Oreochromis niloticus). Environ. Pollut. 2018, 238, 1–9. [Google Scholar] [CrossRef]
- Wang, Y.; Mao, Z.; Zhang, M.; Ding, G.; Sun, J.; Du, M.; Liu, Q.; Cong, Y.; Jin, F.; Zhang, W.; et al. The uptake and elimination of polystyrene microplastics by the brine shrimp, Artemia parthenogenetica, and its impact on its feeding behavior and intestinal histology. Chemosphere 2019, 234, 123–131. [Google Scholar] [CrossRef]
- Pitt, J.A.; Kozal, J.S.; Jayasundara, N.; Massarsky, A.; Trevisan, R.; Geitner, N.; Wiesner, M.; Levin, E.D.; Di Giulio, R.T. Uptake, tissue distribution, and toxicity of polystyrene nanoparticles in developing zebrafish (Danio rerio). Aquat. Toxicol. 2018, 194, 185–194. [Google Scholar] [CrossRef]
- Santana, M.F.M.; Dawson, A.L.; Motti, C.A.; van Herwerden, L.; Lefevre, C.; Kroon, F.J. Ingestion and Depuration of Microplastics by a Planktivorous Coral Reef Fish, Pomacentrus amboinensis. Front. Environ. Sci. 2021, 9, 1–16. [Google Scholar] [CrossRef]
- Qiao, R.; Deng, Y.; Zhang, S.; Wolosker, M.B.; Zhu, Q.; Ren, H.; Zhang, Y. Accumulation of different shapes of microplastics initiates intestinal injury and gut microbiota dysbiosis in the gut of zebrafish. Chemosphere 2019, 236, 124334. [Google Scholar] [CrossRef]
- Hamed, M.; Soliman, H.A.M.; Badrey, A.E.A.; Osman, A.G.M. Microplastics induced histopathological lesions in some tissues of tilapia (Oreochromis niloticus) early juveniles. Tissue Cell 2021, 71, 101512. [Google Scholar] [CrossRef]
- Qiao, R.; Sheng, C.; Lu, Y.; Zhang, Y.; Ren, H.; Lemos, B. Microplastics induce intestinal inflammation, oxidative stress, and disorders of metabolome and microbiome in zebrafish. Sci. Total Environ. 2019, 662, 246–253. [Google Scholar] [CrossRef]
- Liu, Y.; Qiu, X.; Xu, X.; Takai, Y.; Ogawa, H.; Shimasaki, Y.; Oshima, Y. Uptake and depuration kinetics of microplastics with different polymer types and particle sizes in Japanese medaka (Oryzias latipes). Ecotoxicol. Environ. Saf. 2021, 212, 112007. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Sun, M.; Zhou, M.; Chang, Z.; Li, L. Polyvinyl chloride microplastics induce growth inhibition and oxidative stress in Cyprinus carpio var. larvae. Sci. Total Environ. 2020, 716, 136479. [Google Scholar] [CrossRef] [PubMed]
- Mizraji, R.; Ahrendt, C.; Perez-Venegas, D.; Vargas, J.; Pulgar, J.; Ojeda, F.P.; Duarte, C.; Galbán-Malagón, C. Is the feeding type related with the content of microplastics in intertidal fish gut? Mar. Pollut. Bull. 2017, 116, 498–500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, J.; Zhou, A.; Ye, Q.; Feng, Y.; Wang, Z.; Wang, S.; Xu, G.; Zou, J. Species-specific effect of microplastics on fish embryos and observation of toxicity kinetics in larvae. J. Hazard. Mater. 2021, 403, 123948. [Google Scholar] [CrossRef]
- Hamed, M.; Soliman, H.A.M.; Osman, A.G.M.; Sayed, A.E.H. Assessment the effect of exposure to microplastics in Nile Tilapia (Oreochromis niloticus) early juvenile: I. blood biomarkers. Chemosphere 2019, 228, 345–350. [Google Scholar] [CrossRef]
- Tran-Duy, A.; Schrama, J.W.; van Dam, A.A.; Verreth, J.A.J. Effects of oxygen concentration and body weight on maximum feed intake, growth and hematological parameters of Nile tilapia, Oreochromis niloticus. Aquaculture 2008, 275, 152–162. [Google Scholar] [CrossRef]
- Batel, A.; Baumann, L.; Carteny, C.C.; Cormier, B.; Keiter, S.H.; Braunbeck, T. Histological, enzymatic and chemical analyses of the potential effects of differently sized microplastic particles upon long-term ingestion in zebrafish (Danio rerio). Mar. Pollut. Bull. 2020, 153, 111022. [Google Scholar] [CrossRef]
- De Sales-Ribeiro, C.; Brito-Casillas, Y.; Fernandez, A.; Caballero, M.J. An end to the controversy over the microscopic detection and effects of pristine microplastics in fish organs. Sci. Rep. 2020, 10, 12434. [Google Scholar] [CrossRef]
- Huang, J.N.; Wen, B.; Meng, L.J.; Li, X.X.; Wang, M.H.; Gao, J.Z.; Chen, Z.Z. Integrated response of growth, antioxidant defense and isotopic composition to microplastics in juvenile guppy (Poecilia reticulata). J. Hazard. Mater. 2020, 399, 123044. [Google Scholar] [CrossRef]


| Importance Factor | Description |
|---|---|
| 1 | Minimal pathological importance, the lesion is easily reversible as irritant ends |
| 2 | Moderate pathological importance, the lesion is reversible in most cases if the stressor is neutralized |
| 3 | Marked pathological importance, the lesion is generally irreversible |
| Score Value | Description |
|---|---|
| 1 | Unchanged |
| 2 | Mild occurrence |
| 3 | Moderate occurrence |
| 4 | Severe occurrence |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mbugani, J.J.; Machiwa, J.F.; Shilla, D.A.; Kimaro, W.; Joseph, D.; Khan, F.R. Histomorphological Damage in the Small Intestine of Wami Tilapia (Oreochromis urolepis) (Norman, 1922) Exposed to Microplastics Remain Long after Depuration. Microplastics 2022, 1, 240-253. https://doi.org/10.3390/microplastics1020017
Mbugani JJ, Machiwa JF, Shilla DA, Kimaro W, Joseph D, Khan FR. Histomorphological Damage in the Small Intestine of Wami Tilapia (Oreochromis urolepis) (Norman, 1922) Exposed to Microplastics Remain Long after Depuration. Microplastics. 2022; 1(2):240-253. https://doi.org/10.3390/microplastics1020017
Chicago/Turabian StyleMbugani, John J., John F. Machiwa, Daniel A. Shilla, Wahabu Kimaro, Dativa Joseph, and Farhan R. Khan. 2022. "Histomorphological Damage in the Small Intestine of Wami Tilapia (Oreochromis urolepis) (Norman, 1922) Exposed to Microplastics Remain Long after Depuration" Microplastics 1, no. 2: 240-253. https://doi.org/10.3390/microplastics1020017
APA StyleMbugani, J. J., Machiwa, J. F., Shilla, D. A., Kimaro, W., Joseph, D., & Khan, F. R. (2022). Histomorphological Damage in the Small Intestine of Wami Tilapia (Oreochromis urolepis) (Norman, 1922) Exposed to Microplastics Remain Long after Depuration. Microplastics, 1(2), 240-253. https://doi.org/10.3390/microplastics1020017