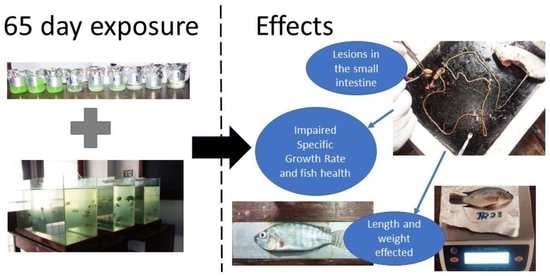
Impaired Growth Performance of Wami Tilapia Juveniles (Oreochromis urolepis) (Norman, 1922) Due to Microplastic Induced Degeneration of the Small Intestine
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Larvae (Fries) Production
2.2. PE MPs Exposure Dose Preparation
2.3. Experimental Exposure Set Up
2.4. Examination of PE MPs Ingestion and Determination of Growth Performance
2.5. Small Intestine Histomorphological Lesion Indices Diagnosis
2.6. Data Analysis
3. Results
3.1. Water Quality Parameters
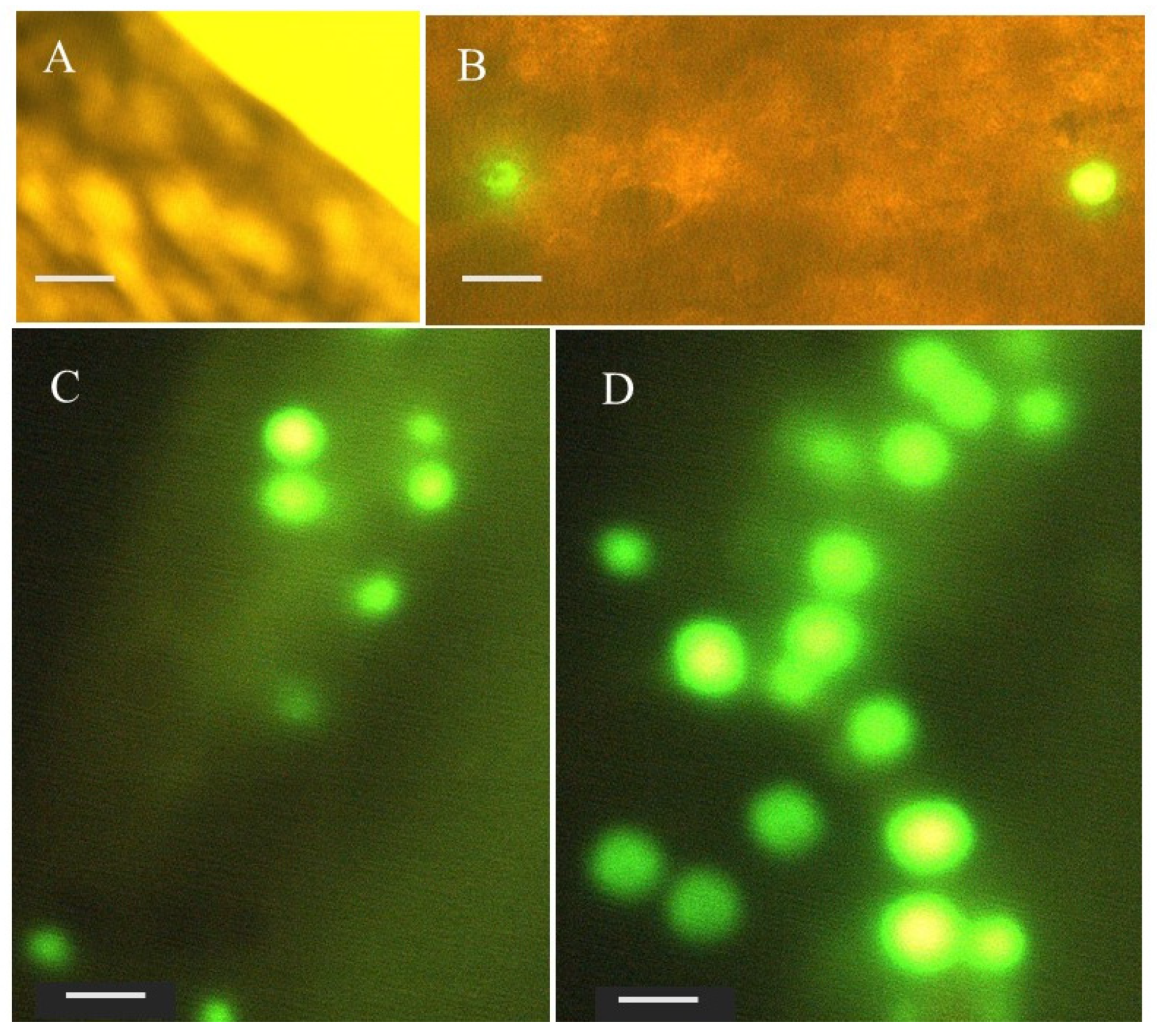
3.2. PE MPs Ingestion and Small Intestines Histomorphological Alteration
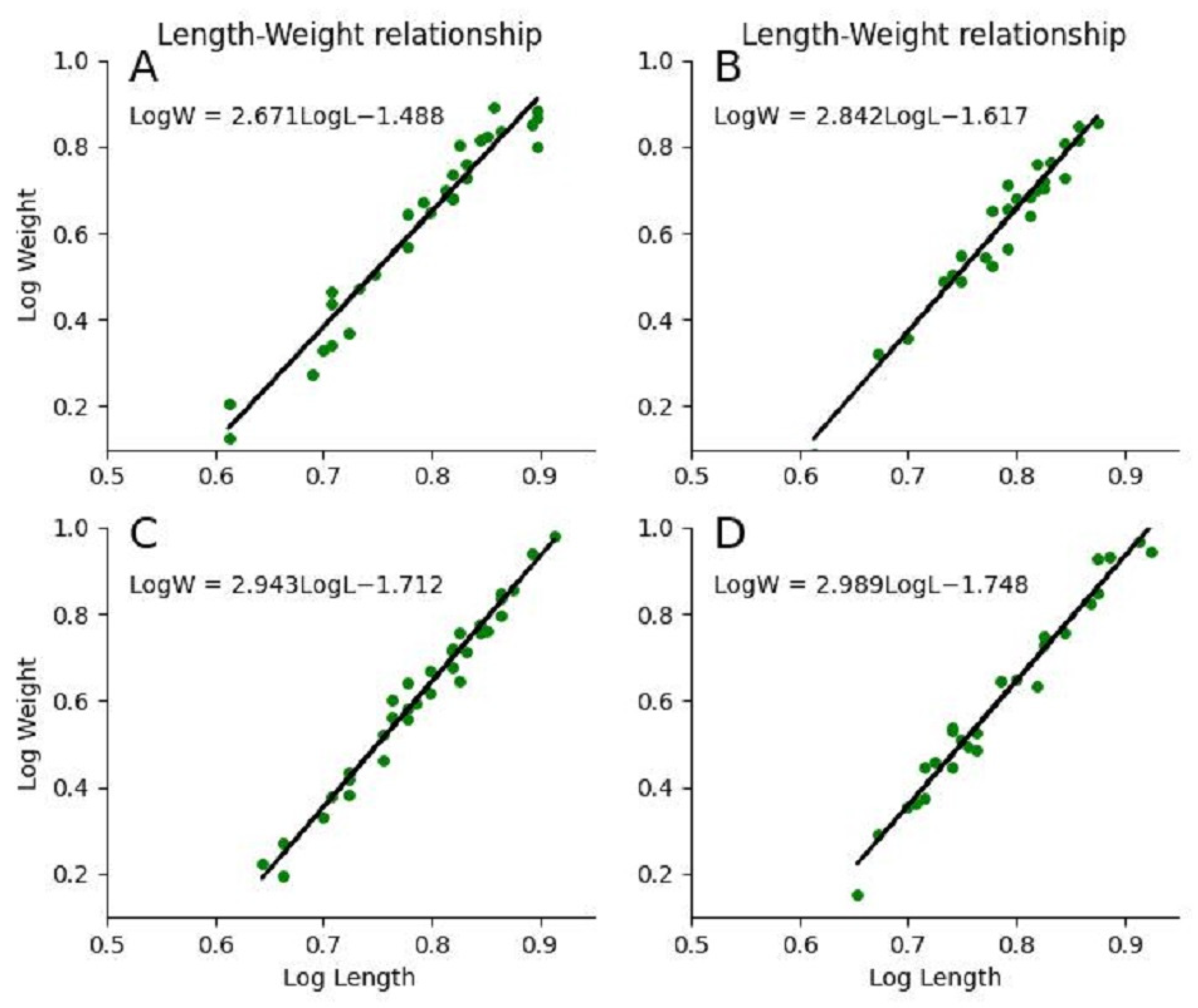
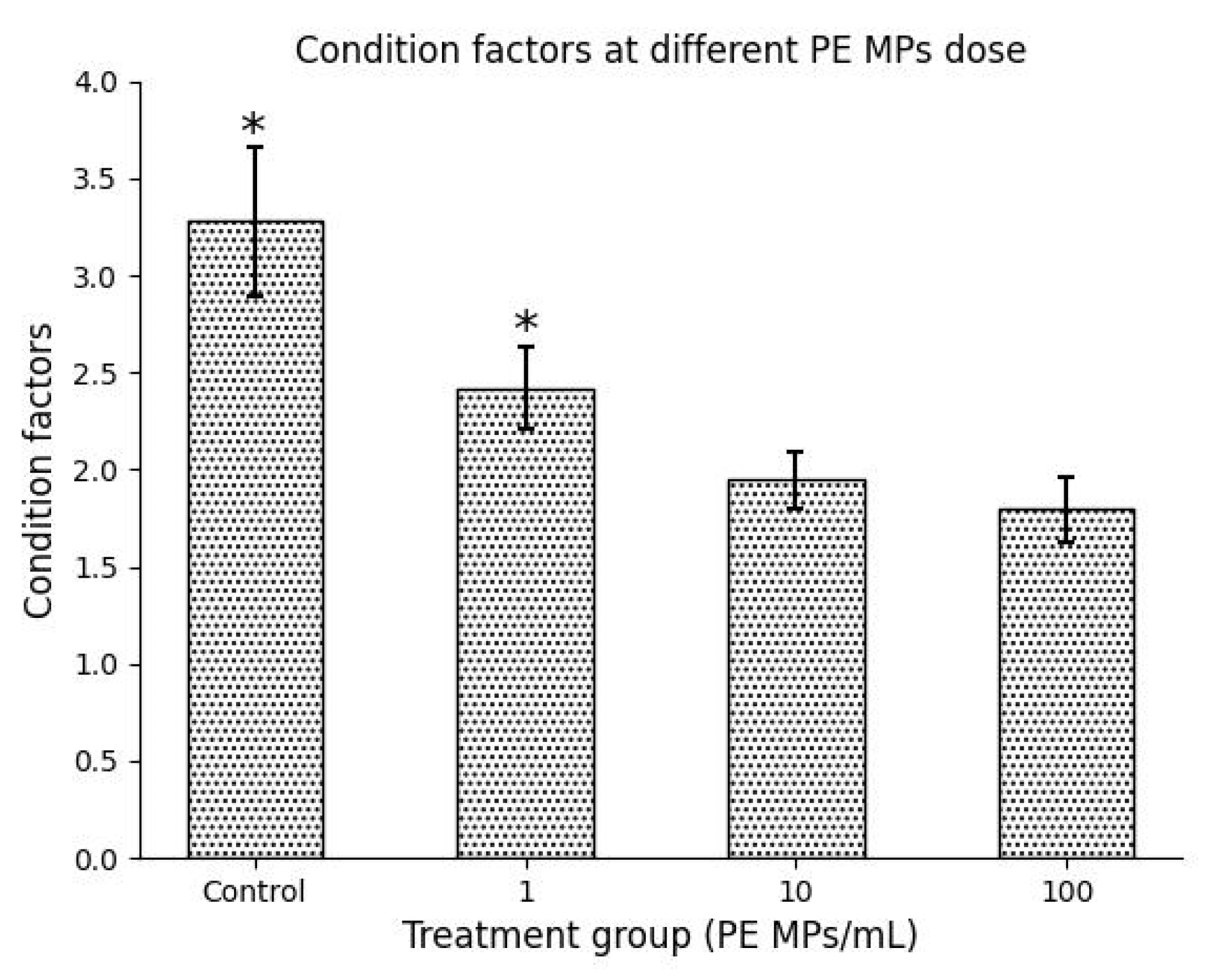
3.3. Effect of Histomorphological Damage on Growth Performance
4. Discussion
4.1. Ingestion of MPs and Associated Histomorphological Changes
4.2. Effect of Histomorphological Alteration on Growth Performance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lusher, A.; Hollman, P.; Mendoza-Hill, J. Microplastics in Fisheries and Aquaculture: Status of Knowledge on Their Occurrence and Implications for Aquatic Organisms and Food Safety; FAO Fisheries and Aquaculture Technical Paper: Rome, Italy, 2017. [Google Scholar]
- Schmidt, C.; Krauth, T.; Wagner, S. Export of Plastic Debris by Rivers into the Sea. Environ. Sci. Technol. 2017, 51, 12246–12253. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.; Ryan, P.G. Micro-plastic ingestion by waterbirds from contaminated wetlands in South Africa. Mar. Pollut. Bull. 2018, 126, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.R.; Shashoua, Y.; Crawford, A.; Drury, A.; Sheppard, K.; Stewart, K.; Sculthorp, T. ‘The Plastic Nile’: First Evidence of Microplastic Contamination in Fish from the Nile River (Cairo, Egypt). Toxics 2020, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Biginagwa, F.J.; Mayoma, B.S.; Shashoua, Y.; Syberg, K.; Khan, F.R. First evidence of microplastics in the African Great Lakes: Recovery from Lake Victoria Nile perch and Nile tilapia. J. Great Lakes Res. 2016, 42, 146–149. [Google Scholar] [CrossRef]
- Yuan, W.; Liu, X.; Wang, W.; Di, M.; Wang, J. Microplastic abundance, distribution and composition in water, sediments, and wild fish from Poyang Lake, China. Ecotoxicol. Environ. Saf. 2019, 170, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Arias, A.H.; Ronda, A.C.; Oliva, A.L.; Marcovecchio, J.E. Evidence of Microplastic Ingestion by Fish from the Bahia Blanca Estuary in Argentina, South America. Bull. Environ. Contam. Toxicol. 2019, 102, 750–756. [Google Scholar] [CrossRef]
- Merga, L.B.; Redondo-Hasselerharm, P.E.; Van den Brink, P.J.; Koelmans, A.A. Distribution of microplastic and small macroplastic particles across four fish species and sediment in an African lake. Sci. Total Environ. 2020, 741, 140527. [Google Scholar] [CrossRef]
- Qiao, R.; Deng, Y.; Zhang, S.; Wolosker, M.B.; Zhu, Q.; Ren, H.; Zhang, Y. Accumulation of different shapes of microplastics initiates intestinal injury and gut microbiota dysbiosis in the gut of zebrafish. Chemosphere 2019, 236, 124334. [Google Scholar] [CrossRef]
- Qiao, R.; Sheng, C.; Lu, Y.; Zhang, Y.; Ren, H.; Lemos, B. Microplastics induce intestinal inflammation, oxidative stress, and disorders of metabolome and microbiome in zebrafish. Sci. Total Environ. 2019, 662, 246–253. [Google Scholar] [CrossRef]
- Solomando, A.; Capo, X.; Alomar, C.; Alvarez, E.; Compa, M.; Valencia, J.M.; Pinya, S.; Deudero, S.; Sureda, A. Long-term exposure to microplastics induces oxidative stress and a pro-inflammatory response in the gut of Sparus aurata Linnaeus, 1758. Environ. Pollut. 2020, 266 Pt 1, 115295. [Google Scholar] [CrossRef]
- Hamed, M.; Soliman, H.A.M.; Osman, A.G.M.; Sayed, A.E.H. Assessment the effect of exposure to microplastics in Nile Tilapia (Oreochromis niloticus) early juvenile: I. blood biomarkers. Chemosphere 2019, 228, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Mbugani, J.J.; Machiwa, J.F.; Shilla, D.A.; Kimaro, W.; Joseph, D.; Khan, F.R. Histomorphological Damage in the Small Intestine of Wami Tilapia (Oreochromis urolepis) (Norman, 1922) Exposed to Microplastics Remain Long after Depuration. Microplastics 2022, 1, 240–253. [Google Scholar] [CrossRef]
- Kosore, C.; Ojwang, L.; Maghanga, J.; Kamau, J.; Kimeli, A.; Omukoto, J.; Ngisiag’e, N.; Mwaluma, J.; Ong’ada, H.; Magori, C.; et al. Occurrence and ingestion of microplastics by zooplankton in Kenya’s marine environment: First documented evidence. Afr. J. Mar. Sci. 2018, 40, 225–234. [Google Scholar] [CrossRef]
- Alessi, E.; Di Carlo, G.; Di Carlo, E.B.; Campogianni, S. Out of the Plastic Trap: Saving the Mediterranean from Plastic Pollution; WWF Mediterranean Marine Initiative: Rome, Italy, 2018. [Google Scholar]
- Zhao, J.; Ran, W.; Teng, J.; Liu, Y.; Liu, H.; Yin, X.; Cao, R.; Wang, Q. Microplastic pollution in sediments from the Bohai Sea and the Yellow Sea, China. Sci. Total Environ. 2018, 640, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Au, S. Toxicity of Microplastics to Aquatic Organisms; TigerPrints: Clemson, SC, USA, 2017. [Google Scholar]
- Zhang, C.; Wang, J.; Zhou, A.; Ye, Q.; Feng, Y.; Wang, Z.; Wang, S.; Xu, G.; Zou, J. Species-specific effect of microplastics on fish embryos and observation of toxicity kinetics in larvae. J. Hazard. Mater. 2021, 403, 123948. [Google Scholar] [CrossRef]
- Popma, T.; Masser, M. Tilapia Life History and Biology. South. Reg. Aquac. Cent. 1999, 283. [Google Scholar] [CrossRef]
- Mizraji, R.; Ahrendt, C.; Perez-Venegas, D.; Vargas, J.; Pulgar, J.; Ojeda, F.P.; Duarte, C.; Galbán-Malagón, C. Is the feeding type related with the content of microplastics in intertidal fish gut? Mar. Pollut. Bull. 2017, 116, 498–500. [Google Scholar] [CrossRef]
- Foley, C.J.; Feiner, Z.S.; Malinich, T.D.; Hook, T.O. A meta-analysis of the effects of exposure to microplastics on fish and aquatic invertebrates. Sci. Total Environ. 2018, 631, 550–559. [Google Scholar] [CrossRef]
- Santillo, D.; Miller, K.; Johnston, P. Microplastics as contaminants in commercially important seafood species. Integr. Environ. Assess. Manag. 2017, 13, 516–521. [Google Scholar] [CrossRef]
- Tran-Duy, A.; Schrama, J.W.; van Dam, A.A.; Verreth, J.A.J. Effects of oxygen concentration and body weight on maximum feed intake, growth and hematological parameters of Nile tilapia, Oreochromis niloticus. Aquaculture 2008, 275, 152–162. [Google Scholar] [CrossRef]
- Anbumani, S.; Kakkar, P. Ecotoxicological effects of microplastics on biota: A review. Environ. Sci. Pollut. Res. 2018, 25, 14373–14396. [Google Scholar] [CrossRef] [PubMed]
- Maganira, J.D.; Nehemia, A.; Rumisha, C. LengthWeight relationship and condition factor of tilapia species grown in marine and freshwater ponds. Agric. Biol. J. N. Am. 2012, 3, 117–124. [Google Scholar] [CrossRef]
- Dadzie, S.; Wangila, B. Reproductive biology, length-weight relationship and relative condition of pond raised Tilapia zilli (Gervais). J. Fish Biol. 1980, 17, 243–253. [Google Scholar] [CrossRef]
- Pegado, T.; Schmid, K.; Winemiller, K.O.; Chelazzi, D.; Cincinelli, A.; Dei, L.; Giarrizzo, T. First evidence of microplastic ingestion by fishes from the Amazon River estuary. Mar. Pollut. Bull. 2018, 133, 814–821. [Google Scholar] [CrossRef]
- Hamed, M.; Soliman, H.A.M.; Badrey, A.E.A.; Osman, A.G.M. Microplastics induced histopathological lesions in some tissues of tilapia (Oreochromis niloticus) early juveniles. Tissue Cell 2021, 71, 101512. [Google Scholar] [CrossRef]
- Ahrendt, C.; Perez-Venegas, D.J.; Urbina, M.; Gonzalez, C.; Echeveste, P.; Aldana, M.; Pulgar, J.; Galban-Malagon, C. Microplastic ingestion cause intestinal lesions in the intertidal fish Girella laevifrons. Mar. Pollut. Bull. 2020, 151, 110795. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Sun, M.; Zhou, M.; Chang, Z.; Li, L. Polyvinyl chloride microplastics induce growth inhibition and oxidative stress in Cyprinus carpio var. larvae. Sci. Total Environ. 2020, 716, 136479. [Google Scholar] [CrossRef]
- Eccles, D.H. A Field Guide to the Freshwater Fishes of Tanzania; FAO: Rome, Italy, 1992. [Google Scholar]
- Watts, A.J.; Lewis, C.; Goodhead, R.M.; Beckett, S.J.; Moger, J.; Tyler, C.R.; Galloway, T.S. Uptake and retention of microplastics by the shore crab Carcinus maenas. Environ. Sci. Technol. 2014, 48, 8823–8830. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Galloway, T.S. The Impact of Polystyrene Microplastics on Feeding, Function and Fecundity in the Marine Copepod Calanus helgolandicus. Environ. Sci. Technol. 2015, 49, 1130–1137. [Google Scholar] [CrossRef]
- Khan, F.R.; Syberg, K.; Shashoua, Y.; Bury, N.R. Influence of polyethylene microplastic beads on the uptake and localization of silver in zebrafish (Danio rerio). Environ. Pollut. 2015, 206, 73–79. [Google Scholar] [CrossRef]
- Crane, D.P.; Ogle, D.H.; Shoup, D.E. Use and misuse of a common growth metric: Guidance for appropriately calculating and reporting specific growth rate. Rev. Aquac. 2019, 12, 1542–1547. [Google Scholar] [CrossRef]
- Karami, A.; Golieskardi, A.; Choo, C.K.; Romano, N.; Ho, Y.B.; Salamatinia, B. A high-performance protocol for extraction of microplastics in fish. Sci. Total Environ. 2017, 578, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Bernet, D.; Schmidt, H.; Meier, W.; Burkhardt-Hom, P.; Wahhli, T. Histopathology in fish: Proposal for a protocol to assess aquatic pollution. Fish Dis. 1999, 22, 25–34. [Google Scholar] [CrossRef]
- Lei, L.; Wu, S.; Lu, S.; Liu, M.; Song, Y.; Fu, Z.; Shi, H.; Raley-Susman, K.M.; He, D. Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Sci. Total Environ. 2018, 619, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pedà, C.; Caccamo, L.; Fossi, M.C.; Gai, F.; Andaloro, F.; Genovese, L.; Perdichizzi, A.; Romeo, T.; Maricchiolo, G. Intestinal alterations in European sea bass Dicentrarchus labrax (Linnaeus, 1758) exposed to microplastics: Preliminary results. Environ. Pollut. 2016, 212, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Mapenzi, L.L.; Mmochi, A.J. Effect of stocking density on growth performance of hybrid of Oreochromis niloticus and Oreochromis urolepis urolepis in saline water. WIO J. Mar. Sci. 2016, 15, 67–74. [Google Scholar]
- Lugert, V.; Thaller, G.; Tetens, J.; Schulz, C.; Krieter, J. A review on fish growth calculation: Multiple functions in fish production and their specific application. Rev. Aquac. 2016, 8, 30–42. [Google Scholar] [CrossRef]
- Froese, R. Cube law, condition factor and weight–length relationships: History, meta-analysis and recommendations. J. Appl. Ichthyol. 2006, 22, 241–253. [Google Scholar] [CrossRef]
- Lamtane, H.; Mgaya, Y.; Bailey, R. Effects of water quality and flooding episode on the yield of Oreochromis urolepis (Norman, 1922) from self-stocked ponds. In Proceedings of the 8th International Symposium on Tilapia in Aquaculture, Cairo, Egypt, 12–14 October 2008. [Google Scholar]
- Critchell, K.; Hoogenboom, M.O. Effects of microplastic exposure on the body condition and behaviour of planktivorous reef fish (Acanthochromis polyacanthus). PLoS ONE 2018, 13, e0193308. [Google Scholar] [CrossRef]
- Naidoo, T.; Glassom, D. Decreased growth and survival in small juvenile fish, after chronic exposure to environmentally relevant concentrations of microplastic. Mar. Pollut. Bull. 2019, 145, 254–259. [Google Scholar] [CrossRef]
- Ighwela, K.A.; Ahmed, A.B.; Abol-Munafi, A.B. Condition Factor as an Indicator of Growth and Feeding Intensity of Nile Tilapia Fingerlings (Oreochromis niloticus) Feed on Different Levels of Maltose. Am.-Eurasian J. Agric. Environ. Sci. 2011, 11, 559–563. [Google Scholar]
- Olurin, K.B.; Aderibigbe, O.A. Length-Weight Relationship and Condition Factor of Pond Reared Juvenile Oreochromis Niloticus. World J. Zool. 2006, 1, 82–85. [Google Scholar]
- Ulotu, E.E.; Mmochi, A.J.; Lamtane, H.A. Effect of Salinity on the Survival and Growth of Rufiji Tilapia (Oreochromis urolepis urolepis) Fry. WIO J. Mar. Sci. 2016, 15, 31–37. [Google Scholar]
- Kasimoglu, C. The effect of fish size, age and condition factor on the contents of seven essential elements in Anguilla anguilla from Tersakan Stream Mugla (Turkey). J. Pollut. Eff. Control 2014, 2, 123. [Google Scholar] [CrossRef]
- Shoko, A.P.; Limbu, S.M.; Mrosso, H.D.J.; Mgaya, Y.D. A comparison of diurnal dynamics of water quality parameters in Nile tilapia (Oreochromis niloticus, Linnaeus, 1758) monoculture and polyculture with African sharp tooth catfish (Clarias gariepinus, Burchell, 1822) in earthen ponds. Int. Aquat. Res. 2014, 6, 56. [Google Scholar] [CrossRef][Green Version]
- Talbot, C. Some aspects of the biology of feeding and growth in fish. Proc. Nutr. Soc. 1993, 52, 403–416. [Google Scholar] [CrossRef]
- Wang, N.; Xu, X.; Kestemont, P. Effect of temperature and feeding frequency on growth performances, feed efficiency and body composition of pikeperch juveniles (Sander lucioperca). Aquaculture 2009, 289, 70–73. [Google Scholar] [CrossRef]
- Mustać, B.; Zoja Cukar, G.; Vidović, A. Comparison of growth parametres between sardine Sardina Pilchardus (Walbaum, 1792) and anchovy Engraulis Encrasicolus (Linnaeus, 1758) from the eastern Adriatic Sea. Pomor. Zb. 2020, 3, 325–333. [Google Scholar] [CrossRef]
- United Nations Environment Programme. Neglected: Environmental Justice Impacts of Marine Litter and Plastic Pollution; UN Environment Programme: Nairobi, Kenya, 2021. [Google Scholar]
- Vanpoucke, M.Z. Impact of Microplastic Uptake: Contamination in Sprat and Microplastic-Mediated Uptake of PAHs by European Shore Crab. Master’s Thesis, Insitute for Agricultural and Fisheries Research (ILVO), Ghent, Belgium, 2015. [Google Scholar]
- Dahms, H.T.J.; van Rensburg, G.J.; Greenfield, R. The microplastic profile of an urban African stream. Sci. Total Environ. 2020, 731, 138893. [Google Scholar] [CrossRef]
- Triebskorn, R.; Braunbeck, T.; Grummt, T.; Hanslik, L.; Huppertsberg, S.; Jekel, M.; Knepper, T.P.; Krais, S.; Müller, Y.K.; Pittroff, M.; et al. Relevance of nano- and microplastics for freshwater ecosystems: A critical review. TrAC Trends Anal. Chem. 2019, 110, 375–392. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, S.; Razanajatovo, R.M.; Zou, H.; Zhu, W. Accumulation, tissue distribution, and biochemical effects of polystyrene microplastics in the freshwater fish red tilapia (Oreochromis niloticus). Environ. Pollut. 2018, 238, 1–9. [Google Scholar] [CrossRef]
- Rochman, C.M.; Brookson, C.; Bikker, J.; Djuric, N.; Earn, A.; Bucci, K.; Athey, S.; Huntington, A.; McIlwraith, H.; Munno, K. Rethinking microplastics as a diverse contaminant suite. Environ. Toxicol. Chem. 2019, 38, 703–711. [Google Scholar] [CrossRef]
- Khan, F.R.; Patsiou, D.; Catarino, A.I. Pollutants bioavailability and toxicological risk from microplastics. In Handbook of Microplastics in the Environment; Springer: Cham, Switzerland, 2020; pp. 697–736. [Google Scholar]

| Water Parameters | Treatment Groups | ||||
|---|---|---|---|---|---|
| Control | 1 PE MPs/mL | 10 PE MPs/mL | 100 PE MPs/mL | p–Value | |
| Temperature | 29.92 ± 1.41 | 28.66 ± 1.54 | 28.90 ± 1.25 | 28.84 ± 1.25 | 0.75 |
| Dissolved oxygen | 5.53 ± 2.66 | 6.64 ± 2.83 | 4.56 ± 2.81 | 5.12 ± 2.31 | 0.59 a |
| pH | 7.78 ± 0.23 | 7.70 ± 0.22 | 7.66 ± 0.20 | 7.67 ± 0.19 | 0.016 a |
| Histomorphological Alteration | Reaction/Alteration Pattern Index | |||
|---|---|---|---|---|
| Control | 1 PE MPS/mL | 10 PE MPs/mL | 100 PE MPs/mL | |
| Villi (height and width) | 0 | 1 | 4 | 6 |
| Epithelial cells (height) | 0 | 2 | 4 | 6 |
| Cryptic and goblet cells | 0 | 1 | 4 | 6 |
| Leucocytic infiltration | 2 | 4 | 8 | 12 |
| Blood congestion | 0 | 4 | 8 | 12 |
| Organ indices | 2 | 12 | 28 | 42 |
| Parameters | Treatment Group | |||
|---|---|---|---|---|
| Control | 1 PE MPs/mL | 10 PE MPs/mL | 100 PE MPs/mL | |
| Total length (cm) ± SD | 6.24 ± 1.10 | 6.21 ± 0.80 | 6.15 ± 1.07 | 6.01 ± 1.21 |
| Initial weight (g) ± SD | 0.015 ± 0.004 | 0.017 ± 0.003 | 0.016 ± 0.004 | 0.015 ± 0.004 |
| Final weight (g) ± SD | 4.626 ± 1.987 | 4.530 ± 1.518 | 4.411 ± 2.055 | 4.246 ± 2.430 |
| Weight gain (g) ± SD | 4.611 ± 1.987 | 4.513 ± 1.518 | 4.396 ± 2.055 | 4.231 ± 2.430 |
| SGR (% day−1) | 8.80 ± 0.78 | 8.42 ± 0.62 | 8.33 ± 0.85 | 8.42 ± 0.95 |
| Condition factor | 3.276 ± 0.379 | 2.424 ± 0.210 | 1.946 ± 0.144 | 1.796 ± 0.170 |
| Growth pattern (b) | 2.671 | 2.842 | 2.943 | 2.989 |
| Coefficient of determination (R2) | 0.947 | 0.954 | 0.982 | 0.977 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mbugani, J.J.; Machiwa, J.F.; Shilla, D.A.; Joseph, D.; Kimaro, W.H.; Khan, F.R. Impaired Growth Performance of Wami Tilapia Juveniles (Oreochromis urolepis) (Norman, 1922) Due to Microplastic Induced Degeneration of the Small Intestine. Microplastics 2022, 1, 334-345. https://doi.org/10.3390/microplastics1030025
Mbugani JJ, Machiwa JF, Shilla DA, Joseph D, Kimaro WH, Khan FR. Impaired Growth Performance of Wami Tilapia Juveniles (Oreochromis urolepis) (Norman, 1922) Due to Microplastic Induced Degeneration of the Small Intestine. Microplastics. 2022; 1(3):334-345. https://doi.org/10.3390/microplastics1030025
Chicago/Turabian StyleMbugani, John J., John F. Machiwa, Daniel A. Shilla, Dativa Joseph, Wahabu H. Kimaro, and Farhan R. Khan. 2022. "Impaired Growth Performance of Wami Tilapia Juveniles (Oreochromis urolepis) (Norman, 1922) Due to Microplastic Induced Degeneration of the Small Intestine" Microplastics 1, no. 3: 334-345. https://doi.org/10.3390/microplastics1030025
APA StyleMbugani, J. J., Machiwa, J. F., Shilla, D. A., Joseph, D., Kimaro, W. H., & Khan, F. R. (2022). Impaired Growth Performance of Wami Tilapia Juveniles (Oreochromis urolepis) (Norman, 1922) Due to Microplastic Induced Degeneration of the Small Intestine. Microplastics, 1(3), 334-345. https://doi.org/10.3390/microplastics1030025