Mitochondrial DNA Alterations in HPV-Related Cancers: Emerging Insights and Future Directions
Abstract
1. Introduction
2. HPV and Mitochondrial Function
2.1. Modulation of Mitochondrial Dynamics
2.2. Impact on Reactive Oxygen Species (ROS) Generation and Oxidative Stress
2.3. Links Between HPV Infection, Metabolic Reprogramming, and Mitochondrial Dysfunction
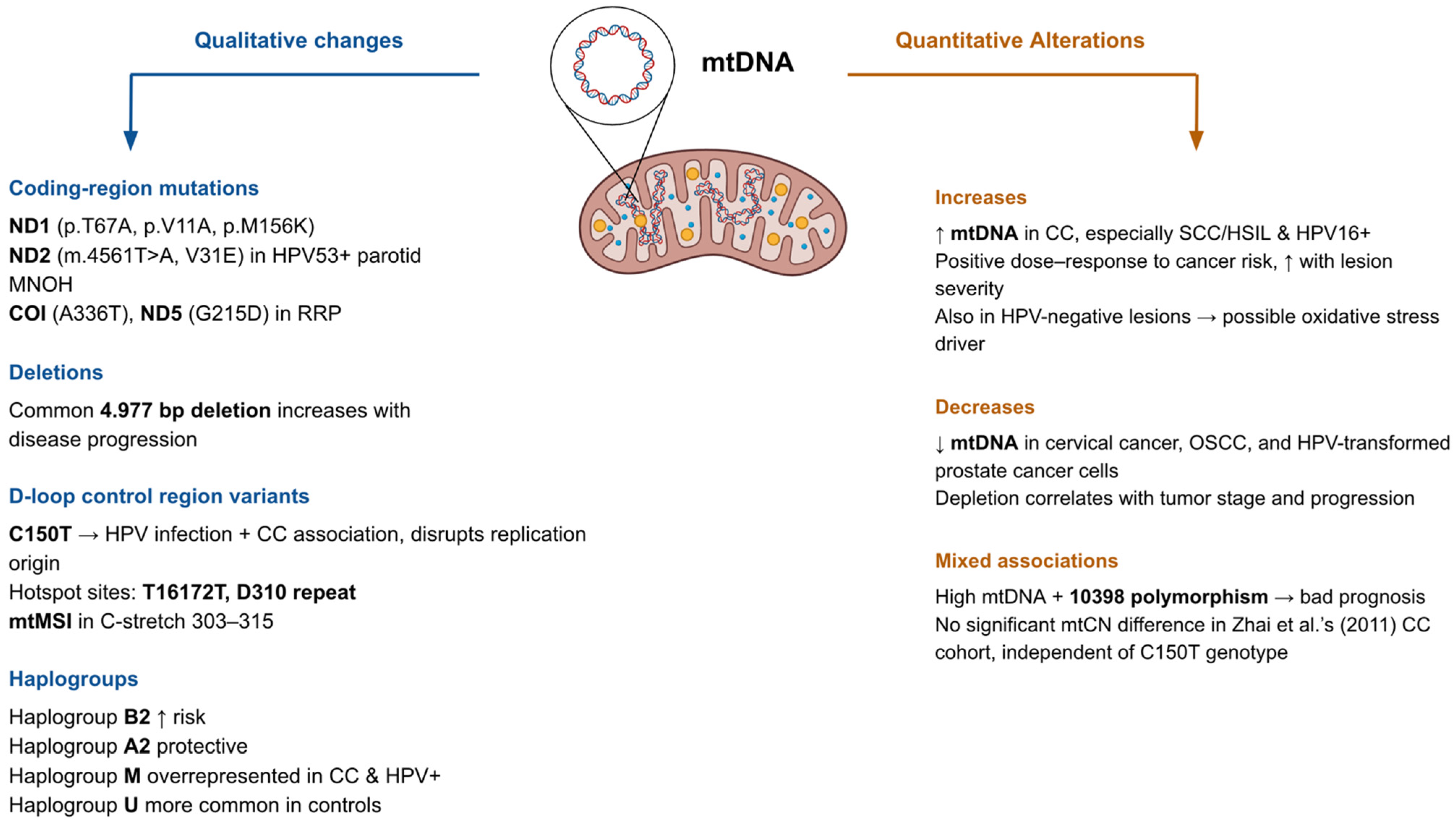
3. Qualitative and Quantitative Alterations in the Mitochondrial Genome
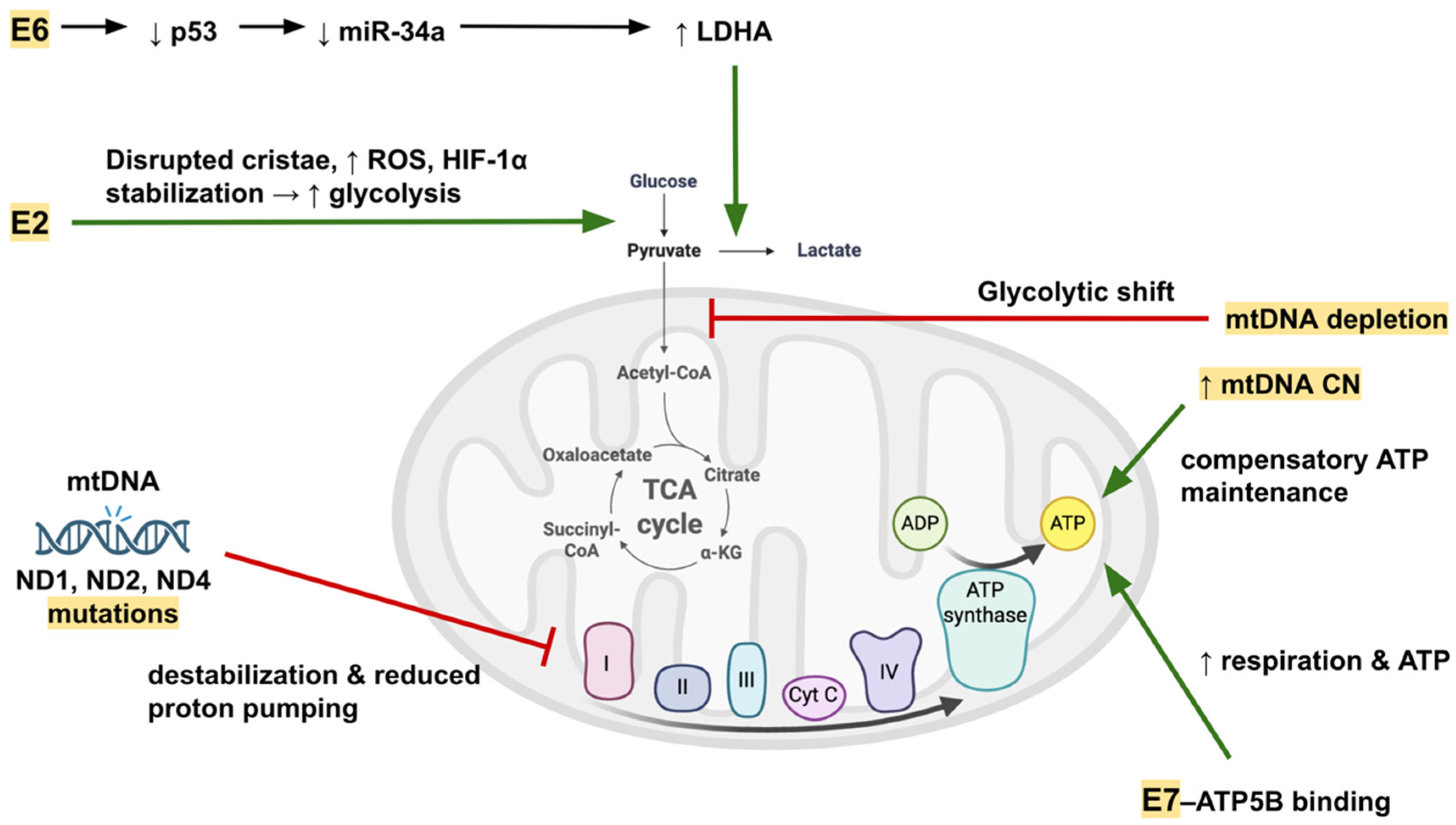
4. Functional Consequences of mtDNA Alterations
4.1. Effects on Oxidative Phosphorylation (OXPHOS) and Cellular Energy Metabolism
4.2. Mitochondrial-Driven Apoptosis Resistance and Cancer Progression
4.3. Crosstalk Between mtDNA Changes and Nuclear Gene Expression
5. Mitochondria-Directed Therapeutic Strategies and Translational Opportunities in HPV-Associated Cancers
5.1. Mitochondria-Targeted Delivery and Metabolic Modulation
5.2. Oxidative Stress, ROS, and Mitochondria-Mediated Apoptosis
5.3. Translational and Advanced Therapeutic Platforms
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Sanjosé, S.; Brotons, M.; Pavón, M.A. The Natural History of Human Papillomavirus Infection. Best. Pract. Res. Clin. Obstet. Gynaecol. 2018, 47, 2–13. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Gillison, M.L.; Chaturvedi, A.K.; Anderson, W.F.; Fakhry, C. Epidemiology of Human Papillomavirus–Positive Head and Neck Squamous Cell Carcinoma. J. Clin. Oncol. 2015, 33, 3235. [Google Scholar] [CrossRef]
- D’Souza, G.; Wentz, A.; Kluz, N.; Zhang, Y.; Sugar, E.; Youngfellow, R.M.; Guo, Y.; Xiao, W.; Gillison, M.L. Sex Differences in Risk Factors and Natural History of Oral Human Papillomavirus Infection. J. Infect. Dis. 2016, 213, 1893–1896. [Google Scholar] [CrossRef]
- McLaughlin-Drubin, M.E.; Münger, K. Oncogenic Activities of Human Papillomaviruses. Virus Res. 2009, 143, 195. [Google Scholar] [CrossRef]
- Thomas, M.; Banks, L. Inhibition of Bak-Induced Apoptosis by HPV-18 E6. Oncogene 1998, 17, 2943–2954. [Google Scholar] [CrossRef] [PubMed]
- Moody, C.A.; Laimins, L.A. Human Papillomavirus Oncoproteins: Pathways to Transformation. Nat. Rev. Cancer 2010, 10, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Pereira, I.O.A.; Silva, N.N.T.; Lima, A.A.; da Silva, G.N. Qualitative and Quantitative Changes in Mitochondrial DNA Associated with Cervical Cancer: A Comprehensive Review. Environ. Mol. Mutagen. 2024, 65, 143–152. [Google Scholar] [CrossRef]
- Cruz-Gregorio, A.; Aranda-Rivera, A.K.; Roviello, G.N.; Pedraza-Chaverri, J. Targeting Mitochondrial Therapy in the Regulation of HPV Infection and HPV-Related Cancers. Pathogens 2023, 12, 402. [Google Scholar] [CrossRef]
- Spinelli, J.B.; Haigis, M.C. The Multifaceted Contributions of Mitochondria to Cellular Metabolism. Nat. Cell Biol. 2018, 20, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Tuppen, H.A.L.; Blakely, E.L.; Turnbull, D.M.; Taylor, R.W. Mitochondrial DNA Mutations and Human Disease. Biochim. Biophys. Acta (BBA) Bioenerg. 2010, 1797, 113–128. [Google Scholar] [CrossRef]
- Alexeyev, M.; Shokolenko, I.; Wilson, G.; LeDoux, S. The Maintenance of Mitochondrial DNA Integrity—Critical Analysis and Update. Cold Spring Harb. Perspect. Biol. 2013, 5, a012641. [Google Scholar] [CrossRef]
- Reznik, E.; Miller, M.L.; Şenbabaoğlu, Y.; Riaz, N.; Sarungbam, J.; Tickoo, S.K.; Al-Ahmadie, H.A.; Lee, W.; Seshan, V.E.; Hakimi, A.A.; et al. Mitochondrial DNA Copy Number Variation across Human Cancers. Elife 2016, 5, e10769. [Google Scholar] [CrossRef]
- Yuan, Y.; Ju, Y.S.; Kim, Y.; Li, J.; Wang, Y.; Yoon, C.J.; Yang, Y.; Martincorena, I.; Creighton, C.J.; Weinstein, J.N.; et al. Comprehensive Molecular Characterization of Mitochondrial Genomes in Human Cancers. Nat. Genet. 2020, 52, 342–352. [Google Scholar] [CrossRef]
- Tindle, R.W. Immune Evasion in Human Papillomavirus-Associated Cervical Cancer. Nat. Rev. Cancer 2002, 2, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Gong, S.; Singh, P.; Lyu, J.; Bai, Y. The Interaction between Mitochondria and Oncoviruses. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1864, 481. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, D.M.; Bernardi, P.; Chieco-Bianchi, L.; Ciminale, V. Mitochondria as Functional Targets of Proteins Coded by Human Tumor Viruses. Adv. Cancer Res. 2005, 94, 87–142. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Gregorio, A.; Aranda-Rivera, A.K.; Pedraza-Chaverri, J. Human Papillomavirus-Related Cancers and Mitochondria. Virus Res. 2020, 286, 198016. [Google Scholar] [CrossRef]
- Warowicka, A.; Kwasniewska, A.; Gozdzicka-Jozefiak, A. Alterations in MtDNA: A Qualitative and Quantitative Study Associated with Cervical Cancer Development. Gynecol. Oncol. 2013, 129, 193–198. [Google Scholar] [CrossRef]
- Vyas, S.; Zaganjor, E.; Haigis, M.C. Mitochondria and Cancer. Cell 2016, 166, 555–566. [Google Scholar] [CrossRef]
- Chatterjee, A.; Mambo, E.; Sidransky, D. Mitochondrial DNA Mutations in Human Cancer. Oncogene 2006, 25, 4663–4674. [Google Scholar] [CrossRef]
- Vlachogiannis, G.; Hedayat, S.; Vatsiou, A.; Jamin, Y.; Fernández-Mateos, J.; Khan, K.; Lampis, A.; Eason, K.; Huntingford, I.; Burke, R.; et al. Patient-Derived Organoids Model Treatment Response of Metastatic Gastrointestinal Cancers. Science 2018, 359, 920. [Google Scholar] [CrossRef]
- Letafati, A.; Taghiabadi, Z.; Zafarian, N.; Tajdini, R.; Mondeali, M.; Aboofazeli, A.; Chichiarelli, S.; Saso, L.; Jazayeri, S.M. Emerging Paradigms: Unmasking the Role of Oxidative Stress in HPV-Induced Carcinogenesis. Infect. Agents Cancer 2024, 19, 1–21. [Google Scholar] [CrossRef]
- Leverrier, S.; Bergamaschi, D.; Ghali, L.; Ola, A.; Warnes, G.; Akgül, B.; Blight, K.; García-Escudero, R.; Penna, A.; Eddaoudi, A.; et al. Role of HPV E6 Proteins in Preventing UVB-Induced Release of pro-Apoptotic Factors from the Mitochondria. Apoptosis 2007, 12, 549–560. [Google Scholar] [CrossRef]
- Zhang, R.; Su, J.; Xue, S.-L.; Yang, H.; Ju, L.-L.; Ji, Y.; Wu, K.-H.; Zhang, Y.-W.; Zhang, Y.-X.; Hu, J.-F.; et al. HPV E6/P53 Mediated down-Regulation of MiR-34a Inhibits Warburg Effect through Targeting LDHA in Cervical Cancer. Am. J. Cancer Res. 2016, 6, 312–320. [Google Scholar]
- Sannigrahi, M.K.; Rajagopalan, P.; Lai, L.; Liu, X.; Sahu, V.; Nakagawa, H.; Jalaly, J.B.; Brody, R.M.; Morgan, I.M.; Windle, B.E.; et al. HPV E6 Regulates Therapy Responses in Oropharyngeal Cancer by Repressing the PGC-1α/ERRα Axis. JCI Insight 2022, 7, e159600. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Gregorio, A.; Aranda-Rivera, A.K.; Aparicio-Trejo, O.E.; Coronado-Martínez, I.; Pedraza-Chaverri, J.; Lizano, M. E6 Oncoproteins from High-Risk Human Papillomavirus Induce Mitochondrial Metabolism in a Head and Neck Squamous Cell Carcinoma Model. Biomolecules 2019, 9, 351. [Google Scholar] [CrossRef]
- Kirschberg, M.; Heuser, S.; Marcuzzi, G.P.; Hufbauer, M.; Seeger, J.M.; Đukić, A.; Tomaić, V.; Majewski, S.; Wagner, S.; Wittekindt, C.; et al. ATP Synthase Modulation Leads to an Increase of Spare Respiratory Capacity in HPV Associated Cancers. Sci. Rep. 2020, 10, 17339. [Google Scholar] [CrossRef]
- Tian, R.; Liu, J.; Fan, W.; Li, R.; Cui, Z.; Jin, Z.; Huang, Z.; Xie, H.; Li, L.; Huang, Z.; et al. Gene Knock-out Chain Reaction Enables High Disruption Efficiency of HPV18 E6/E7 Genes in Cervical Cancer Cells. Mol. Ther. Oncolytics 2022, 24, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Bucha, S.; Mukhopadhyay, D.; Bhattacharyya, N.P. E2F1 Activates MFN2 Expression by Binding to the Promoter and Decreases Mitochondrial Fission and Mitophagy in HeLa Cells. FEBS J. 2019, 286, 4525–4541. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Oleinik, N.; Ogretmen, B. HPV16-E7 Oncoprotein Enhances Ceramide-Mediated Lethal Mitophagy by Regulating the Rb/E2F5/Drp1 Signaling Axis. FASEB J. 2016, 30, 872.6. [Google Scholar] [CrossRef]
- Lai, D.; Tan, C.L.; Gunaratne, J.; Quek, L.S.; Nei, W.; Thierry, F.; Bellanger, S. Localization of HPV-18 E2 at Mitochondrial Membranes Induces ROS Release and Modulates Host Cell Metabolism. PLoS ONE 2013, 8, e75625. [Google Scholar] [CrossRef]
- Villota, C.; Campos, A.; Vidaurre, S.; Oliveira-Cruz, L.; Boccardo, E.; Burzio, V.A.; Varas, M.; Villegas, J.; Villa, L.L.; Valenzuela, P.D.T.; et al. Expression of Mitochondrial Non-Coding RNAs (NcRNAs) Is Modulated by High Risk Human Papillomavirus (HPV) Oncogenes. J. Biol. Chem. 2012, 287, 21303–21315. [Google Scholar] [CrossRef] [PubMed]
- Losón, O.C.; Song, Z.; Chen, H.; Chan, D.C. Fis1, Mff, MiD49, and MiD51 Mediate Drp1 Recruitment in Mitochondrial Fission. Mol. Biol. Cell 2013, 24, 659–667. [Google Scholar] [CrossRef]
- Dutkowska, A.; Domańska-Senderowska, D.; Czarnecka-Chrebelska, K.H.; Pikus, E.; Zielińska, A.; Biskup, L.; Kołodziejska, A.; Madura, P.; Możdżan, M.; Załuska, U.; et al. Mitochondrial Dynamics in Non-Small Cell Lung Cancer. Cancers 2024, 16, 2823. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; He, Q.; Lu, H.; Zhou, X.; Chen, L.; Liu, H.; Lu, Z.; Liu, D.; Liu, Y.; Zuo, D.; et al. Silibinin Induces G2/M Cell Cycle Arrest by Activating Drp1-Dependent Mitochondrial Fission in Cervical Cancer. Front. Pharmacol. 2020, 11, 514620. [Google Scholar] [CrossRef]
- Tomaziu-Todosia Anton, E.; Anton, G.I.; Scripcariu, I.S.; Dumitrașcu, I.; Scripcariu, D.V.; Balmus, I.M.; Ionescu, C.; Visternicu, M.; Socolov, D.G. Oxidative Stress, Inflammation, and Antioxidant Strategies in Cervical Cancer—A Narrative Review. Int. J. Mol. Sci. 2025, 26, 4961. [Google Scholar] [CrossRef]
- Gore, M.; Kabekkodu, S.P.; Chakrabarty, S. Exploring the Metabolic Alterations in Cervical Cancer Induced by HPV Oncoproteins: From Mechanisms to Therapeutic Targets. Biochim. Biophys. Acta (BBA) Rev. Cancer 2025, 1880, 189292. [Google Scholar] [CrossRef]
- You, A.J.; Park, J.; Shin, J.M.; Kim, T.H. Oxidative Stress and Dietary Antioxidants in Head and Neck Cancer. Antioxidants 2025, 14, 508. [Google Scholar] [CrossRef]
- Ma, J.; Yao, Z.; Ma, L.; Zhu, Q.; Zhang, J.; Li, L.; Liu, C. Glucose Metabolism Reprogramming in Gynecologic Malignant Tumors. J. Cancer 2024, 15, 2627–2645. [Google Scholar] [CrossRef]
- Hu, C.; Liu, T.; Han, C.; Xuan, Y.; Jiang, D.; Sun, Y.; Zhang, X.; Zhang, W.; Xu, Y.; Liu, Y.; et al. HPV E6/E7 Promotes Aerobic Glycolysis in Cervical Cancer by Regulating IGF2BP2 to Stabilize M6A-MYC Expression. Int. J. Biol. Sci. 2022, 18, 507–521. [Google Scholar] [CrossRef] [PubMed]
- Hoppe-Seyler, K.; Honegger, A.; Bossler, F.; Sponagel, J.; Bulkescher, J.; Lohrey, C.; Hoppe-Seyler, F. Viral E6/E7 Oncogene and Cellular Hexokinase 2 Expression in HPV-Positive Cancer Cell Lines. Oncotarget 2017, 8, 106342. [Google Scholar] [CrossRef]
- Prakasam, G.; Iqbal, M.A.; Srivastava, A.; Bamezai, R.N.K.; Singh, R.K. HPV18 Oncoproteins Driven Expression of PKM2 Reprograms HeLa Cell Metabolism to Maintain Aerobic Glycolysis and Viability. Virusdisease 2022, 33, 223–235. [Google Scholar] [CrossRef]
- Arizmendi-Izazaga, A.; Navarro-Tito, N.; Jiménez-Wences, H.; Mendoza-Catalán, M.A.; Martínez-Carrillo, D.N.; Zacapala-Gómez, A.E.; Olea-Flores, M.; Dircio-Maldonado, R.; Torres-Rojas, F.I.; Soto-Flores, D.G.; et al. Metabolic Reprogramming in Cancer: Role of HPV 16 Variants. Pathogens 2021, 10, 347. [Google Scholar] [CrossRef]
- Martínez-Ramírez, I.; Carrillo-García, A.; Contreras-Paredes, A.; Ortiz-Sánchez, E.; Cruz-Gregorio, A.; Lizano, M. Regulation of Cellular Metabolism by High-Risk Human Papillomaviruses. Int. J. Mol. Sci. 2018, 19, 1839. [Google Scholar] [CrossRef]
- Du, H.; Xu, T.; Yu, S.; Wu, S.; Zhang, J. Mitochondrial Metabolism and Cancer Therapeutic Innovation. Signal Transduct. Target. Ther. 2025, 10, 245. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Z.; Chen, Y.; Tian, H.; Chai, P.; Shen, Y.; Yao, Y.; Xu, S.; Ge, S.; Jia, R. Lactate and Lactylation in Cancer. Signal Transduct. Target. Ther. 2025, 10, 1–26. [Google Scholar] [CrossRef]
- de la Cruz-López, K.G.; Castro-Muñoz, L.J.; Reyes-Hernández, D.O.; García-Carrancá, A.; Manzo-Merino, J. Lactate in the Regulation of Tumor Microenvironment and Therapeutic Approaches. Front. Oncol. 2019, 9, 1143. [Google Scholar] [CrossRef] [PubMed]
- Abu Shelbayeh, O.; Arroum, T.; Morris, S.; Busch, K.B. PGC-1α Is a Master Regulator of Mitochondrial Lifecycle and ROS Stress Response. Antioxidants 2023, 12, 1075. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Xu, H.; Li, X.; Wei, Y.; Jiang, H.; Xu, H.; Luo, A.; Zhou, F. An Association Analysis between Mitochondrial DNA Content, G10398A Polymorphism, HPV Infection, and the Prognosis of Cervical Cancer in the Chinese Han Population. Tumor Biol. 2016, 37, 5599–5607. [Google Scholar] [CrossRef]
- Guardado-Estrada, M.; Medina-Martínez, I.; Juárez-Torres, E.; Roman-Bassaure, E.; MacÍas, L.; Alfaro, A.; Alcántara-Vázquez, A.; Alonso, P.; Gomez, G.; Cruz-Talonia, F.; et al. The Amerindian MtDNA Haplogroup B2 Enhances the Risk of HPV for Cervical Cancer: De-Regulation of Mitochondrial Genes May Be Involved. J. Hum. Genet. 2012, 57, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Zhai, K.; Chang, L.; Zhang, Q.; Liu, B.; Wu, Y. Mitochondrial C150T Polymorphism Increases the Risk of Cervical Cancer and HPV Infection. Mitochondrion 2011, 11, 559–563. [Google Scholar] [CrossRef]
- Sharma, H.; Singh, A.; Sharma, C.; Jain, S.K.; Singh, N. Mutations in the Mitochondrial DNA D-Loop Region Are Frequent in Cervical Cancer. Cancer Cell Int. 2005, 5, 34. [Google Scholar] [CrossRef][Green Version]
- Sun, W.; Qin, X.; Zhou, J.; Xu, M.; Lyu, Z.; Li, X.; Zhang, K.; Dai, M.; Li, N.; Hang, D. Mitochondrial DNA Copy Number in Cervical Exfoliated Cells and Risk of Cervical Cancer among HPV-Positive Women. BMC Womens Health 2020, 20, 139. [Google Scholar] [CrossRef]
- Al-awadhi, R.; Alroomy, M.; Al-Waheeb, S.; Alwehaidah, M.S. Altered Mitochondrial DNA Copy Number in Cervical Exfoliated Cells among High-risk HPV-positive and HPV-negative Women. Exp. Ther. Med. 2023, 26, 521. [Google Scholar] [CrossRef] [PubMed]
- Kabekkodu, S.P.; Bhat, S.; Mascarenhas, R.; Mallya, S.; Bhat, M.; Pandey, D.; Kushtagi, P.; Thangaraj, K.; Gopinath, P.M.; Satyamoorthy, K. Mitochondrial DNA Variation Analysis in Cervical Cancer. Mitochondrion 2014, 16, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Mondal, R.; Ghosh, S.K.; Choudhury, J.H.; Seram, A.; Sinha, K.; Hussain, M.; Laskar, R.S.; Rabha, B.; Dey, P.; Ganguli, S.; et al. Mitochondrial DNA Copy Number and Risk of Oral Cancer: A Report from Northeast India. PLoS ONE 2013, 8, e57771. [Google Scholar] [CrossRef]
- Mizumachi, T.; Muskhelishvili, L.; Naito, A.; Furusawa, J.; Fan, C.Y.; Siegel, E.R.; Kadlubar, F.F.; Kumar, U.; Higuchi, M. Increased Distributional Variance of Mitochondrial DNA Content Associated with Prostate Cancer Cells as Compared with Normal Prostate Cells. Prostate 2008, 68, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Ruiz, R.; Yang, L.; Neto, A.G.; Amin, M.R.; Kelly, D.; Achlatis, S.; Roof, S.; Bing, R.; Kannan, K.; et al. Mitochondrial Somatic Mutations and the Lack of Viral Genomic Variation in Recurrent Respiratory Papillomatosis. Sci. Rep. 2019, 9, 16625. [Google Scholar] [CrossRef]
- Warowicka, A.; Wołuń-Cholewa, M.; Kwaśniewska, A.; Goździcka-Józefiak, A. Alternations in Mitochondrial Genome in Carcinogenesis of HPV Positive Cervix. Exp. Mol. Pathol. 2020, 117, 104530. [Google Scholar] [CrossRef]
- Kurelac, I.; Salfi, N.C.; Ceccarelli, C.; Alessandrini, F.; Cricca, M.; Caliceti, U.; Gasparre, G. Human Papillomavirus Infection and Pathogenic Mitochondrial DNA Mutation in Bilateral Multinodular Oncocytic Hyperplasia of the Carotid. Pathology 2014, 46, 250–253. [Google Scholar] [CrossRef]
- Singh, M.; Singh, N. Induction of Apoptosis by Hydrogen Peroxide in HPV 16 Positive Human Cervical Cancer Cells: Involvement of Mitochondrial Pathway. Mol. Cell Biochem. 2008, 310, 57–65. [Google Scholar] [CrossRef]
- Matei, C.; Nicolae, I.; Mitran, M.I.; Mitran, C.I.; Ene, C.D.; Nicolae, G.; Georgescu, S.R.; Tampa, M. Biomolecular Dynamics of Nitric Oxide Metabolites and HIF1α in HPV Infection. Biomolecules 2024, 14, 1172. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, G.; Tao, X.; Dong, D.; Liu, J. Targeted Mitochondrial Therapy for Pancreatic Cancer. Transl. Oncol. 2025, 54, 102340. [Google Scholar] [CrossRef]
- Gong, H.; Yu, X.; Zhang, A.; Guan, F.; Li, W.; Han, F.; Wang, Y.; Chen, D. A Novel Supramolecule Combining the Pharmacological Benefits of Berberin and Catechin for the Prevention and Treatment of Cervical Cancer. Colloids Surf. A Physicochem. Eng. Asp. 2024, 698, 134555. [Google Scholar] [CrossRef]
- Alsehli, S.M.; Almutairi, O.A.; Almutairi, S.M.; Almutairi, M.G.; Aljohani, S.A.; Alanazi, N.S.; Almutiri, F.F.; Aloufi, Y.A.; Algohani, A.S.; Alharbi, M.A.; et al. Mitochondrial Dysfunction in Hepatocellular Carcinoma: Insights into the Diagnostic and Therapeutic Implications. Evol. Stud. Imaginative Cult. 2024, 8, 2825–2846. [Google Scholar] [CrossRef]
- Hou, Y.-J.; Yang, X.-X.; Meng, H.-X. Mitochondrial Metabolism in Laryngeal Cancer: Therapeutic Mechanisms and Prospects. Biochim. Biophys. Acta (BBA) Rev. Cancer 2025, 1880, 189335. [Google Scholar] [CrossRef]
- Li, N.; Chamkha, I.; Verma, G.; Swoboda, S.; Lindstedt, M.; Greiff, L.; Elmér, E.; Ehinger, J. Human Papillomavirus-Associated Head and Neck Squamous Cell Carcinoma Cells Rely on Glycolysis and Display Reduced Oxidative Phosphorylation. Front. Oncol. 2023, 13, 1304106. [Google Scholar] [CrossRef]
- Krishnamoorthy, S.; Sabanayagam, R.; Periyasamy, L.; Muruganantham, B.; Muthusami, S. Plumbagin as a Preferential Lead Molecule to Combat EGFR-Driven Matrix Abundance and Migration of Cervical Carcinoma Cells. Med. Oncol. 2024, 41, 89. [Google Scholar] [CrossRef] [PubMed]
- Kurdi, M.; Bamaga, A.; Alkhotani, A.; Alsharif, T.; Abdel-Hamid, G.A.; Selim, M.E.; Alsinani, T.; Albeshri, A.; Badahdah, A.; Basheikh, M.; et al. Mitochondrial DNA Alterations in Glioblastoma and Current Therapeutic Targets. Front. Biosci. Landmark 2024, 29, 367. [Google Scholar] [CrossRef]
- Agarwal, N.R.; Maurya, N.; Pawar, J.S.; Ghosh, I. A Combined Approach against Tumorigenesis Using Glucose Deprivation and Mitochondrial Complex 1 Inhibition by Rotenone. Cell Biol. Int. 2016, 40, 821–831. [Google Scholar] [CrossRef]
- Sharafabad, B.E.; Abdoli, A.; Jamour, P.; Dilmaghani, A. The Ability of Clostridium Novyi-NT Spores to Induce Apoptosis via the Mitochondrial Pathway in Mice with HPV-Positive Cervical Cancer Tumors Derived from the TC-1 Cell Line. BMC Complement. Med. Ther. 2024, 24, 427. [Google Scholar] [CrossRef]
- Mishra, S.R.; Mishra, P.; Dhiman, R.; Bhutia, S.K. The Dual Role of Mitophagy in Cancer and Its Targeting for Effective Anticancer Therapy. In Mitophagy in Health and Disease: Mechanisms, Health Implications, and Therapeutic Opportunities; Elsevier: Amsterdam, The Netherlands, 2025; pp. 187–205. [Google Scholar] [CrossRef]
- van der Pol, Y.; Moldovan, N.; Ramaker, J.; Bootsma, S.; Lenos, K.J.; Vermeulen, L.; Sandhu, S.; Bahce, I.; Pegtel, D.M.; Wong, S.Q.; et al. The Landscape of Cell-Free Mitochondrial DNA in Liquid Biopsy for Cancer Detection. Genome Biol. 2023, 24, 229. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, Y.; Wu, D.; Wang, H. Association between Mitochondrial DNA Copy Number and Head and Neck Squamous Cell Carcinoma: A Systematic Review and Dose-Response Meta-Analysis. Med. Sci. Monit. 2021, 27, e928327-1–e928327-9. [Google Scholar] [CrossRef]
- Aminuddin, A.; Wong, P.K.; Masre, S.F.; Ng, P.Y.; Zakaria, M.A.; Chua, E.W. The Significance of Mitochondrial DNA Changes during the Onset and Progression of Head and Neck Squamous Cell Carcinoma. Future Oncol. 2025. Epub ahead of printing. [Google Scholar] [CrossRef]
- Vichaya, E.G.; Molkentine, J.M.; Vermeer, D.W.; Walker, A.K.; Feng, R.; Holder, G.; Luu, K.; Mason, R.M.; Saligan, L.; Heijnen, C.J.; et al. Sickness Behavior Induced by Cisplatin Chemotherapy and Radiotherapy in a Murine Head and Neck Cancer Model Is Associated with Altered Mitochondrial Gene Expression. Behav. Brain Res. 2016, 297, 241–250. [Google Scholar] [CrossRef]
- Lõhmussaar, K.; Oka, R.; Espejo Valle-Inclan, J.; Smits, M.H.H.; Wardak, H.; Korving, J.; Begthel, H.; Proost, N.; van de Ven, M.; Kranenburg, O.W.; et al. Patient-Derived Organoids Model Cervical Tissue Dynamics and Viral Oncogenesis in Cervical Cancer. Cell Stem Cell 2021, 28, 1380–1396.e6. [Google Scholar] [CrossRef]
| Oncoprotein | Direct Mitochondrial Target/Effect | Indirect Pathway or Regulatory Mechanism | Resulting Mitochondrial or Cellular Effect | Reference(s) |
|---|---|---|---|---|
| E6 | Bak degradation | p53 degradation → ↓ transcription of antioxidant enzymes (SOD2, catalase) | Inhibits MOMP, prevents cytochrome c release, reduces apoptosis; weakens ROS detoxification | [23,24] |
| E6 | — | p53 loss → ↓ miR-34a → ↑ LDHA | Enhances aerobic glycolysis at expense of OXPHOS | [25] |
| E6 | — | p53 loss → ↓ PGC-1α and ERRα | Suppresses mitochondrial biogenesis, reduces oxidative capacity | [26] |
| E6 | — | Activation of Nrf2 and FoxO3a in HPV16 E6 cells | ↑ Expression of antioxidant enzymes (SOD1, SOD2, catalase) → improved oxidative stress tolerance | [27] |
| E7 | ATP5B binding | — | ↑ Mitochondrial respiration, ↑ spare respiratory capacity, ↑ ATP production | [28] |
| E7 | — | pRb degradation → E2F activation → altered expression of mitochondrial dynamics genes | Modifies nuclear-encoded mitochondrial protein expression; impacts mitochondrial morphology and turnover | [29] |
| E7 | — | E2F1 + SP1 complex binds MFN2 promoter | Regulates mitochondrial fusion and mitophagy | [30] |
| E7 | — | — | Associated with increased mitophagy, selective mitochondrial removal | [31] |
| E2 | — | Cristae disruption, ↑ ROS production, HIF-1α stabilization | Shifts metabolism toward glycolysis, promotes angiogenesis and hypoxia adaptation | [32] |
| E2 | — | Alters antisense mitochondrial RNAs (ASncmtRNAs) | Affects cell cycle regulation and nuclear transcription via miRNA sponging (e.g., miR-620 targeting PML) | [33] |
| Alteration Type | Gene/Region | Specific Mutation/Variant | HPV Status Association | Cancer Type/Lesion Stage | Functional Impact/Proposed Mechanism | Reference(s) |
|---|---|---|---|---|---|---|
| Point mutation | ND1 | p.M156K | HPV+ only | Precancerous & cervical cancer | Alters Complex I subunit, reduces proton pumping efficiency | [60] |
| Point mutation | ND4 | T170P | HPV+ | Cervical cancer | Complex I destabilization | [56] |
| Point mutation | ND2 | V31E | HPV53+ | Multinodular oncocytic hyperplasia (parotid) | Complex I destabilization | [61] |
| Point mutation | COI | A336T | HPV+ | Recurrent respiratory papillomatosis | May disrupt ETC function due to conserved residue change | [59] |
| Point mutation | ND5 | G215D | HPV+ | Recurrent respiratory papillomatosis | May disrupt ETC function due to conserved residue change | [59] |
| D-loop polymorphism | Control region | C150T | HPV+ | Cervical cancer | Alters replication origin, modifies OXPHOS | [52] |
| D-loop mutations | Control region | 55 novel mutations | HPV+ | Cervical cancer | May disrupt replication & transcription regulation | [53] |
| D-loop mutations | Control region | 216 variants (29 novel) | Mixed HPV status | Cervical cancer | Multiple substitutions & indels; T16172T & D310 repeat hotspots | [56] |
| mtDNA deletion | Multiple genes | Common 4.977 bp deletion | HPV+ | LSIL → HSIL → CC progression | Loss of ETC genes; ↑ ROS & genomic instability | [19] |
| Copy number ↑ | — | — | HPV16+ (highest in SCC/HSIL) | High-grade lesions & SCC | Possible compensatory mechanism for OXPHOS despite mutations | [19,54,55] |
| Copy number ↓ | — | — | Mixed HPV status | CC, OSCC, HPV-transformed prostate cancer | Favors glycolysis; reduced oxidative capacity | [56,57,58] |
| Haplogroup association | mtDNA haplogroups | Haplogroup B2 ↑, A2 protective | HPV+ | Cervical cancer | Influences mitochondrial tRNA gene expression & replication | [51] |
| Haplogroup association | mtDNA haplogroups | Haplogroup M ↑, Haplogroup U ↓ | HPV+ | Cervical cancer | Overrepresentation of M in HPV+ cases; associated with specific variants (e.g., A73G) | [56] |
| Functional Domain | Mechanism Affected | Key Examples/Alterations | Effect on Cellular Physiology | Reference(s) |
|---|---|---|---|---|
| Oxidative phosphorylation efficiency | Complex I subunit mutations reduce proton pumping efficiency | ND1 p.M156K, ND4 T170P, ND2 V31E | ↓ ETC function, ATP depletion, impaired OXPHOS | [56,60,61] |
| Oxidative phosphorylation efficiency | mtDNA depletion in tumors | CC, OSCC, HPV-transformed prostate cancer | ↓ OXPHOS capacity, ↑ glycolytic phenotype | [56,57,58] |
| Compensatory OXPHOS maintenance | mtDNA copy number increase | HPV16+ high-grade lesions, SCC | Maintains ATP production despite mtDNA mutations/deletions | [19,54,55] |
| ROS homeostasis | mtDNA deletions and missense mutations increase ROS | Common 4.977 bp deletion, ND1 p.M156K | ↑ ROS, oxidative stress loop, promotes genomic instability | [19,60] |
| Apoptosis regulation | Direct oncoprotein targeting of mitochondrial apoptosis factors | E6 → Bak degradation | Prevents MOMP, inhibits cytochrome c release, suppresses caspase activation | [24] |
| Apoptosis regulation | Cell line-specific mitochondrial permeabilization | Cytochrome c release in SiHa but not CaSki | Differential sensitivity to oxidative stress-induced apoptosis | [62] |
| Metabolic reprogramming | Viral oncoprotein regulation of glycolytic enzymes and transporters | E6 → p53 loss → ↓ miR-34a → ↑ LDHA; E2 → HIF-1α stabilization | Favors aerobic glycolysis (Warburg effect), ↑ lactate production | [25,32] |
| Retrograde signaling to nucleus | Suppression of mitochondrial biogenesis regulators | PGC-1α & ERRα repression | ↓ mitochondrial biogenesis, altered oxidative metabolism | [26] |
| Retrograde signaling to nucleus | Activation of nuclear antioxidant programs | Nrf2, FoxO3a activation in HPV16 E6 cells | ↑ SOD1, SOD2, catalase expression, improved oxidative stress tolerance | [27] |
| Angiogenesis & hypoxia adaptation | ROS-mediated HIF-1α stabilization | Induction of VEGF, PDK1, CAIX | Promotes angiogenesis, glycolysis, and tumor adaptation to hypoxia | [32] |
| MicroRNA-mediated regulation | Viral oncoproteins alter miRNA–mitochondria cross-talk | E6-mediated p53 loss → ↓ miR-34a; E2 alters ASncmtRNA | Reinforces glycolysis, affects cell cycle regulation | [25,33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Cakir, M.O.; Selek, M.; Kayhan, G.; Yilmaz, B.; Ozdogan, M.; Ashrafi, G.H. Mitochondrial DNA Alterations in HPV-Related Cancers: Emerging Insights and Future Directions. DNA 2026, 6, 7. https://doi.org/10.3390/dna6010007
Cakir MO, Selek M, Kayhan G, Yilmaz B, Ozdogan M, Ashrafi GH. Mitochondrial DNA Alterations in HPV-Related Cancers: Emerging Insights and Future Directions. DNA. 2026; 6(1):7. https://doi.org/10.3390/dna6010007
Chicago/Turabian StyleCakir, Muharrem Okan, Melis Selek, Guldide Kayhan, Betul Yilmaz, Mustafa Ozdogan, and Gholam Hossein Ashrafi. 2026. "Mitochondrial DNA Alterations in HPV-Related Cancers: Emerging Insights and Future Directions" DNA 6, no. 1: 7. https://doi.org/10.3390/dna6010007
APA StyleCakir, M. O., Selek, M., Kayhan, G., Yilmaz, B., Ozdogan, M., & Ashrafi, G. H. (2026). Mitochondrial DNA Alterations in HPV-Related Cancers: Emerging Insights and Future Directions. DNA, 6(1), 7. https://doi.org/10.3390/dna6010007







