The Multiple DNA-Associated Roles of ASPM and Liquid–Liquid Phase Separation as a Unifying Mechanism of Function
Abstract
1. Introduction
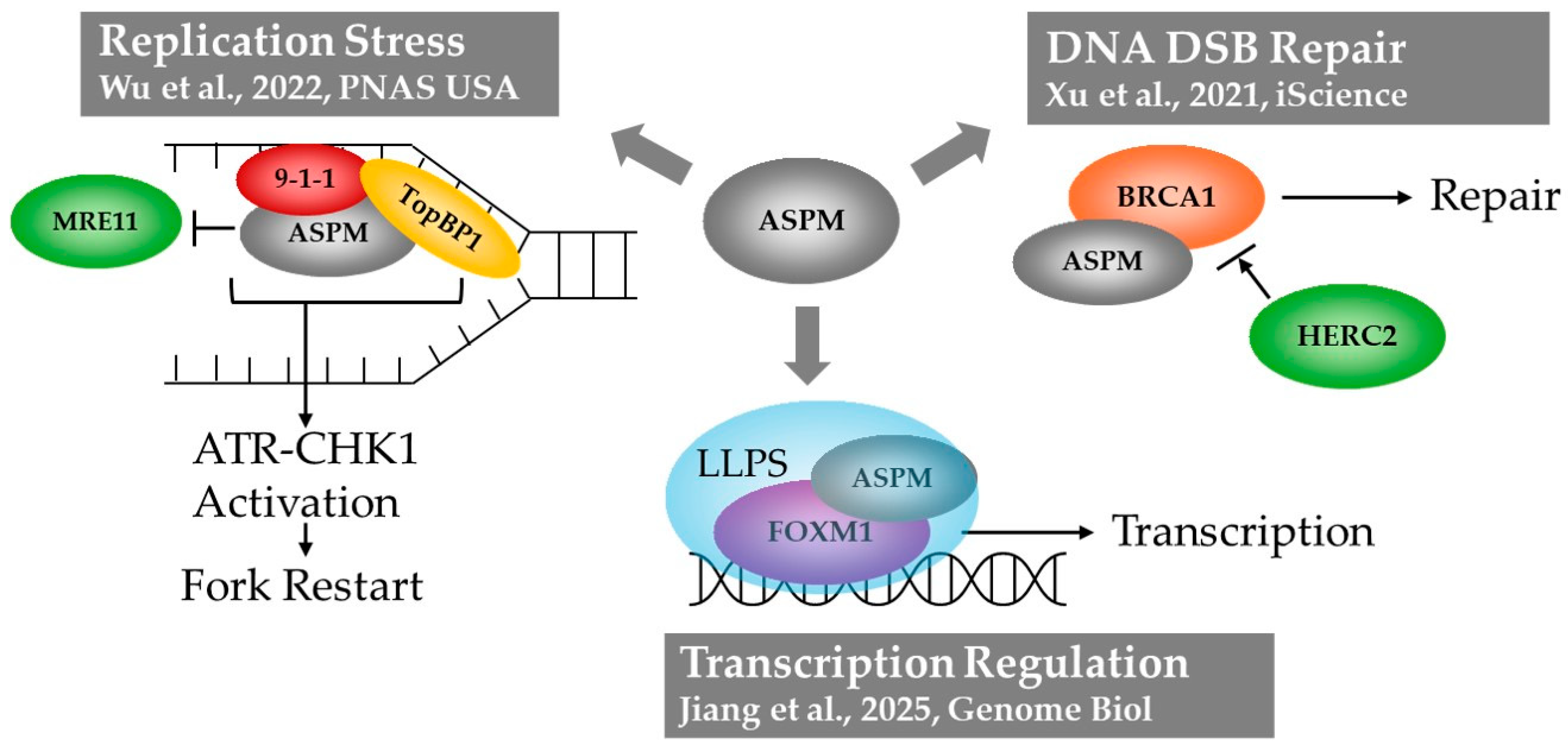
2. The Multiple DNA-Associated Roles of ASPM
2.1. Liquid–Liquid Phase Separation as an Organizer of Cellular Functions
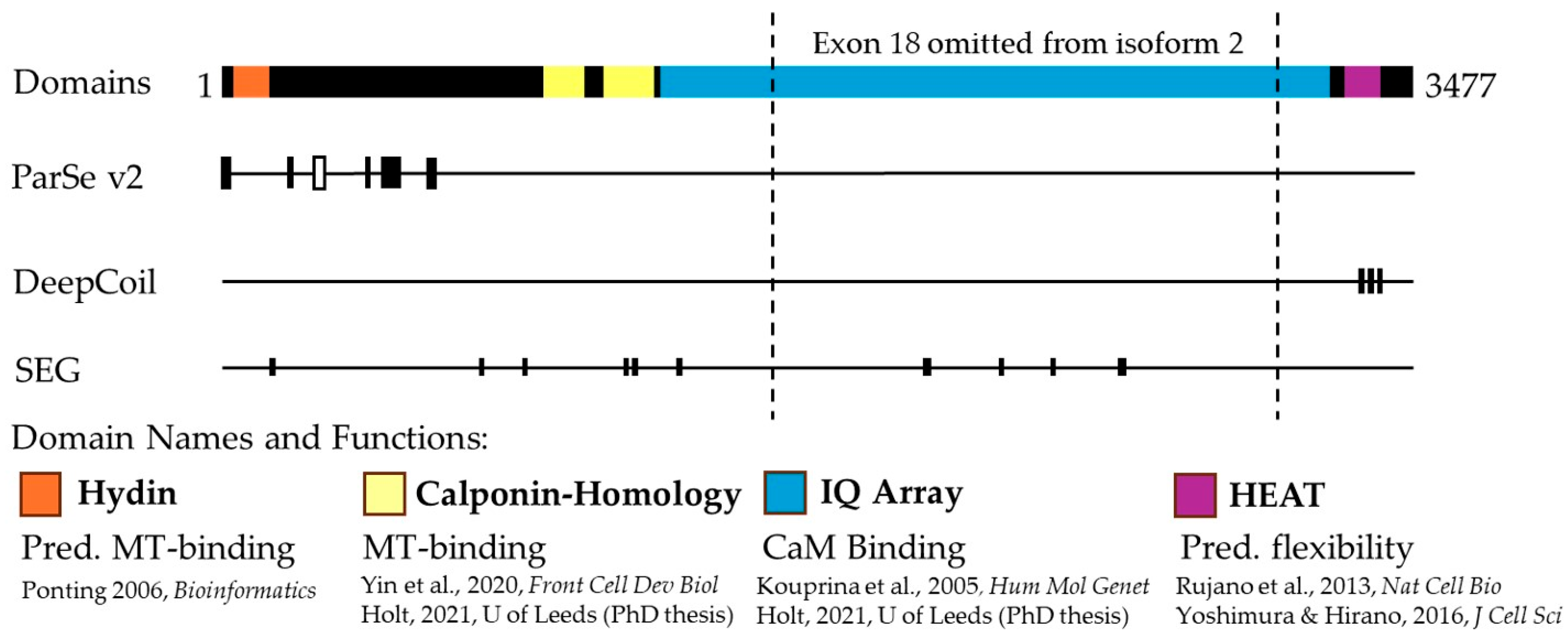
2.2. ASPM Has Characteristics of a Phase-Separating Protein
2.3. ASPM Functions in Areas of Condensate Formation

3. Future Directions: Understanding ASPM Dysfunction in Disease by Considering LLPS
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ripoll, P.; Pimpinelli, S.; Valdivia, M.M.; Avila, J. A Cell Division Mutant of Drosophila with a Functionally Abnormal Spindle. Cell 1985, 41, 907–912. [Google Scholar] [CrossRef]
- Gai, M.; Bianchi, F.T.; Vagnoni, C.; Vernì, F.; Bonaccorsi, S.; Pasquero, S.; Berto, G.E.; Sgrò, F.; Chiotto, A.M.; Annaratone, L.; et al. ASPM and CITK Regulate Spindle Orientation by Affecting the Dynamics of Astral Microtubules. EMBO Rep. 2016, 17, 1396–1409. [Google Scholar] [CrossRef]
- Higgins, J.; Midgley, C.; Bergh, A.-M.; Bell, S.M.; Askham, J.M.; Roberts, E.; Binns, R.K.; Sharif, S.M.; Bennett, C.; Glover, D.M.; et al. Human ASPM Participates in Spindle Organisation, Spindle Orientation and Cytokinesis. BMC Cell Biol. 2010, 11, 85. [Google Scholar] [CrossRef]
- Jiang, K.; Rezabkova, L.; Hua, S.; Liu, Q.; Capitani, G.; Altelaar, A.F.M.; Heck, A.J.R.; Kammerer, R.A.; Steinmetz, M.O.; Akhmanova, A. Microtubule Minus-End Regulation at Spindle Poles by an ASPM-Katanin Complex. Nat. Cell Biol. 2017, 19, 480–492. [Google Scholar] [CrossRef]
- Wakefield, J.G.; Bonaccorsi, S.; Gatti, M. The Drosophila Protein Asp Is Involved in Microtubule Organization during Spindle Formation and Cytokinesis. J. Cell Biol. 2001, 153, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Schoborg, T.; Zajac, A.L.; Fagerstrom, C.J.; Guillen, R.X.; Rusan, N.M. An Asp-CaM Complex Is Required for Centrosome-Pole Cohesion and Centrosome Inheritance in Neural Stem Cells. J. Cell Biol. 2015, 211, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Saunders, R.D.C.; Avides, M.d.C.; Howard, T.; Gonzalez, C.; Glover, D.M. The Drosophila Gene Abnormal Spindle Encodes a Novel Microtubule-Associated Protein That Associates with the Polar Regions of the Mitotic Spindle. J. Cell Biol. 1997, 137, 881–890. [Google Scholar] [CrossRef]
- Ito, A.; Goshima, G. Microcephaly Protein Asp Focuses the Minus Ends of Spindle Microtubules at the Pole and within the Spindle. J. Cell Biol. 2015, 211, 999–1009. [Google Scholar] [CrossRef]
- Razuvaeva, A.V.; Graziadio, L.; Palumbo, V.; Pavlova, G.A.; Popova, J.V.; Pindyurin, A.V.; Bonaccorsi, S.; Somma, M.P.; Gatti, M. The Multiple Mitotic Roles of the ASPM Orthologous Proteins: Insight into the Etiology of ASPM-Dependent Microcephaly. Cells 2023, 12, 922. [Google Scholar] [CrossRef]
- Tsai, K.K.; Bae, B.-I.; Hsu, C.-C.; Cheng, L.-H.; Shaked, Y. Oncogenic ASPM Is a Regulatory Hub of Developmental and Stemness Signaling in Cancers. Cancer Res. 2023, 83, 2993–3000. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.A.; Okayasu, R.; Jeggo, P.A.; Fujimori, A. ASPM Influences DNA Double-Strand Break Repair and Represents a Potential Target for Radiotherapy. Int. J. Radiat. Biol. 2011, 87, 1189–1195. [Google Scholar] [CrossRef]
- Xu, S.; Wu, X.; Wang, P.; Cao, S.-L.; Peng, B.; Xu, X. ASPM Promotes Homologous Recombination-Mediated DNA Repair by Safeguarding BRCA1 Stability. iScience 2021, 24, 102534. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xu, S.; Wang, P.; Wang, Z.-Q.; Chen, H.; Xu, X.; Peng, B. ASPM Promotes ATR-CHK1 Activation and Stabilizes Stalled Replication Forks in Response to Replication Stress. Proc. Natl. Acad. Sci. USA 2022, 119, e2203783119. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, J.; Wang, K.; Sun, J.; Yin, H.; Jiang, Y.; Liu, Y.; Wang, N.; Ding, X.; Gao, P.; et al. ASPM Mediates Nuclear Entrapment of FOXM1 via Liquid-Liquid Phase Separation to Promote Progression of Hepatocarcinoma. Genome Biol. 2025, 26, 68. [Google Scholar] [CrossRef]
- Mannino, M.C.; Cassidy, M.B.; Florez, S.; Rusan, Z.; Chakraborty, S.; Schoborg, T. Mutations in Abnormal Spindle Disrupt Temporal Transcription Factor Expression and Trigger Immune Responses in the Drosophila Brain. Genetics 2023, 225, iyad188. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, Z.; Wang, Z.-Q.; Xu, X. The Neurological and Non-Neurological Roles of the Primary Microcephaly-Associated Protein ASPM. Front. Neurosci. 2023, 17, 1242448. [Google Scholar] [CrossRef] [PubMed]
- Létard, P.; Drunat, S.; Vial, Y.; Duerinckx, S.; Ernault, A.; Amram, D.; Arpin, S.; Bertoli, M.; Busa, T.; Ceulemans, B.; et al. Autosomal Recessive Primary Microcephaly Due to ASPM Mutations: An Update. Hum. Mutat. 2018, 39, 319–332. [Google Scholar] [CrossRef]
- Muhammad, F.; Mahmood Baig, S.; Hansen, L.; Sajid Hussain, M.; Anjum Inayat, I.; Aslam, M.; Anver Qureshi, J.; Toilat, M.; Kirst, E.; Wajid, M.; et al. Compound Heterozygous ASPM Mutations in Pakistani MCPH Families. Am. J. Med. Genet. Part A 2009, 149A, 926–930. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Q.; Luh, F.; Jin, B.; Liu, X. Overexpression of the ASPM Gene Is Associated with Aggressiveness and Poor Outcome in Bladder Cancer. Oncol. Lett. 2019, 17, 1865–1876. [Google Scholar] [CrossRef]
- Lin, P.; Liang, L.; Dong, Y.; Ren, Z.; Zhao, H.; Li, G. Identification of Abnormal Spindle Microtubule Assembly as a Promising Therapeutic Target for Osteosarcoma. Orthop. Surg. 2020, 12, 1963–1970. [Google Scholar] [CrossRef]
- Ibrahim, A.; Atallah, N.M.; Makhlouf, S.; Toss, M.S.; Green, A.; Rakha, E. Deciphering the Role of ASPM in Breast Cancer: A Comprehensive Multicohort Study. Cancers 2024, 16, 3814. [Google Scholar] [CrossRef]
- Cabral de Carvalho Corrêa, D.; Dias Oliveira, I.; Mascaro Cordeiro, B.; Silva, F.A.; de Seixas Alves, M.T.; Saba-Silva, N.; Capellano, A.M.; Dastoli, P.; Cavalheiro, S.; Caminada de Toledo, S.R. Abnormal Spindle-like Microcephaly-Associated (ASPM) Gene Expression in Posterior Fossa Brain Tumors of Childhood and Adolescence. Childs Nerv. Syst. 2021, 37, 137–145. [Google Scholar] [CrossRef]
- Zeman, M.K.; Cimprich, K.A. Causes and Consequences of Replication Stress. Nat. Cell Biol. 2014, 16, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Rundle, S.; Bradbury, A.; Drew, Y.; Curtin, N.J. Targeting the ATR-CHK1 Axis in Cancer Therapy. Cancers 2017, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Bella, L.; Zona, S.; Nestal de Moraes, G.; Lam, E.W.-F. FOXM1: A Key Oncofoetal Transcription Factor in Health and Disease. Semin. Cancer Biol. 2014, 29, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Ryu, K.; Park, G.; Cho, W.-K. Emerging Insights into Transcriptional Condensates. Exp. Mol. Med. 2024, 56, 820–826. [Google Scholar] [CrossRef]
- Altmeyer, M.; Neelsen, K.J.; Teloni, F.; Pozdnyakova, I.; Pellegrino, S.; Grøfte, M.; Rask, M.-B.D.; Streicher, W.; Jungmichel, S.; Nielsen, M.L.; et al. Liquid Demixing of Intrinsically Disordered Proteins Is Seeded by Poly(ADP-Ribose). Nat. Commun. 2015, 6, 8088. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Zhao, W.-W.; Shi, J.; Wan, X.-B.; Zheng, J.; Fan, X.-J. Liquid-Liquid Phase Separation in DNA Double-Strand Breaks Repair. Cell Death Dis. 2023, 14, 746. [Google Scholar] [CrossRef]
- Choi, J.-M.; Holehouse, A.S.; Pappu, R.V. Physical Principles Underlying the Complex Biology of Intracellular Phase Transitions. Annu. Rev. Biophys. 2020, 49, 107–133. [Google Scholar] [CrossRef]
- Banani, S.F.; Lee, H.O.; Hyman, A.A.; Rosen, M.K. Biomolecular Condensates: Organizers of Cellular Biochemistry. Nat. Rev. Mol. Cell Biol. 2017, 18, 285–298. [Google Scholar] [CrossRef]
- Sipko, E.L.; Chappell, G.F.; Berlow, R.B. Multivalency Emerges as a Common Feature of Intrinsically Disordered Protein Interactions. Curr. Opin. Struct. Biol. 2024, 84, 102742. [Google Scholar] [CrossRef]
- Kouprina, N.; Pavlicek, A.; Collins, N.K.; Nakano, M.; Noskov, V.N.; Ohzeki, J.-I.; Mochida, G.H.; Risinger, J.I.; Goldsmith, P.; Gunsior, M.; et al. The Microcephaly ASPM Gene Is Expressed in Proliferating Tissues and Encodes for a Mitotic Spindle Protein. Hum. Mol. Genet. 2005, 14, 2155–2165. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.; Liao, W.; Chan, T.; Chen, W.; Lee, C.; Shan, Y.; Huang, P.; Hou, Y.; Li, C.; Tsai, K.K. The Differential Distributions of ASPM Isoforms and Their Roles in Wnt Signaling, Cell Cycle Progression, and Pancreatic Cancer Prognosis. J. Pathol. 2019, 249, 498–508. [Google Scholar] [CrossRef]
- Ponting, C.P. A Novel Domain Suggests a Ciliary Function for ASPM, a Brain Size Determining Gene. Bioinformatics 2006, 22, 1031–1035. [Google Scholar] [CrossRef]
- Yin, L.-M.; Schnoor, M.; Jun, C.-D. Structural Characteristics, Binding Partners and Related Diseases of the Calponin Homology (CH) Domain. Front. Cell Dev. Biol. 2020, 8, 342. [Google Scholar] [CrossRef]
- Holt, M.J.E. The Role of the Autosomal Recessive Primary Microcephaly Protein ASPM: A Protein Involved in Mitosis. Ph.D. Thesis, University of Leeds, Leeds, UK, 2021. [Google Scholar]
- Rujano, M.A.; Sanchez-Pulido, L.; Pennetier, C.; le Dez, G.; Basto, R. The Microcephaly Protein Asp Regulates Neuroepithelium Morphogenesis by Controlling the Spatial Distribution of Myosin II. Nat. Cell Biol. 2013, 15, 1294–1306. [Google Scholar] [CrossRef]
- Yoshimura, S.H.; Hirano, T. HEAT Repeats—Versatile Arrays of Amphiphilic Helices Working in Crowded Environments? J. Cell Sci. 2016, 129, 3963–3970. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Zhang, Z.; Zong, Z.; Zhang, L.; Zhou, F. Emerging Implications of Phase Separation in Cancer. Adv. Sci. 2022, 9, 2202855. [Google Scholar] [CrossRef]
- Dignon, G.L.; Best, R.B.; Mittal, J. Biomolecular Phase Separation: From Molecular Driving Forces to Macroscopic Properties. Annu. Rev. Phys. Chem. 2020, 71, 53–75. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.Y.; Khaodeuanepheng, N.P.; Amarasekara, D.L.; Correia, J.J.; Lewis, K.A.; Fitzkee, N.C.; Hough, L.E.; Whitten, S.T. Intrinsically Disordered Regions That Drive Phase Separation Form a Robustly Distinct Protein Class. J. Biol. Chem. 2023, 299, 102801. [Google Scholar] [CrossRef]
- Burns, M.C.; Borgal, L. Asp/ASPM Phospho-Regulation throughout the Cell Cycle. Genome 2024, 68, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, M.; Ma, W.; Yang, B.; Lu, H.; Zhou, F.; Zhang, L. Post-Translational Modifications in Liquid-Liquid Phase Separation: A Comprehensive Review. Mol. Biomed. 2022, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Valverde, J.M.; Dubra, G.; Phillips, M.; Haider, A.; Elena-Real, C.; Fournet, A.; Alghoul, E.; Chahar, D.; Andrés-Sanchez, N.; Paloni, M.; et al. A Cyclin-Dependent Kinase-Mediated Phosphorylation Switch of Disordered Protein Condensation. Nat. Commun. 2023, 14, 6316. [Google Scholar] [CrossRef]
- Yamazaki, H.; Takagi, M.; Kosako, H.; Hirano, T.; Yoshimura, S.H. Cell Cycle-Specific Phase Separation Regulated by Protein Charge Blockiness. Nat. Cell Biol. 2022, 24, 625–632. [Google Scholar] [CrossRef]
- Martin, E.W.; Mittag, T. Relationship of Sequence and Phase Separation in Protein Low-Complexity Regions. Biochemistry 2018, 57, 2478–2487. [Google Scholar] [CrossRef]
- Wootton, J.C. Non-Globular Domains in Protein Sequences: Automated Segmentation Using Complexity Measures. Comput. Chem. 1994, 18, 269–285. [Google Scholar] [CrossRef]
- Ramirez, D.A.; Hough, L.E.; Shirts, M.R. Coiled-Coil Domains Are Sufficient to Drive Liquid-Liquid Phase Separation in Protein Models. Biophys. J. 2024, 123, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Ludwiczak, J.; Winski, A.; Szczepaniak, K.; Alva, V.; Dunin-Horkawicz, S. DeepCoil—A Fast and Accurate Prediction of Coiled-Coil Domains in Protein Sequences. Bioinformatics 2019, 35, 2790–2795. [Google Scholar] [CrossRef]
- Chen, Z.; Hou, C.; Wang, L.; Yu, C.; Chen, T.; Shen, B.; Hou, Y.; Li, P.; Li, T. Screening Membraneless Organelle Participants with Machine-Learning Models That Integrate Multimodal Features. Proc. Natl. Acad. Sci. USA 2022, 119, e2115369119. [Google Scholar] [CrossRef]
- Kilic, S.; Lezaja, A.; Gatti, M.; Bianco, E.; Michelena, J.; Imhof, R.; Altmeyer, M. Phase Separation of 53BP1 Determines Liquid-like Behavior of DNA Repair Compartments. EMBO J. 2019, 38, e101379. [Google Scholar] [CrossRef]
- Levone, B.R.; Lenzken, S.C.; Antonaci, M.; Maiser, A.; Rapp, A.; Conte, F.; Reber, S.; Mechtersheimer, J.; Ronchi, A.E.; Mühlemann, O.; et al. FUS-Dependent Liquid-Liquid Phase Separation Is Important for DNA Repair Initiation. J. Cell Biol. 2021, 220, e202008030. [Google Scholar] [CrossRef]
- Chen, Q.; Kassab, M.A.; Dantzer, F.; Yu, X. PARP2 Mediates Branched Poly ADP-Ribosylation in Response to DNA Damage. Nat. Commun. 2018, 9, 3233. [Google Scholar] [CrossRef]
- Singatulina, A.S.; Hamon, L.; Sukhanova, M.V.; Desforges, B.; Joshi, V.; Bouhss, A.; Lavrik, O.I.; Pastré, D. PARP-1 Activation Directs FUS to DNA Damage Sites to Form PARG-Reversible Compartments Enriched in Damaged DNA. Cell Rep. 2019, 27, 1809–1821.e5. [Google Scholar] [CrossRef]
- Frattini, C.; Promonet, A.; Alghoul, E.; Vidal-Eychenie, S.; Lamarque, M.; Blanchard, M.-P.; Urbach, S.; Basbous, J.; Constantinou, A. TopBP1 Assembles Nuclear Condensates to Switch on ATR Signaling. Mol. Cell 2021, 81, 1231–1245.e8. [Google Scholar] [CrossRef] [PubMed]
- Akhmanova, A.; Steinmetz, M.O. Microtubule Minus-End Regulation at a Glance. J. Cell Sci. 2019, 132, jcs227850. [Google Scholar] [CrossRef]
- Borgal, L.; Wakefield, J.G. Context-Dependent Spindle Pole Focusing. Essays Biochem. 2018, 62, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Yang, Y.; Zhou, J.; Liu, P. Liquid–Liquid Phase Separation of Microtubule-Binding Proteins in the Regulation of Spindle Assembly. Cell Prolif. 2024, 57, e13649. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Ho, D.B.T.; Mahe, K.; Mia, J.; Sepulveda, G.; Antkowiak, M.; Jiang, L.; Yamada, S.; Jao, L.-E. Condensation of Pericentrin Proteins in Human Cells Illuminates Phase Separation in Centrosome Assembly. J. Cell Sci. 2021, 134, jcs258897. [Google Scholar] [CrossRef]
- Parra, A.S.; Johnston, C.A. Phase Separation as a Driver of Stem Cell Organization and Function during Development. J. Dev. Biol. 2023, 11, 45. [Google Scholar] [CrossRef]
- Mehta, S.; Zhang, J. Liquid–Liquid Phase Separation Drives Cellular Function and Dysfunction in Cancer. Nat. Rev. Cancer 2022, 22, 239–252. [Google Scholar] [CrossRef]
- Boija, A.; Klein, I.A.; Young, R.A. Biomolecular Condensates and Cancer. Cancer Cell 2021, 39, 174–192. [Google Scholar] [CrossRef]
- Sołtys, K.; Tarczewska, A.; Bystranowska, D. Modulation of Biomolecular Phase Behavior by Metal Ions. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2023, 1870, 119567. [Google Scholar] [CrossRef] [PubMed]
- Bähler, M.; Rhoads, A. Calmodulin Signaling via the IQ Motif. FEBS Lett. 2002, 513, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.G.; Guimarães, E.S.; Andrade, L.M.; Menezes, G.B.; Fatima Leite, M. Decoding Calcium Signaling across the Nucleus. Physiology 2014, 29, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Takuwa, N.; Zhou, W.; Takuwa, Y. Calcium, Calmodulin and Cell Cycle Progression. Cell. Signal. 1995, 7, 93–104. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate Structure Prediction of Biomolecular Interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- Monti, M.; Fiorentino, J.; Miltiadis-Vrachnos, D.; Bini, G.; Cotrufo, T.; Sanchez de Groot, N.; Armaos, A.; Tartaglia, G.G. catGRANULE 2.0: Accurate Predictions of Liquid-Liquid Phase Separating Proteins at Single Amino Acid Resolution. Genome Biol. 2025, 26, 33. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fenwick, G.; Borgal, L. The Multiple DNA-Associated Roles of ASPM and Liquid–Liquid Phase Separation as a Unifying Mechanism of Function. DNA 2025, 5, 55. https://doi.org/10.3390/dna5040055
Fenwick G, Borgal L. The Multiple DNA-Associated Roles of ASPM and Liquid–Liquid Phase Separation as a Unifying Mechanism of Function. DNA. 2025; 5(4):55. https://doi.org/10.3390/dna5040055
Chicago/Turabian StyleFenwick, Gabrielle, and Lori Borgal. 2025. "The Multiple DNA-Associated Roles of ASPM and Liquid–Liquid Phase Separation as a Unifying Mechanism of Function" DNA 5, no. 4: 55. https://doi.org/10.3390/dna5040055
APA StyleFenwick, G., & Borgal, L. (2025). The Multiple DNA-Associated Roles of ASPM and Liquid–Liquid Phase Separation as a Unifying Mechanism of Function. DNA, 5(4), 55. https://doi.org/10.3390/dna5040055





