Captain Tardigrade and Its Shield to Protect DNA
Abstract
1. The Biology of Tardigrades
2. Dsup Protein
3. Dsup’s Mechanism(s) of Binding to DNA
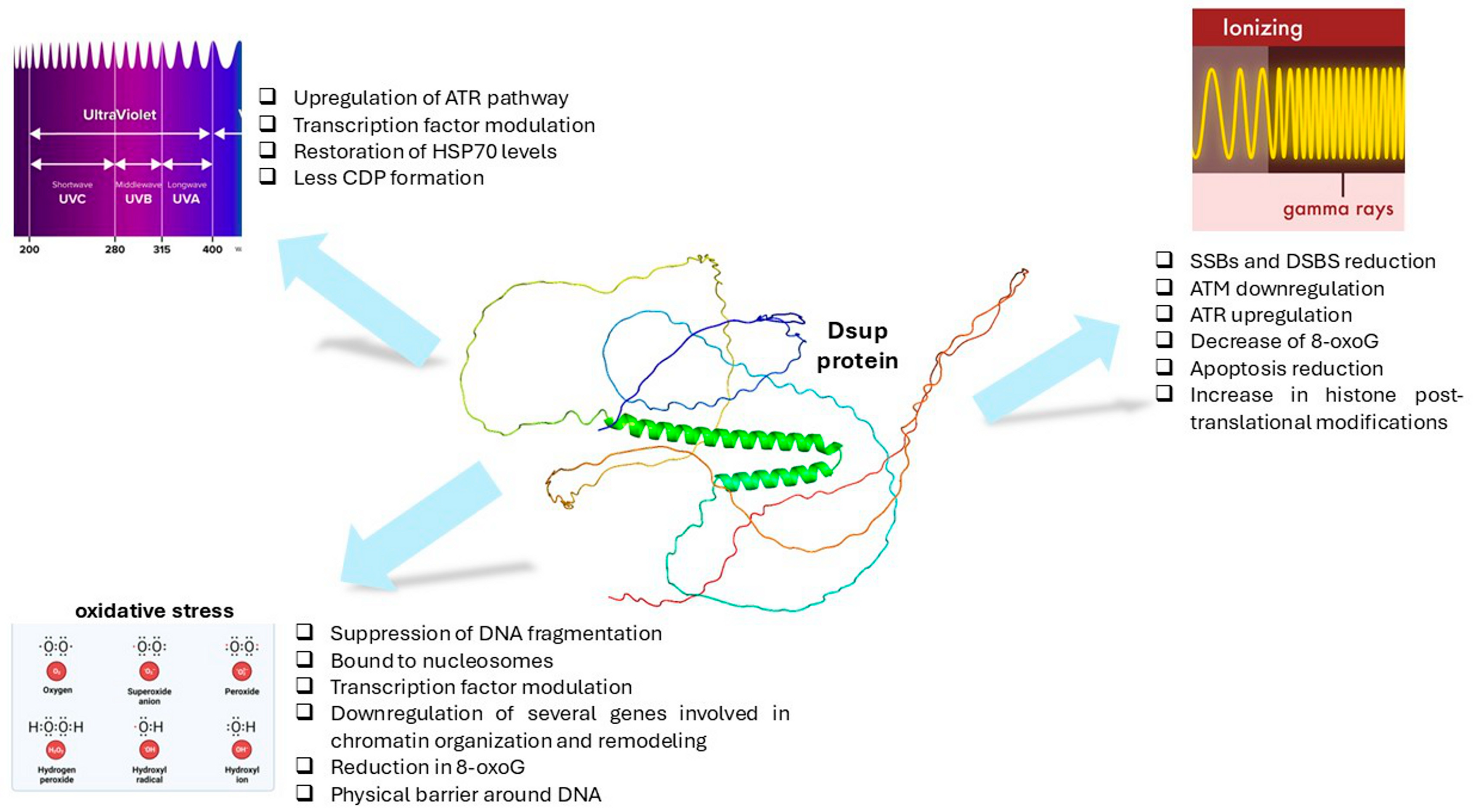
4. DSUP and DNA Protection
4.1. Oxidative Stress
4.1.1. DSUP Role in Response to Oxidative Stress
4.2. UV Radiation
4.2.1. DSUP Role in Response to UV Exposure
4.3. Ionizing Radiation
4.3.1. DSUP Role in Response to Ionizing Radiation
5. Discussion
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schill, R.O. (Ed.) Water Bears: The Biology of Tardigrades; Zoological Monographs; Springer International Publishing: Cham, Switzerland, 2018; Volume 2. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Antioxidant Defense in the Toughest Animals on the Earth: Its Contribution to the Extreme Resistance of Tardigrades. Int. J. Mol. Sci. 2024, 25, 8393. [Google Scholar] [CrossRef]
- Hashimoto, T.; Horikawa, D.D.; Saito, Y.; Kuwahara, H.; Kozuka-Hata, H.; Shin-I, T.; Minakuchi, Y.; Ohishi, K.; Motoyama, A.; Aizu, T.; et al. Extremotolerant Tardigrade Genome and Improved Radiotolerance of Human Cultured Cells by Tardigrade-Unique Protein. Nat. Commun. 2016, 7, 12808. [Google Scholar] [CrossRef]
- Mínguez-Toral, M.; Cuevas-Zuviría, B.; Garrido-Arandia, M.; Pacios, L.F. A Computational Structural Study on the DNA-Protecting Role of the Tardigrade-Unique Dsup Protein. Sci. Rep. 2020, 10, 13424. [Google Scholar] [CrossRef] [PubMed]
- Bemm, F.; Burleigh, L.; Förster, F.; Schmucki, R.; Ebeling, M.; Janzen, C.J.; Dandekar, T.; Schill, R.O.; Certa, U.; Schultz, J. Draft genome of the Eutardigrade Milnesium tardigradum sheds light on ecdysozoan evolution. bioRxiv 2017, bioRxiv: 122309. [Google Scholar] [CrossRef]
- Anoud, M.; Delagoutte, E.; Helleu, Q.; Brion, A.; Duvernois-Berthet, E.; As, M.; Marques, X.; Lamribet, K.; Senamaud-Beaufort, C.; Jourdren, L.; et al. Comparative transcriptomics reveal a novel tardigrade-specific DNA-binding protein induced in response to ionizing radiation. eLife 2024, 13, RP92621. [Google Scholar] [CrossRef] [PubMed]
- Chavez, C.; Cruz-Becerra, G.; Fei, J.; Kassavetis, G.A.; Kadonaga, J.T. The Tardigrade Damage Suppressor Protein Binds to Nucleosomes and Protects DNA from Hydroxyl Radicals. eLife 2019, 8, e47682. [Google Scholar] [CrossRef] [PubMed]
- Zarubin, M.; Murugova, T.; Ryzhykau, Y.; Ivankov, O.; Uversky, V.N.; Kravchenko, E. Structural Study of the Intrinsically Disordered Tardigrade Damage Suppressor Protein (Dsup) and Its Complex with DNA. Sci. Rep. 2024, 14, 22910. [Google Scholar] [CrossRef]
- Receveur-Brechot, V.; Durand, D. How random are intrinsically disordered proteins? A small angle scattering perspective. Curr. Protein Pept. Sci. 2012, 13, 55–75. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, Oxidative Stress and the Biology of Ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Girard, P.M.; Boiteux, S. Repair of Oxidized DNA Bases in the Yeast Saccharomyces Cerevisiae. Biochimie 1997, 79, 559–566. [Google Scholar] [CrossRef]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA Damage, Repair, and Mutagenesis. Environ. Mol. Mutagen. 2017, 58, 235–263. [Google Scholar] [CrossRef] [PubMed]
- Imlay, J.A.; Chin, S.M.; Linn, S. Toxic DNA Damage by Hydrogen Peroxide through the Fenton Reaction in Vivo and in Vitro. Science 1988, 240, 640–642. [Google Scholar] [CrossRef]
- Dizdaroglu, M.; Rao, G.; Halliwell, B.; Gajewski, E. Damage to the DNA Bases in Mammalian Chromatin by Hydrogen Peroxide in the Presence of Ferric and Cupric Ions. Arch. Biochem. Biophys. 1991, 285, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Chetsanga, C.J.; Lozon, M.; Makaroff, C.; Savage, L. Purification and Characterization of Escherichia Coli Formamidopyrimidine-DNA Glycosylase That Excises Damaged 7-Methylguanine from Deoxyribonucleic Acid. Biochemistry 1981, 20, 5201–5207. [Google Scholar] [CrossRef] [PubMed]
- Friedberg, E.C.; Walker, G.C.; Siede, W.; Wood, R.D.; Schultz, R.A.; Ellenberger, T. DNA Repair and Mutagenesis; ASM Press: Washington, DC, USA, 2005. [Google Scholar] [CrossRef]
- Steenken, S.; Jovanovic, S.V. How Easily Oxidizable Is DNA? One-Electron Reduction Potentials of Adenosine and Guanosine Radicals in Aqueous Solution. J. Am. Chem. Soc. 1997, 119, 617–618. [Google Scholar] [CrossRef]
- Kasai, H.; Nishimura, S. Hydroxylation of Deoxyguanosine at the C-8 Position by Ascorbic Acid and Other Reducing Agents. Nucleic Acids Res. 1984, 12, 2137–2145. [Google Scholar] [CrossRef]
- Cheng, K.C.; Cahill, D.S.; Kasai, H.; Nishimura, S.; Loeb, L.A. 8-Hydroxyguanine, an Abundant Form of Oxidative DNA Damage, Causes G-T and A-C Substitutions. J. Biol. Chem. 1992, 267, 166–172. [Google Scholar] [CrossRef]
- Cadet, J.; Delatour, T.; Douki, T.; Gasparutto, D.; Pouget, J.P.; Ravanat, J.L.; Sauvaigo, S. Hydroxyl Radicals and DNA Base Damage. Mutat. Res./Fundam. Mol. Mech. Mutagen. 1999, 424, 9–21. [Google Scholar] [CrossRef]
- Cadet, J.; Douki, T.; Ravanat, J.-L. Oxidatively Generated Base Damage to Cellular DNA. Free Radic. Biol. Med. 2010, 49, 9–21. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Demple, B.; Harrison, L. Repair of Oxidative Damage to DNA: Enzymology and Biology. Annu. Rev. Biochem. 1994, 63, 915–948. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, B.; Pogozelski, W.K.; Tullius, T.D. DNA Strand Breaking by the Hydroxyl Radical Is Governed by the Accessible Surface Areas of the Hydrogen Atoms of the DNA Backbone. Proc. Natl. Acad. Sci. USA 1998, 95, 9738–9743. [Google Scholar] [CrossRef] [PubMed]
- González-Romero, R.; Eirín-López, J.M.; Ausió, J. Evolution of High Mobility Group Nucleosome-Binding Proteins and Its Implications for Vertebrate Chromatin Specialization. Mol. Biol. Evol. 2015, 32, 121–131. [Google Scholar] [CrossRef]
- Ricci, C.; Riolo, G.; Marzocchi, C.; Brunetti, J.; Pini, A.; Cantara, S. The Tardigrade Damage Suppressor Protein Modulates Transcription Factor and DNA Repair Genes in Human Cells Treated with Hydroxyl Radicals and UV-C. Biology 2021, 10, 970. [Google Scholar] [CrossRef] [PubMed]
- Marinho, H.S.; Real, C.; Cyrne, L.; Soares, H.; Antunes, F. Hydrogen Peroxide Sensing, Signaling and Regulation of Transcription Factors. Redox Biol. 2014, 2, 535–562. [Google Scholar] [CrossRef]
- Shi, Y.; Venkataraman, S.L.; Dodson, G.E.; Mabb, A.M.; LeBlanc, S.; Tibbetts, R.S. Direct Regulation of CREB Transcriptional Activity by ATM in Response to Genotoxic Stress. Proc. Natl. Acad. Sci. USA 2004, 101, 5898–5903. [Google Scholar] [CrossRef]
- Pregi, N.; Belluscio, L.M.; Berardino, B.G.; Castillo, D.S.; Cánepa, E.T. Oxidative Stress-Induced CREB Upregulation Promotes DNA Damage Repair Prior to Neuronal Cell Death Protection. Mol. Cell. Biochem. 2017, 425, 9–24. [Google Scholar] [CrossRef]
- Rajabi, H.N.; Baluchamy, S.; Kolli, S.; Nag, A.; Srinivas, R.; Raychaudhuri, P.; Thimmapaya, B. Effects of Depletion of CREB-Binding Protein on c-Myc Regulation and Cell Cycle G1-S Transition. J. Biol. Chem. 2005, 280, 361–374. [Google Scholar] [CrossRef]
- Ali, A.A.E.; Timinszky, G.; Arribas-Bosacoma, R.; Kozlowski, M.; Hassa, P.O.; Hassler, M.; Ladurner, A.G.; Pearl, L.H.; Oliver, A.W. The Zinc-Finger Domains of PARP1 Cooperate to Recognize DNA Strand Breaks. Nat. Struct. Mol. Biol. 2012, 19, 685–692. [Google Scholar] [CrossRef]
- Li, M.; Yu, X. Function of BRCA1 in the DNA Damage Response Is Mediated by ADP-Ribosylation. Cancer Cell 2013, 23, 693–704. [Google Scholar] [CrossRef]
- Indran, I.R.; Hande, M.P.; Pervaiz, S. hTERT Overexpression Alleviates Intracellular ROS Production, Improves Mitochondrial Function, and Inhibits ROS-Mediated Apoptosis in Cancer Cells. Cancer Res. 2011, 71, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Zarubin, M.; Azorskaya, T.; Kuldoshina, O.; Alekseev, S.; Mitrofanov, S.; Kravchenko, E. The Tardigrade Dsup Protein Enhances Radioresistance in Drosophila Melanogaster and Acts as an Unspecific Repressor of Transcription. iScience 2023, 26, 106998. [Google Scholar] [CrossRef]
- Tyler, J.; Aguilar, R.; Arslanovic, N.; Birmingham, K.; Kaliwal, K.; Chakraborty, U.; Postnikoff, S.; Hickman, A.; Kahn, L.; Watson, R.; et al. Multivalent Binding of the Tardigrade Dsup Protein to Chromatin Promotes Yeast Survival and Longevity upon Exposure to Oxidative Damage. Res. Sq. 2023, rs-3, rs-3182883. [Google Scholar] [CrossRef]
- Ni, G.S.; Su, H.; Zhu, Y.; Dhiman, A.; Zhou, H.-X.; Lin, W.; Hao, N. Tardigrade Dsup: Interactions with DNA and Protection of Cells from Oxidative Stress. bioRxiv 2024, bioRxiv:11.06.622393. [Google Scholar] [CrossRef]
- Davies, R.J. Royal Irish Academy Medal Lecture. Ultraviolet Radiation Damage in DNA. Biochem. Soc. Trans. 1995, 23, 407–418. [Google Scholar] [CrossRef]
- Varghese, A.J. Photochemistry of Nucleic Acids and Their Constituents. Photophysiology 1972, 7, 207–274. [Google Scholar]
- Mitchell, D.L.; Nairn, R.S. The Biology of the (6-4) Photoproduct. Photochem. Photobiol. 1989, 49, 805–819. [Google Scholar] [CrossRef] [PubMed]
- You, Y.H.; Szabó, P.E.; Pfeifer, G.P. Cyclobutane Pyrimidine Dimers Form Preferentially at the Major P53 Mutational Hotspot in UVB-Induced Mouse Skin Tumors. Carcinogenesis 2000, 21, 2113–2117. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Richa; Kumar, A.; Tyagi, M.B.; Sinha, R.P. Molecular Mechanisms of Ultraviolet Radiation-Induced DNA Damage and Repair. J. Nucleic Acids 2010, 2010, 592980. [Google Scholar] [CrossRef]
- Peak, M.J.; Peak, J.G. DNA-to-Protein Crosslinks and Backbone Breaks Caused by Far- and near-Ultraviolet, and Visible Light Radiations in Mammalian Cells. Basic Life Sci. 1986, 38, 193–202. [Google Scholar] [CrossRef]
- Sinha, R.P.; Häder, D.P. UV-Induced DNA Damage and Repair: A Review. Photochem. Photobiol. Sci. 2002, 1, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Kirke, J.; Jin, X.-L.; Zhang, X.-H. Expression of a Tardigrade Dsup Gene Enhances Genome Protection in Plants. Mol. Biotechnol. 2020, 62, 563–571. [Google Scholar] [CrossRef]
- Oztas, O.; Selby, C.P.; Sancar, A.; Adebali, O. Genome-Wide Excision Repair in Arabidopsis Is Coupled to Transcription and Reflects Circadian Gene Expression Patterns. Nat. Commun. 2018, 9, 1503. [Google Scholar] [CrossRef] [PubMed]
- Britt, A.B. DNA Damage and Repair in Plants. Annu. Rev. Plant Biol. 1996, 47, 75–100. [Google Scholar] [CrossRef] [PubMed]
- Manova, V.; Gruszka, D. DNA Damage and Repair in Plants—From Models to Crops. Front. Plant Sci. 2015, 6, 885. [Google Scholar] [CrossRef]
- Pathania, S.; Nguyen, J.; Hill, S.J.; Scully, R.; Adelmant, G.O.; Marto, J.A.; Feunteun, J.; Livingston, D.M. BRCA1 Is Required for Postreplication Repair after UV-Induced DNA Damage. Mol. Cell 2011, 44, 235–251. [Google Scholar] [CrossRef]
- Pavey, S.; Pinder, A.; Fernando, W.; D’Arcy, N.; Matigian, N.; Skalamera, D.; Lê Cao, K.-A.; Loo-Oey, D.; Hill, M.M.; Stark, M.; et al. Multiple Interaction Nodes Define the Postreplication Repair Response to UV-Induced DNA Damage That Is Defective in Melanomas and Correlated with UV Signature Mutation Load. Mol. Oncol. 2020, 14, 22–41. [Google Scholar] [CrossRef]
- Lefkofsky, H.B.; Veloso, A.; Ljungman, M. Transcriptional and Post-Transcriptional Regulation of Nucleotide Excision Repair Genes in Human Cells. Mutat. Res. Mol. Mech. Mutagen. 2015, 776, 9–15. [Google Scholar] [CrossRef]
- Marais, T.L.D.; Kluz, T.; Xu, D.; Zhang, X.; Gesumaria, L.; Matsui, M.S.; Costa, M.; Sun, H. Transcription Factors and Stress Response Gene Alterations in Human Keratinocytes Following Solar Simulated Ultra Violet Radiation. Sci. Rep. 2017, 7, 13622. [Google Scholar] [CrossRef]
- Ismail, A.; Yusuf, N. Type I Interferons: Key Players in Normal Skin and Select Cutaneous Malignancies. Dermatol. Res. Pract. 2014, 2014, 847545. [Google Scholar] [CrossRef]
- Shaba, E.; Landi, C.; Marzocchi, C.; Vantaggiato, L.; Bini, L.; Ricci, C.; Cantara, S. Proteomics Reveals How the Tardigrade Damage Suppressor Protein Teaches Transfected Human Cells to Survive UV-C Stress. Int. J. Mol. Sci. 2023, 24, 11463. [Google Scholar] [CrossRef] [PubMed]
- Roh, B.H.; Kim, D.H.; Cho, M.K.; Park, Y.L.; Whang, K.U. Expression of Heat Shock Protein 70 in Human Skin Cells as a Photoprotective Function after UV Exposure. Ann. Dermatol. 2008, 20, 184–189. [Google Scholar] [CrossRef] [PubMed]
- López-Camarillo, C.; Aréchaga Ocampo, E.; López Casamichana, M.; Pérez-Plasencia, C.; Álvarez-Sánchez, E.; Marchat, L.A. Protein Kinases and Transcription Factors Activation in Response to UV-Radiation of Skin: Implications for Carcinogenesis. Int. J. Mol. Sci. 2011, 13, 142–172. [Google Scholar] [CrossRef]
- Andrieux, L.O.; Fautrel, A.; Bessard, A.; Guillouzo, A.; Baffet, G.; Langouët, S. GATA-1 Is Essential in EGF-Mediated Induction of Nucleotide Excision Repair Activity and ERCC1 Expression through ERK2 in Human Hepatoma Cells. Cancer Res. 2007, 67, 2114–2123. [Google Scholar] [CrossRef]
- McAvera, R.M.; Crawford, L.J. TIF1 Proteins in Genome Stability and Cancer. Cancers 2020, 12, 2094. [Google Scholar] [CrossRef]
- Hu, C.; Yang, J.; Qi, Z.; Wu, H.; Wang, B.; Zou, F.; Mei, H.; Liu, J.; Wang, W.; Liu, Q. Heat Shock Proteins: Biological Functions, Pathological Roles, and Therapeutic Opportunities. MedComm 2022, 3, e161. [Google Scholar] [CrossRef] [PubMed]
- Moudry, P.; Lukas, C.; Macurek, L.; Hanzlikova, H.; Hodny, Z.; Lukas, J.; Bartek, J. Ubiquitin-Activating Enzyme UBA1 Is Required for Cellular Response to DNA Damage. Cell Cycle 2012, 11, 1573–1582. [Google Scholar] [CrossRef]
- Wang, Q.; Wani, M.A.; Chen, J.; Zhu, Q.; Wani, G.; El-Mahdy, M.A.; Wani, A.A. Cellular Ubiquitination and Proteasomal Functions Positively Modulate Mammalian Nucleotide Excision Repair. Mol. Carcinog. 2005, 42, 53–64. [Google Scholar] [CrossRef]
- Ye, C.; Guo, J.; Zhou, X.-Q.; Chen, D.-G.; Liu, J.; Peng, X.; Jaremko, M.; Jaremko, Ł.; Guo, T.; Liu, C.-G.; et al. The Dsup Coordinates Grain Development and Abiotic Stress in Rice. Plant Physiol. Biochem. PPB 2023, 205, 108184. [Google Scholar] [CrossRef]
- Del Casino, C.; Conti, V.; Licata, S.; Cai, G.; Cantore, A.; Ricci, C.; Cantara, S. Mitigation of UV-B Radiation Stress in Tobacco Pollen by Expression of the Tardigrade Damage Suppressor Protein (Dsup). Cells 2024, 13, 840. [Google Scholar] [CrossRef]
- Torabinejad, J.; Caldwell, M.M.; Flint, S.D.; Durham, S. Susceptibility of Pollen to UV-B Radiation: An Assay of 34 Taxa. Am. J. Bot. 1998, 85, 360–369. [Google Scholar] [CrossRef]
- Desouky, O.; Ding, N.; Zhou, G. Targeted and Non-Targeted Effects of Ionizing Radiation. J. Radiat. Res. Appl. Sci. 2015, 8, 247–254. [Google Scholar] [CrossRef]
- Wardman, P. The Importance of Radiation Chemistry to Radiation and Free Radical Biology (The 2008 Silvanus Thompson Memorial Lecture). Br. J. Radiol. 2009, 82, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Vignard, J.; Mirey, G.; Salles, B. Ionizing-Radiation Induced DNA Double-Strand Breaks: A Direct and Indirect Lighting Up. Radiother. Oncol. 2013, 108, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Henner, W.D.; Grunberg, S.M.; Haseltine, W.A. Sites and Structure of Gamma Radiation-Induced DNA Strand Breaks. J. Biol. Chem. 1982, 257, 11750–11754. [Google Scholar] [CrossRef] [PubMed]
- Henner, W.D.; Rodriguez, L.O.; Hecht, S.M.; Haseltine, W.A. Gamma Ray Induced Deoxyribonucleic Acid Strand Breaks. 3′ Glycolate Termini. J. Biol. Chem. 1983, 258, 711–713. [Google Scholar] [CrossRef]
- Obe, G.; Johannes, C.; Schulte-Frohlinde, D. DNA Double-Strand Breaks Induced by Sparsely Ionizing Radiation and Endonucleases as Critical Lesions for Cell Death, Chromosomal Aberrations, Mutations and Oncogenic Transformation. Mutagenesis 1992, 7, 3–12. [Google Scholar] [CrossRef]
- Hutchinson, F. Chemical Changes Induced in DNA by Ionizing Radiation. Prog. Nucleic Acid Res. Mol. Biol. 1985, 32, 115–154. [Google Scholar] [CrossRef]
- Iliakis, G. The Role of DNA Double Strand Breaks in Ionizing Radiation-Induced Killing of Eukaryotic Cells. BioEssays 1991, 13, 641–648. [Google Scholar] [CrossRef]
- Lomax, M.E.; Folkes, L.K.; O’Neill, P. Biological Consequences of Radiation-Induced DNA Damage: Relevance to Radiotherapy. Clin. Oncol. 2013, 25, 578–585. [Google Scholar] [CrossRef]
- Andrievski, A.; Wilkins, R.C. The Response of Gamma-H2AX in Human Lymphocytes and Lymphocytes Subsets Measured in Whole Blood Cultures. Int. J. Radiat. Biol. 2009, 85, 369–376. [Google Scholar] [CrossRef]
- Puck, T.T.; Marcus, P.I. Action of X-Rays on Mammalian Cells. J. Exp. Med. 1956, 103, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Westover, C.; Najjar, D.; Meydan, C.; Grigorev, K.; Veling, M.T.; Chang, R.L.; Chin, C.; Butler, D.; Afshin, E.E.; Silver, P.A.; et al. Multi-Omics Analysis of Dsup Expressing Human Cells Reveals Open Chromatin Architectural Dynamics Underyling Radioprotection. bioRxiv 2020, bioRxiv:10.373571. [Google Scholar] [CrossRef]
- Mowery, C.T.; Reyes, J.M.; Cabal-Hierro, L.; Higby, K.J.; Karlin, K.L.; Wang, J.H.; Kimmerling, R.J.; Cejas, P.; Lim, K.; Li, H.; et al. Trisomy of a Down Syndrome Critical Region Globally Amplifies Transcription via HMGN1 Overexpression. Cell Rep. 2018, 25, 1898–1911.e5. [Google Scholar] [CrossRef] [PubMed]
- Puig, J.; Knödlseder, N.; Quera, J.; Algara, M.; Güell, M. DNA Damage Protection for Enhanced Bacterial Survival Under Simulated Low Earth Orbit Environmental Conditions in Escherichia Coli. Front. Microbiol. 2021, 12, 789668. [Google Scholar] [CrossRef]
- Shiotani, B.; Zou, L. ATR Signaling at a Glance. J. Cell Sci. 2009, 122, 301–304. [Google Scholar] [CrossRef]
- Santamaria, N.; Alhothali, M.; Alfonso, M.H.; Breydo, L.; Uversky, V.N. Intrinsic Disorder in Proteins Involved in Amyotrophic Lateral Sclerosis. Cell. Mol. Life Sci. CMLS 2017, 74, 1297–1318. [Google Scholar] [CrossRef]
- Hazelett, D.J.; Chang, J.-C.; Lakeland, D.L.; Morton, D.B. Comparison of Parallel High-Throughput RNA Sequencing Between Knockout of TDP-43 and Its Overexpression Reveals Primarily Nonreciprocal and Nonoverlapping Gene Expression Changes in the Central Nervous System of Drosophila. G3 Genes|Genomes|Genet. 2012, 2, 789–802. [Google Scholar] [CrossRef]
- Nagao, M.; Lanjakornsiripan, D.; Itoh, Y.; Kishi, Y.; Ogata, T.; Gotoh, Y. High Mobility Group Nucleosome-Binding Family Proteins Promote Astrocyte Differentiation of Neural Precursor Cells. Stem Cells 2014, 32, 2983–2997. [Google Scholar] [CrossRef]
- Escarcega, R.D.; Patil, A.A.; Meyer, M.D.; Moruno-Manchon, J.F.; Silvagnoli, A.D.; McCullough, L.D.; Tsvetkov, A.S. The Tardigrade Damage Suppressor Protein Dsup Promotes DNA Damage in Neurons. Mol. Cell. Neurosci. 2023, 125, 103826. [Google Scholar] [CrossRef]
- Nanduri, R.; Furusawa, T.; Bustin, M. Biological Functions of HMGN Chromosomal Proteins. Int. J. Mol. Sci. 2020, 21, 449. [Google Scholar] [CrossRef] [PubMed]
- Kugler, J.; Postnikov, Y.V.; Furusawa, T.; Kimura, S.; Bustin, M. Elevated HMGN4 Expression Potentiates Thyroid Tumorigenesis. Carcinogenesis 2017, 38, 391–401. [Google Scholar] [CrossRef]
- Iyama, T.; Wilson, D.M. DNA Repair Mechanisms in Dividing and Non-Dividing Cells. DNA Repair 2013, 12, 620–636. [Google Scholar] [CrossRef]
- Musselman, C.A.; Kutateladze, T.G. Characterization of Functional Disordered Regions within Chromatin-Associated Proteins. iScience 2021, 24, 102070. [Google Scholar] [CrossRef]
- Miskei, M.; Horvath, A.; Vendruscolo, M.; Fuxreiter, M. Sequence-Based Prediction of Fuzzy Protein Interactions. J. Mol. Biol. 2020, 432, 2289–2303. [Google Scholar] [CrossRef] [PubMed]
- Darling, A.L.; Uversky, V.N. Intrinsic Disorder and Posttranslational Modifications: The Darker Side of the Biological Dark Matter. Front. Genet. 2018, 9, 158. [Google Scholar] [CrossRef]
- van der Lee, R.; Buljan, M.; Lang, B.; Weatheritt, R.J.; Daughdrill, G.W.; Dunker, A.K.; Fuxreiter, M.; Gough, J.; Gsponer, J.; Jones, D.T.; et al. Classification of Intrinsically Disordered Regions and Proteins. Chem. Rev. 2014, 114, 6589–6631. [Google Scholar] [CrossRef] [PubMed]
- Iakoucheva, L.M.; Radivojac, P.; Brown, C.J.; O’Connor, T.R.; Sikes, J.G.; Obradovic, Z.; Dunker, A.K. The Importance of Intrinsic Disorder for Protein Phosphorylation. Nucleic Acids Res. 2004, 32, 1037–1049. [Google Scholar] [CrossRef]
- Kasianchuk, N.; Rzymski, P.; Kaczmarek, Ł. The Biomedical Potential of Tardigrade Proteins: A Review. Biomed. Pharmacother. 2023, 158, 114063. [Google Scholar] [CrossRef]
- Kirtane, A.R.; Bi, J.; Rajesh, N.U.; Tang, C.; Jimenez, M.; Witt, E.; McGovern, M.K.; Cafi, A.B.; Hatfield, S.J.; Rosenstock, L.; et al. Radioprotection of Healthy Tissue via Nanoparticle-Delivered mRNA Encoding for a Damage-Suppressor Protein Found in Tardigrades. Nat. Biomed. Eng. 2025, 1–14. [Google Scholar] [CrossRef]
- Zarubin, M.; Andreev, E.; Kravchenko, E.; Pinaeva, U.; Nechaev, A.; Apel, P. Developing Tardigrade-inspired Material: Track Membranes Functionalized with Dsup Protein for Cell-free DNA Isolation. Biotechnol. Prog. 2024, 40, e3478. [Google Scholar] [CrossRef] [PubMed]
- Klomchitcharoen, S.; Tangwattanasirikun, T.; Gallup, S.; Smerwong, N.; Arunwiriyakit, P.; Tachavises, P.; Tangkijngamwong, J.; Phatthanaanukun, P.; Jirapanyalerd, B.; Chattanupakorn, S.; et al. MINERVA: A CubeSat for Demonstrating DNA Damage Mitigation against Space Radiation in C. Elegans by Using Genetic Modification. Heliyon 2022, 8, e10267. [Google Scholar] [CrossRef] [PubMed]

| Parameters | PBS | H2O | H2O + Urea |
|---|---|---|---|
| Radius of gyration (Rg) (Å) * | 53 | 63 | 80 |
| Flory exponent (ν) ** | 0.5 | 0.58 | |
| Maximum dimension (Dm) | More compact state | Expanded conformation | |
| Shape and 3D model | 200 × 80 Å | 270 × 80 Å | |
| Flexibility (Rflex value) (%) | 89.2 | 88.3 |
| Model | Experimental Conditions | Methods | Results | Ref. |
|---|---|---|---|---|
| HEK293 cells | 100 H2O2 at 4 °C for 30 min. | Comet Assay | DNA fragmentation was significantly suppressed in Dsup-expressing cells compared to control | [3] |
| Purified Dsup | 0.3% (v/v) H2O2 | Gel mobility shift analyses with mononucleosomes; ACF-mediated assembly of periodic nucleosome arrays; Hydroxyl radical-mediated cleavage of nucleosomal DNA | Dsup protected free DNA and chromatin from hydroxyl radicals; Dsup bound specifically to nucleosomes through a conserved region at C-terminal with sequence similarity to vertebrate HMGN proteins. | [7] |
| HEK293 cells | 250, 500 and 1000 μM H2O2 for 4 h or O/N | MTT metabolic assay (viability); ELISA (transcription factors); RT-qPCR (gene expression) | Increase survival; Modulation of transcription factors; Minor impact on DNA repair pathways; Increase of antioxidant mechanisms | [26] |
| Drosophila melanogaster | 9% (v/v) H2O2 added to medium | Measurement of survival and physiological parameters; Comet assay; Microarray (gene expression); gel mobility shift analysis, | Dsup increased the survival rate, but reduced the level of their locomotor activity; Downregulation of several genes involved in chromatin organisation and remodeling, and DNA transcription and regulation; Dsup could bind RNA. | [34] |
| Yeast | 4, 6, or 8 mM H2O2, 90 min at 30 °C for acute exposure, 3 days for chronic exposure | Colony counting Cytometry (Redox assay) ELISA (8-oxoG assay) Cleavage Under Targets & Release Using Nuclease (CUT&RUN) assay dCypher binding assays | Increase in survival in Dsup-expressing yeast compared to controls; Reduction in 8-oxoG in the presence of Dsup; No effect on the redox state of the yeast nucleus; HMGN-like domain was responsible for the interaction with nucleosomes, while the distal C-terminal sequences bound to DNA | [35] |
| HEK293 cells and yeast | 0.125, 0.25, 0.5, 1, 2, 4 mM H2O2 Viability assays: CCK-8 colorimetric assay (HEK293) and stainingwith Propidium Iodide (Yeast) MD simulations FLIM-FRET imaging | Molecular dynamics (MD) simulations and fluorescence lifetime imaging microscopy (FLIM)-Förster resonance energy transfer (FRET) in living cells | Dsup chains wrap around DNA and slows down the melting of DNA, acting as a physical barrier; Evidence of Dsup-DNA interactions in the nuclei of live mammalian cells | [36] |
| Model | Experimental Conditions | Methods | Results | Ref |
|---|---|---|---|---|
| Tobacco plants | UV-C lamp at a distance of 20 cm for 30 min (estimated exposure dosage was 3 kJ/m2) | Comet assay RT-qPCR (gene expression) | Nuclei from the Dsup-expressing plants were more protected from UV than the control plants. Gene expression analysis revealed the upregulation of genes involved in active DNA repair process in Dsup-expressing plants | [44] |
| HEK293 cells | 5 sec or 15 sec exposure to UV-C (source 8 W lamp, 4 mJ/cm2) | MTT assay (viability) T4 Endonuclease V enzyme assay (CDP evaluation) RT-qPCR (gene expression) ELISA (transcription factors) | Dsup-expressing cells showed a reduction in cell death and an increase in cell growth after UV-C exposure No CDP formation was observed Up-regulation of ATR-rescue pathway Modulation of transcription factors | [26] |
| HEK293 cells | 5 sec or 15 sec exposure to UV-C (source 8 W lamp, 4 mJ/cm2) | Proteomic Analysis MALDI-ToF Mass Spectrometry | Dsup activated mechanisms of DNA damage repair, telomere elongation and maintenance, mRNA stability, cytoplasmic stress granule and unfolded protein responses, in addition to metabolic modulation | [53] |
| Rice plants | 400 mW/m2, for continuously treatment over 8 h | electron microscopy RNA-seqencing IP-LC-MS | Dsup enhanced DNA damage resistance at the seed and seedling stages; Dsup increased grain size and altered starch granule structure and cell size; Increased expression of radiation- and abiotic stress-related genes (both after UV exposure and under normal conditions) | [61] |
| Tobacco Pollen | TL20W/12 lamps (UV-B wavelengths), 25 KJ m−2 d−1 | Evaluation of Pollen Tube Length, Position of Callose Plugs and Nuclei Fluorochrome reaction (FCR) test with fluorescein diacetate (FDA) dye (cell viability) ferric reducing antioxidant power (FRAP) assay (total antioxidant power) Folin–Ciocâlteu assay (total polyphenols content) aluminum chloride assay (total flavonoids content) | Dsup expression increased levels of antioxidants and restored the proper distance between the tip and the last callose plug formed, pollen tube length, tubulin, and HSP70 levels. | [62] |
| Model | Experimental Conditions | Methods | Results | Ref |
|---|---|---|---|---|
| HEK293 cells | 10 and 5 Gy for alkaline or neutral conditions, respectively (comet assay) 1 Gy of X-ray (gamma H2AX foci detection) | Comet Assay confocal microscopy (gamma H2AX) PrestoBlue Cell Viability Reagent (viability assay) incorporation of 5-bromo-2-deoxyuridine (BrdU) and flow cytometry (cell cycle analysis) | DNA fragmentation in Dsup-expressing cells was significantly suppressed compared to control Both SSBs and DSBs were reduced Dsup-expressing cells were more viable, able to proliferate and showed a normal morphology after irradiation | [3] |
| Tobacco plants | 80 kilovolts and 44 microamps at a distance of 188 mm for 2 min | Comet assay RT-qPCR (gene expression) | Nuclei from the Dsup-expressing plants were more protected from X-ray than the control plants; Gene expression analysis revealed the downregulation of ATM gene, involved in DSB signalling, and the upregulation of ATR gene, activated by SSBs, in Dsup-expressing plants. | [44] |
| HEK293 cells | 0.5 Gy, 2 Gy, and 4 Gy | BrdU Cell Proliferation ELISA Kit ELISA (8-oxoG assay) Capsase-3 and Annexin V binding assay (apoptosis) RNA-seq (gene expression) ATAC-seq (chromatin accessibility) Cleavage Under Targets & Release Using Nuclease (CUT&RUN) assay (histone modifications and Dsup binding) | Decrease of 8-oxoG; Increase of viability; Reduction of apoptosis; Transcriptomic profile similar to that observed with HMGN1 overexpression; Selective differential opening and closing of the chromatin; Global increase in histone post-translational modifications, indicative of open chromatin. | [75] |
| Drosophila melanogaster | 300 mGy/sec; obtained absorbed dose = 500, 1000 and 1500 Gy | Measurement of survival and physiological parameters Microarray (gene expression) | Dsup increased the survival rate, but reduced the level of their locomotor activity; Downregulation of several genes involved in DNA repair, neurogenesis and proteostasis, but also of genes related to response to irradiation. | [34] |
| E. coli | 0, 500, 1500 and 3000 Gy, at a dose rate of 12.6 Gy/min, up to two times | Colony-Forming Units (CFU) | Increase in survival of more than two orders of magnitude The in Dsup-transformed strain at the 3000 Gy dose, increasing over the course of the exposures; Almost complete survival when exposed up to 500 Gy. | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantara, S.; Regoli, T.; Ricci, C. Captain Tardigrade and Its Shield to Protect DNA. DNA 2025, 5, 27. https://doi.org/10.3390/dna5020027
Cantara S, Regoli T, Ricci C. Captain Tardigrade and Its Shield to Protect DNA. DNA. 2025; 5(2):27. https://doi.org/10.3390/dna5020027
Chicago/Turabian StyleCantara, Silvia, Tommaso Regoli, and Claudia Ricci. 2025. "Captain Tardigrade and Its Shield to Protect DNA" DNA 5, no. 2: 27. https://doi.org/10.3390/dna5020027
APA StyleCantara, S., Regoli, T., & Ricci, C. (2025). Captain Tardigrade and Its Shield to Protect DNA. DNA, 5(2), 27. https://doi.org/10.3390/dna5020027








