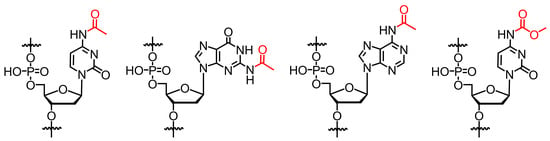
Synthesis of Sensitive Oligodeoxynucleotides Containing Acylated Cytosine, Adenine, and Guanine Nucleobases
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
5. Patent
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
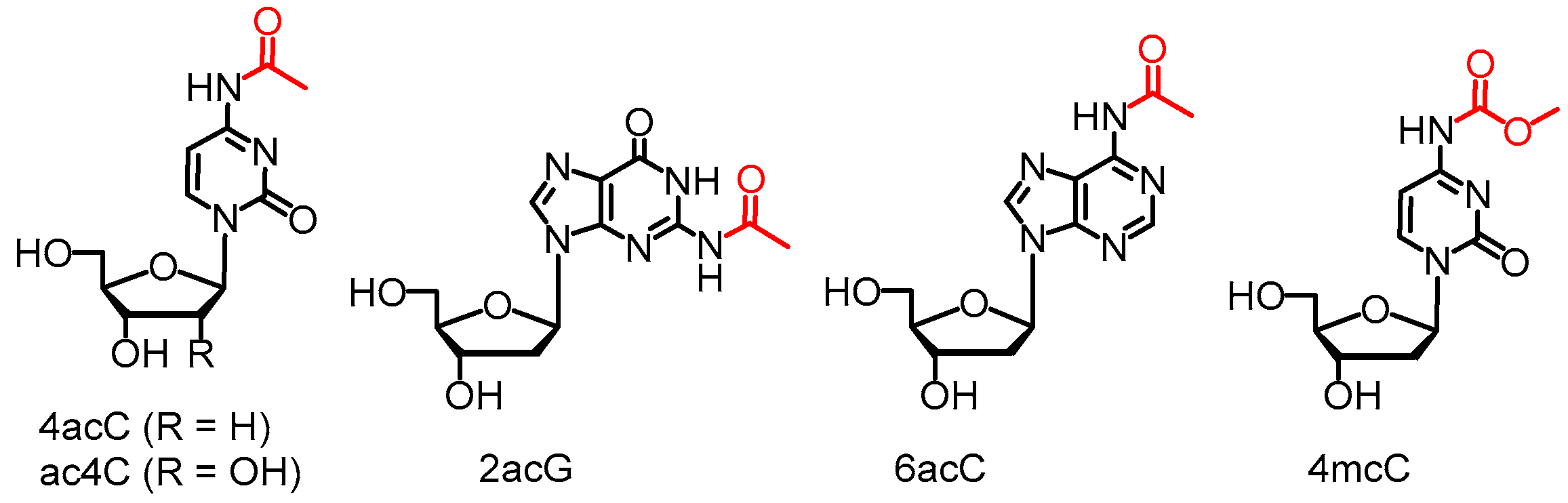
| 2acG | N2-acetylguanosine |
| 4acC | N4-acetyldeoxycytidine |
| 4mcC | N4-methyoxycarbonyldeoxycytidine |
| 6acA | N6-acetyladenosine |
| ACN | Acetonitrile |
| CE | Capillary electrophoresis |
| CPG | Controlled pore glass |
| DBU | 1,8-Diazabicyclo(5.4.0)undec-7-ene |
| DCA | Dichloroacetic acid |
| DCI | 4,5-Dicyanoimidazole |
| DCM | Dichloromethane |
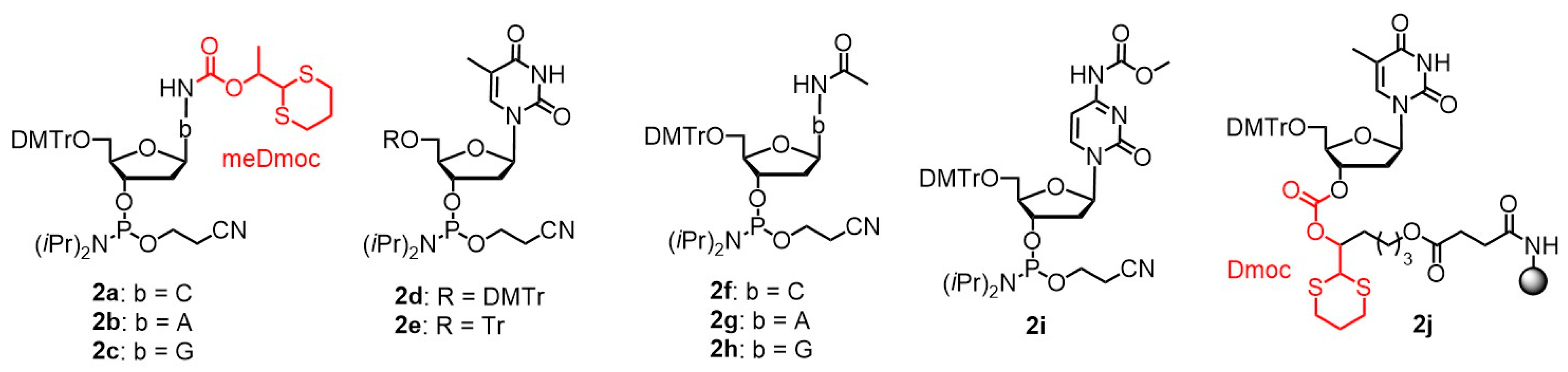
| Dmoc | 1,3-dithian-2-yl-methoxycarbonyl |
| DMTr | Dimethoxytrityl |
| meDmoc | Methyl Dmoc |
| ODN | Oligodeoxynucleotide |
| ON | Oligonucleotide |
| ORN | Oligoribonucleotide |
| Tr | Trityl |
References
- Luo, J.; Cao, J.; Chen, C.; Xie, H. Emerging role of RNA acetylation modification ac4C in diseases: Current advances and future challenges. Biochem. Pharmacol. 2023, 213, 115628. [Google Scholar] [CrossRef]
- Zhang, Y.; Lei, Y.; Dong, Y.; Chen, S.; Sun, S.; Zhou, F.; Zhao, Z.; Chen, B.; Wei, L.; Chen, J.; et al. Emerging roles of RNA ac4C modification and NAT10 in mammalian development and human diseases. Pharmacol. Ther. 2024, 253, 108576. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.H.; Zhong, X.L.; Cao, W.Y.; Liu, J.H.; Zu, X.Y.; Chen, L. Mechanisms of NAT10 as ac4C writer in diseases. Mol. Ther. Nucl. Acids 2023, 32, 359–368. [Google Scholar] [CrossRef]
- Gu, Z.R.; Zou, L.B.; Pan, X.J.; Yu, Y.; Liu, Y.Q.; Zhang, Z.J.; Liu, J.; Mao, S.Y.; Zhang, J.F.; Guo, C.C.; et al. The role and mechanism of NAT10-mediated ac4C modification in tumor development and progression. MedComm 2024, 5, e70026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.L.; Lu, R.H.; Huang, J.Y.; Li, L.; Cao, Y.T.; Huang, C.H.; Chen, R.; Wang, Y.L.; Huang, J.; Zhao, X.; et al. N4-acetylcytidine modifies primary micrornas for processing in cancer cells. Cell. Mol. Life Sci. 2024, 81, 73. [Google Scholar] [CrossRef]
- Wang, S.; Xie, H.; Mao, F.; Wang, H.; Wang, S.; Chen, Z.; Zhang, Y.; Xu, Z.; Xing, J.; Cui, Z.; et al. N(4)-acetyldeoxycytosine DNA modification marks euchromatin regions in arabidopsis thaliana. Genome Biol. 2022, 23, 5. [Google Scholar] [CrossRef]
- Zhou, J.X.; Wang, X.; Wei, Z.; Meng, J.; Huang, D.Y. 4accpred: Weakly supervised prediction of λl4-acetyldeoxycytosine DNA modification from sequences. Mol. Ther. Nucl. Acids 2022, 30, 337–345. [Google Scholar] [CrossRef]
- Meng, W.Y.; Wang, Z.X.; Zhang, Y.F.; Hou, Y.J.; Xue, J.H. Epigenetic marks or not? The discovery of novel DNA modifications in eukaryotes. J. Biol. Chem. 2024, 300, 106791. [Google Scholar] [CrossRef]
- Wada, T.; Kobori, A.; Kawahara, S.; Sekine, M. Synthesis and properties of oligodeoxyribonucleotides containing 4-acetylcytosine bases. Tetrahedron Lett. 1998, 39, 6907–6910. [Google Scholar] [CrossRef]
- Kobori, A.; Miyata, K.; Ushioda, M.; Seio, K.; Sekine, M. A new method for the synthesis of oligodeoxyribonucleotides containing 4-n-alkoxycarbonyldeoxycytidine derivatives and their hybridization properties. J. Org. Chem. 2002, 67, 476–485. [Google Scholar] [CrossRef]
- Bartee, D.; Nance, K.D.; Meier, J.L. Site-specific synthesis of n(4)-acetylcytidine in RNA reveals physiological duplex stabilization. J. Am. Chem. Soc. 2022, 144, 3487–3496. [Google Scholar] [CrossRef] [PubMed]
- Arango, D.; Sturgill, D.; Alhusaini, N.; Dillman, A.A.; Sweet, T.J.; Hanson, G.; Hosogane, M.; Sinclair, W.R.; Nanan, K.K.; Mandler, M.D.; et al. Acetylation of cytidine in mRNA promotes translation efficiency. Cell 2018, 175, 1872–1886.e1824. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.F.; Cui, X.; Min, J.; Lin, Z.G.; Zhou, Y.Y.; Guo, M.J.; An, X.J.; Liu, H.; Janz, S.; Gu, C.Y.; et al. NAT10 promotes cell proliferation by acetylating mRNA to enhance translation efficiency in multiple myeloma. Acta Pharm. Sin. B 2022, 12, 3313–3325. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Yang, C.X.; Zhang, J.; Zhao, K.; Li, P.; Kong, C.Y.; Wu, X.G.; Sun, H.L.; Zheng, R.; Sun, W.; et al. NAT10 is involved in cardiac remodeling through ac4C-mediated transcriptomic regulation. Circ. Res. 2023, 133, 989–1002. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, L.; Chen, S.; Liu, H. A brief review of RNA modification related database resources. Methods 2022, 203, 342–353. [Google Scholar] [CrossRef]
- Xiong, J.; Wu, J.Y.; Liu, Y.; Feng, Y.J.; Yuan, B.F. Quantification and mapping of RNA modifications. Trac-Trends Anal. Chem. 2024, 172, 117606. [Google Scholar] [CrossRef]
- Li, J.X.; Wang, X.S.; Wang, H.S. RNA modifications in long non-coding RNAs and their implications in cancer biology. Bioorg. Med. Chem. 2024, 113, 117922. [Google Scholar] [CrossRef]
- Pan, X.Q.; Bruch, A.; Blango, M.G. Past, present, and future of RNA modifications in infectious disease research. ACS Infect. Dis. 2024, 10, 4017–4029. [Google Scholar] [CrossRef]
- Shahsavari, S.; Eriyagama, D.N.A.M.; Chen, J.S.; Halami, B.; Yin, Y.P.; Chillar, K.; Fang, S.Y. Sensitive oligodeoxynucleotide synthesis using Dim and Dmoc as protecting groups. J. Org. Chem. 2019, 84, 13374–13383. [Google Scholar] [CrossRef]
- Chillar, K.; Eriyagama, A.; Yin, Y.; Shahsavari, S.; Halami, B.; Apostle, A.; Fang, S. Oligonucleotide synthesis under mild deprotection conditions. New J. Chem. 2023, 47, 8714–8722. [Google Scholar] [CrossRef]
- Chillar, K.; Yin, Y.; Apostle, A.; Fang, S. PEGylated Dmoc phosphoramidites for sensitive oligodeoxynucleotide synthesis. Org. Biomol. Chem. 2023, 21, 9005–9010. [Google Scholar] [CrossRef] [PubMed]
- Apostle, A.; Perera, M.; Middleton, D.; Fang, S. Sensitive RNA synthesis using fluoride-cleavable groups for linking and amino protection. ChemRxiv 2024. [Google Scholar] [CrossRef]
- Dreeftromp, C.M.; Hoogerhout, P.; Vandermarel, G.A.; Vanboom, J.H. A new protected acyl protecting group for exocyclic amino functions of nucleobases. Tetrahedron Lett. 1990, 31, 427–430. [Google Scholar] [CrossRef]
- Guerlavais-Dagland, T.; Meyer, A.; Imbach, J.L.; Morvan, F. Fluoride-labile protecting groups for the synthesis of base-sensitive methyl-sate oligonucleotide prodrugs. Eur. J. Org. Chem. 2003, 2003, 2327–2335. [Google Scholar] [CrossRef]
- Lin, X.; Chen, J.; Shahsavari, S.; Green, N.; Goyal, D.; Fang, S. Synthesis of oligodeoxynucleotides containing electrophilic groups. Org. Lett. 2016, 18, 3870–3873. [Google Scholar] [CrossRef]
- Pals, M.J.; Lindberg, A.; Velema, W.A. Chemical strategies for antisense antibiotics. Chem. Soc. Rev. 2024, 53, 11303–11320. [Google Scholar] [CrossRef]
- Kim, Y. Drug discovery perspectives of antisense oligonucleotides. Biomol. Ther. 2023, 31, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Redhwan, M.A.M.; Hariprasad, M.G.; Samaddar, S.; Hard, S.A.A.A.; Yadav, V.; Mukherjee, A.; Kumar, R. Small interference (rnai) technique: Exploring its clinical applications, benefits and limitations. Eur. J. Clin. Investig. 2023, 53, e14039. [Google Scholar] [CrossRef]
- Kara, G.; Calin, G.A.; Ozpolat, B. Rnai-based therapeutics and tumor targeted delivery in cancer. Adv. Drug Deliver. Rev. 2022, 182, 114113. [Google Scholar] [CrossRef]
- Barber, H.M.; Pater, A.A.; Gagnon, K.T.; Damha, M.J.; O’reilly, D. Chemical engineering of CRISPR-cas systems for therapeutic application. Nat. Rev. Drug Discov. 2024, 24, 209–230. [Google Scholar] [CrossRef]
- Sahranavard, T.; Mehrabadi, S.; Pourali, G.; Maftooh, M.; Akbarzade, H.; Hassanian, S.M.; Mobarhan, M.G.; Ferns, G.A.; Khazaei, M.; Avan, A. The potential therapeutic applications of CRISPR/Cas9 in colorectal cancer. Curr. Med. Chem. 2024, 31, 5768–5778. [Google Scholar] [CrossRef] [PubMed]
- He, Y.J.; Johnston, A.P.R.; Pouton, C.W. Therapeutic applications of cell engineering using mRNA technology. Trends Biotechnol. 2025, 43, 83–97. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, H.F.; Gan, J.J.; Liang, G.F.; Li, L.; Zhao, Y.J. Engineered mRNA delivery systems for biomedical applications. Adv. Mater. 2024, 36, e2308029. [Google Scholar] [CrossRef] [PubMed]
- Chillar, K.; Yin, Y.; Eriyagama, A.M.D.N.; Fang, S. Determination of optical density (od) of oligodeoxynucleotide from HPLC peak area. PeerJ Anal. Chem. 2022, 4, e20. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, M.; Berndt, F.; Mahrwald, R.; Ernsting, N.P.; Wagenknecht, H.A. Synthesis of 4-aminophthalimide and 2,4-diaminopyrimidine c-nucleosides as isosteric fluorescent DNA base substitutes. J. Org. Chem. 2013, 78, 2589–2599. [Google Scholar] [CrossRef]
- Miura, F.; Fujino, T.; Kogashi, K.; Shibata, Y.; Miura, M.; Isobe, H.; Ito, T. Triazole linking for preparation of a next-generation sequencing library from single-stranded DNA. Nucleic Acids Res. 2018, 46, e95. [Google Scholar] [CrossRef]
- Alawneh, A.; Caruthers, M. Synthesis and Biological Activity of Phosphoramidate DNA/RNA Duplexes. Patent applicationt WO2019/241729 A1, 19 December 2019. [Google Scholar]
- Raynaud, F.; Asseline, U.; Roig, V.; Thuong, N.T. Synthesis and characterization of o-6-modified deoxyguanosine-containing oligodeoxyribonucleotides for triple-helix formation. Tetrahedron 1996, 52, 2047–2064. [Google Scholar] [CrossRef]
- Fan, Y.; Gaffney, B.L.; Jones, R.A. Transient silylation of the guanosine o6 and amino groups facilitates-acylation. Org. Lett. 2004, 6, 2555–2557. [Google Scholar] [CrossRef]
- Miyata, K.; Kobori, A.; Tamamushi, R.; Ohkubo, A.; Taguchi, H.; Seio, K.; Sekine, M. Conformational studies of 4-carbamoyldeoxycytidine derivatives and synthesis and hybridization properties of oligodeoxyribonucleotides incorporating these modified bases. Eur. J. Org. Chem. 2006, 2006, 3626–3637. [Google Scholar] [CrossRef]
- Palacio, C.M.; Sabaini, M.B.; Iribarren, A.M.; Iglesias, L.E. An efficient and mild access to acetyl protected purine nucleosides based on a chemoselective enzymatic hydrolysis. J. Biotechnol. 2013, 165, 99–101. [Google Scholar] [CrossRef]

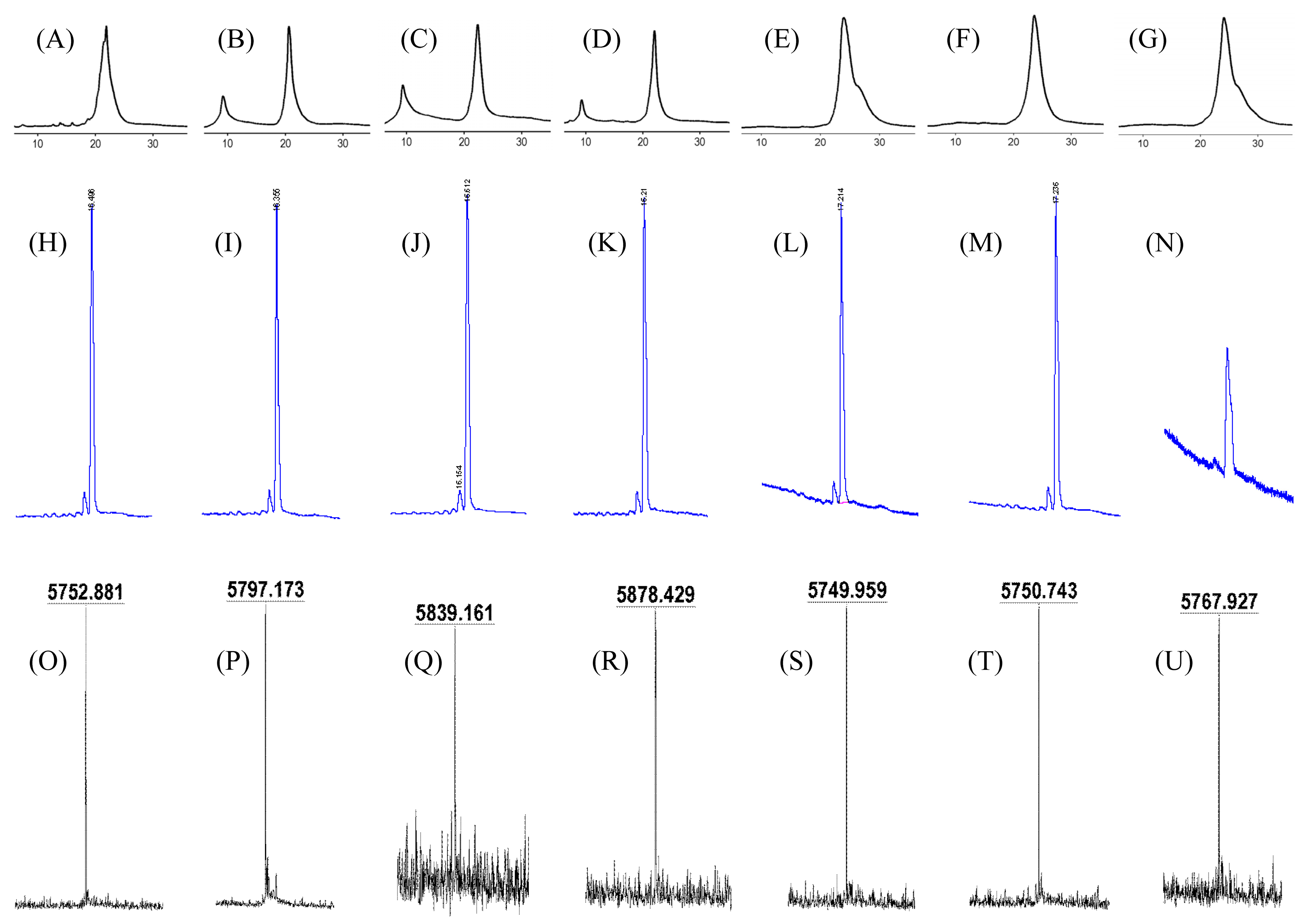
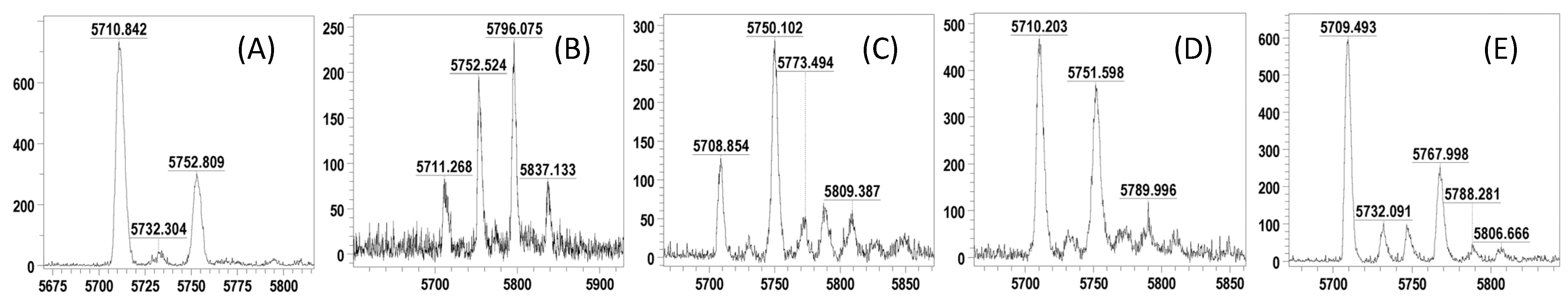
| Entry | ODN | Sequences | OD260 [a] | MALDI MS | |
|---|---|---|---|---|---|
| Calculated | Found | ||||
| 1 | 1a | 5′-TAGTA4acCTTTATCCAACCTT-3′ | 1.13 | [M−H]− 5753.0 | 5752.9 |
| 2 | 1b | 5′-TAGTACTTTAT4acCCAA4acCCTT-3′ | 1.04 | [M+H]+ 5796.0 | 5797.2 |
| 3 | 1c | 5′-TAGTA4acCTTTAT4acCCAA4acCCTT-3′ | 0.53 | [M+H]+ 5839.0 | 5839.2 |
| 4 | 1d | 5′-TAGTA4acCTTTAT4acCCAA4acC4acCTT-3′ | 0.37 | [M−H]− 5879.0 | 5878.4 |
| 5 | 1e | 5′-TA2acGTACTTTATCCAACCTT-3′ | 3.22 | [M−H]− 5753.0 | 5750.0 |
| 6 | 1f | 5′-TAGT6acACTTTATCCAACCTT-3′ | 1.07 | [M−H]− 5753.0 | 5750.7 |
| 7 | 1g | 5′-TAGTA4mcCTTTATCCAACCTT-3′ | 1.84 | [M−H]− 5769.0 | 5767.9 |
| 8 | 1h | 5′-TAGTACTTTATCCAACCTT-3′ | - | [M−H]− 5711.0 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chillar, K.; Awasthy, R.; Tanasova, M.; Fang, S. Synthesis of Sensitive Oligodeoxynucleotides Containing Acylated Cytosine, Adenine, and Guanine Nucleobases. DNA 2025, 5, 25. https://doi.org/10.3390/dna5020025
Chillar K, Awasthy R, Tanasova M, Fang S. Synthesis of Sensitive Oligodeoxynucleotides Containing Acylated Cytosine, Adenine, and Guanine Nucleobases. DNA. 2025; 5(2):25. https://doi.org/10.3390/dna5020025
Chicago/Turabian StyleChillar, Komal, Rohith Awasthy, Marina Tanasova, and Shiyue Fang. 2025. "Synthesis of Sensitive Oligodeoxynucleotides Containing Acylated Cytosine, Adenine, and Guanine Nucleobases" DNA 5, no. 2: 25. https://doi.org/10.3390/dna5020025
APA StyleChillar, K., Awasthy, R., Tanasova, M., & Fang, S. (2025). Synthesis of Sensitive Oligodeoxynucleotides Containing Acylated Cytosine, Adenine, and Guanine Nucleobases. DNA, 5(2), 25. https://doi.org/10.3390/dna5020025